Sox2 Ablation in the Suprachiasmatic Nucleus Perturbs Anxiety- and Depressive-like Behaviors
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Behavioral Paradigms
2.2.1. Forced Swim Test (FST)
2.2.2. Tail Suspension Test (TST)
2.2.3. Elevated Plus Maze (EPM)
2.2.4. Sucrose Preference Test (SPT)
2.3. Brain Tissue Harvest and Processing
2.4. Immunohistochemistry (IHC)
2.5. Image Acquisition and Analysis
2.6. Statistical Analysis
3. Results
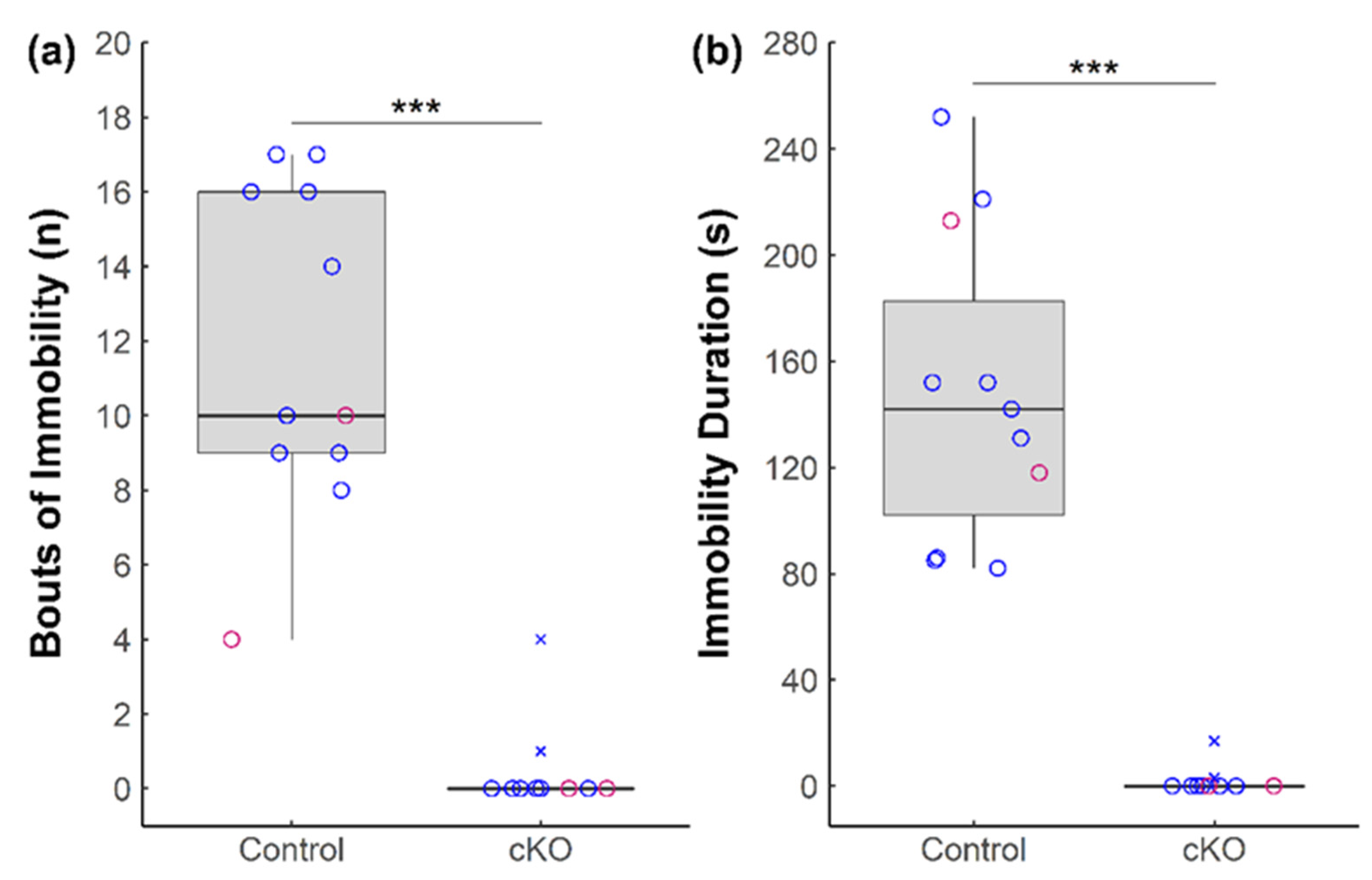
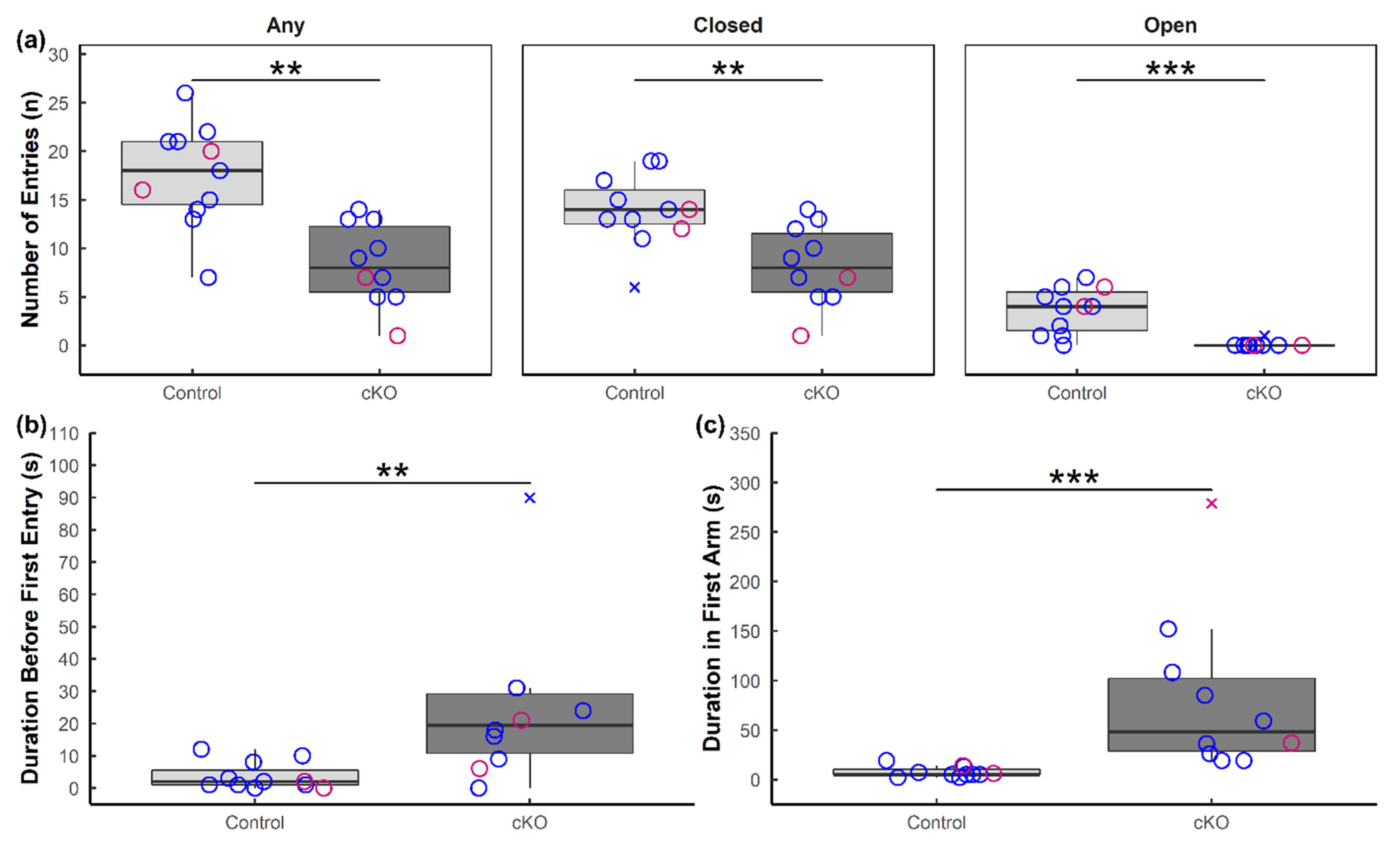
3.1. Ablation of Sox2 in the SCN Severely Alters Anxiety-like and Depressive-like Behaviors in Mice
3.2. Altered Neuronal Activation in Sox2 cKO Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Steel, Z.; Marnane, C.; Iranpour, C.; Chey, T.; Jackson, J.W.; Patel, V.; Silove, D. The global prevalence of common mental disorders: A systematic review and meta-analysis 1980–2013. Int. J. Epidemiol. 2014, 43, 476–493. [Google Scholar] [CrossRef] [PubMed]
- Baxter, A.J.; Scott, K.M.; Vos, T.; Whiteford, H.A. Global prevalence of anxiety disorders: A systematic review and meta-regression. Psychol. Med. 2013, 43, 897–910. [Google Scholar] [CrossRef] [PubMed]
- Case, A.; Deaton, A. Rising morbidity and mortality in midlife among white non-Hispanic Americans in the 21st century. Proc. Natl. Acad. Sci. USA 2015, 112, 15078–15083. [Google Scholar] [CrossRef] [PubMed]
- Mojtabai, R.; Olfson, M.; Han, B. National trends in the prevalence and treatment of depression in adolescents and young adults. Pediatrics 2016, 138. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, R.D.; Weinberger, A.H.; Kim, J.H.; Wu, M.; Galea, S. Trends in anxiety among adults in the United States, 2008–2018: Rapid increases among young adults. J. Psychiatr. Res. 2020, 130, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Patriquin, M.A.; Mathew, S.J. The Neurobiological Mechanisms of Generalized Anxiety Disorder and Chronic Stress. Chronic Stress 2017, 1. [Google Scholar] [CrossRef]
- Fox, M.E.; Lobo, M.K. The molecular and cellular mechanisms of depression: A focus on reward circuitry. Mol. Psychiatry 2019, 24, 1798–1815. [Google Scholar] [CrossRef] [PubMed]
- Pittendrigh, C.S. Temporal Organization: Reflections of a Darwinian Clock-Watcher. Annu. Rev. Physiol. 1993, 55, 17–54. [Google Scholar] [CrossRef]
- Robillard, R.; Carpenter, J.S.; Rogers, N.L.; Fares, S.; Grierson, A.B.; Hermens, D.F.; Naismith, S.L.; Mullin, S.J.; Feilds, K.L.; Glozier, N.; et al. Circadian rhythms and psychiatric profiles in young adults with unipolar depressive disorders. Transl. Psychiatry 2018, 8, 213. [Google Scholar] [CrossRef]
- Barandas, R.; Landgraf, D.; McCarthy, M.J.; Welsh, D.K. Circadian Clocks as Modulators of Metabolic Comorbidity in Psychiatric Disorders. Curr. Psychiatry Rep. 2015, 17. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, J.; Vanotti, G. Circadian neurogenetics of mood disorders. Cell Tissue Res. 2019, 377, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Berson, D.M.; Dunn, F.A.; Takao, M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002, 295, 1070–1073. [Google Scholar] [CrossRef] [PubMed]
- Hegazi, S.; Lowden, C.; Garcia, J.R.; Cheng, A.H.; Obrietan, K.; Levine, J.D.; Cheng, H.Y.M. A symphony of signals: Intercellular and intracellular signaling mechanisms underlying circadian timekeeping in mice and flies. Int. J. Mol. Sci. 2019, 20, 2363. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Viveros, L.; Bouchard-Cannon, P.; Hegazi, S.; Cheng, A.H.; Pastore, S.; Cheng, H.Y.M. Molecular modulators of the circadian clock: Lessons from flies and mice. Cell. Mol. Life Sci. 2017, 74, 1035–1059. [Google Scholar] [CrossRef]
- Hoefflin, S.; Carter, D.A. Neuronal expression of SOX2 is enriched in specific hypothalamic cell groups. J. Chem. Neuroanat. 2014, 61, 153–160. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheng, A.H.; Bouchard-Cannon, P.; Hegazi, S.; Lowden, C.; Fung, S.W.; Chiang, C.K.; Ness, R.W.; Cheng, H.Y.M. SOX2-Dependent Transcription in Clock Neurons Promotes the Robustness of the Central Circadian Pacemaker. Cell Rep. 2019, 26, 3191–3202.e8. [Google Scholar] [CrossRef] [PubMed]
- Can, A.; Dao, D.T.; Arad, M.; Terrillion, C.E.; Piantadosi, S.C.; Gould, T.D. The mouse forced swim test. J. Vis. Exp. 2011, 3638. [Google Scholar] [CrossRef]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology 1985, 85, 367–370. [Google Scholar] [CrossRef]
- Walf, A.A.; Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, G.; Li, H.; Tan, X.; Cheng, H.Y.M. Environmental perturbation of the circadian clock during pregnancy leads to transgenerational mood disorder-like behaviors in mice. Sci. Rep. 2017, 7, 12641. [Google Scholar] [CrossRef]
- Matthews, K.; Forbes, N.; Reid, I.C. Sucrose consumption as an hedonic measure following chronic unpredictable mild stress. Physiol. Behav. 1995, 57, 241–248. [Google Scholar] [CrossRef]
- Der-Avakian, A.; Markou, A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012, 35, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-Y.; Yin, C.-Y.; Zhu, L.-J.; Zhu, X.-H.; Xu, C.; Luo, C.-X.; Chen, H.; Zhu, D.-Y.; Zhou, Q.-G. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat. Protoc. 2018, 13, 1686–1698. [Google Scholar] [CrossRef] [PubMed]
- Scheggi, S.; De Montis, M.G.; Gambarana, C. Making sense of rodent models of anhedonia. Int. J. Neuropsychopharmacol. 2018, 21, 1049–1065. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Gundlach, A.L. Ascending Control of Arousal and Motivation: Role of Nucleus Incertus and its Peptide Neuromodulators in Behavioural Responses to Stress. J. Neuroendocrinol. 2015, 27, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.J.; Ma, S.; Olucha-Bordonau, F.E.; Gundlach, A.L. Nucleus incertus-An emerging modulatory role in arousal, stress and memory. Neurosci. Biobehav. Rev. 2011, 35, 1326–1341. [Google Scholar] [CrossRef]
- Leon-Mercado, L.; Chao, D.H.M.; del Carmen Basualdo, M.; Kawata, M.; Escobar, C.; Buijs, R.M. The arcuate nucleus: A site of fast negative feedback for corticosterone secretion in male rats. eNeuro 2017, 4, 350–366. [Google Scholar] [CrossRef] [PubMed]
- Buijs, F.N.; Guzmán-Ruiz, M.; León-Mercado, L.; Basualdo, M.C.; Escobar, C.; Kalsbeek, A.; Buijs, R.M. Suprachiasmatic nucleus interaction with the arcuate nucleus; Essential for organizing physiological rhythms. eNeuro 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Toda, T.; Parylak, S.L.; Linker, S.B.; Gage, F.H. The role of adult hippocampal neurogenesis in brain health and disease. Mol. Psychiatry 2019, 24, 67–87. [Google Scholar] [CrossRef]
- Vadnie, C.A.; McClung, C.A. Circadian Rhythm Disturbances in Mood Disorders: Insights into the Role of the Suprachiasmatic Nucleus. Neural Plast. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.H.; Walton, J.C.; DeVries, A.C.; Nelson, R.J. Circadian rhythm disruption and mental health. Transl. Psychiatry 2020, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Flo, E.; Pallesen, S.; Magerøy, N.; Moen, B.E.; Grønli, J.; Nordhus, I.H.; Bjorvatn, B. Shift work disorder in nurses—Assessment, prevalence and related health problems. PLoS ONE 2012, 7, e33981. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.J.; Lee, S.H.; Lee, H.S.; Lee, K.J.; Kim, J.J. The association between shift work and depression in hotel workers. Ann. Occup. Environ. Med. 2015, 27, 29. [Google Scholar] [CrossRef]
- Lee, A.; Myung, S.K.; Cho, J.J.; Jung, Y.J.; Yoon, J.L.; Kim, M.Y. Night shift work and risk of depression: Meta-analysis of observational studies. J. Korean Med. Sci. 2017, 32, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Booker, L.A.; Sletten, T.L.; Alvaro, P.K.; Barnes, M.; Collins, A.; Chai-Coetzer, C.L.; Naqvi, A.; McMahon, M.; Lockley, S.W.; Rajaratnam, S.M.W.; et al. Exploring the associations between shift work disorder, depression, anxiety and sick leave taken amongst nurses. J. Sleep Res. 2020, 29, e12872. [Google Scholar] [CrossRef] [PubMed]
- Ben-Hamo, M.; Larson, T.A.; Duge, L.S.; Sikkema, C.; Wilkinson, C.W.; De La Iglesia, H.O.; González, M.M.C. Circadian Forced Desynchrony of the Master Clock Leads to Phenotypic Manifestation of Depression in Rats. eNeuro 2017, 3. [Google Scholar] [CrossRef]
- Landgraf, D.; Long, J.E.; Proulx, C.D.; Barandas, R.; Malinow, R.; Welsh, D.K. Genetic Disruption of Circadian Rhythms in the Suprachiasmatic Nucleus Causes Helplessness, Behavioral Despair, and Anxiety-like Behavior in Mice. Biol. Psychiatry 2016, 80, 827–835. [Google Scholar] [CrossRef] [PubMed]
- De Bundel, D.; Gangarossa, G.; Biever, A.; Bonnefont, X.; Valjent, E. Cognitive dysfunction, elevated anxiety, and reduced cocaine response in circadian clock-deficient cryptochrome knockout mice. Front. Behav. Neurosci. 2013, 7, 152. [Google Scholar] [CrossRef] [PubMed]
- Spencer, S.; Falcon, E.; Kumar, J.; Krishnan, V.; Mukherjee, S.; Birnbaum, S.G.; Mcclung, C.A. Circadian genes Period 1 and Period 2 in the nucleus accumbens regulate anxiety-related behavior. Eur. J. Neurosci. 2013, 37, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiao, X.; Wang, L.; Wang, Q.; Liang, R.; Zheng, C.; Yang, J.; Ming, D. Comparison effects of chronic sleep deprivation on juvenile and young adult mice. J. Sleep Res. 2021, e13399. [Google Scholar] [CrossRef]
- Yankelevitch-Yahav, R.; Franko, M.; Huly, A.; Doron, R. The forced swim test as a model of depressive-like behavior. J. Vis. Exp. 2015, 2015, 52587. [Google Scholar] [CrossRef] [PubMed]
- de Kloet, E.R.; Molendijk, M.L. Coping with the Forced Swim Stressor: Towards Understanding an Adaptive Mechanism. Neural Plast. 2016, 2016, 6503162. [Google Scholar] [CrossRef]
- Anyan, J.; Amir, S. Too Depressed to Swim or Too Afraid to Stop? A Reinterpretation of the Forced Swim Test as a Measure of Anxiety-Like Behavior. Neuropsychopharmacology 2018, 43, 931–933. [Google Scholar] [CrossRef] [PubMed]
- Shankman, S.A.; Katz, A.C.; DeLizza, A.A.; Sarapas, C.; Gorka, S.M.; Campbell, M.L. The Different Facets of Anhedonia and Their Associations with Different Psychopathologies. Anhedonia A Compr. Handb. Vol. I Concept. Issues Neurobiol. Adv. 2014, 3–22. [Google Scholar] [CrossRef]
- Liu, J.; Garza, J.C.; Truong, H.V.; Henschel, J.; Zhang, W.; Lu, X.Y. The melanocortinergic pathway is rapidly recruited by emotional stress and contributes to stress-induced anorexia and anxiety-like behavior. Endocrinology 2007, 148, 5531–5540. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Shen, P.J.; Ma, S.; Bathgate, R.A.D.; Gundlach, A.L. Swim stress excitation of nucleus incertus and rapid induction of relaxin-3 expression via CRF1 activation. Neuropharmacology 2010, 58, 145–155. [Google Scholar] [CrossRef]
- Walker, L.C.; Lawrence, A.J. CRF and the nucleus incertus: A node for integration of stress signals. Nat. Rev. Neurosci. 2017, 18, 158. [Google Scholar] [CrossRef] [PubMed]
- de Ávila, C.; Chometton, S.; Ma, S.; Pedersen, L.T.; Timofeeva, E.; Cifani, C.; Gundlach, A.L. Effects of chronic silencing of relaxin-3 production in nucleus incertus neurons on food intake, body weight, anxiety-like behaviour and limbic brain activity in female rats. Psychopharmacology 2020, 237, 1091–1106. [Google Scholar] [CrossRef]
- Zhang, C.; Chua, B.E.; Yang, A.; Shabanpoor, F.; Hossain, M.A.; Wade, J.D.; Rosengren, K.J.; Smith, C.M.; Gundlach, A.L. Central relaxin-3 receptor (RXFP3) activation reduces elevated, but not basal, anxiety-like behaviour in C57BL/6J mice. Behav. Brain Res. 2015, 292, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.J.; Büchler, E.; Shabanpoor, F.; Hossain, M.A.; Wade, J.D.; Lawrence, A.J.; Gundlach, A.L. Central relaxin-3 receptor (RXFP3) activation decreases anxiety- and depressive-like behaviours in the rat. Behav. Brain Res. 2013, 244, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.R.; Rajkumar, R.; Jayakody, T.; Marwari, S.; Hong, J.M.; Ma, S.; Gundlach, A.L.; Lai, M.K.P.; Dawe, G.S. Relaxin’ the brain: A case for targeting the nucleus incertus network and relaxin-3/RXFP3 system in neuropsychiatric disorders. Br. J. Pharmacol. 2017, 174, 1061–1076. [Google Scholar] [CrossRef] [PubMed]
- Qu, N.; He, Y.; Wang, C.; Xu, P.; Yang, Y.; Cai, X.; Liu, H.; Yu, K.; Pei, Z.; Hyseni, I.; et al. A POMC-originated circuit regulates stress-induced hypophagia, depression, and anhedonia. Mol. Psychiatry 2020, 25, 1006–1021. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hou, Y.; Zhang, J.; Sui, G.; Du, X.; Licinio, J.; Wong, M.L.; Yang, Y. AGRP neurons modulate fasting-induced anxiolytic effects. Transl. Psychiatry 2019, 9, 111. [Google Scholar] [CrossRef]
- Cheng, A.H.; Bouchard-Cannon, P.; Ness, R.W.; Cheng, H.Y.M. RNA-sequencing data highlighting the time-of-day-dependent transcriptome of the central circadian pacemaker in Sox2-deficient mice. Data Br. 2019, 24, 103909. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Hernández, R.; Escobar, C.; Buijs, R.M. Suprachiasmatic Nucleus–Arcuate Nucleus Axis: Interaction Between Time and Metabolism Essential for Health. Obesity 2020, 28, S10–S17. [Google Scholar] [CrossRef] [PubMed]
- Da Li, J.; Hu, W.P.; Zhou, Q.Y. Disruption of the circadian output molecule prokineticin 2 results in anxiolytic and antidepressant-like effects in mice. Neuropsychopharmacology 2009, 34, 367–373. [Google Scholar] [CrossRef]
- Kishi, T.; Kitajima, T.; Tsunoka, T.; Okumura, T.; Ikeda, M.; Okochi, T.; Kinoshita, Y.; Kawashima, K.; Yamanouchi, Y.; Ozaki, N.; et al. Possible Association of Prokineticin 2 Receptor Gene (PROKR2) with Mood Disorders in the Japanese Population. NeuroMol. Med. 2009, 11, 114–122. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boehler, N.A.; Fung, S.W.; Hegazi, S.; Cheng, A.H.; Cheng, H.-Y.M. Sox2 Ablation in the Suprachiasmatic Nucleus Perturbs Anxiety- and Depressive-like Behaviors. Neurol. Int. 2021, 13, 541-554. https://doi.org/10.3390/neurolint13040054
Boehler NA, Fung SW, Hegazi S, Cheng AH, Cheng H-YM. Sox2 Ablation in the Suprachiasmatic Nucleus Perturbs Anxiety- and Depressive-like Behaviors. Neurology International. 2021; 13(4):541-554. https://doi.org/10.3390/neurolint13040054
Chicago/Turabian StyleBoehler, Nicholas A., Samuel W. Fung, Sara Hegazi, Arthur H. Cheng, and Hai-Ying Mary Cheng. 2021. "Sox2 Ablation in the Suprachiasmatic Nucleus Perturbs Anxiety- and Depressive-like Behaviors" Neurology International 13, no. 4: 541-554. https://doi.org/10.3390/neurolint13040054
APA StyleBoehler, N. A., Fung, S. W., Hegazi, S., Cheng, A. H., & Cheng, H.-Y. M. (2021). Sox2 Ablation in the Suprachiasmatic Nucleus Perturbs Anxiety- and Depressive-like Behaviors. Neurology International, 13(4), 541-554. https://doi.org/10.3390/neurolint13040054





