Hydrocephalus and Cerebrospinal Fluid Analysis Following Severe Traumatic Brain Injury: Evaluation of a Prospective Cohort
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Acquisition and Clinical Outcomes
2.3. Statistical Analysis
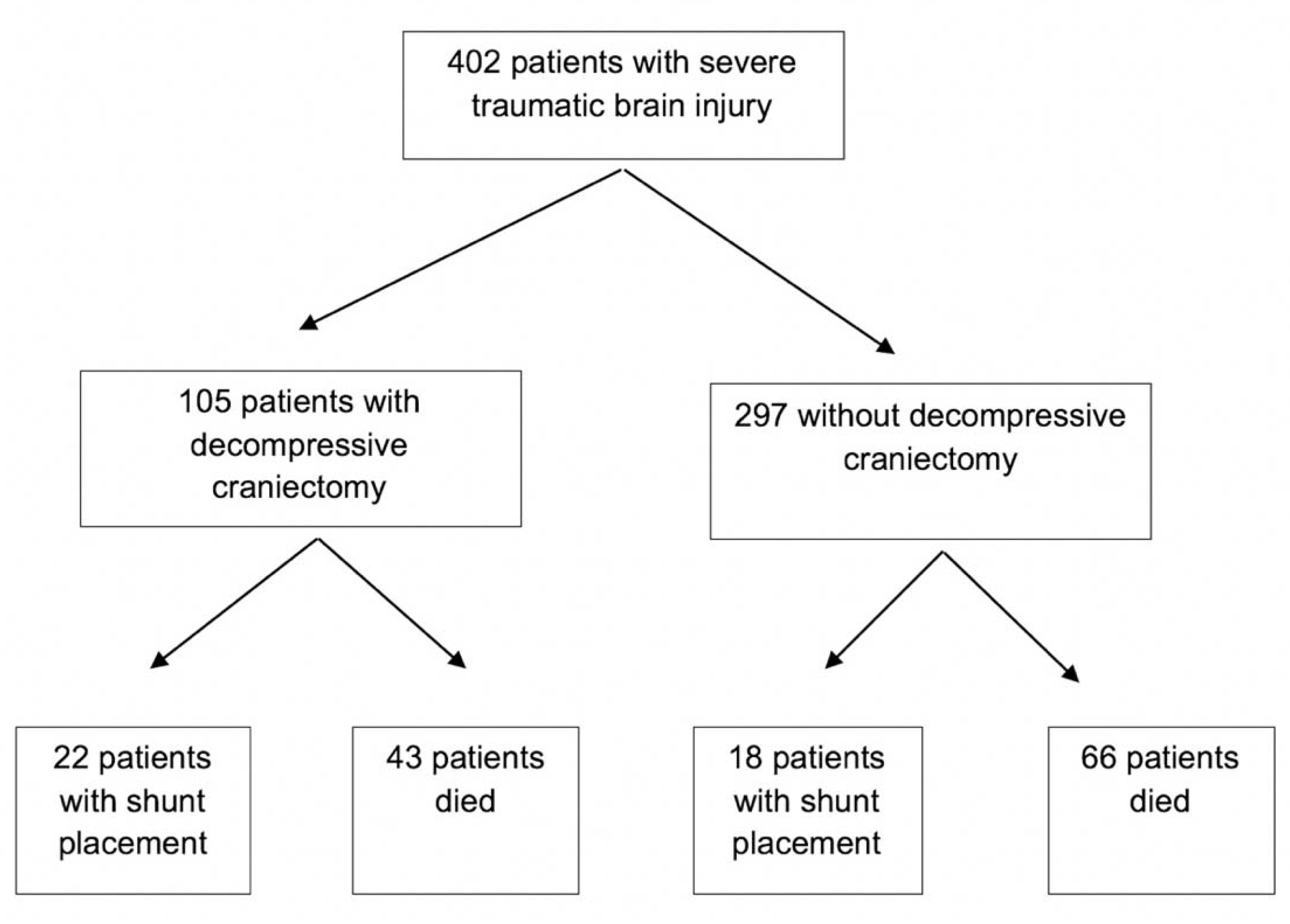
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vedantam, A.; Yamal, J.-M.; Hwang, H.; Robertson, C.S.; Gopinath, S.P. Factors associated with shunt-dependent hydrocephalus after decompressive craniectomy for traumatic brain injury. J. Neurosurg. 2018, 128, 1547–1552. [Google Scholar] [CrossRef] [PubMed]
- Di, G.; Hu, Q.; Liu, D.; Jiang, X.; Chen, J.; Liu, H. Risk Factors Predicting Posttraumatic Hydrocephalus After Decompressive Craniectomy in Traumatic Brain Injury. World Neurosurg. 2018, 116, e406–e413. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, A.H.; Gerber, D.J.; Kowalski, R.G. Posttraumatic Hydrocephalus as a Confounding Influence on Brain Injury Rehabilitation: Incidence, Clinical Characteristics, and Outcomes. Arch. Phys. Med. Rehabil. 2017, 98, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt, E.; Deng, H.; Puccio, A.M.; Okonkwo, D.O. Post-traumatic hydrocephalus following decompressive hemicraniectomy: Incidence and risk factors in a prospective cohort of severe TBI patients. J. Clin. Neurosci. 2020, 73, 85–88. [Google Scholar] [CrossRef]
- Aarabi, B.; Chesler, D.; Maulucci, C.; Blacklock, T.; Alexander, M. Dynamics of subdural hygroma following decompressive craniectomy: A comparative study. Neurosurg. Focus 2009, 26, E8. [Google Scholar] [CrossRef]
- Nasi, D.; Gladi, M.; Di Rienzo, A.; di Somma, L.; Moriconi, E.; Iacoangeli, M.; Dobran, M. Risk factors for post-traumatic hydrocephalus following decompressive craniectomy. Acta Neurochir. 2018, 160, 1691–1698. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, R.G.; Weintraub, A.H.; Rubin, B.A.; Gerber, D.J.; Olsen, A.J. Impact of timing of ventriculoperitoneal shunt placement on outcome in posttraumatic hydrocephalus. J. Neurosurg. 2018, 130, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Marmarou, A.; Abd-Elfattah Foda, M.A.; Bandoh, K.; Yoshihara, M.; Yamamoto, T.; Tsuji, O.; Zasler, N.; Ward, J.D.; Young, H.F. Posttraumatic ventriculomegaly: Hydrocephalus or atrophy? A new approach for diagnosis using CSF dynamics. J. Neurosurg. 1996, 85, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Honeybul, S.; Ho, K.M. Incidence and Risk Factors for Post-Traumatic Hydrocephalus following Decompressive Craniectomy for Intractable Intracranial Hypertension and Evacuation of Mass Lesions. J. Neurotrauma 2012, 29, 1872–1878. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.-L.; Xu, T.; Hu, J.; Cui, Y.H.; Chen, H.; Zhou, L.F. Risk factors related to hydrocephalus after traumatic subarachnoid hemorrhage. Surg. Neurol. 2008, 69, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Poca, M.A.; Sahuquillo, J.; Mataró, M.; Benejam, B.; Arikan, F.; Báguena, M. Ventricular enlargement after moderate or severe head injury: A frequent and neglected problem. J. Neurotrauma 2005, 22, 1303–1310. [Google Scholar] [CrossRef]
- Mazzini, L.; Campini, R.; Angelino, E.; Rognone, F.; Pastore, I.; Oliveri, G. Posttraumatic hydrocephalus: A clinical, neuroradiologic, and neuropsychologic assessment of long-term outcome 11No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the author(s) or upon any organization with which the author(s) is/are associated. Arch. Phys. Med. Rehabilit. 2003, 84, 1637–1641. [Google Scholar]
- Tribl, G.; Oder, W. Outcome after shunt implantation in severe head injury with post-traumatic hydrocephalus. Brain Inj. 2000, 14, 345–354. [Google Scholar] [PubMed]
- Stocchetti, N.; Maas, A.I.R. Traumatic intracranial hypertension. N. Engl. J. Med. 2014, 371, 972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Güiza, F.; Depreitere, B.; Piper, I.; Citerio, G.; Chambers, I.; Jones, P.A.; Lo, T.-Y.M.; Enblad, P.; Nillson, P.; Feyen, B.; et al. Visualizing the pressure and time burden of intracranial hypertension in adult and paediatric traumatic brain injury. Intensive Care Med. 2015, 41, 1067–1076. [Google Scholar] [CrossRef]
- Carney, N.; Totten, A.M.; O’Reilly, C.; Ullman, J.S.; Hawryluk, G.W.; Bell, M.J.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2017, 80, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Guyot, L.L.; Michael, D.B. Post-traumatic hydrocephalus. Neurol. Res. 2000, 22, 25–28. [Google Scholar] [CrossRef] [PubMed]
- De Bonis, P.; Pompucci, A.; Mangiola, A.; Rigante, L.; Anile, C. Post-traumatic hydrocephalus after decompressive craniectomy: An underestimated risk factor. J. Neurotrauma 2010, 27, 1965–1970. [Google Scholar] [CrossRef]
- Cooper, D.J.; Rosenfeld, J.V.; Murray, L.; Arabi, Y.M.; Davies, A.R.; D’Urso, P.; Kossmann, T.; Ponsford, J.; Seppelt, I.; Reilly, P.; et al. Decompressive craniectomy in diffuse traumatic brain injury. N. Engl. J. Med. 2011, 364, 1493–1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutchinson, P.J.; Kolias, A.G.; Timofeev, I.S.; Corteen, E.A.; Czosnyka, M.; Timothy, J.; Anderson, I.; Bulters, D.O.; Belli, A.; Eynon, A.; et al. Trial of Decompressive Craniectomy for Traumatic Intracranial Hypertension. N. Engl. J. Med. 2016, 375, 1119–1130. [Google Scholar] [CrossRef] [Green Version]
- Gopalakrishnan, M.S.; Shanbhag, N.C.; Shukla, D.P.; Konar, S.K.; Bhat, D.I.; Devi, B.I. Complications of Decompressive Craniectomy. Front. Neurol. 2018, 9, 977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waziri, A.; Fusco, D.; Mayer, S.A.; McKhann, G.M.; Connolly, E.S., Jr. Postoperative Hydrocephalus in Patients Undergoing Decompressive Hemicraniectomy For Ischemic or Hemorrhagic Stroke. Neurosurgery 2007, 61, 489–494. [Google Scholar] [CrossRef]
- Hu, Q.; Di, G.; Shao, X.; Zhou, W.; Jiang, X. Predictors Associated with Post-Traumatic Hydrocephalus in Patients with Head Injury Undergoing Unilateral Decompressive Craniectomy. Front. Neurol. 2018, 9, 337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, H.; Zusman, B.E.; Nwachuku, E.L.; Yue, J.K.; Chang, Y.-F.; Conley, Y.P.; Okonkwo, D.O.; Puccio, A.M. B-Cell Lymphoma 2 (Bcl-2) Gene Is Associated with Intracranial Hypertension after Severe Traumatic Brain Injury. J. Neurotrauma 2021, 38, 291–299. [Google Scholar] [PubMed]
- Deng, H.; Yue, J.K.; Zusman, B.E.; Nwachuku, E.L.; Abou-Al-Shaar, H.; Upadhyayula, P.S.; Okonkwo, D.O.; Puccio, A.M. B-Cell Lymphoma 2 (Bcl-2) and Regulation of Apoptosis after Traumatic Brain Injury: A Clinical Perspective. Medicina 2020, 56, 300. [Google Scholar] [CrossRef]
- De Bonis, P.; Sturiale, C.L.; Anile, C.; Gaudino, S.; Mangiola, A.; Martucci, M.; Colosimo, C.; Rigante, L.; Pompucci, A. Decompressive craniectomy, interhemispheric hygroma and hydrocephalus: A timeline of events? Clin. Neurol. Neurosurg. 2013, 115, 1308–1312. [Google Scholar] [CrossRef]
- Stiver, S.I. Complications of decompressive craniectomy for traumatic brain injury. Neurosurg Focus 2009, 26, E7. [Google Scholar] [CrossRef] [PubMed]
- Honeybul, S.; Ho, K.M. Decompressive craniectomy for severe traumatic brain injury: The relationship between surgical complications and the prediction of an unfavourable outcome. Injury 2014, 45, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, K.; Fried, A.; Takei, F.; Kohn, I. Effect of the skull and dura on neural axis pressure-volume relationships and CSF hydrodynamics. J. Neurosurg. 1985, 63, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Czosnyka, M.; Whitehouse, H.; Smielewski, P.; Gaudino, S.; Mangiola, A.; Martucci, M.; Colosimo, C.; Rigante, L.; Pompucci, A. Testing of cerebrospinal compensatory reserve in shunted and non-shunted patients: A guide to interpretation based on an observational study. J. Neurol. Neurosurg. Psychiatry 1996, 60, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Anderson, I.A.; Saukila, L.F.; Robins, J.M.W.; Akhunbay-Fudge, C.Y.; Goodden, J.R.; Tyagi, A.K.; Phillips, N.; Chumas, P.D. Factors Associated With 30-day Ventriculoperitoneal Shunt Failure in Pediatric and Adult Patients. J. Neurosurg. 2018, 130, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Rehman, A.; Shamim, M.S.; Bari, M.E. Factors affecting ventriculoperitoneal shunt survival in adult patients. Surg. Neurol. Int. 2015, 6, 25. [Google Scholar]
- Lund-Johansen, M.; Svendsen, F.; Wester, K. Shunt failures and complications in adults as related to shunt type, diagnosis, and the experience of the surgeon. Neurosurgery 1994, 35, 839–844. [Google Scholar] [CrossRef]
- Giordan, E.; Palandri, G.; Lanzino, G.; Murad, M.H.; Elder, B.D. Outcomes and complications of different surgical treatments for idiopathic normal pressure hydrocephalus: A systematic review and meta-analysis. J. Neurosurg. 2018, 131, 1024–1036. [Google Scholar] [CrossRef] [Green Version]
- Bloomfield, I.G.; Johnston, I.H.; Bilston, L.E. Effects of proteins, blood cells and glucose on the viscosity of cerebrospinal fluid. Pediatr. Neurosurg. 1998, 28, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Fulkerson, D.H.; Vachhrajani, S.; Bohnstedt, B.N.; Patel, N.B.; Patel, A.J.; Fox, B.D.; Jea, A.; Boaz, J.C. Analysis of the risk of shunt failure or infection related to cerebrospinal fluid cell count, protein level, and glucose levels in low-birth-weight premature infants with posthemorrhagic hydrocephalus. J. Neurosurg. Pediatr. 2011, 7, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Rammos, S.; Klopfenstein, J.; Augspurger, L.; Wang, H.; Wagenbach, A.; Poston, J.; Lanzino, G. Conversion of external ventricular drains to ventriculoperitoneal shunts after aneurysmal subarachnoid hemorrhage: Effects of site and protein/red blood cell counts on shunt infection and malfunction. J. Neurosurg. 2008, 109, 1001–1004. [Google Scholar] [CrossRef] [Green Version]
| Parameters | Total (n = 402) | No Shunt (n = 362) | Shunt for PTH (n = 40) | p Value |
|---|---|---|---|---|
| Age, years (mean, SD) | 38.0 (16.7) | 38.4 (16.8) | 34.5 (16.2) | 0.16 |
| Male gender, % | 78.4 | 77.9 | 82.5 | 0.69 |
| Marshall score, median (IQR) | 3.0 (4.0) | 3.0 (4.0) | 3.0 (1.0) | 0.31 |
| ISS, median (IQR) | 30.0 (13.0) | 30.0 (13.0) | 35.0 (12.0) | 0.32 |
| GCS, median (IQR) | 6.0 (1.0) | 6.0 (2.0) | 6.0 (2.0) | 0.29 |
| Primary intracranial bleed, % | ||||
| EDH | 7.2 | 6.9 | 10.0 | 0.32 |
| SDH | 32.6 | 31.8 | 40.0 | 0.19 |
| SAH | 18.2 | 19.3 | 7.5 | 0.04 |
| ICH | 31.3 | 30.9 | 35.0 | 0.36 |
| IVH | 2.7 | 2.8 | 2.5 | 1.00 |
| Diffuse axonal injury, % | 36.4 | 35.7 | 42.3 | 0.33 |
| Neurosurgical intervention, % | ||||
| EVD | 58.5 | 57.7 | 65.0 | 0.24 |
| DHC | 27.6 | 24.6 | 55.0 | <0.01 |
| Neuromonitoring, mean (SD) | ||||
| ICP, mmHg | 10.1 (8.6) | 9.9 (7.9) | 10.1 (8.8) | 0.90 |
| PbtO2, mmHg | 22.6 (14.4) | 22.8 (14.4) | 21.6 (14.7) | 0.69 |
| CPP, mmHg | 78.9 (14.3) | 78.6 (14.1) | 80.7 (15.4) | 0.51 |
| Parameters | Total with Shunt (n = 40) | Shunt Failure (n = 18) | No Shunt Failure (n = 22) | p Value |
|---|---|---|---|---|
| Age, years mean (SD) | 34.5 (16.2) | 29.7 (23.2) | 36.9 (14.4) | 0.14 |
| Male gender, % | 82.5 | 83.3 | 87.5 | 0.56 |
| ISS, median (IQR) | 35.0 (12.0) | 30.0 (10.0) | 38.0 (15.0) | 0.06 |
| GCS, median (IQR) | 6.0 (2.0) | 6.0 (1.0) | 6.5 (2.0) | 0.26 |
| Trauma to shunt, days median (IQR) | 35.0 (53.5) | 32.0 (16.0) | 33.5 (1163) | 0.29 |
| Follow up, days median (IQR) | 1814 (2803) | 1871 (2047) | 1819 (3510) | 0.82 |
| Primary bleed, % | 0.17 | |||
| EDH | 10.0 | 22.2 | 0.0 | |
| SDH | 40.0 | 33.3 | 50.0 | |
| SAH | 7.5 | 11.1 | 0.0 | |
| ICH | 35.0 | 27.8 | 43.8 | |
| Diffuse axonal injury, % | 42.3 | 54.5 | 30.0 | 0.25 |
| Programmable valve, % | 75.0 | 88.9 | 68.8 | 0.15 |
| GOS, median (IQR) | ||||
| 6 months | 3.0 (1.5) | 3.0 (1.0) | 3.0 (0.5) | 0.55 |
| 12 months | 3.0 (2.0) | 3.0 (1.0) | 3.0 (1.5) | 0.47 |
| CSF Content | Shunt Failure | No Shunt Failure | p Value |
|---|---|---|---|
| RBC, cells/mm3 | 2376 ± 9312 | 840.9 ± 1883 | 0.48 |
| WBC, cells/mm3 | 3.9 ± 11.1 | 1.7 ± 1.6 | 0.40 |
| Glucose, mg/dL | 66.5 ± 9.9 | 62.1 ± 19.7 | 0.41 |
| Protein, mg/dL | 57.4 ± 83.3 | 51.0 ± 38.28 | 0.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, H.; Goldschmidt, E.; Nwachuku, E.; Yue, J.K.; Angriman, F.; Wei, Z.; Agarwal, N.; Puccio, A.M.; Okonkwo, D.O. Hydrocephalus and Cerebrospinal Fluid Analysis Following Severe Traumatic Brain Injury: Evaluation of a Prospective Cohort. Neurol. Int. 2021, 13, 527-534. https://doi.org/10.3390/neurolint13040052
Deng H, Goldschmidt E, Nwachuku E, Yue JK, Angriman F, Wei Z, Agarwal N, Puccio AM, Okonkwo DO. Hydrocephalus and Cerebrospinal Fluid Analysis Following Severe Traumatic Brain Injury: Evaluation of a Prospective Cohort. Neurology International. 2021; 13(4):527-534. https://doi.org/10.3390/neurolint13040052
Chicago/Turabian StyleDeng, Hansen, Ezequiel Goldschmidt, Enyinna Nwachuku, John K. Yue, Federico Angriman, Zhishuo Wei, Nitin Agarwal, Ava M. Puccio, and David O. Okonkwo. 2021. "Hydrocephalus and Cerebrospinal Fluid Analysis Following Severe Traumatic Brain Injury: Evaluation of a Prospective Cohort" Neurology International 13, no. 4: 527-534. https://doi.org/10.3390/neurolint13040052
APA StyleDeng, H., Goldschmidt, E., Nwachuku, E., Yue, J. K., Angriman, F., Wei, Z., Agarwal, N., Puccio, A. M., & Okonkwo, D. O. (2021). Hydrocephalus and Cerebrospinal Fluid Analysis Following Severe Traumatic Brain Injury: Evaluation of a Prospective Cohort. Neurology International, 13(4), 527-534. https://doi.org/10.3390/neurolint13040052








