Ice Pack Test Eased Ptosis in a Patient Presenting with a Possible Oculomotor Nerve Schwannoma: A Case Report
Abstract
:1. Introduction
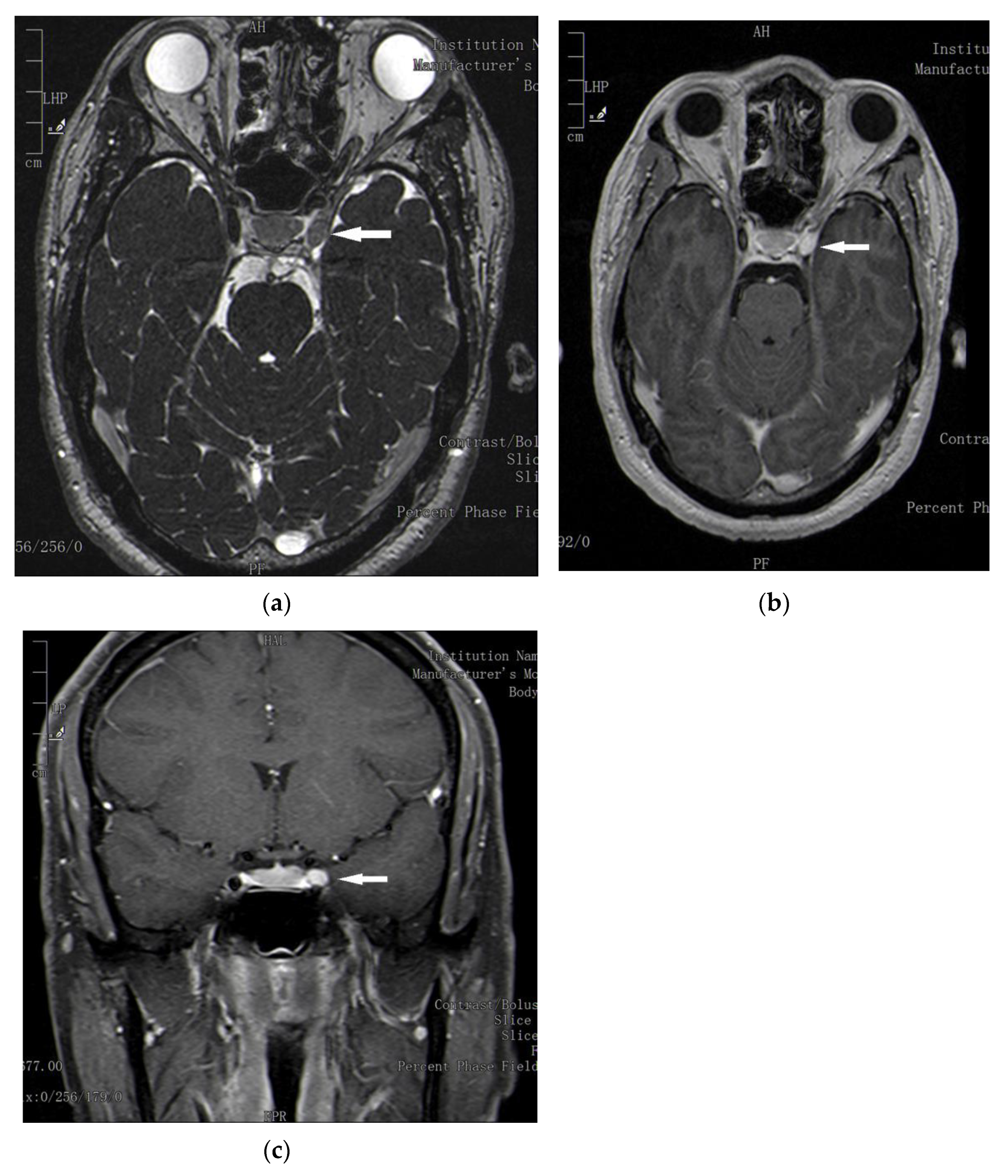
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AChR | acetylcholine receptor |
| Gad | gadolinium |
| GKRS | Gamma Knife radiosurgery |
| IPT | ice pack test |
| LPSM | Levator palpebrae superioris muscle |
| MG | myasthenia gravis |
| MRD | margin reflex distance |
| MRI | magnetic resonance imaging |
| ONS | oculomotor nerve schwannoma |
| PFH | palpebral fissure height |
| STM | superior tarsal muscle |
References
- Dias, L.; Araújo, R. Ice pack test in myasthenia gravis: A cool investigation at the bedside. Lancet 2020, 396, e82. [Google Scholar] [CrossRef]
- Natarajan, B.; Saifudheen, K.; Gafoor, V.A.; Jose, J. Accuracy of the ice test in the diagnosis of myasthenic ptosis. Neurol India 2016, 64, 1169–1172. [Google Scholar] [PubMed]
- Fakiri, M.O.; Tavy, D.L.J.; Hama-Amin, A.D.; Wirtz, P.W. Accuracy of the ice test in the diagnosis of myasthenia gravis in patients with ptosis. Muscle Nerve 2013, 48, 902–904. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J. Ice Pack Test eased eyelid drooping in man with LEMS, case report finds. Lambert Eaton News. 10 December 2020. Available online: lamberteatonnews.com/new-posts/2020/12/10/ice-pack-test-eased-eyelid-drooping-in-lems-patient-case-report-finds (accessed on 16 December 2020).
- Kumar, L.P.; Monica, I.; Uppin, M.S.; Naidu Kotiyala, V.J. Large oculomotor nerve schwannoma—Rare entity: A case report with review of literature. J. Cancer Res. Ther. 2014, 10, 1098–1100. [Google Scholar] [CrossRef]
- Prabhu, S.S.; Bruner, J.M. Large oculomotor schwannoma presenting as a parasellar mass: A case report and literature review. Surg. Neurol. Int. 2010, 1, 15. [Google Scholar] [CrossRef] [Green Version]
- Ramsey, A.E.; Gaillard, F. Radiopaedia.org. Intracranial Schwannoma. Available online: https://radiopaedia.org (accessed on 20 August 2021).
- Skolnik, A.D.; Loevner, L.A.; Sampathu, D.M.; Newman, J.G.; Bagley, L.J.; Learned, K.O. Cranial Nerve Schwannomas: Diagnostic Imaging Approach. RadioGraphics 2016, 36, 1463–1477. [Google Scholar] [CrossRef] [Green Version]
- Wippold, F.J.; Lubner, M.; Perrin, R.J.; Lämmle, M.; Perry, A. Neuropathology for the Neuroradiologist: Antoni A and Antoni B Tissue Patterns. Am. J. Neuroradiol. 2007, 28, 1633–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, R.D.; Victor, M.; Ropper, A.H. Principi di Neurologia, 6th ed.; McGraw-Hill Libri Italia: Milano, Italy, 1998; p. 649. [Google Scholar]
- Newton, H.B. Primary brain tumors: Review of etiology, diagnosis and treatment. Am. Fam.Physician 1994, 49, 787–797. [Google Scholar]
- Ohata, K.; Takami, T.; Goto, T.; Ishibashi, K. Schwannomaof theoculomotor nerve. Neurol India 2006, 54, 437–439. [Google Scholar] [CrossRef] [Green Version]
- Fisher, L.M.; Doherty, J.K.; Lev, M.H.; Slattery, W.H., 3rd. Distributionof nonvestibularcranial nerve schwannomasinneurofibromatosis 2. Otol. Neurotol. 2007, 28, 1083–1090. [Google Scholar] [CrossRef]
- Choi, Y.S.; Sung, K.S.; Song, Y.J.; Kim, H.D. Olfactory schwannoma-case report. J. Korean Neurosurg. Soc. 2009, 45, 103–106. [Google Scholar] [CrossRef]
- Kovacs, W. Über ein solitäres Neurom des nervus oculomotorius. Zentralbl. Allg. Pathol. 1927, 40, 518–522. [Google Scholar]
- PubMed®. Oculomotor Nerve Schwannoma and Case Report. Available online: https://pubmed.ncbi.nlm.nih.gov (accessed on 20 May 2021).
- Muhammad, S.; Niemelä, M. Management of oculomotor nerve schwannoma: Systemic review of literature and illustrative case. Surg. Neurol. Int. 2019, 10, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Celli, P.; Ferrante, L.; Acqui, M.; Mastronardi, L.; Fortuna, A.; Palma, L. Neurinoma of the third, fourth, and sixth cranial nerves: A survey and report of a new fourth nerve case. Surg. Neurol. 1992, 38, 216–224. [Google Scholar] [CrossRef]
- Mehta, V.S.; Singh, R.V.; Misra, N.K.; Choudhary, C. Schwannoma of the oculomotor nerve. Br. J. Neurosurg. 1990, 4, 69–72. [Google Scholar] [CrossRef]
- Katoh, M.; Kawamoto, T.; Ohnishi, K.; Sawamura, Y.; Abe, H. Asymptomatic schwannoma of the oculomotor nerve: Case report. J. Clin. Neurosci. 2000, 7, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Asaoka, K.; Sawamura, Y.; Murai, H.; Satoh, M. Schwannoma of the oculomotor nerve: A case report with consideration of the surgical treatment. Neurosurgery 1999, 45, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Elsharkawy, M.; Xu, Z.; Schlesinger, D.; Sheehan, J.P. Gamma knife surgery for nonvestibular schwannomas: Radiological and clinical outcomes. J. Neurosurg. 2012, 116, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; Kondziolka, D.; Niranjan, A.; Flickinger, J.C.; Lunsford, L.D. Gamma knife surgery for schwannomas originating from cranial nerves III, IV, and VI. J. Neurosurg. 2008, 109, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.D.; Victor, M.; Ropper, A.H. Principi di Neurologia, 6th ed.; McGraw-Hill Libri Italia: Milano, Italy, 1998; pp. 1387–1398. [Google Scholar]
- Sanders, D.B.; Howard, J.F., Jr. AAEE minimonograph #25: Single-fiber electromyography in myasthenia gravis. Muscle Nerve 1986, 9, 809–819. [Google Scholar]
- Borenstein, S.; Desmedt, J.E. Local cooling in myasthenia. Improvement of neuromuscular failure. Arch. Neurol. 1975, 32, 152–157. [Google Scholar] [CrossRef]
- Sener, H.O.; Yaman, A. Effect of high temperature on neuromuscular jitter in myasthenia gravis. Eur. Neurol. 2008, 59, 179–182. [Google Scholar] [CrossRef]
- Koka, K.; Patel, B.C. Ptosis Correction. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021; [Updated 25 February 2021]. [Google Scholar]
- Giannoccaro, M.P.; Paolucci, M.; Zenesini, C.; Di Stasi, V.; Donadio, V.; Avoni, P.; Liguori, R. Comparison of ice pack test and single-fiber EMG diagnostic accuracy in patients referred for myasthenic ptosis. Neurology 2020, 95, e1800–e1806. [Google Scholar] [CrossRef] [PubMed]
- Kee, H.J.; Yang, H.K.; Hwang, J.-M.; Park, K.S. Evaluation and validation of sustained upgaze combined with the ice-pack test for ocular myasthenia gravis in Asians. Neuromuscul. Disord. 2019, 29, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Gray, H.; Carter, H.V. Gray’s Anatomy, 15th ed.; Barnes & Noble Books: New York, NY, USA, 1995; pp. 35–36, 275–277. [Google Scholar]
- Esperidião-Antonio, V.; Conceição-Silva, F.; De-Ary-Pires, B.; Pires-Neto, M.A.; de Ary-Pires, R. The human superior tarsal muscle (Müller’s muscle): A morphological classification with surgical correlations. Anat. Sci. Int. 2010, 85, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Squire, L.R. Encyclopedia of Neuroscience, 1st ed.; Academic Press: London, UK; Burlington, MA, USA; San Diego, CA, USA, 2009; Volume 1, p. 684. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buechner, S.; Capone, L. Ice Pack Test Eased Ptosis in a Patient Presenting with a Possible Oculomotor Nerve Schwannoma: A Case Report. Neurol. Int. 2021, 13, 510-516. https://doi.org/10.3390/neurolint13040050
Buechner S, Capone L. Ice Pack Test Eased Ptosis in a Patient Presenting with a Possible Oculomotor Nerve Schwannoma: A Case Report. Neurology International. 2021; 13(4):510-516. https://doi.org/10.3390/neurolint13040050
Chicago/Turabian StyleBuechner, Susanne, and Loredana Capone. 2021. "Ice Pack Test Eased Ptosis in a Patient Presenting with a Possible Oculomotor Nerve Schwannoma: A Case Report" Neurology International 13, no. 4: 510-516. https://doi.org/10.3390/neurolint13040050
APA StyleBuechner, S., & Capone, L. (2021). Ice Pack Test Eased Ptosis in a Patient Presenting with a Possible Oculomotor Nerve Schwannoma: A Case Report. Neurology International, 13(4), 510-516. https://doi.org/10.3390/neurolint13040050





