Unilateral Trigeminal Sensory Neuropathy with Sjögren’s Syndrome with Liver and Renal Impairment
Abstract
:1. Introduction
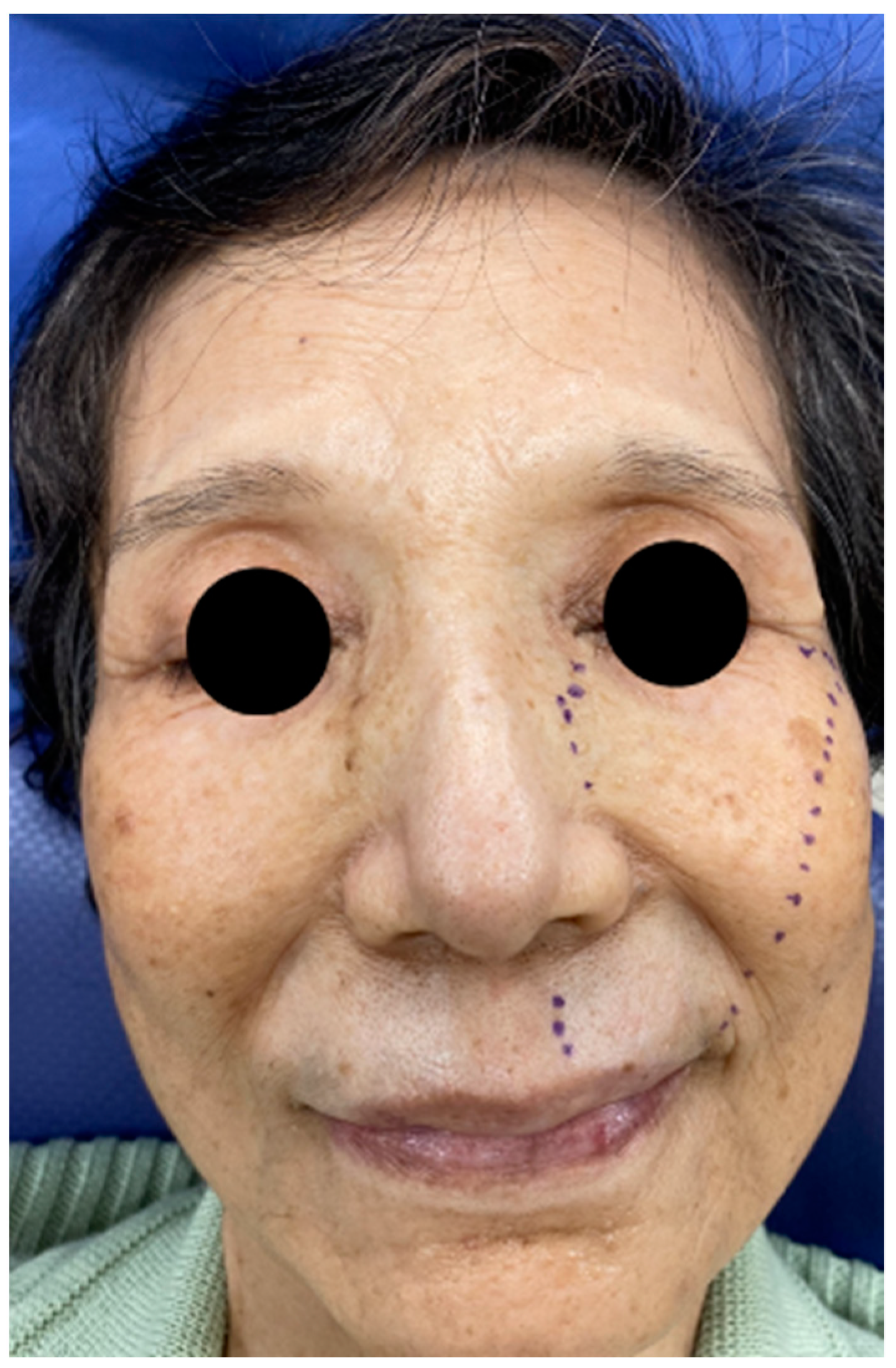
2. Case
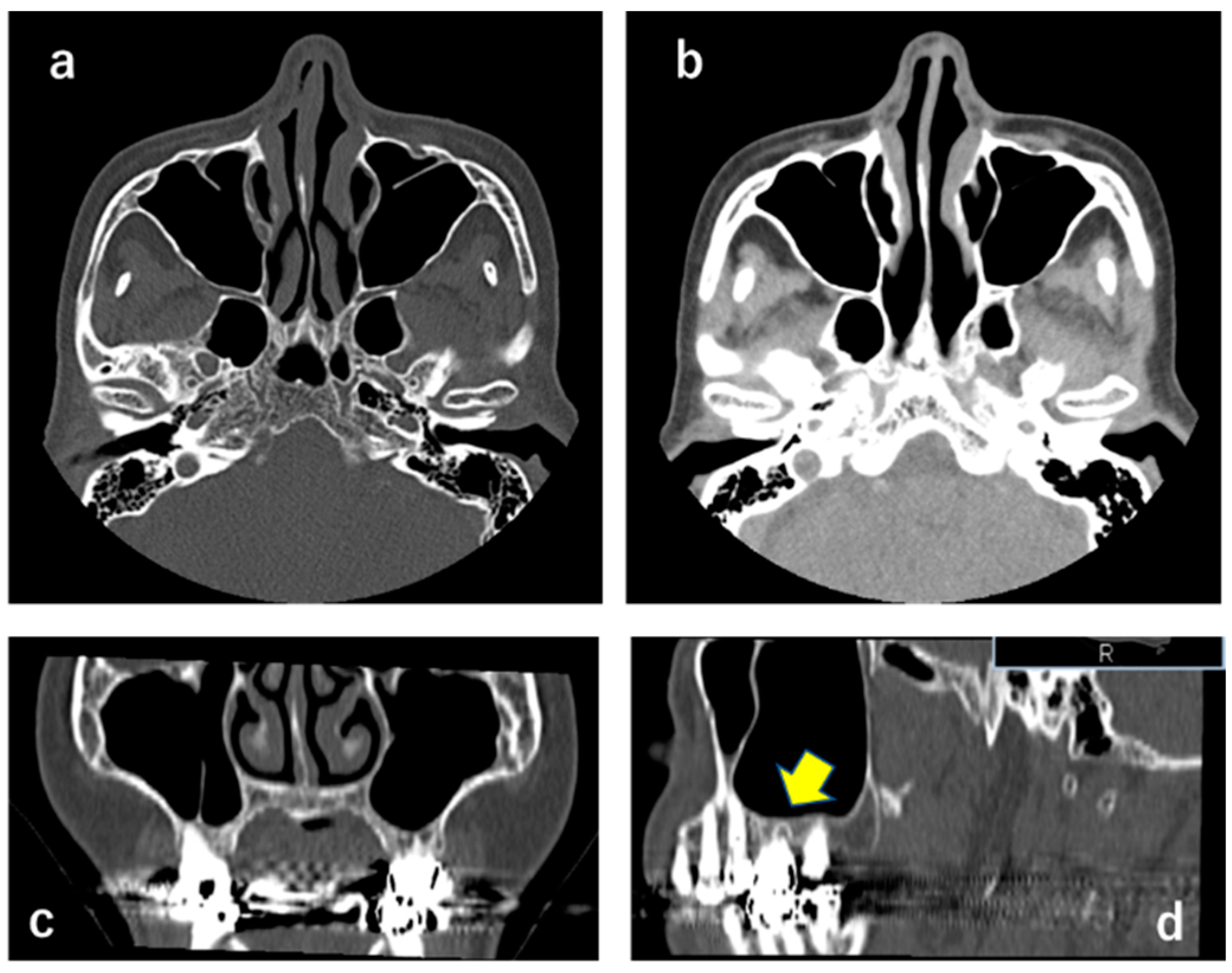
2.1. CT X-ray-Findings
2.2. QST
2.3. Diagnosis
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Valls-Sole, J.; Graus, F.; Font, J.; Pou, A.; Tolosa, E.S. Normal proprioceptive trigeminal afferents in patients with Sjögren’s syndrome and sensory neuronopathy. Ann. Neurol. 1990, 28, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Alexander, E.L.; Provost, T.T.; Stevens, M.B.; Alexander, G.E. Neurologic complications of primary Sjogren’s syndrome. Medicine 1982, 61, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Malinow, K.; Yannakakis, G.D.; Glusman, S.M.; Edlow, D.W.; Griffin, J.; Pestronk, A.; Powell, D.L.; Ramsey-Goldman, R.; Eidelman, B.H.; Medsger, T.A., Jr.; et al. Subacute sensory neuronopathy secondary to dorsal root ganglionitis in primary Sjögren’s syndrome. Ann. Neurol. 1986, 20, 535–537. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.; Logigian, E.L. Neurological manifestations of primary Sjogren’s syndrome. Curr. Opin. Neurol. 2010, 23, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Pou-Serradell, A.; Vinas-Gaya, J. Trois cas de neuropathies périphériques rares associées au syndrome de Gougerot–Sjögren primitif. Rev. Neurol. 1993, 149, 481–484. [Google Scholar] [PubMed]
- Kuhl, V.; Urban, P.P.; Mayet, W.J.; Hopf, H.C. Isolated trochlear nerve palsy and repetitive Raynaud’s phenomenon of the tongue in primary Sjögren’s syndrome. J. Neurol. 1999, 246, 974–975. [Google Scholar] [CrossRef] [PubMed]
- Graus, F.; Pou, A.; Kanterewicz, E.; Anderson, N.E. Sensory neuronopathy and Sjögren’s syndrome: Clinical and immunological study of two patients. Neurology 1988, 38, 1637–1639. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, Y.; Nishinarita, S.; Morie, T. Abducent and trochlear palsies in a patient with Sjögren’s syndrome. Br. J. Rheumatol. 1995, 34, 484–485. [Google Scholar] [CrossRef] [PubMed]
- Urban, P.P.; Keilmann, A.; Teichmann, E.M.; Hopf, H.C. Sensory neuropathy of the trigeminal, glossopharyngeal, and vagal nerves in Sjogren’s syndrome. J. Neurol. Sci. 2001, 186, 59–63. [Google Scholar] [CrossRef]
- Rolke, R.; Baron, R.; Maier, C.; Tölle, T.R.; Treede, D.R.; Beyer, A.; Binder, A.; Birbaumer, N.; Birklein, F.; Botefur, I.C.; et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): Standardized protocol and reference values. Pain 2006, 123, 231–243. [Google Scholar] [CrossRef] [PubMed]
- ICOP. International Classification of Orofacial Pain (ICOP), 1st ed.; Cephalalgia: Thousand Oaks, CA, USA, 2020; Volume 40, pp. 129–221. [Google Scholar]
- Yuan, J.; Gong, L.; Wu, H.; Chen, Q.; Wang, J.; Chen, W.; Wang, X.; Ren, C. Case report of primary Sjögren Syndrome with simple trigeminal lesion as initial symptom. J. Neuroimmunol. 2018, 324, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Iijima, M.; Koike, H.; Hattori, N.; Tanaka, F.; Watanabe, H.; Katsuno, M.; Fujita, A.; Aiba, I.; Ogata, A.; et al. The wide spectrum of clinical manifestations in Sjogren’s syndrome-associated neuropathy. Brain 2005, 128, 2518–2534. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Casals, M.; Solans, R.; Rosas, J.; Camps, M.T.; Gil, A.; Del Pino-Montes, J.; Calvo-Alen, J.; Jimenez-Alonso, J.; Mico, M.L.; Beltran, J.; et al. Primary Sjögren syndrome in Spain: Clinical and immunologic expression in 1010 patients. Medicine 2008, 87, 210–219. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozasa, K.; Noma, N.; Nakata, J.; Imamura, Y. Unilateral Trigeminal Sensory Neuropathy with Sjögren’s Syndrome with Liver and Renal Impairment. Neurol. Int. 2021, 13, 464-468. https://doi.org/10.3390/neurolint13030045
Ozasa K, Noma N, Nakata J, Imamura Y. Unilateral Trigeminal Sensory Neuropathy with Sjögren’s Syndrome with Liver and Renal Impairment. Neurology International. 2021; 13(3):464-468. https://doi.org/10.3390/neurolint13030045
Chicago/Turabian StyleOzasa, Kana, Noboru Noma, Jumi Nakata, and Yoshiki Imamura. 2021. "Unilateral Trigeminal Sensory Neuropathy with Sjögren’s Syndrome with Liver and Renal Impairment" Neurology International 13, no. 3: 464-468. https://doi.org/10.3390/neurolint13030045
APA StyleOzasa, K., Noma, N., Nakata, J., & Imamura, Y. (2021). Unilateral Trigeminal Sensory Neuropathy with Sjögren’s Syndrome with Liver and Renal Impairment. Neurology International, 13(3), 464-468. https://doi.org/10.3390/neurolint13030045




