Abstract
Introduction: This paper explores the potential influence of a single nucleotide variant in the ANK-2 gene on COVID-19 myocarditis-related ventricular tachycardia. Case Description: A 53-year-old female with a history of Crohn’s disease and asthma developed COVID-19. Shortly after infection, she experienced symptoms of chest pressure, palpitations, and shortness of breath, leading to the eventual diagnosis of myocarditis complicated by recurrent ventricular tachycardia. Treatment with mechanistically driven anti-arrhythmic therapy and beta-blockers suppressed this highly symptomatic ventricular tachycardia. Genetic testing to further risk stratify and influence long term care identified a single nucleotide variant in the ANK-2 gene, which is known to be associated with arrhythmic risk. Discussion: This case study highlights the use of rationally selected anti-arrhythmic therapy, mexiletine, in the management of ventricular tachycardia associated with COVID-19 myocarditis and the presence of a single nucleotide variant in ANK-2, raising the possibility of its contribution to VT susceptibility and severity. Our patient demonstrated significant improvement with administered therapeutics, including the resolution of myocarditis and ventricular tachycardia. The normalization of the QT interval during the resolution phase further supports the potential influence of the genetic variant in ANK-2 on potassium channel activity.
1. Introduction
The COVID-19 pandemic persists, as hospitalizations and deaths due to the virus remain prevalent [1]. The COVID-19 disease state can vary in clinical intensity in patients, ranging from asymptomatic to cardiac manifestations including myocarditis, which has been associated with mortality [2]. COVID-induced myocarditis occurs due to an immune-mediated inflammation of the myocardium after infection with SARS-CoV-2. In general, myocarditis due to COVID-19 development can present with no symptoms, or with chest pains, dyspnea, and/or arrhythmias [3]. The currently hypothesized mechanisms of these myocardial injuries are related to a dysregulated immune response, renin–angiotensin–aldosterone system dysregulation, and/or direct myocardial injury [4]. Furthermore, the resultant severity of the clinical manifestation, particularly in COVID-induced fulminant myocarditis, may arise from patient-specific immunogenetic features or genetic variants within myocardial cells that predispose to arrhythmias and cardiomyopathies.
In this report, we present a case of COVID-19 myocarditis-associated ventricular tachycardia in a patient with a single nucleotide variant in ANK-2. Though we believe that her clinical manifestation may likely be attributed to well-established mechanisms of re-entrant VT in myocarditis, we are intrigued by the possibility that her single nucleotide variant in ANK-2, which is known to be associated with arrhythmic risk [5], may have contributed to her disease state. This furthers the well-established understanding that underlying genetic architecture may serve as a basis for driving the severity of disease states.
2. Clinical History
A 53-year-old Caucasian female with a past medical history significant for Crohn’s disease and asthma developed COVID-19 in February of 2021. Approximately three weeks after her COVID-19 diagnosis, she began to experience chest pressure, rapid palpitations, and shortness of breath. At an outside institution, she underwent a standard evaluation, consisting of an EKG, an echocardiogram, and a Holter monitor. Her echo revealed a normal LVEF, no valve concerns, and RVSP, and her Holter revealed salvos of ventricular tachycardia. She underwent cardiac catheterization, which demonstrated normal angiographic coronaries (Figure 1A,B), and subsequently underwent a cardiac MRI (Figure 1C) that revealed diffuse myocardial edema without fibrosis in a pattern diagnostic for myocarditis. At the outside institution, she was started on flecainide 50 mg twice a day. Despite these interventions, she continued to have frequent episodes of highly symptomatic ventricular tachycardia, prompting her referral to our program. Despite her diagnosis, the patient does not take medication to control her Crohn’s disease.
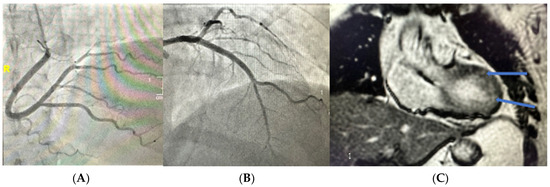
Figure 1.
(A) Angiography of right coronary artery, RAO caudal projection. (B) Angiography of left coronary artery, RAO cranial projection. (C) Cardiac MRI. Arrows highlighting myocardial edema consistent with acute phase myocarditis.
3. Myocarditis and Ventricular Tachycardia (VT)
Myocarditis is an immune-mediated inflammation of the heart muscle that can result in an electrical storm during the acute inflammatory phase, which is a state that can be resistant to anti-arrhythmic therapy. An electrical storm is defined as three or more separate episodes of sustained ventricular tachycardia (VT) within 24 h. Sustained VT with hemodynamic instability calls for prompt cardioversion due to it being a state of poor perfusion. Amiodarone may be used both as a preventative measure and for treatment of recurrent monomorphic VT [6]. A recent study showed that polymorphic and irregular ventricular arrhythmias are more common during the active inflammatory phase, whereas monomorphic and regular ventricular arrhythmias are associated with healed myocarditis [7]. There are a variety of hypotheses as to what could be triggering viral-induced myocarditis and subsequent ventricular arrhythmia periods. Myocardial fibrosis and secondary hypertrophy could favor a regional slowing of action potentials, resulting in re-entry circuits. Small focal inflammation could also explain electrically sensitive myocardial areas, or abnormal function of myocardial ion channels, all leading to VT [8].
Upon our review and evaluation of external records, we discontinued flecainide given the presence of myocardial edema/injury on cardiac MRI, and advised a Class IB agent, mexiletine. We rationalized this agent specifically due to our suspicion that her VT originated from depolarized myocarditis associated tissue, resembling a state of injury that can be seen in ischemia.
Mexiletine is a Class IB anti-arrhythmic drug (AAD). Its anti-arrhythmic properties are due to its ability to block fast sodium channels, thereby reducing phase 0 of the cardiac myocyte action potential. Oral mexiletine has proven to be an effective option for the long-term management and prevention of ventricular arrhythmia from various etiologies, including ischemic cardiomyopathy and acute coronary syndrome [9,10]. Class IB anti-arrhythmics bind with the highest affinity and avidity to sodium channels in their depolarized state, which elucidates their effectiveness in preventing ventricular arrhythmias associated with ischemia. Specifically, class IB AADs cause prolongation of the effective refractory period (ERP) and, consequently, prolongation of post-repolarization refractoriness in ischemic cells [11]. Mexiletine is classified as a Class IB anti-arrhythmic agent, according to the Vaughan-Williams classification system. Class IB drugs, including mexiletine, primarily work by blocking fast sodium channels in cardiomyocytes, preferentially the Nav1.5 in the inactivated state. This reduces the influx of sodium ions into cardiomyocytes during phase 0 of the cardiac myocyte action potential [12]. This allows more time for the refractory period and decreases the upstroke velocity, which decreases the action potential duration. The end effect is helping to stabilize the heart rhythm [13]. Given the case features, mexiletine offered a logical alternative for the management of ventricular arrhythmias in our patient with ischemic-like tissue who had failed her first anti-arrhythmic.
Along with initiation of mexiletine, we started a low-dose beta blocker for adrenergic attenuation and titrated slowly without any exacerbation of her reactive airway disease. We monitored her progress using mobile cardiac telemetry (MCOT) and were pleased to see complete amelioration of ventricular tachycardia. We initiated Aldactone 25 mg once daily to further manage concomitant hypertension while providing potential anti-fibrotic effects for her at-risk myocardium. Aldactone is a mineralocorticoid receptor (MR) antagonist that impacts fluid, electrolyte, and hemodynamic homeostasis, and plays an important role in tissue remodeling. When mineralocorticoids are hyperactive, there is an increase in tissue inflammation leading to fibrosis. Blockade of these MRs plays a substantial role in limiting fibrotic effects in those with an at-risk myocardium [14].
In her external data, we noted QT prolongation on her index/initial presenting EKG (Figure 2A). Further Kardia and holter monitor data demonstrated salvos of VT (Figure 2B,C). There was no discernible family history of sudden death or known LQTS, and there were no contributions from iatrogenic pharmacologic agents. Though we believed that her ventricular tachycardia was likely secondary to a re-entrant mechanism related to myocarditis-associated injury, her prolonged QT on initial EKG raised suspicion that the severity of her VT was partly driven by underlying genetically mediated arrhythmogenic susceptibility. To probe this further and to guide our long-term management, we pursued clinical genetic testing.
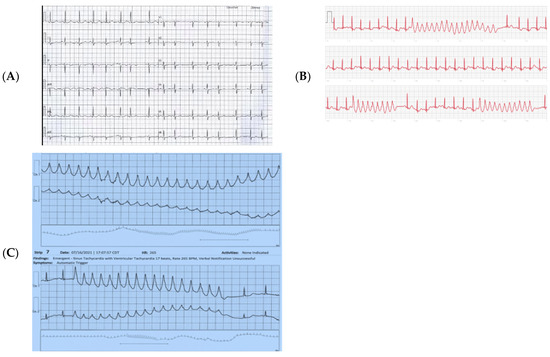
Figure 2.
(A) Index EKG. NSR with QT 50% of the RR interval, 470 msec. (B) KardiaTM Tracings demonstrating salvos of VT. (C) Patch Holter demonstrating salvos of VT.
4. Genetic Testing Methods
The patient provided a saliva sample from which genomic deoxyribonucleic acid (gDNA) was isolated using a standardized methodology, and quantified though a commercial sequencing partner. Genes analyzed (42 total) included: AKAP9, ANK2, CACNA1C, CACNA2D1, CACNB2, CALM1, CALM2, CALM3, CASQ2, CAV3, DES, DSC2, DSG2, DSP, GPD1L, HCN4, JUP, KCND3, KCNE1, KCNE2, KCNE3, KCNH2, KCNJ2, KCNJ5, KCNJ8, KCNQ1, LMNA, NKX2-5, PKP2, PLN, RYR2, SCN10A, SCN1B, SCN3B, SCN4B, SCN5A, SNTA1, TBX5, TECRL, TMEM43, TRDN, and TRPM4 (sequencing and deletion/duplication) [15,16,17]. Sequence enrichment of the targeted coding exons and adjacent intronic nucleotides was carried out by a bait-capture methodology using long biotinylated oligonucleotide probes, and was followed by polymerase chain reaction (PCR) and next-generation sequencing [15,16,17]. Additional Sanger sequencing was performed for any regions that were missing or that had insufficient read depth coverage for reliable heterozygous variant detection [15,16,17]. Variants in regions complicated by pseudogene interference, variant calls not satisfying depth of coverage and variant allele frequency quality thresholds, and potentially homozygous variants were verified by Sanger sequencing. Gross deletion/duplication analysis was performed for all genes using a custom pipeline based on read depth from NGS data, followed by a confirmatory orthogonal method, as needed [15,16,17]. Exon-level resolution was not necessarily achieved for every gene [15,16,17]. The corresponding NCBI reference sequences are as follows: AKAP9 NM_005751.4, ANK2 NM_001148.4, CACNA1C NM_000719.6, CACNA2D1 NM_000722.2, CACNB2 NM_201590.2, CALM1 NM_006888.4, CALM2 NM_001743.4, CALM3 NM_006888.4, CASQ2 NM_001232.3, CAV3 NM_033337.2, DES NM_001927.3, DSC2 NM_024422.3, DSG2 NM_001943.3, DSP NM_004415.2, GPD1L NM_015141.3, HCN4 NM_005477.2, JUP NM_002230.2, KCND3 NM_004980.4, KCNE1 NM_000219.3, KCNE2 NM_172201.1, KCNE3 NM_005472.4, KCNH2 NM_000238.3, KCNJ2 NM_000891.2, KCNJ5 NM_000890.3, KCNJ8 NM_004982.2, KCNQ1 NM_000218.2, LMNA NM_170707.2, NKX2-5 NM_004387.3, PKP2 NM_004572.3, PLN NM_002667.3, RYR2 NM_001035.2, SCN1B NM_001037.4, SCN2B NM_004588.4, SCN3B NM_018400.3, SCN4B NM_174934.3, SCN5A NM_198056.2, SCN10A NM_006514.2, SNTA1 NM_003098.2, TBX5 NM_000192.3, TECRL NM_001010874.4, TMEM43 NM_024334.2, TRDN NM_006073.2, and TRPM4 NM_017636.3 [15,16,17]. The molecular diagnostic methods for genetic analysis were performed by Ambry Genetics. We subsequently provided 1:1 genetic counseling.
5. Genetic Testing Results
Sequencing demonstrated a novel single nucleotide variant in ANK2: c.3074G>C p.G1025A. This change results in a glycine to alanine amino acid change, two amino acids with differing properties (Table 1).

Table 1.
Amino acid characteristics. Frequency gnomAD: 0.02% (47/282430) total alleles studied, 0.04% (46/128804) European (non-Finnish) alleles. Grantham Score 60 (similar amino acid substitution) [18].
6. Clinical Outcome
After 6 months of mexiletine, b-blocker, and Aldactone therapy, demonstration of myocarditis resolution by repeat cardiac MRI (Figure 3A), and complete absence of any VT by extended Holter monitoring, mexiletine was weaned off (Figure 3B). Interestingly, as shown in Figure 3A, an EKG in this resolution phase demonstrated normalization of her QT interval. We suspect this normalization occurred due to the complete healing of the myocardium, thereby removing this “inducible” factor that appears to have interacted with her ANK-2 susceptibility genotype during acute myocarditis. The patient remains symptom-free with a normal QT on B-blockers.
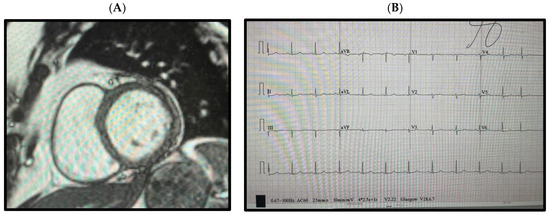
Figure 3.
(A) Cardiac MRI demonstrating a normal T1/T2 weighted cardiac myocardium without fibrosis or edema. (B) EKG 6 months post-treatment, showing normalization of the QT interval.
7. Ankyrin-2
The ANK2 gene is located on the long arm of chromosome 4 (4q25-q276) in humans. The gene spans over 500 kb and contains 61 exons that can be alternatively spliced to generate multiple isoforms of the ankyrin-B protein (Figure 4). Ankyrin-B is a relatively large protein, consisting of over 2400 amino acids. It plays an important role in the regulation of the sodium/calcium exchanger in cardiomyocytes [19].
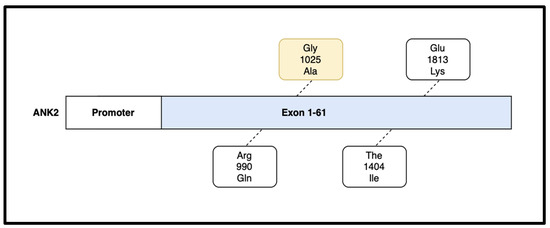
Figure 4.
The ANK2 gene contains 61 exons and is responsible for the formation of the large Ankyrin-B protein product. Various mutations have been isolated on the ANK2 gene including R990Q, T1404I, and E1813K. The G1025A mutation, highlighted in yellow, was present in our patient [18].
Ankyrin-B plays a critical role in the regulation of ion channels in cardiomyocytes, including those for potassium, sodium, and calcium ions. Ankyrin-B helps to anchor and regulate the activity of potassium channels, including the KCNQ1 and HERG channels. Mutations in the ANK2 gene can disturb the activity of potassium channels, which can cause prolongation of the QT interval and increase the risk of arrhythmias, including LQTS [17].
Ankyrin-B affects cardiac contraction by ensuring that the sodium/calcium exchanger, Na/K ATPase, and InsP3 receptors are located in the sarcoplasmic reticulum [20]. Mutations in the ANK2 gene can cause mis-localization or altered activity of sodium channels, which can impair electrical signaling and increase the risk of arrhythmias. Ankyrin-B also interacts with several calcium channels, including L-type calcium channels. Changes to L-type calcium channels can decrease heart contractility [15].
Some ANK2 variants are associated with increased susceptibility to certain cardiovascular disorders such as LQTS and arrhythmias, while others may have protective effects. For example, the SNP rs180432 on the ANK2 gene is associated with an increased risk of developing ventricular arrhythmia [21].
8. Discussion: Myocarditis, ANK2, and VT
Our patient’s genetic testing revealed a single nucleotide variant in ANK2 c.3074G>C p.G1025A. We suspect that this missense mutation exerted an effect on the activity of the ion channels in her cardiomyocytes during the stress of her myocarditis. The COVID-19-induced myocarditis served as a “second hit” compounding the effects of the change in ion channel activity and led to an “inducible” long QT syndrome, which ultimately manifested in her as highly symptomatic VT. To our knowledge, this is the first description of a c.3074G>C p.G1025A nucleotide variant of ANK2 associated with myocarditis-related VT.
Though VT was present in this patient case, myocarditis has been previously associated with various cardiac rhythm irregularities, including both bradyarrhythmias and tachyarrhythmias. These abnormal rhythms may present at any time during the disease course, and all offer a variety of presenting symptoms. Of note, unexplained premature ventricular complexes (PVCs) in children or adults are frequently secondary to underlying myocarditis [22]. These ventricular beats may be an asymptomatic finding on an ECG, or may be clinically recognized as palpitations, chest discomfort, and dizziness [23]. Novel ECG strategies have been utilized to isolate the origin of PVCs in patients, with the most common sites of origin being the left ventricular outflow tract (LVOT) and the right ventricular outflow tract (RVOT). These strategies revealed that with respect to PVCs that originate specifically from the RVOT, subclinical myocarditis appears to be the underlying mechanism [23]. In our case, we unfortunately did not have a PVC captured on a 12 lead EKG during the acute myocarditis phase to aid conclusions regarding a PVC axis, i.e., RVOT basal, LVOT basal, or elsewhere. These known arrhythmogenic properties of myocarditis, paired with the known cause–effect relationship of COVID-19 with cardiac arrythmias [24], further support the hypothesis of a multifactorial etiology responsible for our patient’s presentation.
Emerging studies have looked at the genetic susceptibility in humans to COVID-19. Various human leukocyte antigen (HLA) types have been associated with worse or better prognosis in the setting of COVID-19 infection. This is due to the ability of certain HLA types to be more or less efficient at presenting the SARS-CoV-2 peptide [25]. In addition, non-classical HLA ligands can be upregulated by the virus and cause an inhibitory effect on the immune system. This leads to a decrease in T cells, NK cells, B cells, and macrophages [26]. Other HLA types were also found to have a role in response to the COVID-19 vaccine and may indicate an important finding for vaccine selection [27]. Other genetic polymorphisms, some of which are on chromosomes 3 and 9, were found to impact COVID-19 susceptibility as well. These genetic differences allowed the SARS-CoV-2 virus enhanced access to host cells and increased viral production, along with a decreased immune response to the virus [25,28]. These genes affect immune regulation, emphasizing the importance of immunogenetics on COVID-19 susceptibility. Interestingly, our subject had an underlying history of established Crohn’s disease, and we are intrigued by the possibility that this underlying autoimmune condition may have influenced her susceptibility to myocarditis in the setting of her acute SARS-CoV-2, with her ANK2 SNV influencing the manifestation of her VT. This provides an alluring “multi-hit” hypothesis that should prompt further studies to explore the intersection of the effects of immunogenicity and genetically mediated arrhythmic risk on COVID-19 myocarditis.
9. Conclusions
These findings further advance the literature surrounding disease phenotypes being influenced by underlying genetic modifications. They also introduce new variants in ANK-2 as a potential source of susceptibility for the development of ventricular tachycardia in patients that are suffering from myocarditis secondary to various etiologies, including infection with SARS-CoV-2. Mutations in ANK2 can disturb the activity of potassium channels, sodium channels, and calcium channels, leading to increased arrhythmia risk. The potential mechanisms behind myocardial injuries and ventricular arrhythmias were explored, including dysregulated immune response, renin–angiotensin–aldosterone system dysregulation, and myocardial injury. This case study suggested that the patient’s ventricular tachycardia was likely due to a re-entrant mechanism related to myocarditis-associated injury. The patient’s single nucleotide variant in ANK2 (c.3074G>C p.G1025A) was described, and its potential influence on ion channel activity during myocarditis was suggested as a contributing factor to the severity of ventricular tachycardia. The normalization of the QT interval during the resolution phase further supported the potential influence of the genetic variant in ANK2 on potassium channel activity.
This report emphasizes the importance of rationally selected anti-arrhythmic therapy in managing ventricular tachycardia associated with COVID-19 myocarditis, and suggests a potential role for a genetic variant in ion channel genes enhancing the myocarditis disease state to VT susceptibility and severity. We anticipate that this report will lead to further research exploring the genetic and immunogenetic factors contributing to COVID-19 outcomes, and their implications for personalized treatment approaches.
Author Contributions
P.M., C.K., E.H. and A.R. all contributed equally to the authorship of this entire manuscript. M.S. conceived the hypothesis, oversaw clinical and genetic care, supervised manuscript development and editing, and was responsible for the final version. All authors have read and agreed to the published version of the manuscript.
Funding
Though this work was not specifically funded, the authors acknowledge the generous support of the Potishman Foundation, Fort Worth, Texas via restricted grants to support the broad research activity of the Sathyamoorthy Lab.
Institutional Review Board Statement
Ethical review and approval were waived for this study per TCU IRB guidelines due to the number of participants and their individual willingness to provide fully informed written consent for this report. These consents are on file at CCMS-FW.
Informed Consent Statement
Obtained and on file at CCMS-FW.
Data Availability Statement
The participant of this study provided informed written consent for publication of this report, but did not give written consent for their data to be shared publicly. Due to the sensitive nature of the research protected by US privacy laws, supporting data are not publicly available and are retained at CCSM-FW.
Acknowledgments
In addition, we would like to express our gratitude to the Consultants in Cardiovascular Medicine and Science Fort Worth for their invaluable contribution in providing us with access to medical records, obtaining records, and ensuring the accuracy and reliability of the data used in our analysis. Their support was instrumental in advancing our understanding of this case.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- del Rio, C.; Omer, S.B.; Malani, P.N. Winter of Omicron—The Evolving COVID-19 Pandemic. JAMA 2022, 327, 319–320. [Google Scholar] [CrossRef] [PubMed]
- Siripanthong, B.; Nazarian, S.; Muser, D.; Deo, R.; Santangeli, P.; Khanji, M.Y.; Cooper, L.T., Jr.; Chahal, C.A.A. Recognizing COVID-19–related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020, 17, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Kociol, R.; Cooper, L.; Fang, J.; Moslehi, J. Recognition and Initial Management of Fulminant Myocarditis|Circulation. Available online: https://www.ahajournals.org/doi/10.1161/CIR.0000000000000745 (accessed on 2 October 2022).
- Rezkalla, S.H.; Kloner, R.A. Viral myocarditis: 1917–2020: From the Influenza A to the COVID-19 pandemics. Trends Cardiovasc. Med. 2021, 31, 163–169. [Google Scholar] [CrossRef] [PubMed]
- An International, Multicentered, Evidence-Based Reappraisal of Genes Reported to Cause Congenital Long QT Syndrome|Circulation. Available online: https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.119.043132 (accessed on 20 March 2023).
- Doodnauth, A.V.; Goel, R.; Chen, L.; Uppin, V.; Malik, Z.R.; Patel, K.H.; McFarlane, S.I. Electrical Storm with Incessant Ventricular Tachycardia in a COVID-19 Patient: Review of Current Evidence. Cureus 2021, 13, e15604. [Google Scholar] [CrossRef] [PubMed]
- Peretto, G.; Sala, S.; Rizzo, S.; Palmisano, A.; Esposito, A.; De Cobelli, F.; Campochiaro, C.; De Luca, G.; Foppoli, L.; Dagna, L.; et al. Ventricular Arrhythmias in Myocarditis: Characterization and Relationships With Myocardial Inflammation. J. Am. Coll. Cardiol. 2020, 75, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Scridon, A.; Chevalier, P. Ventricular Arrhythmias Complicating Acute Myocarditis. Available online: https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-9/Ventricular-arrhythmias-complicating-acute-myocarditis (accessed on 20 March 2023).
- Farkowski, M.M.; Karlinski, M.; Pytkowski, M.; de Asmundis, C.; Lewandowski, M.; Mugnai, G.; Conte, G.; Marijon, E.; Anic, A.; Boveda, S.; et al. Mexiletine for recurrent ventricular tachycardia in adult patients with structural heart disease and implantable cardioverter defibrillator: An EHRA systematic review. EP Eur. 2022, 24, 1504–1511. [Google Scholar] [CrossRef] [PubMed]
- Mugnai, G.; Paolini, C.; Cavedon, S.; Mecenero, A.; Perrone, C.; Bilato, C. Mexiletine for ventricular arrhythmias in patients with chronic coronary syndrome: A cohort study. Acta Cardiol. 2022, 77, 264–270. [Google Scholar] [CrossRef]
- Lei, M.; Wu, L.; Terrar, D.A.; Huang, C.L.H. Modernized Classification of Cardiac Antiarrhythmic Drugs. Circulation 2018, 138, 1879–1896. [Google Scholar] [CrossRef]
- Snyder, D.W. Class IB antiarrhythmic drugs: Tocainide, mexiletine, and moricizine. J. La. State Med. Soc. Off. Organ La. State Med. Soc. 1989, 141, 21–25. [Google Scholar]
- Singh, S.; Kerndt, C.; Chauhan, S.; Zeltser, R. Mexiletine. StatPearls. Published online 12 October 2022. Available online: https://www.statpearls.com/ArticleLibrary/viewarticle/36481 (accessed on 28 March 2023).
- Agarwal, R.; Kolkhof, P.; Bakris, G.; Bauersachs, J.; Haller, H.; Wada, T.; Zannad, F. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur. Heart J. 2020, 42, 152–161. [Google Scholar] [CrossRef]
- Mohler, P.J.; Rivolta, I.; Napolitano, C.; LeMaillet, G.; Lambert, S.; Priori, S.G.; Bennett, V. Nav1.5 E1053K mutation causing Brugada syndrome blocks binding to ankyrin-G and expression of Nav1.5 on the surface of cardiomyocytes. Proc. Natl. Acad. Sci. USA 2004, 101, 17533–17538. [Google Scholar] [CrossRef]
- Mohler, P.J.; Schott, J.J.; Gramolini, A.O.; Dilly, K.W.; Guatimosim, S.; DuBell, W.H.; Song, L.S.; Haurogné, K.; Kyndt, F.; Ali, M.E.; et al. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature 2003, 421, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Mohler, P.J.; Le Scouarnec, S.; Denjoy, I.; Lowe, J.S.; Guicheney, P.; Caron, L.; Driskell, I.M.; Schott, J.J.; Norris, K.; Leenhardt, A.; et al. Defining the Cellular Phenotype of “Ankyrin-B Syndrome” Variants: Human ANK2 Variants Associated With Clinical Phenotypes Display a Spectrum of Activities in Cardiomyocytes. Circulation 2007, 115, 432–441. [Google Scholar] [CrossRef] [PubMed]
- TAAD Syndrome Genetic Testing|TAADNext|Ambry Genetics. Available online: https://www.ambrygen.com/providers/genetic-testing/12/cardiology/taadnext (accessed on 23 May 2023).
- ANK2 Ankyrin 2 [Homo Sapiens (Human)]—Gene—NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/287 (accessed on 28 March 2023).
- Lowe, J.S.; Palygin, O.; Bhasin, N.; Hund, T.J.; Boyden, P.A.; Shibata, E.; Anderson, M.E.; Mohler, P.J. Voltage-gated Nav channel targeting in the heart requires an ankyrin-G–dependent cellular pathway. J. Cell Biol. 2008, 180, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Mohler, P.J.; Splawski, I.; Napolitano, C.; Bottelli, G.; Sharpe, L.; Timothy, K.; Priori, S.G.; Keating, M.T.; Bennett, V. A cardiac arrhythmia syndrome caused by loss of ankyrin-B function. Proc. Natl. Acad. Sci. USA 2004, 101, 9137–9142. [Google Scholar] [CrossRef] [PubMed]
- Peretto, G.; Sala, S.; Rizzo, S.; De Luca, G.; Campochiaro, C.; Sartorelli, S.; Benedetti, G.; Palmisano, A.; Esposito, A.; Tresoldi, M.; et al. Arrhythmias in myocarditis: State of the art. Heart Rhythm 2019, 16, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Tsiachris, D.; Botis, M.; Doundoulakis, I.; Bartsioka, L.I.; Tsioufis, P.; Kordalis, A.; Antoniou, C.K.; Tsioufis, K.; Gatzoulis, K.A. Electrocardiographic Characteristics, Identification, and Management of Frequent Premature Ventricular Contractions. Diagnostics 2023, 13, 3094. [Google Scholar] [CrossRef] [PubMed]
- Manolis, A.S.; Manolis, A.A.; Manolis, T.A.; Apostolopoulos, E.J.; Papatheou, D.; Melita, H. COVID-19 infection and cardiac arrhythmias. Trends Cardiovasc. Med. 2020, 30, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Hollenbach, J.A. The immunogenetics of COVID-19. Immunogenetics 2023, 75, 309–320. [Google Scholar] [CrossRef]
- Liu, Y.; Ning, Z.; Chen, Y.; Guo, M.; Liu, Y.; Gali, N.K.; Sun, L.; Duan, Y.; Cai, J.; Westerdahl, D.; et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature 2020, 582, 557–560. [Google Scholar] [CrossRef]
- Bolze, A.; Neveux, I.; Barrett, K.M.S.; White, S.; Isaksson, M.; Dabe, S.; Lee, W.; Grzymski, J.J.; Washington, N.L.; Cirulli, E.T. HLA-A∗03:01 is associated with increased risk of fever, chills, and stronger side effects from Pfizer-BioNTech COVID-19 vaccination. HGG Adv. 2022, 3, 100084. [Google Scholar] [CrossRef] [PubMed]
- Ishak, A.; Mehendale, M.; AlRawashdeh, M.M.; Sestacovschi, C.; Sharath, M.; Pandav, K.; Marzban, S. The association of COVID-19 severity and susceptibility and genetic risk factors: A systematic review of the literature. Gene 2022, 836, 146674. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).