An Artificial Neural Network-Based Battery Management System for LiFePO4 Batteries
Abstract
1. Introduction
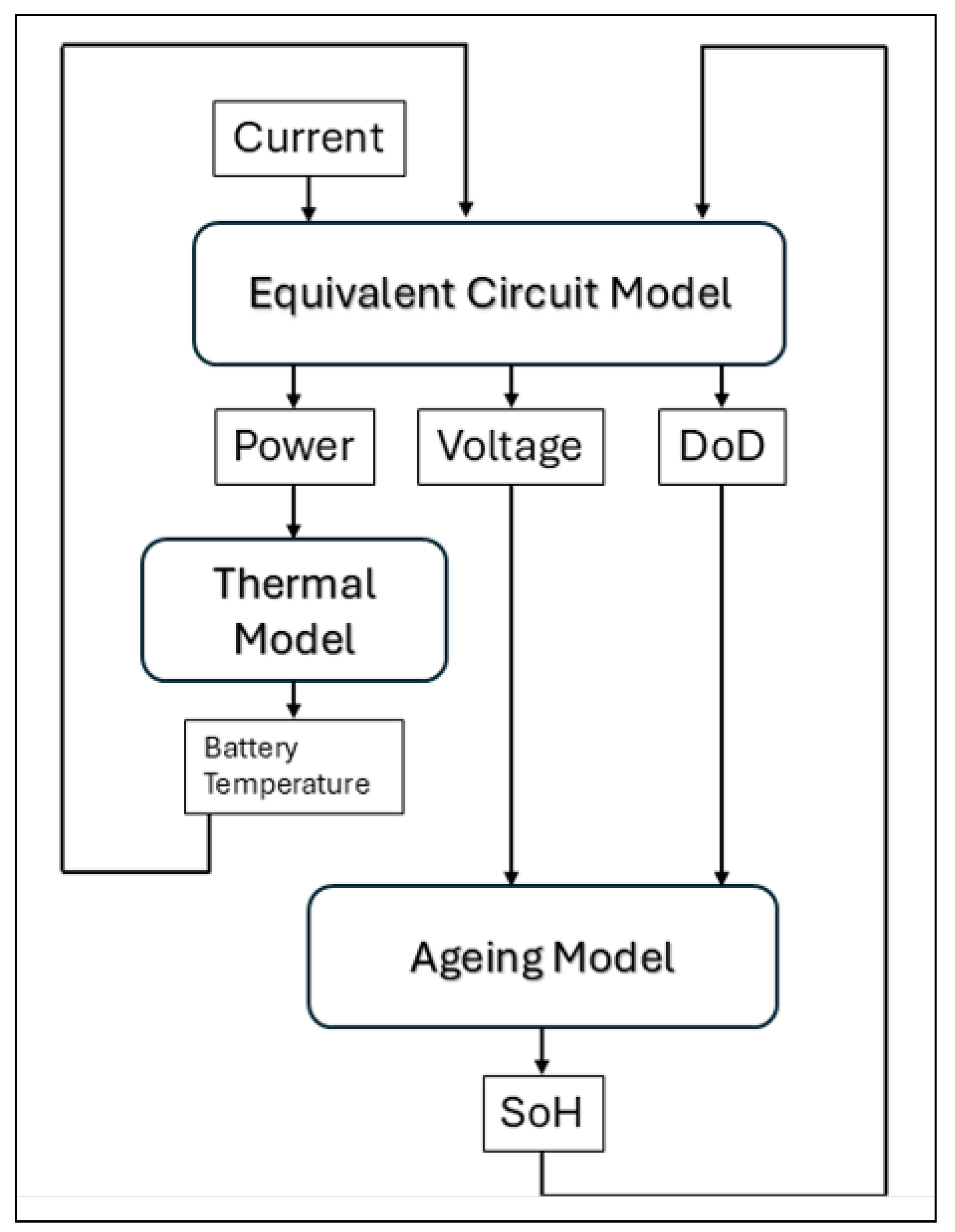
2. Materials and Methods
2.1. Coupling the Heat-Transfer Model to the Cahn–Hilliard Equation
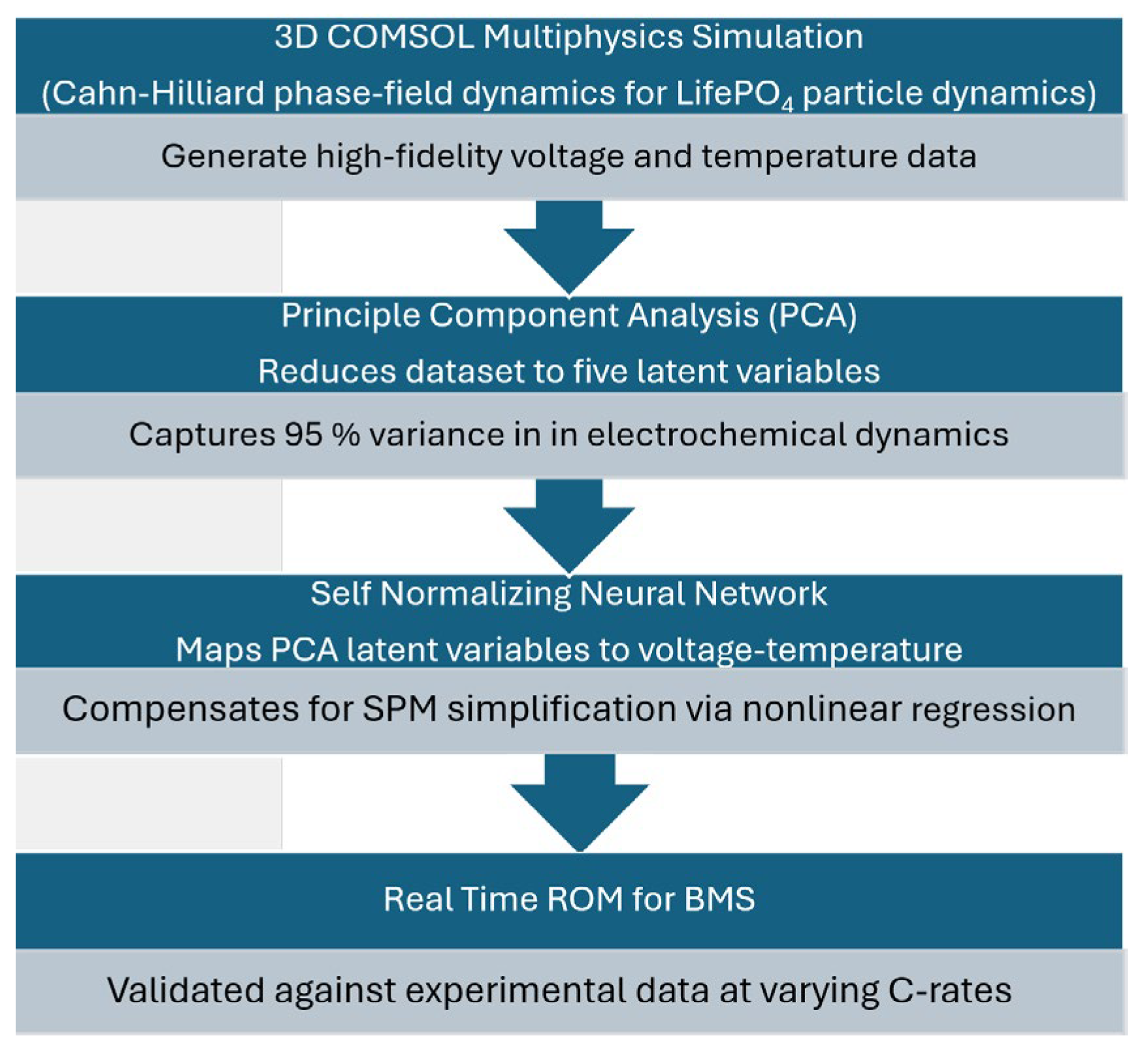
2.2. Generating the Dataset from the COMSOL® Multiphysics Simulation
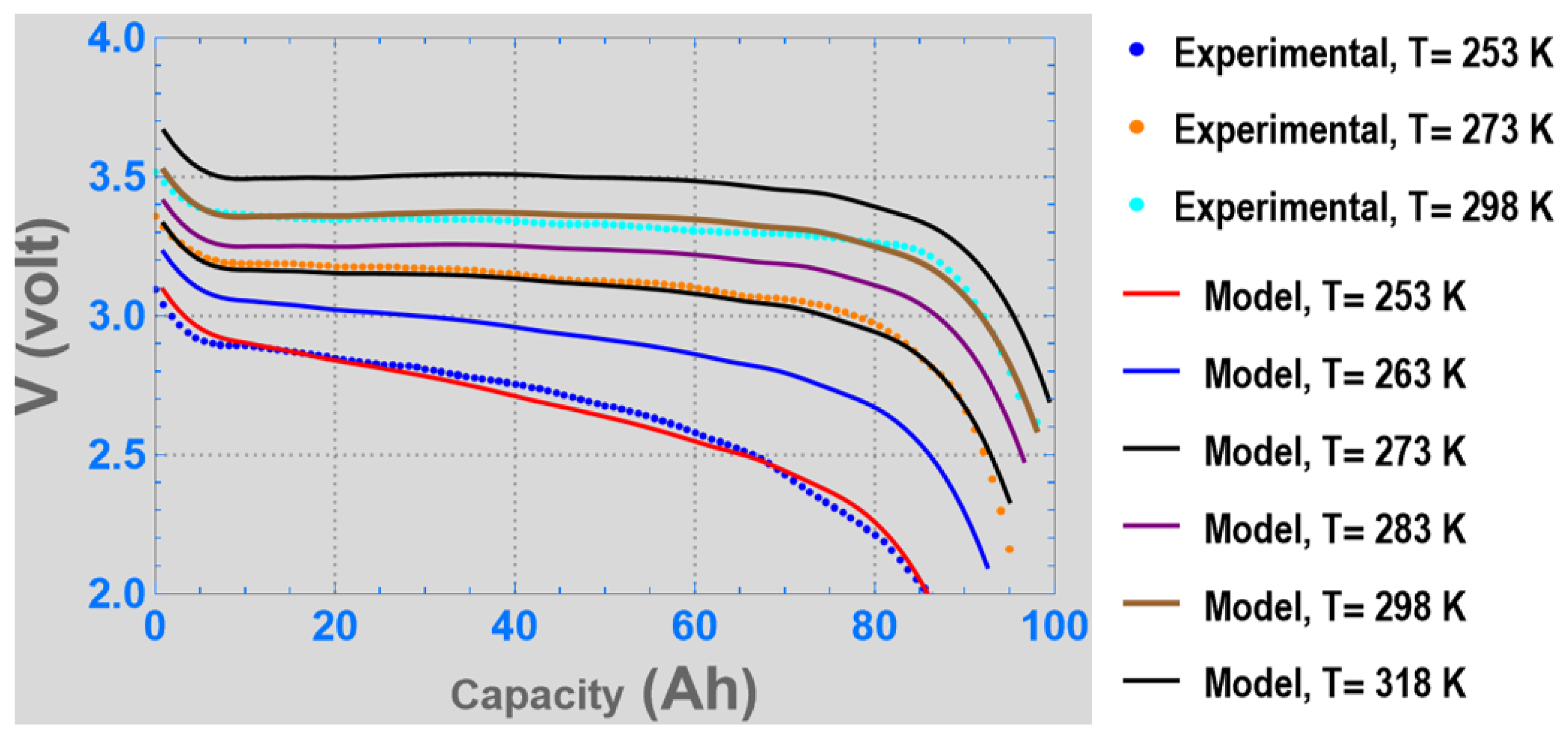
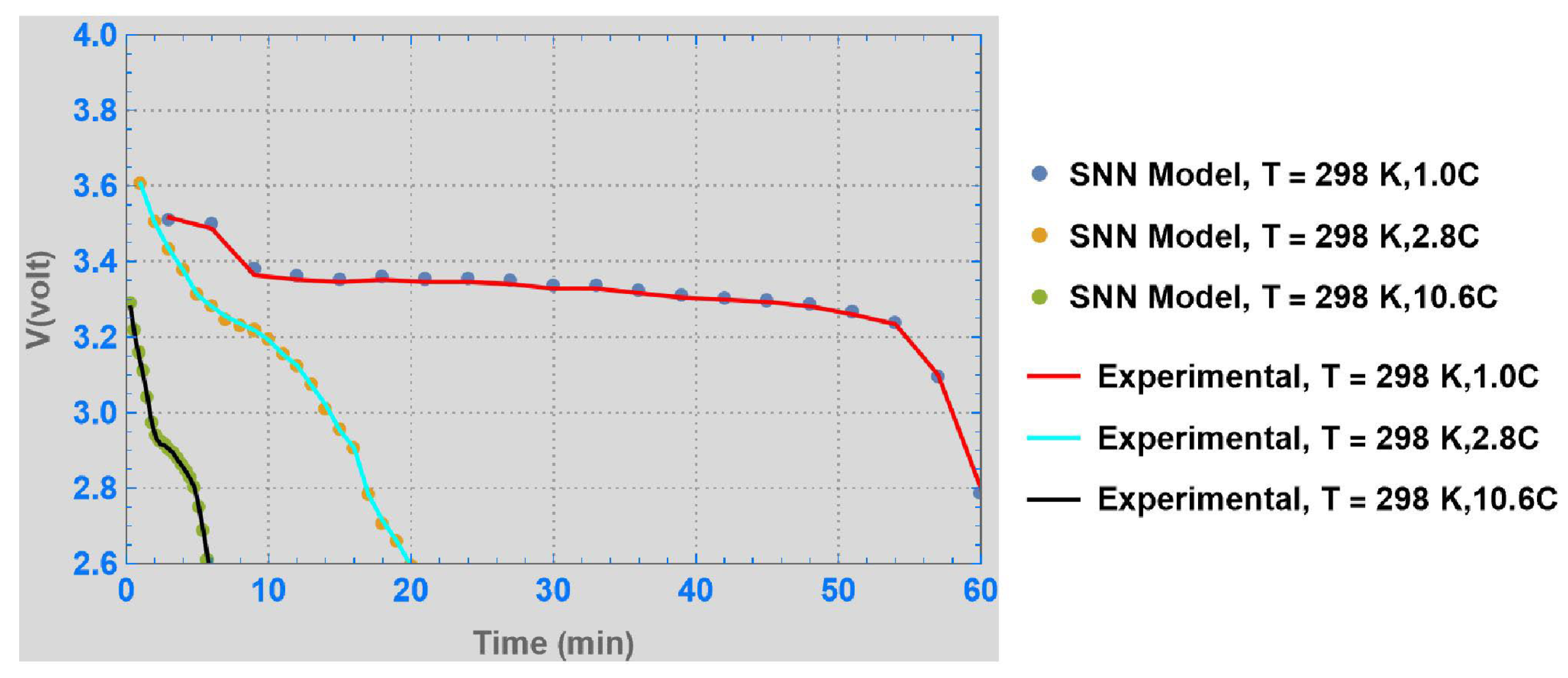
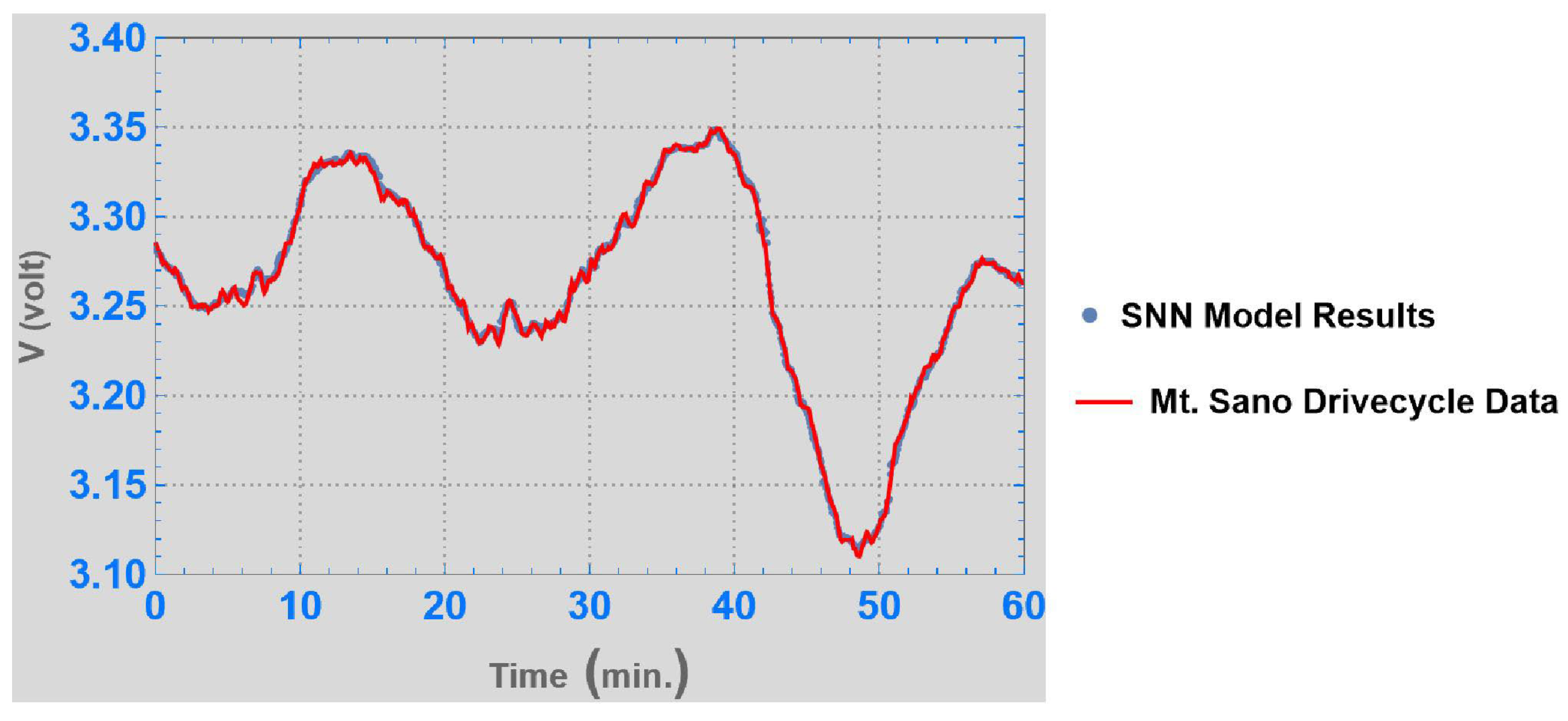
3. Results
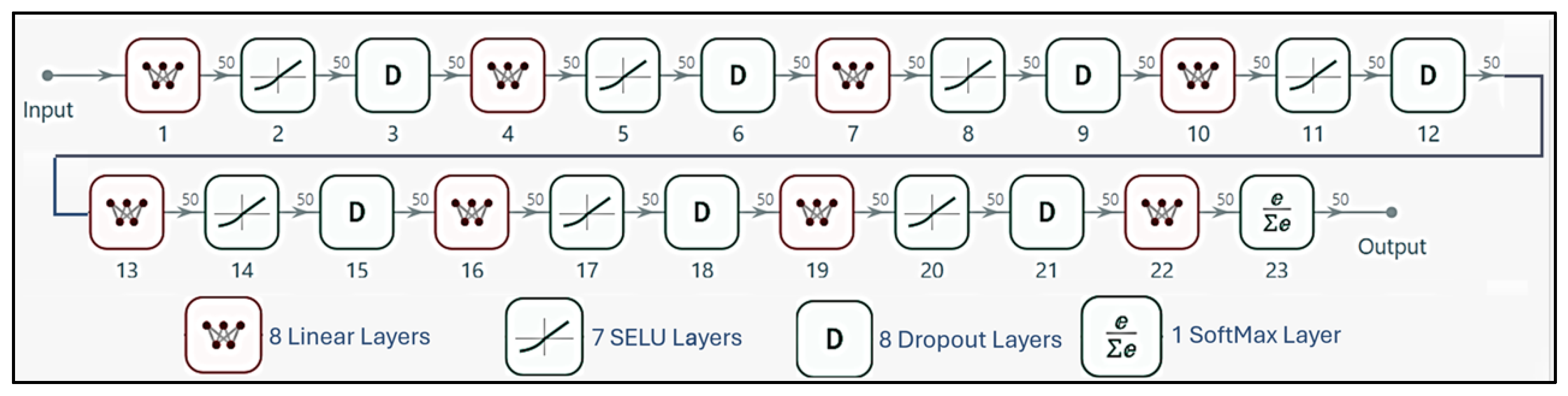
- Five nodes derived from PCA of the COMSOL® simulation data.
- Hidden layers with three nodes each, utilizing SELU activation functions;
- Alpha Dropout layers (seven total), with a 10% dropout rate between hidden layers to maintain self-normalizing properties and prevent overfitting;
- Weight initialization employing LeCun normal distribution to ensure zero mean and unit variance in initial activations.
- One node with linear activation, providing scalar voltage predictions.
- Critical Design Aspects:
- Self-Normalization Mechanism:
- The three-node width per hidden layer was optimized to preserve information from the five PCA latent variables, prevent overfitting given the limited experimental dataset, and balance computational efficiency with nonlinear representation capacity.
- Unlike conventional dropout, Alpha Dropout maintains the mean and variance of activations, ensuring compatibility with SELU’s normalization properties.
- Loss function: mean squared error (MSE);
- Optimizer: Adam with specified learning rate;
- Batch size: 32;
- Early stopping on validation split (20% of data).
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Battery surface area | Parameter | 4.12 × 10−2 [m2] | |
| Concentration | Variable | [mol m−3] | |
| Dimensionless concentration | Variable | ||
| Maximum concentration | Parameter | 1.379 × 1028 [m−3] | |
| Specific heat coefficient | Parameter | 825 J [kg−1 K−1] | |
| Convection heat-transfer coefficient | Parameter | 5.0 [W m−2 K−1] | |
| Current | Variable | [A] | |
| Dimensionless current | Variable | ||
| Current density | Parameter | 1.6 × 10−4 [A m−2] | |
| Dimensionless current density | Parameter | ||
| Current from online sensors | Variable | [A] | |
| Boltzmann constant | Constant | 3.13 × 109 [eV K−1] | |
| Radial flux | Variable | [s−1 m−2] | |
| Dimensionless radial flux | Variable | ||
| Heat loss to surroundings | Variable | [W] | |
| Particle diameter | Parameter | 1 × 10−7 [m] | |
| Temperature | Variable | [K] | |
| Surroundings temperature | Parameter | (253–298) [K] | |
| Temperature from on-board sensor | Variable | [K] | |
| Voltage | Variable | [V] | |
| Dimensionless voltage | Variable | ||
| Single-particle voltage for a half-filled particle | Variable | [V] | |
| Cell voltage at 50 % SOC | Variable | [V] | |
| Reference voltage | Constant | 3.42 [V] | |
| Voltage simulated by the SPM | Variable | [V] | |
| Dimensionless reference voltage | Constant | ||
| Electron transfer symmetry factor | Parameter | 0.5 | |
| Activation potential | Variable | - | |
| Dimensionless activation potential | Variable | ||
| Near-equilibrium voltage | Variable | [V] | |
| Chemical potential | Variable | [eV] | |
| Dimensionless chemical potential | Variable | ||
| Potential energy term from the SPM | Variable | - | |
| Potential energy estimated from Equation with 7 | Variable | - | |
| Open-circuit voltage | |||
| Battery volume | Parameter | 3.42 × 10−5 [m3] | |
| Battery density | Parameter | 1824 [kg m−3] | |
| Enthalpy of mixing | Parameter | 0.115 [eV] |
References
- Tian, J.; Xiong, R.; Shen, W. Electrode aging estimation and open circuit voltage reconstruction for lithium-ion batteries. Energy Storage Mater. 2021, 37, 283–295. [Google Scholar] [CrossRef]
- Tian, J.; Xiong, R.; Shen, W.; Lu, J. State-of-charge estimation of LiFePO4 batteries in electric vehicles: A deep-learning enabled approach. Appl. Energy 2021, 291, 116812. [Google Scholar] [CrossRef]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Dai, H.; Jiang, B.; Hu, X.; Lin, X.; Wei, X.; Pecht, M. Advanced battery management strategies for a sustainable energy future: Multilayer design concepts and research trends. Renew. Sustain. Energy Rev. 2021, 138, 110480. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, J.; Sun, Z.; Wang, L.; Xu, R.; Li, M.; Chen, Z. A comprehensive review of battery modeling and state estimation approaches for advanced battery management systems. Renew. Sustain. Energy Rev. 2020, 131, 110015. [Google Scholar] [CrossRef]
- Scrosati, B.; Garche, J. Lithium Batteries: Status, Prospects and Future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Tanim, T.R.; Rahn, C.D. State of charge estimation of a lithium ion cell based on a temperature dependent and electrolyte en- hanced single particle model. J. Power Sources 2015, 294, 638–649. [Google Scholar] [CrossRef]
- Fergus, J. Recent Developments in Cathode Materials for Lithium-Ion Batteries. J. Power Sources 2010, 195, 939–954. [Google Scholar] [CrossRef]
- Shim, J. Electrochemical analysis for cycle performance and capacity fading of a lithium-ion battery cycled at elevated temperature. J. Power Sources 2002, 112, 222–230. [Google Scholar] [CrossRef]
- Ning, G.; Popov, B. Cycle Life Modeling of Lithium-Ion Batteries. J. Electrochem. Soc. 2004, 151, A1584. [Google Scholar] [CrossRef]
- Spotnitz, R.; Franklin, J. Abuse behavior of high-power, lithium-ion cells. J. Power Sources 2003, 113, 81–100. [Google Scholar] [CrossRef]
- Krewer, U.; Roder, F.; Harinath, E.; Braatz, R.; Bedurftig, B.; Findeisen, R. Review—Dynamic Models of Li-Ion Batteries for Diagnosis and Operation: A Review and Perspective. J. Electrochem. Soc. 2018, 165, A3656–A3673. [Google Scholar] [CrossRef]
- Sakar, S.; Halim, Z.; El-Halwagi, M.; Khan, F. Electrochemical models: Methods and applications for safer lithium-ion battery operation. J. Electrochem. Soc. 2022, 169, 100501. [Google Scholar] [CrossRef]
- Li, Y.; Karunathilake, D.; Vilathgamuwa, D.M.; Mishra, Y.; Farrell, T.W.; Zou, C. Model Order Reduction Techniques for Physics-Based Lithium-Ion Battery Management. IEEE Ind. Electron. Mag. 2022, 16, 36–51. [Google Scholar] [CrossRef]
- Yannliaw, B. Modeling of lithium-ion cells? A simple equivalent-circuit model approach. Solid State Ion. 2004, 175, 835–839. [Google Scholar] [CrossRef]
- Xie, Y.; Li, W.; Hu, X.; Tran, M.K.; Panchal, S.; Fowler, M.; Liu, K. Co-estimation of SOC and three-dimensional SOT for lithium-ion batteries based on distributed spatial-temporal online correction. IEEE Trans. Ind. Electron. 2022, 70, 5937–5948. [Google Scholar] [CrossRef]
- Sekhar, R.; Shah, P.; Panchal, S.; Fowler, M.; Fraser, R. Distance to empty soft sensor for ford escape electric vehicle. Results Control. Optim. 2022, 9, 100168. [Google Scholar] [CrossRef]
- Verbrugge, M. Adaptive, multi-parameter battery state estimator with optimized time-weighting factors. J. Appl. Electrochem. 2007, 37, 605–616. [Google Scholar] [CrossRef]
- Hu, Y.; Yurkovich, S.; Guezennec, Y.; Yurkovich, B. Electro-thermal battery model identification for automotive applications. J. Power Sources 2011, 196, 449–457. [Google Scholar] [CrossRef]
- Perez, H.E.; Siegel, J.B.; Lin, X.; Stefanopoulou, A.G.; Ding, Y.; Castanier, M.P. Parameterization and Validation of an Integrated Electro-Thermal Cylindrical LFP Battery Model. In Dynamic Systems and Control Conference; American Society of Mechanical Engineers: New York, NY, USA, 2012. [Google Scholar]
- Torabi, F.; Ahmadi, P. Simulation of Battery Systems; Academic Press: London, UK, 2019. [Google Scholar]
- Barreras, J.; Schaltz, E.; Andreasen, S. Datasheet-Based Modeling of Li-Ion Batteries. In Proceedings of the 2012 IEEE Vehicle Power and Propulsion Conference, Seoul, Republic of Korea, 9–12 October 2012. [Google Scholar]
- Zou, Z.; Xu, J.; Mi, C.; Cao, B.; Chen, Z. Evaluation of Model Based State of Charge Estimation Methods for Lithium-Ion Batteries. Energies 2014, 7, 5065–5082. [Google Scholar] [CrossRef]
- Suri, R.; Onori, S. A control-oriented cycle-life model for hybrid electric vehicle lithium-ion batteries. Energy 2017, 120, 549–561. [Google Scholar] [CrossRef]
- Liu, K.; Li, K.; Peng, Q.; Zhang, C. A brief review on key technologies in the battery management system of electric vehicles. Front. Mech. Eng. 2018, 13, 251–267. [Google Scholar] [CrossRef]
- Wei, Z.; Hu, J.; He, H.; Yu, Y.; Marco, J. Embedded distributed temperature sensing enabled multistate joint observation of smart lithium-ion battery. IEEE Trans. Ind. Electron. 2022, 70, 555–565. [Google Scholar] [CrossRef]
- Hu, X.; Sun, F.; Zou, Y. Estimation of state of charge of a lithium-ion battery pack for electric vehicles using an adaptive Luenberger observer. Energies 2010, 3, 1586–1603. [Google Scholar] [CrossRef]
- Kim, I. The novel state of charge estimation method for lithium battery using sliding mode observer. J. Power Sources 2006, 163, 584–590. [Google Scholar] [CrossRef]
- Sun, F.; Xiong, R.; He, H.; Li, W.; Aussems, J. Model-based dynamic multi-parameter method for peak power estimation of lithium-ion batteries. Appl. Energy 2012, 96, 378–386. [Google Scholar] [CrossRef]
- Xu, J.; Mi, C.; Cao, B.; Deng, J.; Chen, Z.; Li, S. The state of charge estimation of lithium-ion batteries based on a proportional integral observer. IEEE Trans. Veh. Technol. 2014, 63, 1614–1621. [Google Scholar] [CrossRef]
- Yu, Z.; Xiao, L.; Li, H.; Zhu, X.; Huai, R. Model parameter identification for lithium batteries using the coevolutionary particle swarm optimization method. IEEE Trans. Ind. Electron. 2017, 64, 5690–5700. [Google Scholar] [CrossRef]
- Dong, G.; Wei, J.; Zhang, C.; Chen, Z. Online state of charge estimation and open circuit voltage hysteresis modeling of LiFePO4 battery using invariant imbedding method. Appl. Energy 2016, 162, 163–171. [Google Scholar] [CrossRef]
- Rahimi-Eichi, H.; Baronti, F.; Chow, M.-Y. Online adaptive parameter identification and state-of-charge coestimation for lithium-polymer battery cells. IEEE Trans. Ind. Electron. 2014, 61, 2053–2061. [Google Scholar] [CrossRef]
- Xia, B.; Zhao, X.; De Callafon, R.; Garnier, H.; Nguyen, T.; Mi, C. Accurate lithium-ion battery parameter estimation with continuous-time system identification methods. Appl. Energy 2016, 179, 426–436. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, R.; He, H.; Shen, W. A lithium-ion battery pack state of charge and state of energy estimation algorithms using a hardware-inthe-loop validation. IEEE Trans. Power Electron. 2017, 32, 4421–4431. [Google Scholar] [CrossRef]
- Wei, Z.; Dong, G.; Zhang, X.; Pou, J.; Quan, Z.; He, H. Noise-immune model identification and state-of-charge estimation for lithium-ion battery using bilinear parameterization. IEEE Trans. Ind. Electron. 2021, 68, 312–323. [Google Scholar] [CrossRef]
- Padhi, A.K. Phospho-olivines as Positive-Electrode Materials for Rechargeable Lithium Batteries. J. Electrochem. Soc. 1997, 144, 1188. [Google Scholar] [CrossRef]
- Yamada, A.; Chung, S.C.; Hinokuma, K. ChemInform Abstract: Optimized LiFePO4 for Lithium Battery Cathodes. ChemInform 2010, 32, 17. [Google Scholar] [CrossRef]
- Huang, H.; Yin, S.; Nazar, L.F. Approaching Theoretical Capacity of LiFePO4 at Room Temperature at High Rates. Electrochem. Solid-State Lett. 2001, 4, A170. [Google Scholar] [CrossRef]
- Ravet, N.; Chouinard, Y.; Magnan, J.; Besner, S.; Gauthier, M.; Armand, M. Electroactivity of natural and synthetic triphylite. J. Power Sources 2001, 97–98, 503–507. [Google Scholar] [CrossRef]
- Chung, S.; Bloking, J.T.; Chiang, Y. Electronically conductive phospho-olivines as lithium storage electrodes. Nat. Mater. 2002, 1, 123–128. [Google Scholar] [CrossRef]
- Newman, J.; Tiedemann, W. Porous-Electrode Theory with Battery Applications. AIChE J. 1975, 21, 25–41. [Google Scholar] [CrossRef]
- Doyle, M. Modeling of Galvanostatic Charge and Discharge of the Lithium/Polymer/Insertion Cell. J. Electrochem. Soc. 1993, 140, 1526. [Google Scholar] [CrossRef]
- Bazant, M. Theory of Chemical Kinetics and Charge Transfer Based on Nonequilibrium Thermodynamics. Acc. Chem. Res. 2013, 46, 1144–1160. [Google Scholar] [CrossRef]
- Singh, G.; Ceder; Bazant, M. Intercalation dynamics in rechargeable battery materials: General theory and phase-transformation waves in LiFePO4. Electrochim. Acta 2008, 53, 7599–7613. [Google Scholar] [CrossRef]
- Painter, R.; Embry, I.; Sharpe, L.; Hargrove, S.K. A Reduced Order Thermal Model for Lithium-Ion Batteries Derived from the Cahn-Hilliard Equation. In Proceedings of the 2020 COMSOL® Conference, Boston, MA, USA, 7–8 October 2020. [Google Scholar]
- Bai, P.; Cogswell, D.; Bazant, M.Z. Suppression of phase separation in LiFePO4 nanoparticles during battery discharge. Nano Lett. 2011, 11, 4890–4896. [Google Scholar] [CrossRef]
- Malik, R.; Abdellahi, A.; Ceder, G. A Critical Review of the Li Insertion Mechanisms in LiFePO4 Electrodes. J. Electrochem. Soc. 2013, 160, A3179–A3197. [Google Scholar] [CrossRef]
- Chen, L. Phase-Field Models for Microstructure Evolution, Annu. Rev. Mater. Res. 2002, 32, 113–140. [Google Scholar] [CrossRef]
- Yuan, C.; Gao, X.; Jia, Y.; Zhang, W.; Wu, Q.; Xu, J. Coupled crack propagation and dendrite growth in solid electrolyte of all-solid-state battery. Nano Energy 2021, 86, 106057. [Google Scholar] [CrossRef]
- Yuan, C.; Lu, W.; Xu, J. Unlocking the electrochemical–mechanical coupling behaviors of dendrite growth and crack propagation in all-solid-state batteries. Adv. Energy Mater. 2021, 11, 2101807. [Google Scholar] [CrossRef]
- Guo, M.; Sikha, G.; White, R. Single-Particle Model for A Lithium-Ion Cell: Thermal Behavior. Electrochem. Soc. 2011, 158, A122, Erratum in J. Electrochem. Soc. 2011, 158, S11. [Google Scholar] [CrossRef]
- Cai, L.; White, R. Mathematical Modeling of a Lithium Ion Battery with Thermal Effects in COMSOL® Inc. Multiphysics (MP) Software. J. Power Sources 2011, 196, 5985–5989. [Google Scholar] [CrossRef]
- Painter, R.; Berryhill, B.; Sharpe, L.; Hargrove, S.K. A Single Particle Thermal Model for Lithium-Ion Batteries. In Proceedings of the 2012 COMSOL® Conference, Boston, MA, USA, 3–5 October 2020. [Google Scholar]
- Painter, R.; Sharpe, L.; Hargrove, S.K. A Phase Field Model for Lithium Batteries. In Proceedings of the 2012 COMSOL® Conference, Boston, MA, USA, 3–5 October 2020. [Google Scholar]
- Sullivan, U.V. Published 2019. Available online: http://www.sullivanuv.com/wp-content/uploads/2014/06/A123-Cell-Datasheet-2008-08.pdf (accessed on 1 December 2019).
- Zeng, Y.; Bazant, M. Cahn-Hilliard Reaction Model for Isotropic Li-Ion Battery Particles. MRS Proc. 2013, 1542, 0201. [Google Scholar] [CrossRef]
- Forgez, C.; Vinh Do, D.; Friedrich, G.; Morcrette, M.; Delacourt, C. Thermal modeling of a cylindrical LiFePO4/graphite lithium-ion battery. J. Power Sources 2010, 195, 2961–2968. [Google Scholar] [CrossRef]
- Youngki, K.; Shanka, M.; Siegel, J.; Stefanopoulou, A. The Estimation of Temperature Distribution in Cylindrical Battery Cells Under Unknown Cooling Conditions. IEEE Trans. Control. Syst. Technol. 2014 22, 2277–2286. [CrossRef]
- Youngki, K. Power Capability Estimation Accounting for Thermal and Electrical Constraints for Lithium-Ion Batteries. Ph.D. Thesis, University of Michigan, Ann Arbor, MI, USA, 2014. [Google Scholar]
- Li, Y.; Li, K.; Liu, X.; Zhang, L. Fast battery capacity estimation using convolutional neural networks. Trans. Inst. Meas. Control 2020, 42, 1–14. [Google Scholar] [CrossRef]
- Available online: https://docplayer.net/20772679-Managing-a123-cells-with-fma-cell-balancing-technologies.html (accessed on 1 March 2022).
- Pivelait, B.; Rentel, C.; Plett, G.; Marcel, M.; Carmen, D. An advanced battery management system for lithium-ion batteries. In Proceedings of the 2011 NDIA Ground Vehicle Systems Engineering and Technology Symposium, Dearborn, MI, USA, 9–11 August 2011. [Google Scholar]
| Nominal capacity and voltage | 2.3 Ah, 3.3 V |
| Internal impedance (1 kHz AC) | 8 mΩ typical |
| Internal resistance 10 A, 1 s DC | 10 mΩ typical |
| Recommended charge method | 3 A to 3.6 V CCCV, 45 min |
| Recommended fast charge current | 10 A to 3.6 CCCV |
| Maximum continuous discharge | 70 A |
| Pulse discharge at 10 s | 120 A |
| Cycle life at 10 C discharge | Over 1000 cycles |
| Recommended pulse charge/discharge cutoff | 3.8 V to 1.6 V |
| Operating temperature range | 243 K to 333 K |
| Core cell weight | 70 g |
| Parameter | Symbol | Value | Unit |
|---|---|---|---|
| Density | ρ | 1824 | kg/m3 |
| Specific heat coefficient | Cp | 825 | J/kg K |
| Thermal conductivity | kt | 0.488 | W/m K |
| Convection coefficient | h | 5.0 | W/m2-K |
| Radius | R | 12.93 × 10−3 | m |
| Height | L | 65.15 × 10−3 | m |
| Volume | V | 3.42 × 10−5 | m3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the World Electric Vehicle Association. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Painter, R.; Parthasarathy, R.; Li, L.; Embry, I.; Sharpe, L.; Hargrove, S.K. An Artificial Neural Network-Based Battery Management System for LiFePO4 Batteries. World Electr. Veh. J. 2025, 16, 282. https://doi.org/10.3390/wevj16050282
Painter R, Parthasarathy R, Li L, Embry I, Sharpe L, Hargrove SK. An Artificial Neural Network-Based Battery Management System for LiFePO4 Batteries. World Electric Vehicle Journal. 2025; 16(5):282. https://doi.org/10.3390/wevj16050282
Chicago/Turabian StylePainter, Roger, Ranganathan Parthasarathy, Lin Li, Irucka Embry, Lonnie Sharpe, and S. Keith Hargrove. 2025. "An Artificial Neural Network-Based Battery Management System for LiFePO4 Batteries" World Electric Vehicle Journal 16, no. 5: 282. https://doi.org/10.3390/wevj16050282
APA StylePainter, R., Parthasarathy, R., Li, L., Embry, I., Sharpe, L., & Hargrove, S. K. (2025). An Artificial Neural Network-Based Battery Management System for LiFePO4 Batteries. World Electric Vehicle Journal, 16(5), 282. https://doi.org/10.3390/wevj16050282







