The Overlooked Role of Battery Cell Relaxation: How Reversible Effects Manipulate Accelerated Aging Characterization †
Abstract
1. Introduction
1.1. Fundamental Electrochemical and Physical Processes Impacting Cycle Life
1.2. Contributions and Layout
- (1)
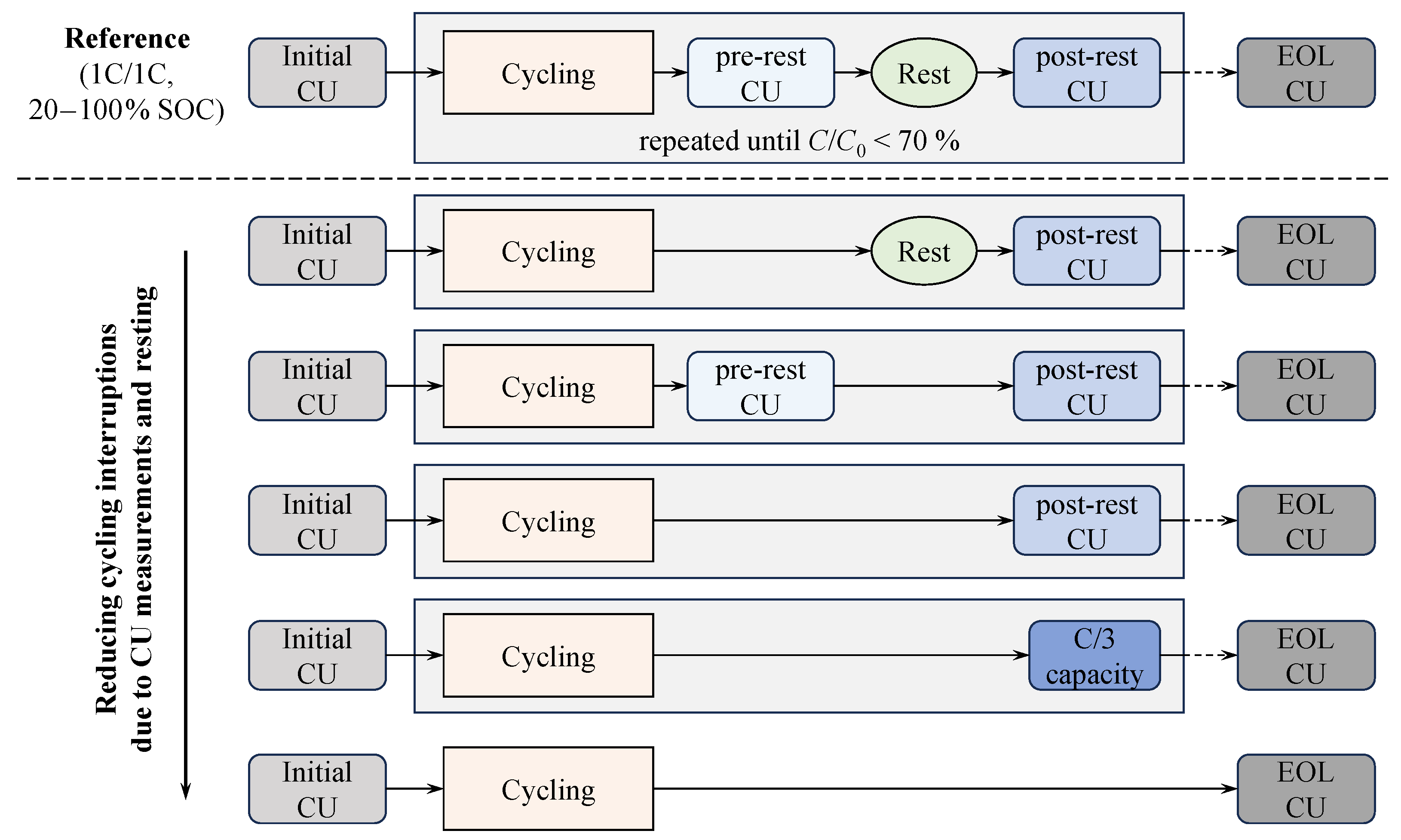
- Understanding the impact of the test procedure on cell inhomogeneity:We address how the test procedure induces cell inhomogeneity, how it manifests, and how it affects both short- and long-term cell behavior as well as cycle stability. For this purpose, cell conditions before and after regular rest phases in the cycle aging tests are captured, and capacity differences, indicating equalization processes, are analyzed.
- (2)
- Investigating the impact of various stress factors on cell inhomogeneity:The impact of DOD and C-rate during cycling is assessed. We investigate the correlation between stress levels, the degree of inhomogenization and equalization, and the occurrence of lithium plating.
- (3)
- Supporting cell relaxation and rehomogenization during cycling:Based on these findings, we focus on rehomogenization behavior. We investigate static and dynamic methods to actively promote the equalization of inhomogeneities during accelerated cycle aging tests, aiming to minimize their impact on cell performance and degradation.
2. Materials and Methods
2.1. Cell Specifications
2.2. Measurement Equipment
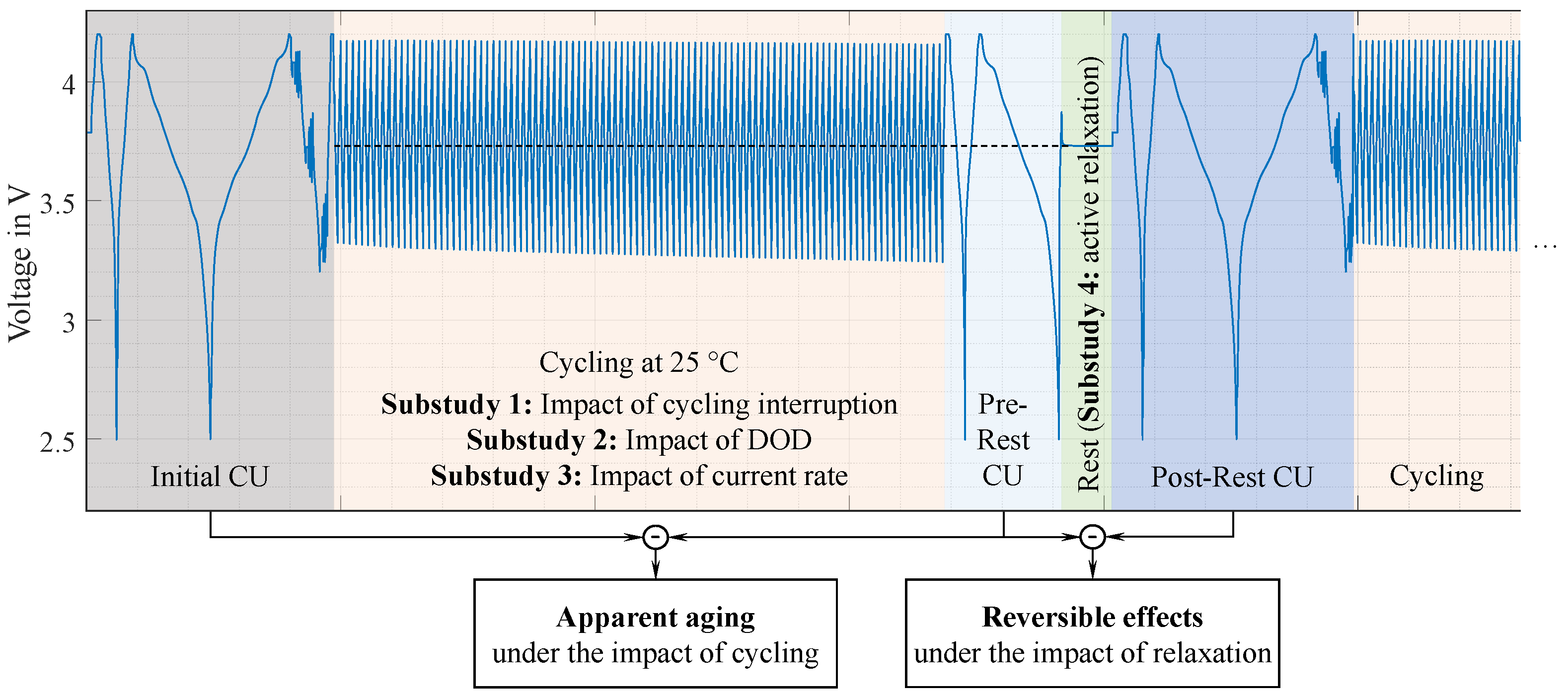
2.3. Test Design and Measurement Procedures
2.3.1. Check-Up Procedures
2.3.2. Cycle Aging Test Procedures
2.4. Analysis Methods
3. Results and Discussion
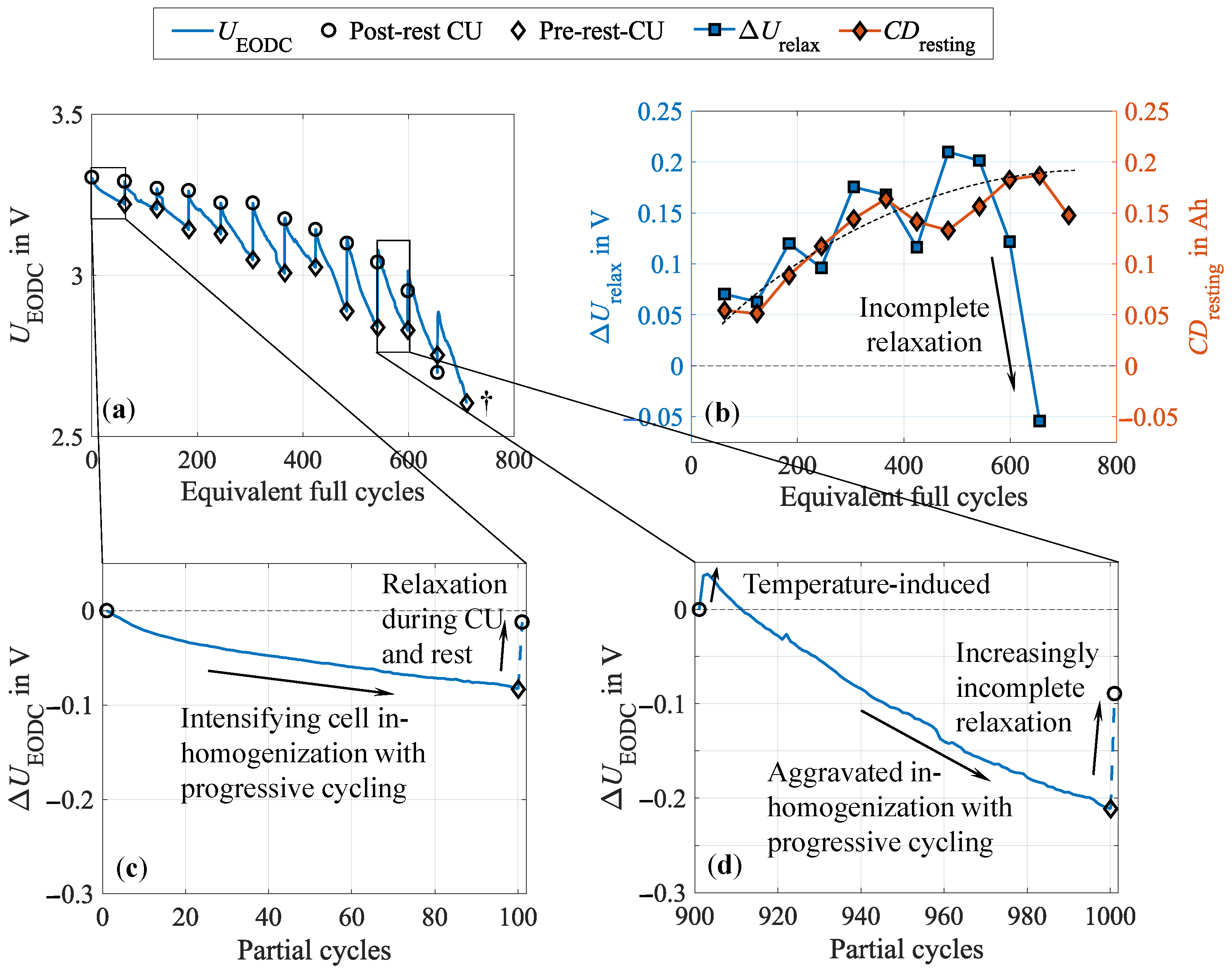
3.1. Formation of Cell Inhomogeneities During Cycling
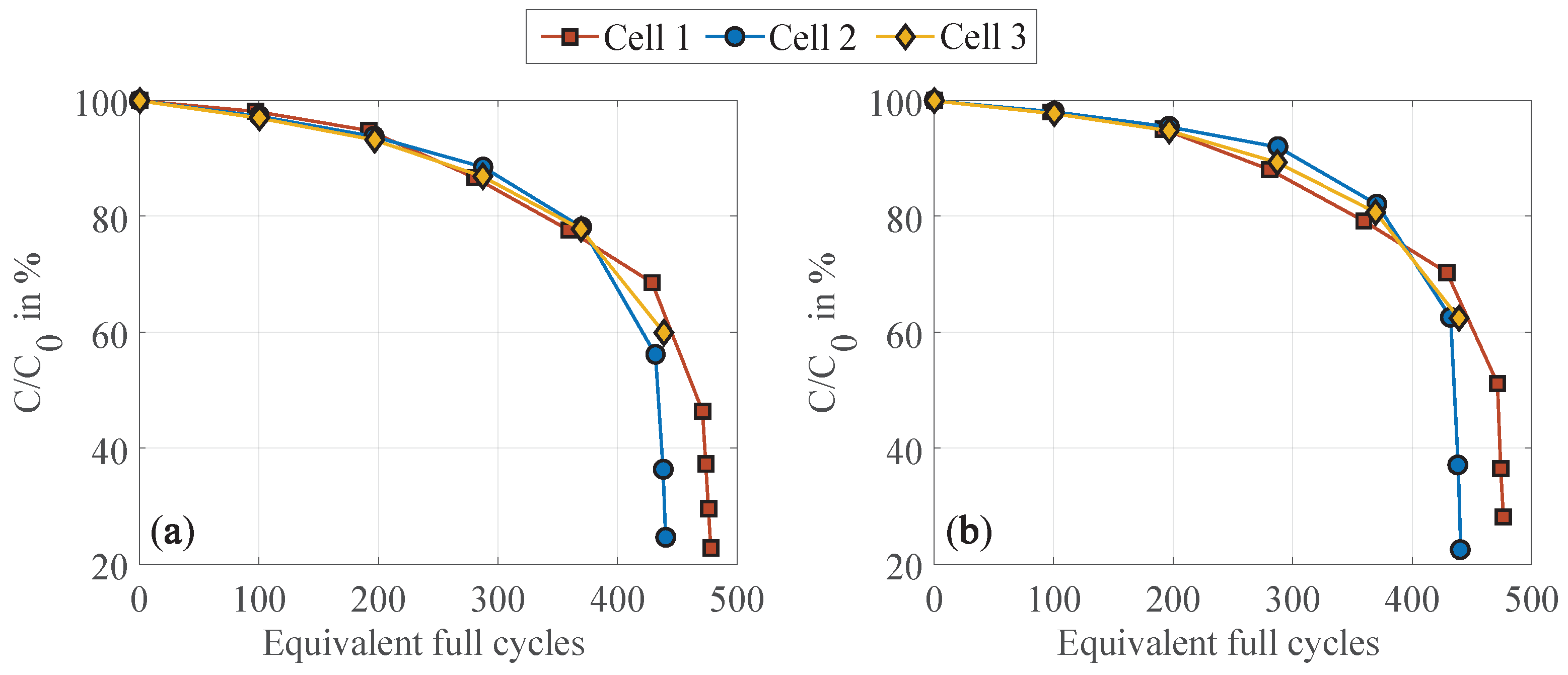
3.2. Statistical Significance and Robustness
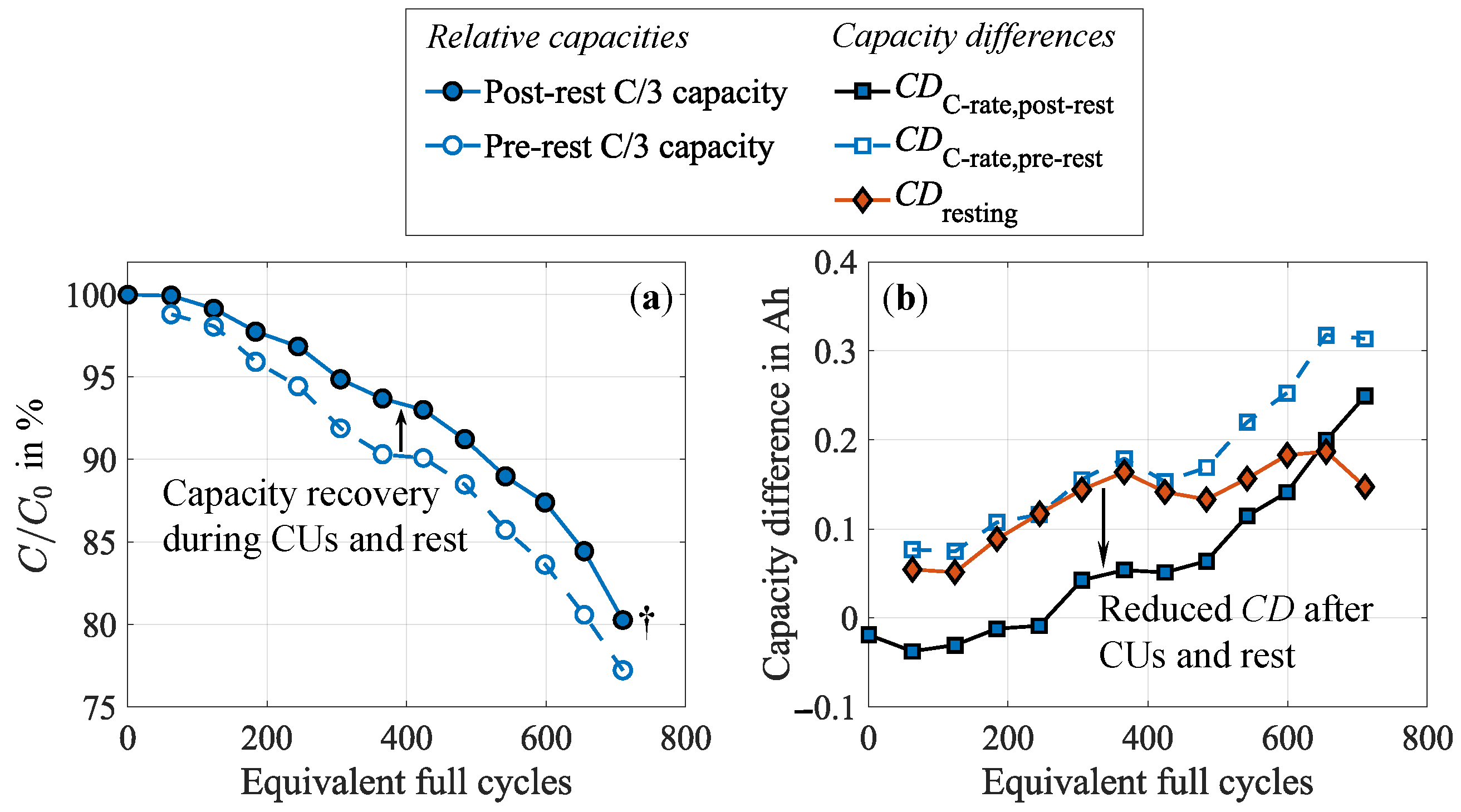
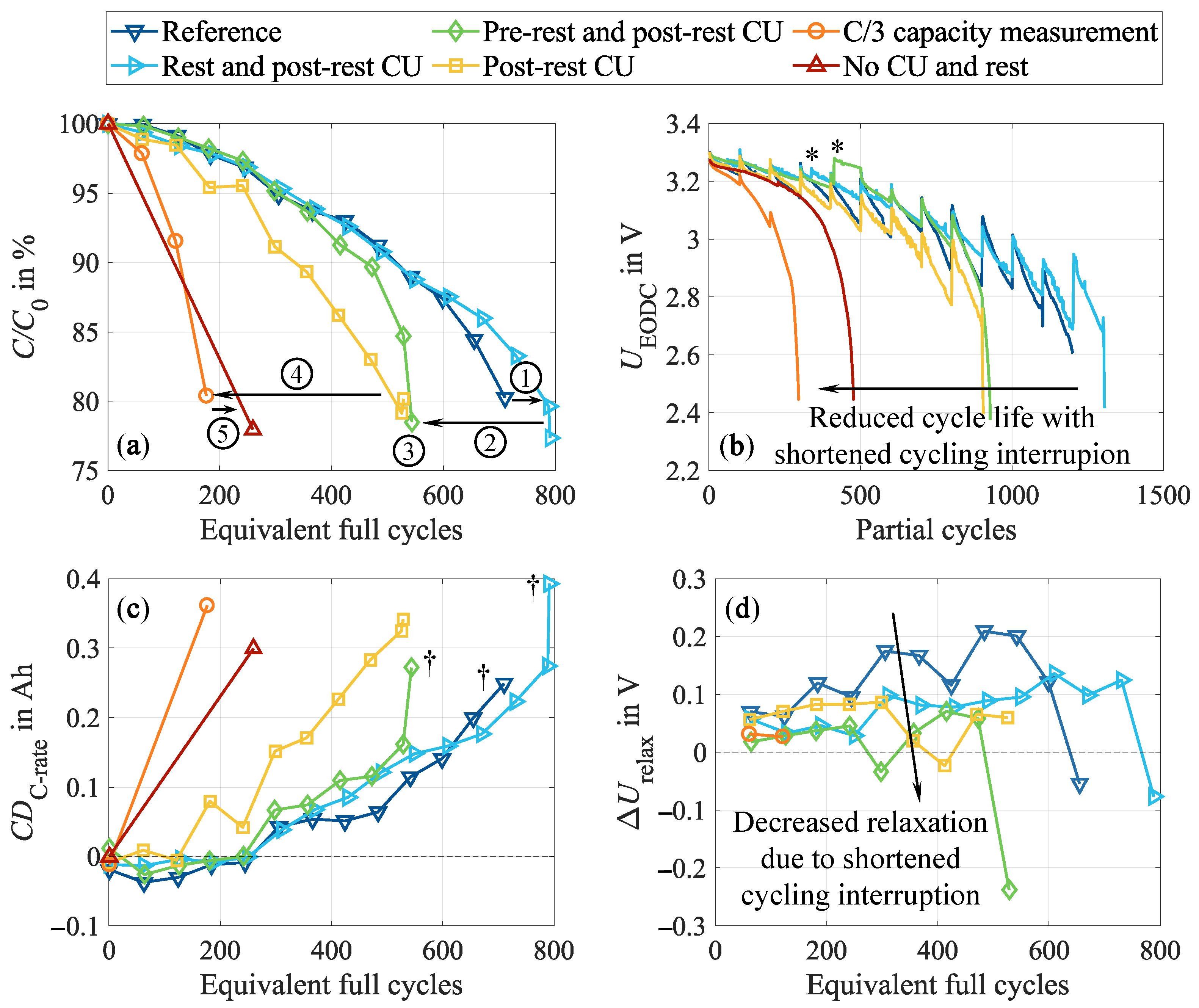
3.3. Impact of the Test Procedure on Cell Inhomogeneities
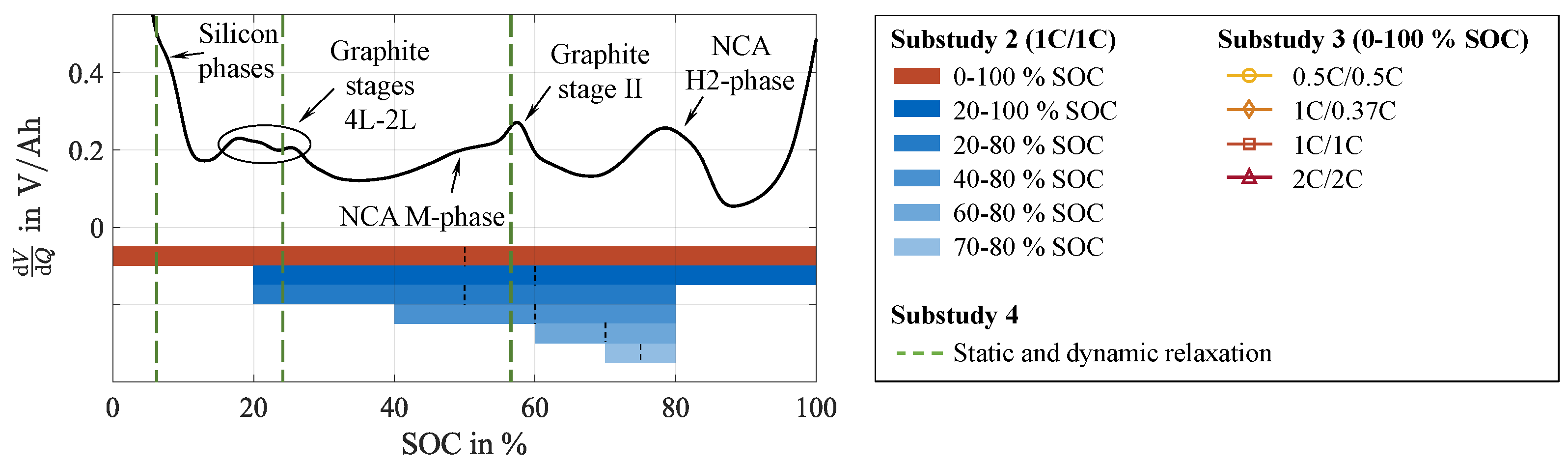
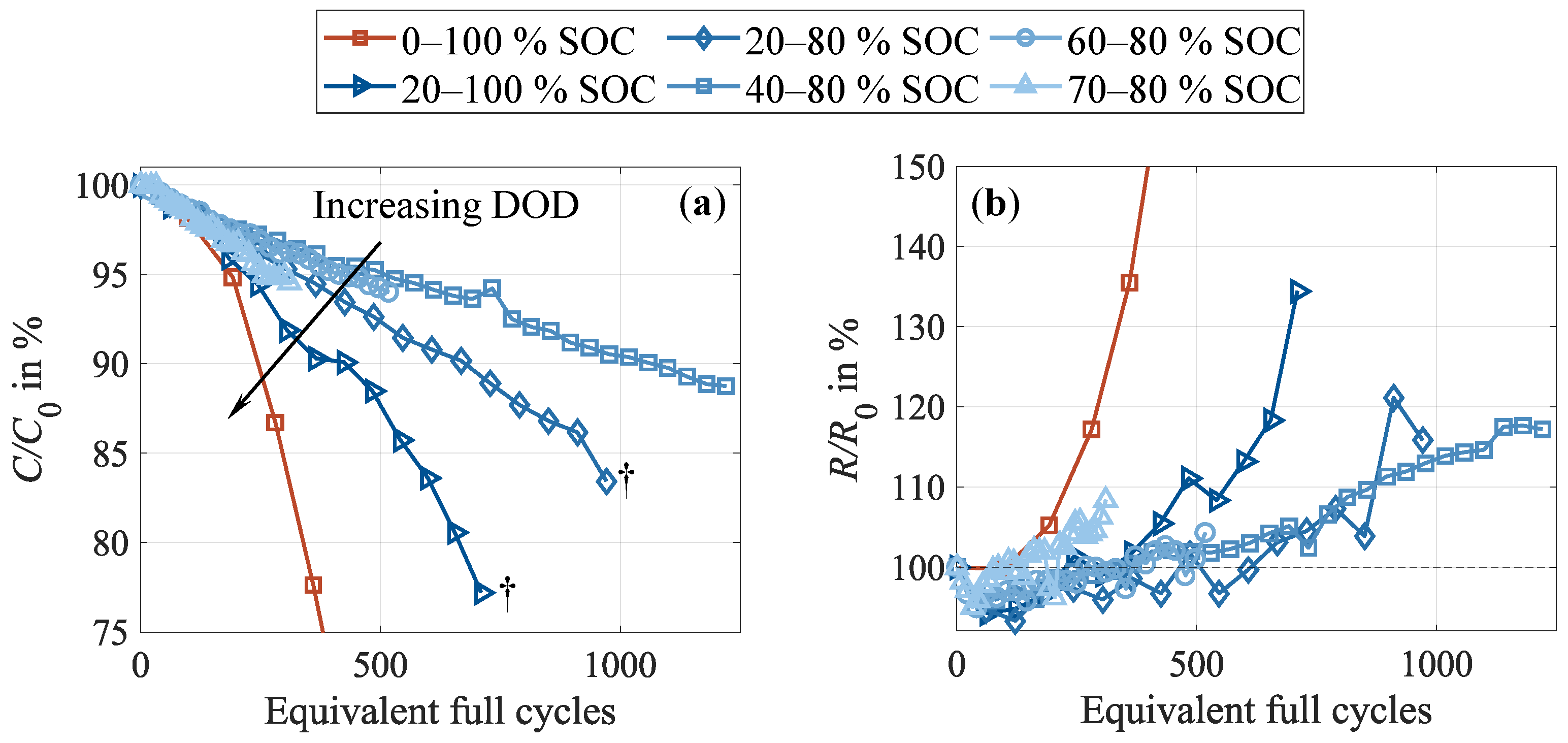
3.4. Impact of Stress Factors During Cycling
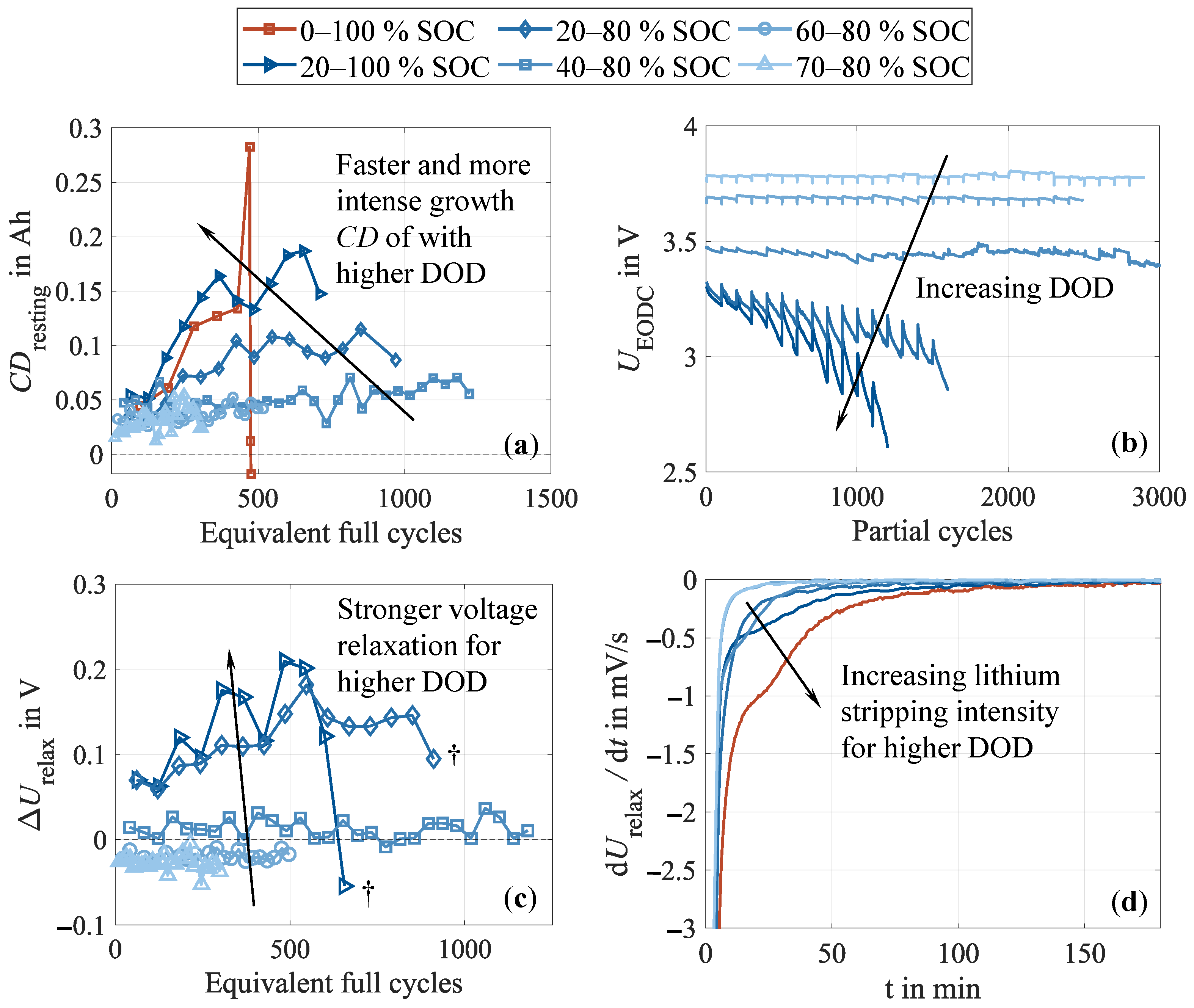
3.4.1. Depth of Discharge
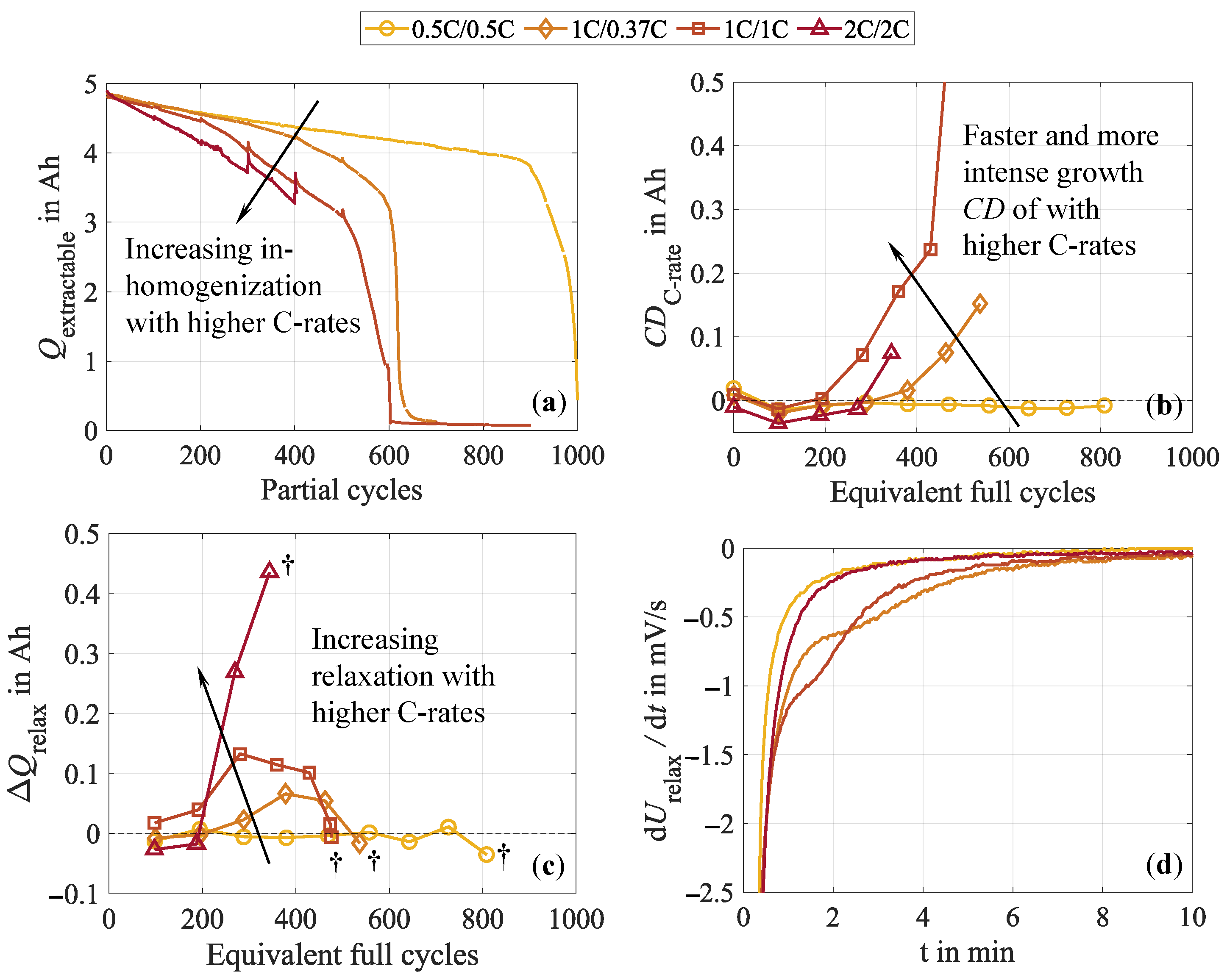
3.4.2. Current Rate
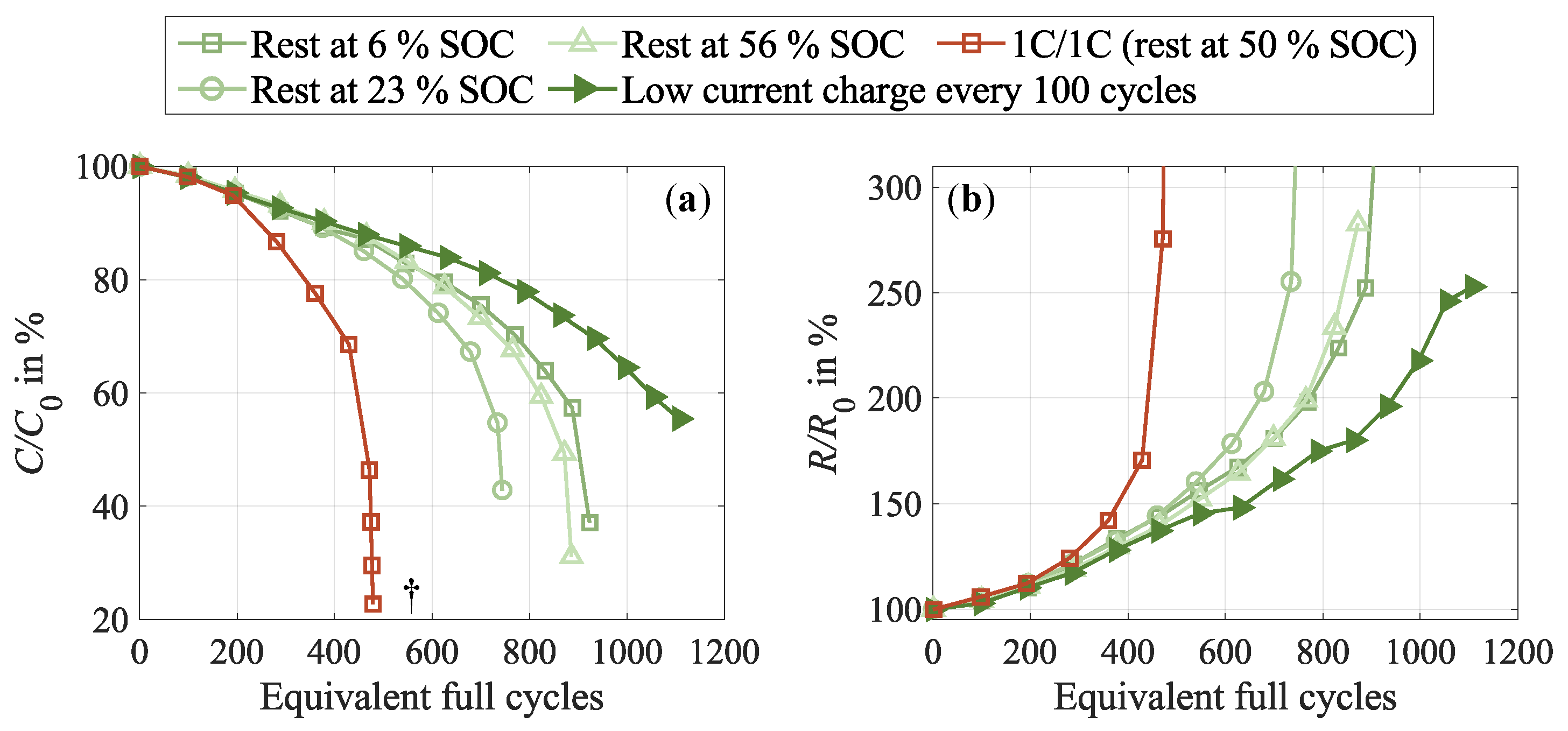
3.5. Supporting Cell Recovery During Cycling
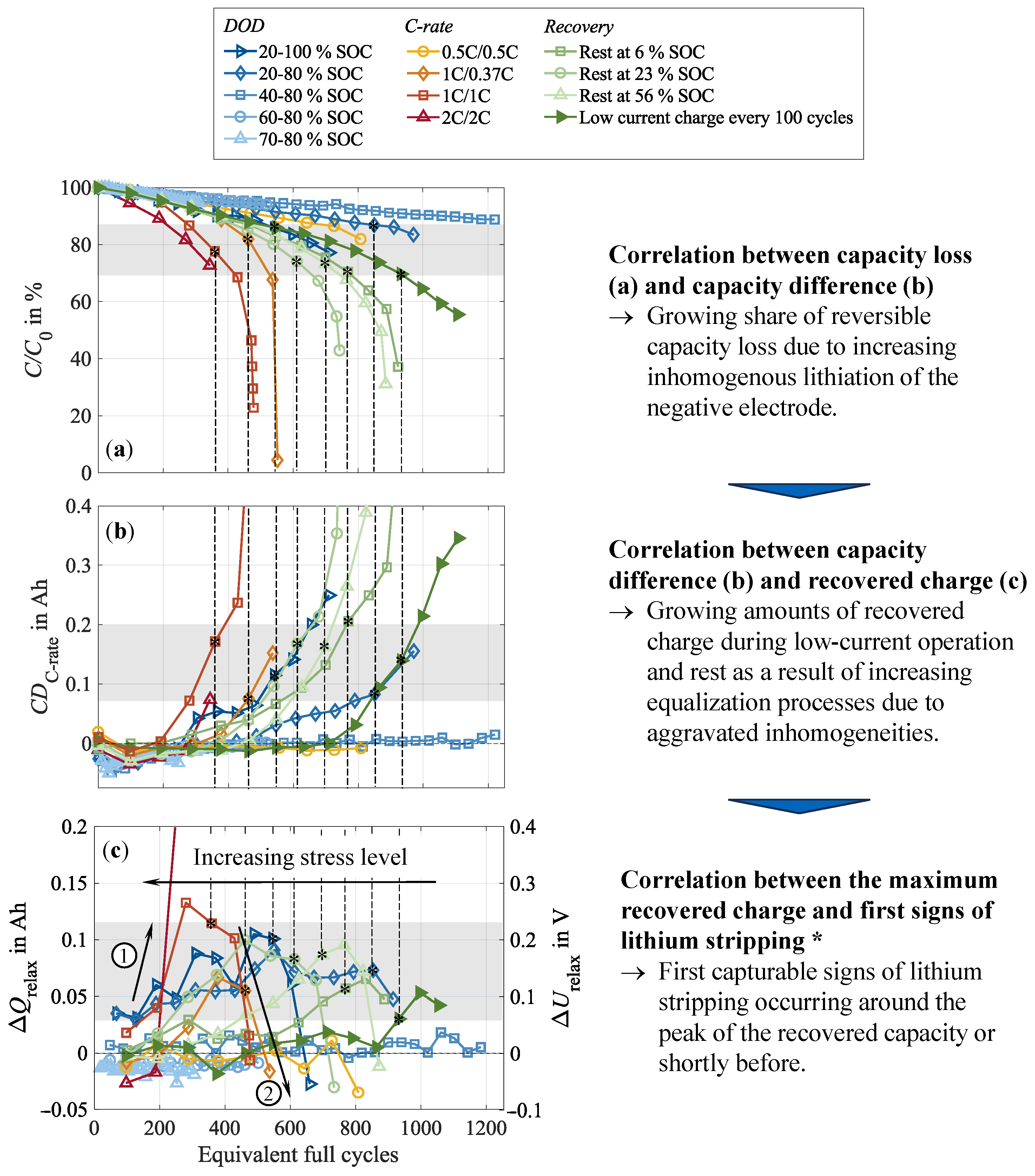
3.6. Implications of Cell Inhomogenization and Relaxation on Cycle Life
3.7. Implications for Accelerated Aging Characterization and BEV Applications
4. Summary and Conclusions
- (1)
- Impact of the test procedure and stress factors on cell inhomogeneities.Continuous cycling leads to the inhomogenization of the lithium distribution within the negative electrode. During CUs, which are commonly performed at low current rates, as well as rest phases, these inhomogeneities partly equalize. Consequently, shortening these cycling interruptions by reducing the CU procedures or eliminating the rest period accelerates cell inhomogenization. This is furthermore influenced by the stress factors applied during cycling, with increasing stress levels supporting cell inhomogenization. High current rates have been found to be particularly detrimental. No dependency on the cycle depth was observed below 40% DOD when cycling between 40 and 80% SOC. While initially reversible, this inhomogenization progressively promotes permanent degradation in the form of lithium plating. A correlation between increasing cell inhomogeneity, reduced equalization intensity, and lithium plating has been proven shortly before the knee point, followed by immediate cell failure.
- (2)
- Implications for aging characterization in vehicle applications.A distinctive feature of vehicle applications is the prevalence of frequent and extended intermediate rest phases, combined with relatively short sequences of continuous cycling compared to laboratory tests. This characteristic, on the one hand, leads to marginal cell inhomogenization during operation, as well as to frequent relaxation and equalization during the pauses on the other. Neglecting such conditions in aging tests unintentionally intensifies cell aging, resulting in manipulation and superelevation of cell degradation compared to real-world usage and, consequently, a high likelihood of underestimating the real battery lifetime.
- (3)
- Dedicated strategies for cell relaxation as a countermeasure.Moreover, our study provides viable insights for optimizing LIB performance for vehicle applications. Regular pauses during operation emerge as a beneficial strategy for preserving cycle life performance over the long term and extending battery lifespan. Even better results were achieved with intermediate low-current charge cycles, which promoted cell homogeneity and doubled the cycle life. Implementing these insights into the design of cycle aging tests holds the potential to enhance their overall reliability by maintaining short test periods.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
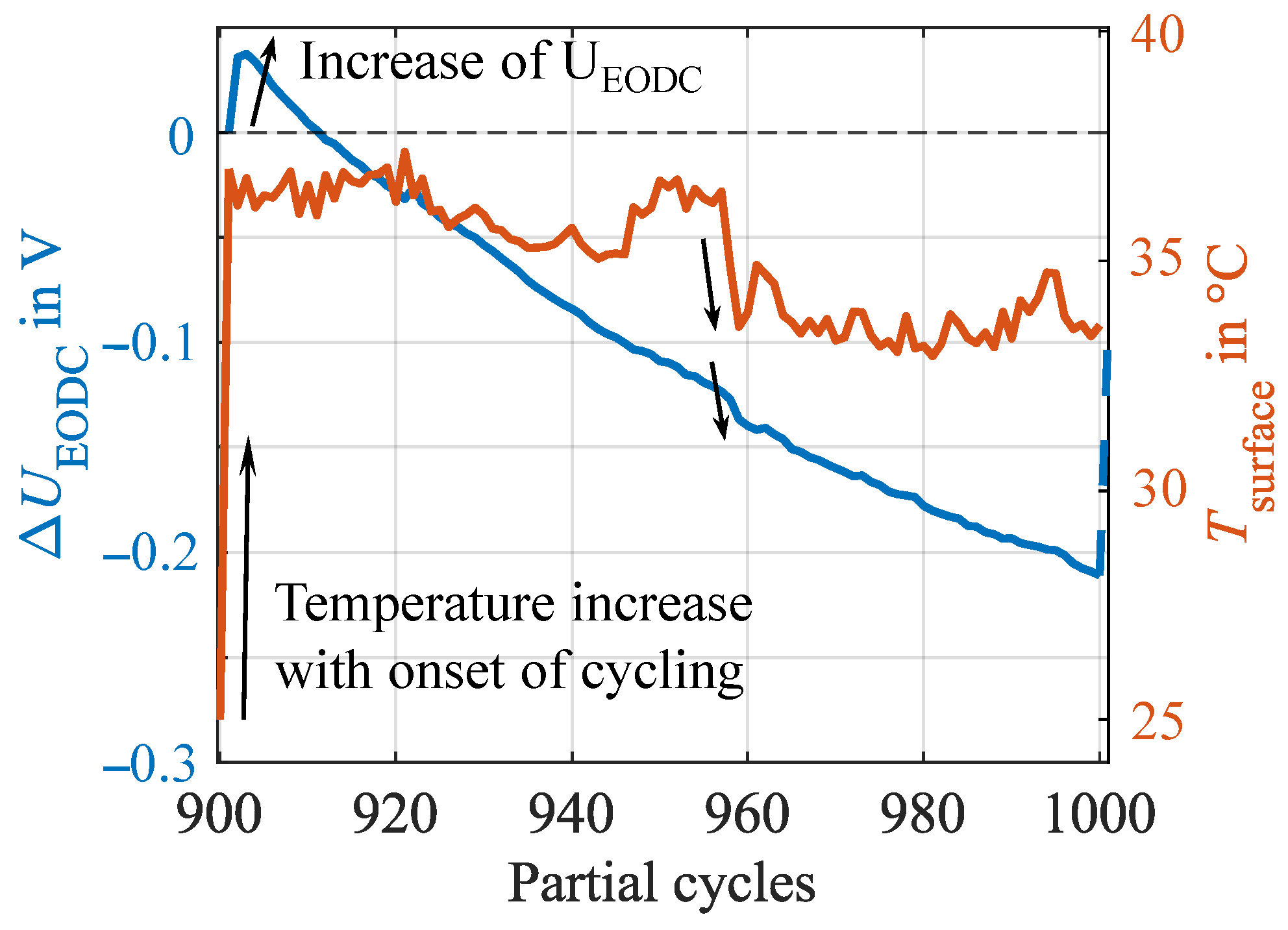
Appendix A. Correlation Between End of Discharge Voltage and Cell Temperature
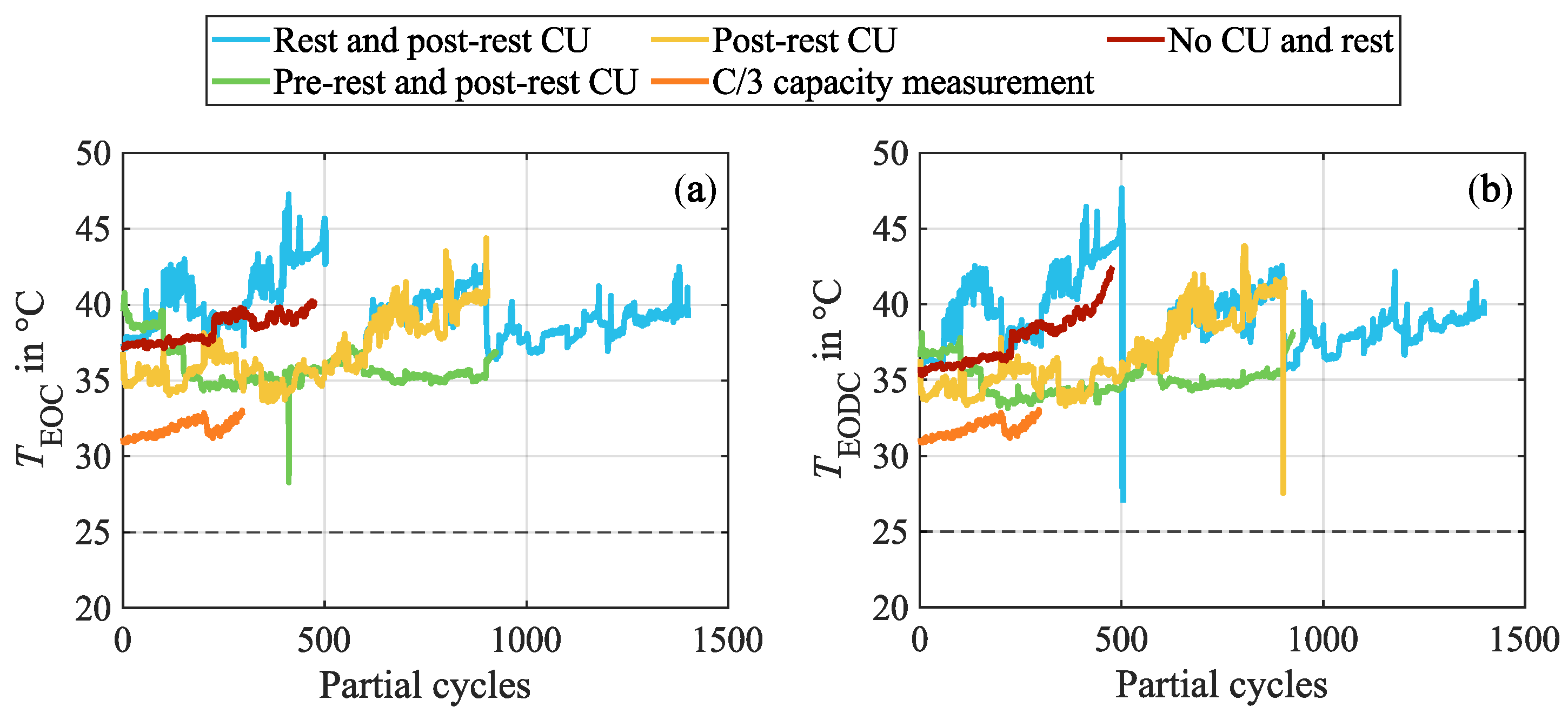
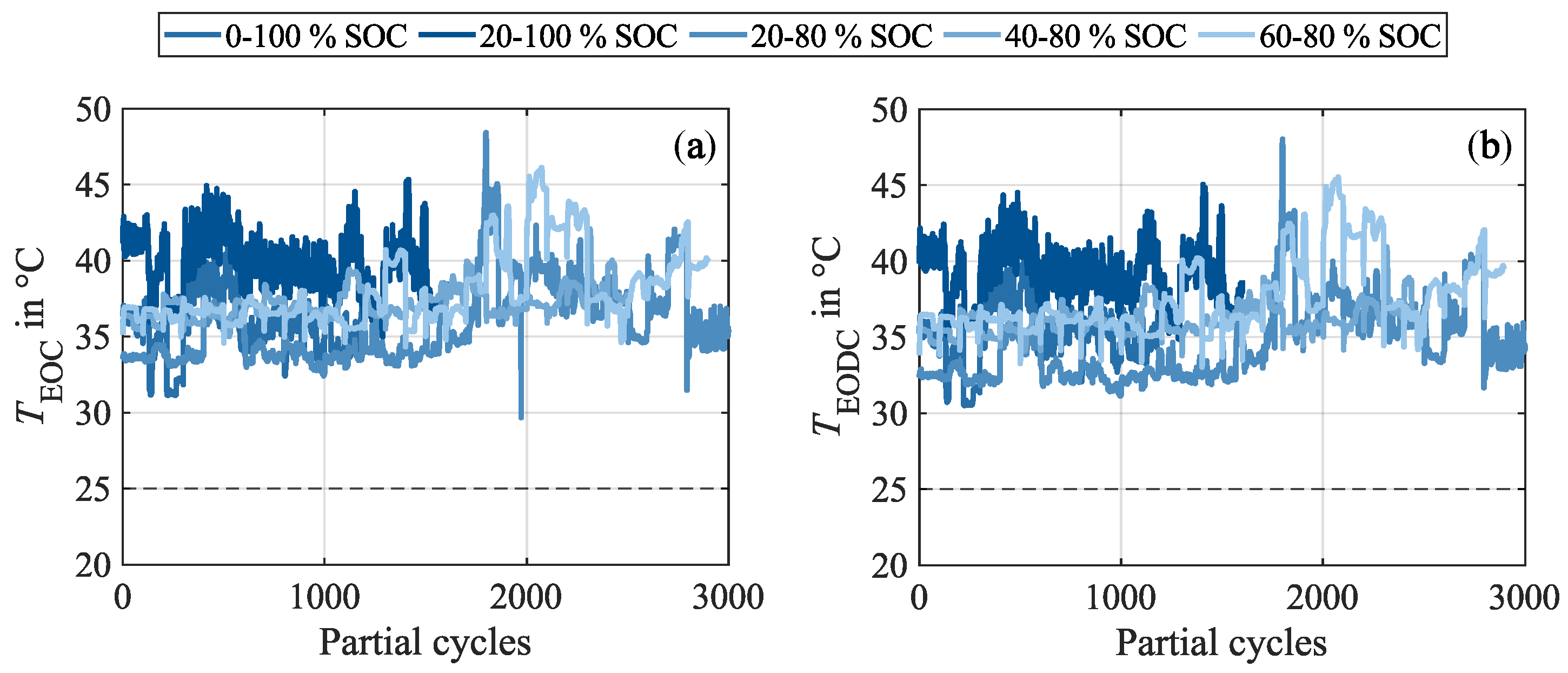
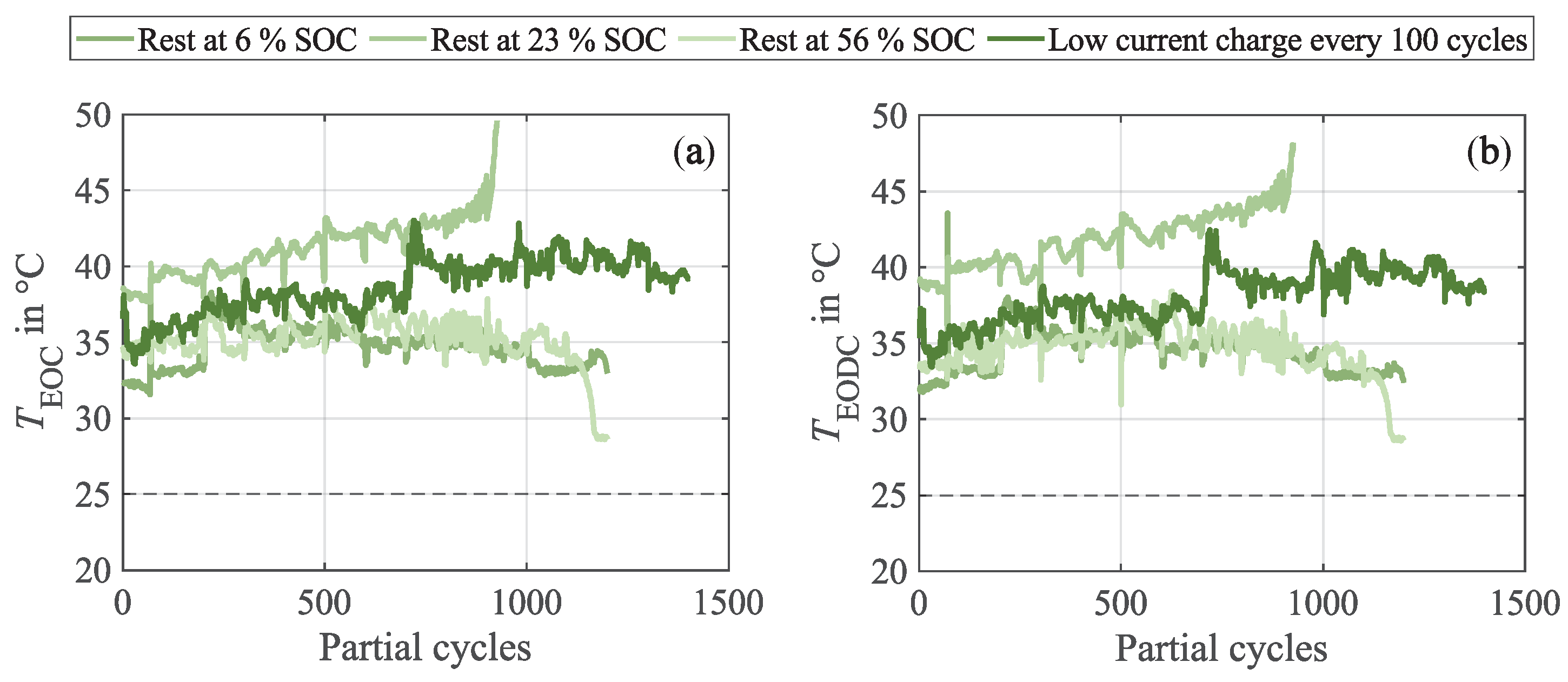
Appendix B. Progression of the Cell Surface Temperatures During Cycling

References
- Braess, H.H.; Seiffert, U. Vieweg Handbuch Kraftfahrzeugtechnik, 7. auflage ed.; Springer Fachmedien: Wiesbaden, Germany, 2021. [Google Scholar] [CrossRef]
- Masias, A.; Marcicki, J.; Paxton, W.A. Opportunities and Challenges of Lithium Ion Batteries in Automotive Applications. ACS Energy Lett. 2021, 6, 621–630. [Google Scholar] [CrossRef]
- Gewald, T.; Lienkamp, M. A systematic method for accelerated aging characterization of lithium-Ion cells in automotive applications. Forsch Ingenieurwes 2019, 83, 831–841. [Google Scholar] [CrossRef]
- Epding, B.; Rumberg, B.; Jahnke, H.; Stradtmann, I.; Kwade, A. Investigation of significant capacity recovery effects due to long rest periods during high current cyclic aging tests in automotive lithium ion cells and their influence on lifetime. J. Energy Storage 2019, 22, 249–256. [Google Scholar] [CrossRef]
- Nobis, C.; Kuhnimhof, T. Mobilität in Deutschland-MiD Ergebnisbericht. Study Conducted by Infas, DLR, IVT, and Infas 360 on Behalf of the Federal Ministry for Digital and Transport (Research Project No. 70.904/15). Bonn, Berlin. Available online: http://www.mobilitaet-in-deutschland.de (accessed on 5 March 2025).
- Kondor, D.; Zhang, H.; Tachet, R.; Santi, P.; Ratti, C. Estimating Savings in Parking Demand Using Shared Vehicles for Home—Work Commuting. IEEE Trans. Intell. Transp. Syst. 2019, 20, 2903–2912. [Google Scholar] [CrossRef]
- Reichert, M.; Andre, D.; Rösmann, A.; Janssen, P.; Bremes, H.G.; Sauer, D.U.; Passerini, S.; Winter, M. Influence of relaxation time on the lifetime of commercial lithium-ion cells. J. Power Sources 2013, 239, 45–53. [Google Scholar] [CrossRef]
- de Hoog, J.; Timmermans, J.M.; Ioan-Stroe, D.; Swierczynski, M.; Jaguemont, J.; Goutam, S.; Omar, N.; van Mierlo, J.; van den Bossche, P. Combined cycling and calendar capacity fade modeling of a Nickel-Manganese-Cobalt Oxide Cell with real-life profile validation. Appl. Energy 2017, 200, 47–61. [Google Scholar] [CrossRef]
- Morales Torricos, P.; Endisch, C.; Lewerenz, M. Apparent Aging during Accelerated Cycling Aging Test of Cylindrical Silicon Containing Li-Ion Cells. Batteries 2023, 9, 230. [Google Scholar] [CrossRef]
- Vetter, J.; Novák, P.; Wagner, M.R.; Veit, C.; Möller, K.C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing mechanisms in lithium-ion batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Paarmann, S.; Schreiber, M.; Chahbaz, A.; Hildenbrand, F.; Stahl, G.; Rogge, M.; Dechent, P.; Queisser, O.; Frankl, S.D.; Morales Torricos, P.; et al. Short–Term Tests, Long–Term Predictions–Accelerating Ageing Characterisation of Lithium–Ion Batteries. Batter. Supercaps 2024, 7, e202300594. [Google Scholar] [CrossRef]
- Rogge, M.; Jossen, A. Path–Dependent Ageing of Li–Ion Batteries and Implications on the Ageing Assessment of Accelerated Ageing Tests. Batter. Supercaps 2024, 7, e202300313. [Google Scholar] [CrossRef]
- Chahbaz, A.; Luo, Y.; Stahl, G.; Ditler, H.; Jaumann, T.; Glinka, M.; Lingen, C.; Sauer, D.U.; Li, W. Pressure–Induced Capacity Recovery and Performance Enhancements in LTO/NMC–LCO Batteries. Adv. Funct. Mater. 2024, 35, 2419229. [Google Scholar] [CrossRef]
- Schreiber, M.; Lin, Y.; Sommer, A.; Wassiliadis, N.; Morales Torricos, P.; Rogge, M.; Lewerenz, M.; Grosu, C.; Endisch, C.; Jossen, A.; et al. Apparent vs. True Battery Aging: Impact of Various Load Characteristics on Accelerated Aging Tests. 2025. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=5137196 (accessed on 5 March 2025). [CrossRef]
- Lewerenz, M.; Dechent, P.; Sauer, D.U. Investigation of capacity recovery during rest period at different states-of-charge after cycle life test for prismatic Li(Ni1/3Mn1/3Co1/3)O2-graphite cells. J. Energy Storage 2019, 21, 680–690. [Google Scholar] [CrossRef]
- Lewerenz, M.; Sauer, D.U. Evaluation of cyclic aging tests of prismatic automotive LiNiMnCoO2-Graphite cells considering influence of homogeneity and anode overhang. J. Energy Storage 2018, 18, 421–434. [Google Scholar] [CrossRef]
- Fath, J.P.; Alsheimer, L.; Storch, M.; Stadler, J.; Bandlow, J.; Hahn, S.; Riedel, R.; Wetzel, T. The influence of the anode overhang effect on the capacity of lithium-ion cells—A 0D-modeling approach. J. Energy Storage 2020, 29, 101344. [Google Scholar] [CrossRef]
- Käbitz, S.; Gerschler, J.B.; Ecker, M.; Yurdagel, Y.; Emmermacher, B.; André, D.; Mitsch, T.; Sauer, D.U. Cycle and calendar life study of a graphite|LiNi1/3Mn1/3Co1/3O2 Li-ion high energy system. Part A: Full cell characterization. J. Power Sources 2013, 239, 572–583. [Google Scholar] [CrossRef]
- Solchenbach, S.; Tacconis, C.; Gomez Martin, A.; Peters, V.; Wallisch, L.; Stanke, A.; Hofer, J.; Renz, D.; Lewerich, B.; Bauer, G.; et al. Electrolyte motion induced salt inhomogeneity—A novel aging mechanism in large-format lithium-ion cells. Energy Environ. Sci. 2024, 17, 7294–7317. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Krause, L.J. Reversible Cycling of Crystalline Silicon Powder. J. Electrochem. Soc. 2007, 154, A103. [Google Scholar] [CrossRef]
- Lewerenz, M.; Warnecke, A.; Sauer, D.U. Introduction of capacity difference analysis (CDA) for analyzing lateral lithium-ion flow to determine the state of covering layer evolution. J. Power Sources 2017, 354, 157–166. [Google Scholar] [CrossRef]
- Spingler, F.B.; Naumann, M.; Jossen, A. Capacity Recovery Effect in Commercial LiFePO4/Graphite Cells. J. Electrochem. Soc. 2020, 167, 040526. [Google Scholar] [CrossRef]
- Stadler, J.; Groch, T.K.; García, M.; Storch, M.; Fath, J.; Ecker, M.; Latz, A. Comprehensive analysis of lithium-ion cells and their aging trajectory toward nonlinear aging. J. Energy Storage 2023, 65, 107247. [Google Scholar] [CrossRef]
- Hogrefe, C.; Paul, N.; Boveleth, L.; Bolsinger, M.; Flügel, M.; Danner, T.; Latz, A.; Gilles, R.; Knoblauch, V.; Wohlfahrt-Mehrens, M.; et al. Lithium Redistribution Mechanism within Silicon-Graphite Electrodes: Multi-Method Approach and Method Validation. J. Electrochem. Soc. 2024, 171, 070503. [Google Scholar] [CrossRef]
- Fuller, T.F.; Doyle, M.; Newman, J. Relaxation Phenomena in Lithium–Ion–Insertion Cells. J. Electrochem. Soc. 1994, 141, 982–990. [Google Scholar] [CrossRef]
- Berhaut, C.L.; Mirolo, M.; Dominguez, D.Z.; Martens, I.; Pouget, S.; Herlin-Boime, N.; Chandesris, M.; Tardif, S.; Drnec, J.; Lyonnard, S. Charge Dynamics Induced by Lithiation Heterogeneity in Silicon–Graphite Composite Anodes. Adv. Energy Mater. 2023, 13, 2301874. [Google Scholar] [CrossRef]
- Kindermann, F.M.; Noel, A.; Erhard, S.V.; Jossen, A. Long-term equalization effects in Li-ion batteries due to local state of charge inhomogeneities and their impact on impedance measurements. Electrochim. Acta 2015, 185, 107–116. [Google Scholar] [CrossRef]
- von Lüders, C.; Zinth, V.; Erhard, S.V.; Osswald, P.J.; Hofmann, M.; Gilles, R.; Jossen, A. Lithium plating in lithium-ion batteries investigated by voltage relaxation and in situ neutron diffraction. J. Power Sources 2017, 342, 17–23. [Google Scholar] [CrossRef]
- Wilhelm, J.; Seidlmayer, S.; Keil, P.; Schuster, J.; Kriele, A.; Gilles, R.; Jossen, A. Cycling capacity recovery effect: A coulombic efficiency and post-mortem study. J. Power Sources 2017, 365, 327–338. [Google Scholar] [CrossRef]
- Farmann, A.; Sauer, D.U. A study on the dependency of the open-circuit voltage on temperature and actual aging state of lithium-ion batteries. J. Power Sources 2017, 347, 1–13. [Google Scholar] [CrossRef]
- Yao, K.P.C.; Okasinski, J.S.; Kalaga, K.; Almer, J.D.; Abraham, D.P. Operando Quantification of (De)Lithiation Behavior of Silicon–Graphite Blended Electrodes for Lithium–Ion Batteries. Adv. Energy Mater. 2019, 9, 1803380. [Google Scholar] [CrossRef]
- Richter, K.; Waldmann, T.; Paul, N.; Jobst, N.; Scurtu, R.G.; Hofmann, M.; Gilles, R.; Wohlfahrt-Mehrens, M. Low-Temperature Charging and Aging Mechanisms of Si/C Composite Anodes in Li-Ion Batteries: An Operando Neutron Scattering Study. ChemSusChem 2020, 13, 529–538. [Google Scholar] [CrossRef]
- Osswald, P.J.; Erhard, S.V.; Rheinfeld, A.; Rieger, B.; Hoster, H.E.; Jossen, A. Temperature dependency of state of charge inhomogeneities and their equalization in cylindrical lithium-ion cells. J. Power Sources 2016, 329, 546–552. [Google Scholar] [CrossRef]
- Schreiber, M.; Steiner, T.; Lienkamp, M. Cell Relaxation during Accelerated Aging Characterization: Impact of Depth of Discharge on the Aging Behavior. In Proceedings of the EVS37—International Electric Vehicle Symposium and Exhibition, Seoul, Republic of Korea, 23–26 April 2024. [Google Scholar]
- Kim, T.; Park, S.; Bae, J.; Jung, D.; Cheon, H.; Lee, W.G.; Choi, Y. Diagnosis of high-Ni NCA/Gr-Si cells before rapid capacity drop by monitoring the heterogeneous degradation. Mater. Horizons 2023, 11, 1008–1013. [Google Scholar] [CrossRef]
- Samsung SDI Co., Ltd. Data Sheet—INR21700-50G. Available online: https://www.batteryexpert.org/wp-content/uploads/2021/03/INR21700-50G.pdf (accessed on 5 March 2025).
- Bonakdarpour, A.; Stoševski, I.; Tiwari, A.; Smith, S.R.; Way, B.M.; Wilkinson, D.P. Impact of Electrolyte Volume on the Cycling Performance and Impedance Growth of 18650 Li-ion Cells. J. Electrochem. Soc. 2024, 171, 020543. [Google Scholar] [CrossRef]
- Hildenbrand, F.; Ditscheid, D.; Barbers, E.; Sauer, D.U. Influence of the anode overhang on the open-circuit voltage and the ageing of lithium-ion batteries—A model based study. Appl. Energy 2023, 332, 120395. [Google Scholar] [CrossRef]
- Li, X.; Colclasure, A.M.; Finegan, D.P.; Ren, D.; Shi, Y.; Feng, X.; Cao, L.; Yang, Y.; Smith, K. Degradation mechanisms of high capacity 18650 cells containing Si-graphite anode and nickel-rich NMC cathode. Electrochim. Acta 2019, 297, 1109–1120. [Google Scholar] [CrossRef]
- Harlow, J.E.; Glazier, S.L.; Li, J.; Dahn, J.R. Use of Asymmetric Average Charge- and Average Discharge- Voltages as an Indicator of the Onset of Unwanted Lithium Deposition in Lithium-Ion Cells. J. Electrochem. Soc. 2018, 165, A3595–A3601. [Google Scholar] [CrossRef]
- Cheng, J.H.; Harlow, J.E.; Johnson, M.B.; Gauthier, R.; Dahn, J.R. Effect of Duty Cycle on the Lifetime of Single Crystal LiNi0.5Mn0.3Co0.2O2/Graphite Lithium-Ion Cells. J. Electrochem. Soc. 2020, 167, 130529. [Google Scholar] [CrossRef]
- Maheshwari, A.; Heck, M.; Santarelli, M. Cycle aging studies of lithium nickel manganese cobalt oxide-based batteries using electrochemical impedance spectroscopy. Electrochim. Acta 2018, 273, 335–348. [Google Scholar] [CrossRef]
- Laszczynski, N.; Solchenbach, S.; Gasteiger, H.A.; Lucht, B.L. Understanding Electrolyte Decomposition of Graphite/NCM811 Cells at Elevated Operating Voltage. J. Electrochem. Soc. 2019, 166, A1853–A1859. [Google Scholar] [CrossRef]
- Broussely, M.; Biensan, P.; Bonhomme, F.; Blanchard, P.; Herreyre, S.; Nechev, K.; Staniewicz, R.J. Main aging mechanisms in Li ion batteries. J. Power Sources 2005, 146, 90–96. [Google Scholar] [CrossRef]
- Harlow, J.E.; Ma, X.; Li, J.; Logan, E.; Liu, Y.; Zhang, N.; Ma, L.; Glazier, S.L.; Cormier, M.M.E.; Genovese, M.; et al. A Wide Range of Testing Results on an Excellent Lithium-Ion Cell Chemistry to be used as Benchmarks for New Battery Technologies. J. Electrochem. Soc. 2019, 166, A3031–A3044. [Google Scholar] [CrossRef]
- Heugel, P.; Märkle, W.; Deich, T.; von Kessel, O.; Tübke, J. Thickness change and jelly roll deformation and its impact on the aging and lifetime of commercial 18650 cylindrical Li-ion cells with silicon containing anodes and nickel-rich cathodes. J. Energy Storage 2022, 53, 105101. [Google Scholar] [CrossRef]
- Schreiber, M.; Abo Gamra, K.; Bilfinger, P.; Teichert, O.; Schneider, J.; Kröger, T.; Wassiliadis, N.; Ank, M.; Rogge, M.; Schöberl, J.; et al. Understanding lithium-ion battery degradation in vehicle applications: Insights from realistic and accelerated aging tests using Volkswagen ID.3 pouch cells. J. Energy Storage 2025, 112, 115357. [Google Scholar] [CrossRef]
- Laresgoiti, I.; Käbitz, S.; Ecker, M.; Sauer, D.U. Modeling mechanical degradation in lithium ion batteries during cycling: Solid electrolyte interphase fracture. J. Power Sources 2015, 300, 112–122. [Google Scholar] [CrossRef]
- Lewerenz, M.; Münnix, J.; Schmalstieg, J.; Käbitz, S.; Knips, M.; Sauer, D.U. Systematic aging of commercial LiFePO4|Graphite cylindrical cells including a theory explaining rise of capacity during aging. J. Power Sources 2017, 345, 254–263. [Google Scholar] [CrossRef]
- Keil, P.; Jossen, A. Impact of Dynamic Driving Loads and Regenerative Braking on the Aging of Lithium-Ion Batteries in Electric Vehicles. J. Electrochem. Soc. 2017, 164, A3081–A3092. [Google Scholar] [CrossRef]
- Hackmann, M.; Knoerzer, H.; Pfeuffer, J.; Jeckel, P. Battery Aging in Practice: Analysis of over 7000 Vehicles Provide Deep Insights into Battery Life and Vehicle Residual Value. P3 Group. Whitepaper 2024. Available online: https://www.p3-group.com/en/p3-updates/battery-aging-in-practice/ (accessed on 5 March 2025).


| Parameter | Value |
|---|---|
| Manufacturer | Samsung SDI |
| Cell type | INR21700-50G |
| Cell format | 21700 cylindrical |
| Positive electrode material | NCA (LiNixCoyAlzO2, stoichiometry unknown) |
| Negative electrode material | Silicon–graphite (stoichiometry unknown) |
| Nominal capacity 1 | |
| Nominal voltage | |
| Minimum voltage | |
| Maximum voltage |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the World Electric Vehicle Association. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schreiber, M.; Steiner, T.; Kayl, J.; Schönberger, B.; Grosu, C.; Lienkamp, M. The Overlooked Role of Battery Cell Relaxation: How Reversible Effects Manipulate Accelerated Aging Characterization. World Electr. Veh. J. 2025, 16, 255. https://doi.org/10.3390/wevj16050255
Schreiber M, Steiner T, Kayl J, Schönberger B, Grosu C, Lienkamp M. The Overlooked Role of Battery Cell Relaxation: How Reversible Effects Manipulate Accelerated Aging Characterization. World Electric Vehicle Journal. 2025; 16(5):255. https://doi.org/10.3390/wevj16050255
Chicago/Turabian StyleSchreiber, Markus, Theresa Steiner, Jonas Kayl, Benedikt Schönberger, Cristina Grosu, and Markus Lienkamp. 2025. "The Overlooked Role of Battery Cell Relaxation: How Reversible Effects Manipulate Accelerated Aging Characterization" World Electric Vehicle Journal 16, no. 5: 255. https://doi.org/10.3390/wevj16050255
APA StyleSchreiber, M., Steiner, T., Kayl, J., Schönberger, B., Grosu, C., & Lienkamp, M. (2025). The Overlooked Role of Battery Cell Relaxation: How Reversible Effects Manipulate Accelerated Aging Characterization. World Electric Vehicle Journal, 16(5), 255. https://doi.org/10.3390/wevj16050255








