Energy Management of a 1 MW Photovoltaic Power-to-Electricity and Power-to-Gas for Green Hydrogen Storage Station
Abstract
1. Introduction
2. Related Works
- -
- Better management of the H2 value chain by implementing an energy management and optimization algorithm (EMS) which has a direct impact on cost reduction, the reliability of the installation, and its efficiency.
- -
- Flexible, resilient, and sustainable infrastructure by integrating different energy sources and two structures: P2E and P2G strategies in the same coherent energy system. The first one is the PtE (Power-to-Electricity) which allows electricity production via the PV–electrolyzer–PEMFC chain, while the second P2G (Power-to-Gas) allows the hydrogen production to be injected into the natural gas network, either directly or indirectly by methanation.
3. Hydrogen Production and Storage Processes
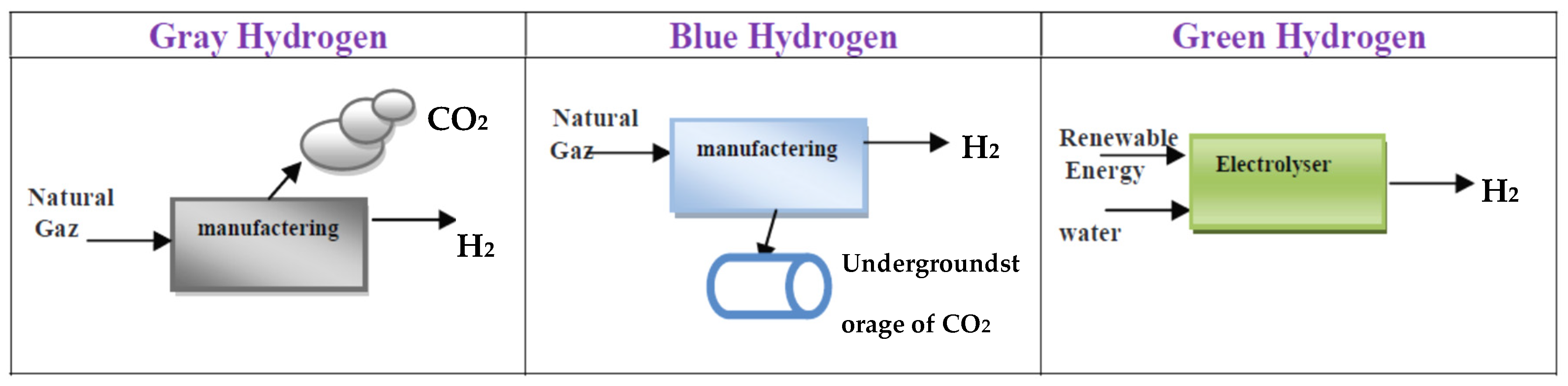
3.1. Hydrogen Production Methods
3.2. Hydrogen Production by Steam Reforming
3.3. H2 Storage Methods
- In tanks or bottles which allow hydrogen to be transported by trucks;
- In service stations and storage tanks of hydrogen vehicles, where it will then be used to power a hydrogen fuel cell which generates electricity;
- In massive underground storage facilities.
- Transport by dedicated long-distance pipelines;
- Road or rail transport in pressure cylinders, made of steel;
- Maritime transport.
3.4. Storage of Renewable Energies in the Form of Hydrogen
- -
- Store hydrogen in pressurized gaseous chemical form;
- -
- Store hydrogen in electrical form via PEMFC cells;
- -
- Inject H2 into the natural gas transport network;
- -
- Inject H2 into the electricity network via PEMFC.
4. Conception and Modeling of the PV Green Hydrogen Production and Storage
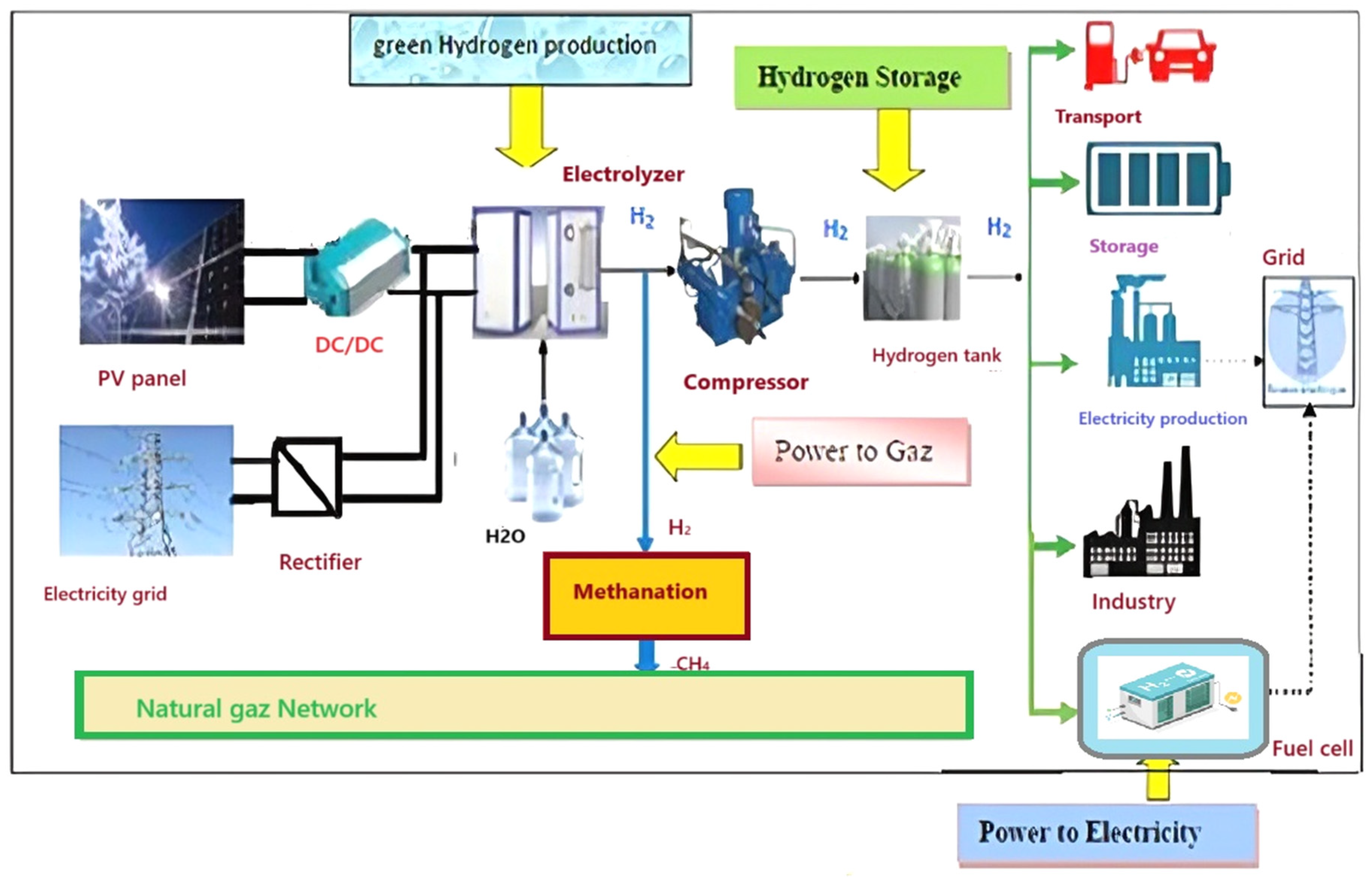
4.1. Description of the Proposed Hydrogen PtE and PtG Structure
- -
- Injected into the natural gas network (CH4) either directly or after methanization;
- -
- Compressed between 350 and 700 bars in bottles, tanks to be transported, and then sent to users or service stations.
- Power-to-Electricity (PtE): The hydrogen is converted into electricity via a PEMFC fuel cell;
- Power-to-Mobility (PtM): the hydrogen aliment the electric vehicles, the hydro and hybrid ones as well as the refueling and recharging stations;
- Power-to-Gas (PtG): The hydrogen is combined with CO2 to produce the gas of methane (CH4) which is injected into the natural gas outlet (heating, …).
4.2. Modeling
- Model of PV generators
- Is: the reverse saturation current of the diode at a temperature of 25 °C;
- Eg: the gap energy of the semiconductor used in the cell (eV);
- A: the ideal factor which depends on the PV technology;
- Isc: the short-circuit current of the PV cell at a temperature of 25 °C;
- Ki: coefficient of a temperature of the PV cell;
- Tc: temperature of the PV cell in °K;
- q: load of an electron= 1.6 · 10−19;
- K: Boltzmann constant = 1.38 · 10−23
- Tref: reference temperature = 298 °K (25 °C);
- G: current sunshine in W/m2;
- Gref: reference sunshine = 1000 W/m2;
- Iph: photodiode current;
- VPV, IPV: output voltage and output current of the PV cell
- Rs, Rp: serial and parallel resistances
- PEM electrolyzer model
- -
- Electrolysis yield
- -
- Electric power
- -
- Quantity of H2 produced
- -
- Efficiency
- Vin: open circuit voltage;
- Vanode(t): voltage losses at the anodes;
- Vcathode(t): voltage losses at the cathodes;
- Rel(t): ohmic resistance of the electrolytes;
- Iel(t): electrolyzer current;
- Ianode1(t), Ianode2(t): currents in the resistive and capacitive anode branches;
- Icathode1(t), Icathode2(t): currents in the resistive and capacitive cathode branches;
- nc: the number of cells in the electrolyzer;
- F: the Faraday constant (96,485.33 C/mol);
- T: the temperature of the electrolyzer (in Kelvin);
- ηF: the Faraday efficiency.
- Compressor model
- Cp: the specific heat of hydrogen at constant pressure, (14.3 kJ/kg/K);
- Tin: the temperature of hydrogen at the compressor inlet, (293 K);
- ηcomp: the mechanical efficiency of the compressor;
- Pin, Pout: are, respectively, the inlet pressure (30 MPa) and outlet pressure (70 to 700 bars) of the compressor;
- r: the ratio of the specific heats of hydrogen, (r = 1.4);
- mcomp(t): the mass flow rate of gas through the compressor [kg/s].
- Hydrogenstorage model
- P: gas pressure in pascals (Pa);
- Pinitial: the initial pressure (Pa);
- : the mass of the hydrogen quantity;
- R: the gas constant equal to 8.314 J/Kmol;
- Ttank: the absolute temperature of the gas in Kelvin (K);
- Vtank: the gas volume (m3);
- z: compressibility factor;
- M: the molar mass of the hydrogen gas (2.016 × 10−3 kg/mol).
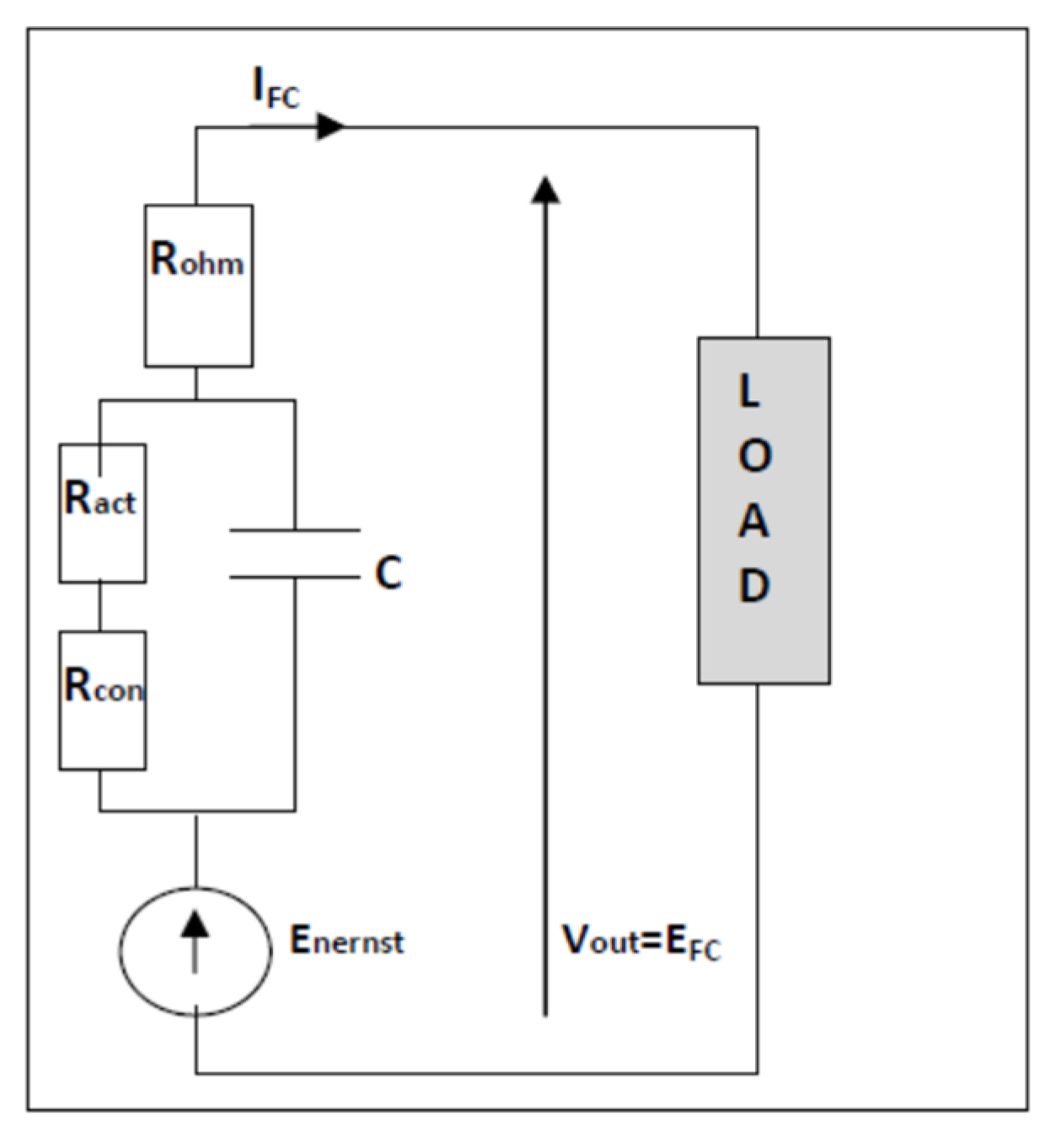
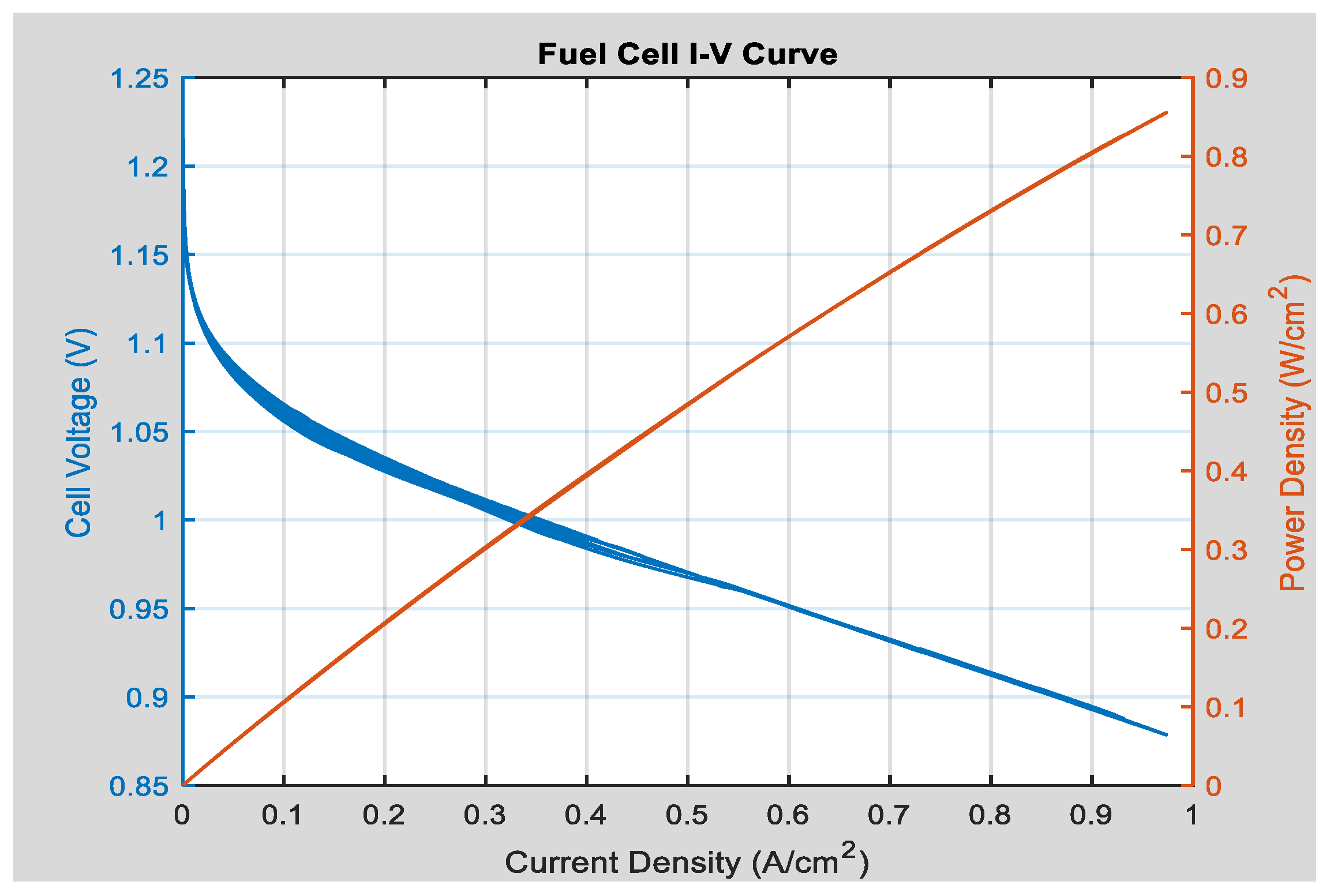
- Fuel cell model
- IFC is the fuel cell current;
- ξ1, ξ2, ξ3 and ξ4 are the empirical parameters of the fuel cell;
- CO2 is the concentration of oxygen (mol/cm3).
- RM: is the resistance of the membrane;
- RC: is the contact resistance to the conduction of electrons.
- Vstack: the output voltage of the PEMFC (all cells);
- Ncell: number of cells in series;
- Enernst: the thermodynamic potential of a single cell;
- Vact: the activation voltage;
- Vohm: the ohmic voltage;
- Vcon: the concentration voltage;
- T: cell temperature (in °K);
- : hydrogen pressure (in atm);
- : oxygen pressure (atm);
- J: the current density of the cell (A/cm2);
- Jmax: the maximum current density of the battery (A/cm2);
- B: a weighting constant.
- dmout(t): the hydrogen mass consumption by the fuel cell PEMFC (kg/h);
- PFC: the power of the PEM fuel cell systems
- PCI: the Lower Hydrogen Calorific Value;
- ηFC: the efficiency of fuel cell.
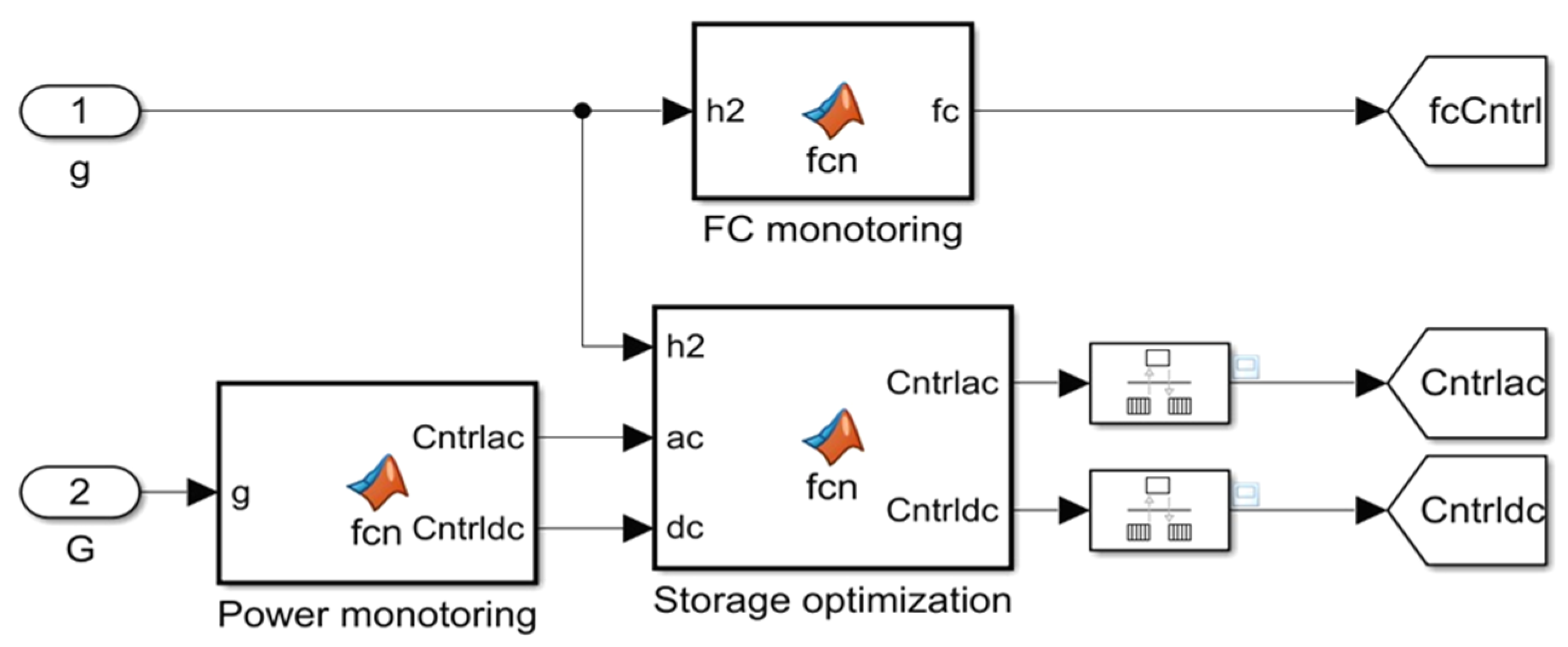
5. Energy Control and Management Strategy of the Proposed System
5.1. Description
5.2. System Control Strategy
5.3. Energy Management of the Hydrogen Production and Storage System
- Energy management algorithm
- Analysis of the energy management scenarios.
- ➢
- Initializing Data Inputs: Configure the initial parameters required for the algorithm, including Ppv, grid availability, H2 storage tank capacity, minimum PV power thresholds, and minimum variation for H2 storage.
- ➢
- PV Power Evaluation.
- If the generated PV power exceeds a minimum threshold (Pmin) and if the PV power is sufficient, then the power generated is directed by PV to the PEM electrolyzer for H2 production.
- If the PV power is insufficient then the energy is imported from the electricity grid.
- ➢
- H2 Storage Tank Monitoring: Continuously monitor the status of the H2 storage tank and ensure that the tank is neither overfilled or underfilled for safe operating conditions.
- ➢
- Check if the current H2 storage level is within a predefined operational range and is not close to the maximum capacity of the tank.
- ➢
- Optimization of H2 Charge.
- If the current variation in dmh2(t) is less than a predefined minimum variation (Delt mH2 max) then interrupt the filling process to maintain system stability.
- If the H2 variation allows additional charging then continue to charge the H2 storage tank until it reaches an optimal level.
- ➢
- Management of Excess PV Power: When the H2 storage tank is full, the solar power generation is checked if it is PV and not zero.
- If excess PV power is available then activate the system to export excess PV power to the grid, ensuring efficient use of the generated energy.
- ➢
- Iteration of the Algorithm: The algorithm evaluates and repeats these steps on an hourly basis to adapt to changing conditions and optimize energy management throughout the system.
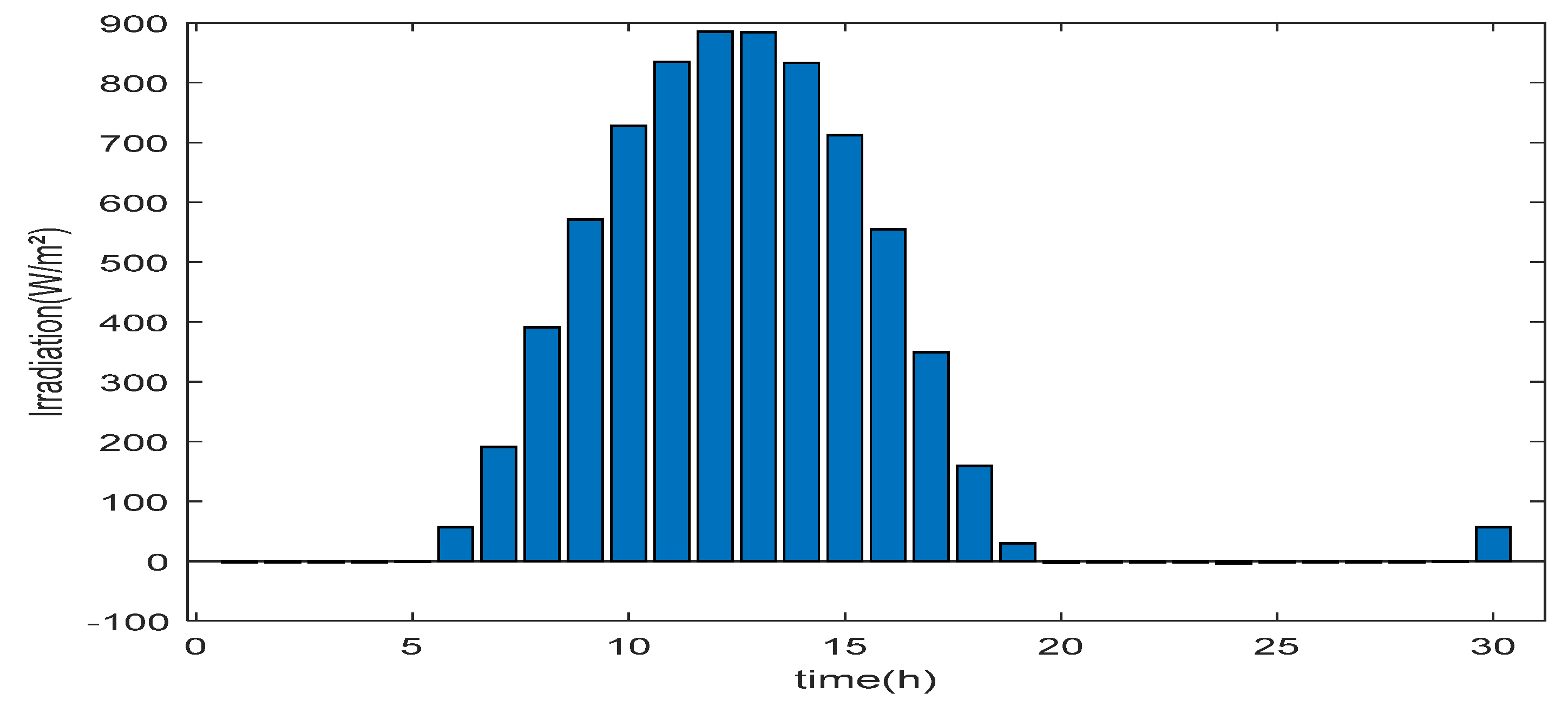
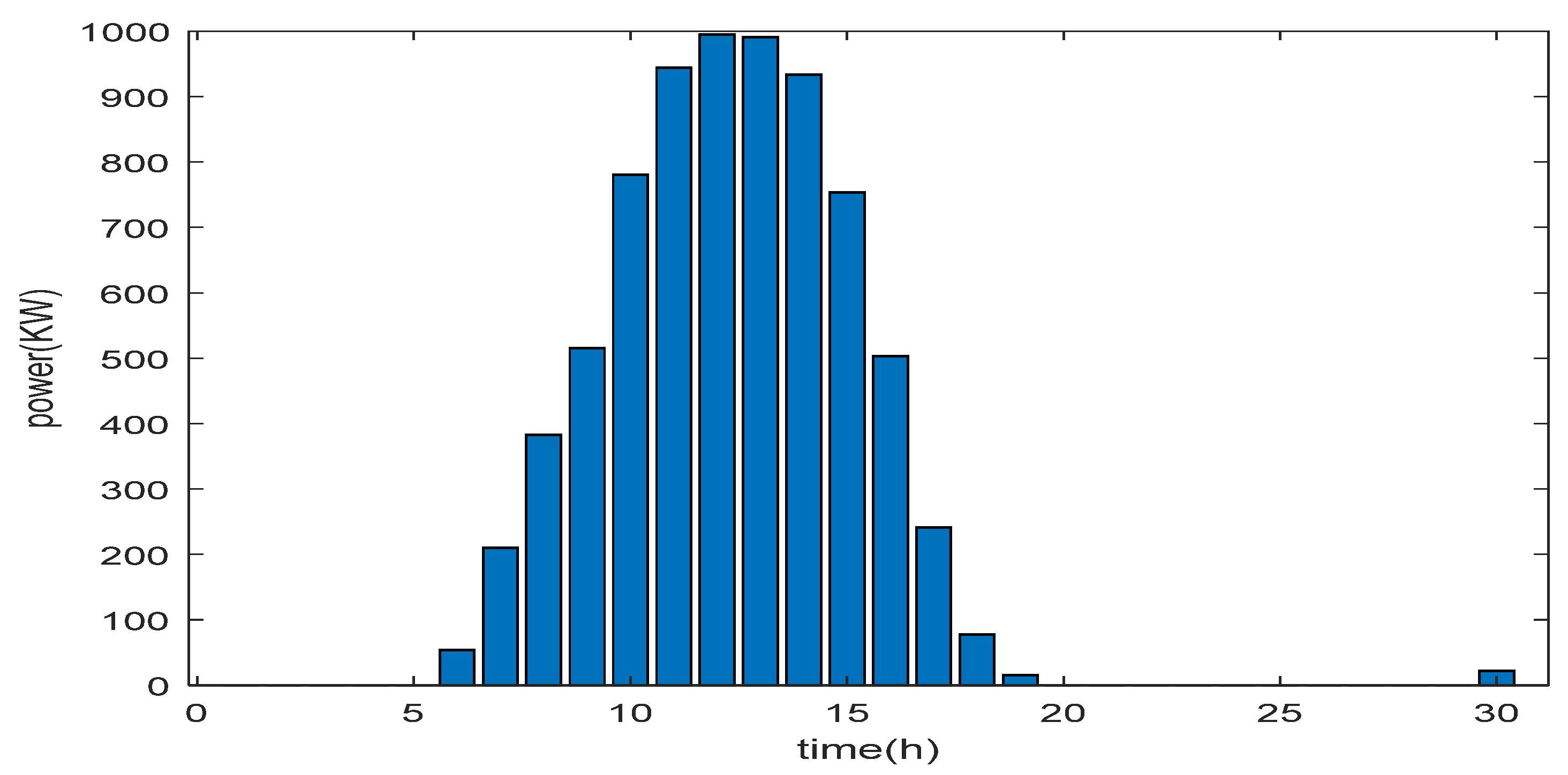
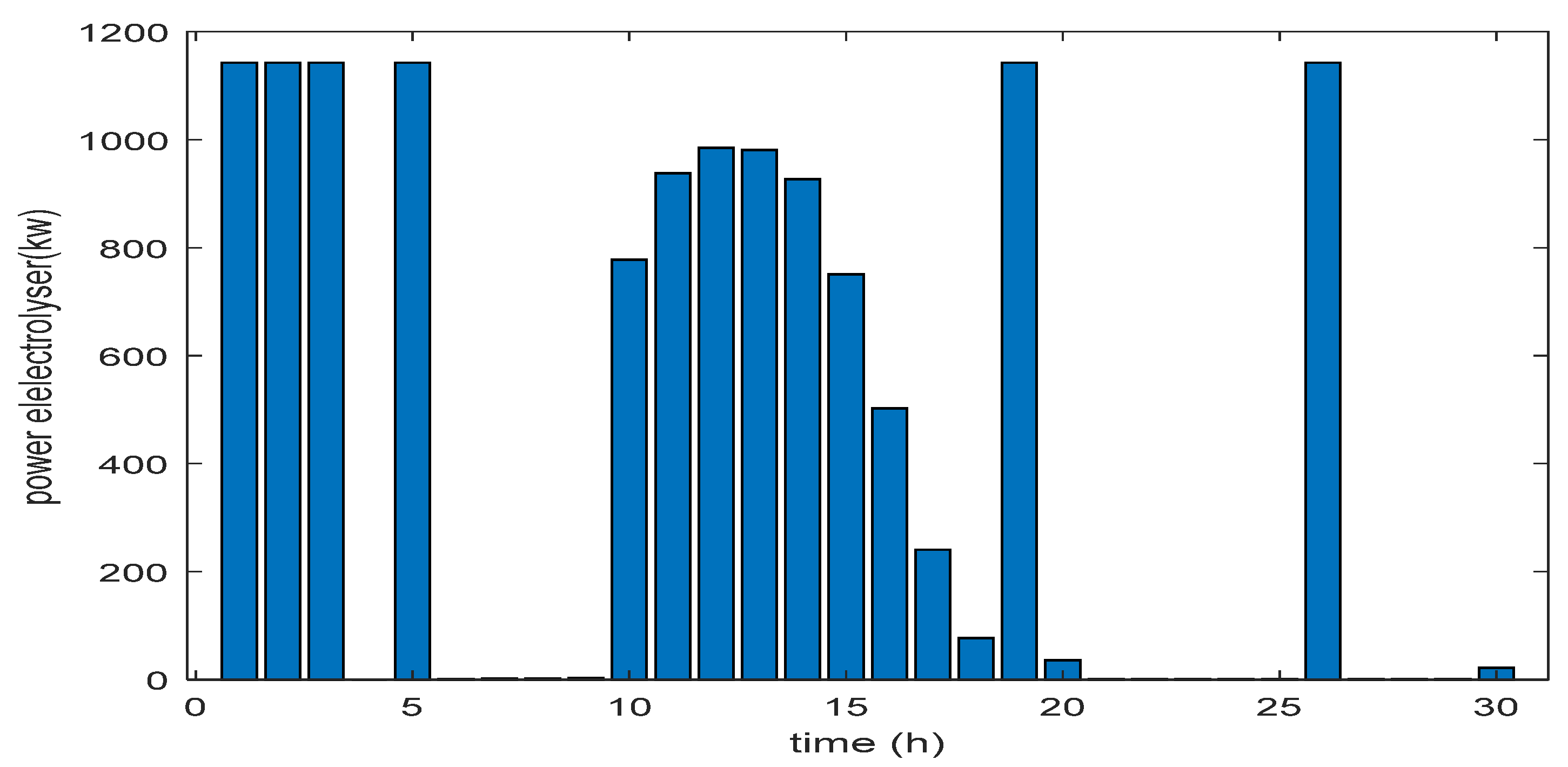
6. Results and Discussions
6.1. Simulation Parameters
- Evaluation of excess energy mode
- Evaluation of energy deficit mode
6.2. Evaluation of CO2 Emissions
- : produced quantity of carbon dioxide CO2 (kg CO2);
- : emission Factor of green H2 (kg CO2/kg H2);
- :produced quantity of Hydrogen (kg H2);
- P: power of the plant (kW);
- T: average functioning time of the plant (Hours);
- : efficiency of H2;
- :calorific coefficient of H2 (Kwh/kg).
6.3. Comparison Study of CO2 Emission Between Hydrogen and Fuel
- Case of Hydrogen station
- Case of fuel (Diesel) station
- Gain in reduction of CO2 emissions
6.4. Results Discussion
- -
- Economic viability: High initial costs could hinder adoption, particularly in economically constrained regions.
- -
- System dependence on weather fluctuations in solar energy production can affect hydrogen production during poor sunlight conditions, especially in regions not connected to the power grid.
- -
- The country’s lack of hydrogen transportation and distribution infrastructure, which smooths hydrogen away from refueling stations or users.
- -
- Regulatory framework and public acceptance.
- -
- For sustainability, it was ensured by the hybridization between solar and green hydrogen, which are two sources without greenhouse gas emissions and complementary.
- -
- For reliability, it is the proposed energy management algorithm that allows scenarios to be managed and energy and thermal transfers to be optimized and therefore, the efficiency of daily hydrogen production.
- -
- For operating costs, it is the amortization of the actualized cost of selling hydrogen that reduces the cost price. This cost is reduced by the integration of solar on the one hand and by the algorithmic optimization of management and supervision of the installation on the other.
- -
- For resilience, it is improved by an integrated approach that combines energy diversification (PtE and PtG strategies), optimized, and proactive management as well as flexible and multiple storage.
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- García-Triviño, P.; Torreglosa, J.P.; Jurado, F.; Fernández Ramírez, L.M. Optimized operation of power sources of a PV/battery/hydrogen-powered hybrid charging station for electric and fuel cell vehicles. IET Renew. Power Gener. 2019, 13, 3022–3032. [Google Scholar] [CrossRef]
- European Commission: The Strategic Energy Technology (SET). Available online: https://www.bluemed-initiative.eu/wp-content/uploads/2018/11/Strategic-Energy-Technology-Plan.pdf (accessed on 10 June 2019).
- Feliciano, D. Net Zero Technical Report Committee on Climate Change; Committee on Climate Change: London, UK, 2019. [Google Scholar] [CrossRef]
- Narayanan, A.; Mets, K.; Strobbe, M.; Develder, C. Feasibility of 100% renewable energy-based electricity production for cities with storage and flexibility. Renew. Energy 2019, 134, 698–709. [Google Scholar]
- Widera, B. Renewable hydrogen implementations for combined energy storage, transportation and stationary applications. Therm. Sci. Eng. Prog. 2020, 16, 100460. [Google Scholar]
- Dahmane, N.; Djerioui, A.; Zeghlache, S.; Dahmane, Z.E.; Houari, A.; Hadjkaddour, A. Energy Management of PV/FC/BAT/SC and PEM Electrolyzer. In Proceedings of the 2022 IEEE International Conference on Electrical Sciences and Technologies in Maghreb (CISTEM), Tunis, Tunisia, 26–28 October 2022; pp. 1–6. [Google Scholar] [CrossRef]
- Javadpoor, S.; Nazarpour, D. Modeling a PV-FC-Hydrogen Hybrid Power Generation System. Eng. Technol. Appl. Sci. Res. 2017, 7, 1455–1459. [Google Scholar] [CrossRef]
- Parra, D.; Valverde, L.; Pino, F.J.; Martin, K. A review on the role, cost and value of hydrogen energy systems for deep decarbonization. Renew. Sustain. Energy Rev. 2019, 101, 279–294. [Google Scholar] [CrossRef]
- Tang, D.; Zio, E.; Yuan, Y.; Zhao, J.; Yan, X. The energy management and optimization strategy for fuel cell hybrid ships. In Proceedings of the 2nd International Conference on System Reliability and Safety (ICSRS), Milan, Italy, 20–22 December 2017; Available online: https://api.semanticscholar.org/CorpusID:46292092 (accessed on 31 December 2017).
- Han, J.; Charpentier, J.F.; Tang, T. An Energy Management System of a Fuel Cell/Battery Hybrid Boat. Energies 2014, 7, 2799–2820. [Google Scholar] [CrossRef]
- Okundamiya, M.S. Size optimization of a hybrid photovoltaic/fuel cell grid connected power system including hydrogen storage. Int. J. Hydrogen Energy 2021, 46, 30539–30546. [Google Scholar] [CrossRef]
- Bukar, A.L.; Tan, C.W. A review on stand-alone photovoltaic-wind energy system with fuel cell: System optimization and energy management strategy. J. Clean. Prod. 2019, 221, 73–88. [Google Scholar]
- Alhasnawi, B.; Jasim, B.H.; Bures, V.; Sedhom, B.E.; Alhasnawi, A.N.; Abbasi, R.; Razaq, M.M.; Siano, P.; Guerrero, J.M. A novel economic dispatch in the stand-alone system using improved butterfly optimization algorithm. Energy Strategy Rev. 2023, 49, 101135. [Google Scholar] [CrossRef]
- Yawale, S.K.; Hanaoka, T.; Kapshe, M.; Deshpande, A. Direct and indirect air pollutant reductions as co-benefits of the energy transition toward carbon-neutrality in India’s residential sector. Energy Strategy Rev. 2023, 49, 101143. [Google Scholar] [CrossRef]
- Bai, Y.X.; Wang, C.; Zeng, M.; Chen, Y.H.; Wen, H.X.; Nie, P.Y. Does carbon trading mechanism improve the efficiency of green innovation: Evidence from China. Energy Strategy Rev. 2023, 49, 101170. [Google Scholar] [CrossRef]
- Jia, J.; Xin, L.; Lu, C.; Wu, B.; Zhong, Y. China’s CO2 emissions: A systematic decomposition concurrently from multi-sectors and multi-stages since 1980 by an extended logarithmic mean divisia index. Energy Strategy Rev. 2023, 49, 101141. [Google Scholar] [CrossRef]
- Balsamo, F.; Capasso, C.; Miccione, G.; Veneri, O. Hybrid Storage System Control Strategy for All-Electric Powered Ships. Energy Procedia 2017, 126, 1083–1090. [Google Scholar] [CrossRef]
- Brekken, T.K.; Yokochi, A.; Von Jouanne, A.; Yen, Z.Z.; Hapke, H.M.; Halamay, D.A. Optimal energy storage sizing and control for wind power applications. Sustain. Energy, IEEE Trans. 2011, 2, 69–77. [Google Scholar]
- Zhang, Y.; Chowdhury, A.A.; Koval, D.O. Probabilistic wind energy modeling in electric generation system reliability assessment. Ind. Appl. IEEE Trans. 2011, 47, 1507–1514. [Google Scholar] [CrossRef]
- Skrúcaný, T.; Kendra, M.; Stopka, O.; Milojević, S.; Figlus, T.; Csiszár, C. Impact of the electric mobility implementation on the greenhouse gases production in central European countries. Sustainability 2019, 11, 4948. [Google Scholar] [CrossRef]
- Hasan, T.; Hassan, N.M.; Shah, R.; Emami, K.; Anderson, J. A hydrogen supply-chain model powering Australian isolated communities. Energy Rep. 2023, 9, 209–214. [Google Scholar]
- Marin, J. Lhyfewillproduce Green Hydrogen with Wind Turbines. Volume 17. 2020. Available online: https://fr.wikipedia.org/wiki/La_Tribune_(France,_1985) (accessed on 22 April 2023).
- Rauline, N. Hydrogen: The World Bank Calls for Investments in the Countries of the South. Volume 20. 2023. Available online: https://fr.wikipedia.org/wiki/Les_%C3%89chos (accessed on 1 May 2023).
- Wang, Y.; Fenton, J.M.; Brooker, P. Dynamic Modeling and Operation of a Green Hydrogen Fueling Station for Heavy-Duty Fuel Cell Vehicles. Proc. Meet. 2024, MA2023-01, 1976. Available online: https://iopscience.iop.org/article/10.1149/MA2023-01361976mtgabs (accessed on 21 February 2025).
- Rizk-Allah, R.M.; Hassan, I.A.; Snasel, V.; Hassanien, A.E. An optimal standalone wind-photovoltaic power plant system for green hydrogen generation: Case study for hydrogen refueling station. Results Eng. 2024, 22, 102234. [Google Scholar] [CrossRef]
- Anastasiadis, A.G.; Papadimitriou, P.; Vlachou, P.; Vokas, G.A. Management of Hybrid Wind and Photovoltaic System Electrolyzer for Green Hydrogen Production and Storage in the Presence of a Small Fleet of Hydrogen Vehicles. Energies 2023, 16, 7990. [Google Scholar] [CrossRef]
- Sayed, K. Feasibility Study and Economic Analysis of PV/Wind-Powered Hydrogen Production Plant. IEEE Access 2024, 12, 76304–76318. [Google Scholar] [CrossRef]
- Lin, Y.C.; Wyżga, P.; Macyk, J.; Macyk, W.; Guzik, M.N. Solar-driven (photo) electrochemical devices for green hydrogen production and storage: Working principles and design. J. Energy Storage 2024, 82, 110484. [Google Scholar]
- Atteya, A.I.; Ali, D.; Sellami, N. Precise Dynamic Modeling of Real-World Hybrid Solar—Hydrogen Energy Systems for Grid-Connected Buildings. Energies 2023, 16, 5449. [Google Scholar] [CrossRef]
- Essakhi, H.; Seddik, F. Modeling and photovoltaic simulation. In Proceedings of the Conference JIME 2019, Agadir, Morroco, 14–15 March 2019. [Google Scholar]
- Boulmrharj, S.; Khaidar, M.; Bakhouya, M.; Ouladsine, R.; Siniti, M.; Zine-dine, K. Performance Assessment of a Hybrid System with Hydrogen Storage and Fuel Cell for Cogeneration in Buildings. Sustainability 2020, 12, 4832. [Google Scholar] [CrossRef]
- Benchouia, N.; Hadjadj, A.E.; Derghal, A.; Khochemane, L.; Mahmah, B. Modeling and validation of fuel cell PEMFC. Rev. Des Energ. Renouvelables 2013, 16, 365–377. [Google Scholar] [CrossRef]
- Amrouch, F.; Mahmah, B.; Belhamel, M.; Benmoussa, H. Modeling of a PEMFC fuel cell supplied directly with hydrogen-oxygen and experimental validation. Review. Energy Ren. 2005, 8, 109–121. [Google Scholar]
- Saleh, Z.H. Simplified mathematical model of proton exchange membrane fuel cell based on horizon fuel cell stack. J. Mod. Power Syst. Clean Energy 2016, 4, 668–679. [Google Scholar]
- Bhuyan, S.K.; Hota, P.K.; Panda, B. Modeling, Control and Power Management Strategy of a Grid connected Hybrid Energy System. Int. J. Electr. Comput. Eng. (IJECE) 2018, 8, 1345–1356. [Google Scholar] [CrossRef]
- Mohammad, S.S.; Iqbal, S.J. Optimization and Power Management of Solar PV-based Integrated Energy System for Distributed Green Hydrogen Production. Distrib. Gener. Altern. Energy J. 2022, 37, 865–898. [Google Scholar] [CrossRef]
- Naseri, N.; El Hani, S.; Machmoum, M.; Elbouchikhi, E.; Daghouri, A. Energy Management Strategy for a Net Zero Emission Islanded Photovoltaic Microgrid-Based Green Hydrogen System. Energies 2024, 17, 2111. [Google Scholar] [CrossRef]
- Shaker, F.N.; Obed, A.A.; Abid, A.J.; Saleh, A.L.; Hassoon, R.J. Energy Management Strategy for PV PSO MPPT/Fuel Cell/Battery Hybrid System with Hydrogen Production and Storage. J. Tech. 2023, 5, 52–60. [Google Scholar] [CrossRef]
- Hollmuller, P.; Joubert, J.-M. Evaluation of a 5 kWp photovoltaic hydrogen production and storage installation for a residential home in Switzerland. Int. J. Hydrogen Energy 2000, 25, 97–109. [Google Scholar] [CrossRef]






| Settings | Value/Type |
|---|---|
| PV power | 1200 kWpic |
| Total number of PV modules | 3042 |
| PV Connection Modules | 78 strings × 39 in series |
| PV Connection Modules | 400 W× (78 strings × 39 in series) |
| Short-circuit current (Isc) | 7.66 A (each one) |
| Open-circuit voltage (Voc) | 48.5 V (each one) |
| Settings | Values |
|---|---|
| Rated electrical power | 355 W |
| Rated steak voltage | 8 V |
| Stack current range | 0–50 A |
| Operating temperature range | 298 °K |
| Hydrogen outlet pressure | 10.5 bar |
| Cells numbers | 90 cells in Series and 25 in Parallel |
| Settings | Values |
|---|---|
| Rated output power | 500 kW |
| Electrical efficiency | 49% |
| H2 purity | 99% |
| Fuel consumption | 335 nm3/h |
| Settings | Value/Type |
|---|---|
| Total PV installed capacity | 1200 kW |
| Total number of PV modules | 3042 |
| PV Connection Modules | 78 strings × 39 in series |
| Nominal power of each PV Module | 400 W |
| Short-circuit current (Isc) | 7.66 A |
| Open-circuit voltage (Voc) | 48.5 V |
| PEM Electrolyze power | 700 kW |
| Hydrogen tank | 800 Kg |
| PEM Fuel Cell | 500 kW |
| Efficiency of Fuel Cell | 46% |
| Study | Results |
|---|---|
| Sheikh Suhail M [36] | PV power = 150 kW Electrolyzer power = 100 kW Battery power: 47 kW Produced energy = 650 kWh/day H2 production = 12 kg/day cost of H2 production = USD 4.8 per kg Total Costs = USD 749,904 |
| N. Naseri and Al [37] | Equivalent hydrogen efficiency (EHC): 30% Battery State of Charge (SOC): 20% to 80% Power Sharing Efficiency: 50% Maximum Power Point Tracking (MPPT) with fuzzy logic controller = Efficiency 95% |
| F. N. Shaker [38] | PV power = 18 kW Battery = 3.7 kW Fuell cell = 4.9 kW PEM electrolyzer = 7 KW H2 production = 400 kg/day Global Efficiency = 0.35 |
| P. Hollmuller, J-M. Joubert [39] | PV power = 5 kW Energy production = 30 kWh/day Electrolyzer power 5 kW (37 V, 250 A), 2 bar Battery capacity: 22 kWh H2 production = 1100 Nm3 per year = 100 kg/year 1 Nm3 of hydrogen produces 3 kWh. 1 kg of hydrogen contains 33.3 kWh of energy. |
| Our study Hidouri D and Al. | PV power = 1200 kW PEM electrolyzer = 750 kW Fuell cell = 500 kW Battery = 75 kW H2 production = 250 to 400 kg/day Global Efficiency = 0.49 EMS with SCADA and supervision interface |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the World Electric Vehicle Association. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidouri, D.; Ben Omrane, I.; Khalil, K.; Cherif, A. Energy Management of a 1 MW Photovoltaic Power-to-Electricity and Power-to-Gas for Green Hydrogen Storage Station. World Electr. Veh. J. 2025, 16, 227. https://doi.org/10.3390/wevj16040227
Hidouri D, Ben Omrane I, Khalil K, Cherif A. Energy Management of a 1 MW Photovoltaic Power-to-Electricity and Power-to-Gas for Green Hydrogen Storage Station. World Electric Vehicle Journal. 2025; 16(4):227. https://doi.org/10.3390/wevj16040227
Chicago/Turabian StyleHidouri, Dalila, Ines Ben Omrane, Kassmi Khalil, and Adnen Cherif. 2025. "Energy Management of a 1 MW Photovoltaic Power-to-Electricity and Power-to-Gas for Green Hydrogen Storage Station" World Electric Vehicle Journal 16, no. 4: 227. https://doi.org/10.3390/wevj16040227
APA StyleHidouri, D., Ben Omrane, I., Khalil, K., & Cherif, A. (2025). Energy Management of a 1 MW Photovoltaic Power-to-Electricity and Power-to-Gas for Green Hydrogen Storage Station. World Electric Vehicle Journal, 16(4), 227. https://doi.org/10.3390/wevj16040227





