Investigation of Inlet Gas Relative Humidity on Performance Characteristics of PEMFC Operating at Elevated Temperature
Abstract
1. Introduction
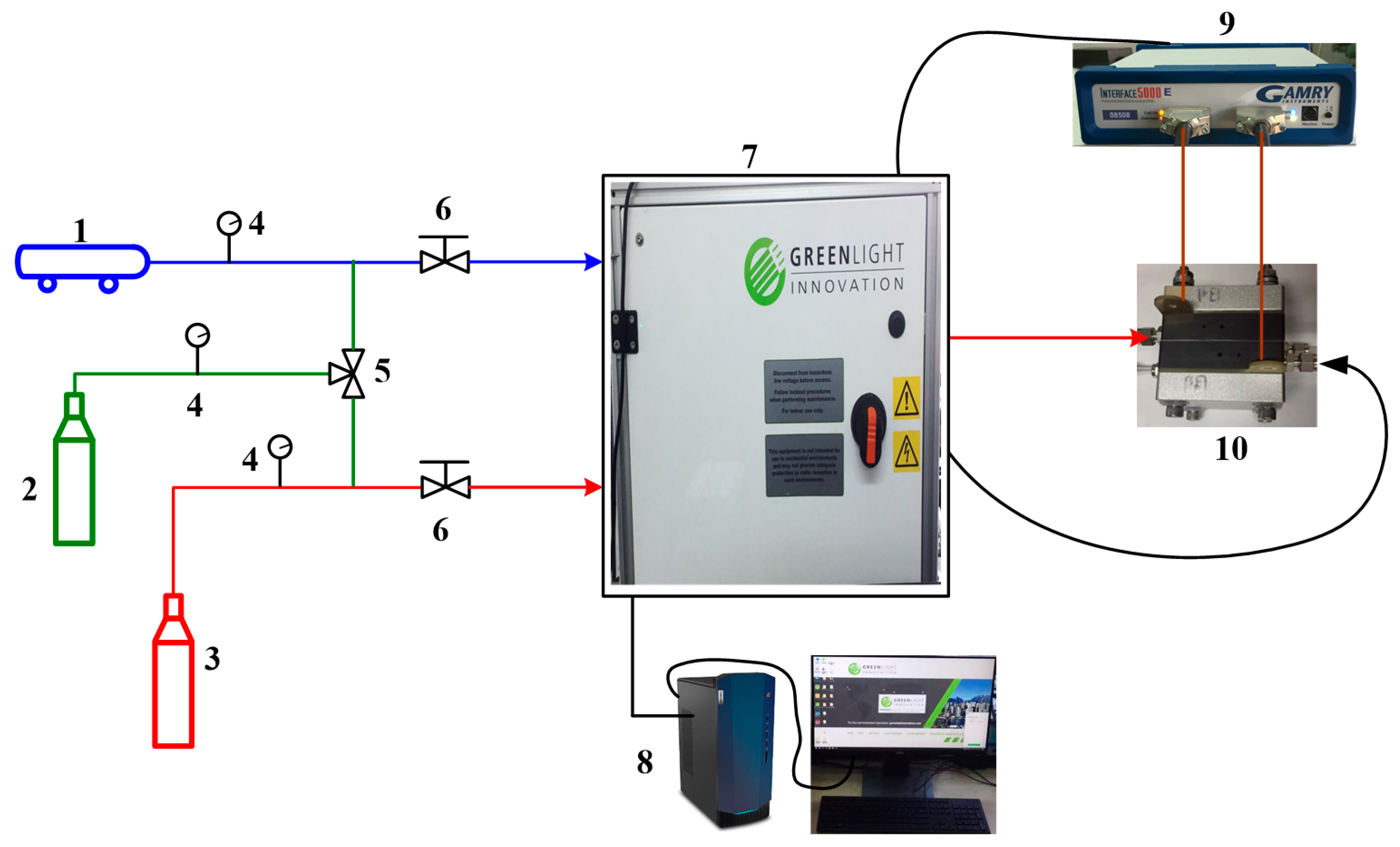
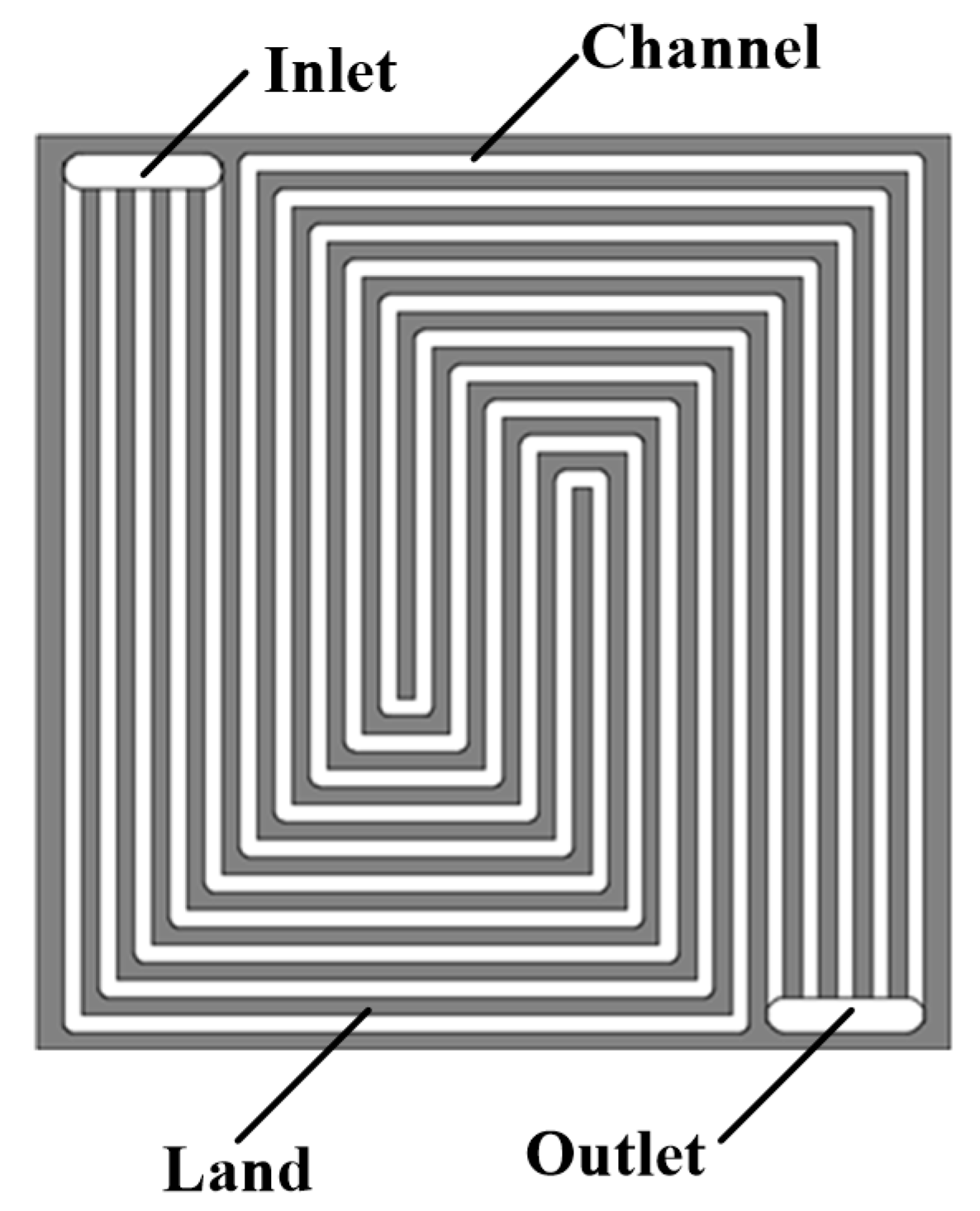
2. Experimental Setup
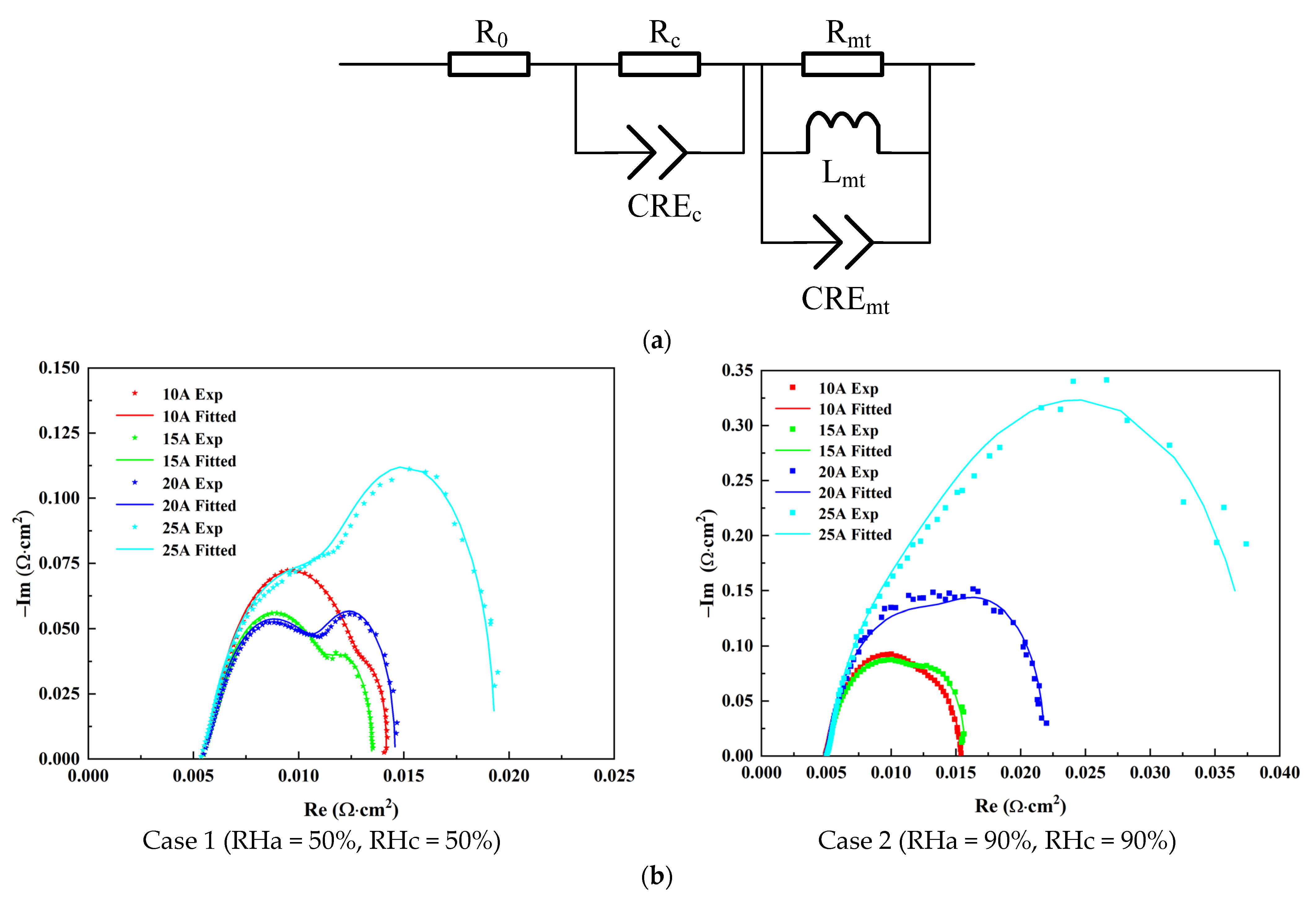
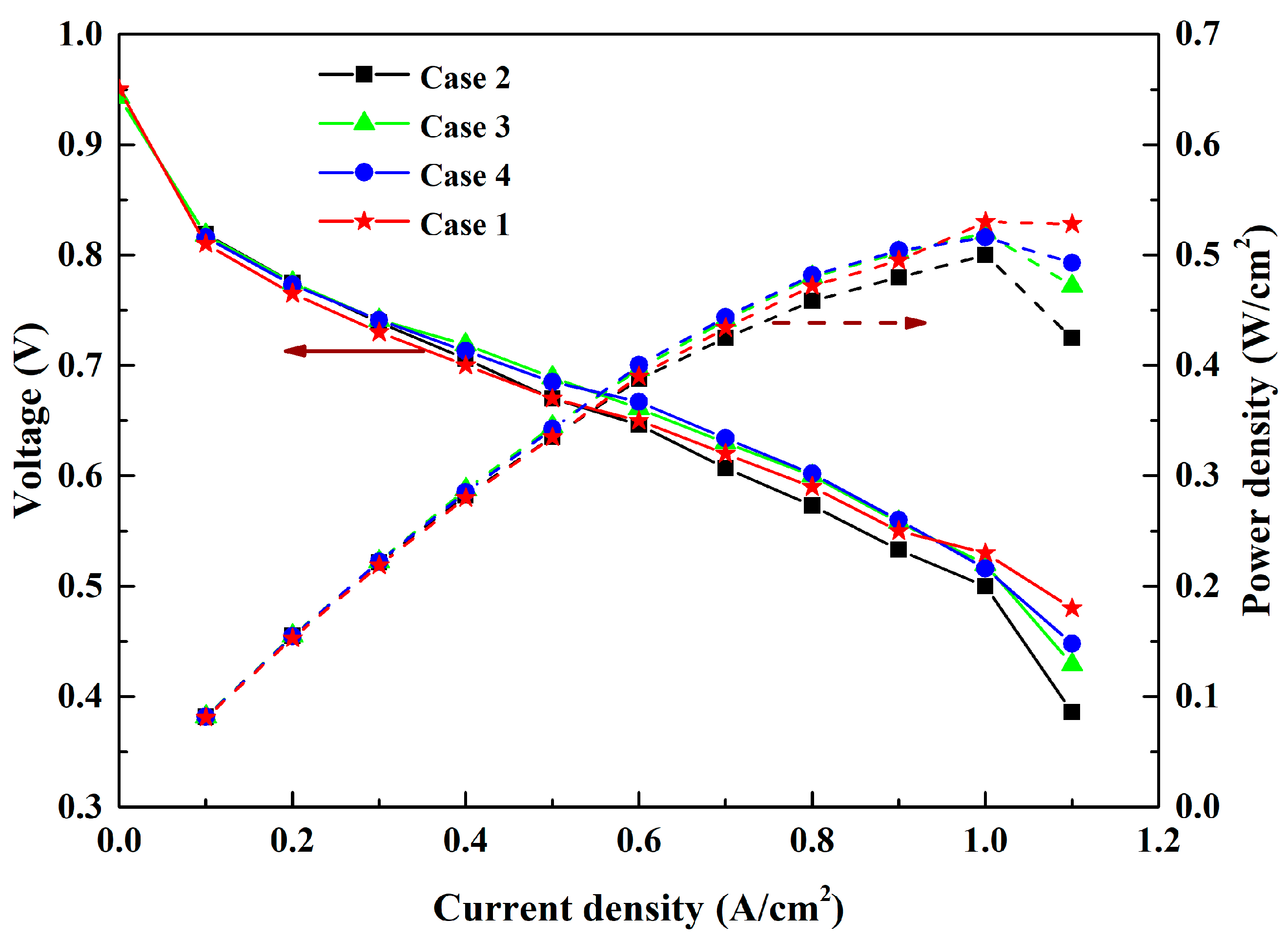
3. Results and Discussion
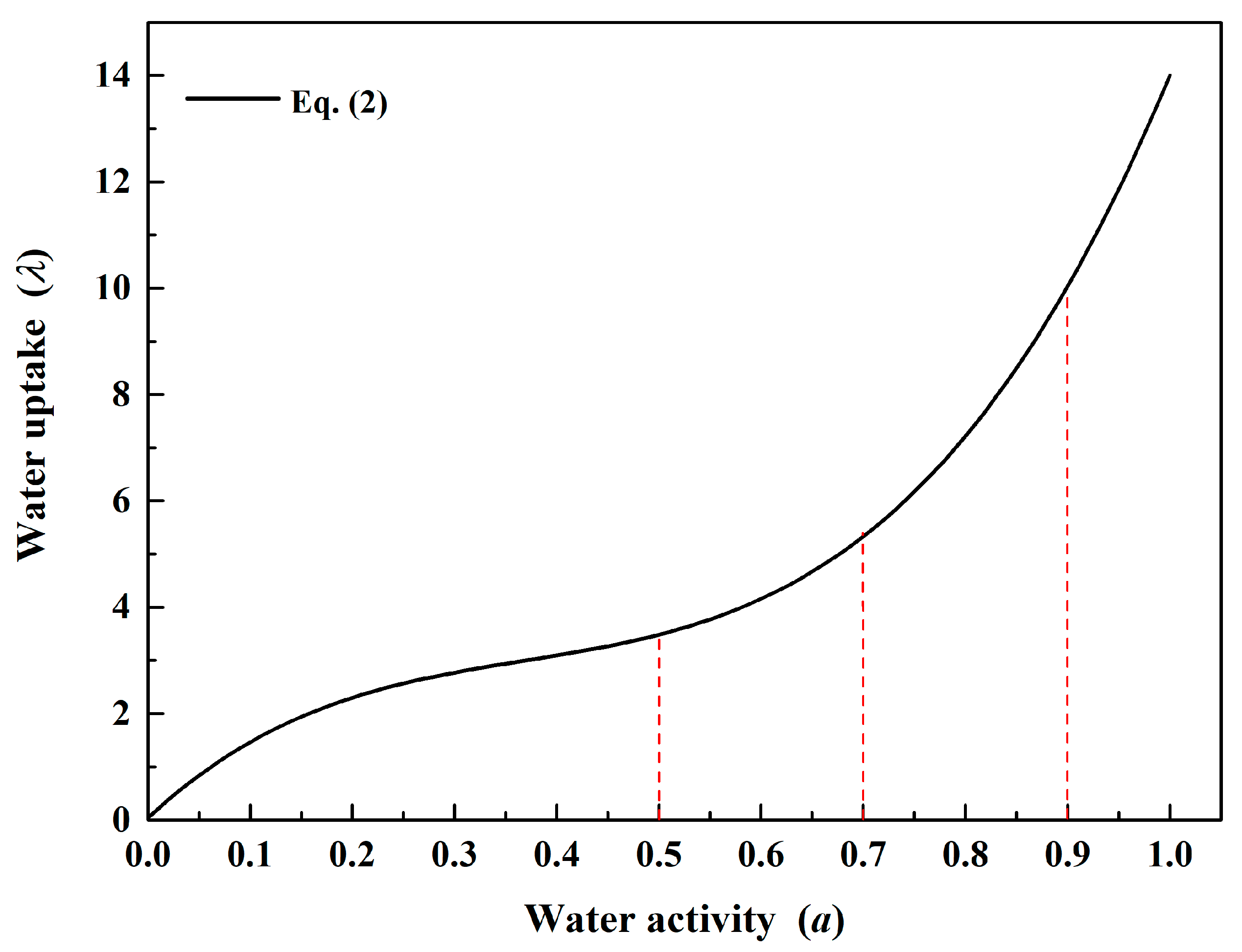
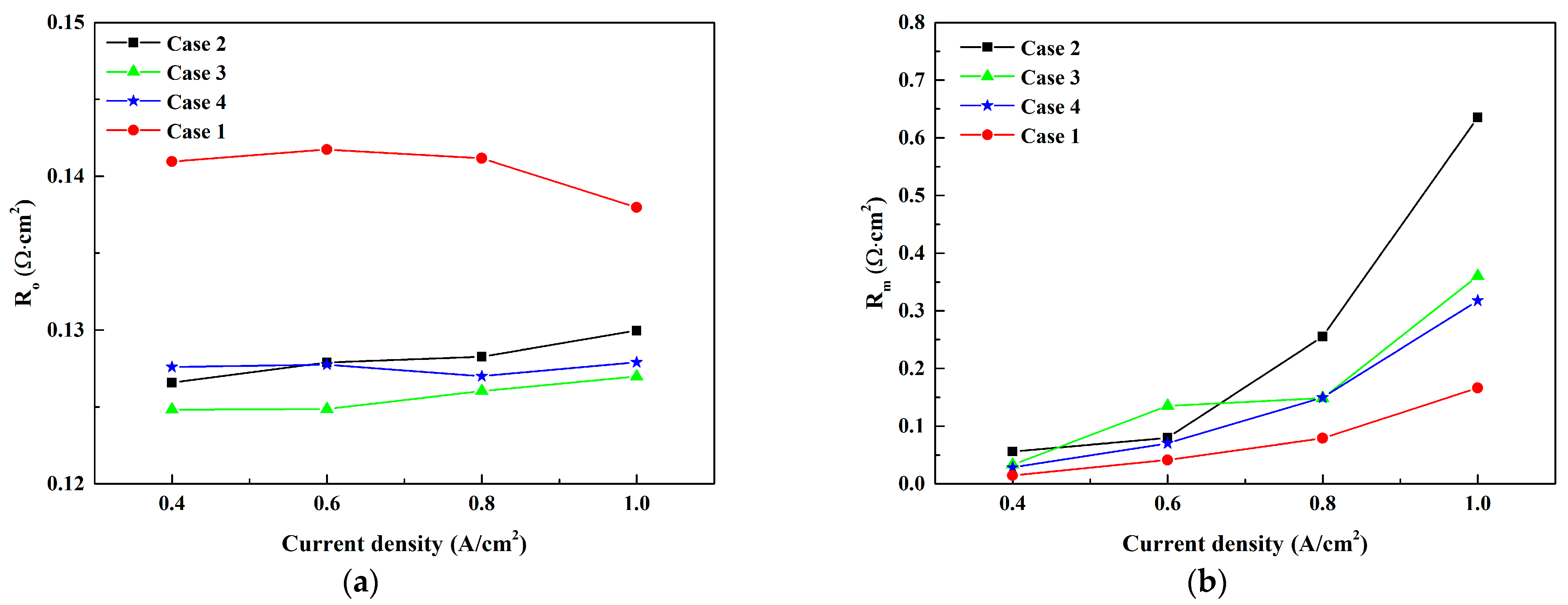
3.1. Effect of Anode Inlet RH
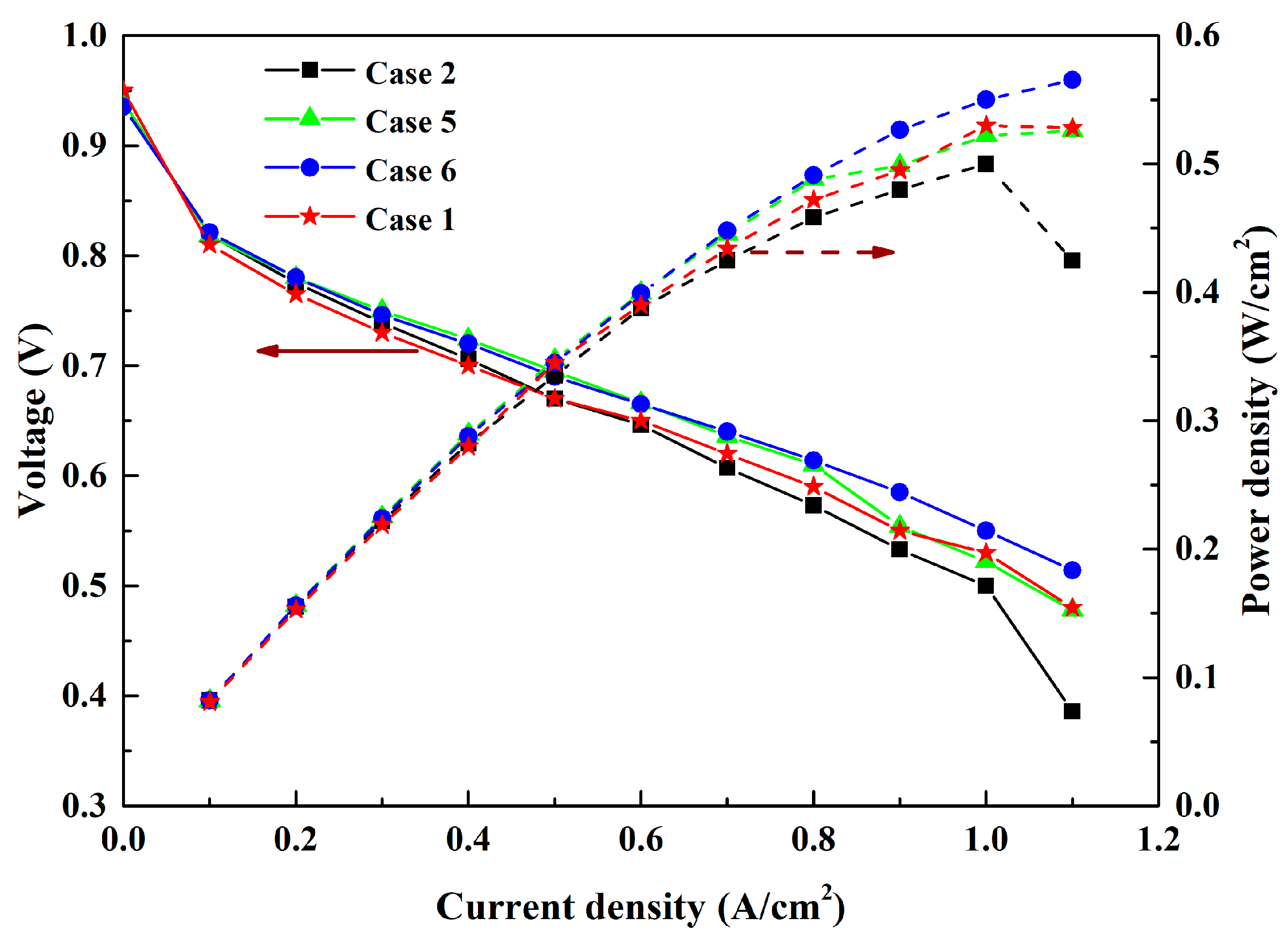
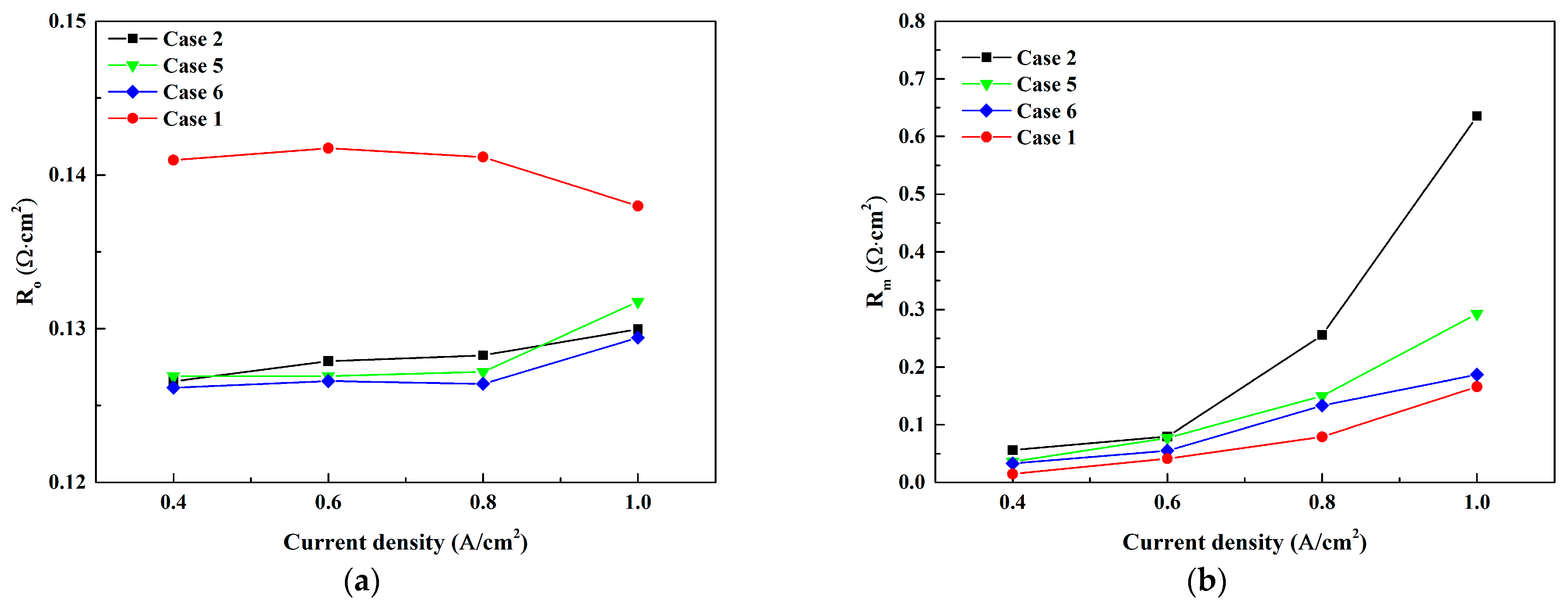
3.2. Effect of Cathode Inlet RH
3.3. Effect of RH on the Efficiency
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tran, M.; Bhatti, A.; Vrolyk, R.; Wong, D.; Panchal, S.; Fowler, M.; Fraser, R. A Review of Range Extenders in Battery Electric Vehicles: Current Progress and Future Perspectives. World Electr. Veh. J. 2021, 12, 54. [Google Scholar] [CrossRef]
- Tanc, B.; Arat, H.T.; Baltacioglu, E.; Aydin, K. Overview of the next quarter century vision of hydrogen fuel cell electric vehicles. Int. J. Hydrogen Energy 2019, 44, 10120–10128. [Google Scholar] [CrossRef]
- New Energy and Industrial Technology Development Organization (NEDO). Road Map 2010 of NEDO Fuel Cell and Hydrogen Technology Development. Department of Fuel Cell and Hydrogen Technology, 2010. Available online: http://www.nedo.go.jp/content/100642949.pdf (accessed on 30 June 2010).
- Atiyeh, H.K.; Karan, K.; Peppley, B.; Phoenix, A.; Halliop, E.; Pharoah, J. Experimental investigation of the role of a microporous layer on the water transport and performance of a PEM fuel cell. J. Power Sources 2007, 170, 111–121. [Google Scholar] [CrossRef]
- Li, H.; Tang, Y.H.; Wang, Z.W.; Shi, Z.; Wu, S.; Song, D.; Zhang, J.; Fatih, K.; Zhang, J.; Wang, H.; et al. A review of water flooding issues in the proton exchange membrane fuel cell. J. Power Sources 2008, 178, 103–117. [Google Scholar] [CrossRef]
- Judith, O.; Manikandan, R.; Murat, A. In situ detection of anode flooding of a PEM fuel cell. Int. J. Hydrogen Energy 2009, 34, 6765–6770. [Google Scholar]
- Ozen, D.N.; Timurkutluk, B.; Altinisik, K. Effects of operation temperature and reactant gas humidity levels on performance of PEM fuel cells. Renew. Sustain. Energy Rev. 2016, 59, 1298–1306. [Google Scholar] [CrossRef]
- Zhang, Q.; Lin, R.; Técher, L.; Cui, X. Experimental study of variable operating parameters effects on overall PEMFC performance and spatial performance distribution. Energy 2016, 115, 550–560. [Google Scholar] [CrossRef]
- Ge, N.; Banerjee, R.; Muirhead, D.; Lee, J.; Liu, H.; Shrestha, P.; Wong, A.; Jankovic, J.; Tam, M.; Susac, D.; et al. Membrane dehydration with increasing current density at high inlet gas relative humidity in polymer electrolyte membrane fuel cells. J. Power Sources 2019, 422, 163–174. [Google Scholar] [CrossRef]
- Janicka, E.; Mielniczek, M.; Gawel, L.; Darowicki, K.; Landowska, P. The impact of air humidity on the operation of proton exchange membrane fuel cells determined using dynamic electrochemical impedance spectroscopy. Electrochim. Acta 2020, 341, 136036. [Google Scholar] [CrossRef]
- Mohsin, M.; Raza, R.; Mohsin-ul-Mulk, M.; Yousaf, A.; Yousaf, A.; Hacker, V. Electrochemical characterization of polymer electrolyte membrane fuel cells and polarization curve analysis. Int. J. Hydrogen Energy 2020, 45, 24093–24107. [Google Scholar] [CrossRef]
- Ou, K.; Yuan, W.; Choi, M.; Yang, S.; Kim, Y. Performance increase for an open-cathode PEM fuel cell with humidity and temperature control. Int. J. Hydrogen Energy 2017, 42, 1–11. [Google Scholar] [CrossRef]
- Askaripour, H. Effect of operating conditions on the performance of a PEM fuel cell. Int. J. Heat Mass Transf. 2019, 144, 118705. [Google Scholar] [CrossRef]
- Chen, H.; Liu, B.; Zhang, T.; Pei, P. Influencing sensitivities of critical operating parameters on PEMFC output performance and gas distribution quality under different electrical load conditions. Appl. Energy 2019, 255, 113849. [Google Scholar] [CrossRef]
- Xing, L.; Cai, Q.; Xu, C.; Liu, C.; Scott, K.; Yan, Y. Numerical study of the effect of relative humidity and stoichiometric flow ratio on PEM (proton exchange membrane) fuel cell performance with various channel lengths: An anode partial flooding modelling. Energy 2016, 106, 631–645. [Google Scholar] [CrossRef]
- Nishimura, A.; Kamiya, S.; Okado, T.; Sato, Y.; Hirota, M.; Kolhe, M.L. Heat and mass transfer analysis in single cell of PEFC using different PEM and GDL at higher temperature. Int. J. Hydrogen Energy 2019, 44, 29631–29640. [Google Scholar] [CrossRef]
- Nishimura, A.; Yoshimura, M.; Kamiya, S.; Hirota, M.; Hu, E. Impact of Relative Humidity of Supply Gas on Temperature Distributions in Single Cell of Polymer Electrolyte Fuel Cell When Operated at High Temperature. J. Energy Power Eng. 2017, 11, 706–718. [Google Scholar] [CrossRef][Green Version]
- Akitomo, F.; Sasabe, T.; Yoshida, T.; Naito, H.; Kawamura, K.; Hirai, S. Investigation of effects of high temperature and pressure on a polymer electrolyte fuel cell with polarization analysis and X-ray imaging of liquid water. J. Power Sources 2019, 431, 205–209. [Google Scholar] [CrossRef]
- Jeon, S.W.; Cha, D.; Kim, H.S.; Kim, Y. Analysis of the system efficiency of an intermediate temperature proton exchange membrane fuel cell at elevated temperature and relative humidity conditions. Appl. Energy 2016, 166, 165–173. [Google Scholar] [CrossRef]
- Chang, Y.; Qin, Y.; Yin, Y.; Zhang, J.; Li, X. Humidification strategy for polymer electrolyte membrane fuel cells—A review. Appl. Energy 2018, 230, 643–662. [Google Scholar] [CrossRef]
- Springer, T.E.; Zawodzinski, T.A.; Gottesfeld, S. Polymer electrolyte fuel cell model. J. Electrochem. Soc. 1991, 138, 2334–2342. [Google Scholar] [CrossRef]
- Yoshiyuki, H.; Hiroshi, D.; Tomoaki, N. Effects of environmental conditions on cathode degradation of polymer electrolyte fuel cell during potential cycle. World Electr. Veh. J. 2019, 10, 24. [Google Scholar]
- Yuan, X.Z.; Li, H.; Gu, E.; Qian, W.; Girard, F.; Wang, Q.; Biggs, T.; Jaeggle, M. Measurements of GDL Properties for Quality Control in Fuel Cell Mass Production Line. World Electr. Veh. J. 2016, 8, 422–430. [Google Scholar] [CrossRef]
- Chuang, P.A.; Rahman, M.A.; Mojica, F.; Hussey, D.S.; Jacobson, D.L.; Lamanna, J.M. The interactive effect of heat and mass transport on water condensation in the gas diffusion layer of a proton exchange membrane fuel cell. J. Power Sources 2020, 480, 229121. [Google Scholar] [CrossRef]
- Hosseini, M.; Afrouzi, H.H.; Arasteh, H.; Toghraie, D. Energy analysis of a proton exchange membrane fuel cell (PEMFC) with an open-ended anode using agglomerate model: A CFD study. Energy 2019, 188, 116090. [Google Scholar] [CrossRef]
- Moein-Jahromi, M.; Kermani, M.J. Three-dimensional multiphase simulation and multi-objective optimization of PEM fuel cells degradation under automotive cyclic loads. Energy Convers. Manag. 2021, 231, 113837. [Google Scholar] [CrossRef]
- Liu, Z.; Zeng, X.; Ge, Y.; Shen, J.; Liu, W. Multi-objective optimization of operating conditions and channel structure for a proton exchange membrane fuel cell. Int. J. Heat Mass Transf. 2017, 111, 289–298. [Google Scholar] [CrossRef]
- Khaki, B.; Das, P. Multi-objective optimal charging current and flow management of Vanadium Redox Flow Batteries for fast charging and energy-efficient operation. J. Power Sources 2021, 506, 230199. [Google Scholar] [CrossRef]
- Jiaqiang, E.; Han, D.; Qiu, A.; Zhu, H.; Deng, Y.; Chen, J.; Zhao, X.; Zuo, W.; Wang, H.; Chen, J.; et al. Orthogonal experimental design of liquid-cooling structure on the cooling effect of a liquid-cooled battery thermal management system. Appl. Therm. Eng. 2018, 132, 508–520. [Google Scholar]

| Part | Characteristics | Size |
|---|---|---|
| Membrane | GORE-SELECT 735.18 | 5 cm ∗ 5 cm ∗ 18 μm |
| Catalyst layer (CL) | Pt loading (anode: 0.1 mg/cm2; cathode: 0.35 mg/cm2) | 5 cm ∗ 5 cm ∗ 5 μm |
| Microporous layer (MPL) | attached with GDL | 5 cm ∗ 5 cm ∗ 30 μm |
| Gas diffusion layer (GDL) | SGL 28BC | 5 cm ∗ 5 cm ∗ 235 μm |
| Flow field plate | Carbon graphite | 9 cm ∗ 9 cm ∗ 2 cm |
| Current collector | Copper coated with gold | 9 cm ∗ 9 cm ∗ 3 mm |
| Endplate | Aluminum | 9 cm ∗ 9 cm ∗ 2 cm |
| Operation Condition | Anode | Cathode |
|---|---|---|
| Gas type and flow rate | Hydrogen: 300 mL/min | Air: 900 mL/min |
| Temperature of gas feed | 90 °C | 90 °C |
| Absolute pressure of gas | 1.5 atm | 1.5 atm |
| Relative humidity | Case 1 (RHa50%/RHc50%); Case 2 (RHa90%/RHc90%) Case 3 (RHa70%/RHc90%); Case 4 (RHa50%/RHc90%) Case 5 (RHa90%/RHc70%); Case 6 (RHa90%/RHc50%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Chang, G.; Zhang, J.; Li, Y.; Xu, S. Investigation of Inlet Gas Relative Humidity on Performance Characteristics of PEMFC Operating at Elevated Temperature. World Electr. Veh. J. 2021, 12, 110. https://doi.org/10.3390/wevj12030110
Xu Y, Chang G, Zhang J, Li Y, Xu S. Investigation of Inlet Gas Relative Humidity on Performance Characteristics of PEMFC Operating at Elevated Temperature. World Electric Vehicle Journal. 2021; 12(3):110. https://doi.org/10.3390/wevj12030110
Chicago/Turabian StyleXu, Yiming, Guofeng Chang, Jienan Zhang, Yuyang Li, and Sichuan Xu. 2021. "Investigation of Inlet Gas Relative Humidity on Performance Characteristics of PEMFC Operating at Elevated Temperature" World Electric Vehicle Journal 12, no. 3: 110. https://doi.org/10.3390/wevj12030110
APA StyleXu, Y., Chang, G., Zhang, J., Li, Y., & Xu, S. (2021). Investigation of Inlet Gas Relative Humidity on Performance Characteristics of PEMFC Operating at Elevated Temperature. World Electric Vehicle Journal, 12(3), 110. https://doi.org/10.3390/wevj12030110





