Dual Closed-Loops Capacity Evolution Prediction for Energy Storage Batteries Integrated with Coupled Electrochemical Model
Abstract
:1. Introduction
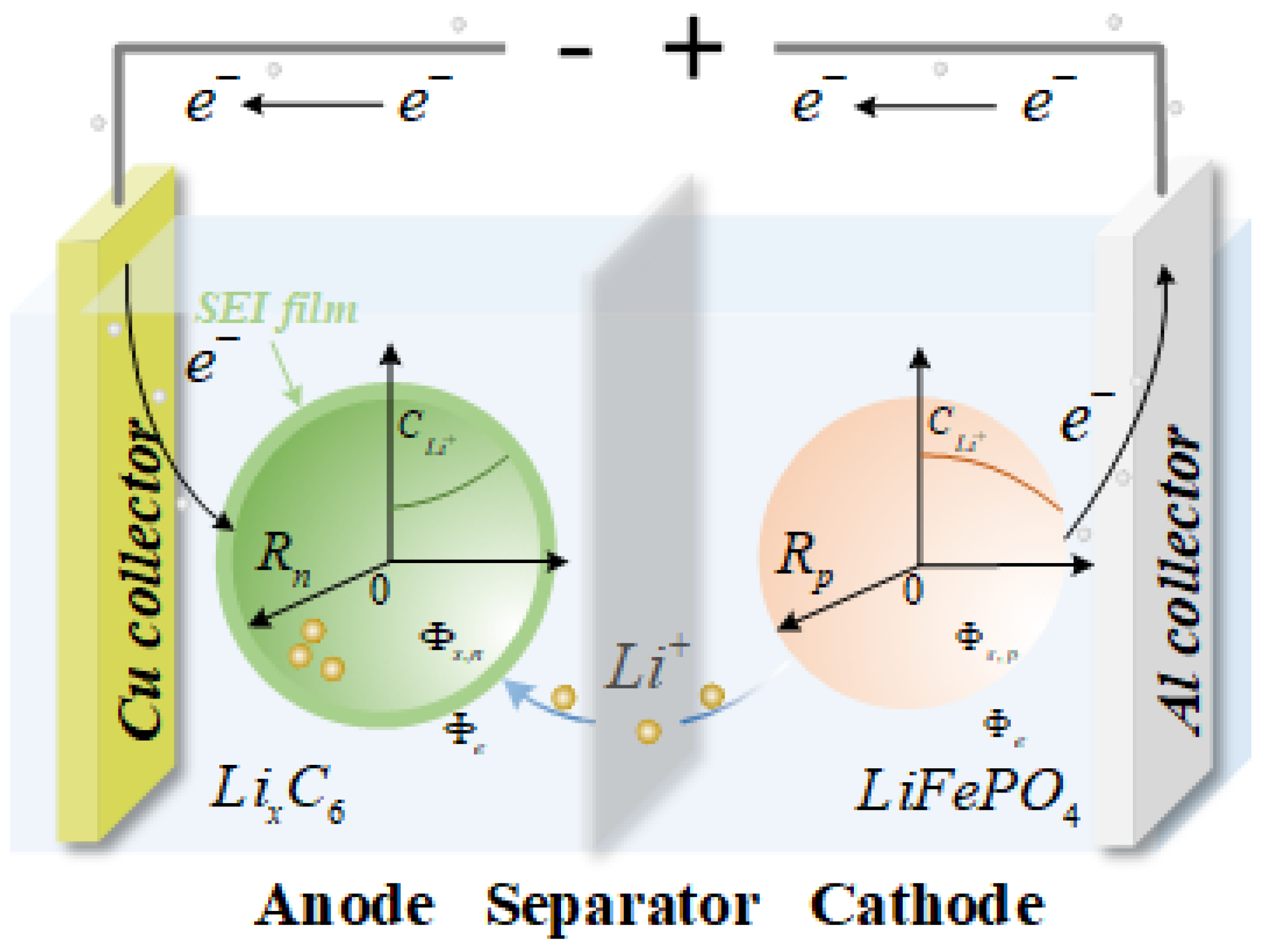
2. Reduced-Order Physical Degradation Model
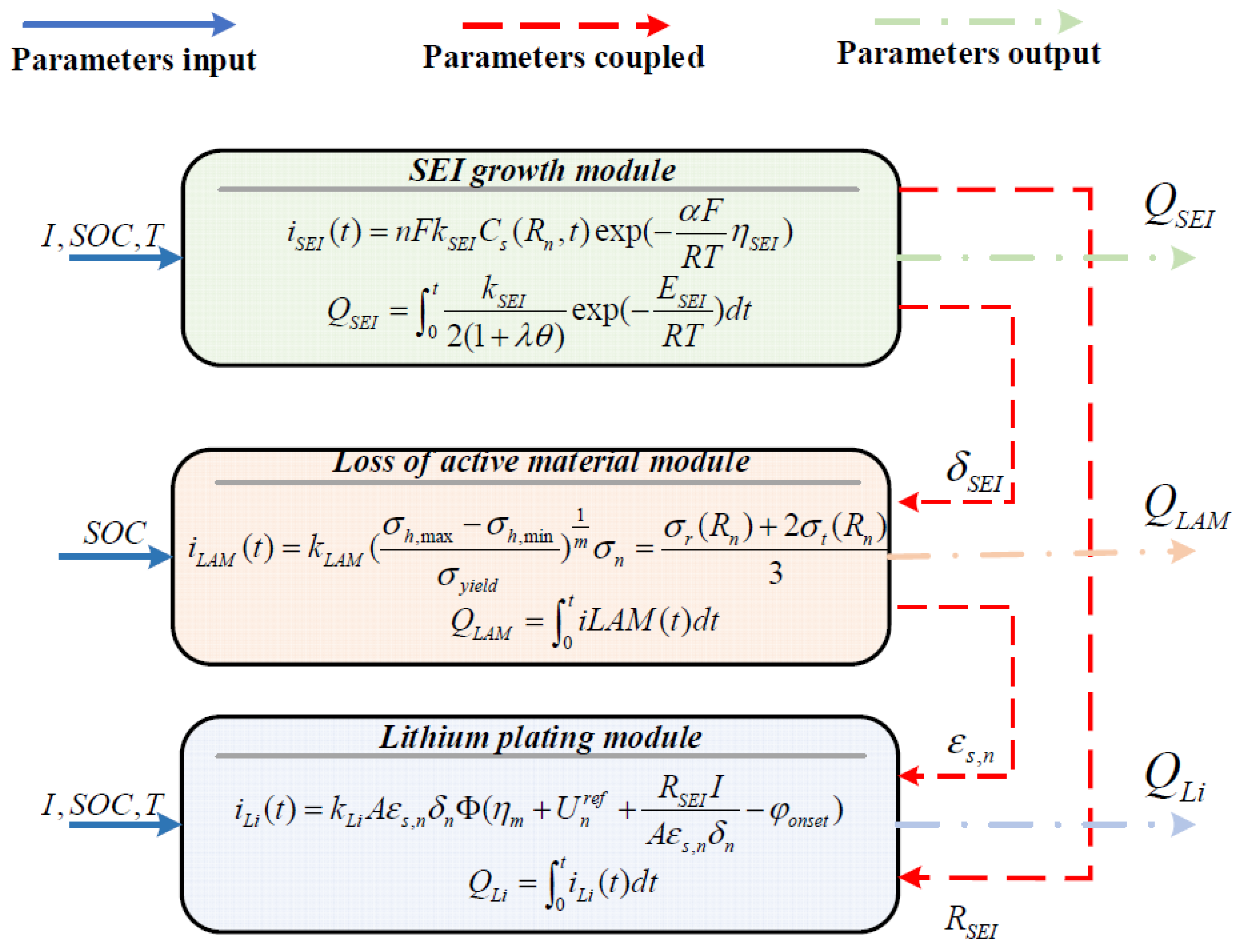
2.1. SEI Growth Sub-Model
2.2. LAM Sub-Model
2.3. Lithium Plating Sub-Model
2.4. Coupling Principle for Sub-Models
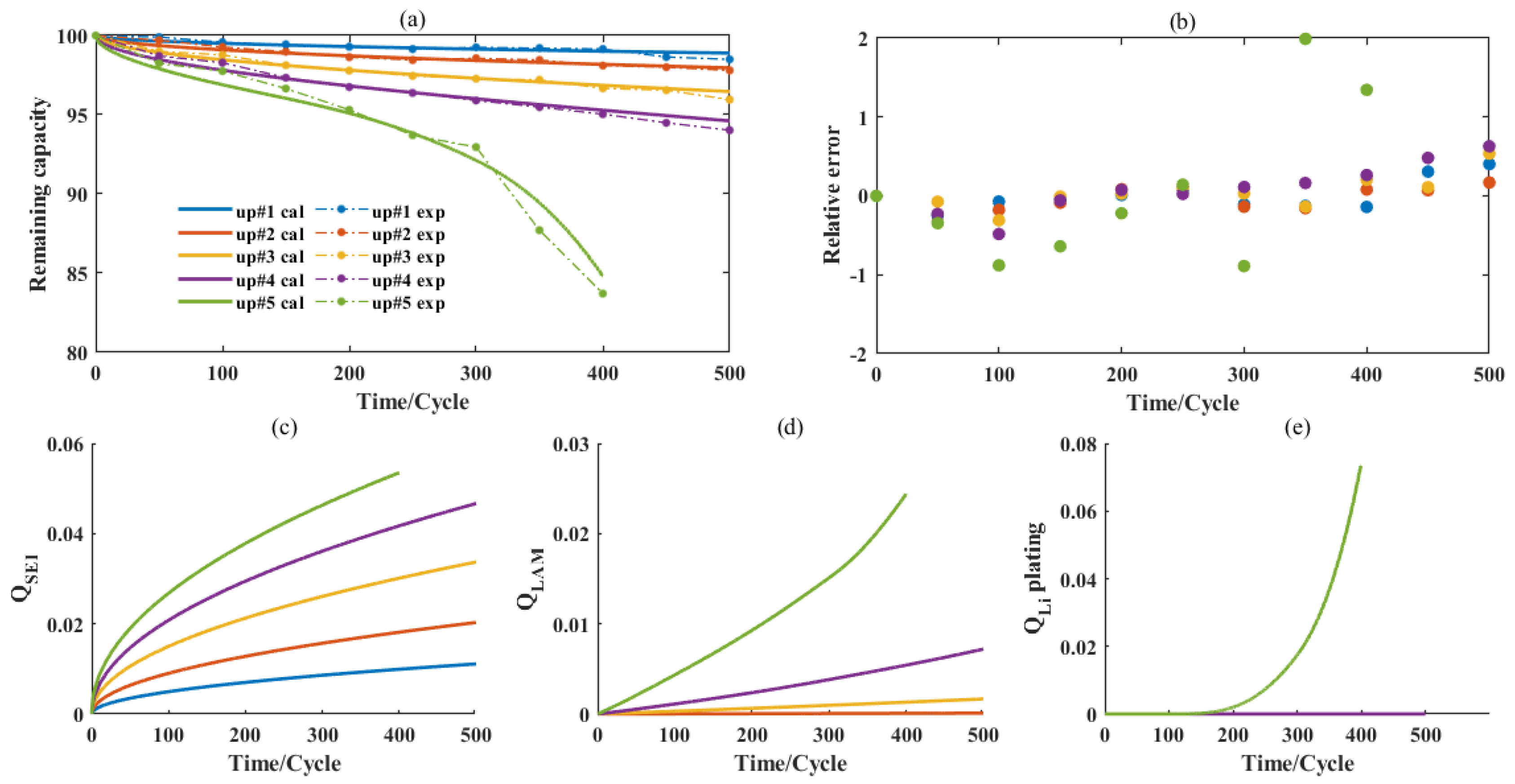
3. Calibration and Verification
3.1. Microgrid Operations Simplified for Accelerated Degradation
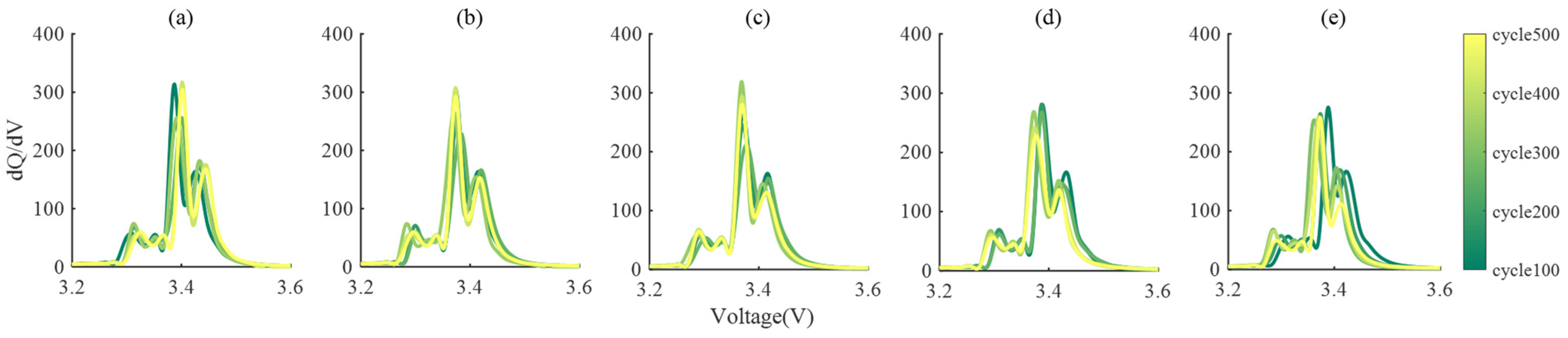
3.2. Result Analysis and Model Verification
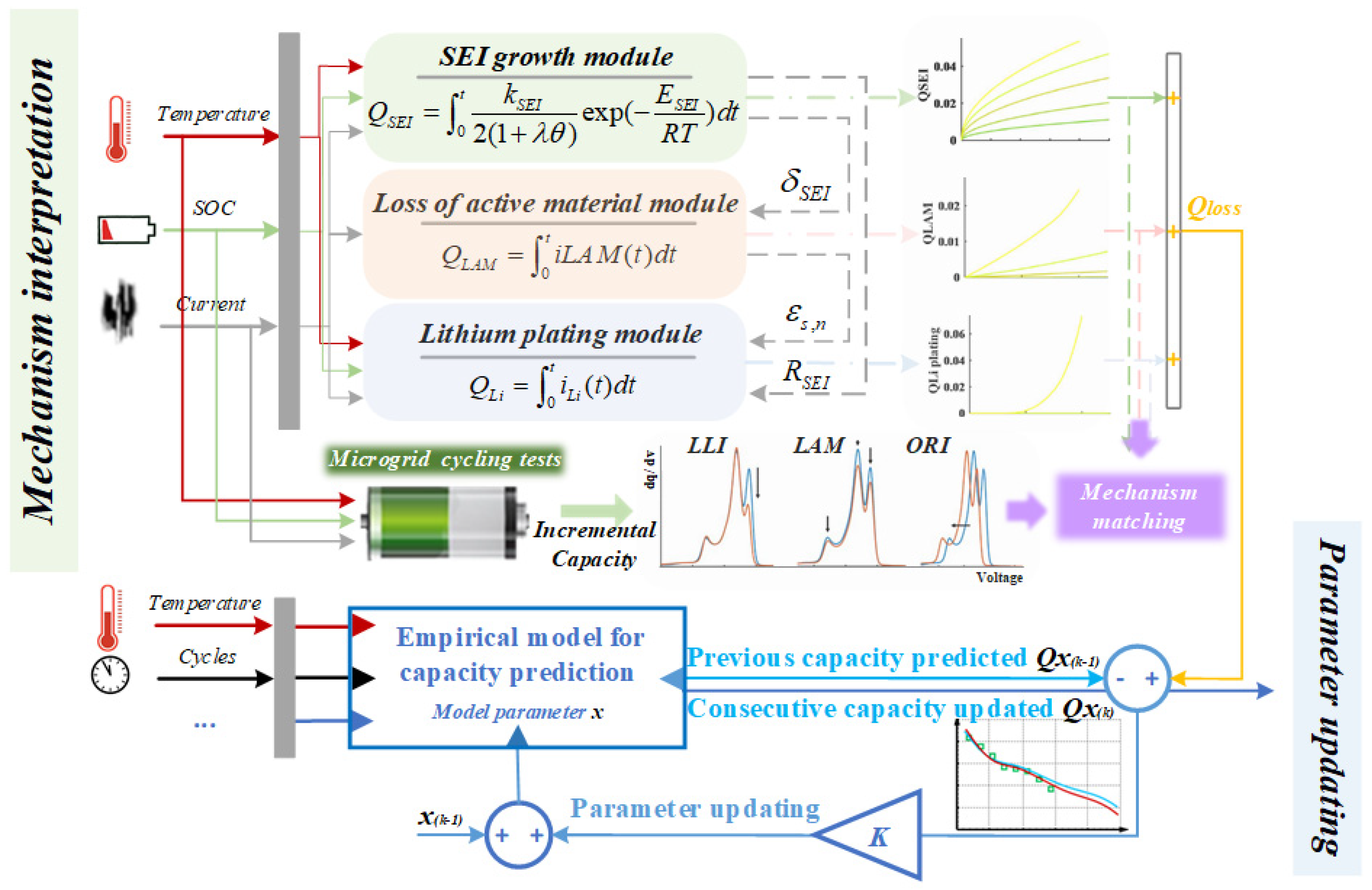
4. Dual Closed-Loops Capacity Prediction Framework
4.1. The First Closed Loop: Mechanism Interpretation
4.2. Second Closed Loop: Parameter Updating
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Khalid, M.R.; Alam, M.S.; Sarwar, A.; Jamil Asghar, M.S. A Comprehensive Review on Electric Vehicles Charging Infrastructures and Their Impacts on Power-Quality of the Utility Grid. eTransportation 2019, 1, 100006. [Google Scholar] [CrossRef]
- Tian, J.; Xiong, R.; Shen, W. A Review on State of Health Estimation for Lithium-Ion Batteries in Photovoltaic Systems. eTransportation 2019, 2, 100028. [Google Scholar] [CrossRef]
- Han, X.; Lu, L.; Zheng, Y.; Feng, X.; Li, Z.; Li, J.; Ouyang, M. A Review on the Key Issues of the Lithium-Ion Battery Degradation Among the Whole Life Cycle. eTransportation 2019, 1, 100005. [Google Scholar] [CrossRef]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A Review on the Key Issues for Lithium-Ion Battery Management in Electric Vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Xiong, R.; Li, L.; Tian, J. Towards a Smarter Battery Management System: A Critical Review on Battery State of Health Monitoring Methods. J. Power Sources 2018, 405, 18–29. [Google Scholar] [CrossRef]
- Sarasketa-Zabala, E.; Gandiaga, I.; Martinez-Laserna, E.; Rodriguez-Martinez, L.M.; Villarreal, I. Cycle Ageing Analysis of a LiFePO4/Graphite Cell with Dynamic Model Validations: Towards Realistic Lifetime Predictions. J. Power Sources 2015, 275, 573–587. [Google Scholar] [CrossRef]
- Zheng, Y.; Qin, C.; Lai, X.; Han, X.; Xie, Y. A Novel Capacity Estimation Method for Lithium-Ion Batteries Using Fusion Estimation of Charging Curve Sections and Discrete Arrhenius Aging Model. Appl. Energy 2019, 251, 113327. [Google Scholar] [CrossRef]
- Yang, S.; Hua, Y.; Qiao, D.; Lian, Y.-b.; Pan, Y.; He, Y.-l. A Coupled Electrochemical-Thermal-Mechanical Degradation Modelling Approach for Lifetime Assessment of Lithium-Ion Batteries. Electrochim. Acta 2019, 326, 134928. [Google Scholar] [CrossRef]
- Pang, H.; Mou, L.; Guo, L.; Zhang, F. Parameter Identification and Systematic Validation of an Enhanced Single-Particle Model with Aging Degradation Physics for Li-Ion Batteries. Electrochim. Acta 2019, 307, 474–487. [Google Scholar] [CrossRef]
- Jin, X.; Vora, A.; Hoshing, V.; Saha, T.; Shaver, G.; García, R.E.; Wasynczuk, O.; Varigonda, S. Physically-Based Reduced-Order Capacity Loss Model for Graphite Anodes in Li-Ion Battery Cells. J. Power Sources 2017, 342, 750–761. [Google Scholar] [CrossRef] [Green Version]
- Reniers, J.M.; Mulder, G.; Howey, D.A. Review and Performance Comparison of Mechanical-Chemical Degradation Models for Lithium-Ion Batteries. J. Electrochem. Soc. 2019, 166, 3189–3200. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.; Liu, C. Physics-Based Control-Oriented Reduced-Order Degradation Model for LiNiMnCoO2-Graphite Cell. Electrochim. Acta 2019, 312, 188–201. [Google Scholar] [CrossRef]
- Wang, S.; Kuang, K.; Han, X.; Chu, Z.; Lu, L.; Ouyang, M. A Model-Based Continuous Differentiable Current Charging Approach for Electric Vehicles in Direct Current Microgrids. J. Power Sources 2021, 482, 229019. [Google Scholar] [CrossRef]
- Laresgoiti, I.; Käbitz, S.; Ecker, M.; Sauer, D.U. Modeling Mechanical Degradation in Lithium-Ion Batteries During Cycling: Solid Electrolyte Interphase Fracture. J. Power Sources 2015, 300, 112–122. [Google Scholar] [CrossRef]
- Li, Y.; Feng, X.; Ren, D.; Ouyang, M.; Lu, L.; Han, X. Thermal Runaway Triggered by Plated Lithium on the Anode After Fast Charging. ACS Appl. Mater. Interfaces 2019, 11, 46839–46850. [Google Scholar] [CrossRef]
- Tomaszewska, A.; Chu, Z.; Feng, X.; O’Kane, S.; Liu, X.; Chen, J.; Ji, C.; Endler, E.; Li, R.; Liu, L.; et al. Lithium-Ion Battery Fast Charging: A Review. eTransportation 2019, 1, 100011. [Google Scholar] [CrossRef]
- Wang, S.; Lu, L.; Han, X.; Ouyang, M.; Feng, X. Virtual-Battery Based Droop Control and Energy Storage System Size Optimization of a DC Microgrid for Electric Vehicle Fast Charging Station. Appl. Energy 2020, 259, 114146. [Google Scholar] [CrossRef]
- Wang, S.; Guo, D.; Han, X.; Lu, L.; Sun, K.; Li, W.; Sauer, D.U.; Ouyang, M. Impact of Battery Degradation Models on Energy Management of a Grid-Connected DC Microgrid. Energy 2020, 207, 118228. [Google Scholar] [CrossRef]
- Wu, B.; Yufit, V.; Merla, Y.; Martinez-Botas, R.F.; Brandon, N.P.; Offer, G.J. Differential Thermal Voltammetry for Tracking of Degradation in Lithium-Ion Batteries. J. Power Sources 2015, 273, 495–501. [Google Scholar] [CrossRef]
- Feng, X.; Merla, Y.; Weng, C.; Ouyang, M.; He, X.; Liaw, B.Y.; Santhanagopalan, S.; Li, X.; Liu, P.; Lu, L.; et al. A Reliable Approach of Differentiating Discrete Sampled-Data for Battery Diagnosis. eTransportation 2020, 3, 100051. [Google Scholar] [CrossRef]
- Song, Z.; Yang, X.-G.; Yang, N.; Delgado, F.P.; Hofmann, H.; Sun, J. A Study of Cell-to-Cell Variation of Capacity in Parallel-Connected Lithium-Ion Battery Cells. eTransportation 2021, 7, 100091. [Google Scholar] [CrossRef]
- Cui, H.; Miao, Q.; Liang, W.; Wang, Z. Application of Unscented Particle Filter in Remaining Useful Life Prediction of Lithium-Ion Batteries. Progn. Syst. Health Manag. Conf. 2012, 2012. [Google Scholar]
- Sun, T.; Xu, B.; Cui, Y.; Feng, X.; Han, X.; Zheng, Y. A Sequential Capacity Estimation for the Lithium-Ion Batteries Combining Incremental Capacity Curve and Discrete Arrhenius Fading Model. J. Power Sources 2020, 484, 229248. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, C.; Chen, Z. An Online Method for Lithium-Ion Battery Remaining Useful Life Estimation Using Importance Sampling and Neural Networks. Appl. Energy 2016, 173, 134–140. [Google Scholar] [CrossRef]
- Chen, L.; Wang, H.; Chen, J.; An, J.; Ji, B.; Lyu, Z.; Cao, W.; Pan, H. A Novel Remaining Useful Life Prediction Framework for Lithium-Ion Battery Using Grey Model and Particle Filtering. Int. J. Energy Res. 2020, 44, 7435–7449. [Google Scholar] [CrossRef]


| Parameters | Value | Parameters | Value |
|---|---|---|---|
| 86,995 | 787.41 | ||
| 15.55 | 0.23 | ||
| 27,219 | 4.20 | ||
| 1.61 | −5.5 |
| Parameters | Value | Parameters | Value | Parameters | Value |
|---|---|---|---|---|---|
| 1 | 16.7 | 15 | |||
| 96,487 | 0.59 | 0.5 | |||
| 0.5 | 0.02 | 0.3 | |||
| 8.314 | 4 | 0.2 | |||
| 9 | 0.162 | 31.92 | |||
| 2 | 1690 | 3.1 | |||
| 1 | 5 × 10−6 | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, B.; Sun, T.; Wang, S.; Wei, Y.; Han, X.; Zheng, Y. Dual Closed-Loops Capacity Evolution Prediction for Energy Storage Batteries Integrated with Coupled Electrochemical Model. World Electr. Veh. J. 2021, 12, 109. https://doi.org/10.3390/wevj12030109
Xu B, Sun T, Wang S, Wei Y, Han X, Zheng Y. Dual Closed-Loops Capacity Evolution Prediction for Energy Storage Batteries Integrated with Coupled Electrochemical Model. World Electric Vehicle Journal. 2021; 12(3):109. https://doi.org/10.3390/wevj12030109
Chicago/Turabian StyleXu, Bowen, Tao Sun, Shuoqi Wang, Yifan Wei, Xuebing Han, and Yuejiu Zheng. 2021. "Dual Closed-Loops Capacity Evolution Prediction for Energy Storage Batteries Integrated with Coupled Electrochemical Model" World Electric Vehicle Journal 12, no. 3: 109. https://doi.org/10.3390/wevj12030109
APA StyleXu, B., Sun, T., Wang, S., Wei, Y., Han, X., & Zheng, Y. (2021). Dual Closed-Loops Capacity Evolution Prediction for Energy Storage Batteries Integrated with Coupled Electrochemical Model. World Electric Vehicle Journal, 12(3), 109. https://doi.org/10.3390/wevj12030109





