Material Characterization and Analysis on the Effect of Vibration and Nail Penetration on Lithium Ion Battery
Abstract
1. Introduction
2. Experimental Setup
3. Results and Discussion
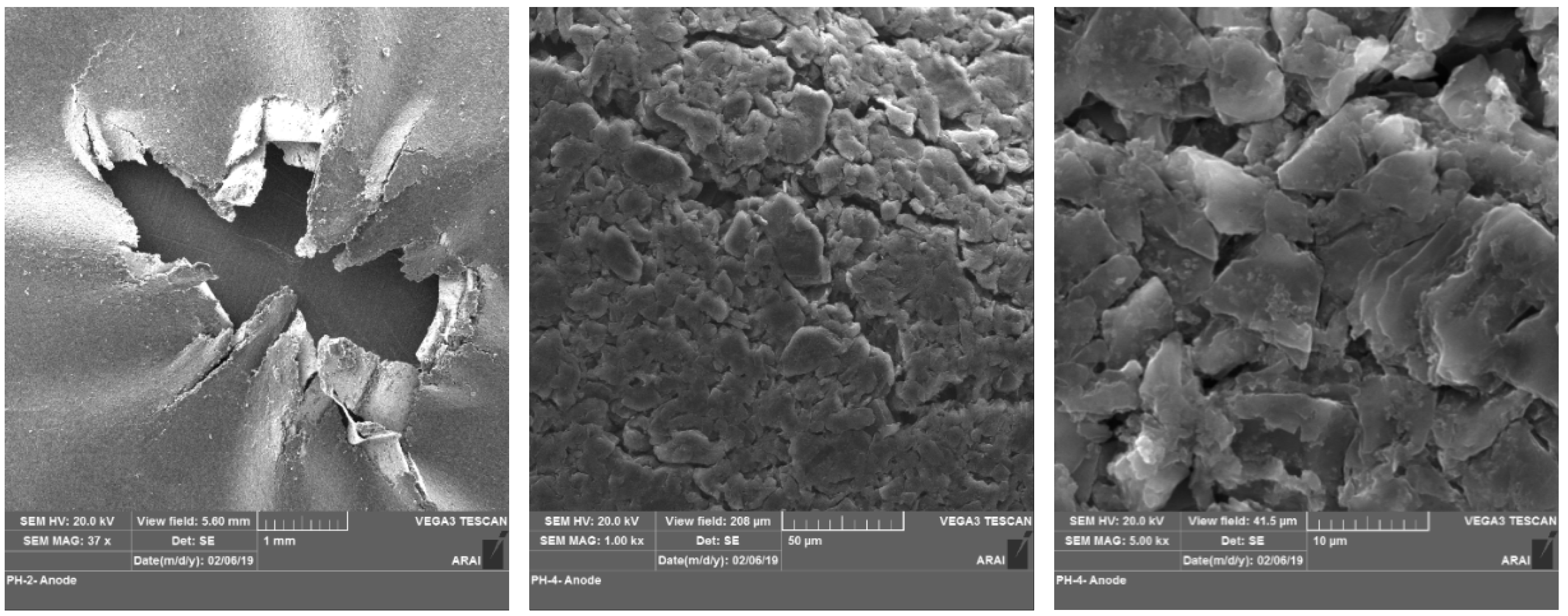
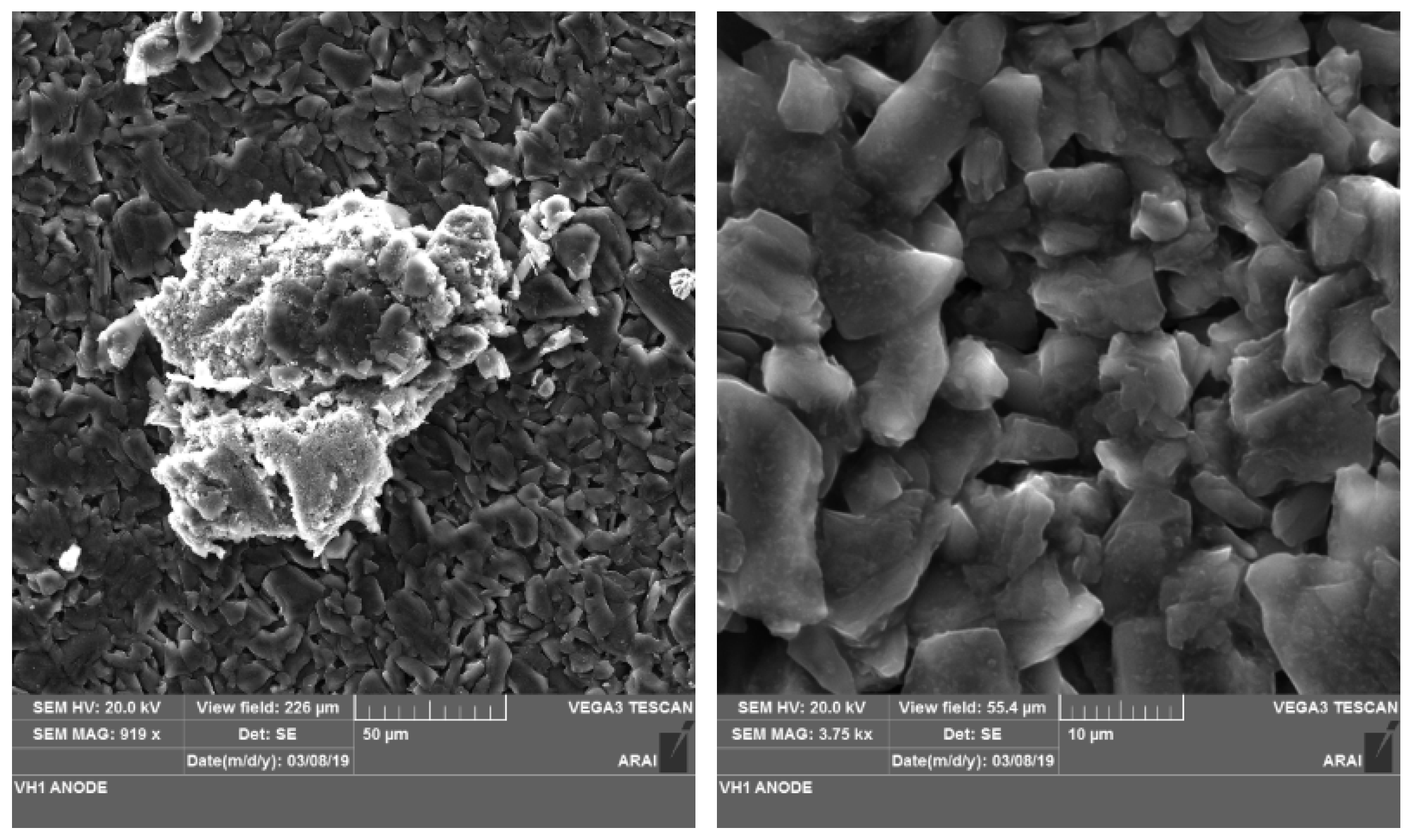
3.1. Morphology Analysis
3.2. Composition Analysis
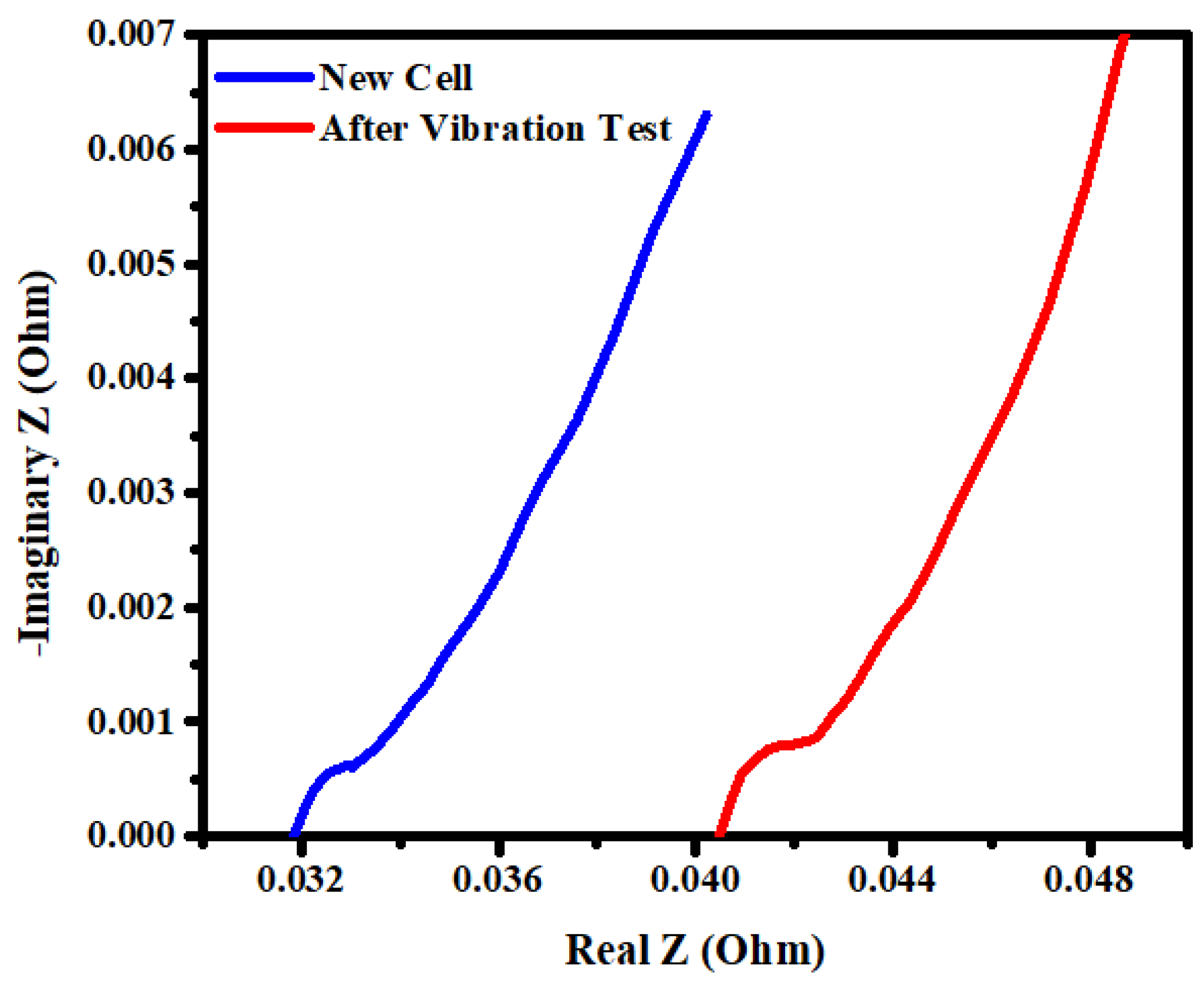
3.3. Internal Resistance Analysis
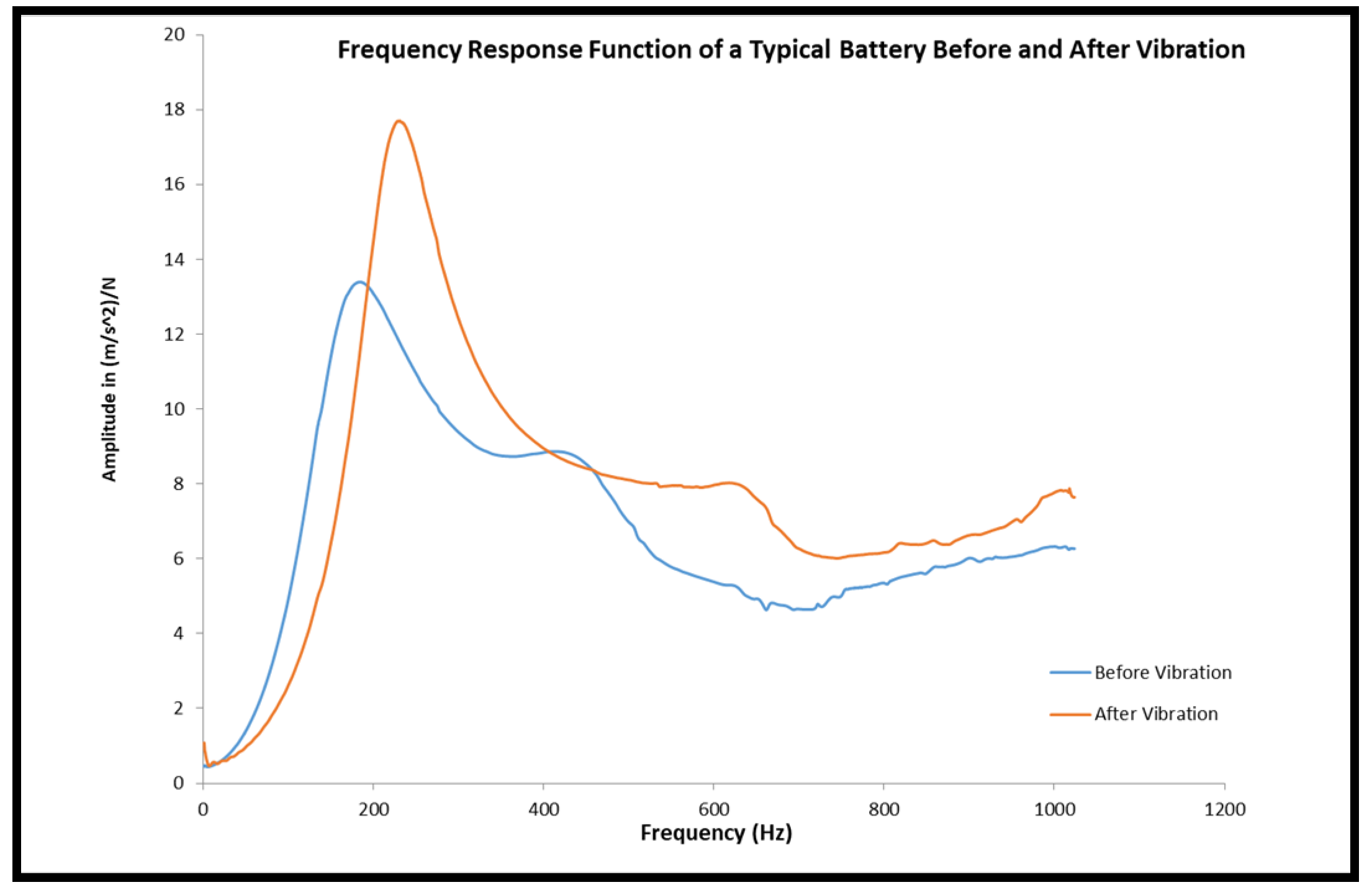
3.4. Dynamic Stiffness Analysis
4. Conclusions
- Morphology analysis of vibration-tested anode showed the presence of a tower-like structure which had grown due to deposition of Fe and P elements from the cathode.
- Compositional analysis showed that, when the LiB undergoes localized mechanical damage without a thermal runaway then that leads to uneven distribution of the elements within the electrode surface.
- In the top side location away from centre, for the cathode at 50% SoC in nail-penetrated samples, the elemental Wt.% for C, O, P and Fe was similar before and after testing and thus there was no effect of abuse on the distribution of the elements.
- Capacity fade happened in the cells that were in 100% SoC to 50% SoC as the voltage dropped by 0.3 to 0.4 V.
- After the cell was tested for its sinusoidal vibration the average max amplitude, resonance frequency, dynamic stiffness and internal resistance increased by 14%, 38%, 70% and 25% respectively.
- FRF analysis of LiB can inform us about the state of the cell and can be used as a non-destructive evaluation technique.
- It is important to validate the performance of an LiB for its application and the vibrational load during the lifetime of cell should be carefully considered while designing the battery pack.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kasnatscheew, J.; Evertz, M.; Kloepsch, R.; Streipert, B.; Wagner, R.; Cekic Laskovic, I.; Winter, M. Learning from Electrochemical Data: Simple Evaluation and Classification of LiMO2-type-based Positive Electrodes for Li-Ion Batteries. Energy Technol. 2017, 5, 1670–1679. [Google Scholar] [CrossRef]
- Kasnatscheew, J.; Evertz, M.; Streipert, B.; Wagner, R.; Nowak, S.; Laskovic, I.C.; Winter, M. Improving cycle life of layered lithium transition metal oxide (LiMO2) based positive electrodes for Li ion batteries by smart selection of the electrochemical charge conditions. J. Power Sources 2017, 359, 458–467. [Google Scholar] [CrossRef]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Choi, N.S.; Chen, Z.; Freunberger, S.A.; Ji, X.; Sun, Y.K.; Amine, K.; Yushin, G.; Nazar, L.F.; Cho, J.; Bruce, P.G. Challenges facing lithium batteries and electrical double-layer capacitors. Angew. Chem. Int. Ed. 2012, 51, 9994–10024. [Google Scholar] [CrossRef] [PubMed]
- Owen, J.R. Rechargeable lithium batteries. Chem. Soc. Rev. 1997, 26, 259–267. [Google Scholar] [CrossRef]
- Roberts, M.; Johns, P.; Owen, J.; Brandell, D.; Edstrom, K.; El Enany, G.; Guery, C.; Golodnitsky, D.; Lacey, M.; Lecoeur, C.; et al. 3D lithium ion batteries—From fundamentals to fabrication. J. Mater. Chem. 2011, 21, 9876–9890. [Google Scholar] [CrossRef]
- Kasnatscheew, J.; Wagner, R.; Winter, M.; Cekic-Laskovic, I. Interfaces and Materials in Lithium Ion Batteries: Challenges for Theoretical Electrochemistry. In Modeling Electrochemical Energy Storage at the Atomic Scale; Springer: Cham, Germany, 2018; pp. 23–51. [Google Scholar]
- Janek, J.; Zeier, W.G. A solid future for battery development. Energy 2016, 500, 300. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable batteries. J. Power Sources 2011, 196, 6688–6694. [Google Scholar] [CrossRef]
- Padhi, A.K.; Nanjundaswamy, K.S.; Goodenough, J.B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J. Electrochem. Soc. 1997, 144, 1188–1194. [Google Scholar] [CrossRef]
- Liu, H.; Cao, Q.; Fu, L.J.; Li, C.; Wu, Y.P.; Wu, H.Q. Doping effects of zinc on LiFePO4 cathode material for lithium ion batteries. Electrochem. Commun. 2006, 8, 1553–1557. [Google Scholar] [CrossRef]
- Chung, S.Y.; Bloking, J.T.; Chiang, Y.M. Electronically conductive phospho-olivines as lithium storage electrodes. Nat. Mater. 2002, 1, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Ellis, B.L.; Lee, K.T.; Nazar, L.F. Positive electrode materials for Li-ion and Li-batteries. Chem. Mater. 2010, 22, 691–714. [Google Scholar] [CrossRef]
- Whittingham, M.S. Lithium batteries and cathode materials. Chem. Rev. 2004, 104, 4271–4302. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Yu, H.; Zhou, H. Layered lithium transition metal oxide cathodes towards high energy lithium-ion batteries. J. Mater. Chem. 2012, 22, 3680–3695. [Google Scholar] [CrossRef]
- Lang, J.F.; Kjell, G. Comparing vibration measurements in an electric vehicle with standard vibration requirements for Li-ion batteries using power spectral density analysis. Int. J. Electr. Hybrid Veh. 2015, 7, 272–286. [Google Scholar] [CrossRef]
- Hooper, J.M.; Marco, J. Characterising the in-vehicle vibration inputs to the high voltage battery of an electric vehicle. J. Power Sources 2014, 245, 510–519. [Google Scholar] [CrossRef]
- Unkelhaeuser, T.; Smallwood, D. Electrochemical Storage System Abuse Test Procedure Manual; USABC/SNL CRADA No. SC961447; United States Advanced Battery Consortium (USABC): Washington, WA, USA, 1999. [Google Scholar]
- ECE R100.2. Uniform Provisions Concerning the Approval of Vehicles with Regard to Specific Requirements for the Electric Power Train; UN/ECE: Geneva, Switzerland, 2013. [Google Scholar]
- Automotive Industry Standard 048—Battery Operated Vehicles—Safety Requirements of Traction Batteries; Automotive Research Association of India (ARAI): Pune, India, 2009.
- Golla-Schindler, U.; Zeibig, D.; Prickler, L.; Behn, S.; Bernthaler, T.; Schneider, G. Characterization of degeneration phenomena in lithium-ion batteries by combined microscopic techniques. Micron 2018, 113, 10–19. [Google Scholar] [CrossRef]
- Liu, S.; Hong, X.; Wang, D.; Li, Y.; Xu, J.; Zheng, C.; Xie, K. Hollow carbon spheres with nanoporous shells and tailored chemical interfaces as sulfur host for long cycle life of lithium sulfur batteries. Electrochim. Acta 2018, 279, 10–18. [Google Scholar] [CrossRef]
- Babu, P.A.; Waghmare, A.S.; Mulla, S.M.; Karle, U.S.; Saraf, M.R. Material characterization of Lithium-ion battery cells by scanning electron microscopy & X-ray diffraction techniques. In Proceedings of the 2017 IEEE Transportation Electrification Conference (ITEC-India), Pune, India, 13–15 December 2017; pp. 1–5. [Google Scholar]
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy: Theory, Experiment, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Huet, F. A review of impedance measurements for determination of the state-of-charge or state-of-health of secondary batteries. J. Power Sources 1998, 70, 59–69. [Google Scholar] [CrossRef]
- Buller, S. Impedance Based Simulation Models for Energy Storage Devices in Advanced Automotive Power Systems; Shaker Verlag: Herzogenrath, Germany, 2002. [Google Scholar]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 48. [Google Scholar]
- Farhat, D.; Maibach, J.; Eriksson, H.; Edström, K.; Lemordant, D.; Ghamouss, F. Towards high-voltage Li-ion batteries: Reversible cycling of graphite anodes and Li-ion batteries in adiponitrile-based electrolytes. Electrochim. Acta 2018, 281, 299–311. [Google Scholar] [CrossRef]
- Illig, J.; Schmidt, J.P.; Weiss, M.; Weber, A.; Ivers-Tiffée, E. Understanding the impedance spectrum of 18650 LiFePO4-cells. J. Power Sources 2013, 239, 670–679. [Google Scholar] [CrossRef]
- Samad, N.A.; Kim, Y.; Siegel, J.B.; Stefanopoulou, A.G. Battery capacity fading estimation using a force-based incremental capacity analysis. J. Electrochem. Soc. 2016, 163, A1584–A1594. [Google Scholar] [CrossRef]
- Dai, H.; Yu, C.; Wei, X.; Sun, Z. State of charge estimation for lithium-ion pouch batteries based on stress measurement. Energy 2017, 129, 16–27. [Google Scholar] [CrossRef]
- Cannarella, J.; Arnold, C.B. State of health and charge measurements in lithium-ion batteries using mechanical stress. J. Power Sources 2014, 269, 7–14. [Google Scholar] [CrossRef]
- Hooper, J.M.; Marco, J. Experimental modal analysis of lithium-ion pouch cells. J. Power Sources 2015, 285, 247–259. [Google Scholar] [CrossRef]
- Pham, H.L.; Dietz, J.E.; Adams, D.E.; Sharp, N.D. Lithium-ion battery cell health monitoring using vibration diagnostic test. In Proceedings of the ASME 2013 International Mechanical Engineering Congress and Exposition, San Diego, CA, USA, 15–21 November 2013; American Society of Mechanical Engineers: New York, NY, USA, 2013; p. V04BT04A048. [Google Scholar]










| Frequency Range (Hz) | Peak Acceleration (g) | Duration (min) |
|---|---|---|
| 20–10 | 3 | 72 |
| 20–40 | 2 | 72 |
| 40–90 | 1.5 | 72 |
| 90–140 | 1 | 72 |
| 140–190 | 0.75 | 72 |
| Curve | Max Amplitude (m/s2)/N | Max Amplitude’s Freq, (Hz) | Dynamic Stiffness at Max Amplitude Freq (N/mm) |
|---|---|---|---|
| Cell 1 | 15.3 | 156.50 | 63.17 |
| Cell 2 | 15.1 | 177.25 | 82.18 |
| Cell 3 | 13.4 | 183.75 | 99.53 |
| Average | 14.6 | 172.50 | 81.63 |
| Curve | Max Amplitude (m/s2)/N | Max Amplitude’s Freq, (Hz) | Dynamic Stiffness at Max Amplitude Freq (N/mm) |
|---|---|---|---|
| Cell 1 | 17.0 | 216.5 | 109.09 |
| Cell 2 | 15.3 | 270.5 | 188.45 |
| Cell 3 | 17.7 | 231.0 | 118.96 |
| Average | 16.7 | 239.3 | 138.83 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parasumanna, A.B.K.; Karle, U.S.; Saraf, M.R. Material Characterization and Analysis on the Effect of Vibration and Nail Penetration on Lithium Ion Battery. World Electr. Veh. J. 2019, 10, 69. https://doi.org/10.3390/wevj10040069
Parasumanna ABK, Karle US, Saraf MR. Material Characterization and Analysis on the Effect of Vibration and Nail Penetration on Lithium Ion Battery. World Electric Vehicle Journal. 2019; 10(4):69. https://doi.org/10.3390/wevj10040069
Chicago/Turabian StyleParasumanna, Ajeet Babu K., Ujjwala S. Karle, and Mangesh R. Saraf. 2019. "Material Characterization and Analysis on the Effect of Vibration and Nail Penetration on Lithium Ion Battery" World Electric Vehicle Journal 10, no. 4: 69. https://doi.org/10.3390/wevj10040069
APA StyleParasumanna, A. B. K., Karle, U. S., & Saraf, M. R. (2019). Material Characterization and Analysis on the Effect of Vibration and Nail Penetration on Lithium Ion Battery. World Electric Vehicle Journal, 10(4), 69. https://doi.org/10.3390/wevj10040069




