Blinatumomab in Relapsed/Refractory Burkitt Lymphoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatment
2.3. Assessment of Safety and Efficacy
2.4. Measurements and Definitions
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics at First Diagnosis
3.2. Previous Lines of Treatment before Blinatumomab
3.3. Blinatumomab Therapy
3.4. Adverse Effects Infection, CRS and Neurotoxicity
3.5. Outcome of Blinatumomab Therapy
3.6. Treatment after Blinatumomab Therapy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chamuleau, M.E.D.; Stenner, F.; Chitu, D.; Novak, U.; Minnema, M.; Visser, O.; Stevens, W.; Zenz, T.; van Imhoff, G.; Wu, K.L.; et al. R-CODOX-M/R-IVAC Versus DA-EPOCH-R in Patients with Newly Diagnosed High-Risk Burkitt Lymphoma: First Results of a Multi-Center Randomized HOVON/SAKK Trial. In Proceedings of the EHA Congress Vienna AAL, Vienna, Austria, 9–12 June 2022. [Google Scholar]
- Oosten, L.E.M.; Chamuleau, M.E.D.; Thielen, F.W.; de Wreede, L.C.; Siemes, C.; Doorduijn, J.K.; Smeekes, O.S.; Kersten, M.J.; Hardi, L.; Baars, J.W.; et al. Treatment of sporadic Burkitt lymphoma in adults, a retrospective comparison of four treatment regimens. Ann. Hematol. 2018, 97, 255–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crombie, J.; LaCasce, A. The treatment of Burkitt lymphoma in adults. Blood. 2021, 137, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Evens, A.M.; Danilov, A.; Jagadeesh, D.; Sperling, A.; Kim, S.H.; Vaca, R.; Wei, C.; Rector, D.; Sundaram, S.; Reddy, N.; et al. Burkitt lymphoma in the modern era: Real-world outcomes and prognostication across 30 US cancer centers. Blood 2021, 137, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.L.; Toothaker, S.R.; Ciminello, L.; Hoelzer, D.; Holte, H.; LaCasce, A.S.; Mead, G.; Thomas, D.; Van Imhoff, G.W.; Kahl, B.S.; et al. Outcomes of patients with Burkitt lymphoma older than age 40 treated with intensive chemotherapeutic regimens. Clin. Lymphoma Myeloma 2009, 9, 307–310. [Google Scholar] [CrossRef] [Green Version]
- Zayac, A.S.; Olszewski, A.J. Burkitt lymphoma: Bridging the gap between advances in molecular biology and therapy. Leuk Lymphoma 2020, 61, 1784–1796. [Google Scholar] [CrossRef]
- Short, N.J.; Kantarjian, H.M.; Ko, H.; Khoury, J.D.; Ravandi, F.; Thomas, D.A.; Garcia-Manero, G.; Khouri, M.; Cortes, J.E.; Wierda, W.G.; et al. Outcomes of adults with relapsed or refractory Burkitt and high-grade B-cell leukemia/lymphoma. Am. J. Hematol. 2017, 92, 114–117. [Google Scholar] [CrossRef] [Green Version]
- Sweetenham, J.W.; Pearce, R.; Taghipour, G.; Blaise, D.; Gisselbrecht, C.; Goldstone, A.H. Adult Burkitt’s and Burkitt-like non-Hodgkin’s lymphoma-outcome for patients treated with high-dose therapy and autologous stem-cell transplantation in first remission or at relapse: Results from the European Group for Blood and Marrow Transplantation. J. Clin. Oncol. 1996, 14, 2465–2472. [Google Scholar] [CrossRef]
- Saleh, K.; Michot, J.M.; Camara-Clayette, V.; Vassetsky, Y.; Ribrag, V. Burkitt and Burkitt-Like Lymphomas: A Systematic Review. Cur. Oncol. Rep. 2020, 22, 33. [Google Scholar] [CrossRef]
- Wu, J.; Cao, Y.; Zhang, Q.; Liu, W.; Zhou, X.; Ming, X.; Meng, F.; Zhang, Y.; Li, C.; Huang, L.; et al. Chimeric Antigen Receptor-Modified T Cell Immunotherapy for Relapsed and Refractory Adult Burkitt Lymphoma. Front. Immunol. 2022, 13, 879983. [Google Scholar] [CrossRef]
- Nagorsen, D.; Kufer, P.; Baeuerle, P.A.; Bargou, R. Blinatumomab: A historical perspective. Pharmacol. Ther. 2012, 136, 334–342. [Google Scholar] [CrossRef]
- Burt, R.; Warcel, D.; Fielding, A.K. Blinatumomab, a bispecific B-cell and T-cell engaging antibody, in the treatment of B-cell malignancies. Hum. Vaccin. Immunother. 2019, 15, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Sigmund, A.M.; Sahasrabudhe, K.D.; Bhatnagar, B. Evaluating Blinatumomab for the Treatment of Relapsed/Refractory ALL: Design, Development, and Place in Therapy. Blood Lymphat. Cancer 2020, 10, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, W.; Huang, H. Efficacy, and safety of bispecific T-cell engager (BiTE) antibody blinatumomab for the treatment of relapsed/refractory acute lymphoblastic leukemia and non-Hodgkin’s lymphoma: A systemic review and meta-analysis. Hematology 2019, 24, 199–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linch, D.C. Burkitt lymphoma in adults. Br. J. Haematol. 2012, 156, 693–703. [Google Scholar] [CrossRef]
- Awasthi, A.; Edani, D.; Azmy, C.; Ayello, J.; Cairo, M.S. Blinatumomab Significantly Enhanced Cytotoxicity and T-Cell Cytokine Secretion Against Burkitt Lymphoma (BL) and Primary Mediastinal B-Cell Lymphoma (PMBL). Br. J. Haematol. 2018, 182, 67. [Google Scholar] [CrossRef] [Green Version]
- Duell, J.; Zugmaier, G.; Eisele, F.; Brüggemann, M.; Kufer, P.; Einsele, H.; Topp, M. Treatment of R/R Burkitt lymphoma with blinatumomab is feasible and induced a long lasting complete remission. HemaSphere 2019, 3, 816–817. [Google Scholar] [CrossRef]
- Viardot, A.; Goebeler, M.E.; Hess, G.; Neumann, S.; Pfreundschuh, M.; Adrian, N.; Zettl, F.; Libicher, M.; Sayehli, C.; Stieglmaier, J.; et al. Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood 2016, 127, 1410–1416. [Google Scholar] [CrossRef] [Green Version]
- Greene, F.L.; Page, D.L.; Fleming, I.D.; Fritz, A.G.; Balch, C.M.; Haller, D.G.; Morrow, M. Lymphoid Neoplasms. In AJCC Cancer Staging Manual, 6th ed.; Springer New York: New York, NY, USA, 2002. [Google Scholar]
- International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N. Engl. J. Med. 1993, 329, 987–994. [Google Scholar] [CrossRef]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638. [Google Scholar] [CrossRef] [Green Version]
- Jaffe, E.S. The 2008 WHO classification of lymphomas: Implications for clinical practice and translational research. Hematology Am. Soc. Hematol. Educ. Program. 2009, 1, 523–531. [Google Scholar] [CrossRef]
- Molyneux, E.M.; Rochford, R.; Griffin, B.; Newton, R.; Jackson, G.; Menon, G.; Harrison, C.J.; Israels, T.; Bailey, S. Burkitt’s lymphoma. Lancet 2012, 379, 1234–1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blum, K.A.; Lozanski, G.; Byrd, J.C. Adult Burkitt leukemia and lymphoma. Blood 2004, 104, 3009–3020. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.; Stein, A.; Gökbuget, N.; Fielding, A.K.; Schuh, A.C.; Ribera, J.M.; Wei, A.; Dombret, H.; Foà, R.; Bassan, R.; et al. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2017, 376, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Topp, M.S.; Gökbuget, N.; Stein, A.S.; Zugmaier, G.; O’Brien, S.; Bargou, R.C.; Dombret, H.; Fielding, A.K.; Heffner, L.; Larson, R.A.; et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: A multicentre, single-arm, phase 2 study. Lancet Oncol. 2015, 16, 57–66. [Google Scholar] [CrossRef]
- Topp, M.S.; Kufer, P.; Gökbuget, N.; Goebeler, M.; Klinger, M.; Neumann, S.; Horst, H.A.; Raff, T.; Viardot, A.; Schmid, M.; et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J. Clin. Oncol. 2011, 29, 2493–2498. [Google Scholar] [CrossRef]
- Gökbuget, N.; Dombret, H.; Bonifacio, M.; Reichle, A.; Graux, C.; Faul, C.; Diedrich, H.; Topp, M.S.; Brüggemann, M.; Horst, H.A.; et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood 2018, 131, 1522–1531. [Google Scholar] [CrossRef] [Green Version]
- Martinelli, G.; Boissel, N.; Chevallier, P.; Ottmann, O.; Gökbuget, N.; Rambaldi, A.; Ritchie, E.K.; Papayannidis, C.; Tuglus, C.A.; Morris, J.D.; et al. Long-term follow-up of blinatumomab in patients with relapsed/refractory Philadelphia chromosome-positive B-cell precursor acute lymphoblastic leukaemia: Final analysis of ALCANTARA study. Eur. J. Cancer 2021, 146, 107–114. [Google Scholar] [CrossRef]
- Topp, M.S.; Gökbuget, N.; Zugmaier, G.; Klappers, P.; Stelljes, M.; Neumann, S.; Viardot, A.; Marks, R.; Diedrich, H.; Faul, C.; et al. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J. Clin. Oncol. 2014, 32, 4134–4140. [Google Scholar] [CrossRef]
- Cremer, M.; Schwarzbich, M.A.; Schöning, T.; Lisenko, K.; Ho, A.D.; Witzens-Harig, M. In Burkitt lymphoma patients who relapse after induction with a short-intensive chemoimmunotherapy protocol, aggressive salvage chemotherapy therapy is ineffective: A single-center retrospective study. Ann. Hematol. 2017, 96, 1573–1575. [Google Scholar] [CrossRef]
- Viardot, A.; Locatelli, F.; Stieglmaier, J.; Zaman, F.; Jabbour, E. Concepts in immuno-oncology: Tackling B cell malignancies with CD19-directed bispecific T cell engager therapies. Ann. Hematol. 2020, 99, 2215–2229. [Google Scholar] [CrossRef]
| Case/Characteristic | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| Age | 27 | 33 | 43 | 52 | 25 | 50 | 28 | 30 | 62 |
| Gender | m | f | m | f | m | m | m | f | f |
| Subtype BL | sporadic | ||||||||
| MYC translocation | detected | ||||||||
| Ann Arbor stage | IV | IV | IV | II | II | IV | IV | IV | II |
| IPI | High | High | High | Intermediate | Low-intermediate | High | High-intermediate | High-intermediate | High-intermediate |
| ECOG performance status | 1 | 2 | 1 | 0 | 2 | 1 | 1 | 3 | 2 |
| Extranodal involvement | Yes | yes | yes | no | yes | yes | yes | yes | yes |
| Bone marrow involvement (%) | 90% | 95% | 45% | No | No | 40% | 85% | No | no |
| CNS involvement | No | No | No | No | No | No | No | No | no |
| B-symptoms | Yes | Yes | Yes | no | Yes | Yes | Yes | Yes | Yes |
| Bulky disease | No | No | No | Yes | Yes | No | No | Yes | yes |
| Peripheral blood parameters LDH (U/L) Hemoglobin (g/L) Leukocytes (G/L) Thrombocytes (G/L) Peripheral Burkitt cells (%) | 982 U/L 72 g/L 17.2 (G/L) 42 (G/L) 44% | 1012 U/L 68 g/L 1.2 (G/L) 24 (G/L) 0% | 689 U/L 82 g/L 8.2 (G/L) 68 (G/L) 4% | 712 U/L 109 g/L 5.6 (G/L) 155 (G/L) 0% | 160 U/L 100 g/L 5.2 (G/L) 235 (G/L) 0% | 5596 U/L 136 g/L 6.2 (G/L) 70 (G/L) 8% | 11276 U/L 72 g/L 89.2 (G/L) 31 (G/L) 72% | 4649 U/L 133 g/L 5.9 (G/L) 236 (G/L) 0% | 1479 U/L 82 g/L 5.8 (G/L) 79 (G/L) 0% |
| Case | Number of Previous Lines of Treatment | Details on Previous Lines of Treatment 1st Line 2nd Line 3rd Line | Best Response to 1st-Line Therapy | Relapse/Refractory to/after 1st-Line Treatment | Median Duration from 1st-Line Therapy until Relapse/Refractoriness (Months) |
|---|---|---|---|---|---|
| 1 | 1 | R-DA-EPOCH | CR | Relapse | 8 |
| 2 | 1 | R-CODOX-M/R-IVAC | CR | Relapse | 7 |
| 3 | 1 | R-DA-EPOCH | CR | Relapse | 11 |
| 4 | 1 | R-CODOX-M/R-IVAC | PR | Relapse | 4 |
| 5 | 2 | R-CODOX-M/R-IVAC R-DHAP | PR | Relapse | 3.5 |
| 6 | 2 | R-CODOX-M/R-IVAC CLAG-Ida-Rituximab | PR | Relapse | 4.5 |
| 7 | 3 | R-CHOP R-CODOX-M/R-IVAC CLAG-Ida-Rituximab | PR | Refractory | 3 |
| 8 | 3 | R-CHOP R-DA-EPOCH radiotherapy | PR | Relapse | 6 |
| 9 | 1 | R-CHOP | PR | Refractory | 4 |
| Case | Remission Status at Start of Blinatumomab | Maximum Dose Blinatumomab Given (mcg/day) | Number of Cycles of Blinatumomab | Additional Radiotherapy during Blinatumomab | End of Blinatumomab Therapy |
|---|---|---|---|---|---|
| 1 | PD | 112 | 3 | no | Due to progression |
| 2 | PD | 112 | 4 | No | As planned |
| 3 | PD | 112 | 5 | No | As planned |
| 4 | PD | 112 | 2 | No | Due to progression |
| 5 | PD | 9 | 1 * | No | Due to progression |
| 6 | PR | 28 | 3 | Yes | As planned |
| 7 | PD | 9 | 1 * | No | Due to progression |
| 8 | PD | 112 | 3 | Yes | Due to progression |
| 9 | PD | 112 | 1 | No | Due to progression |
| Case | Infection, Pathogen and Manifestation | CRS | Tocilizumab Given for CRS | Neurotoxicity | Steroids Given | Hospitalization |
|---|---|---|---|---|---|---|
| 1 | Klebsiella pneumoniae, pneumonia with bacteremia | Grade 2 | Yes | Grade 1 | Due to CRS and neurotoxicity | Due to CRS |
| 2 | Enterobacter cloacae, bacteriemia | Grade 2 | Yes | No | Due to CRS | Due to CRS |
| 3 | CMV, reactivation in colon, colitis | Grade 2 | Yes | Grade 1 | Due to CRS | Due to CRS |
| 4 | No | Grade 1 | Yes | No | Due to CRS | Due to CRS |
| 5 | No | No | - | No | - | - |
| 6 | No | No | - | No | - | - |
| 7 | No | No | - | No | - | - |
| 8 | No | No | - | Grade 1 and Grade 2 | Due to neurotoxicity | Due to neurotoxicity |
| 9 | Clostridium difficile, colitis | Grade 2 | Yes | No | Due to CRS | Due to CRS |
| Case | Best Response to Blinatumomab | Remission Status at End of Blinatumomab | Relapse/Progress during/after Blinatumomab | Duration until Relapse/Progression* | Death | Duration until Death * | Last Follow Up * |
|---|---|---|---|---|---|---|---|
| 1 | CR | PD | Yes | 5 m | Due to progression | 6 m | 6 m |
| 2 | CR | CR | No | - | No | - | 32 m |
| 3 | CR | CR | Yes | 13 m | Due to progression | 16 m | 16 m |
| 4 | PD | PD | Yes | 2 m | Due to progression | 5 m | 5 m |
| 5 | PD | PD | Yes | 5 d | Due to progression | 11 d | 11 d |
| 6 | PR | PR | Yes | 51 d | Due to acute liver failure GvHD-related | 6 m | 6 m |
| 7 | PD | PD | Yes | 7 d | Due to progression | 14 d | 14 d |
| 8 | PR | PD | Yes | 3 m | Due to progression | 10 m | 10 m |
| 9 | PD | PD | Yes | 11 d | Due to progression | 46 d | 46 d |
| Parameter | Duration |
|---|---|
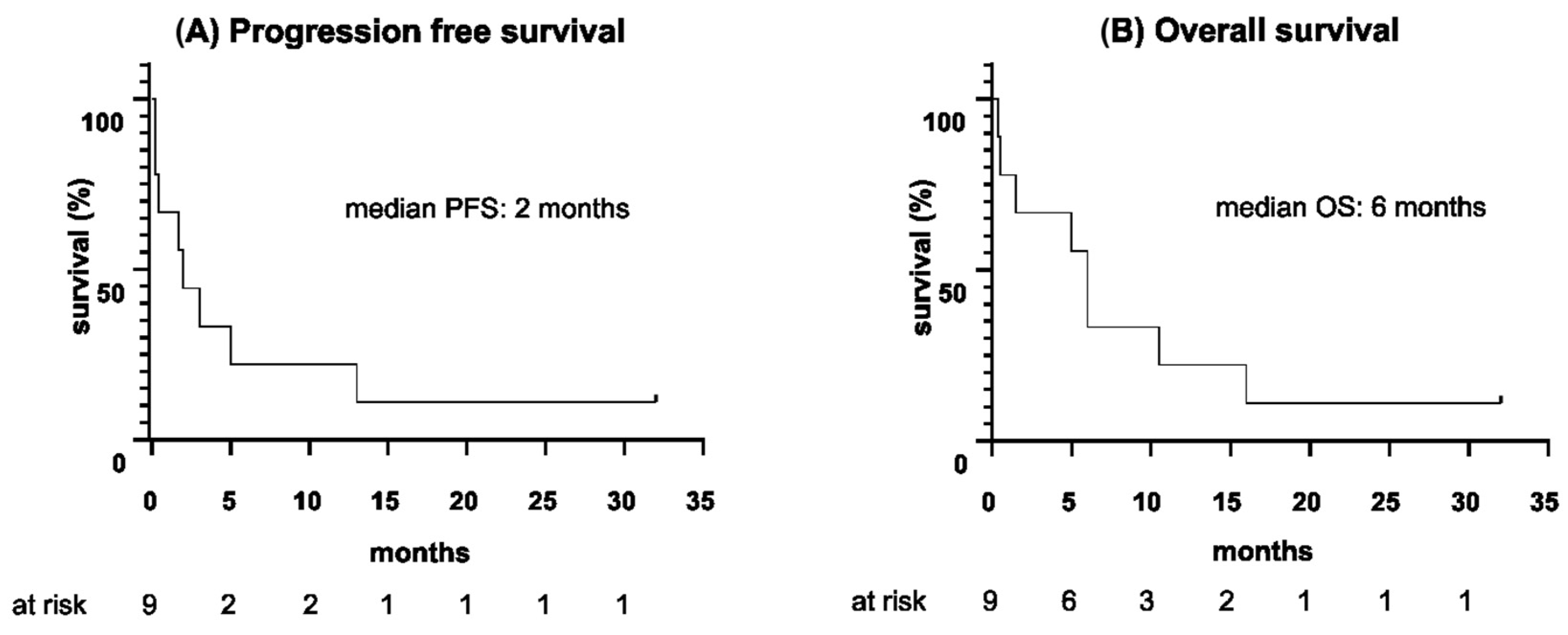
| Median duration until relapse since start of blinatumomab, m (range) | 2 (5 d–13 m) |
| Median PFS, m (range) | 2 (5 d–32 m) |
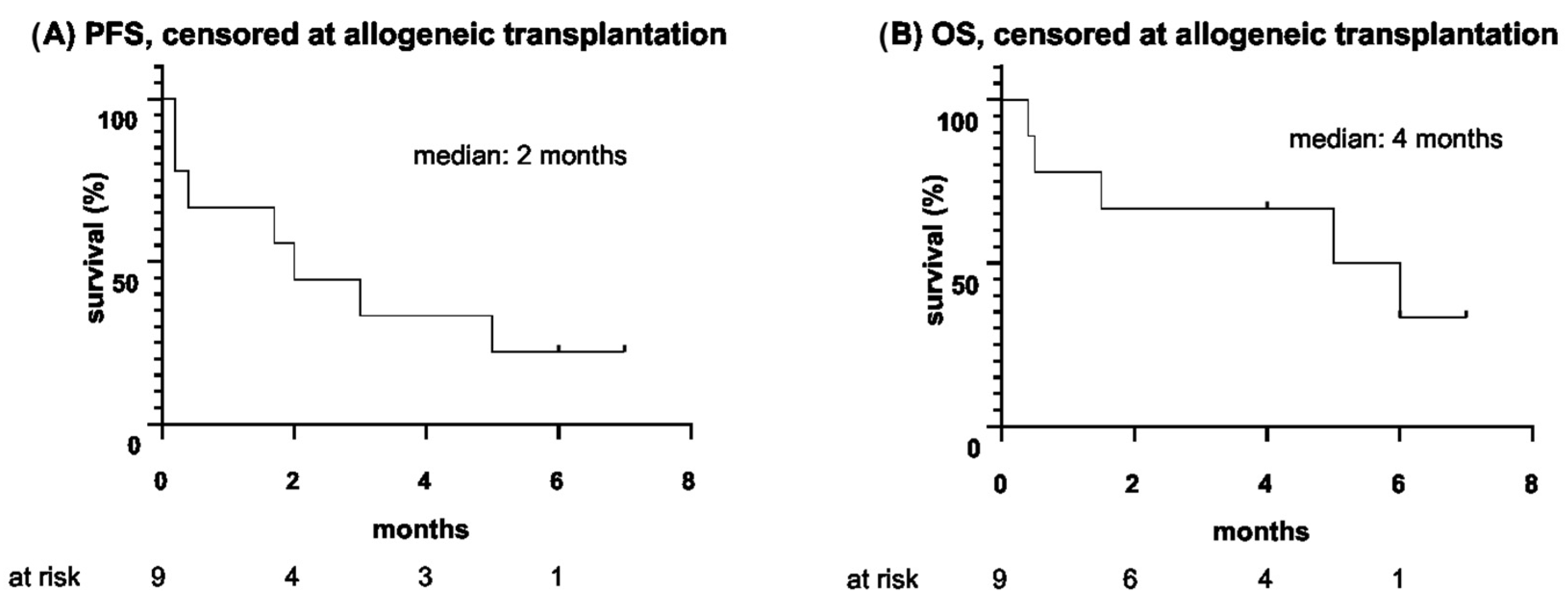
| Median PFS, censored at allogeneic HSCT, m (range) | 2 (5 d–7 m) |
| Median duration until death since start of blinatumomab, m (range) | 6 (11 d–16 m) |
| Median OS, m (range) | 6 (11 d–32 m) |
| Median OS, censored at allogeneic HSCT, m (range) | 4 (11 d–7 m) |
| Median follow up since start of blinatumomab, m (range) | 6 (11 d–32 m) |
| Case | Further Therapy after Blinatumomab | Status of Remission at Start of Further Therapy | Relapse/Death Following Further Therapy |
|---|---|---|---|
| 1 | No further therapy | ||
| 2 | Allogeneic HSCT | CR | no |
| 3 | Allogeneic HSCT | CR | Relapse and death |
| 4 | Radiotherapy | PD | Relapse and death |
| 5 | No further therapy | ||
| 6 | Allogeneic HSCT | PR | Relapse and death |
| 7 | No further therapy | ||
| 8 | Allogeneic HSCT | PD | Relapse and death |
| 9 | No further therapy | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bohler, J.; Bacher, U.; Banz, Y.; Stadelmann, R.; Medinger, M.; Zander, T.; Pabst, T. Blinatumomab in Relapsed/Refractory Burkitt Lymphoma. Cancers 2023, 15, 44. https://doi.org/10.3390/cancers15010044
Bohler J, Bacher U, Banz Y, Stadelmann R, Medinger M, Zander T, Pabst T. Blinatumomab in Relapsed/Refractory Burkitt Lymphoma. Cancers. 2023; 15(1):44. https://doi.org/10.3390/cancers15010044
Chicago/Turabian StyleBohler, Jeanne, Ulrike Bacher, Yara Banz, Raphael Stadelmann, Michael Medinger, Thilo Zander, and Thomas Pabst. 2023. "Blinatumomab in Relapsed/Refractory Burkitt Lymphoma" Cancers 15, no. 1: 44. https://doi.org/10.3390/cancers15010044





