Timosaponin AIII Inhibits Migration and Invasion Abilities in Human Cervical Cancer Cells through Inactivation of p38 MAPK-Mediated uPA Expression In Vitro and In Vivo
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents and Antibodies
2.2. Cell Culture
2.3. Western Blotting Analysis
2.4. Cell Growth Assay
2.5. Three-Dimensional Sphere Formation
2.6. Analysis of Cell Cycle and Cell Apoptisis
2.7. In Vitro Migration and Invasion Assay
2.8. Total RNA Extraction and Real-Time PCR Assay
2.9. Lung Metastasis Animal Experiment
2.10. Statistical Analysis
3. Results
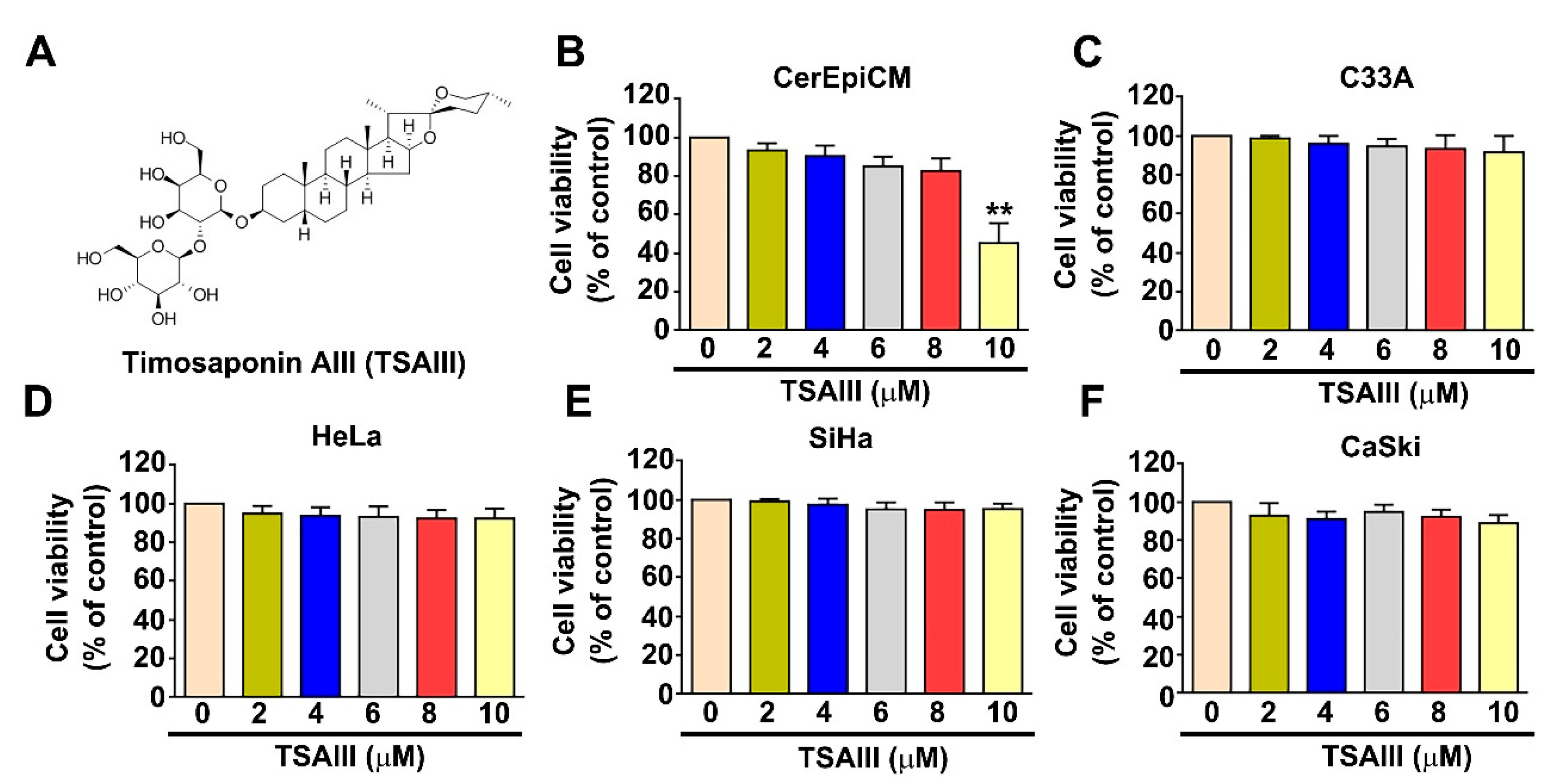
3.1. Effect of TSAIII on Cell Viability and Cytotoxicity in Human Cervical Cancer Cells
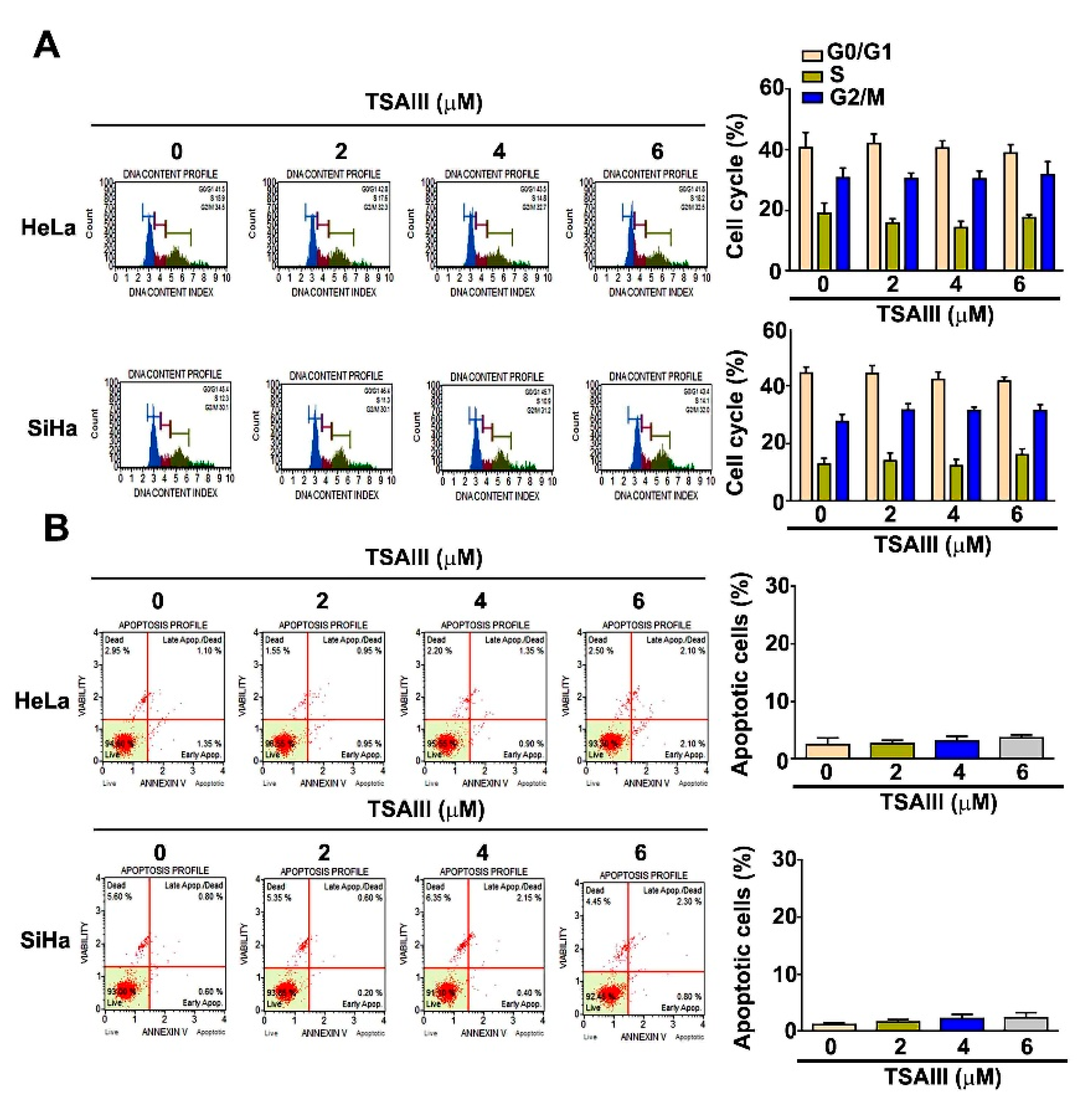
3.2. Effect of TSAIII on Cell Cycle Distribution and Apoptosis Induction in Human Cervical Cancer Cells
3.3. TSAIII Inhibits Cell Migration and Invasion in Human Cervical Cancer Cells
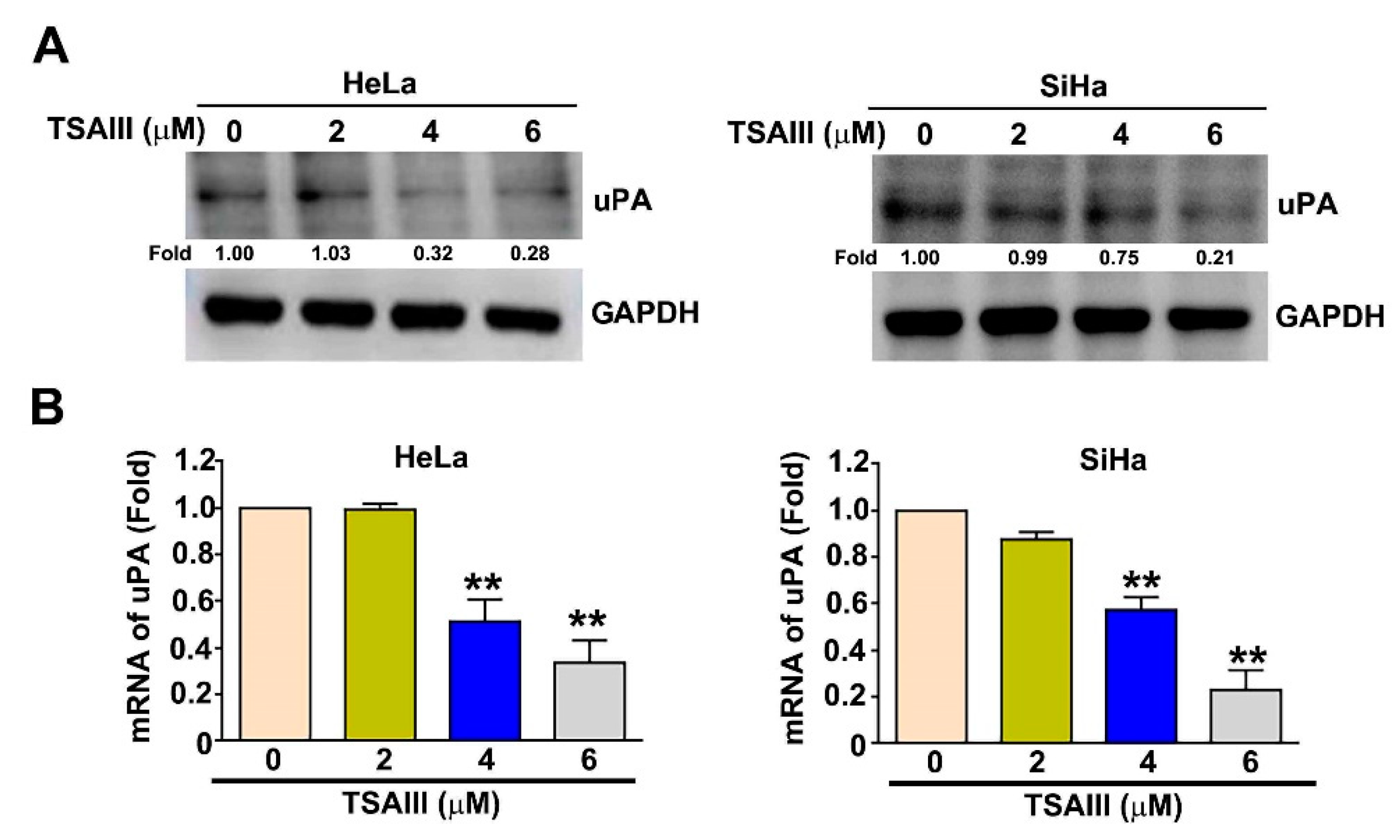
3.4. TSAIII Inhibits the uPA Expression in Human Cervical Cancer Cells
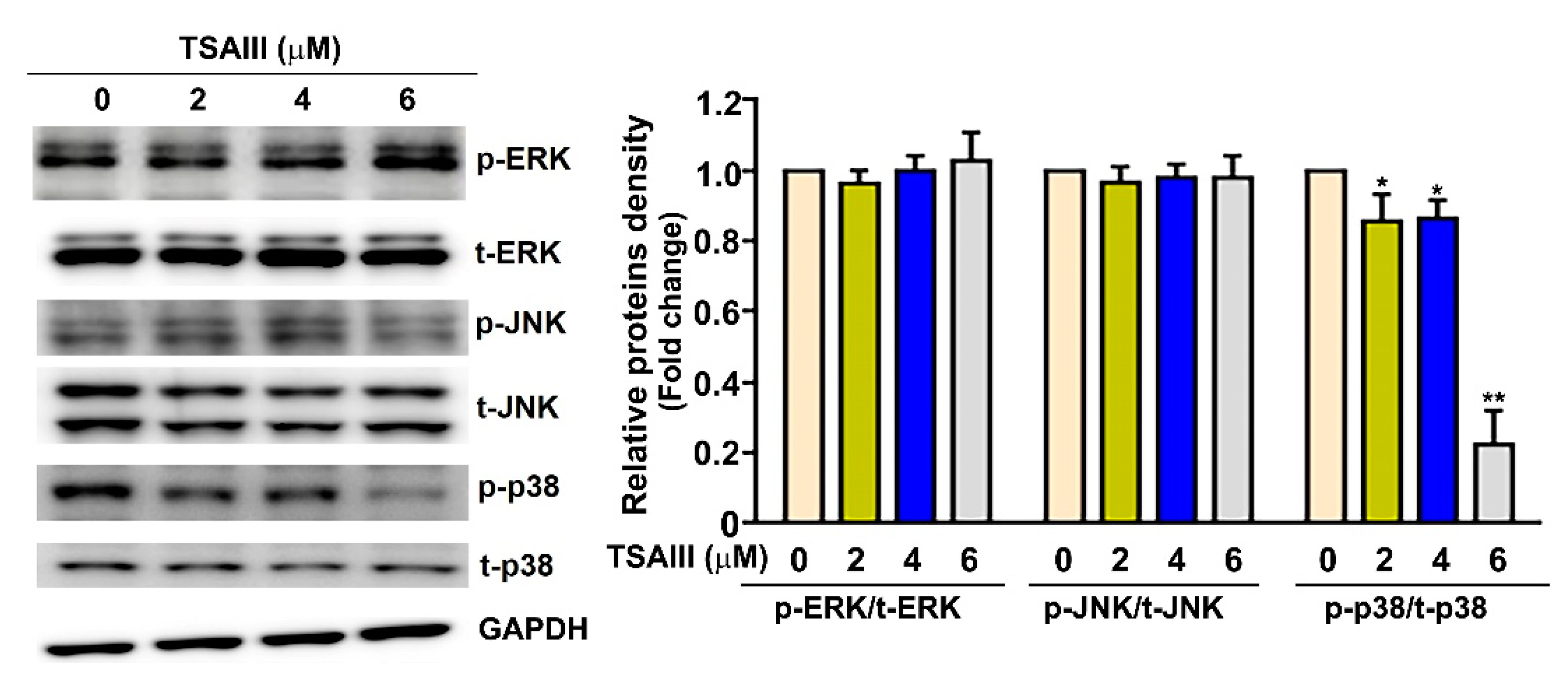
3.5. TSAIII Inhibits the Activation of p38 MAPK Pathway in Human Cervical Cancer Cells
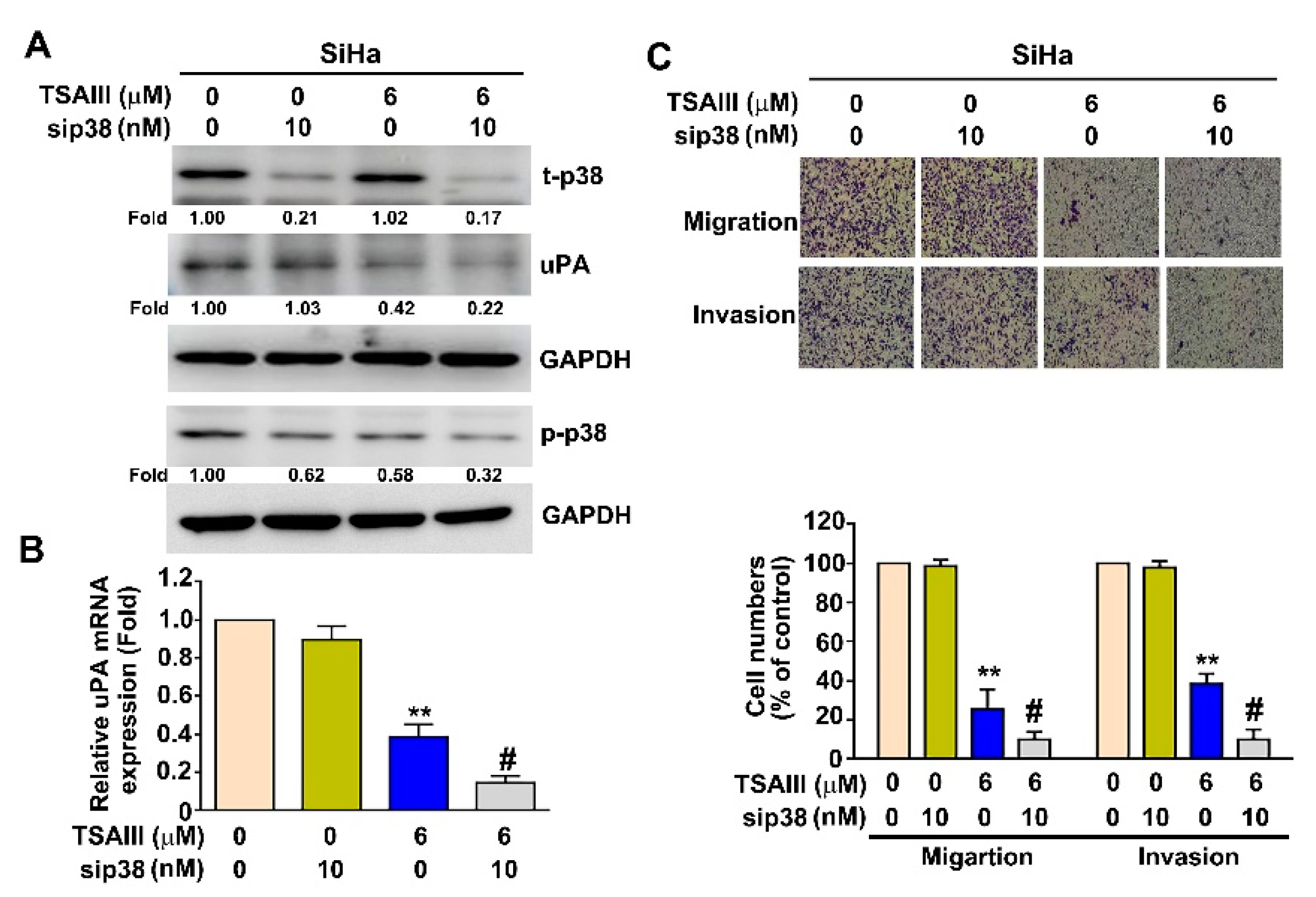
3.6. p38 MAPK Pathway Involved in TSAIII Inhibits uPA Expression, Cell Migration and Invasion in Human Cervical Cancer Cells
3.7. TSAIII Inhibits Cancer Stemness, p38 Activation, uPA Expression and Invasion in Human Cervical Cancer Stem Cells
3.8. TSAIII Inhibits Lung Metastasis of Human Cervical Cancer Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Eskander, R.N.; Tewari, K.S. Chemotherapy in the treatment of metastatic, persistent, and recurrent cervical cancer. Curr. Opin. Obstet. Gynecol. 2014, 26, 314–321. [Google Scholar] [CrossRef]
- Feng, H.; Hu, Y.; Jin, P.; Meng, X.; Chen, Y.; Zhang, H. Intensity-modulated radiotherapy combined with iodine-125 seed implantation in non-central recurrence of cervical cancer: A case report and literature review. Oncol. Lett. 2017, 14, 4085–4091. [Google Scholar] [CrossRef]
- Liotta, L.A.; Stetler-Stevenson, W.G. Tumor invasion and metastasis: An imbalance of positive and negative regulation. Cancer Res. 1991, 51, 5054s–5059s. [Google Scholar]
- Takeuchi, H.; Kitajima, M.; Kitagawa, Y. Sentinel lymph node as a target of molecular diagnosis of lymphatic micrometastasis and local immunoresponse to malignant cells. Cancer Sci. 2008, 99, 441–450. [Google Scholar] [CrossRef]
- Yamamoto, K.; Yoshikawa, H.; Shiromizu, K.; Saito, T.; Kuzuya, K.; Tsunematsu, R.; Kamura, T. Pulmonary metastasectomy for uterine cervical cancer: A multivariate analysis. Ann. Thorac. Surg. 2004, 77, 1179–1182. [Google Scholar] [CrossRef]
- Thanapprapasr, D.; Nartthanarung, A.; Likittanasombut, P.; Na Ayudhya, N.I.; Charakorn, C.; Udomsubpayakul, U.; Subhadarbandhu, T.; Wilailak, S. Bone metastasis in cervical cancer patients over a 10-year period. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2010, 20, 373–378. [Google Scholar] [CrossRef]
- Park, J.Y.; Lim, M.C.; Lim, S.Y.; Bae, J.M.; Yoo, C.W.; Seo, S.S.; Kang, S.; Park, S.Y. Port-site and liver metastases after laparoscopic pelvic and para-aortic lymph node dissection for surgical staging of locally advanced cervical cancer. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2008, 18, 176–180. [Google Scholar] [CrossRef]
- Kanthan, R.; Senger, J.L.; Diudea, D.; Kanthan, S. A review of duodenal metastases from squamous cell carcinoma of the cervix presenting as an upper gastrointestinal bleed. World J. Surg. Oncol. 2011, 9, 113. [Google Scholar] [CrossRef]
- Ying, T.H.; Lee, C.H.; Chiou, H.L.; Yang, S.F.; Lin, C.L.; Hung, C.H.; Tsai, J.P.; Hsieh, Y.H. Knockdown of Pentraxin 3 suppresses tumorigenicity and metastasis of human cervical cancer cells. Sci. Rep. 2016, 6, 29385. [Google Scholar] [CrossRef]
- Chou, C.H.; Lu, K.H.; Yang, J.S.; Hsieh, Y.H.; Lin, C.W.; Yang, S.F. Dihydromyricetin suppresses cell metastasis in human osteosarcoma through SP-1- and NF-kappaB-modulated urokinase plasminogen activator inhibition. Phytomed. Int. J. Phytother. Phytopharm. 2021, 90, 153642. [Google Scholar] [CrossRef]
- Wu, M.H.; Chiu, Y.F.; Wu, W.J.; Wu, P.L.; Lin, C.Y.; Lin, C.L.; Hsieh, Y.H.; Liu, C.J. Synergistic antimetastatic effect of cotreatment with licochalcone A and sorafenib on human hepatocellular carcinoma cells through the inactivation of MKK4/JNK and uPA expression. Environ. Toxicol. 2018, 33, 1237–1244. [Google Scholar] [CrossRef]
- Xu, X.H.; Li, T.; Fong, C.M.; Chen, X.; Chen, X.J.; Wang, Y.T.; Huang, M.Q.; Lu, J.J. Saponins from Chinese Medicines as Anticancer Agents. Molecules 2016, 21, 1326. [Google Scholar] [CrossRef]
- Yin, L.; Qi, Y.; Xu, Y.; Xu, L.; Han, X.; Tao, X.; Song, S.; Peng, J. Dioscin Inhibits HSC-T6 Cell Migration via Adjusting SDC-4 Expression: Insights from iTRAQ-Based Quantitative Proteomics. Front. Pharm. 2017, 8, 665. [Google Scholar] [CrossRef]
- Jung, O.; Lee, J.; Lee, Y.J.; Yun, J.M.; Son, Y.J.; Cho, J.Y.; Ryou, C.; Lee, S.Y. Timosaponin AIII inhibits migration and invasion of A549 human non-small-cell lung cancer cells via attenuations of MMP-2 and MMP-9 by inhibitions of ERK1/2, Src/FAK and beta-catenin signaling pathways. Bioorg. Med. Chem. Lett. 2016, 26, 3963–3967. [Google Scholar] [CrossRef]
- Kang, Y.J.; Chung, H.J.; Nam, J.W.; Park, H.J.; Seo, E.K.; Kim, Y.S.; Lee, D.; Lee, S.K. Cytotoxic and antineoplastic activity of timosaponin A-III for human colon cancer cells. J. Nat. Prod. 2011, 74, 701–706. [Google Scholar] [CrossRef]
- Kim, K.M.; Im, A.R.; Kim, S.H.; Hyun, J.W.; Chae, S. Timosaponin AIII inhibits melanoma cell migration by suppressing COX-2 and in vivo tumor metastasis. Cancer Sci. 2016, 107, 181–188. [Google Scholar] [CrossRef]
- Tsai, C.H.; Yang, C.W.; Wang, J.Y.; Tsai, Y.F.; Tseng, L.M.; King, K.L.; Chen, W.S.; Chiu, J.H.; Shyr, Y.M. Timosaponin AIII Suppresses Hepatocyte Growth Factor-Induced Invasive Activity through Sustained ERK Activation in Breast Cancer MDA-MB-231 Cells. Evid. Based Complement. Altern. Med. 2013, 2013, 421051. [Google Scholar] [CrossRef]
- Hsieh, Y.H.; Hsu, W.H.; Yang, S.F.; Liu, C.J.; Lu, K.H.; Wang, P.H.; Lin, R.C. Potential Antimetastatic Effect of Timosaponin AIII against Human Osteosarcoma Cells through Regulating the Integrin/FAK/Cofilin Axis. Pharmaceuticals 2021, 14, 260. [Google Scholar] [CrossRef]
- Lin, C.S.; Lin, C.L.; Ying, T.H.; Chiou, H.L.; Hung, C.H.; Liao, W.S.; Hsieh, Y.H.; Kao, S.H. beta-Mangostin inhibits the metastatic power of cervical cancer cells attributing to suppression of JNK2/AP-1/Snail cascade. J. Cell. Physiol. 2020, 235, 8446–8460. [Google Scholar] [CrossRef]
- Lee, C.Y.; Hsin, M.C.; Chen, P.N.; Lin, C.W.; Wang, P.H.; Yang, S.F.; Hsiao, Y.H. Arctiin Inhibits Cervical Cancer Cell Migration and Invasion through Suppression of S100A4 Expression via PI3K/Akt Pathway. Pharmaceutics 2022, 14, 365. [Google Scholar] [CrossRef]
- Wu, M.H.; Lin, C.L.; Chiou, H.L.; Yang, S.F.; Lin, C.Y.; Liu, C.J.; Hsieh, Y.H. Praeruptorin A Inhibits Human Cervical Cancer Cell Growth and Invasion by Suppressing MMP-2 Expression and ERK1/2 Signaling. Int. J. Mol. Sci. 2017, 19, 10. [Google Scholar] [CrossRef]
- Masucci, M.T.; Minopoli, M.; Di Carluccio, G.; Motti, M.L.; Carriero, M.V. Therapeutic Strategies Targeting Urokinase and Its Receptor in Cancer. Cancers 2022, 14, 498. [Google Scholar] [CrossRef]
- Chiang, K.C.; Lai, C.Y.; Chiou, H.L.; Lin, C.L.; Chen, Y.S.; Kao, S.H.; Hsieh, Y.H. Timosaponin AIII inhibits metastasis of renal carcinoma cells through suppressing cathepsin C expression by AKT/miR-129-5p axis. J. Cell. Physiol. 2019, 234, 13332–13341. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Zhao, W.R.; Xiao, Y.; Zhou, X.M.; Huang, C.; Shi, W.T.; Zhang, J.; Ye, Q.; Chen, X.L.; Tang, J.Y. Antiangiogenesis effect of timosaponin AIII on HUVECs in vitro and zebrafish embryos in vivo. Acta Pharm. Sin. 2020, 41, 260–269. [Google Scholar] [CrossRef]
- Wang, N.; Feng, Y.; Zhu, M.; Siu, F.M.; Ng, K.M.; Che, C.M. A novel mechanism of XIAP degradation induced by timosaponin AIII in hepatocellular carcinoma. Biochim. Biophys. Acta 2013, 1833, 2890–2899. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, K.H.; Lee, I.S.; Park, J.Y.; Na, Y.C.; Chung, W.S.; Jang, H.J. Apoptosis and G2/M cell cycle arrest induced by a timosaponin A3 from Anemarrhena asphodeloides Bunge on AsPC-1 pancreatic cancer cells. Phytomedicine 2019, 56, 48–56. [Google Scholar] [CrossRef]
- Peng, Y.; Pan, J.; Ou, F.; Wang, W.; Hu, H.; Chen, L.; Zeng, S.; Zeng, K.; Yu, L. Fenbendazole and its synthetic analog interfere with HeLa cells’ proliferation and energy metabolism via inducing oxidative stress and modulating MEK3/6-p38-MAPK pathway. Chem. Biol. Interact. 2022, 361, 109983. [Google Scholar] [CrossRef]
- Katopodis, P.; Kerslake, R.; Zikopoulos, A.; Beri, N.; Anikin, V. p38beta-MAPK11 and its role in female cancers. J. Ovarian. Res. 2021, 14, 84. [Google Scholar] [CrossRef]
- Grossi, V.; Peserico, A.; Tezil, T.; Simone, C. p38alpha MAPK pathway: A key factor in colorectal cancer therapy and chemoresistance. World J. Gastroenterol. 2014, 20, 9744–9758. [Google Scholar] [CrossRef]
- Tsai, J.P.; Hsiao, P.C.; Yang, S.F.; Hsieh, S.C.; Bau, D.T.; Ling, C.L.; Pai, C.L.; Hsieh, Y.H. Licochalcone A suppresses migration and invasion of human hepatocellular carcinoma cells through downregulation of MKK4/JNK via NF-kappaB mediated urokinase plasminogen activator expression. PLoS ONE 2014, 9, e86537. [Google Scholar] [CrossRef]
- Chou, R.H.; Hsieh, S.C.; Yu, Y.L.; Huang, M.H.; Huang, Y.C.; Hsieh, Y.H. Fisetin inhibits migration and invasion of human cervical cancer cells by down-regulating urokinase plasminogen activator expression through suppressing the p38 MAPK-dependent NF-kappaB signaling pathway. PLoS ONE 2013, 8, e71983. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.J.; Peng, S.F.; Chueh, F.S.; Tsai, C.H.; Tsai, F.J.; Huang, C.Y.; Tang, C.H.; Yang, J.S.; Hsu, Y.M.; Huang, W.W.; et al. Lupeol suppresses migration and invasion via p38/MAPK and PI3K/Akt signaling pathways in human osteosarcoma U-2 OS cells. Biosci. Biotechnol. Biochem. 2019, 83, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, M.; Kobayashi, H.; Kanayama, N.; Terao, T. Clinical significance of urokinase-type plasminogen activator (uPA) in invasive cervical cancer of the uterus. Gynecol. Oncol. 1992, 46, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Moquet-Torcy, G.; Tolza, C.; Piechaczyk, M.; Jariel-Encontre, I. Transcriptional complexity and roles of Fra-1/AP-1 at the uPA/Plau locus in aggressive breast cancer. Nucleic Acids Res. 2014, 42, 11011–11024. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, V.; Jayaraman, P.S.; Zaitsev, S.V.; Lebedeva, T.; Bdeir, K.; Kershaw, R.; Holman, K.R.; Parfyonova, Y.V.; Semina, E.V.; Beloglazova, I.B.; et al. Urokinase-type Plasminogen Activator (uPA) Promotes Angiogenesis by Attenuating Proline-rich Homeodomain Protein (PRH) Transcription Factor Activity and De-repressing Vascular Endothelial Growth Factor (VEGF) Receptor Expression. J. Biol. Chem. 2016, 291, 15029–15045. [Google Scholar] [CrossRef]
- Di Fiore, R.; Suleiman, S.; Drago-Ferrante, R.; Subbannayya, Y.; Pentimalli, F.; Giordano, A.; Calleja-Agius, J. Cancer Stem Cells and Their Possible Implications in Cervical Cancer: A Short Review. Int. J. Mol. Sci. 2022, 23, 5167. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Kawana, K.; Fujimoto, A.; Yoshida, M.; Nakamura, H.; Nishida, H.; Inoue, T.; Taguchi, A.; Takahashi, J.; Adachi, K.; et al. Clinical significance of Gremlin 1 in cervical cancer and its effects on cancer stem cell maintenance. Oncol. Rep. 2016, 35, 391–397. [Google Scholar] [CrossRef]
- Chien, H.J.; Ying, T.H.; Hsieh, S.C.; Lin, C.L.; Yu, Y.L.; Kao, S.H.; Hsieh, Y.H. alpha-Mangostin attenuates stemness and enhances cisplatin-induced cell death in cervical cancer stem-like cells through induction of mitochondrial-mediated apoptosis. J. Cell. Physiol. 2020, 235, 5590–5601. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chien, H.-J.; Liu, C.-J.; Ying, T.-H.; Wu, P.-J.; Wang, J.-W.; Ting, Y.-H.; Hsieh, Y.-H.; Wang, S.-C. Timosaponin AIII Inhibits Migration and Invasion Abilities in Human Cervical Cancer Cells through Inactivation of p38 MAPK-Mediated uPA Expression In Vitro and In Vivo. Cancers 2023, 15, 37. https://doi.org/10.3390/cancers15010037
Chien H-J, Liu C-J, Ying T-H, Wu P-J, Wang J-W, Ting Y-H, Hsieh Y-H, Wang S-C. Timosaponin AIII Inhibits Migration and Invasion Abilities in Human Cervical Cancer Cells through Inactivation of p38 MAPK-Mediated uPA Expression In Vitro and In Vivo. Cancers. 2023; 15(1):37. https://doi.org/10.3390/cancers15010037
Chicago/Turabian StyleChien, Hung-Ju, Chung-Jung Liu, Tsung-Ho Ying, Pei-Ju Wu, Jiunn-Wei Wang, Yi-Hsuan Ting, Yi-Hsien Hsieh, and Shih-Chiang Wang. 2023. "Timosaponin AIII Inhibits Migration and Invasion Abilities in Human Cervical Cancer Cells through Inactivation of p38 MAPK-Mediated uPA Expression In Vitro and In Vivo" Cancers 15, no. 1: 37. https://doi.org/10.3390/cancers15010037
APA StyleChien, H.-J., Liu, C.-J., Ying, T.-H., Wu, P.-J., Wang, J.-W., Ting, Y.-H., Hsieh, Y.-H., & Wang, S.-C. (2023). Timosaponin AIII Inhibits Migration and Invasion Abilities in Human Cervical Cancer Cells through Inactivation of p38 MAPK-Mediated uPA Expression In Vitro and In Vivo. Cancers, 15(1), 37. https://doi.org/10.3390/cancers15010037






