Purification of Two Taxanes from Taxus cuspidata by Preparative High-Performance Liquid Chromatography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Ultrasonic Extraction Experiment
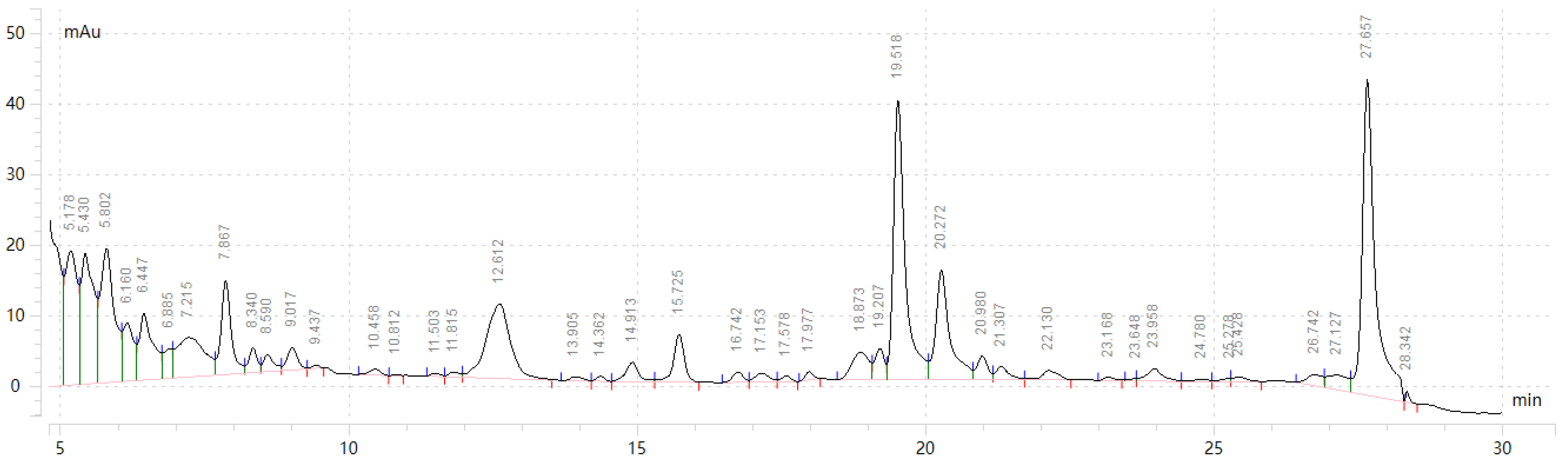
2.3. Separation of Taxanes by HPLC
2.4. Purification Experiment of Preparative HPLC
2.5. Single-Factor Experiments
2.6. Orthogonal Experimental Design
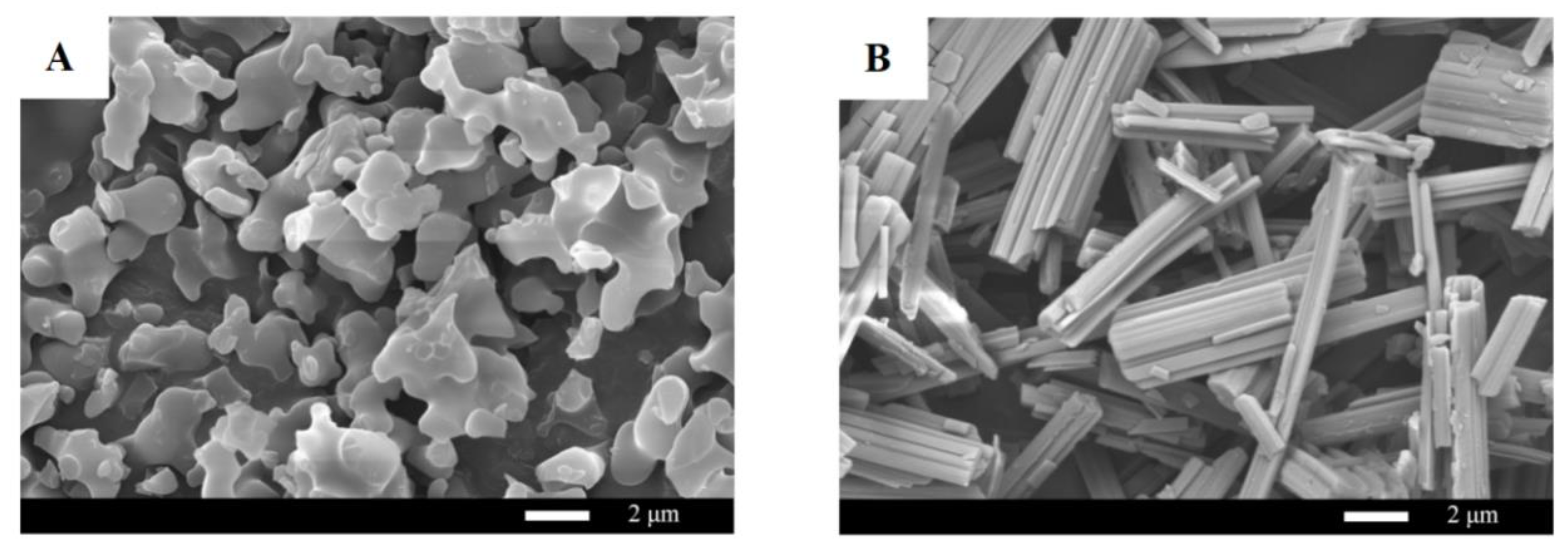
2.7. SEM
2.8. ESI-HRMS
3. Results
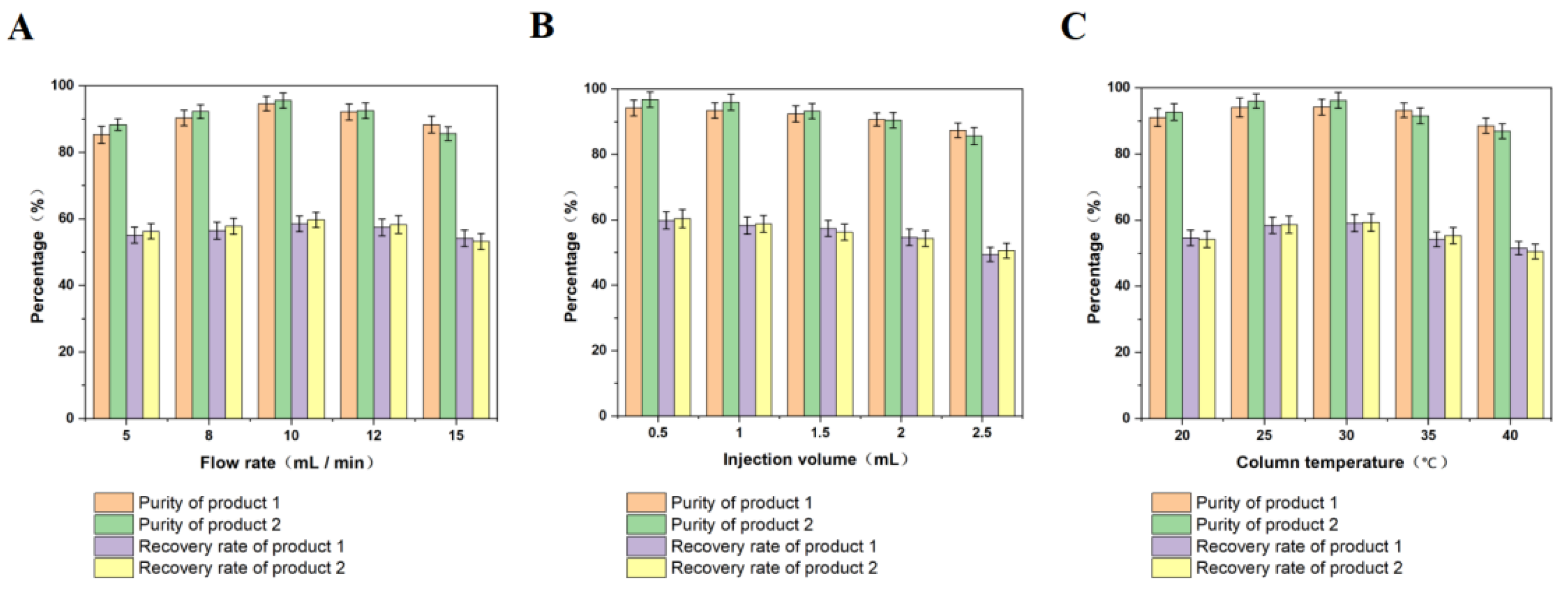
3.1. Single-Factor Experiments
3.1.1. Effect of Flow Rate on Purity and Recovery of the Two Products
3.1.2. Effect of Injection Volume on Purity and Recovery of the Two Products
3.1.3. Effect of Column Temperature on Purity and Recovery of the Two Products
3.2. Optimization of Preparation Conditions for Purification of the Two Products by Orthogonal Experimental Design
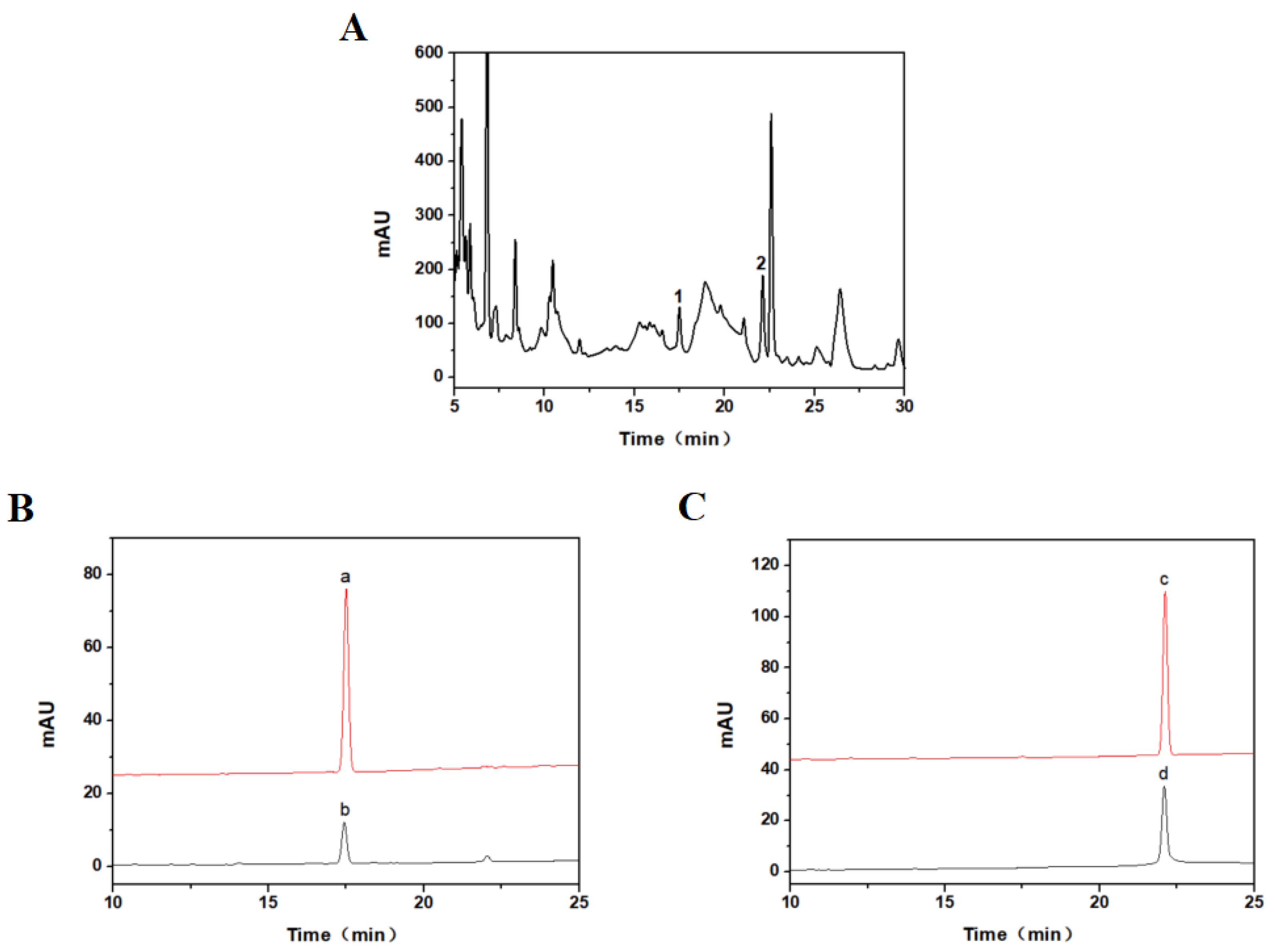
3.3. Results and Analysis of Optimized Preparative HPLC
3.4. SEM Analysis of Purified Products
3.5. HPLC Detection of Purified Products
3.6. ESI-HRMS Detection of Preparative High-Performance Liquid Chromatography Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nan, D.; Zhe, X.; Bing, H.; Lijuan, H.; Shaomei, L.; Chunyan, Y. Optimization of callus induction and subculture conditions of Taxus chinensis. J. Pharm. Sci. Innov. 2015, 41, 2058–2062. [Google Scholar] [CrossRef]
- Kim, H.-G.; Kim, J.-H. Elucidation of the Mechanism and Kinetics of Ultrasonic Extraction of Paclitaxel from Plant Cell Cultures of Taxus chinensis. Biotechnol. Bioprocess Eng. 2022, 27, 668–677. [Google Scholar] [CrossRef]
- Charpin, D.; Pichot, C.; Belmonte, J.; Sutra, J.-P.; Zidkova, J.; Chanez, P.; Shahali, Y.; Sénéchal, H.; Poncet, P. Cypress Pollinosis: From Tree to Clinic. Clin. Rev. Allergy Immunol. 2019, 56, 174–195. [Google Scholar] [CrossRef] [PubMed]
- Meng, A.-P.; Li, J.; Pu, S.-B. Chemical Constituents of Leaves of Taxus chinensis. Chem. Nat. Compd. 2018, 54, 841–845. [Google Scholar] [CrossRef]
- Gao, L.-M.; Tan, S.-L.; Zhang, G.-L.; Thomas, P. A new species of Amentotaxus (Taxaceae) from China, Vietnam, and Laos. PhytoKeys 2019, 130, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Tang, D.F.; Liu, D.H.; Jiang, L.W.; Wang, S.Z.; Li, L.; Xiang, B.W. Tissue culture of Taxus chinensis var. mairei. Hunan Agric. Sci. 2008, 47, 21–23. [Google Scholar] [CrossRef]
- Hook, I.; Poupat, C.; Ahond, A.; Guénard, D.; Guéritte, F.; Adeline, M.-T.; Wang, X.-P.; Dempsey, D.; Breuillet, S.; Potier, P. Seasonal variation of neutral and basic taxoid contents in shoots of European Yew (Taxus baccata). Phytochemistry 1999, 52, 1041–1045. [Google Scholar] [CrossRef]
- Wu, M.; Wu, Y.; Zhou, J.; Pan, Y. Structural characterisation of a water-soluble polysaccharide with high branches from the leaves of Taxus chinensis var. mairei. Food Chem. 2009, 113, 1020–1024. [Google Scholar] [CrossRef]
- Vidensek, N.; Lim, P.; Campbell, A.; Carlson, C. Taxol Content in Bark, Wood, Root, Leaf, Twig, and Seedling from Several Taxus Species. J. Nat. Prod. 1990, 53, 1609–1610. [Google Scholar] [CrossRef]
- Ehrsam, D.; Sieber, S.; Oufir, M.; Porta, F.; Hamburger, M.; Huwyler, J.; Zu Schwabedissen, H.E.M. Design, Synthesis, and Characterization of a Paclitaxel Formulation Activated by Extracellular MMP9. Bioconjugate Chem. 2020, 31, 781–793. [Google Scholar] [CrossRef]
- Zhu, J.; Sun, H.; Callmann, C.E.; Thompson, M.P.; Battistella, C.; Proetto, M.T.; Carlini, A.S.; Gianneschi, N.C. Paclitaxel-terminated peptide brush polymers. Chem. Commun. 2020, 56, 6778–6781. [Google Scholar] [CrossRef] [PubMed]
- Perdue, J.D.; Seaton, P.J.; Tyrell, J.A.; DeVido, D.R. The removal of Cremophor® EL from paclitaxel for quantitative analysis by HPLC–UV. J. Pharm. Biomed. Anal. 2006, 41, 117–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamal, M.M.; Nazzal, S. Development and validation of a HPLC-UV method for the simultaneous detection and quantification of paclitaxel and sulforaphane in lipid based self-microemulsifying formulation. J. Chromatogr. Sci. 2020, 57, 931–938. [Google Scholar] [CrossRef]
- Chen, T.S.; Li, X.; Bollag, D.; Liu, Y.-C.; Chang, C.-J. Biotransformation of taxol. Tetrahedron Lett. 2001, 42, 3787–3789. [Google Scholar] [CrossRef]
- Sanchez-Muñoz, R.; Perez-Mata, E.; Almagro, L.; Cusido, R.M.; Bonfill, M.; Palazon, J.; Moyano, E. A Novel Hydroxylation Step in the Taxane Biosynthetic Pathway: A New Approach to Paclitaxel Production by Synthetic Biology. Front. Bioeng. Biotechnol. 2020, 8, 410. [Google Scholar] [CrossRef] [PubMed]
- Papas, S.; Akoumianaki, T.; Kalogiros, C.; Hadjiarapoglou, L.; Theodoropoulos, P.A.; Tsikaris, V. Synthesis and antitumor activity of peptide-paclitaxel conjugates. J. Pept. Sci. 2007, 13, 662–671. [Google Scholar] [CrossRef]
- Li, H.; Dou, M.; Wang, X.; Guo, N.; Kou, P.; Jiao, J.; Fu, Y. Optimization of Cellulase Production by a Novel Endophytic Fungus Penicillium oxalicum R4 Isolated from Taxus cuspidata. Sustainability 2021, 13, 6006. [Google Scholar] [CrossRef]
- Tong, X.-J.; Fang, W.-S.; Zhou, J.-Y.; He, C.-H.; Chen, W.-M.; Fang, Q.-C. Three New Taxane Diterpenoids from Needles and Stems of Taxus cuspidata. J. Nat. Prod. 2004, 58, 233–238. [Google Scholar] [CrossRef]
- Yu, S.-H.; Zhang, M.-L.; Wang, Y.-F.; Huo, C.-H.; Gu, Y.-C.; Shi, Q.-W. A New Taxane from the Hard Wood of Taxus cuspidata. Z. Für Nat. B 2010, 65, 635–638. [Google Scholar] [CrossRef]
- Kim, B.J.; Gibson, D.M.; Shuler, M.L. Effect of the plant peptide regulator, phytosulfokine-α, on the growth and Taxol production from Taxus sp. suspension cultures. Biotechnol. Bioeng. 2006, 95, 8–14. [Google Scholar] [CrossRef]
- Huo, C.; Zhang, X.; Li, C.; Wang, Y.; Shi, Q.; Kiyota, H. A new taxol analogue from the leaves of Taxus cuspidata. Biochem. Syst. Ecol. 2007, 35, 704–708. [Google Scholar] [CrossRef]
- Bai, J.; Kitabatake, M.; Toyoizumi, K.; Fu, L.; Zhang, S.; Dai, J.; Sakai, J.; Hirose, K.; Yamori, T.; Tomida, A. Production of biologically active taxoids by a callus culture of Taxus cuspidata. J. Nat. Prod. 2004, 67, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Yuan, Y.-J. Responses of Taxus cuspidata to hydrodynamics in bubble column bioreactors with different sparging nozzle sizes. Biochem. Eng. J. 2009, 45, 100–106. [Google Scholar] [CrossRef]
- Ge, L.; Qi, Q.; Zhang, Q.; Guo, C.; Shi, B. Content changes of endogenous hormones in Taxus cuspidata during the flower development. Wit Trans. Built Environ. 2014, 145, 683–690. [Google Scholar] [CrossRef]
- Naill, M.C.; Kolewe, M.E.; Roberts, S.C. Paclitaxel uptake and transport in Taxus cell suspension cultures. Biochem. Eng. J. 2012, 63, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Kingston, D.; Newman, D.J. Taxoids: Cancer-fighting compounds from nature. Curr. Opin. Drug Discov. Dev. 2007, 10, 130. [Google Scholar] [CrossRef] [Green Version]
- Yadava, U.; Gupta, H.; Roychoudhury, M. Stabilization of Microtubules by Taxane Diterpenoids: Insight from Docking and MD simulations. J. Biol. Phys. 2015, 41, 117–133. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Liu, K.; Xie, M. Purification of taxol by industrial preparative liquid chromatography. J. Chromatogr. A 1998, 813, 201–204. [Google Scholar] [CrossRef]
- Ke, C.; Ren, Y.; Gao, P.; Han, J.; Tao, Y.; Huang, J.; Yang, X. Separation and purification of pyrroloquinoline quinone from fermentation broth by pretreatment coupled with macroporous resin adsorption. Sep. Purif. Technol. 2021, 257, 117962. [Google Scholar] [CrossRef]
- Ulusoy, M.; Aslıyüce, S.; Keskin, N.; Denizli, A. Beauvericin purification from fungal strain using molecularly imprinted cryogels. Process Biochem. 2022, 113, 185–193. [Google Scholar] [CrossRef]
- Gast, M.; Wondany, F.; Raabe, B.; Michaelis, J.; Sobek, H.; Mizaikoff, B. Use of Super-Resolution Optical Microscopy to Reveal Direct Virus Binding at Hybrid Core–Shell Matrixes. Anal. Chem. 2020, 92, 3050–3057. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Wang, Y.; Shi, J.; Zhou, S.; Tang, W.; Chen, Z. Enhanced boron removal by crmnfeni-based high-entropy alloys during purification of silicon. Sep. Purif. Technol. 2021, 279, 119682. [Google Scholar] [CrossRef]
- Hu, C.; Mei, H.; Guo, H.; Yu, Z.; Zhu, J. Purification of ammonium nitrate via recrystallization for isotopic profiling using isotope ratio mass spectrometry. Forensic Sci. Int. 2021, 328, 111009. [Google Scholar] [CrossRef]
- Bailey, G.G.; Needham, L.L. Simultaneous quantification of erythrocyte zinc protoporphyrin and protoporphyrin IX by liquid chromatography. Clin. Chem. 2020, 32, 2137–2142. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, S.-P.R.; Jia, Z.; Monteiro, M.J. Analysis of cyclic polymer purity by size exclusion chromatography: A model system. Polym. Chem. 2020, 11, 7354–7361. [Google Scholar] [CrossRef]
- Vanova, J.; Malinak, D.; Andrys, R.; Kubat, M.; Mikysek, T.; Rousarova, E.; Musilek, K.; Rousar, T.; Cesla, P. Optimization of gradient reversed phase high performance liquid chromatography analysis of acetaminophen oxidation metabolites using linear and non-linear retention model. J. Chromatogr. A 2022, 1669, 462956. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.; Liu, Q.; Xie, S.; Zhu, F.; Chen, X. Preparative isolation and purification of 12 main antioxidants from the roots of Polygonum multiflorum Thunb. using high-speed countercurrent chromatography and preparative HPLC guided by 1,1′-diphenyl-2-picrylhydrazyl-HPLC. J. Sep. Sci. 2020, 43, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Fernando, I.P.S.; Lee, S.-H.; Ko, S.-C.; Kang, M.C.; Ahn, G.; Je, J.-G.; Sanjeewa, K.; Rho, J.-R.; Shin, H.J.; et al. Isolation and characterization of anti-inflammatory compounds from Sargassum horneri via high-performance centrifugal partition chromatography and high-performance liquid chromatography. Algal Res. 2021, 54, 102209. [Google Scholar] [CrossRef]
- Nugroho, T.T.; Puja, K.; Eryanti, Y.; Miranti, M. Fractionation of Garcinia Mangostana Fruit Pericarp Cellulase Assisted Extracts by Preparative Thin Layer Chromatography and High Performance Liquid Chromatography. J. Nat. Indones. 2020, 18, 31. [Google Scholar] [CrossRef]
- Dufour, A.; Thiébaut, D.; Loriau, M.; Ligiero, L.; Vial, J. Corona charged aerosol detector non-uniform response factors of purified alcohol ethoxylated homologues using liquid chromatography. J. Chromatogr. A 2020, 1627, 461402. [Google Scholar] [CrossRef]
- Jiang, T.; Ghosh, R.; Charcosset, C. Extraction, purification and applications of curcumin from plant materials—A comprehensive review. Trends Food Sci. Technol. 2021, 112, 419–430. [Google Scholar] [CrossRef]
- Bredahl, E.K.; Kjaerulff, L.; Ndi, C.; Semple, S.; Buirchell, B.; Møller, B.L.; Staerk, D. Isolation and structure elucidation of caryophyllane sesquiterpenoids from leaves of Eremophila spathulata. Phytochem. Lett. 2022, 47, 156–163. [Google Scholar] [CrossRef]
- Liu, Y.; Murakami, N.; Wang, L.; Zhang, S. Preparative high-performance liquid chromatography for the purification of natural acylated anthocyanins from red radish (Raphanus sativus L.). J. Chromatogr. Sci. 2008, 46, 743–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferretti, R.; Carradori, S.; Guglielmi, P.; Pierini, M.; Casulli, A.; Cirilli, R. Enantiomers of triclabendazole sulfoxide: Analytical and semipreparative HPLC separation, absolute configuration assignment, and transformation into sodium salt. J. Pharm. Biomed. Anal. 2017, 140, 38–44. [Google Scholar] [CrossRef]
- Liu, R.; Choi, H.S.; Kim, S.-L.; Kim, J.-H.; Yun, B.-S.; Lee, D.-S. 6-Methoxymellein Isolated from Carrot (Daucus carota L.) Targets Breast Cancer Stem Cells by Regulating NF-κB Signaling. Molecules 2020, 25, 4374. [Google Scholar] [CrossRef]
- Gritti, F. Rebirth of recycling liquid chromatography with modern chromatographic columns: Extension to gradient elution. J. Chromatogr. A 2021, 1653, 462424. [Google Scholar] [CrossRef]
- Zhai, L.; Xu, X.; Liu, J.; Jing, C.; Yang, X.; Zhao, D.; Jiang, R.; Sun, L.-W. A Novel Biochemical Study of Anti-Dermal Fibroblast Replicative Senescence Potential of Panax Notoginseng Oligosaccharides. Front. Pharmacol. 2021, 12, 690538. [Google Scholar] [CrossRef]
- Wu, Z.; Ren, S.; Chen, T.; Hui, A.; Zhang, W. Separation and purification of six isoflavones from Iris tectorum Maxim by macroporous resin-based column chromatography coupled with preparative high-performance liquid chromatography. Sep. Sci. Technol. 2020, 55, 1686–1694. [Google Scholar] [CrossRef]
- Li, H.; Shi, J.; Li, Y.; Wang, C.; Hou, G.; Cong, W.; Zhao, F. Purification of spinosin from Ziziphi Spinosae Semen using macroporous resins followed by preparative high-performance liquid chromatography. J. Sep. Sci. 2019, 42, 3134–3140. [Google Scholar] [CrossRef]
- Arzanlou, M.; Bohlooli, S.; Omid, M.R. Purification of Allicin From Garlic Extract Using Semi-Preparative High Performance Liquid Chromatography. Jundishapur J. Nat. Pharm. Prod. 2015, 10, e17424. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, B.; Thakur, V.; Thakur, A.; Thakur, N.; Pandey, D.; Chand, D. Hyper-production of taxol from Aspergillus fumigatus, an endophytic fungus isolated from Taxus sp. of the Northern Himalayan region. Biotechnol. Rep. 2019, 24, e00395. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, N.; Mayer, G.; Has, C.; Kopczynski, D.; Al Machot, F.; Schwudke, D.; Ahrends, R.; Marcus, K.; Eisenacher, M.; Turewicz, M. A Current Encyclopedia of Bioinformatics Tools, Data Formats and Resources for Mass Spectrometry Lipidomics. Metabolites 2022, 12, 584. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xue, A.; Li, X.; Huang, X.; Ning, F.; Zhang, X.; Liu, T.; Chen, H.; Luo, L. Analysis of chemical composition of nectars and honeys from Citrus by extractive electrospray ionization high resolution mass spectrometry. LWT-Food Sci. Technol. 2020, 131, 109748. [Google Scholar] [CrossRef]
- Nan, Q.; Hejian, W.; Ping, X.; Baohua, S.; Junbo, Z.; Hongxiao, D.; Huosheng, Q.; Fenyun, S.; Yan, S. Investigation of Fragmentation Pathways of Fentanyl Analogues and Novel Synthetic Opioids by Electron Ionization High-Resolution Mass Spectrometry and Electrospray Ionization High-Resolution Tandem Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2020, 31, 277–291. [Google Scholar] [CrossRef]
- Dang, D.H.; Evans, R.D. Application of ESI-HRMS for molybdenum speciation in natural waters: An investigation of molybdate-halide reactions. Talanta 2018, 179, 221–229. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Z.; Meng, H.; Li, W.; Wang, S. Ultrasonic Extraction and Separation of Taxanes from Taxus cuspidata Optimized by Response Surface Methodology. Separations 2022, 9, 193. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Z.; Li, W.; Tang, Y.; Meng, H.; Wang, S. Separation and Purification of Taxanes from Crude Taxus cuspidata Extract by Antisolvent Recrystallization Method. Separations 2022, 9, 304. [Google Scholar] [CrossRef]
- Petrásková, L.; Káňová, K.; Brodsky, K.; Hetman, A.; Petránková, B.; Pelantová, H.; Křen, V.; Valentová, K. Sulfated Phenolic Substances: Preparation and Optimized HPLC Analysis. Int. J. Mol. Sci. 2022, 23, 5743. [Google Scholar] [CrossRef]
- Wang, H.-J.; Pan, M.-C.; Chang, C.-K.; Chang, S.-W.; Hsieh, C. Optimization of Ultrasonic-Assisted Extraction of Cordycepin from Cordyceps militaris Using Orthogonal Experimental Design. Molecules 2014, 19, 20808–20820. [Google Scholar] [CrossRef] [Green Version]
- Suzer, S.; Strelcov, E.; Kolmakov, A. Comparative Operando XPS and SEM Spatiotemporal Potential Mapping of Ionic Liquid Polarization in a Coplanar Electrochemical Device. Anal. Chem. 2021, 93, 13268–13273. [Google Scholar] [CrossRef]
- Bourhim, T.; Villareal, M.O.; Couderc, F.; Hafidi, A.; Isoda, H.; Gadhi, C. Melanogenesis Promoting Effect, Antioxidant Activity, and UPLC-ESI-HRMS Characterization of Phenolic Compounds of Argan Leaves Extract. Molecules 2021, 26, 371. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.-X.; Quan, C.; Zhang, W.; Li, H.-M.; Huang, T.; Sun, Y.-Y.; Yan, T.; Liu, J.-B. Purification of folic acid candidate reference material by preparative high-performance liquid chromatography. J. Liq. Chromatogr. Relat. Technol. 2017, 40, 419–427. [Google Scholar] [CrossRef]
- Stevens, J.M.; Hamstra, A.; Hegeman, T.; Scharrer, G. Factors and parameters that directly affect recovery of collected fractions in preparative hplc. LC-GC Asia Pacific. 2006, 24, 102–105. [Google Scholar] [CrossRef] [Green Version]
- Brandt, A.; Mann, G.; Arlt, W. Temperature gradients in preparative high-performance liquid chromatography columns. J. Chromatogr. A 1997, 769, 109–117. [Google Scholar] [CrossRef]
- Fang, D.S.; Wang, Y.W.; Gan, Y.J.; Gao, H.W.; Chen, D.H. Study on Separation and Purification of Taxane Diterpenoids Compound 10-DAB III. China Pharm. 2012, 23, 3879–3881. [Google Scholar]
- Lee, J.-Y.; Kim, J.-H. Influence of crude extract purity and pure paclitaxel content on fractional precipitation for purification of paclitaxel. Sep. Purif. Technol. 2013, 103, 8–14. [Google Scholar] [CrossRef]
- Liang, Z.; Huang, Y.; Xie, Z.; Xu, X. Application of High-Speed Counter-Current Chromatography for Isolation and Purification of Paclitaxel and Related Taxanes from Taxus chinensis Cell Culture. Sep. Sci. Technol. 2015, 50, 851–858. [Google Scholar] [CrossRef]
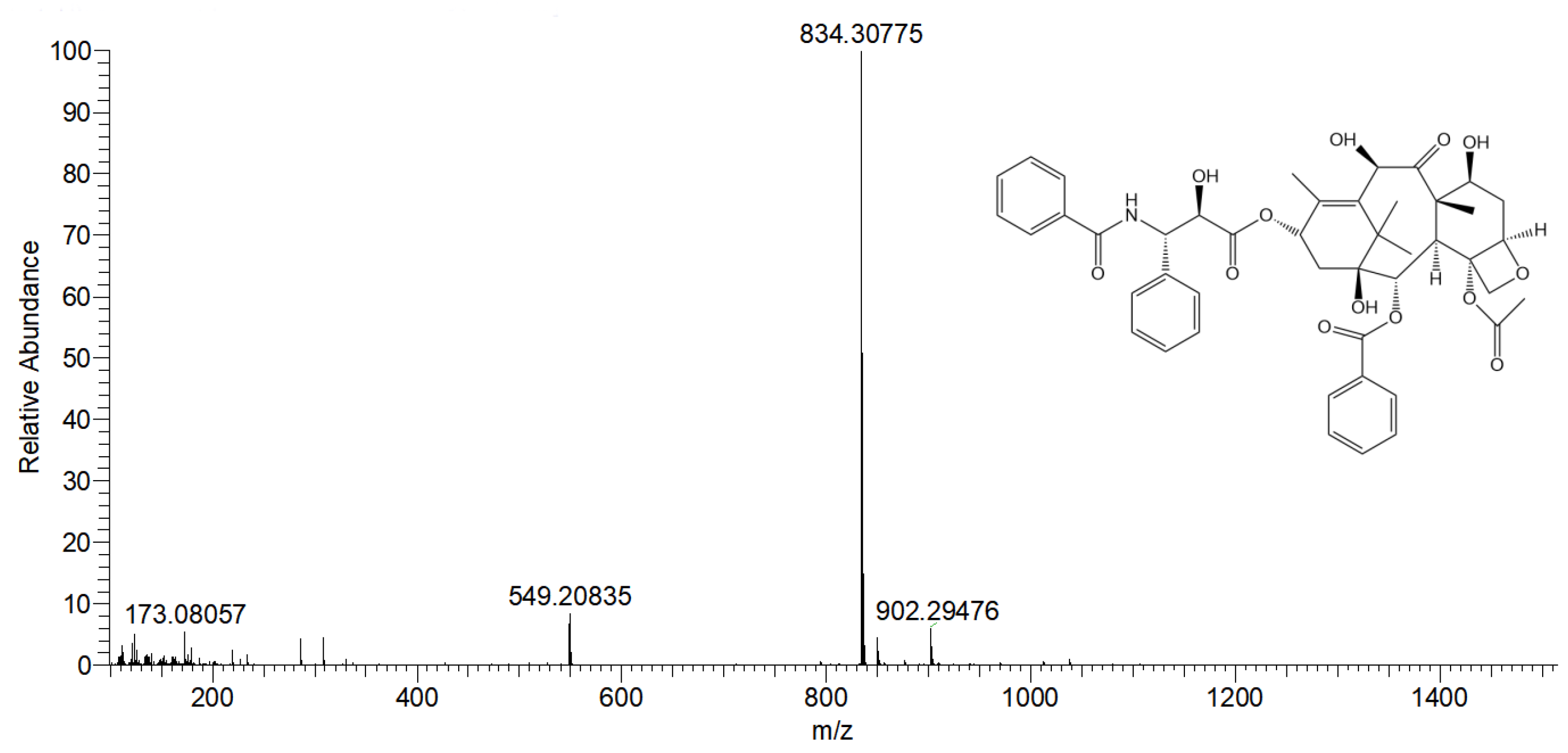
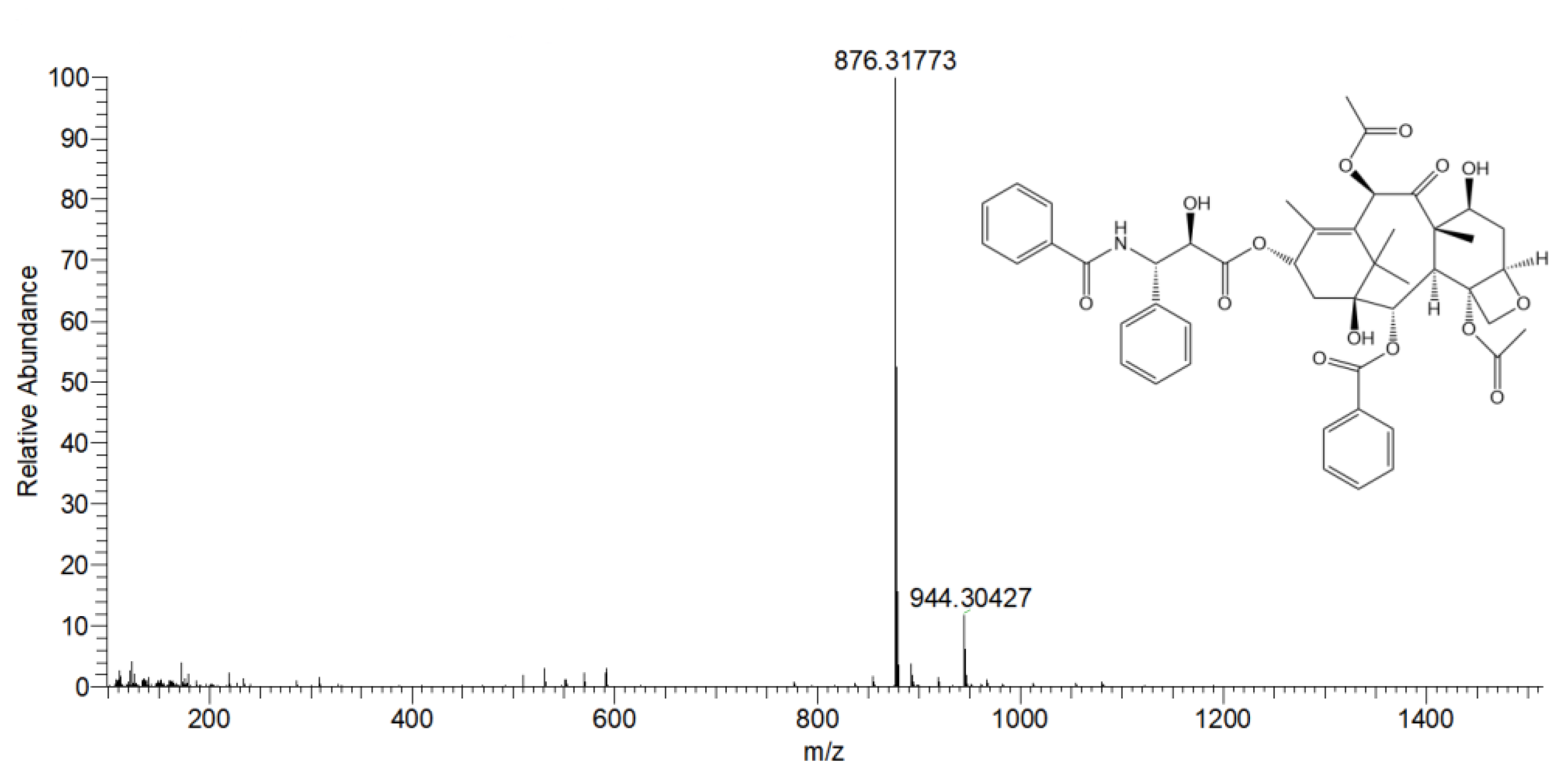
| Factor Level | X1 Flow Rate (mL/min) | X2 Injection Volume (mL) | X3 Column Temperature (°C) |
|---|---|---|---|
| 1 | 8 | 0.5 | 25 |
| 2 | 10 | 1.0 | 30 |
| 3 | 12 | 1.5 | 35 |
| Run | X1 | X2 | X3 | Yi (%) | yi (%) |
|---|---|---|---|---|---|
| 1 | 3 | 3 | 1 | 85.79 | 87.28 |
| 2 | 1 | 2 | 3 | 92.43 | 93.37 |
| 3 | 3 | 1 | 3 | 90.05 | 91.26 |
| 4 | 1 | 3 | 2 | 90.58 | 90.48 |
| 5 | 2 | 3 | 3 | 92.61 | 94.31 |
| 6 | 3 | 2 | 2 | 89.35 | 90.92 |
| 7 | 2 | 2 | 1 | 94.17 | 98.67 |
| 8 | 2 | 1 | 2 | 95.33 | 99.15 |
| 9 | 1 | 1 | 1 | 92.96 | 93.64 |
| j1 | 91.99 | 92.78 | 90.97 | X1 > X2 > X3 | |
| j2 | 94.04 | 91.98 | 91.75 | ||
| j3 | 88.40 | 89.66 | 91.70 | ||
| Rj | 5.64 | 3.12 | 0.78 | ||
| j1 | 92.50 | 94.68 | 93.20 | X1 > X2 > X3 | |
| j2 | 97.38 | 94.32 | 93.52 | ||
| j3 | 89.82 | 90.69 | 92.98 | ||
| Rj | 7.56 | 3.99 | 0.54 | ||
| Source of Variation | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| X1 | 48.910 | 2 | 24.455 | 127.452 | 0.008 |
| X2 | 15.767 | 2 | 7.883 | 41.086 | 0.024 |
| X3 | 1.135 | 2 | 0.567 | 2.957 | 0.253 |
| Pure error | 0.384 | 2 | 0.192 | ||
| Total | 75,374.362 | 9 |
| Source of Variation | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| X1 | 88.082 | 2 | 44.041 | 219.681 | 0.005 |
| X2 | 29.256 | 2 | 14.628 | 72.965 | 0.014 |
| X3 | 0.437 | 2 | 0.219 | 1.091 | 0.478 |
| Pure error | 0.401 | 2 | 0.200 | ||
| Total | 78,346.537 | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhao, Z.; Li, W.; Tang, Y.; Meng, H.; Wang, S. Purification of Two Taxanes from Taxus cuspidata by Preparative High-Performance Liquid Chromatography. Separations 2022, 9, 446. https://doi.org/10.3390/separations9120446
Zhang Y, Zhao Z, Li W, Tang Y, Meng H, Wang S. Purification of Two Taxanes from Taxus cuspidata by Preparative High-Performance Liquid Chromatography. Separations. 2022; 9(12):446. https://doi.org/10.3390/separations9120446
Chicago/Turabian StyleZhang, Yajing, Zirui Zhao, Wenlong Li, Yuanhu Tang, Huiwen Meng, and Shujie Wang. 2022. "Purification of Two Taxanes from Taxus cuspidata by Preparative High-Performance Liquid Chromatography" Separations 9, no. 12: 446. https://doi.org/10.3390/separations9120446
APA StyleZhang, Y., Zhao, Z., Li, W., Tang, Y., Meng, H., & Wang, S. (2022). Purification of Two Taxanes from Taxus cuspidata by Preparative High-Performance Liquid Chromatography. Separations, 9(12), 446. https://doi.org/10.3390/separations9120446




