Abstract
Rape pollen has always been considered as a research hotspot in health foods and pharmaceuticals due to its abundance of natural active ingredients. In this work, a compound with antioxidant activity was directly isolated from the methanol extract of rape pollen using a two-step procedure, under the supervision of online HPLC−1,1-diphenyl-2-picrylhydrazyl (HPLC-DPPH) detection. Firstly, online HPLC−DPPH detection was used to identify the active peaks in the methanol extract of rape pollen, and then the methanol extract was pretreated via medium-pressure liquid chromatography (MPLC) to obtain the target fraction 3 (Fr3). Fr3 was further purified using HPLC to finally obtain the target fraction 3-1, which was identified as kaempferol 3,4′-di-O-β-D-glucopyranoside through NMR and mass spectrometry. To further explore the free radical scavenging activity of this compound, its DPPH scavenging ability was determined, and two proteins related to the antioxidant pathway were used for molecular docking. The results revealed that the chromatographic strategy used in this study was efficient and reliable in separating high−purity antioxidants from rape pollen. A strategy such as this, meanwhile, also holds promise for qualitatively identifying and specifically isolating active compounds from other natural products.
1. Introduction
Rape (Brassica rapa var. oleifera), a species of the Brassica genus Gruciferous, is the largest cultivated and most widely distributed nectar crop in China, as well as one of the major oil crops [1,2,3]. Its by-product, rape pollen, is recognized as a natural health food. Rape pollen contains a large number of proteins, vitamins, lipids, and active small molecule compounds, which have various effects, such as anti-atherogenic, antioxidant, inhibiting prostate enlargement, and promoting the function of the nervous system [4,5,6,7]. Modern phytochemical technology analysis shows that rape pollen is rich in polyphenols and flavonoids and has a significant antioxidant capacity, making it an ideal research target for health foods and pharmaceuticals [8,9]. However, few studies have been reported on the antioxidant active components of rape pollen. In order to further promote the development of deep processing of rapeseed pollen and related industries, a suitable chromatographic purification method for exploring the chemical composition of rapeseed pollen is particularly essential [10,11,12].
The health industry has been growing quickly in recent years based on a variety of human health demands, and concepts such as “health care”, “oxidative stress”, and “antioxidant” have received more attention [13]. Studies have shown that aging, cancer, and cardiovascular and neurodegenerative diseases of the human body are all related to oxidative stress [14,15,16,17]. The condition known as oxidative stress occurs when the body’s oxidation and antioxidant systems are out of balance, which is a negative effect of excessive levels of free radicals in the body [18,19,20]. Endogenous free radical inhibitors are often insufficient to cope with this state, and the intervention of exogenous free radical inhibitors is very important at this time [21]. Natural products with large resources and many active ingredients are an important source for obtaining exogenous free radical inhibitors [22]. Currently, there are various ways for identifying antioxidants from natural products, among which online HPLC−DPPH detection has gained popularity among researchers due to its stability, online access, and high efficiency [23,24,25].
Not surprisingly, some non-target components (pigments, macromolecular polymers, etc.) will be co-eluted with active ingredients when extracting chemical constituents from natural products. If the crude extract is directly prepared via HPLC, the preparative column will be irreparably damaged, thereby shortening the service life of the preparative column and reducing the column efficiency [26]. Therefore, a suitable pretreatment approach is needed to remove these non-target components prior to HPLC preparation. MPLC is now able to perform such a function. MPLC is formed by connecting a medium-pressure column with liquid chromatography. By setting different elution conditions and column packing, the objectives of online separation, automatic operation, and high-throughput processing can be achieved. With a low column pressure, large particle size of packing (>20 µm), and adjustable column length, MPLC is ideal for the pretreatment of natural products [27,28]. At the same time, suitable MPLC pretreatment techniques also have the potential to form a two-dimensional liquid chromatography system with subsequent HPLC preparation, which facilitates the acquisition of high-purity compounds.
Considering the multiple reports listed previously, a rapid chromatographic strategy for the preparation of antioxidants from rape pollen was developed in this paper. An online HPLC−DPPH detection was used for the qualitative identification of antioxidants, MPLC was used for sample pretreatment, and HPLC was utilized to obtain free radical inhibitors directly from the target fraction. In addition, a DPPH scavenging assay and molecular docking were used to further explore the antioxidant ability of the isolated high-purity compound. The results showed that this combined chromatographic strategy is reliable and efficient, and the isolated free radical inhibitor was beneficial for future research on deep processing and high value-added products of rape pollen. It is also worth mentioning that the chromatographic strategy established in this paper is expected to make achievements in the future development of food and pharmaceutical products.
2. Materials and Methods
2.1. Instrumentation and Reagents
NMR spectra were obtained on a Bruker Avance 600 MHz (Bruker, Karlsruhe, Germany), and ESI-MS spectra were collected from a Waters QDa ESI mass spectrometer (Waters, Milford, MA, USA). The online HPLC-DPPH detection was performed using LC-10AD (Shimadzu, Kyoto, Japan) and LC-16 (Shimadzu, Shanghai, China) instruments. A triple valve and an 18 m long reaction coil (polyetheretherketone) were used to connect these instruments. Sample separation was performed via a preparative HPLC system (Hanbon, Huai’an, China). For this work, the following chromatographic columns and separation materials were employed: MCI GEL®CHP20P (120 μm) used in a medium-pressure column (49 × 460 mm) was purchased from Mitsubishi Chemical Corporation (Kyoto, Japan). ReproSil-Pur C18 AQ analytical column (4.6 × 250 mm, 5 μm) and ReproSil-Pur C18 AQ preparative column (20 × 250 mm, 5 μm) were supplied by Maisch Corporation (Munich, Germany). Additionally, the UV absorbance values of the samples were read with a ReadMax 1900 microplate reader (Flash, Shanghai, China).
DPPH was purchased from Sigma-Aldrich (Steinheim, Germany). All reagents used in the experiments, including analytical methanol (MeOH), HPLC-grade acetonitrile (ACN), and MeOH were obtained from Xinlanjing Chemical Industry (Yuxi, China). HPLC-grade H2O was prepared using a Moore water purification instrument (Chongqing, China).
2.2. Sample Extraction, MPLC Pretreatment and Active Peaks Recognition
A total of 1 kg of rape pollen gifted by Qinghai Ruihu Bioresource Development Co., Ltd. was extracted directly with 8 L of MeOH (extracted twice for 24 h, room temperature). The extract was combined, filtered, and concentrated using a rotary evaporator at 40 °C. After the volume of this extract solution was reduced to about 0.3 L, it was mixed with 100 g of dried amorphous silica gel and dried in an oven at a temperature of 40 °C.
A total of 182.4 g of extract−silica gel mixture was obtained after drying, which was calculated to be about 82.4 g of crude sample (yield of about 8.24%). Then the generated mixture was added to an empty chromatographic tower (49 × 100 mm) and coupled to an MCI medium-pressure column (49 × 460 mm) and liquid chromatography for pretreatment. MeOH and H2O were utilized as mobile phases, and the elution procedure was set as follows: 0–30 min, 100% H2O; 30–150 min, 0–100% MeOH; 150–240 min, 100% MeOH. The flow rate was 30 mL/min, and the absorbance was recorded at 254 nm. After 18 repeated elution procedures, five fractions were recovered (labeled Fr1, Fr2, Fr3, Fr4, and Fr5).
An additional 3 mg of rape pollen was weighed and dissolved in 1 mL of MeOH, then passed through an organic filter membrane (0.45 μm) for subsequent activity determination. The online HPLC−DPPH detection system was used to screen for active peaks in crude sample on ReproSil-Pur C18 AQ analytical column. This system consists of two HPLCs connected by a triple valve and reaction coils. The sample is detected through the first HPLC and then pumped to the second HPLC, where it is mixed with the DPPH solution (pumped in as mobile phase) and detected again. The active ingredients reacting with DPPH decrease the absorption at 517 nm, thus forming observable inverted peaks. The conditions for activity determination were as follows: for the first HPLC analysis, the flow rate was 1 mL/min, H2O and ACN made up the mobile phase, and 5–50% ACN was linearly eluted for 60 min. Simultaneously, for the second HPLC, the flow rate of DPPH solution was 0.8 mL/min, and the DPPH concentration was 50 μg/mL. Chromatographic information of rape pollen was gathered at 254 nm. The data of free radical scavenging activity were recorded at 517 nm. In addition, the five recovered fractions as well as the crude sample were analyzed under the same chromatographic conditions.
2.3. Directed Separation of Antioxidants from the Target Fraction
A total of 1 g of Fr3 was completely dissolved in 8 mL of MeOH and passed through a 0.45 µm organic membrane for subsequent activity determination and separation. The conditions for activity determination were as follows: the mobile phase consisted of H2O and ACN, with a flow rate of 1 mL/min, and the isocratic elution was 0–60 min at 13% ACN. As for online HPLC-DPPH detection, the flow rate of DPPH solution was 0.8 mL/min, and the DPPH concentration was 50 μg/mL. Chromatographic information was gathered at 254 nm. The data of free radical scavenging activity were recorded at 517 nm.
The antioxidant in Fr3 was purified using a ReproSil-Pur C18 AQ preparative column with a mobile phase composed of ACN and H2O. The isocratic elution mode was used with the ACN content kept at 13% for 60 min. The eluent flow rate was constant at 19 mL/min, and chromatograms were collected at 254 nm. After 16 cycles of preparation, the target compound Fr3-1 was obtained, which was concentrated under reduced pressure and weighed as 406.7 mg.
2.4. Assessment of Activity and Purity of Antioxidants
Through the online HPLC−DPPH detection, the extracted compound’s purity and free radical scavenging ability were verified; ACN and H2O were utilized as the mobile phases, respectively. An amount of 5–50% ACN was eluted linearly over 60 min at 1 mL/min. At the same time, the concentration of DPPH solution was 50 μg/mL, and the flow rate was 0.8 mL/min. The data of free radical scavenging activity were measured at 517 nm.
Furthermore, the DPPH scavenging assay method was utilized to further explore the free radical scavenging activity of the isolated compound. Sample solutions containing compounds were prepared at various concentrations (0.1, 1, 10, 50, 200, and 500 μg/mL). In a 96-well microtiter plate, 30 μL sample solutions of various concentrations were mixed with 70 μL DPPH (0.25 mg/mL) solution and incubated in a shaker for 30 min. Finally, the absorbance at 517 nm of each well was measured using a microplate reader. The antioxidant capacity of the compound was obtained using the following formula:
Inhibitory activity (%) = [1 − (A1 − A2)/A3] × 100%
A1 indicates the sample solution’s absorbance, A2 indicates the blank’s absorbance, and A3 indicates the control solution’s absorbance.
2.5. Molecular Docking
The 3D structure of Fr3−1 was drawn and output via ChemBio3D software (16.0, CambridgeSoft, Cambridge, MA, USA). The crystal structures of Nrf2 protein (PDB ID: 4IQK) and HO-1 protein (PDB ID: 1N3U) were retrieved from the RCSB Protein Data Bank (https://www.rcsb.org/ (accessed on 2 October 2022)). After preparing these files of ligand and receptor, the receptor proteins were dehydrated and ligands were removed via PyMOL software (2.3.0, DeLano Scientific LLC, Shanghai, China). Employing the AutoDock Tools, the charge and hydrogen bonding of receptor proteins were calculated and added. Moreover, the active pocket locations where small molecule ligands attach was defined as characteristics of the receptor protein docking site, and the molecular docking between ligand and receptor proteins was displayed using AutoDock software (https://autodock.scripps.edu/ (accessed on 10 November 2022)). Eventually, a total of 100 peptide conformation results were obtained, and then the results with the lowest binding energy were visualized with PyMOL.
2.6. Statistical Analysis
The statistical analysis was conducted using SPSS 20.0 (SPSS, Chicago, IL, USA), and values were expressed as mean ± SD. All data given represented three repeat experiments. The nonlinear regression was used to calculate the concentration of the isolated compound that caused 50% inhibition (IC50) of the scavenging activities. The curve representing free radical scavenging activities was created using Prism 8.0.
3. Results and Discussion
3.1. MPLC Pretreatment and Active Peaks Recognition
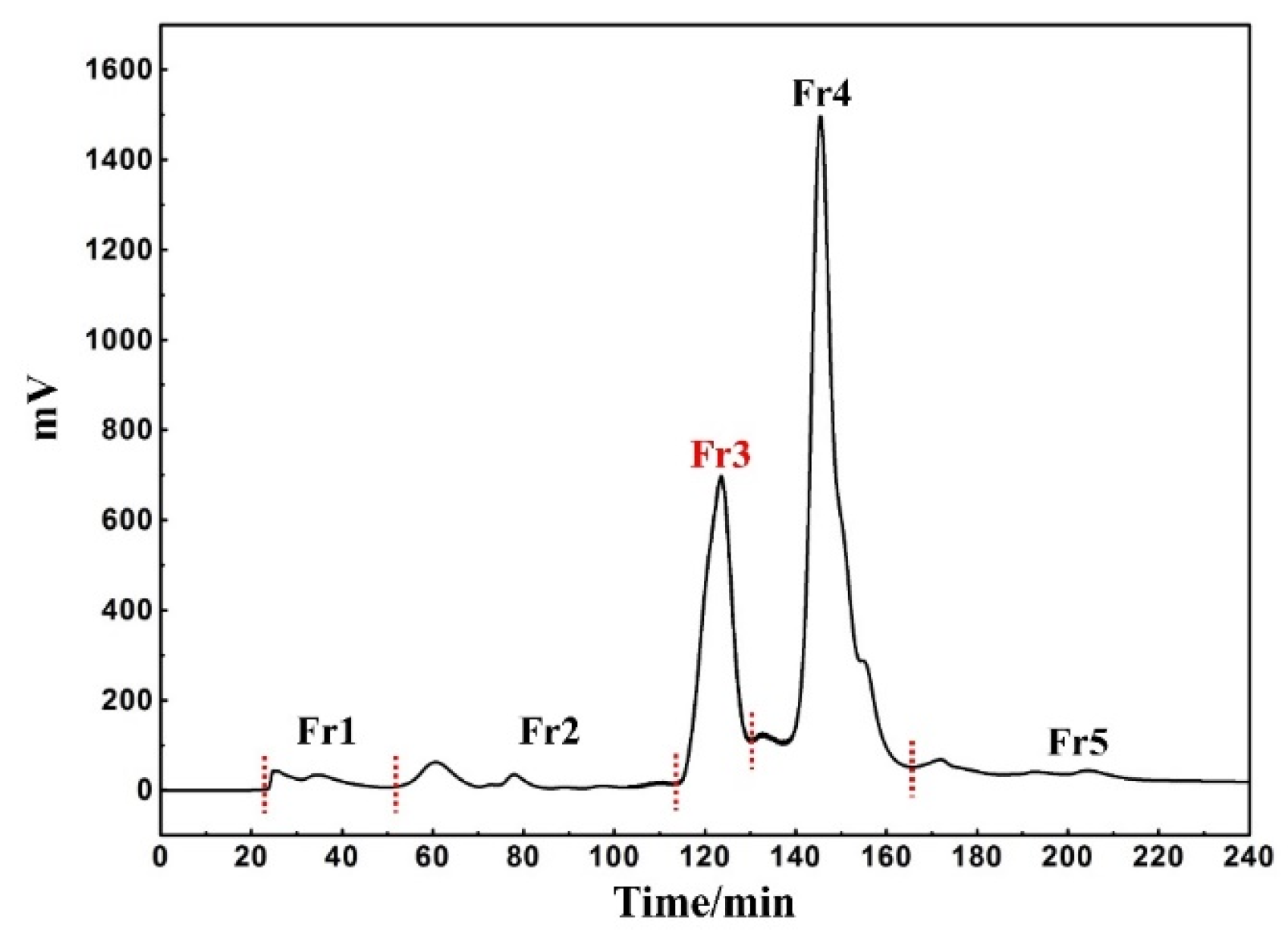
Considering its inexpensive cost and relative environmental friendliness, MeOH was chosen as the extraction solvent. Mixing and drying the concentrated extract with amorphous silica gel can maintain the stability and reproducibility of subsequent sample pretreatment as much as possible. At the same time, this form of dry loading is also useful for exploring the maximum sample loading during the pretreatment process. The weights of the five fractions recovered were as follows: Fr1 1.4 g, Fr2 1.3 g, Fr3 18.7 g, Fr4 37.4 g, and Fr5 1.3 g, with a recovery of 72.9%. According to the preparation diagram in Figure 1, it is found that under such elution conditions, the baseline separation between each fraction was largely achieved without having an impact on the sample recovery.
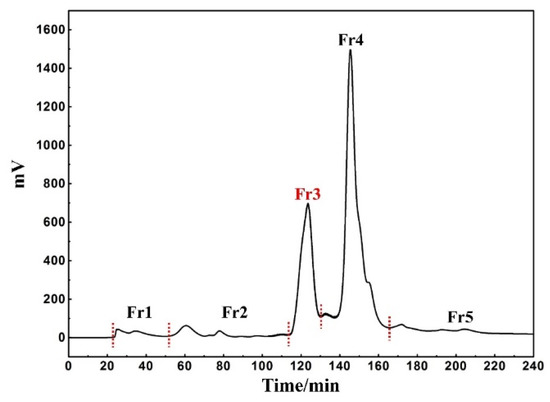
Figure 1.
Chromatogram of rape pollen extract pretreatment with MCI-MPLC.
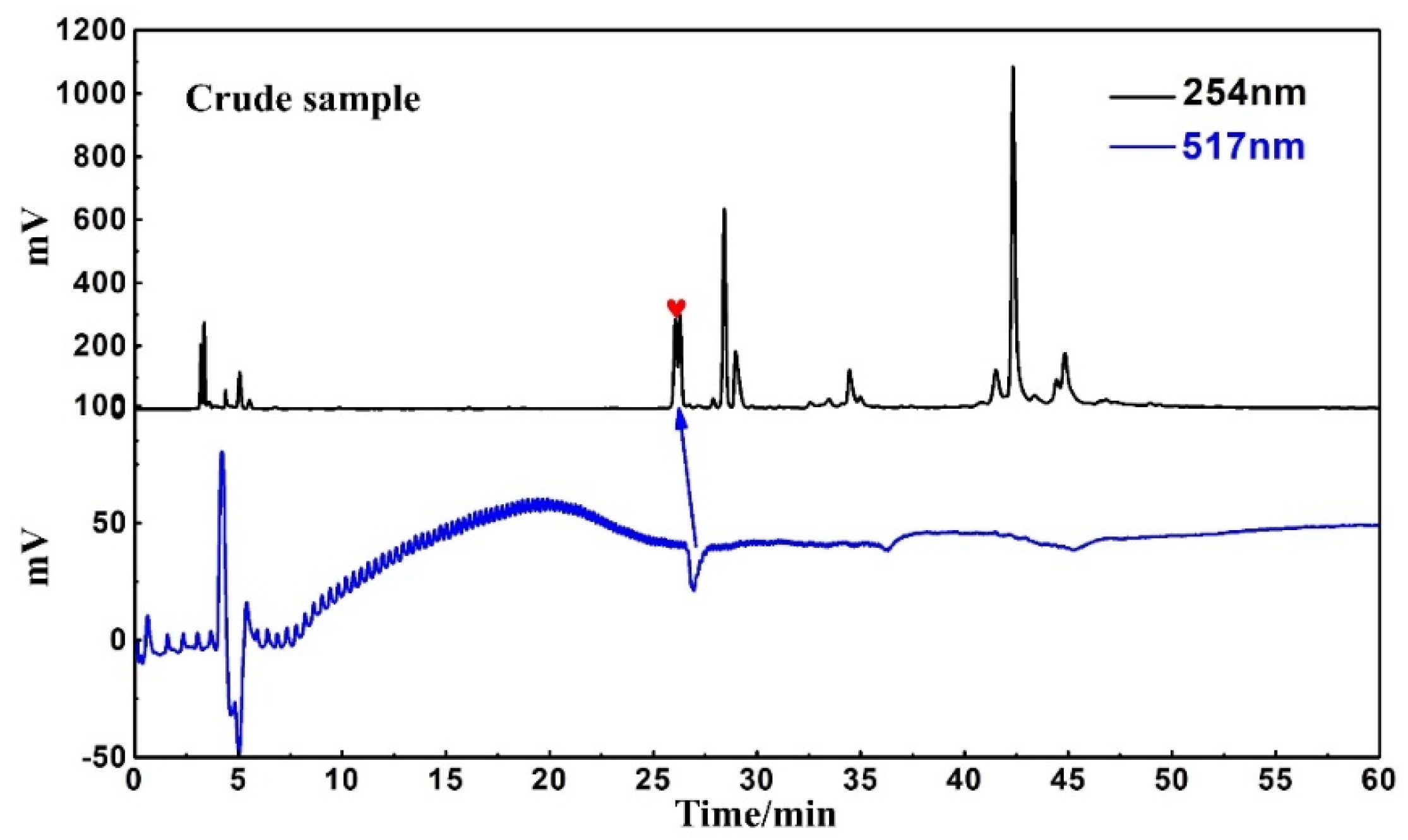
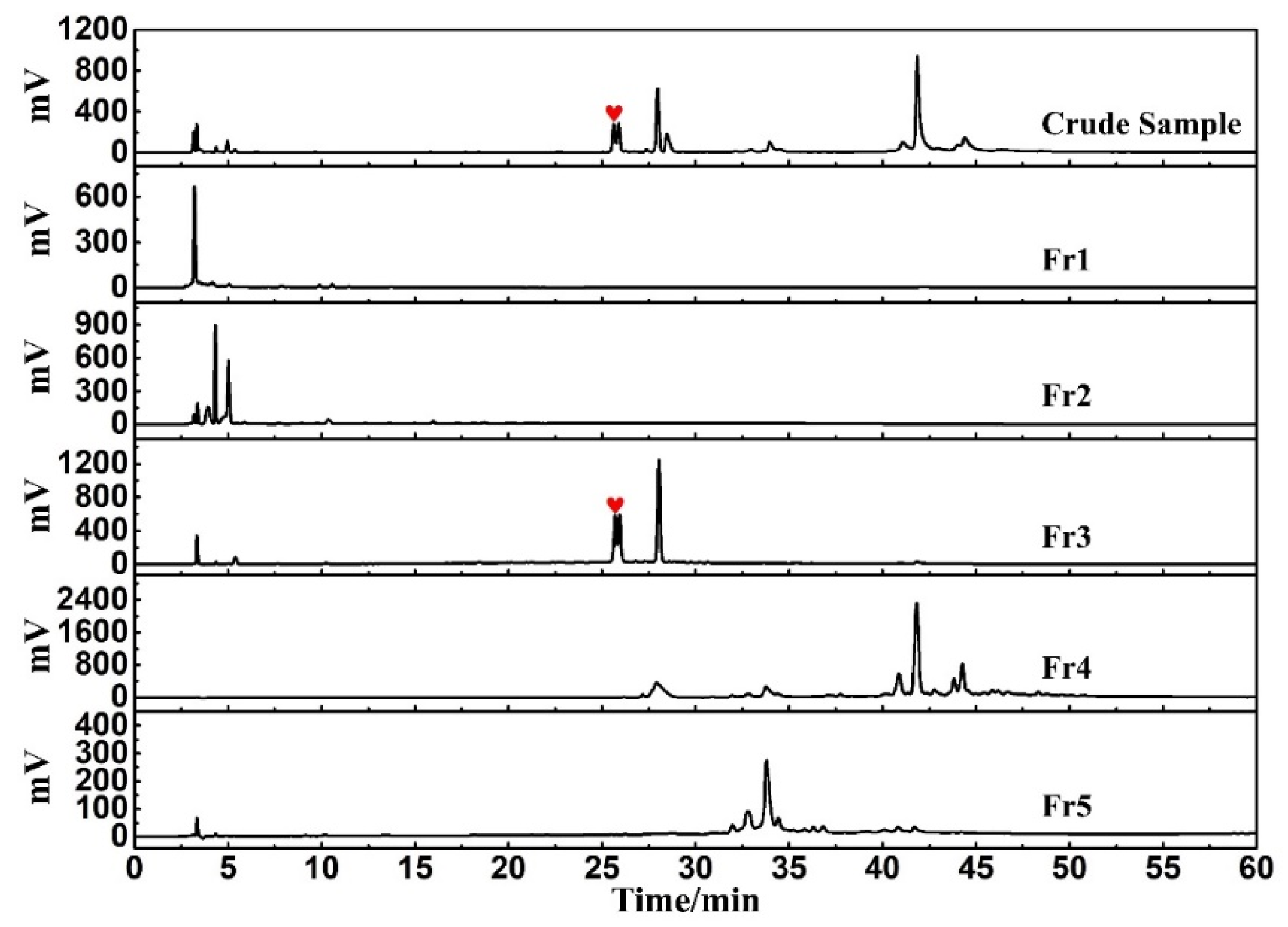
An online HPLC−DPPH detection was used to identify active peaks from rape pollen crude samples. In addition, HPLC analysis was performed on five fractions as well as the crude sample to observe the pretreatment effect. As shown in Figure 2, a clear active peak (inverted peak at 517 nm) can be detected at a retention time of roughly 26 min. From Figure 3, it can be seen that there is basically no overlap between the five fractions, encompassing all chromatographic peaks of the crude sample. At the same time, analysis of Figure 2 in conjunction with Figure 3 reveals that the active peak identified through the online HPLC−DPPH detection was effectively enriched in Fr3. Taken together, these chromatographic behaviors indicated that the MCI−MPLC pretreatment was satisfactory and saved the workload of the next separation step. In order to further explore the antioxidant active components in rape pollen, Fr3 was selected as the target fraction for the next separation.
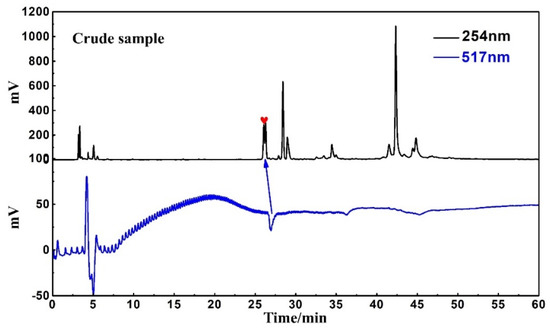
Figure 2.
Online HPLC−DPPH detection chromatogram of crude sample.
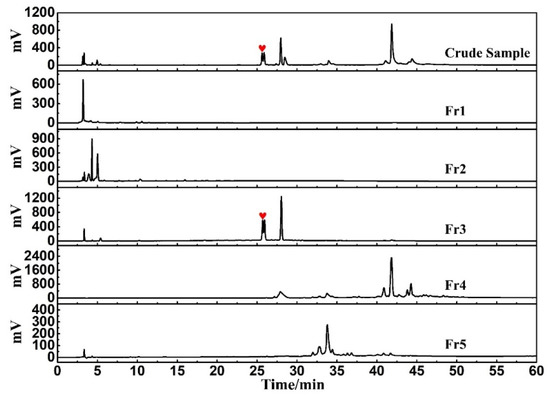
Figure 3.
Analysis chromatograms of crude sample, Fr1, Fr2, Fr3, Fr4, and Fr5 under the same chromatographic condition.
3.2. Directed Separation of Active Compound from the Target Fraction
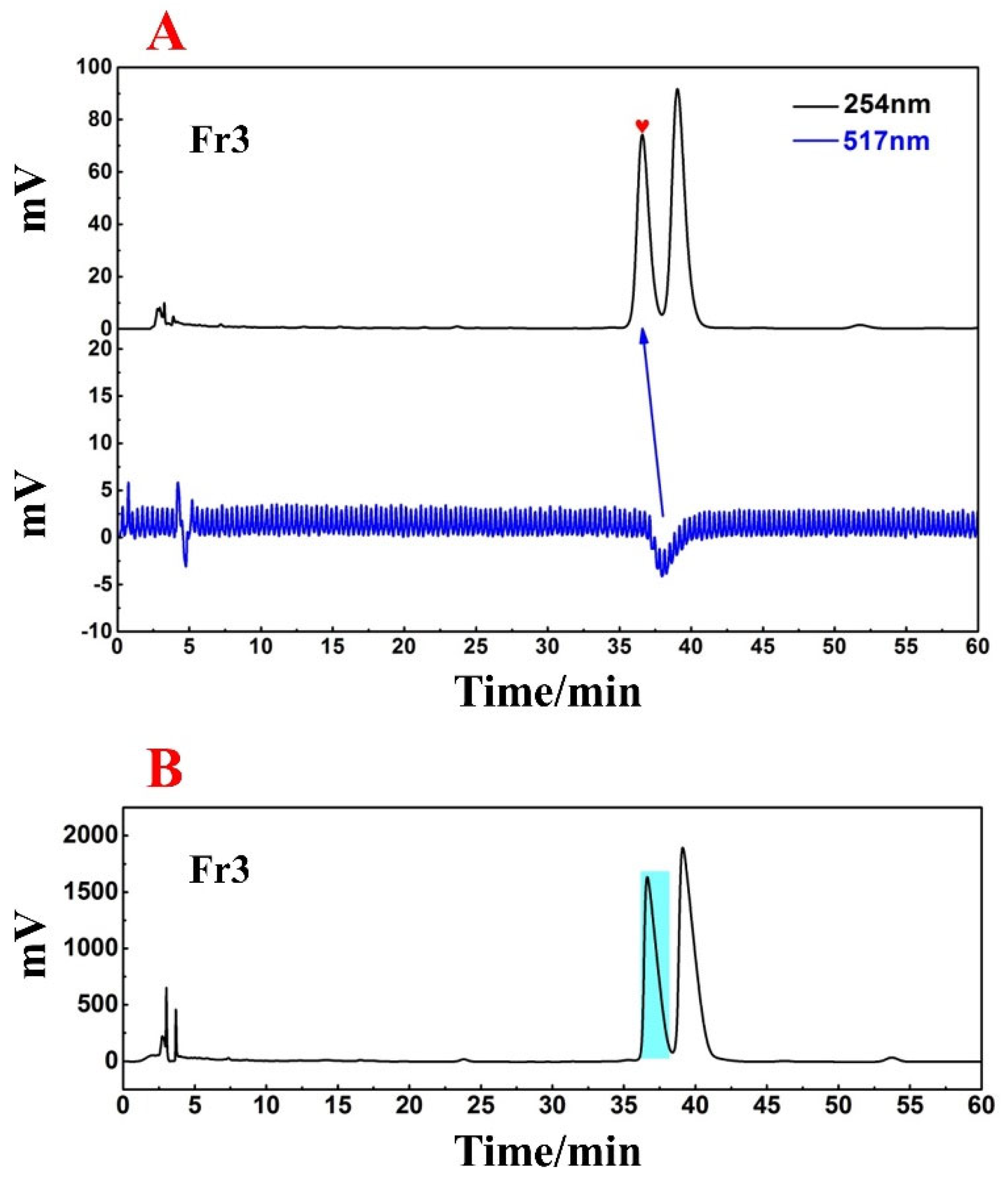
It can be seen from Figure 3 that Fr3 has two chromatographic peaks at a retention time of 26 min. To obtain greater peak resolution during the following HPLC preparation, the chromatographic conditions for Fr3 were further optimized to a 13% ACN isocratic elution for 60 min. Considering the directional separation of the high-purity free radical inhibitor in the next step, online HPLC−DPPH detection was again used to screen the active peaks under the optimized chromatographic conditions (Figure 4A). Based on the order in which the peaks appear in Figure 4A, the first peak was identified as the active peak (marked with a red graphic), and subsequent preparations will be focused on recovering this peak.
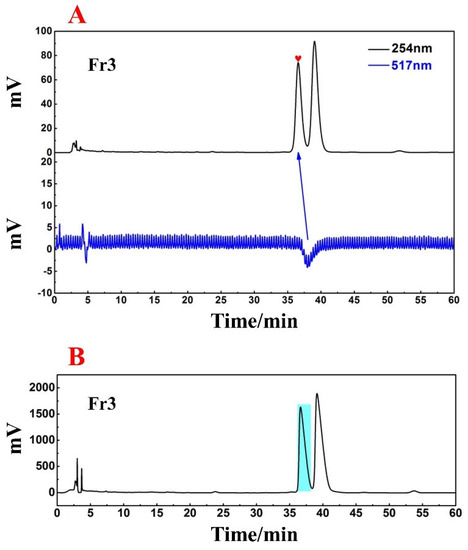
Figure 4.
Online HPLC−DPPH detection chromatogram of Fr3 (A); preparation chromatogram of Fr3 (B).
A high-pressure preparation of the active peak in Fr3 via final optimized chromatographic conditions with suitable parameters such as injection volume and flow rate were selected to obtain good reproducibility. Finally, the preparative chromatogram is shown in Figure 4B, and a light blue label was placed on the prepared target peak. A comparison of Figure 4A and B makes it evident that active chromatographic peaks have nearly identical retention times on the ReproSil-Pur C18 AQ analytical column and the ReproSil-Pur C18 AQ preparative column, ensuring an accurate and efficient recovery of target peaks. After 16 cycles of preparation, the eluted solutions were combined and concentrated to finally obtain the target compound, named Fr3−1 (406.7 mg, with a yield of approximately 40.7%).
3.3. Purity, Structural Characterization and Activity of the Isolated Compound
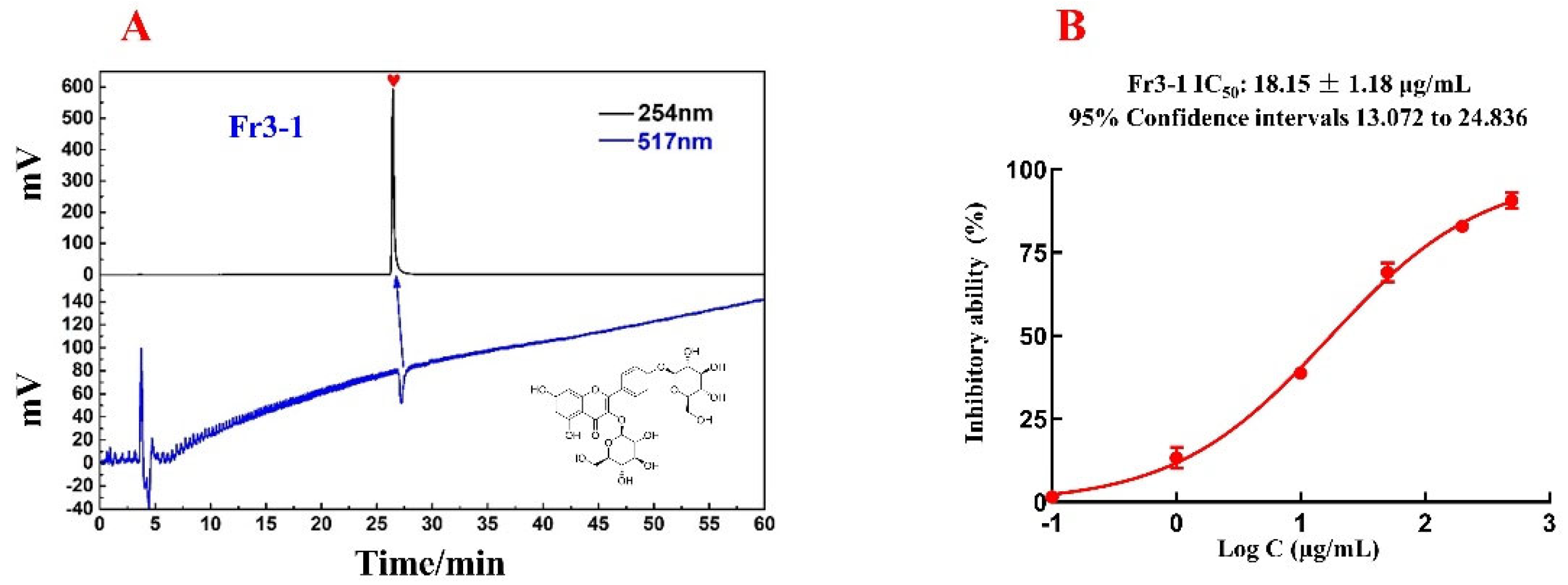
The activity and purity of the isolated compound Fr3−1 were determined via online HPLC-DPPH detection with the ReproSil-Pur C18 AQ analytical column. According to Figure 5A, the purity of Fr3−1 is over 95%.
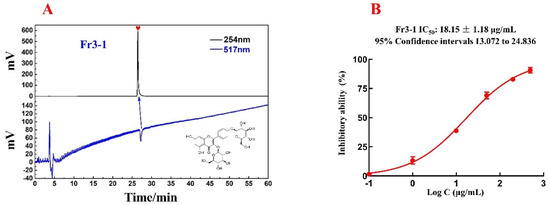
Figure 5.
Purity and activity verification chromatogram of Fr3−1, as well as its chemical structure (A); DPPH inhibition activity of Fr3−1 at different concentrations were fitted with a logistic function to count the IC50 value (B).
NMR and ESI-MS data were obtained to elucidate the structure of the isolated compound and were compared with the data reported in the literature. More detailed spectral data on this compound are available in the Supporting Information.
Fr3-1 (kaempferol 3,4′-di-O-β-D-glucopyranoside, yellow powder, 406.7 mg, ESI-MS m/z 609.45, [M-H]−, calc. For C27H30O16, m/z 610.15); 1H NMR (600 MHz, DMSO-d6): 8.12 (2H, d, J = 9.0 Hz, H-2′, 6′), 7.15 (2H, d, J = 9.0 Hz, H-3′, 5′), 6.46 (1H, d, J = 2.1 Hz, H-8), 6.22 (1H, d, J = 2.1 Hz, H-6), 5.48 (1H, d, J = 7.6 Hz, H-1″), 5.02 (1H, d, J = 7.4 Hz, H-1‴); 13C NMR(151 MHz, DMSO-d6): 177.6 (C-4), 164.3 (C-7), 161.3 (C-5), 159.3 (C-4′), 156.5 (C-2), 155.6 (C-9), 133.7 (C-3), 130.6 (C-2′, 6′), 123.7 (C-1′), 115.8 (C-3′, 5′), 104.1 (C-10), 100.8 (C-1″), 100.0 (C-1‴), 98.8 (C-6), 93.8 (C-8), 77.6 (C-5″), 77.1 (C-5‴), 76.5 (C-3″), 76.4 (C-3‴), 74.2 (C-2″), 73.2(C-2‴), 69.9 (C-4″), 69.6 (C-4‴), 60.9 (C-6″), 60.6 (C-6‴). The data were consistent with kaempferol 3,4′-di-O-β-D-glucopyranoside [29].
With the aim to evaluate in vitro DPPH scavenging activities of Fr3−1, a DPPH scavenging assay was used in this paper with minor modifications [30]. As shown in Figure 5B, the IC50 value of Fr3−1 was 18.15 μg/mL. Flavonoids are a well-recognized class of compounds with a wide range of biological activities. As a flavonoid with the parent nucleus kaempferol, kaempferol 3,4′-di-O-β-D-glucopyranoside showed significant antioxidant activity in this experiment, validating the reliability of the online HPLC−DPPH assay. In view of the correlation between oxidative stress and multiple diseases, this compound is expected to be actively explored in future studies for its potential to treat other diseases.
3.4. Molecular Docking
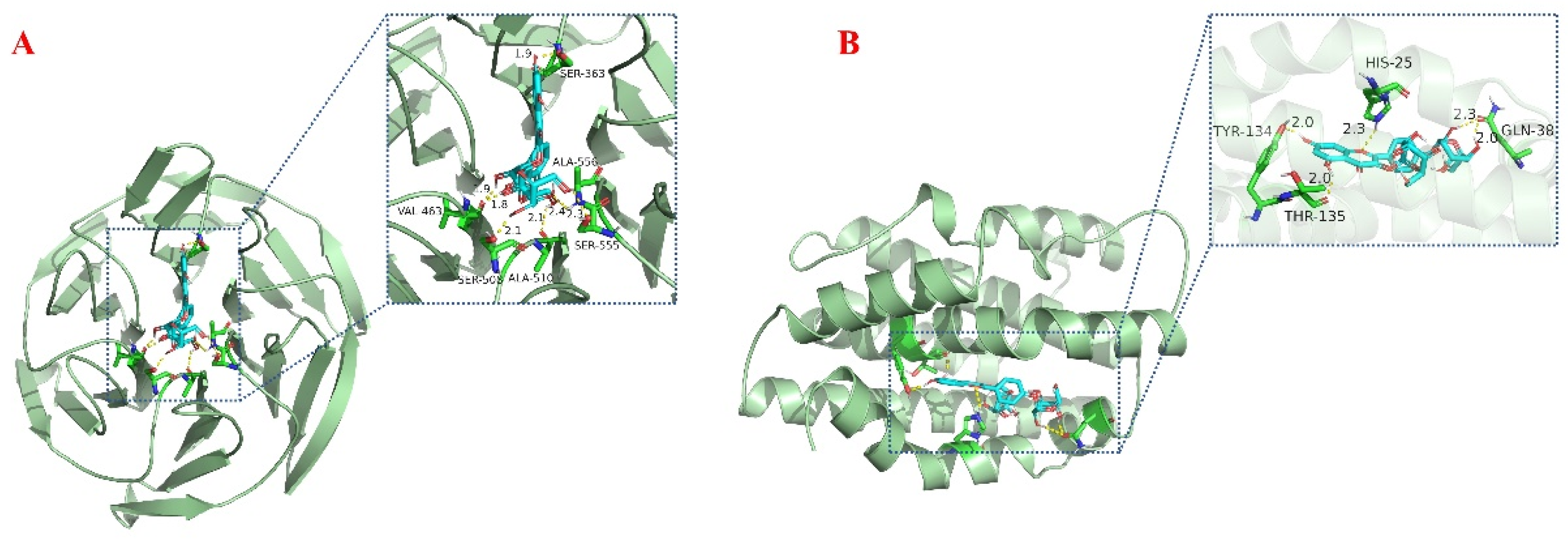
Oxidative stress is a complex pathological process involving several signaling pathways and related proteins. As one of the vital antioxidant pathways, Nrf2/HO-1 can regulate the expression of antioxidant enzymes that contribute to the restoration of the cellular redox state, and this pathway is also engaged in the regulation of inflammation, epilepsy, cerebral ischemia, and other related pathological processes. In this study, Fr3−1 was molecularly docked to Nrf2 and HO-1, and the results are visualized in Figure 6. The minimum binding energies of Fr3−1 to Nrf2 and HO-1 were −6.1 Kcal/mol and −5.34 Kcal/mol. For Nrf2, Fr3−1 was bound to a pocket with Ser363, Ala556, Ser555, Ala510, Ser 508, and Val463 as amino acid residues. For HO-1, Fr3−1 was located in a pocket of activity surrounded by multiple amino acids, His25, Gln38, Thr135, and Tyr134. Therefore, it is reasonable to speculate that Fr3−1 exerts its free radical scavenging activity through the Nrf2/HO-1 pathway. The antioxidant activity of this compound has been verified through chromatography, in vitro assays, and molecular docking. However, considering the complexity of the organism, its detailed and rigorous antioxidant mechanism needs to be elucidated by further cellular or animal experiments.
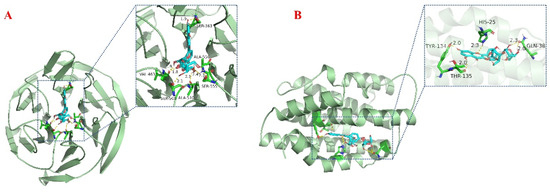
Figure 6.
Molecular docking analysis of Nrf2 (A) and HO-1 (B) during binding to Fr3-1.
4. Conclusions
The appropriate chromatographic strategy has a significant effect in coping with the isolation and purification of natural products’ active ingredients. In this study, online HPLC-DPPH detection was conducted for the qualitative identification of free radical inhibitors in rape pollen; MPLC pretreatment and HPLC preparation were employed for the isolation and purification of antioxidants from rape pollen. Finally, a free radical inhibitor (kaempferol 3,4′-di-O-β-D-glucopyranoside) was obtained from Fr3, its antioxidant capacity was detected through the DPPH method, and then the interaction between this compound and related proteins was theoretically investigated through a molecular docking experiment. This paper has demonstrated that the integrated chromatographic strategy of “online recognition, targeted enrichment and directed separation” is reliable and efficient for the rapid isolation of antioxidants from rape pollen and will provide theoretical support for future research on the deep processing and high value-added products of rape pollen. At the same time, the recognition and purification techniques of active compounds in other natural products can also be learned from this paper in the future.
Author Contributions
Methodology, L.J., H.L. and X.L.; data curation, L.J. and H.L.; funding acquisition, L.J., H.L. and J.H.; writing—original draft preparation, L.J. and H.L.; visualization, X.L. and K.G.; writing—review and editing, L.J. and M.W.; supervision, M.W. and J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Wuxi Taihu Talent Training Project (Double hundred Medical Youth Professionals Program, No. HB2020054), Scientific Research Project of Jiangsu Commission of Health (M2020005), Qinghai Basic Research Project (2020-ZJ747).
Data Availability Statement
All data are available on request from the authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, Z.; Cao, H.G.; Chen, C.; Chen, X.; Wei, Q.; Zhao, F.Y. Effects of fermentation by Ganoderma lucidum and Saccharomyces cerevisiae on rape pollen morphology and its wall. J. Food. Sci. Technol. 2017, 54, 4026–4034. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wang, S.; Wang, L.B.; Huang, D.J.; Chen, S.W. Identification and characterization of an angiotensin-I converting enzyme inhibitory peptide from enzymatic hydrolysate of rape (Brassica napus L.) bee pollen. LWT Food. Sci. Technol. 2021, 147, 111502. [Google Scholar] [CrossRef]
- Ares, A.M.; Valverde, S.; Bernal, J.L.; Nozal, M.J.; Bernal, J. Extraction and determination of bioactive compounds from bee pollen. J. Pharmaceut. Biomed. 2018, 147, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.L.; Yang, Y.N.; He, J.; Zhang, Z.W.; Zhang, P.C. Two new furan derivatives from bee-collected rape pollen. J. Asian Nat. Prod. Res. 2011, 13, 930–933. [Google Scholar] [CrossRef] [PubMed]
- Rzepecka-Stojko, A.; Stojko, J.; Jasik, K.; Buszman, E. Anti-atherogenic activity of polyphenol-rich extract from bee pollen. Nutrients 2017, 12, 1369. [Google Scholar] [CrossRef]
- Cheng, N.; Chen, S.N.; Liu, X.Y.; Zhao, H.A.; Cao, W. Impact of schisandrachinensis bee pollen on nonalcoholic fatty liver disease and gut microbiota in high fat diet induced obese mice. Nutrients 2019, 2, 346. [Google Scholar] [CrossRef]
- Liao, Y.L.; Bae, H.J.; Zhang, J.B.; Kwon, Y.B.; Koo, B.; Jung, I.H.; Kim, H.M.; Park, J.H.; Lew, J.H.; Ryu, J.H. The ameliorating effects of bee pollen on scopolamine-induced cognitive impairment in mice. Biol. Pharm. Bull. 2019, 3, 379–388. [Google Scholar] [CrossRef]
- Lv, H.H.; Wang, X.Y.; He, Y.F.; Wang, H.L.; Suo, Y.R. Identification and quantification of flavonoid aglycones in rape bee pollen from Qinghai-Tibetan Plateau by HPLC-DAD-APCI/MS. J. Food Compos. Anal. 2015, 38, 49–54. [Google Scholar] [CrossRef]
- Fang, H.L.; Zhang, J.; Zhao, Y.W.; Han, N.J.; Wang, W.D.; Li, Y. Antioxidant and anti-aging mechanism of wall broken rape pollen. Sci. Technol. Food Ind. 2022, 43, 378–384. [Google Scholar] [CrossRef]
- Hao, Z. The effect and research progress of rape pollen. Cereal. Oil 2020, 8, 4–6. Available online: https://lsyy.cbpt.cnki.net/WKC2/WebPublication/paperDigest.aspx?paperID=8dc2904d-5c5a-4f17-b76d-5f3d6b709ec0# (accessed on 20 October 2022).
- Yao, Q.P.; Li, J.; Hu, J.; Huang, Y.F. Studies on quality evaluation of rape pollen based on HPLC fingerprint. Zhongguo Shipin Xuebao 2012, 8, 203–209. [Google Scholar] [CrossRef]
- Xu, X.; Sun, L.P.; Dong, J.; Zhang, H.C. Breaking the cells of rape bee pollen and consecutive extraction of functional oil with supercritical carbon dioxide. Innov. Food. Sci. Emerg. 2009, 10, 42–46. [Google Scholar] [CrossRef]
- Aceto, G.; Persico, V.; Pescapé, A. Industry 4.0 and health: Internet of things, big data, and cloud computing for healthcare 4.0. J. Ind. Inf. Integr. 2020, 18, 100129. [Google Scholar] [CrossRef]
- Jadalla, B.M.I.S.; Moser, J.J.; Sharma, R.; Etsassala, N.G.E.R.; Egieyeh, S.A.; Badmus, J.A.; Marnewick, J.L.; Beukes, D.; Cupido, C.N.; Hussein, A.A. In vitro alpha-glucosidase and alpha-amylase inhibitory activities and antioxidant capacity of Helichrysum cymosum and Helichrysum pandurifolium schrank constituents. Separations 2022, 8, 190. [Google Scholar] [CrossRef]
- Viñas, G.; Puig, T.; Porta, R. Oxidative stress in patients with cancer: Two sides of the same coin. Med. Clin. 2012, 4, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Zhou, Y.X.; Gao, Q.N.; Ping, D.N.; Wang, Y.L.; Wu, W.; Lin, X.; Fang, Y.J.; Zhang, J.M.; Shao, A.W. The role of exosomal microRNAs and oxidative stress in neurodegenerative diseases. Oxid. Med. Cell Longev. 2020, 4, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Salmon, A.B.; Richardson, A.; Pérez, V.I. Update on the oxidative stress theory of aging: Does oxidative stress play a role in aging or healthy aging? Free. Radic. Biol. Med. 2010, 5, 642–655. [Google Scholar] [CrossRef]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.; Harakeh, S.; et al. Oxidative stress in human pathology and aging: Molecular mechanisms and perspectives. Cells 2022, 3, 552. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. Oxidative stress-induced hypertension of developmental origins: Preventive aspects of antioxidant therapy. Antioxidants 2022, 3, 511. [Google Scholar] [CrossRef]
- Hirschenson, J.; Melgar-Bermudez, E.; Mailloux, R.J. The uncoupling proteins: A systematic review on the mechanism used in the prevention of oxidative stress. Antioxidants 2022, 2, 322. [Google Scholar] [CrossRef]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free radical properties, source and targets, antioxidant consumption and health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Agraharam, G.; Girigoswami, A.; Girigoswami, K. Myricetin: A multifunctional flavonol in biomedicine. Curr. Pharmacol. Rep. 2022, 8, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Dawa, Y.; Du, Y.R.; Wang, Q.; Chen, C.B.; Zou, D.L.; Qi, D.S.; Ma, J.B.; Dang, J. Targeted isolation of 1, 1-diphenyl-2-picrylhydrazyl inhibitors from Saxifraga atrata using medium-and high-pressure liquid chromatography combined with online high performance liquid chromatography-1,1-diphenyl-2-picrylhydrazyl detection. J. Chromatogr. A 2021, 1635, 461690. [Google Scholar] [CrossRef]
- Liu, C.; Lei, Y.Q.; Dang, J.; Wang, W.D.; Zhang, L.; Mei, L.J.; Liu, Z.G.; Tao, Y.D.; Shao, Y. Preparative isolation of 1, 1-diphenyl-2-picrylhydrazyl inhibitors from Ribes himalense using medium-pressure and two-dimensional reversed-phase/reversed-phase liquid chromatography guided by an online HPLC-1,1-diphenyl-2-picrylhydrazyl assay. J. Sep. Sci. 2021, 7, 1345–1352. [Google Scholar] [CrossRef]
- Dang, J.; Ma, J.B.; Dawa, Y.; Liu, C.; Ji, T.F.; Wang, Q.L. Preparative separation of 1, 1-diphenyl-2-picrylhydrazyl inhibitors originating from Saxifraga sinomontana employing medium-pressure liquid chromatography in combination with reversed-phase liquid chromatography. RSC Adv. 2021, 61, 38739–38749. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Dang, J.; Han, Y.; Liu, C.; Yu, S.; Lv, Y.; Cui, Y.B.; Wang, Z.H.; Li, G. Preparative isolation of maltol glycoside from Dianthus superbus and its anti-inflammatory activity in vitro. RSC Adv. 2022, 8, 5031–5041. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Liang, Q.L.; Ping, H.; Wang, Y.M.; Jun, F.W.; Luo, G.A. Combination of normal-phase medium-pressure liquid chromatography and high-performance counter-current chromatography for preparation of ginsenoside-Ro from panax ginseng with high recovery and efficiency. Sep. Purif. Technol. 2010, 3, 397–402. [Google Scholar] [CrossRef]
- Li, Y.J.; Lin, H.M.; Zhang, J.B.; Deng, X.; Li, J.A. An efficient procedure for preparing high-purity pingyangmycin and boanmycin from Streptomyces verticillus var. pingyangensis fermentation broth via macroporous cation-exchange resin and subsequent reversed-phase preparative chromatography. J. Chromatogr. B 2020, 1136, 121883. [Google Scholar] [CrossRef]
- Feng, Z.F.; Chen, X.F.; Di, D.L. Online extraction and isolation of highly polar chemical constituents from Brassica napus L. pollen by high shear technique coupled with high-performance counter-current chromatography. J. Sep. Sci. 2012, 35, 625–632. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, Z.; Wu, Q.; Fang, Y.; Wang, Q.L.; Li, G.; Dang, J. Preparation and Antioxidant Activities of Phenylethanoids from Dracocephalum heterophyllum. Separations 2022, 5, 111. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).