Nutrient Composition, Antioxidant Activities and Glycaemic Response of Instant Noodles with Wood Ear Mushroom (Auricularia cornea) Powder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Proximate Composition Analysis
2.3. Total Dietary Fibre (TDF)
2.4. Mineral Analysis
2.5. Antioxidant Activities Analysis
2.6. Glycaemic Analysis (Blood Glucose Measurement and Calculation of Glycaemic Index)
2.7. Data Analysis
3. Results and Discussion
3.1. Proximate Composition
3.2. Total Dietary Fibre (TDF)
3.3. Mineral Analysis
3.4. Antioxidant Activities and Total Phenolic Contents (TPC)
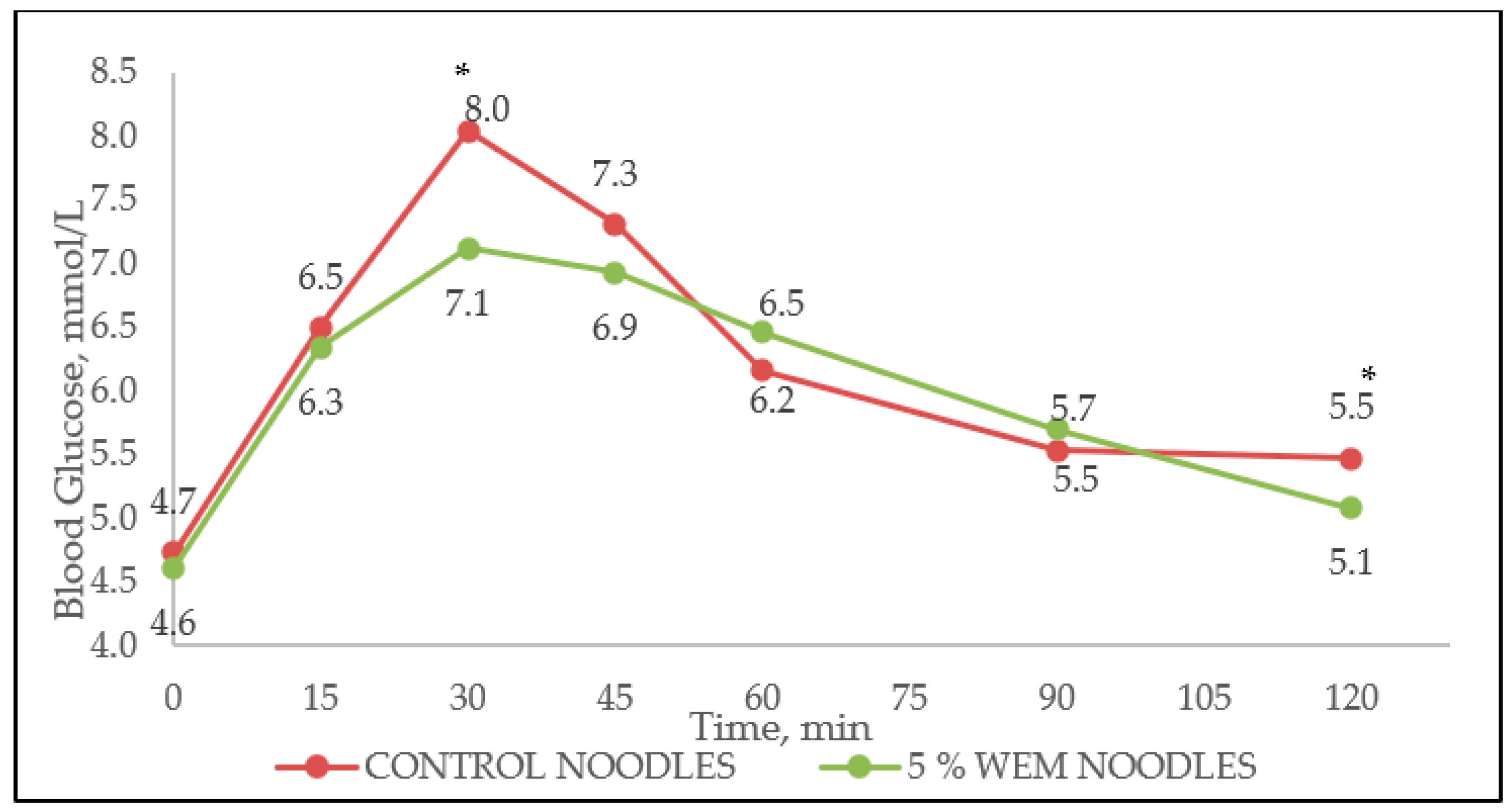
3.5. Glycaemic Index
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Priya, R.U.; Gethaa, D.; Darshan, S. Biology and Cultivation of Black Ear Mushroom—Auricularia spp. Adv. Life Sci. 2016, 5, 10252–10254. [Google Scholar]
- Wu, F.; Tohtirjap, A.; Fan, L.F.; Zhou, L.W.; Alvarenga, R.L.M.; Gibertoni, T.B.; Dai, Y.C. Global Diversity and Updated Phylogeny of Auricularia (Auriculariales, Basidiomycota). J. Fungi 2021, 7, 933. [Google Scholar] [CrossRef] [PubMed]
- Bandara, A.R.; Rapior, S.; Mortimer, P.E.; Kakumyan, P.; Hyde, K.D.; Xu, J. A Review of the Polysaccharide, Protein and Selected Nutrient Content of Auricularia, and Their Potential Pharmacological Value. Mycosphere 2019, 10, 579–607. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Jakovljevic, D.; Todorovic, N.; Vunduk, J.; Petrović, P.; Niksic, M.; Vrvic, M.M.; van Griensven, L. Antioxidants of Edible Mushrooms. Molecules 2015, 20, 19489–19525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avci, E.; Cagatay, G.; Alp Avci, G.; Suicmez, M.; Coskun Cevher, S. An Edible Mushroom With Medicinal Significance; Auricularia polytricha. Hittite J. Sci. Eng. 2016, 3, 111–116. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Yao, X.-P.; Liu, B.; Reheman, H.N.; Gao, Y.; Sun, Z.; Ma, Q. Auricularia auricula-judae Polysaccharide Attenuates Lipopolysaccharide-Induced Acute Lung Injury by Inhibiting Oxidative Stress and Inflammation. Biomed. Rep. 2015, 3, 478–482. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zeng, Y.; Men, Y.; Zhang, J.; Liu, H.; Sun, Y. Structural Characterization and Immunomodulatory Activity of Exopolysaccharides from Submerged Culture of Auricularia auricula-judae. Int. J. Biol. Macromol. 2018, 115, 978–984. [Google Scholar] [CrossRef]
- De Silva, D.D.; Rapior, S.; Hyde, K.D.; Bahkali, A.H. Medicinal Mushrooms in Prevention and Control of Diabetes Mellitus. Fungal Divers 2012, 56, 1–29. [Google Scholar] [CrossRef]
- Chiu, W.C.; Yang, H.H.; Chiang, S.C.; Chou, Y.X.; Yang, H.T. Auricularia polytricha Aqueous Extract Supplementation Decreases Hepatic Lipid Accumulation and Improves Antioxidative Status in Animal Model of Nonalcoholic Fatty Liver. Biomed. Pharmacother. 2014, 4, 29–38. [Google Scholar] [CrossRef]
- Hyde, K.D.; Xu, J.; Rapior, S.; Jeewon, R.; Lumyong, S.; Niego, A.G.T.; Abeywickrama, P.D.; Aluthmuhandiram, J.V.S.; Brahamanage, R.S.; Brooks, S.; et al. The Amazing Potential of Fungi: 50 Ways We Can Exploit Fungi Industrially. Fungal Divers. 2019, 97, 1–136. [Google Scholar] [CrossRef] [Green Version]
- Gulia, N.; Dhaka, V.; Khatkar, B.S. Instant Noodles: Processing, Quality, and Nutritional Aspects. Crit Rev Food Sci Nutr 2014, 54, 1386–1399. [Google Scholar] [CrossRef] [PubMed]
- Adejuwon, O.H.; Jideani, A.I.O.; Falade, K.O. Quality and Public Health Concerns of Instant Noodles as Influenced by Raw Materials and Processing Technology. Food Rev. Int. 2020, 36, 276–317. [Google Scholar] [CrossRef]
- Arora, B.; Kamal, S.; Sharma, V.P. Nutritional and Quality Characteristics of Instant Noodles Supplemented with Oyster Mushroom (P. ostreatus). J Food Process. Preserv. 2018, 42, e13521. [Google Scholar] [CrossRef]
- Wahyono, A.; Novianti; Bakri, A. Kasutjianingati Physicochemical and Sensorial Characteristics of Noodle Enriched with Oyster Mushroom (Pleorotus ostreatus) Powder. J. Phys. Conf. Ser. 2018, 953, 012120. [Google Scholar] [CrossRef]
- Parvin, R.; Farzana, T.; Mohajan, S.; Rahman, H.; Rahman, S.S. Quality Improvement of Noodles with Mushroom Fortified and Its Comparison with Local Branded Noodles. NFS J. 2020, 20, 37–42. [Google Scholar] [CrossRef]
- Heo, S.; Jeon, S.; Lee, S. Utilization of Lentinus edodes Mushroom β-Glucan to Enhance the Functional Properties of Gluten-Free Rice Noodles. LWT Food Sci. Technol. 2014, 55, 627–631. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, H.; Brennan, M.; Guan, W.; Liu, J.; Wang, M.; Wen, X.; He, J.; Brennan, C. In Vitro Gastric Digestion Antioxidant and Cellular Radical Scavenging Activities of Wheat-Shiitake Noodles; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; Volume 330, ISBN 0000000242943. [Google Scholar]
- Wang, J.; Brennan, M.A.; Serventi, L.; Stephen, C.; Wang, J.; Brennan, M.A.; Serventi, L.; Stephen, C. Impact of Functional Vegetable Ingredients on the Technical and Nutritional Quality of Pasta. Crit. Rev. Food Sci. Nutr. 2022, 62, 6069–6080. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, H.; Zong, X.; Li, S.; Wang, J.; Wang, Y.; Jin, M. Polysaccharides from Auricularia auricula: Preparation, Structural Features and Biological Activities. Carbohydr. Polym. 2020, 247, 116750. [Google Scholar] [CrossRef]
- Hu, X.; Liu, C.; Wang, X.; Jia, D.; Lu, W.; Sun, X.; Liu, Y.; Yuan, L. Hpyerglycemic and Anti-Diabetic Nephritis Activities of Polysaccharides Separated from Auricularia auricular in Diet-Streptozotocin-Induced Diabetic Rats. Exp. Med. 2017, 13, 352–358. [Google Scholar] [CrossRef] [Green Version]
- Lu, A.; Yu, M.; Shen, M.; Xu, S.; Xu, Z.; Zhang, Y.; Lin, Z.; Wang, W. Preparation of the Auricularia auricular Polysaccharides Simulated Hydrolysates and Their Hypoglycaemic Effect. Int. J. Biol. Macromol. 2018, 106, 1139–1145. [Google Scholar] [CrossRef]
- Shen, M.; Fang, Z.; Chen, Y.; Xiao, B.; Guo, L.; Xu, Y.; Wang, G.; Wang, W.; Zhang, Y. Hypoglycemic Effect of the Degraded Polysaccharides from the Wood Ear Medicinal Mushroom Auricularia auricula-judae ( Agaricomycetes). Int. J. Med. Mushrooms 2019, 21, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Vallée, M.; Lu, X.; Narciso, J.O.; Li, W.; Qin, Y.; Brennan, M.A.; Brennan, C.S. Physical, Predictive Glycaemic Response and Antioxidative Properties of Black Ear Mushroom (Auricularia auricula) Extrudates. Plant Foods Hum. Nutr. 2017, 72, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.J.; Chiou, F.J.; Weng, Y.M.; Yu, Z.R.; Wang, B.J. In Vitro Hypoglycemic Effects of Hot Water Extract from Auricularia polytricha (Wood Ear Mushroom). Int. J. Food Sci. Nutr. 2014, 65, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, D.S.; Zakaria, M.K.; George, R.; Matanjun, P. Sensory Evaluation and Nutrient Composition of Noodles Enriched with Wood Ear Mushroom (Auricularia polytricha) Powder. Trans. Sci. Technol. 2021, 8, 172–177. [Google Scholar]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Analytical Communities: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Kalač, P. A Review of Chemical Composition and Nutritional Value of Wild-Growing and Cultivated Mushrooms. J. Sci. Food Agric. 2013, 93, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Wen Yuh, L.; Mars, J.Y.; Lien Tsung, H.; Liang Chuan, L. Antioxidant Properties of Methanol Extract of a New Commercial Gelatinous Mushrooms (White Variety of Auricularia fuscosuccinea) of Taiwan. Afr. J. Biotechnol. 2013, 12, 6210–6221. [Google Scholar] [CrossRef] [Green Version]
- Teoh, H.L.; Ahmad, I.S.; Johari, N.M.K.; Aminudin, N.; Abdullah, N. Antioxidant Properties and Yield of Wood Ear Mushroom, Auricularia polytricha (Agaricomycetes), Cultivated on Rubberwood Sawdust. Int. J. Med. Mushrooms 2018, 20, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Yim, H.S.; Chye, F.Y.; Koo, S.M.; Matanjun, P.; How, S.E.; Ho, C.W. Optimization of Extraction Time and Temperature for Antioxidant Activity of Edible Wild Mushroom, Pleurotus porrigens. Food Bioprod. Process. 2012, 90, 235–242. [Google Scholar] [CrossRef]
- Wolever, T.M.S.; Meynier, A.; Jenkins, A.L.; Brand-Miller, J.C.; Atkinson, F.S.; Gendre, D.; Leuillet, S.; Cazaubiel, M.; Housez, B.; Vinoy, S. Glycemic Index and Insulinemic Index of Foods: An Interlaboratory Study Using the ISO 2010 Method. Nutrients 2019, 11, 2218. [Google Scholar] [CrossRef] [Green Version]
- Urbaniak, G.C.; Plous, S. Research Randomizer (Version 4.0) [Computer Software]. Available online: http://www.randomizer.org/ (accessed on 1 June 2021).
- Ballance, S.; Knutsen, S.H.; Fosvold, Ø.W.; Fernandez, A.S.; Monro, J. Predicting Mixed-Meal Measured Glycaemic Index in Healthy Subjects. Eur. J. Nutr. 2019, 58, 2657–2667. [Google Scholar] [CrossRef]
- Cheung, P.C.K. Mini-Review on Edible Mushrooms as Source of Dietary Fiber: Preparation and Health Benefits. Food Sci. Hum. Wellness 2013, 2, 162–166. [Google Scholar] [CrossRef] [Green Version]
- Stachowiak, B.; Reguła, J. Health-Promoting Potential of Edible Macromycetes under Special Consideration of Polysaccharides: A Review. Eur. Food Res. Technol. 2012, 234, 369–380. [Google Scholar] [CrossRef]
- Assemie, A.; Abaya, G. The Effect of Edible Mushroom on Health and Their Biochemistry. Int. J. Microbiol. 2022, 2022, 8744788. [Google Scholar] [CrossRef] [PubMed]
- Samsudin, N.I.P.; Abdullah, N. Edible Mushrooms from Malaysia; a Literature Review on Their Nutritional and Medicinal Properties. Int Food Res J 2019, 26, 11–31. [Google Scholar]
- Islam, T.; Ganesan, K.; Xu, B. Insights into Health-Promoting Effects of Jew’s Ear (Auricularia auricula-judae). Trends Food Sci. Technol. 2021, 114, 552–569. [Google Scholar] [CrossRef]
- Yao, H.; Liu, Y.; Ma, Z.F.; Zhang, H.; Fu, T.; Li, Z.; Li, Y.; Hu, W.; Han, S.; Zhao, F.; et al. Analysis of Nutritional Quality of Black Fungus Cultivated with Corn Stalks. J. Food Qual. 2019, 2019, 9590251. [Google Scholar] [CrossRef]
- Kadnikova, I.A.; Costa, R.; Kalenik, T.K.; Guruleva, O.N.; Yanguo, S. Chemical Composition and Nutritional Value of the Mushroom Auricularia auricula-judae. J. Food Nutr. Res. 2015, 3, 478–482. [Google Scholar] [CrossRef]
- Afiukwa, C.A.; Ebem, E.C.; Igwe, D.O. Characterization of the Proximate and Amino Acid Composition of Edible Wild Mushroom Species in Abakaliki, Nigeria. Am. Assoc. Sci. Technol. J. Biosci. 2015, 1, 20–25. [Google Scholar]
- Liu, Y.T.; Sun, J.; Luo, Z.Y.; Rao, S.Q.; Su, Y.J.; Xu, R.R.; Yang, Y.J. Chemical Composition of Five Wild Edible Mushrooms Collected from Southwest China and Their Antihyperglycemic and Antioxidant Activity. Food Chem. Toxicol. 2012, 50, 1238–1244. [Google Scholar] [CrossRef]
- Hung, P.V.; Nhi, N.N.Y. Nutritional Composition and Antioxidant Capacity of Several Edible Mushrooms Grown in the Southern Vietnam. Int. Food Res. J. 2012, 19, 611–615. [Google Scholar]
- Ao, T.; Deb, C.R. Nutritional and Antioxidant Potential of Some Wild Edible Mushrooms of Nagaland, India. J. Food Sci. Technol. 2019, 56, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Dong Sun, X.; Chun Yang, H.; Yuan Liu, F.; Wang, B.; Chai, L.; Luan, J. Proximate Compositions and Bioactive Compounds of Cultivated and Wild Auricularia auricular from Northeastern China. Eur. J. Nutr. Food Saf. 2019, 11, 175–186. [Google Scholar] [CrossRef]
- Obodai, M.; Ferreira, I.C.F.R.; Fernandes, Â.; Barros, L.; Narh Mensah, D.L.; Dzomeku, M.; Urben, A.F.; Prempeh, J.; Takli, R.K. Evaluation of the Chemical and Antioxidant Properties of Wild and Cultivated Mushrooms of Ghana. Molecules 2014, 19, 19532–19548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.; Lou, H.; Hu, J.; Liu, Z.; Chen, Q. Macrofungi: A Review of Cultivation Strategies, Bioactivity, and Application of Mushrooms. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2333–2356. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Yao, F.; Idrees, M.; Lu, L.; Fang, M.; Wang, P.; Jiang, W.Z.; Zhang, Y.M. A Comparative Study of Growth, Biological Efficiency, Antioxidant Activity and Molecular Structure in Wild and Commercially Cultivated Auricularia cornea Strains. Folia Hortic. 2020, 32, 255–264. [Google Scholar] [CrossRef]
- Srikram, A.; Supapvanich, S. Proximate Compositions and Bioactive Compounds of Edible Wild and Cultivated Mushrooms from Northeast Thailand. Agric. Nat. Resour. 2016, 50, 432–436. [Google Scholar] [CrossRef]
- Thatoi, H.; Singdevsachan, S.K. Diversity, Nutritional Composition and Medicinal Potential of Indian Mushrooms: A Review. Afr J Biotechnol 2014, 13, 523–545. [Google Scholar] [CrossRef]
- Yadav, D.; Negi, P.S. Bioactive Components of Mushrooms: Processing Effects and Health Benefits. Food Res. Int. 2021, 148, 110599. [Google Scholar] [CrossRef]
- Sikander, M.; Malik, A.; Khan, M.S.G.; Qurratul-ain; Khan, R.G. Instant Noodles: Are They Really Good for Health? A Review. A Review. Electron. J Biol 2017, 13, 222–227. [Google Scholar]
- Shams, R.; Jammu, T.; Singh, J.; Rajaram, S.; Hospital, E.; Dar, A.H. Assessment of Shelf Stability of Noodles Fortified with Button Mushroom and Chickpea Starch. J. Postharvest. Technol. 2022, 10, 122–133. [Google Scholar]
- Wandee, Y.; Uttapap, D.; Puncha-arnon, S.; Puttanlek, C.; Rungsardthong, V.; Wetprasit, N. Enrichment of Rice Noodles with Fibre-Rich Fractions Derived from Cassava Pulp and Pomelo Peel. Int. J. Food Sci. Technol. 2014, 49, 2348–2355. [Google Scholar] [CrossRef]
- Khursheed, R.; Singh, S.K.; Wadhwa, S.; Gulati, M.; Awasthi, A. Therapeutic Potential of Mushrooms in Diabetes Mellitus: Role of Polysaccharides. Int. J. Biol. Macromol. 2020, 164, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary Fibre in Foods: A Review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Ekissi, A.C.; Kouame, K.B.; Niaba, K.P.V.; Beugre, G.A.M.; Kati-Coulibaly, S. Physicochemical Characterization of Two Species of Wild Edible Mushrooms: Lentinus brunneofloccosus pegler and Auricularia Auricularia-judae. Food Nutr. Sci. 2021, 12, 319–331. [Google Scholar] [CrossRef]
- Bandara, A.R.; Karunarathna, S.C.; Mortimer, P.E.; Hyde, K.D.; Khan, S.; Kakumyan, P.; Xu, J. First Successful Domestication and Determination of Nutritional and Antioxidant Properties of the Red Ear Mushroom Auricularia thailandica (Auriculariales, Basidiomycota). Mycol. Prog. 2017, 16, 1029–1039. [Google Scholar] [CrossRef]
- Ache, T.N.; Bi, M.E.; Ndam, L.M.; Kinge, T.R. Nutrient and Mineral Components of Wild Edible Mushrooms from the Kilum-Ijim Forest, Cameroon. Afr. J. Food Sci. 2021, 15, 152–161. [Google Scholar] [CrossRef]
- Ibrahium, M.I.; Hegazy, A.I. Effect of Replacement of Wheat Flour with Mushroom Powder and Sweet Potato Flour on Nutritional Composition and Sensory Characteristics of Biscuits. Researchgate.Net 2014, 3, 8. [Google Scholar]
- Liu, E.; Ji, Y.; Zhang, F.; Liu, B.; Meng, X. Review on Auricularia auricula-judae as a Functional Food: Growth, Chemical Composition, and Biological Activities. J. Agric. Food Chem. 2021, 69, 1739–1750. [Google Scholar] [CrossRef]
- Mohammadifard, N.; Humphries, K.H.; Gotay, C.; Mena-Sánchez, G.; Salas-Salvadó, J.; Esmaillzadeh, A.; Ignaszewski, A.; Sarrafzadegan, N. Trace Minerals Intake: Risks and Benefits for Cardiovascular Health. Crit. Rev. Food Sci. Nutr. 2019, 59, 1334–1346. [Google Scholar] [CrossRef]
- Freeland-Graves, J.H.; Sachdev, P.K.; Binderberger, A.Z.; Sosanya, M.E. Global Diversity of Dietary Intakes and Standards for Zinc, Iron, and Copper. J. Trace Elem. Med. Biol. 2020, 61, 126515. [Google Scholar] [CrossRef]
- Chan, P.T.; Matanjun, P.; Yasir, S.M.; Tan, T.S. Antioxidant Activities and Polyphenolics of Various Solvent Extracts of Red Seaweed, Gracilaria changii. J. Appl. Phycol. 2015, 27, 2377–2386. [Google Scholar] [CrossRef]
- Lu, X.; Brennan, M.A.; Serventi, L.; Liu, J.; Guan, W.; Brennan, C.S. Addition of Mushroom Powder to Pasta Enhances the Antioxidant Content and Modulates the Predictive Glycaemic Response of Pasta. Food Chem. 2018, 264, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Boonsong, S.; Klaypradit, W.; Wilaipun, P. Antioxidant Activities of Extracts from Five Edible Mushrooms Using Different Extractants. Agric. Nat. Resour. 2016, 50, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Kumar, H.; Bhardwaj, K.; Sharma, R.; Nepovimova, E.; Cruz-martins, N.; Dhanjal, D.S.; Singh, R.; Chopra, C.; Verma, R.; Abd-elsalam, K.A.; et al. Potential Usage of Edible Mushrooms and Their Residues to Retrieve Valuable Supplies for Industrial Applications. J. Fungi 2021, 7, 427. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Vargas, E.; Rodriguez, J.A.; Domínguez, R.; Lorenzo, J.M.; Sosa, M.E.; Andrés, S.C.; Rosmini, M.; Pérez-Alvarez, J.A.; Teixeira, A.; Santos, E.M. Edible Mushrooms as a Natural Source of Food Ingredient/Additive Replacer. Foods 2021, 10, 2687. [Google Scholar] [CrossRef]
- Gull, A.; Prasad, K.; Kumar, P. Nutritional, Antioxidant, Microstructural and Pasting Properties of Functional Pasta. J. Saudi Soc. Agric. Sci. 2018, 17, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Choy, A.; Morrison, P.D.; Hughes, J.G.; Marriott, P.J.; Small, D.M. Quality and Antioxidant Properties of Instant Noodles Enhanced with Common Buckwheat Flour. J Cereal Sci 2013, 57, 281–287. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Chen, J.; Hu, Y.; Wang, D.; Fan, Y.; Wang, J.; Abula, S.; Zhang, J.; Qin, T.; Chen, X.; et al. In Vitro Antiviral Activity of Sulfated Auricularia auricula Polysaccharides. Carbohydr. Polym. 2012, 90, 1254–1258. [Google Scholar] [CrossRef]
- Su, Y.; Li, L. Structural Characterization and Antioxidant Activity of Polysaccharide from Four Auriculariales. Carbohydr Polym 2020, 229, 115407. [Google Scholar] [CrossRef]
- Giacco, R.; Costabile, G.; Riccardi, G. Metabolic Effects of Dietary Carbohydrates: The Importance of Food Digestion. Food Res. Int. 2016, 88, 336–341. [Google Scholar] [CrossRef]
- Scazzina, F.; Siebenhandl-Ehn, S.; Pellegrini, N. The Effect of Dietary Fibre on Reducing the Glycaemic Index of Bread. Br. J. Nutr. 2013, 109, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Ngugi, M.P.; Njagi, J.; Kibiti, C.; Ngeranwa, J.J.N. The Role of Vitamins and Mineral Elements in Management of Type 2 Diabetes Mellitus: A Review. South As. J. Biol. Sci. 2012, 2, 107–115. [Google Scholar]
- Roupas, P.; Keogh, J.; Noakes, M.; Margetts, C.; Taylor, P. The Role of Edible Mushrooms in Health: Evaluation of the Evidence. J. Funct. Foods 2012, 4, 687–709. [Google Scholar] [CrossRef]
- Matthan, N.R.; Ausman, L.M.; Meng, H.; Tighiouart, H.; Lichtenstein, A.H. Estimating the Reliability of Glycemic Index Values and Potential Sources of Methodological and Biological Variability. Am. Soc. Nutr. Downloaded 2016, 104, 1004–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, H.L.; Bae, I.Y.; Lee, H.G. In Vitro Starch Digestibility of Noodles with Various Cereal Flours and Hydrocolloids. LWT Food Sci. Technol. 2015, 63, 122–128. [Google Scholar] [CrossRef]
- Bharath Kumar, S.; Prabhasankar, P. A Study on Noodle Dough Rheology and Product Quality Characteristics of Fresh and Dried Noodles as Influenced by Low Glycemic Index Ingredient. J. Food Sci. Technol. 2015, 52, 1404–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bello-perez, L.A.; Flores-Silva, P.C.; Sifuentes-Nieves, I.; Agama-Acevedo, E. Controlling Starch Digestibility and Glycaemic Response in Maize-Based Foods. J. Cereal. Sci. 2021, 99, 103222. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hobl, L.P.; Hoffmann, G. Effects of Low Glycaemic Index/Low Glycaemic Load vs. High Glycaemic Index/ High Glycaemic Load Diets on Overweight/Obesity and Associated Risk Factors in Children and Adolescents: A Systematic Review and Meta-Analysis. Nutr. J. 2015, 14, 87. [Google Scholar] [CrossRef] [Green Version]
- Mirrahimi, A.; Chiavaroli, L.; Srichaikul, K.; Augustin, L.S.A.; Sievenpiper, J.L.; Kendall, C.W.C.; Jenkins, D.J.A. The Role of Glycemic Index and Glycemic Load In Cardiovascular Disease And Its Risk Factors: A Review of The Recent Literature. Curr. Atheroscler. Rep. 2014, 16, 381. [Google Scholar] [CrossRef]
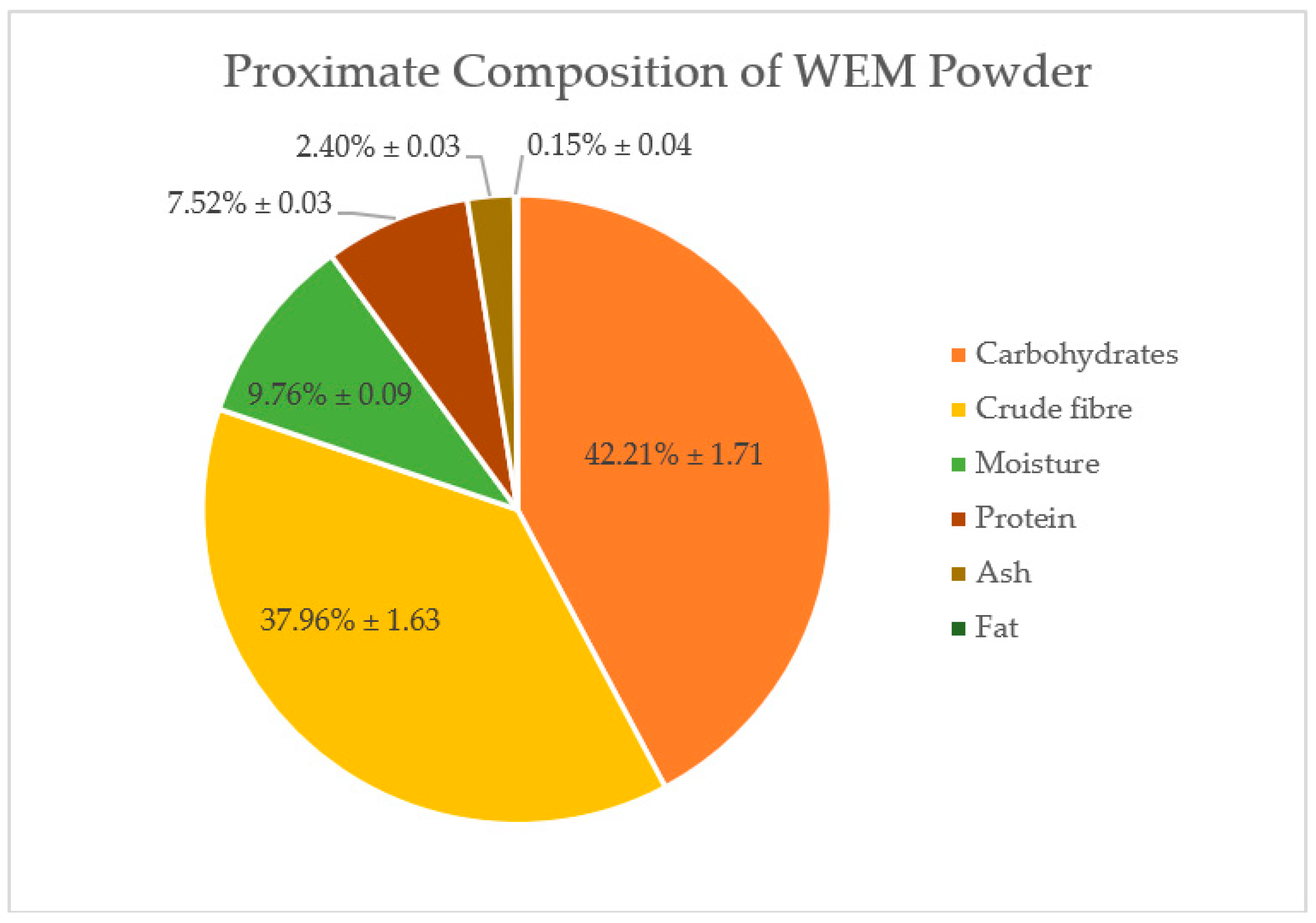
| Instant Noodles | Proximate Composition (%) | |||||
|---|---|---|---|---|---|---|
| Moisture | Ash | Protein | Fat | Crude Fibre | Carbohydrate | |
| Control | 10.61 ± 0.18 a | 1.36 ± 0.02 b | 11.86± 0.01 a | 0.21 ± 0.04 a | 3.36 ± 0.25 b | 72.70 ± 0.32 a |
| 5% WEM | 10.47 ± 0.45 a | 2.87 ± 0.03 a | 11.37 ± 0.06 a | 0.16 ± 0.06 a | 5.68 ± 1.29 a | 68.96 ± 1.34 b |
| Instant Noodles | Dietary Fibre (%) |
|---|---|
| Control | 4.06 ± 0.34 b |
| 5% WEM | 13.30 ± 3.06 a |
| Concentration of Macro Element (mg/kg dw) | ||||||
| Sodium | Potassium | Calcium | Magnesium | |||
| WEM | 68.929 ± 1.72 | 10,136.303 ± 4.70 | 750.315 ± 0.02 | 504.305 ± 0.02 | ||
| Concentration of Micro Element (mg/kg dw) | ||||||
| Iron | Zinc | Manganese | Copper | Chromium | Selenium | |
| WEM | 32.015 ± 0.80 | 11.499 ± 0.29 | 3.790 ± 0.09 | 2.104 ± 0.07 | 0.149 ± 0.00 | 0.131 ± 0.00 |
| Concentration of Macro Element (mg/kg dw) | ||||||
| Sodium | Potassium | Calcium | Magnesium | |||
| Control | 1.61 × 104 ± 3.551 a | 1442.932 ± 1.323 b | 418.604 ± 0.384 b | 269.131 ± 0.247 b | ||
| 5% WEM | 1.52 × 104 ± 4.450 b | 1896.633 ± 2.448 a | 507.511 ± 0.031 a | 303.160 ± 1.976 a | ||
| Concentration of Micro Element (mg/kg dw) | ||||||
| Iron | Zinc | Manganese | Copper | Chromium | Selenium | |
| Control | 61.122 ± 0.285 b | 8.768 ± 0.118 b | 7.197 ± 0.097 a | 5.234 ± 0.070 a | 0.262 ± 0.004 a | 0.174 ± 0.004 a |
| 5% WEM | 77.584 ± 0.195 a | 10.475 ± 0.218 a | 7.281 ± 0.151 a | 4.215 ± 0.088 a | 0.255 ± 0.005 a | 0.141 ± 0.003 b |
| Control Noodles | 5% WEM Noodles | WEM Powder | |
|---|---|---|---|
| IC50 DPPH (mg/mL) | 19.53 ± 0.51 c | 13.00 ± 0.36 b | 0.85 ± 0.11 a |
| FRAP (µM FeSO4/g) | 4.01 ± 0.04 c | 7.64 ± 0.08 b | 17.73 ± 0.83 a |
| TPC (mg GAE/g) | 0.93 ± 0.08 c | 1.19 ± 0.24 b | 5.81 ± 0.09 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakaria, M.K.; Matanjun, P.; George, R.; Pindi, W.; Mamat, H.; Surugau, N.; Seelan, J.S.S. Nutrient Composition, Antioxidant Activities and Glycaemic Response of Instant Noodles with Wood Ear Mushroom (Auricularia cornea) Powder. Appl. Sci. 2022, 12, 12671. https://doi.org/10.3390/app122412671
Zakaria MK, Matanjun P, George R, Pindi W, Mamat H, Surugau N, Seelan JSS. Nutrient Composition, Antioxidant Activities and Glycaemic Response of Instant Noodles with Wood Ear Mushroom (Auricularia cornea) Powder. Applied Sciences. 2022; 12(24):12671. https://doi.org/10.3390/app122412671
Chicago/Turabian StyleZakaria, Muhammad Kamil, Patricia Matanjun, Ramlah George, Wolyna Pindi, Hasmadi Mamat, Noumie Surugau, and Jaya Seelan Sathiya Seelan. 2022. "Nutrient Composition, Antioxidant Activities and Glycaemic Response of Instant Noodles with Wood Ear Mushroom (Auricularia cornea) Powder" Applied Sciences 12, no. 24: 12671. https://doi.org/10.3390/app122412671








