Delivery of Cancer Therapeutics Using Nanotechnology
Abstract
:1. Introduction
2. Common Nanostructures for Drug Carriers: Their Structures and Drug-Loading Principles
2.1. Supramolecular Organic Architectures
2.1.1. Polymeric Micelles
2.1.2. Bilayer Vesicles
2.1.3. Cross-Linked Nanogels

2.1.4. Solid Lipid Nanoparticles
2.2. Inorganic Cargoes: Simple Particles, Porous Materials, Hollow Structures
2.2.1. Simple Sphere Nanoparticles
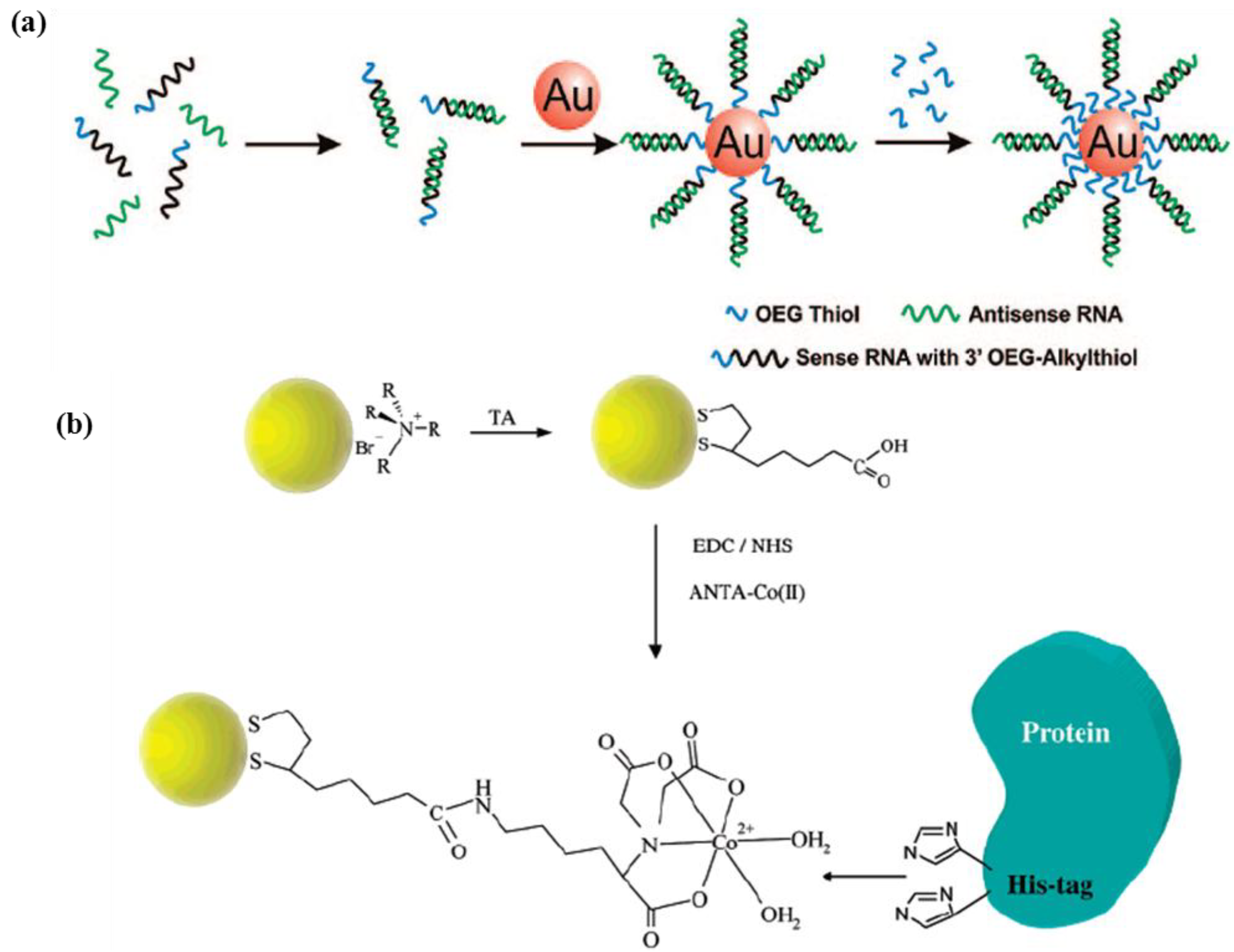
2.2.2. Porous Nanoparticles
2.2.3. Hollow Nanoparticles

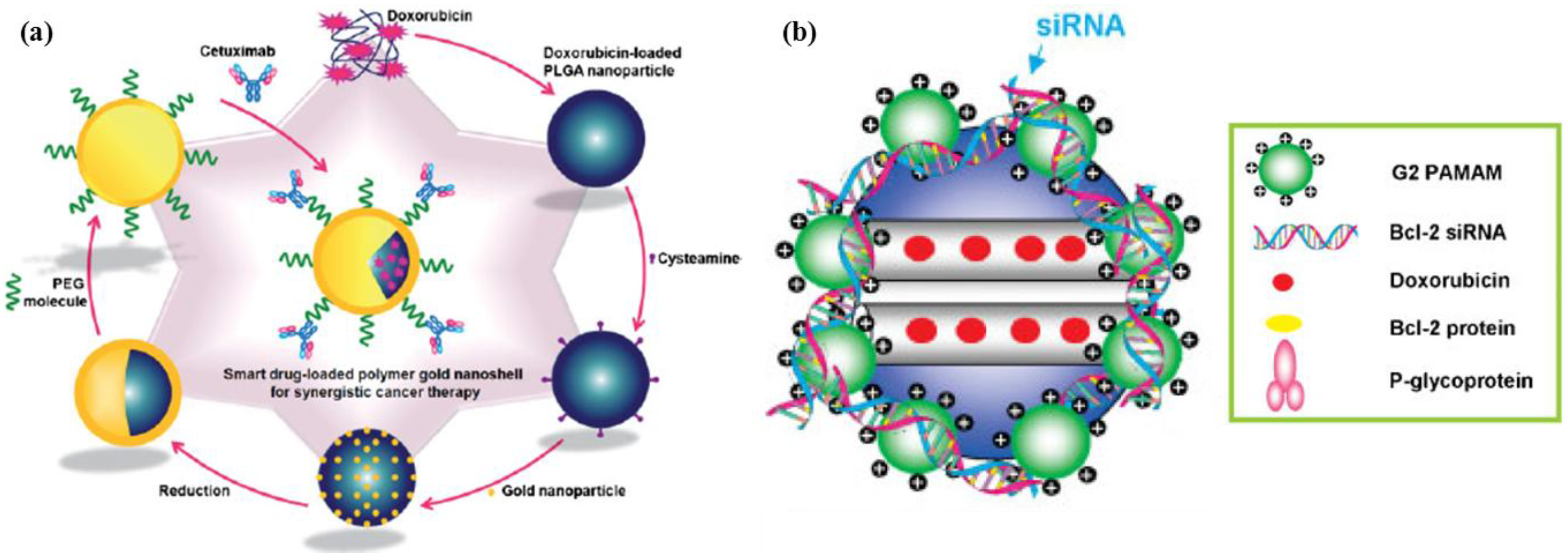
2.3. Hierarchical Organic/Inorganic Hybrids
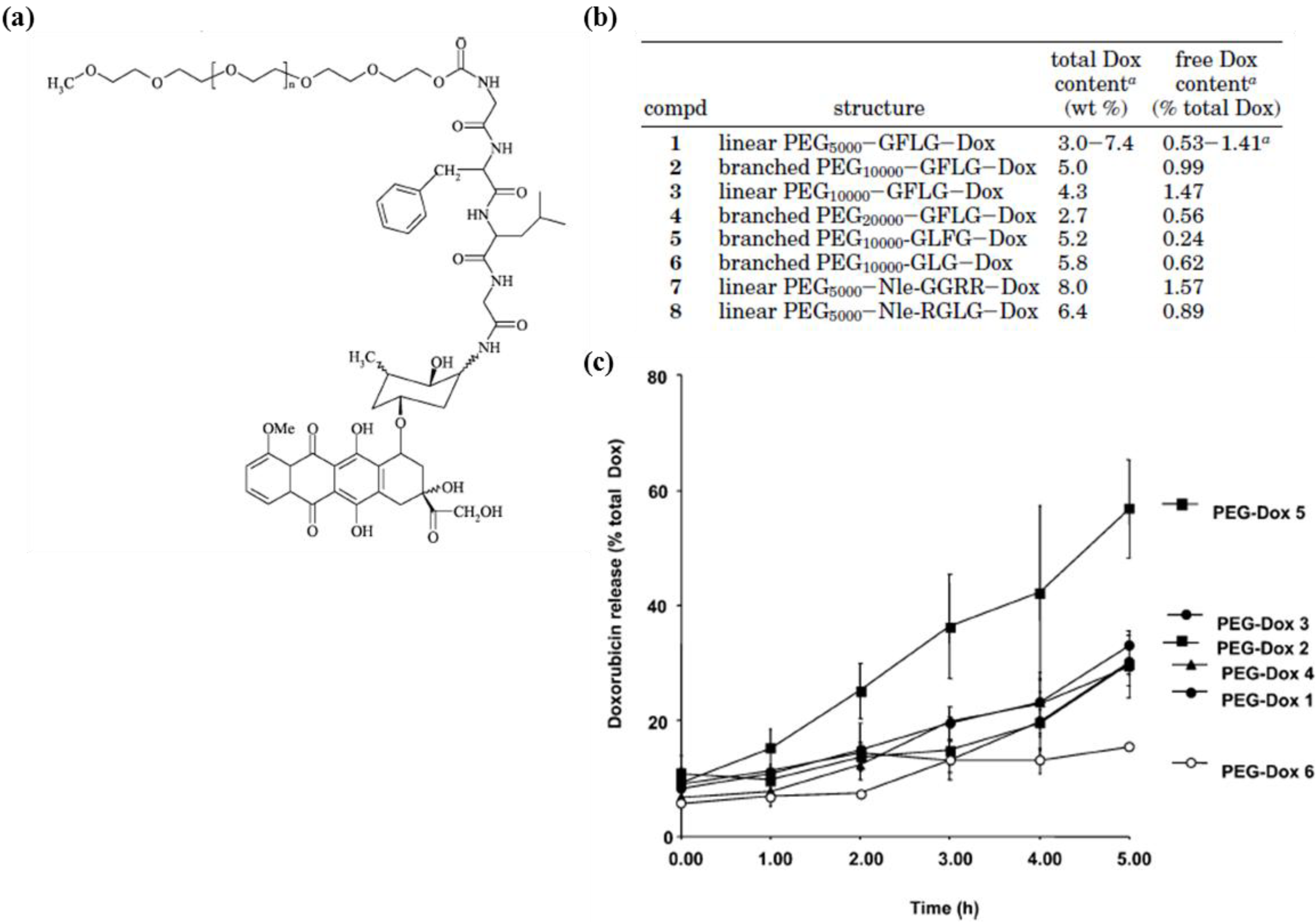
3. Enhanced Drug Accumulation at Target Sites: Pharmacokinetics and Biodistribution
3.1. General Physiological Strategies: Size Modulation for Enhanced Circulation Half-Life, Reticuloendothelial system (RES) Avoidance by Surface Modification (PEG)
3.2. Cancer-Specific Physiological Strategies
3.2.1. Passive Targeting by EPR Effects
3.2.2. Active Targeting by Molecular Binding Receptor (Antibody, Targetable Polymer, etc.)
3.2.3. Active Targeting by Magnetic Guidance

4. Ongoing Advances in Drug Delivery Systems
4.1. Stimuli-Responsive Drug Delivery
4.1.1. Light-responsive Drug Delivery Systems
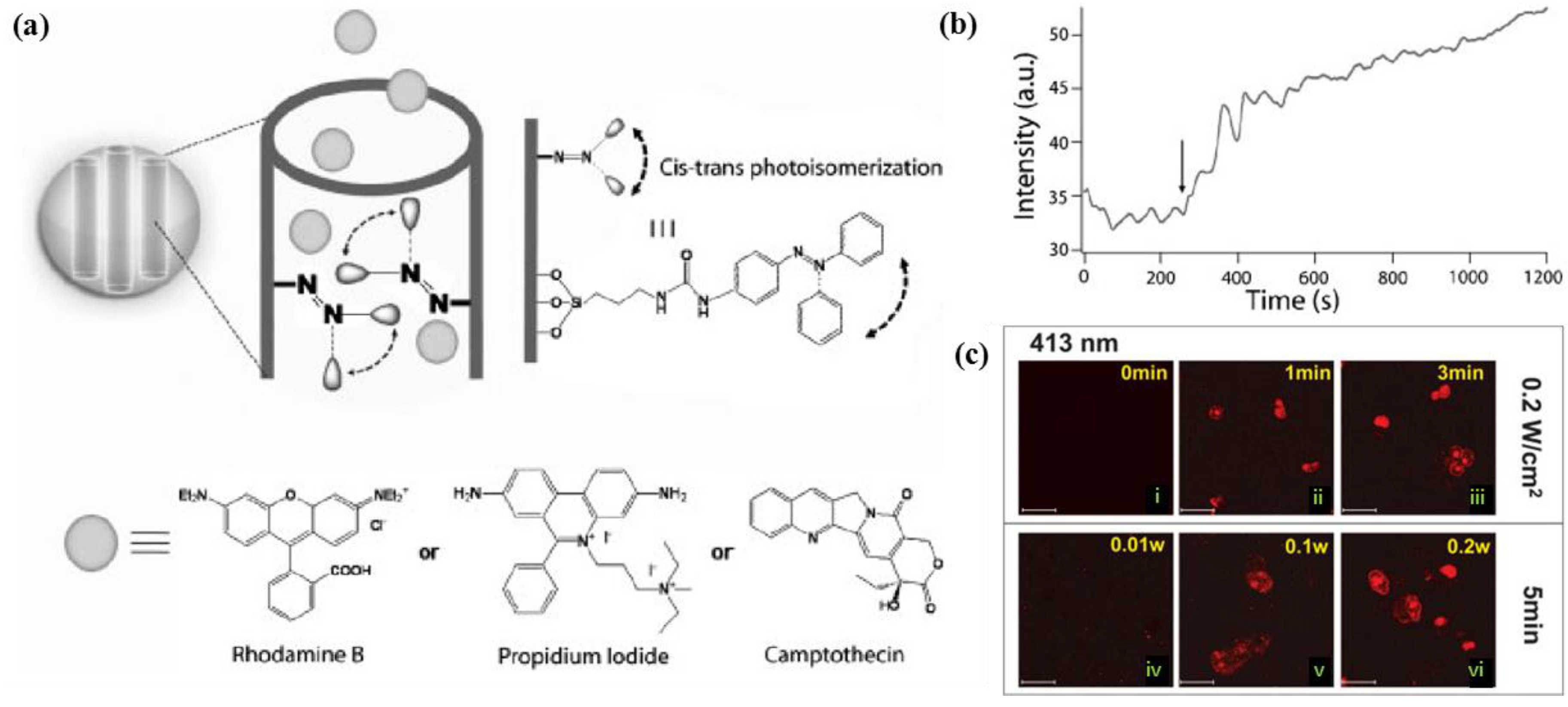
4.1.2. Magnetically-Triggered Drug Delivery Systems

4.1.3. Ultrasound-Mediated Drug Delivery Systems
4.1.4. pH-Responsive Drug Delivery Systems
4.1.5. Enzyme-Responsive Drug Delivery Systems
4.2. Theranostic Nanoparticles
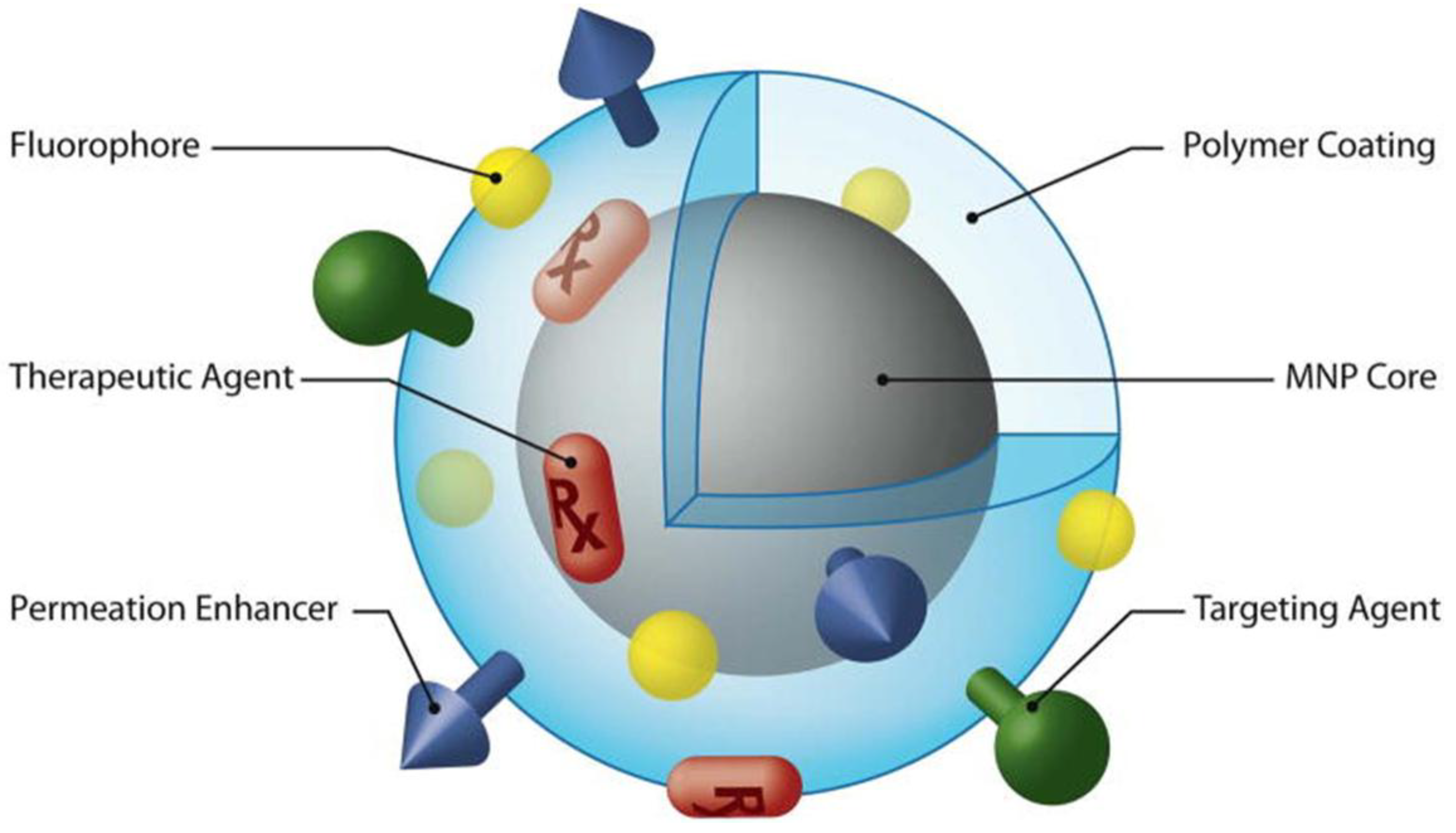
5. Conclusion
Acknowledgements
References
- Kreuter, J. Nanoparticles—A hsitorical perspective. Int. J. Pharm. 2007, 331, 1–10. [Google Scholar] [CrossRef]
- Duncan, R. Polymer conjugates as anticancer nanomedicines. Nat. Rev. Cancer 2006, 6, 688–791. [Google Scholar]
- Lobatto, M.E.; Fuster, V.; Fayad, Z.A.; Mulder, W.J.M. Perspectives and opportunities for nanomedicine in the management of atherosclerosis. Nat. Rev. Drug Discov. 2011, 10, 835–852. [Google Scholar]
- Mitragotri, S.; Lahann, J. Physical approaches to biomaterial design. Nat. Mater. 2009, 8, 15–23. [Google Scholar] [CrossRef]
- Minchinton, A.I.; Tannock, I.F. Drug penetration in solid tumours. Nat. Rev. Cancer 2006, 583–592. [Google Scholar] [CrossRef]
- Allen, T.M. Ligand-targeted therapeutics in anticancer therapy. Nat. Rev. Cancer 2002, 2, 750–763. [Google Scholar] [CrossRef]
- Davis, M.E.; Chen, Z.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008, 8, 771–782. [Google Scholar] [CrossRef]
- Haag, R. Supramolecular drug-delivery systems based on polymeric core–shell architectures. Angew. Chem. Int. Ed. 2004, 43, 278–282. [Google Scholar] [CrossRef]
- Veronese, F.M.; Schiavon, O.; Pasut, G.; Mendichi, R.; Andersson, L.; Tsirk, A.; Ford, J.; Wu, G.; Kneller, S.; Davies, J.; et al. PEG-doxorubicin conjugates: Influence of polymer structure on drug release, in vitro cytotoxicity, biodistribution, and antitumor activity. Bioconjugate Chem. 2005, 16, 775–784. [Google Scholar] [CrossRef]
- Acharya, S.; Sahoo, S.K. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 170–183. [Google Scholar] [CrossRef]
- Hyung, W.; Ko, H.; Park, J.; Lim, E.; Park, S.B.; Park, Y.; Yoon, H.G.; Suh, J.S.; Haam, S.; Huh, Y. Novel hyaluronic acid (HA) coated drug carriers (HCDCs) for human breast cancer treatment. Biotechnol. Bioeng. 2008, 99, 422–454. [Google Scholar]
- Jung, S.W.; Jeong, Y.I.; Kim, Y.H.; Choi, K.C.; Kim, S.H. Drug release from core-shell type nanoparticles of poly(DL-lactide-co-glycolide)-grafted dextran. J. Microencapsul. 2005, 22, 901–911. [Google Scholar] [CrossRef]
- Chung, Y.; Kim, J.C.; Kim, Y.H.; Tae, G.; Lee, S.; Kim, K.; Kwon, I.C. The effect of surface functionalization of PLGA nanoparticles by heparin- or chitosan-conjugated Pluronic on tumor targeting. J. Control. Release 2010, 10, 374–382. [Google Scholar]
- Lim, E.; Huh, Y.; Yang, J.; Lee, K.; Suh, J.; Haam, S. pH-triggered drug-releasing magnetic nanoparticles for cancer therapy guided by molecular imaging by MRI. Adv. Mater. 2011, 23, 2436–2442. [Google Scholar] [CrossRef]
- Miele, E.; Spinelli, G.P.; Miele, E.; Tomao, F.; Tomao, S. Albumin-bound formulation of paclitaxel (Abraxane® ABI-007) in the treatment of breast cancer. Int. J. Nanomedicine 2009, 4, 99–105. [Google Scholar]
- Micha, J.P.; Goldstein, B.H.; Birk, C.L.; Rettenmaier, M.A.; Brown, J.V., III. Abraxane in the treatment of ovarian cancer: The absence of hypersensitivity reactions. Gynecol. Oncol. 2006, 100, 437–438. [Google Scholar] [CrossRef]
- Green, M.R.; Manikhas, G.M.; Orlov, S.; Afanasyev, B.; Makhson, A.M.; Bhar, P.; Hawkins, M.J. Abraxane®, a novel Cremophor®—Free, albumin-bound particle form of paclitaxel for the treatment of advanced non-small-cell lung cancer. Ann. Oncol. 2006, 17, 1263–1268. [Google Scholar] [CrossRef]
- Lasic, D.D. Sterically stabilized vesicles. Angew. Chem. Int. Ed. 1994, 33, 1685–1698. [Google Scholar] [CrossRef]
- Tanner, P.; Baumann, P.; Enea, R.; Onaca, O.; Palivan, C.; Meier, W. Polymeric vesicles: From drug carriers to nanoreactors and artificial organelles. Acc. Chem. Res. 2011, 44, 1039–1049. [Google Scholar] [CrossRef]
- Dhanikula, A.B.; Panchagnula, R. Preparation and characterization of water-soluble prodrug, liposomes and micelles of paclitaxel. Curr. Drug Deliv. 2005, 2, 75–91. [Google Scholar] [CrossRef]
- Levine, D.H.; Ghoroghchian, P.P.; Freudenberg, J.; Zhang, G.; Therien, M.J.; Greene, M.I.; Hammer, D.A.; Murali, R. Polymersomes: A new multi-functional tool for cancer diagnosis and therapy. Methods 2008, 46, 25–32. [Google Scholar] [CrossRef]
- Chang, H.-I.; Yeh, M.-K. Clinical development of liposome-based drugs: Formulation, characterization, and therapeutic efficacy. Int. J. Nanomed. 2012, 7, 49–60. [Google Scholar]
- Haley, B.; Frenkel, E. Nanoparticles for drug delivery in cancer treatment. Urol. Oncol. Semin. Orig. Investig. 2008, 26, 57–64. [Google Scholar] [CrossRef]
- Chacko, R.T.; Ventura, J.; Zhuang, J.; Thayumanavan, S. Polymer nanogels: A versatile nanoscopic drug delivery platform. Adv. Drug Deliv. Rev. 2012, 64, 836–851. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as pharmaceutical carriers: Finite networks of infinite capabilities. Angew. Chem. Int. Ed. 2009, 48, 5418–5429. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, W.; Lin, L.; Chen, D.; Stenzel, M.H. Degradable disulfide core-cross-linked micelles as a drug delivery system prepared from vinyl functionalized nucleosides via the RAFT process. Biomacromolecules 2008, 9, 3321–3331. [Google Scholar] [CrossRef]
- Mühlen, A.Z.; Schwarz, C.; Mehnert, W. Slid lipid nanoparticles (SLN) for controlled drug delivery—Drug release and release mechanism. Eur. J. Pharm. Biopharm. 1998, 45, 149–155. [Google Scholar] [CrossRef]
- Almeida, A.J.; Souto, E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv. Drug Deliv. Rev. 2007, 57, 478–490. [Google Scholar] [CrossRef]
- Wissing, S.A.; Kayse, O.; Müller, R.H. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef]
- Blasi, P.; Giovagnoli, S.; Schoubben, A.; Ricci, M.; Rossi, C. Solid lipid nanoparticles for targeted brain drug delivery. Adv. Drug Deliv. Rev. 2007, 59, 454–477. [Google Scholar] [CrossRef]
- Jenning, V.; Thünemann, A.F.; Gohla, S.H. Characterisation of a novel solid lipid nanoparticle carrier system based on binary mixtures of liquid and solid lipids. Int. J. Pharm. 2000, 199, 167–177. [Google Scholar] [CrossRef]
- Schwarz, C.; Mehnert, W. Solid lipid nanoparticles (SLN) for controlled drug delivery II. Drug incorporation and physicochemical characterization. J. Microencapsul. 1999, 16, 205–213. [Google Scholar] [CrossRef]
- Kang, K.W.; Chun, M.-K.; Lim, O.; Subedi, R.K.; Ahn, S.-G.; Yoon, J.-H.; Choi, H.-K. Doxorubicin-loaded solid lipid nanoparticles to overcome multidrug resistance in cancer therapy. Nanomedicine 2010, 6, 210–213. [Google Scholar] [CrossRef]
- Sekhon, B.S.; Kamboj, S.R. Inorganic nanomedicine—Part 1. Nanomed. Nanotech. Biol. Med. 2010, 6, 516–522. [Google Scholar] [CrossRef]
- Sekhon, B.S.; Kamboj, S.R. Inorganic nanomedicine—Part 2. Nanomed. Nanotech. Biol. Med. 2010, 6, 612–618. [Google Scholar] [CrossRef]
- Duncan, B.; Kim, C.; Rotello, V.M. Gold nanoparticle platforms as drug and biomacromolecule delivery systems. J. Control. Release 2010, 148, 122–127. [Google Scholar] [CrossRef]
- Rana, S.; Bajaj, A.; Mout, R.; Rotello, V.M. Monolayer coated gold nanoparticles for delivery applications. Adv. Drug Deliv. Rev. 2012, 64, 200–216. [Google Scholar] [CrossRef]
- Giljohann, D.A.; Seferos, D.S.; Prigodich, A.E.; Patel, P.C.; Mirkin, C.A. Gene regulationwith polyvalent siRNA-nanoparticle conjugates. J. Am. Chem. Soc. 2009, 131, 2072–2073. [Google Scholar]
- Ghosh, P.S.; Kim, C.K.; Han, G.; Forbes, N.S.; Rotello, V.M. Efficient gene delivery vectors by tuning the surface charge density of amino acid-functionalized gold nanoparticles. ACS Nano 2008, 2, 2213–2218. [Google Scholar] [CrossRef]
- Kim, C.K.; Ghosh, P.; Pagliuca, C.; Zhu, Z.J.; Menichetti, S.; Rotello, V.M. Entrapment of hydrophobic drugs in nanoparticle monolayers with efficient release into cancer cells. J. Am. Chem. Soc. 2009, 131, 1360–1361. [Google Scholar]
- Serizawa, T.; Hirai, Y.; Aizawa, M. Novel synthetic route to peptide-capped gold nanoparticles. Langmuir 2009, 25, 12229–12234. [Google Scholar] [CrossRef]
- Abad, J.M.; Mertens, S.F.L.; Pita, M.; Fernandez, V.M.; Schiffrin, D.J. Functionalization of thioctic acid-capped gold nanoparticles for specific immobilization of histidine-tagged proteins. J. Am. Chem. Soc. 2005, 127, 5689–5694. [Google Scholar]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Balas, F.; Arcos, D. Mesoporous materials for drug delivery. Angew. Chem. Int. Ed. 2007, 46, 7548–7558. [Google Scholar] [CrossRef]
- Andersson, J.; Rosenholm, J.; Areva, S.; Linden, M. Influences of material characteristics on ibuprofen drug loading and release profiles from ordered micro- and mesoporous silica matrices. Chem. Mater. 2004, 16, 4160–4167. [Google Scholar] [CrossRef]
- Yanes, R.E.; Tamanoi, F. Development og mesoporous silica nanomaterials as a vehicle for anticancer drug delivery. Ther. Deliv. 2012, 3, 389–404. [Google Scholar] [CrossRef]
- Lu, J.; Liong, M.; Li, Z.; Zink, J.I.; Tamanoi, F. Biocompatibility, biodistribution, and drug-delivery efficiency of mesoposrous silica nanoparticles for cancer therapy in animals. Small 2010, 6, 1794–1805. [Google Scholar] [CrossRef]
- Vivero-Escoto, J.L.; Slowing, I.I.; Trewyn, B.G.; Lin, V.S.-Y. Mesoporous silica nanoparticles for intracellular controlled drug delivery. Small 2010, 6, 1952–1967. [Google Scholar] [CrossRef]
- Ambrogio, M.W.; Thomas, C.R.; Zhao, Y.-L.; Zink, J.I.; Stoddart, J.F. Mechanized silica nanoparticles: A new frontier in theranostic nanomedicine. Acc. Chem. Res. 2011, 44, 903–913. [Google Scholar]
- Park, J.H.; Gu, L.; Maltzahn, G.; Ruoslahti, E.; Nhatia, S.N.; Sailor, M.J. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nat. Mater. 2009, 8, 331–336. [Google Scholar] [CrossRef]
- Khanal, A.; Inoue, Y.; Yada, M.; Nakashima, K. Synthesis of silica hollow nanoparticles templated by polymeric micelle with core-shell-corona structure. J. Am. Chem. Soc. 2007, 129, 1534–1535. [Google Scholar] [CrossRef]
- Yang, J.; Lee, J.; Kang, J.; Lee, K.; Suh, J.; Yoon, H.; Huh, Y.; Haam, S. Hollow silica nanocontainers as drug delivery vehicles. Langmuir 2008, 24, 3417–3421. [Google Scholar]
- Yang, J.; Lind, J.U.; Trogler, W.C. Synthesis of hollow silica and titania nanospheres. Chem. Mater. 2008, 20, 2875–2877. [Google Scholar] [CrossRef]
- Yavuz, M.S.; Cheng, Y.; Chen, J.; Cobley, C.M.; Zhang, Q.; Rycenga, M.; Xie, J.; Kime, C.; Song, K.H.; Schwartz, A.G.; et al. Gold nanocages covered by smart polymers for controlled release with near-infrared light. N. Nat. Mater. 2009, 8, 935–939. [Google Scholar]
- Adeli, M.; Kalantari, M.; Parsamanesh, M.; Sadeghi, E.; Mahmoudi, M. Synthesis of new hybrid nanomaterials: Promising systems for cancer therapy. Nanomed. Nanotech. Biol. Med. 2011, 7, 806–817. [Google Scholar] [CrossRef]
- Yang, J.; Lee, J.; Kang, J.; Oh, S.J.; Ko, H.; Son, J.; Lee, K.; Suh, J.; Huh, Y.; Haam, S. Smart drug-loaded polymer gold nanoshells for systemic and localized therapy of human epithelial cancer. Adv. Mater. 2009, 21, 4339–4342. [Google Scholar] [CrossRef]
- Chen, A.M.; Zhang, M.; Wei, D.; Stueber, D.; Taratula, O.; Minko, T.; He, H. Co-delivery of doxorubicin and Bcl-2 siRNA by mesoporous silica nanoparticles enhances the efficacy of chemotherapy in multidrug-resistant cancer cells. Small 2009, 5, 2673–2677. [Google Scholar]
- Pasut, G.; Veronese, F.M. PEG conjugates in clinical development or use as anticancer agents: An overview. Adv. Drug Deliv. Rev. 2009, 61, 1177–1188. [Google Scholar] [CrossRef]
- Veronese, F.M.; Pasut, G. PEGylation, successful approach to drug delivery. Drug Discov. Today 2005, 10, 1451–1458. [Google Scholar] [CrossRef]
- Torchilin, V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 131–135. [Google Scholar] [CrossRef]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef]
- Maruyama, K. Intracellular targeting delivery of liposomal drugs to solid tumors based on EPR effects. Adv. Drug Deliv. Rev. 2011, 63, 161–169. [Google Scholar] [CrossRef]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. 2010, 9, 6288–6308. [Google Scholar]
- Yang, J.; Park, S.-G.; Yoon, H.G.; Huh, Y.-M.; Haam, S. Preparation of poly ε-caprolactone nanoparticles containing magnetite for magnetic drug carrier. Int. J. Pharm. 2006, 324, 185–190. [Google Scholar] [CrossRef]
- Kim, E.; Jung, Y.; Choi, H.; Yang, J.; Suh, J.-S.; Huh, Y.-M.; Kim, K.; Haam, S. Prostate cancer cell death produced by the co-delivery of Bcl-xL shRNA and doxorubicin using an aptamer-conjugated polplex. Biomaterials 2010, 31, 4592–4599. [Google Scholar] [CrossRef]
- Yang, J.; Lee, C.-H.; Park, J.; Seo, S.; Lim, E.-K.; Song, Y.J.; Suh, J.-S.; Yoon, H.-G.; Huh, Y.-M.; Haam, S. Antibody conjugated magnetic PLGA nanoparticles for diagnosis and treatment of breast cancer. J. Mater. Chem. 2007, 17, 2695–2699. [Google Scholar]
- Koevit, M.F.; Zhang, M. Cancer nanotheranostics: Improving imaging and therapy by targeted delivery across biological barriers. Adv. Mater. 2011, 23, H217–H247. [Google Scholar] [CrossRef]
- Sun, C.; Lee, J.S.H.; Zhang, M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef]
- Choi, J.; Yang, J.; Jang, E.; Suh, J.-S.; Huh, Y.-M.; Lee, K.; Haam, S. Gold nanostructures as photothermal therapy agent for cancer. Anticancer Agents. Med. Chem. 2011, 11, 953–964. [Google Scholar]
- Yang, J.; Lee, C.-H.; Ko, H.-J.; Suh, J.-S.; Yoon, H.-G.; Lee, K.; Huh, Y.-M.; Haam, S. Multifunctional magneto-polymeric nanohybrids for targeted detection and synergistic therapeutic effects on breast cancer. Angew. Chem. Int. Ed. 2007, 46, 8836–8839. [Google Scholar]
- Manish, C.; Vimukta, S. Targeted drug delivery system: A review. Res. J. Chem. Sci. 2011, 1, 135–138. [Google Scholar]
- Andreani, T.; Doktorovová, S.; Lopes, C.M.; Souto, E.B. Nanobiotechnology approaches for targeteddelivery of pharmaceutics and cosmetics ingredients. Int. J. Nanotechnol. 2011, 8, 66–77. [Google Scholar]
- Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Targeting of drugs and nanoparticles to tumors. J. Cell Biol. 2010, 188, 759–768. [Google Scholar] [CrossRef]
- Yang, J.; Cho, E.-J.; Seo, S.; Lee, J.-W.; Yoon, H.-G.; Suh, J.-S.; Huh, Y.-M.; Haam, S. Enhancement of cellular binding efficiency and cytotoxicity using polyethylene glycol base triblock copolymeric nanoparticles for targeted drug delivery. J. Biomed. Mater. Res. A 2008, 84, 273–280. [Google Scholar]
- Choi, K.Y.; Jeon, E.J.; Yoon, H.Y.; Lee, B.S.; Na, J.H.; Min, J.H.; Kim, S.Y.; Myung, S.-J.; Chen, X.; Kwon, I.C.; et al. Theranostic nanoparticles based on PEGylated hyaluronic acid for the diagnosis, therapy and monitoring of colon cancer. Biomaterials 2012, 33, 6186–6193. [Google Scholar] [CrossRef]
- Ruoslahti, E. The RGD story: A personal account. Matrix Biol. 2003, 22, 459–465. [Google Scholar] [CrossRef]
- Chen, K.; Chen, X. Integrin targeted delivery of chemotherapeutics. Theranostics 2011, 1, 189–200. [Google Scholar] [CrossRef]
- Ruoslahti, E. Specialization of tumour vasculature. Nat. Rev. Cancer 2002, 2, 83–90. [Google Scholar] [CrossRef]
- Choi, J.; Yang, J.; Park, J.; Kim, E.; Suh, J.-S.; Huh, Y.-M.; Haam, S. Specific near-IR absorption imaging of glioblastomas using integrin-targeting gold nanorods. Adv. Funct. Mater. 2011, 21, 1082–1088. [Google Scholar] [CrossRef]
- Ren, Y.; Cheung, H.W.; von Maltzhan, G.; Agrawal, A.; Cowley, G.S.; Weir, B.A.; Boehm, J.S.; Tamayo, P.; Karst, A.M.; Liu, J.F.; et al. Targeted tumor-penetrating siRNA nanocomplexes for credentialing the ovarian cancer oncogene ID4. Sci. Transl. Med. 2012, 15, 147ra112. [Google Scholar]
- Sugahara, K.N.; Teesalu, T.; Karmali, P.P.; Kotamraju, V.R.; Agemy, L.; Girard, O.M.; Hanahan, D.; Mattrey, R.F.; Ruoslahti, E. Tissue-penetrating delivery of compounds and nanoparticles into tumors. Cancer Cell 2009, 16, 510–520. [Google Scholar] [CrossRef]
- Lim, Y.-B.; Kwon, O.-J.; Lee, E.; Kim, P.-H.; Yun, C.-O.; Lee, M. A cyclicRGD-coated peptide nanoribbon as a selective intracellular nanocarrier. Org. Biomol. Chem. 2008, 6, 1944–1948. [Google Scholar] [CrossRef]
- Flanagan, P.A.; Duncan, R.; Subr, V.; Ulbrich, K.; Kopeckov, P.; Kopecek, J. Evaluation of protein-N-(2-hydroxypropyl) methacrylamide copolymer conjugates as targetable drug-carriers. 2. Body distribution of conjugates containing transferrin, antitransferrin receptor antibody or anti-Thy 1.2 antibody and effectiveness of transferrin-containing daunomycin conjugates against mouse L1210 leukaemia in vivo. J. Contr. Release 1992, 18, 25–30. [Google Scholar] [CrossRef]
- Li, J.-L.; Wang, L.; Liu, X.-Y.; Zhang, Z.-P.; Guo, H.-C.; Liu, W.-M.; Tang, S.-H. In vitro cancer cell imaging and therapy using transferrin-conjugated gold nanoparticles. Cancer Lett. 2009, 274, 319–326. [Google Scholar] [CrossRef]
- Li, H.; Qian, Z.M. Transferrin/transferrin receptor-mediated drug delivery. Med Res Rev. 2002, 22, 225–250. [Google Scholar] [CrossRef]
- Seo, S.-B.; Yang, J.; Hyung, W.; Cho, E.-J.; Lee, T.-I.; Song, Y.J.; Yoon, H.-G.; Suh, J.-S.; Huh, Y.-M.; Haam, S. Novel multifunctional PHDCA/PEI nano-drug carriers for simultaneous magnetically targeted cancer therapy and diagnosis via magnetic resonance imaging. Nanotechnology 2007, 18, 475105. [Google Scholar] [CrossRef]
- Kim, H.-J.; Ahn, J.-E.; Haam, S.; Shul, Y.-G.; Song, S.-Y.; Tatsumi, T. Synthesis and characterization of mesoporous Fe/SiO2 for magnetic drug targeting. J. Mater. Chem. 2006, 16, 1617–1621. [Google Scholar] [CrossRef]
- Gang, J.; Park, S.-B.; Hyung, W.; Choi, E.H.; Wen, J.; Kim, H.-S.; Shul, Y.-G.; Haam, S.; Song, S.Y. Magnetic poly ε-caprolactone nanoparticles containing Fe3O4 and gemcitabine enhance anti-tumor effect in pancreatic cancer xenograft mouse model. J. Drug Target. 2007, 15, 445–453. [Google Scholar] [CrossRef]
- Agasti, S.S.; Chompoosor, A.; You, C.-C.; Ghosh, P.; Kim, C.K.; Rotello, V.M. Photoregulated release of caged anticancer drugs from gold nanoparticles. J. Am. Chem. Soc. 2009, 131, 5728–5729. [Google Scholar]
- Braun, G.B.; Pallaoro, A.; Wu, G.; Missirlis, D.; Zasadzinski, J.A.; Tirrell, M.; Reich, N.O. Laser-activated gene silencing via gold nanoshell-siRNA conjugates. ACS Nano 2009, 3, 2007–2015. [Google Scholar] [CrossRef]
- Vivero-Escoto, J.L.; Slowing, I.I.; Wu, C.-W.; Lin, V.S.-Y. Photoinduced intracellular controlled release drug delivery in human cells by gold-capped mesoporous silica nanosphere. J. Am. Chem. Soc. 2009, 131, 3462–3463. [Google Scholar] [CrossRef]
- Han, G.; You, C.-C.; Kim, B.-J.; Turingan, R.S.; Forbes, N.S.; Martin, C.T.; Rotello, V.M. Light-regulated release of DNA and its delivery to nuclei by means of photolabile gold nanoparticles. Angew. Chem. Int. Ed. 2006, 118, 3237–3241. [Google Scholar] [CrossRef]
- Lu, J.; Choi, E.; Tamanoi, F.; Zink, J.I. Light-activated nanoimpeller-controlled drug release in cancer cells. Small 2008, 4, 421–426. [Google Scholar] [CrossRef]
- Gary-Bobo, M.; Mir, Y.; Rouxel, C.; Brevet, D.; Hocine, O.; Maynadier, M.; Gallud, A.; da Silva, A.; Mongin, O.; Blanchard-Desce, M.; et al. Multifunctionalized mesoporous silica nanoparticles for the in vitro treatment of retinoblastoma: Drug delivery, one and two-photon photodynamic therapy. Int. J. Pharm. 2012, 432, 99–104. [Google Scholar]
- Kumar, C.S.S.R.; Mohammad, F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 789–808. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S.; Müller, R.; Zeisberger, M. Magnetic particle hyperthermia: Nanoparticle magnetism and materials development for cancer therapy. J. Phys. Condens. Matter. 2006, S2919–S2934. [Google Scholar]
- Yoo, D.; Jeong, H.; Preihs, C.; Choi, J.-S.; Shin, T.-H.; Sessler, J.L.; Cheon, J. Double-effector nanoparticles: A synergistic approach to apoptotic hyperthermia. Angew. Chem. Int. Ed. 2012, 51, 1–5. [Google Scholar]
- Lee, J.-H.; Jang, J.-T.; Choi, J.-S.; Moon, S.H.; Noh, S.-H.; Kim, J.W.; Kim, J.-G.; Kim, I.-S.; Park, K.I.; Cheon, J. Exchange-coupled magnetic nanoparticles for efficient heat induction. Nat. Nanotech. 2011, 6, 418–422. [Google Scholar] [CrossRef]
- Lee, J.-H.; Chen, K.-J.; Noh, S.-H.; Garcia, M.A.; Wang, H.; Lin, W.-Y.; Jeong, H.; Kong, B.J.; Stout, D.B.; Cheon, J.; et al. On-demand drug release systems for in vivo cancer treatment through self-assembled magnetic nanoparticles. Angew. Chem. Int. Ed. 2013, 52, 1–6. [Google Scholar] [CrossRef]
- Cho, M.H.; Lee, E.J.; Son, M.; Lee, J.-H.; Yoo, D.; Kim, J.-W.; Park, S.W.; Shin, J.-S.; Cheon, J. A magnetic switch for the control of cell death signaling in in vitro and in vivo systems. Nat. Mater. 2012, 11, 1038–1043. [Google Scholar]
- Thomas, C.R.; Ferris, D.P.; Lee, J.H.; Choi, E.; Cho, M.H.; Kim, E.S.; Stoddart, J.F.; Shin, J.S.; Cheon, J.; Zink, J.I. Noninvasive remote-controlled release of drug molecules in vitro using magnetic actuation of mechanized nanoparticles. J. Am. Chem. Soc. 2010, 132, 10623–10625. [Google Scholar] [CrossRef]
- Katagiri, K.; Imai, Y.; Kounoto, K.; Kaiden, T.; Kono, K.; Aoshima, S. Magnetoresponsive on-demand release of hybrid liposomes formed from Fe3O4 nanoparticles and thermosensitive block copolymer. Small 2011, 7, 1683–1689. [Google Scholar] [CrossRef]
- Hernot, S.; Klibanov, A.L. Microbubbles in ultrasound-triggered drug and gene delivery. Adv. Drug Deliv. Rev. 2008, 60, 1153–1166. [Google Scholar] [CrossRef]
- Kost, J.; Leong, K.; Langer, R. Ultrasound-enhaced polymer degradation and release of incorporated substances. Proc. Natl. Acad. Sci. USA 1989, 86, 7663–7666. [Google Scholar] [CrossRef]
- Ferrara, K.; Pollard, R.; Borden, M. Ultrasound microbubble contrast agents: Fundamentals and application to gene and drug delivery. Annu. Rev. Biomed. Eng. 2007, 9, 415–447. [Google Scholar] [CrossRef]
- Tachibana, K.; Tachibana, S. The use of ultrasound for drug delivery. Echocardiography 2001, 18, 323–328. [Google Scholar]
- Hosseinkhani, H.; Tabata, Y. Ultrasound enhances in vivo tumor expression of plasmid DNA by PEG-introduced cationized dextran. J. Contr. Release 2005, 108, 540–556. [Google Scholar] [CrossRef]
- Chumakova, O.V.; Liopo, A.V.; Andreev, V.G.; Cicenaite, I.; Mark Evers, B.; Chakrabart, S.; Pappas, T.C.; Esenaliev, R.O. Composition of PLGA and PEI/DNA nanoparticles improves ultrasound-mediated gene delivery in solid tumors in vivo. Cancer Lett. 2008, 261, 215–225. [Google Scholar] [CrossRef]
- Lum, A.F.H.; Borden, M.A.; Dayton, P.A.; Kruse, D.E.; Simon, S.I.; Ferrara, K.W. Ultrasound radiation force enables targeted deposition of model drug carriers loaded on microbubbles. J. Contr. Release 2006, 111, 128–134. [Google Scholar] [CrossRef]
- Felber, A.E.; Dufresne, M.-H.; Lerous, J.C. pH-Sensitive vesicles, polymeric micelles, and nanospheres prepared with polycarboxylates. Adv. Drug Deliv. Rev. 2012, 64, 979–992. [Google Scholar] [CrossRef]
- Makhlof, A.; Tozuka, Y.; Takeuchi, H. pH-Sensitive nanospheres for colon-specific drug delivery in experimentally induced colitis rat model. Eur. J. Pharm. Biopharm. 2009, 72, 1–8. [Google Scholar] [CrossRef]
- Wang, Z.-C.; Xu, X.-D.; Chen, C.-S.; Wang, G.-R.; Wang, B.; Zhang, X.-Z.; Zhuo, R.-X. Study on novel hydrogels based on thermosensitive PNIPAAm with pH sensitive PDMAEMA grafts. Colloid Surf. B Biointerfaces 2008, 67, 245–252. [Google Scholar]
- Etrych, T.; Jelínková, M.A.; Ríhová, B.; Ulbrich, K. New HPMA copolymers containing doxorubicin bound via pH-sensitive linkage: Synthesis and preliminary in vitro and in vivo biological properties. J. Contr. Release 2001, 73, 89–102. [Google Scholar] [CrossRef]
- Soppimath, K.S.; Liu, L.-H.; Seow, W.Y.; Liu, S.-Q.; Powell, R.; Chan, P.; Yang, Y.Y. Multifunctional core/shell nanoparticles self-assembled from pH-induced thermosensitive polymers for targeted intracellular anticancer drug delivery. Adv. Funct. Mater. 2007, 17, 355–362. [Google Scholar] [CrossRef]
- Lee, S.; Cha, E.-J.; Park, K.; Lee, S.-Y.; Hong, J.-K.; Sun, I.-C.; Kim, S.; Choi, K.; Kwon, I.C.; Kim, K.; et al. A near-infrared-fluorescence-quenched gold-nanoparticle imaging probe for in vivo drug screening and protease activity determination. Angew. Chem. Int. Ed. 2008, 47, 2804–2807. [Google Scholar] [CrossRef]
- Egeblad, M.; Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2002, 2, 161–174. [Google Scholar] [CrossRef]
- Oishi, M.; Tamura, A.; Nakamura, T.; Nagasaki, Y. A smart nanoprobe based on fluorescence-quenching pegylated nanogels containing gold nanoparticles for monitoring the response to cancer therapy. Adv. Funct. Mater. 2009, 19, 827–834. [Google Scholar] [CrossRef]
- Xia, X.; Yang, M.; Oetjen, L.K.; Zhang, Y.; Li, Q.; Chen, J.; Xia, Y. An enzyme-sensitive probe for photoacoustic imaging and fluorescence detection of protease activity. Nanoscale 2011, 3, 950–953. [Google Scholar] [CrossRef]
- Kim, T.; Huh, Y.-M.; Haam, S.; Lee, K. Activatable nanomaterials at the forefront of biomedical sciences. J. Mater. Chem. 2010, 20, 8194–8206. [Google Scholar] [CrossRef]
- Lammers, T.; Aime, S.; Hennink, W.E.; Storm, G.; Kiessling, F. Theranostic Nanomedicine. Acc. Chem. Res. 2011, 44, 1029–1038. [Google Scholar] [CrossRef]
- Sailor, M.J.; Park, J.-H. Hybrid nanoparticles for detection and treatment of cancer. Adv. Mater. 2012, 24, 3779–3802. [Google Scholar] [CrossRef]
- Koo, H.; Huh, M.S.; Sun, I.-C.; Yuk, S.H.; Choi, K.; Kim, K.; Kwon, I.C. In vivo targeted delivery of nanoparticles for theranosis. Acc. Chem. Res. 2011, 44, 1018–1028. [Google Scholar] [CrossRef]
- Kim, E.; Lee, K.; Huh, Y.-M.; Haam, S. Magnetic nanocomplexes and the physiologicalchallenges associated with their use for cancer imagingand therapy. J. Mater. Chem. B 2013, 1, 729–739. [Google Scholar] [CrossRef]
- Janib, S.M.; Moses, A.S.; Mackay, J.A. Imaging and drug delivery using theranostic nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 1052–1063. [Google Scholar] [CrossRef]
- Xie, J.; Lee, S.; Chen, X. Nanoparticle-based theranostic agents. Adv. Drug Deliv. Rev. 2010, 62, 1064–1079. [Google Scholar] [CrossRef]
- Sing, A.K.; Han, M.A.; Gutwein, L.G.; Rule, M.C.; Knapik, J.A.; Moudgil, B.M.; Grobmyer, S.R.; Brown, S.C. Multi-dye theranostic nanoparticle platform for bioimaging and cancer therapy. Int. J. Nanomed. 2012, 7, 2739–2750. [Google Scholar] [Green Version]
- Fonkong, S.; Theek, B.; Wu, Z.; Koczera, P.; Appold, L.; Jorge, S.; Resch-Genger, U.; Zandvoort, M.; Storm, G.; Kiessling, F.; et al. Image-guided targeted and triggered drug delivery to tumors using polymer-based microbubbles. J. Contr. Release 2012, 163, 75–81. [Google Scholar] [CrossRef]
- Liu, Y.; Ibricevic, A.; Cohen, J.A.; Cohen, J.L.; Gunsten, S.P.; Fréchet, J.M.; Walter, M.J.; Welch, M.J.; Brody, S.L. Impact of hydrogel nanoparticle size and functionalization on in vivo behavior for lung imaging and therapeutics. Mol. Pharm. 1891, 6, 1891–1902. [Google Scholar]
- Park, H.; Yang, J.; Seo, S.; Kim, K.; Suh, J.-S.; Kim, D.; Haam, S.; Yoo, K.-H. Multifunctional nanoparticles for photothermally controlled drug delivery and magnetic resonance imaging enhancement. Small 2008, 4, 192–196. [Google Scholar] [CrossRef]
- Rai, P.; Mallidi, S.; Zheng, X.; Rahmanzadeh, R.; Mir, Y.; Elrington, S.; Jhurshid, A.; Hasan, T. Development and applications of photo-triggered agents. Adv. Drug Deliv. Rev. 2010, 62, 1094–1124. [Google Scholar]
- Chang, Y.-T.; Liao, P.Y.; Cheu, H.-S.; Tseng, Y.-J.; Cheng, F.-Y.; Yeh, C.-S. Near-infrared light-responsive intracellular drug and siRNA release using au nanoensembles with oligonucleotides-capped silica shell. Adv. Mater. 2012, 24, 3309–3314. [Google Scholar]
- Gupta, M.K.; Meyer, T.A.; Nelson, C.E.; Duvall, C.L. Poly(PS-b-DMA) micelles for reactive oxygen species triggered drug release. J. Contr. Release 2012, 162, 591–598. [Google Scholar]
- Graf, N.; Lippard, S.J. ReDOX activation of metal-based prodrugs as a strategy for drug delivery. Adv. Drug Deliv. 2012, 64, 993–1004. [Google Scholar] [CrossRef]
- Gatenby, R.A.; Gawlinski, E.T.; Gmitro, A.F.; Kaylor, B.; Gillies, R.J. A reaction-diffusion model of cancer invasion. Cancer Res. 1996, 56, 5745–5753. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lim, E.-K.; Jang, E.; Lee, K.; Haam, S.; Huh, Y.-M. Delivery of Cancer Therapeutics Using Nanotechnology. Pharmaceutics 2013, 5, 294-317. https://doi.org/10.3390/pharmaceutics5020294
Lim E-K, Jang E, Lee K, Haam S, Huh Y-M. Delivery of Cancer Therapeutics Using Nanotechnology. Pharmaceutics. 2013; 5(2):294-317. https://doi.org/10.3390/pharmaceutics5020294
Chicago/Turabian StyleLim, Eun-Kyung, Eunji Jang, Kwangyeol Lee, Seungjoo Haam, and Yong-Min Huh. 2013. "Delivery of Cancer Therapeutics Using Nanotechnology" Pharmaceutics 5, no. 2: 294-317. https://doi.org/10.3390/pharmaceutics5020294
APA StyleLim, E.-K., Jang, E., Lee, K., Haam, S., & Huh, Y.-M. (2013). Delivery of Cancer Therapeutics Using Nanotechnology. Pharmaceutics, 5(2), 294-317. https://doi.org/10.3390/pharmaceutics5020294



