Recent Advances in Supramolecular Systems for Precision Medicine: Structural Design, Functional Integration, and Clinical Translation Challenges
Abstract
1. Introduction
2. Classification and Design Strategies for Supramolecular Systems
2.1. Dynamic Covalent Supramolecular Systems
2.2. Dynamic Non-Covalent Supramolecular Systems
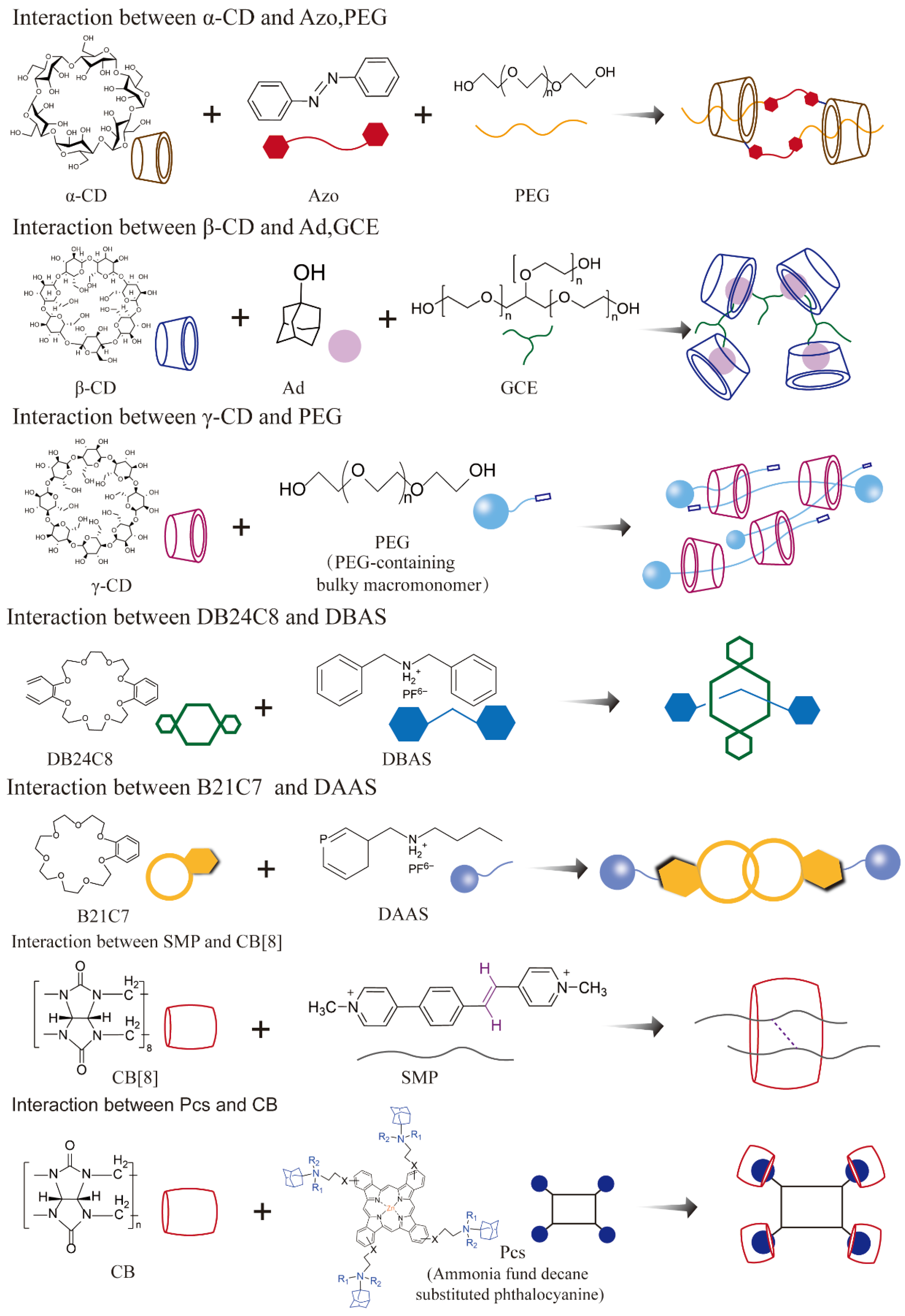
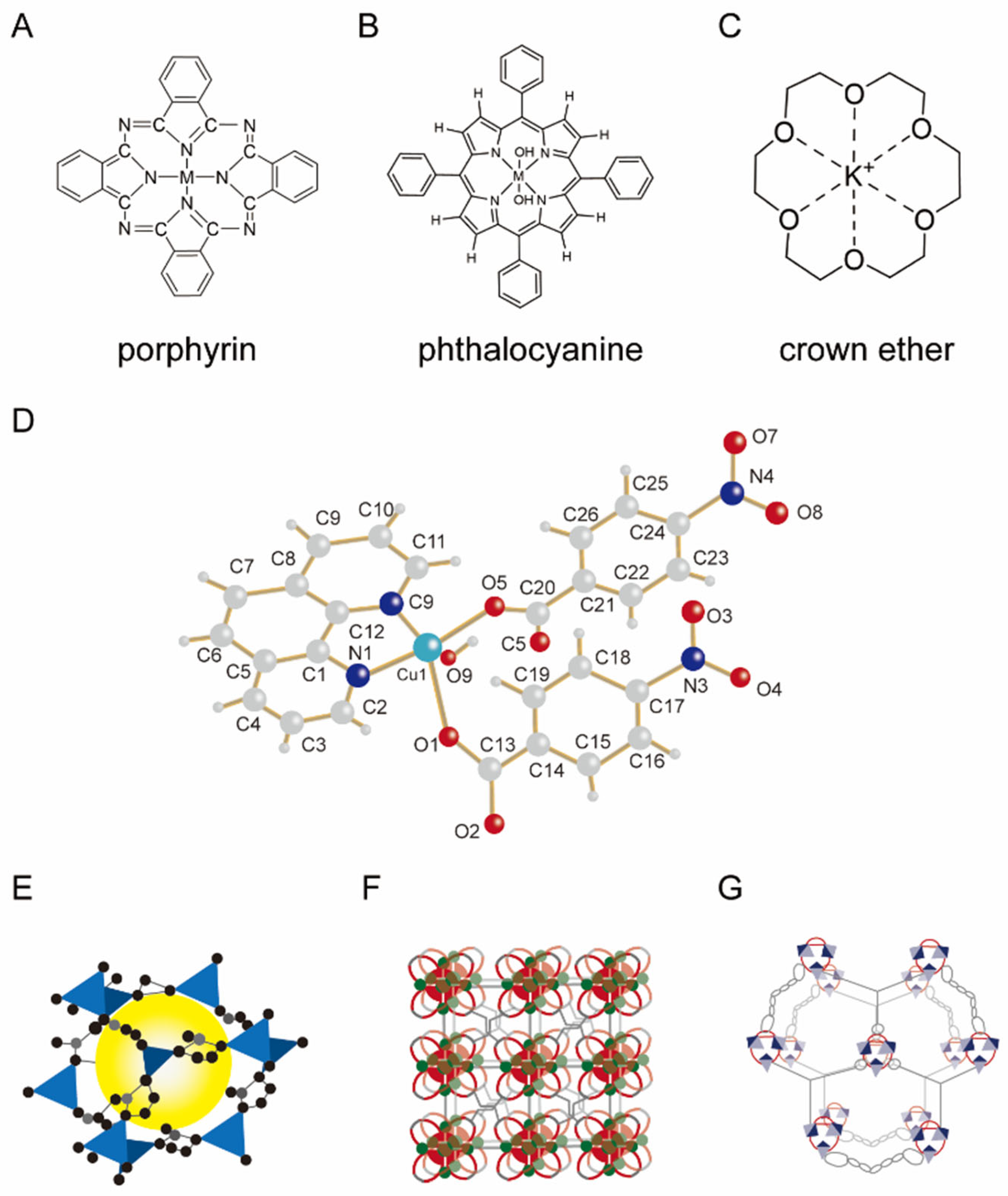
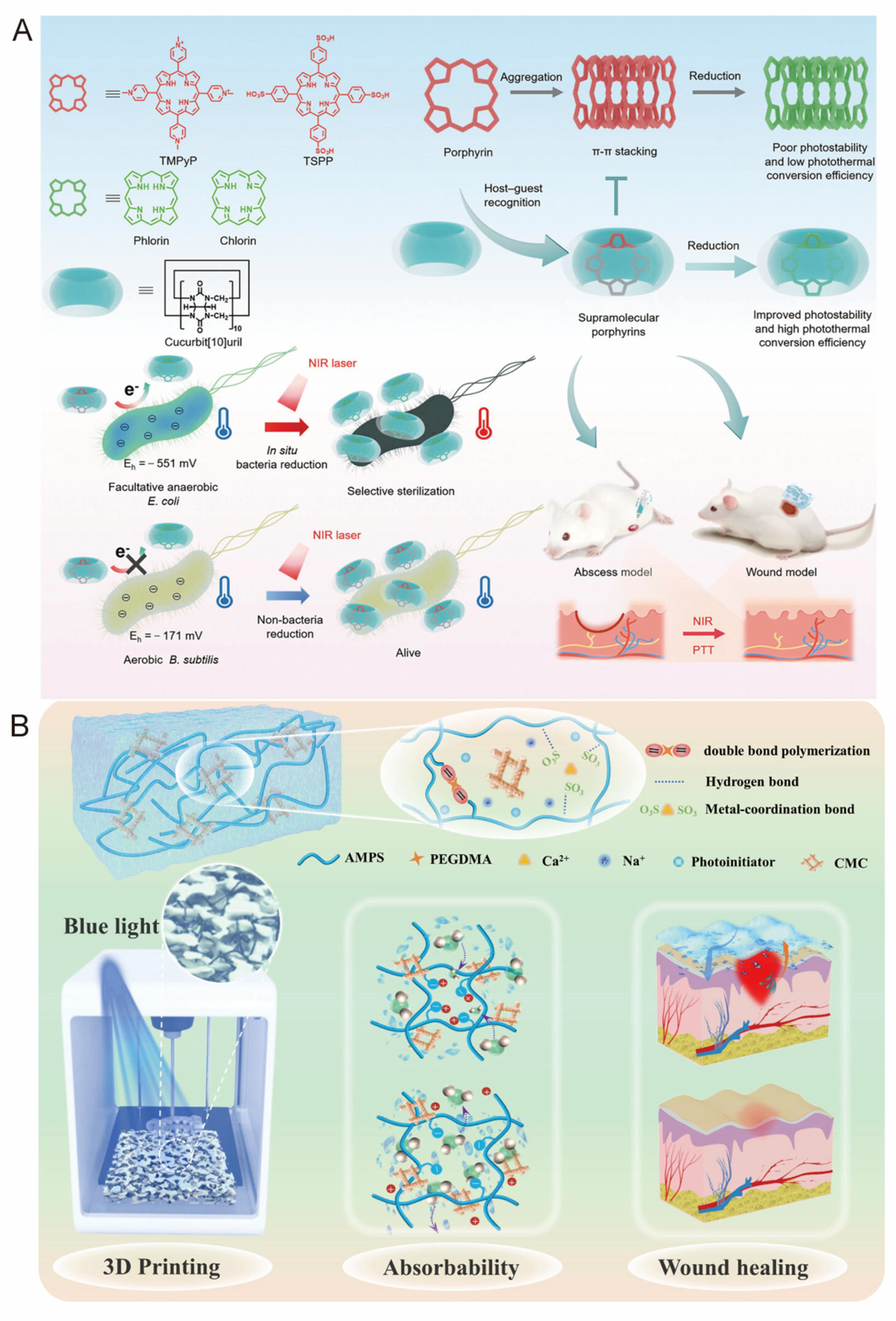
2.2.1. Host–Guest Supramolecular Systems
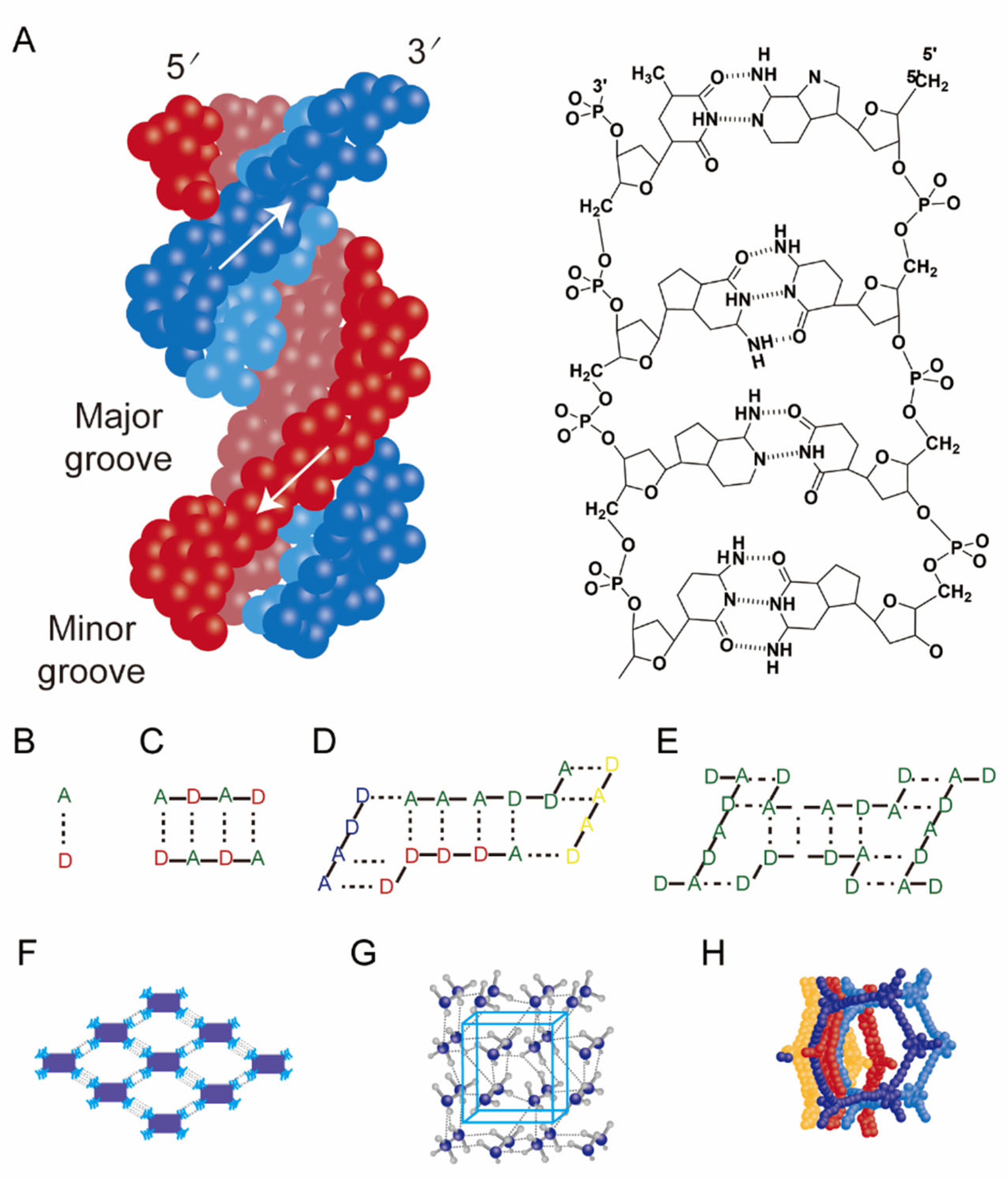
2.2.2. Dynamic Systems Based on Hydrogen-Bonding Networks
2.2.3. Metal-Coordinated Supramolecular Systems
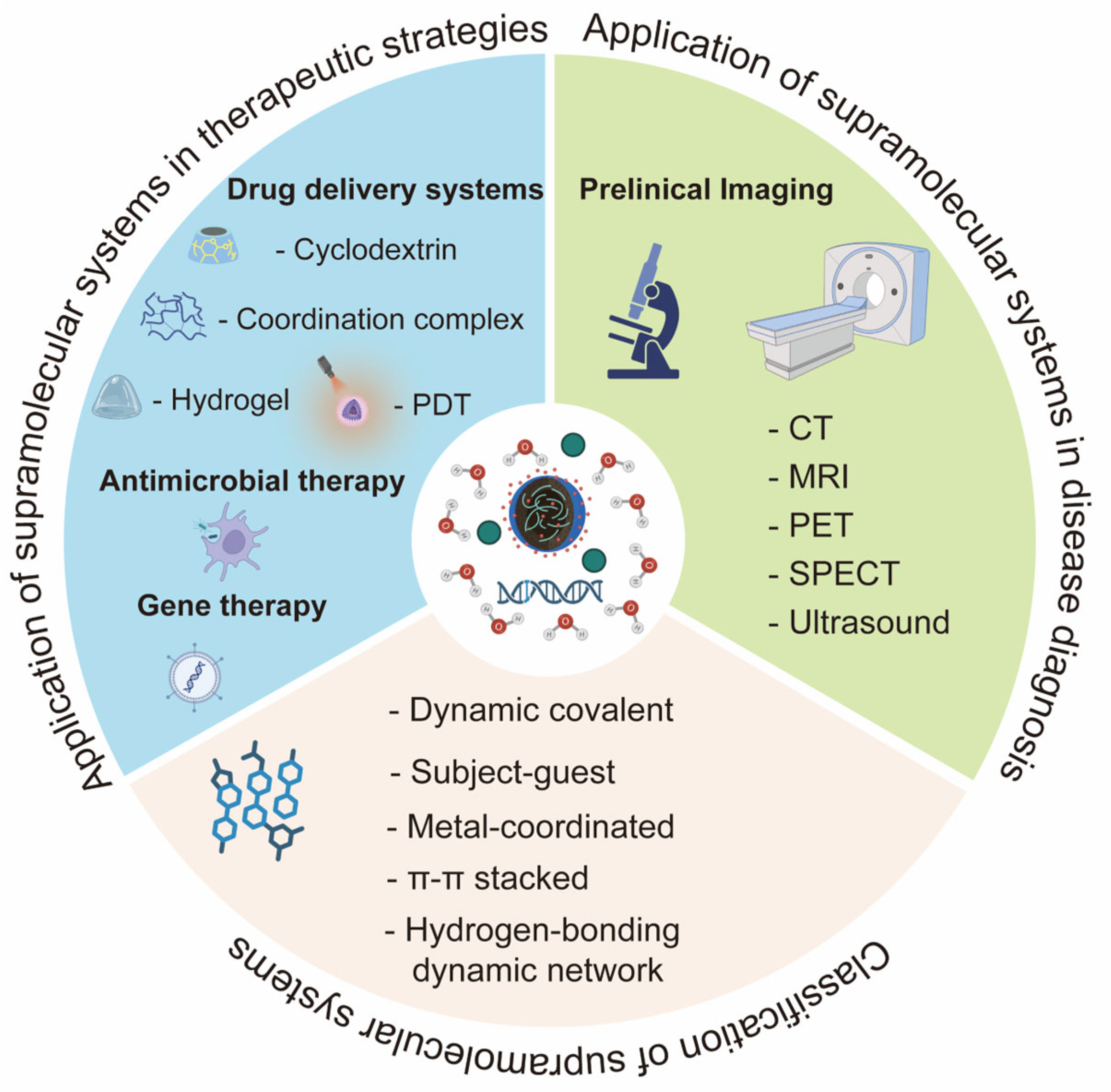
3. Current Research Progress
3.1. Applications of Supramolecular Systems in Disease Diagnosis
3.2. Applications of Supramolecular Systems in Therapeutic Strategies
3.2.1. Supramolecular Drug Delivery Systems
3.2.2. Applications of Supramolecular Systems in Antimicrobial Therapy
3.2.3. Applications of Supramolecular Systems in Gene Therapy
4. Challenges and Future Directions
4.1. Current Challenges
4.2. Future Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lehn, J.-M. Supramolecular chemistry—Scope and perspectives molecules, supermolecules, and molecular devices (nobel lecture). Angew. Chem. Int. Ed. 1988, 27, 89–112. [Google Scholar] [CrossRef]
- Lehn, J.M. Cryptates: The chemistry of macropolycyclic inclusion complexes. Acc. Chem. Res. 1978, 11, 49–57. [Google Scholar] [CrossRef]
- Lehn, J.-M. Supramolecular chemistry: Receptors, catalysts, and carriers. Science 1985, 227, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.-R.; Wang, Y.; Cui, P.-F.; Xing, L.; Lee, J.; Kim, D.; Jiang, H.-L.; Oh, Y.-K. Applications of π–π stacking interactions in the design of drug-delivery systems. J. Control Release 2019, 294, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.J.; Zhou, Y.F.; Huang, X.H.; Zhu, X.Y.; Lu, Y.F.; Shen, J. Functional supramolecular polymers for biomedical applications. Adv. Mater. 2015, 27, 498–526. [Google Scholar] [CrossRef]
- Lundberg, D.J.; Brown, C.M.; Bobylev, E.O.; Oldenhuis, N.J.; Alfaraj, Y.S.; Zhao, J.; Kevlishvili, I.; Kulik, H.J.; Johnson, J.A. Nested non-covalent interactions expand the functions of supramolecular polymer networks. Nat. Commun. 2024, 15, 3951. [Google Scholar] [CrossRef]
- Thakor, A.S. The third pillar of precision medicine—Precision delivery. Medcomm 2025, 6, e70200. [Google Scholar] [CrossRef]
- Chen, Q.; Sun, P.; Zhou, J.; Long, T.; Xiao, A.; Liu, Z.; Xu, S.; Lei, W.; Zhang, R.; Tian, J.; et al. Renal tubular gsdme protects cisplatin nephrotoxicity by impeding ogt-stat3-s100a7a axis in male mice. Nat. Commun. 2025, 16, 6807. [Google Scholar] [CrossRef]
- Lu, P.; Ruan, D.; Huang, M.; Tian, M.; Zhu, K.; Gan, Z.; Xiao, Z. Harnessing the potential of hydrogels for advanced therapeutic applications: Current achievements and future directions. Signal Transduct. Target. 2024, 9, 166. [Google Scholar] [CrossRef]
- Liu, L.; Huang, F.; Liu, J.; Xiao, M. Recent advances of supramolecular systems in precise cancer theranostics. Supramol. Mater. 2025, 4, 100116. [Google Scholar] [CrossRef]
- Yang, X.; Yang, C.; Zhang, S.; Geng, H.; Zhu, A.X.; Bernards, R.; Qin, W.; Fan, J.; Wang, C.; Gao, Q. Precision treatment in advanced hepatocellular carcinoma. Cancer Cell 2024, 42, 180–197. [Google Scholar] [CrossRef]
- Joseph, K.; De Waal, B.; Jansen, S.A.H.; Van Der Tol, J.J.B.; Vantomme, G.; Meijer, E.W. Consequences of vibrational strong coupling on supramolecular polymerization of porphyrins. J. Am. Chem. Soc. 2024, 146, 12130–12137. [Google Scholar] [CrossRef]
- Nuthanakanti, A.; Srivatsan, S.G. Multi-stimuli responsive heterotypic hydrogels based on nucleolipids show selective dye adsorption. Nanoscale Adv. 2020, 2, 4161–4171. [Google Scholar] [CrossRef]
- Bai, H.; Wang, J.; Phan, C.U.; Chen, Q.; Hu, X.; Shao, G.; Zhou, J.; Lai, L.; Tang, G. Cyclodextrin-based host-guest complexes loaded with regorafenib for colorectal cancer treatment. Nat. Commun. 2021, 12, 759. [Google Scholar] [CrossRef]
- Li, S.; Zou, Q.; Li, Y.; Yuan, C.; Xing, R.; Yan, X. Smart peptide-based supramolecular photodynamic metallo-nanodrugs designed by multicomponent coordination self-assembly. J. Am. Chem. Soc. 2018, 140, 10794–10802. [Google Scholar] [CrossRef]
- Lai, J.-C.; Li, L.; Wang, D.-P.; Zhang, M.-H.; Mo, S.-R.; Wang, X.; Zeng, K.-Y.; Li, C.-H.; Jiang, Q.; You, X.-Z.; et al. A rigid and healable polymer cross-linked by weak but abundant zn(ii)-carboxylate interactions. Nat. Commun. 2018, 9, 2725. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Chen, T.; Jin, L.; Zhang, P.; Xie, L.; Zhou, S.; Fan, L.; Wang, L.; Zhang, C.; Tang, N.; et al. A carrier-free supramolecular nano-twin-drug for overcoming irinotecan-resistance and enhancing efficacy against colorectal cancer. J. Nanobiotechnol. 2023, 21, 393. [Google Scholar] [CrossRef] [PubMed]
- D’orchymont, F.; Holland, J.P. Supramolecular rotaxane-based multi-modal probes for cancer biomarker imaging. Angew. Chem. Int. Ed. 2022, 134, e202204072. [Google Scholar] [CrossRef]
- Ooi, Y.J.; Wen, Y.T.; Zhu, J.L.; Song, X.; Li, J. Codelivery of doxorubicin and p53 gene by β-cyclodextrin-based supramolecular nanoparticles formed via host-guest complexation and electrostatic interaction. Biomacromolecules 2024, 25, 2980–2989. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, G.; Shen, J.; Matai, I.; Sachdev, A.; Boy, R.; Tonelli, A.E. Cyclodextrin-based nanostructures. Prog. Mater. Sci. 2022, 124, 100869. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Cheng, P.; Zaworotko, M.J.; Chen, Y.; Zhang, Z. Post-synthetic modifications of metal–organic cages. Nat. Rev. Chem. 2022, 6, 339–356. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, C.; Wang, J.; Feng, Y.; Zhu, Y.; Pan, Y.; Yuan, Y.; Chen, C.; Cao, J.; Lin, J.; et al. Antiadhesive, antibacterial, and anti-inflammatory sandwich-structured zif8-containing gauze for enhanced wound healing. Chem. Eng. J. 2024, 491, 152060. [Google Scholar] [CrossRef]
- Karimi, M.; Eslami, M.; Sahandi-Zangabad, P.; Mirab, F.; Farajisafiloo, N.; Shafaei, Z.; Ghosh, D.; Bozorgomid, M.; Dashkhaneh, F.; Hamblin, M.R. Ph-sensitive stimulus-responsive nanocarriers for targeted delivery of therapeutic agents. WIREs Nanomed. Nanobiotechnol. 2016, 8, 696–716. [Google Scholar] [CrossRef] [PubMed]
- Dheeraj, S.; Chandana, M.; Rupa, M.; Soumya, M.; Chhaya, A.; Deep Shikha, S. Insight into the recent developments of nanoparticles in treatment of cancer and neuro-degenerative disease: A review. Curr. Top. Med. Chem. 2025, 25, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Leng, J.; Wang, N.; Chang, X.L.; Zhang, X.P.; Xu, J.; Yang, Z.L.; Qian, K.L.; Zheng, Z.Q.; Tao, G.H.; Jia, X.D.; et al. Neodymium nitrate promotes the apoptosis of mouse liver cells via bcl2l1/caspase 3 pathway. Toxicol. Mech. Methods 2025, 18:16, 3911. [Google Scholar] [CrossRef]
- Oh, J.Y.; Kim, H.S.; Palanikumar, L.; Go, E.M.; Jana, B.; Park, S.A.; Kim, H.Y.; Kim, K.; Seo, J.K.; Kwak, S.K.; et al. Cloaking nanoparticles with protein corona shield for targeted drug delivery. Nat. Commun. 2018, 9, 4548. [Google Scholar] [CrossRef]
- Zheng, Y.; Baidya, A.; Annabi, N. Molecular design of an ultra-strong tissue adhesive hydrogel with tunable multifunctionality. Bioact. Mater. 2023, 29, 214–229. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, X.; Zhang, D.; Cui, H.; Tian, X.; Du, W.; Yang, Z.; Wan, D.; Qiu, Z.; Liu, C.; et al. Precision-guided stealth missiles in biomedicine: Biological carrier-mediated nanomedicine hitchhiking strategy. Adv. Sci. 2025, 12, 2504672. [Google Scholar] [CrossRef]
- Zhao, F.; Yin, H.; Li, J. Supramolecular self-assembly forming a multifunctional synergistic system for targeted co-delivery of gene and drug. Biomaterials 2014, 35, 1050–1062. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Bai, X.; Li, P.; Su, D.; Zhang, W.; Zhang, W.; Tang, B. Highly specific cys fluorescence probe for living mouse brain imaging via evading reaction with other biothiols. Anal. Chem. 2019, 91, 8591–8594. [Google Scholar] [CrossRef]
- Lee, H.; Shahrivarkevishahi, A.; Lumata, J.L.; Luzuriaga, M.A.; Hagge, L.M.; Benjamin, C.E.; Brohlin, O.R.; Parish, C.R.; Firouzi, H.R.; Nielsen, S.O.; et al. Supramolecular and biomacromolecular enhancement of metal-free magnetic resonance imaging contrast agents. Chem. Sci. 2020, 11, 2045–2050. [Google Scholar] [CrossRef]
- Li, Y.; Huang, F.; Stang, P.J.; Yin, S. Supramolecular coordination complexes for synergistic cancer therapy. Acc. Chem. Res. 2024, 57, 1174–1187. [Google Scholar] [CrossRef] [PubMed]
- António, J.P.M.; Gandioso, A.; Nemati, F.; Soliman, N.; Vinck, R.; Sun, F.; Robert, C.; Burckel, P.; Decaudin, D.; Thomas, C.M.; et al. Polymeric encapsulation of a ruthenium(ii) polypyridyl complex: From synthesis to in vivo studies against high-grade epithelial ovarian cancer. Chem. Sci. 2023, 14, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, T.; Sun, Y.; Wang, S.; Wang, W.; Kuang, Z.; Duan, M.; Du, T.; Liu, M.; Wu, L.; et al. Carbon-spaced tandem-disulfide bond bridge design addresses limitations of homodimer prodrug nanoassemblies: Enhancing both stability and activatability. J. Am. Chem. Soc. 2024, 146, 22675–22688. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Luo, C.; Yu, H.; Zhang, X.; Chen, Q.; Yang, W.; Wang, M.; Kan, Q.; Zhang, H.; Wang, Y.; et al. Disulfide bond-driven oxidation- and reduction-responsive prodrug nanoassemblies for cancer therapy. Nano Lett. 2018, 18, 3643–3650. [Google Scholar] [CrossRef]
- Ma, W.; Wang, X.; Zhang, D.; Mu, X. Research progress of disulfide bond based tumor microenvironment targeted drug delivery system. Int. J. Nanomed. 2024, 19, 7547–7566. [Google Scholar] [CrossRef]
- Cho, S.; Hwang, S.Y.; Oh, D.X.; Park, J. Recent progress in self-healing polymers and hydrogels based on reversible dynamic b–o bonds: Boronic/boronate esters, borax, and benzoxaborole. J. Mater. Chem. A 2021, 9, 14630–14655. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, H.; In, I.; Park, S.Y. Ph/redox/photo responsive polymeric micelle via boronate ester and disulfide bonds with spiropyran-based photochromic polymer for cell imaging and anticancer drug delivery. Eur. Polym. J. 2014, 57, 1–10. [Google Scholar] [CrossRef]
- Guo, X.; Shi, C.; Wang, J.; Di, S.; Zhou, S. Ph-triggered intracellular release from actively targeting polymer micelles. Biomaterials 2013, 34, 4544–4554. [Google Scholar] [CrossRef]
- Shi, Y.; Pan, X.; Xu, S.; Zhu, H.; Zhao, B.; Sun, Z.; Dong, R.; Li, N.; Hou, X.; Yang, X. Synthesis of the ph-sensitive nanoparticles based on the acylhydrazone bonds conjugated doxorubicin and studies on their in vivo anti-tumor effects. Eur. J. Med. Chem. 2023, 260, 115715. [Google Scholar] [CrossRef]
- Liu, Y.; Si, L.; Jiang, Y.; Jiang, S.; Zhang, X.; Li, S.; Chen, J.; Hu, J. Design of ph-responsive nanomaterials based on the tumor microenvironment. Int. J. Nanomed. 2025, 20, 705–721. [Google Scholar] [CrossRef]
- Hai, Y.; Ye, H.; Li, Z.; Zou, H.; Lu, H.; You, L. Light-induced formation/scission of c–n, c–o, and c–s bonds enables switchable stability/degradability in covalent systems. J. Am. Chem. Soc. 2021, 143, 20368–20376. [Google Scholar] [CrossRef] [PubMed]
- Angelova, S.; Nikolova, V.; Pereva, S.; Spassov, T.; Dudev, T. A-cyclodextrin: How effectively can its hydrophobic cavity be hydrated? J. Phys. Chem. B 2017, 121, 9260–9267. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Zhou, J. Pillararene-based supramolecular polymers for cancer therapy. Molecules 2023, 28, 1470. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Tian, J.; Yue, T.; Cao, H.; Chu, J.; Cai, H.; Zhang, W. Pillar[5]arene-based acid-triggered supramolecular porphyrin photosensitizer for combating bacterial infections and biofilm dispersion. Adv. Healthc. Mater. 2022, 11, 2102015. [Google Scholar] [CrossRef]
- Zhou, J.; Li, J.; Du, X.W.; Xu, B. Supramolecular biofunctional materials. Biomaterials 2017, 129, 1–27. [Google Scholar] [CrossRef]
- Ren, Y.; Dong, X. Dynamic polymeric materials via hydrogen-bond cross-linking: Effect of multiple network topologies. Prog. Polym. Sci. 2024, 158, 101890. [Google Scholar] [CrossRef]
- Annabi, N.; Tamayol, A.; Uquillas, J.A.; Akbari, M.; Bertassoni, L.E.; Cha, C.; Camci-Unal, G.; Dokmeci, M.R.; Peppas, N.A.; Khademhosseini, A. 25th anniversary article: Rational design and applications of hydrogels in regenerative medicine. Adv. Mater. 2014, 26, 85–124. [Google Scholar] [CrossRef]
- Lin, Z.-J.; Mahammed, S.A.R.; Liu, T.-F.; Cao, R. Multifunctional porous hydrogen-bonded organic frameworks: Current status and future perspectives. ACS Cent. Sci. 2022, 8, 1589–1608. [Google Scholar] [CrossRef]
- Pan, Y.; Zeng, F.; Luan, X.; He, G.; Qin, S.; Lu, Q.; He, B.; Han, X.; Song, Y. Polyamine-depleting hydrogen-bond organic frameworks unleash dendritic cell and t cell vigor for targeted crispr/cas-assisted cancer immunotherapy. Adv. Mater. 2025, 37, 2411886. [Google Scholar] [CrossRef]
- Wu, J.; Zeng, F.; Fan, Z.; Xuan, S.; Hua, Z.; Liu, G. Hierarchical hydrogen bonds endow supramolecular polymers with high strength, toughness, and self-healing properties. Adv. Funct. Mater. 2024, 34, 2410518. [Google Scholar]
- Song, P.; Wang, H. High-performance polymeric materials through hydrogen-bond cross-linking. Adv. Mater. 2020, 32, 1901244. [Google Scholar]
- Wang, F.; Yu, X.; Cao, Z.; Liu, Y.; Jiang, X.; Gu, X. Synergic enhancement of hydrogel upon multi-level hydrogen bonds via macromolecular design for dual-mode electronic skin. Chem. Eng. J. 2024, 489, 151249. [Google Scholar]
- Huang, A.; Yang, H.; Huang, S.; Chen, G.; Ouyang, G. Hydrogen-bonded supramolecular crystal: A manual exoskeleton for bioentity. Matter 2023, 6, 2635–2646. [Google Scholar] [CrossRef]
- Luo, J.; Wang, J.-W.; Zhang, J.-H.; Lai, S.; Zhong, D.-C. Hydrogen-bonded organic frameworks: Design, structures and potential applications. Crystengcomm 2018, 20, 5884–5898. [Google Scholar] [CrossRef]
- Rubio-Martinez, M.; Avci-Camur, C.; Thornton, A.W.; Imaz, I.; Maspoch, D.; Hill, M.R. New synthetic routes towards mof production at scale. Chem. Soc. Rev. 2017, 46, 3453–3480. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y.-W. Metal–organic frameworks for biomedical applications. Small 2020, 16, 1906846. [Google Scholar] [CrossRef]
- Chen, J.; Gao, Y.; Shi, L.; Yu, W.; Sun, Z.; Zhou, Y.; Liu, S.; Mao, H.; Zhang, D.; Lu, T.; et al. Phase-locked constructing dynamic supramolecular ionic conductive elastomers with superior toughness, autonomous self-healing and recyclability. Nat. Commun. 2022, 13, 4868. [Google Scholar] [CrossRef]
- Wang, R.; Xu, T.; Yang, Y.; Zhang, M.; Xie, R.; Cheng, Y.; Zhang, Y. Tough polyurethane hydrogels with a multiple hydrogen-bond interlocked bicontinuous phase structure prepared by in situ water-induced microphase separation. Adv. Mater. 2025, 37, 2412083. [Google Scholar]
- Ranji-Burachaloo, H.; Gurr, P.A.; Dunstan, D.E.; Qiao, G.G. Cancer treatment through nanoparticle-facilitated fenton reaction. ACS Nano 2018, 12, 11819–11837. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, N.; Cheng, S.-C.; Xu, K.; Deng, Z.; Chen, S.; Xu, Z.; Xie, K.; Tse, M.-K.; Shi, P.; et al. Phorbiplatin, a highly potent pt(iv) antitumor prodrug that can be controllably activated by red light. Chem 2019, 5, 3151–3165. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, G.; Tan, C.; Liu, Y.; Wang, X.-F. Concentration gradient-induced syntheses and crystal structures of two copper(ii) coordination polymer based on phthalic acid and 2,2′-bipyridine. Molecules 2025, 30, 1953. [Google Scholar] [CrossRef]
- Mckinlay, A.C.; Morris, R.E.; Horcajada, P.; Férey, G.; Gref, R.; Couvreur, P.; Serre, C. Biomofs: Metal–organic frameworks for biological and medical applications. Angew. Chem. Int. Ed. 2010, 49, 6260–6266. [Google Scholar]
- Sukur, S.; Ranc, V. Magnetic 2d transition-metal-based nanomaterials in biomedicine: Opportunities and challenges in cancer therapy. Materials 2025, 18, 2570. [Google Scholar] [PubMed]
- Cao, C.; Wang, X.; Yang, N.; Song, X.; Dong, X. Recent advances of cancer chemodynamic therapy based on fenton/fenton-like chemistry. Chem. Sci. 2022, 13, 863–889. [Google Scholar] [PubMed]
- Liu, Z.; Zhou, W.; Li, J.; Zhang, H.; Dai, X.; Liu, Y.; Liu, Y. High-efficiency dynamic sensing of biothiols in cancer cells with a fluorescent β-cyclodextrin supramolecular assembly. Chem. Sci. 2020, 11, 4791–4800. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liang, C.; Song, X.; Yi, X.; Yang, K.; Feng, L.; Liu, Z. Hybrid protein nano-reactors enable simultaneous increments of tumor oxygenation and iodine-131 delivery for enhanced radionuclide therapy. Small 2019, 15, e1903628. [Google Scholar] [CrossRef]
- Zeng, X.; Xiao, Y.; Lin, J.; Li, S.; Zhou, H.; Nong, J.; Xu, G.; Wang, H.; Xu, F.; Wu, J.; et al. Near-infrared ii dye-protein complex for biomedical imaging and imaging-guided photothermal therapy. Adv. Healthc. Mater. 2018, 7, 1800589. [Google Scholar]
- Zengin, A.; Hafeez, S.; Habibovic, P.; Baker, M.; Van Rijt, S. Extracellular matrix mimetic supramolecular hydrogels reinforced with covalent crosslinked mesoporous silica nanoparticles. J. Mater. Chem. B 2024, 12, 12577–12588. [Google Scholar] [CrossRef]
- Yu, R.; Yang, Y.; He, J.; Li, M.; Guo, B. Novel supramolecular self-healing silk fibroin-based hydrogel via host–guest interaction as wound dressing to enhance wound healing. Chem. Eng. J. 2021, 417, 128278. [Google Scholar]
- Sollenberger, C.H.; Qiu, R.; Sai, H.; Carrow, J.K.; Fyrner, T.; Gao, Z.; Palmer, L.C.; Stupp, S.I. Boosting chondrocyte bioactivity with ultra-sulfated glycopeptide supramolecular polymers. Acta Biomater. 2024, 189, 103–115. [Google Scholar] [CrossRef]
- Tang, M.-D.Q.; Tran, N.B.; Nguyen, T.-H.T.; Nguyen, K.-U.H.; Trinh, N.-T.; Van Vo, T.; Kobayashi, M.; Yoshitomi, T.; Nagasaki, Y.; Vong, L.B. Development of oral ph-sensitive redox nanotherapeutics for gastric ulcer therapy. J. Control Release 2024, 375, 758–766. [Google Scholar] [CrossRef]
- Wu, D.; Du, X.; Xue, Q.; Zhou, J.; Ping, K.; Cao, Y.; Liu, S.; Zhu, Q. Supramolecular porphyrin photosensitizers based on host-guest recognition for in situ bacteria-responsive near-infrared photothermal therapy. Adv. Healthc. Mater. 2024, 13, e2401662. [Google Scholar] [CrossRef]
- Pan, Y.; Fan, Z.; Yu, S.; Xia, L.; Li, J. Ros-responsive supramolecular antimicrobial peptides-based nanoprodrugs for cervical cancer therapy. Colloid Surf. B. 2025, 247, 114411. [Google Scholar] [CrossRef] [PubMed]
- He, X.-C.; Chen, X.-N.; Liu, Y.-H.; Zhong, X.; Qiang, L.; Wang, H.-Q.; Wang, F.-Z.; Wang, J.-S.; Li, C.-H.; Zheng, P.-F. A blue light 3d printable hydrogel with water absorption, antibacterial, and hemostatic properties for skin wound healing. Chem. Eng. J. 2024, 493, 152439. [Google Scholar] [CrossRef]
- Wang, H.; Feng, Z.; Xu, B. Supramolecular assemblies of peptides or nucleopeptides for gene delivery. Theranostics 2019, 9, 3213–3222. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Jiang, H.; Cao, J.; Wu, J.; Ge, F. Biobased reversible acid-sensitive colorimetric fabric sensor based on natural polyphenols and amino acid. Chem. Eng. J. 2025, 520, 166396. [Google Scholar] [CrossRef]
- Nguyen, M.T.N.; Nguyen, T.D.; Han, J.-H.; Lee, J.S. Synthesis of pdms chain structure with introduced dynamic covalent bonding for high-performance rehealable tactile sensor application. Small Methods 2024, 8, 2400163. [Google Scholar] [CrossRef]
- Carden, G.P.; Martins, M.L.; Toleutay, G.; Ge, S.; Li, B.; Zhao, S.; Sokolov, A.P. Critical role of free amine groups in the imine bonds exchange in dynamic covalent networks. Macromolecules 2024, 57, 9920. [Google Scholar] [CrossRef]
- Liu, H.; Lu, H.-H.; Zhuang, J.; Thayumanavan, S. Three-component dynamic covalent chemistry: From janus small molecules to functional polymers. J. Am. Chem. Soc. 2021, 143, 20735–20746. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Zhao, L.; Zhang, Y.; Li, Z.-T.; Huang, F. Multiple hydrogen bonding driven supramolecular architectures and their biomedical applications. Chem. Soc. Rev. 2024, 53, 1592–1623. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Win, K.Y.; Liu, S.; Teng, C.P.; Zheng, Y.; Han, M.Y. Surface-functionalized nanoparticles for biosensing and imaging-guided therapeutics. Nanoscale 2013, 5, 3127–3148. [Google Scholar] [CrossRef]
- Maity, S.; Deb, V.K.; Mondal, S.; Chakraborty, A.; Pramanick, K.; Adhikari, S. Leveraging supramolecular systems in biomedical breakthroughs. Biofactors 2025, 51, e70005. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhou, Z.; Xu, L.; Zhong, H.; Xiong, B.; Ren, T.; Li, Z.; Yuan, L.; Zhang, X.B. Multivalent supramolecular fluorescent probes for accurate disease imaging. Sci. Adv. 2024, 10, eadp8719. [Google Scholar] [CrossRef]
- Xu, X.D.; Lin, B.B.; Feng, J.; Wang, Y.; Cheng, S.X.; Zhang, X.Z.; Zhuo, R.X. Biological glucose metabolism regulated peptide self-assembly as a simple visual biosensor for glucose detection. Macromol. Rapid Commun. 2012, 33, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Chagri, S.; Ng, D.Y.W.; Weil, T. Designing bioresponsive nanomaterials for intracellular self-assembly. Nat. Rev. Chem. 2022, 6, 320–338. [Google Scholar] [CrossRef]
- Han, J.; Li, H.; Yoon, J. Activated supramolecular nano-agents: From diagnosis to imaging-guided tumor treatment. Nano Today 2022, 43, 101392. [Google Scholar] [CrossRef]
- Hong, L.; Li, W.; Li, Y.; Yin, S. Nanoparticle-based drug delivery systems targeting cancer cell surfaces. RSC Adv. 2023, 13, 21365–21382. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Z. Albumin carriers for cancer theranostics: A conventional platform with new promise. Adv. Mater. 2016, 28, 10557–10566. [Google Scholar] [CrossRef]
- Hazarika, B.; Singh, V.P. Macrocyclic supramolecular biomaterials in anti-cancer therapeutics. Chin. Chem. Lett. 2023, 34, 108220. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, F.H.; Li, Z.T.; Liu, Y. Controllable macrocyclic supramolecular assemblies in aqueous solution. Sci. China Chem. 2018, 61, 979–992. [Google Scholar] [CrossRef]
- Nanda, J.; Banerjee, A. Β-amino acid containing proteolitically stable dipeptide based hydrogels: Encapsulation and sustained release of some important biomolecules at physiological ph and temperature. Soft Matter 2012, 8, 3380–3386. [Google Scholar] [CrossRef]
- Wei, X.; Yu, C.Y.; Wei, H. Application of cyclodextrin for cancer immunotherapy. Molecules 2023, 28, 5610. [Google Scholar] [CrossRef] [PubMed]
- Abou-Elkacem, L.; Arns, S.; Brix, G.; Gremse, F.; Zopf, D.; Kiessling, F.; Lederle, W. Regorafenib inhibits growth, angiogenesis, and metastasis in a highly aggressive, orthotopic colon cancer model. Mol. Cancer Ther. 2013, 12, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Mir, O.; Brodowicz, T.; Italiano, A.; Wallet, J.; Blay, J.Y.; Bertucci, F.; Chevreau, C.; Piperno-Neumann, S.; Bompas, E.; Salas, S.; et al. Safety and efficacy of regorafenib in patients with advanced soft tissue sarcoma (regosarc): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2016, 17, 1732–1742. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Misra, S.K.; Saha, M.L.; Lahiri, N.; Louie, J.; Pan, D.; Stang, P.J. Orthogonal self-assembly of an organoplatinum(ii) metallacycle and cucurbit[8]uril that delivers curcumin to cancer cells. Proc. Natl. Acad. Sci. USA 2018, 115, 8087–8092. [Google Scholar] [CrossRef]
- Annane, D.; Bellissant, E.; Cavaillon, J.M. Septic shock. Lancet 2005, 365, 63–78. [Google Scholar] [CrossRef]
- Fricker, S.P.; Slade, E.; Powell, N.A.; Vaughan, O.J.; Henderson, G.R.; Murrer, B.A.; Megson, I.L.; Bisland, S.K.; Flitney, F.W. Ruthenium complexes as nitric oxide scavengers: A potential therapeutic approach to nitric oxide-mediated diseases. Br. J. Pharmacol. 1997, 122, 1441–1449. [Google Scholar] [CrossRef]
- He, C.; Duan, X.; Guo, N.; Chan, C.; Poon, C.; Weichselbaum, R.R.; Lin, W. Core-shell nanoscale coordination polymers combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. Nat. Commun. 2016, 7, 12499. [Google Scholar] [CrossRef]
- Santamaria-Garcia, V.J.; Flores-Hernandez, D.R.; Contreras-Torres, F.F.; Cué-Sampedro, R.; Sánchez-Fernández, J.A. Advances in the structural strategies of the self-assembly of photoresponsive supramolecular systems. Int. J. Mol. Sci. 2022, 23, 7998. [Google Scholar] [CrossRef]
- Kouwer, P.H.; Koepf, M.; Le Sage, V.A.; Jaspers, M.; Van Buul, A.M.; Eksteen-Akeroyd, Z.H.; Woltinge, T.; Schwartz, E.; Kitto, H.J.; Hoogenboom, R.; et al. Responsive biomimetic networks from polyisocyanopeptide hydrogels. Nature 2013, 493, 651–655. [Google Scholar] [CrossRef]
- Chan, G.; Mooney, D.J. New materials for tissue engineering: Towards greater control over the biological response. Trends Biotechnol. 2008, 26, 382–392. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Hubbell, J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef]
- Rijns, L.; Baker, M.B.; Dankers, P.Y.W. Using chemistry to recreate the complexity of the extracellular matrix: Guidelines for supramolecular hydrogel-cell interactions. J. Am. Chem. Soc. 2024, 146, 17539–17558. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Wang, H.; Ma, P.X.; Guo, B. Self-healing conductive hydrogels: Preparation, properties and applications. Nanoscale 2020, 12, 1224–1246. [Google Scholar] [CrossRef]
- Chouhan, D.; Chakraborty, B.; Nandi, S.K.; Mandal, B.B. Role of non-mulberry silk fibroin in deposition and regulation of extracellular matrix towards accelerated wound healing. Acta Biomater. 2017, 48, 157–174. [Google Scholar] [CrossRef]
- Gou, G.H.; Tseng, F.J.; Wang, S.H.; Chen, P.J.; Shyu, J.F.; Weng, C.F.; Pan, R.Y. Autologous chondrocyte implantation versus microfracture in the knee: A meta-analysis and systematic review. Arthroscopy 2020, 36, 289–303. [Google Scholar] [CrossRef]
- Niethammer, T.R.; Loitzsch, A.; Horng, A.; Baur-Melnyk, A.; Bendiks, M.; Gülecyüz, M.F.; Müller, P.E.; Pietschmann, M.F. Graft hypertrophy after third-generation autologous chondrocyte implantation has no correlation with reduced cartilage quality: Matched-pair analysis using t2-weighted mapping. Am. J. Sports Med. 2018, 46, 2414–2421. [Google Scholar] [CrossRef]
- Fagundes, F.L.; Pereira, Q.C.; Zarricueta, M.L.; Dos Santos, R.D.C. Malvidin protects against and repairs peptic ulcers in mice by alleviating oxidative stress and inflammation. Nutrients 2021, 13, 3312. [Google Scholar] [CrossRef] [PubMed]
- Yeomans, N.; Lanas, A.; Labenz, J.; Van Zanten, S.V.; Van Rensburg, C.; Rácz, I.; Tchernev, K.; Karamanolis, D.; Roda, E.; Hawkey, C.; et al. Efficacy of esomeprazole (20 mg once daily) for reducing the risk of gastroduodenal ulcers associated with continuous use of low-dose aspirin. Off. J. Am. Coll. Gastroenterol.|ACG 2008, 103, 2465–2473. [Google Scholar] [CrossRef] [PubMed]
- Ünal, A.T.; Navruz, F.Z.; Korcan, S.E.; İnce, S.; Göçer, E.U. Research on genotoxicity evaluation of the fungal alpha-amylase enzyme on drosophila melanogaster. Biology 2025, 14, 219. [Google Scholar] [CrossRef]
- Qi, Z.; Bharate, P.; Lai, C.H.; Ziem, B.; Böttcher, C.; Schulz, A.; Beckert, F.; Hatting, B.; Mülhaupt, R.; Seeberger, P.H.; et al. Multivalency at interfaces: Supramolecular carbohydrate-functionalized graphene derivatives for bacterial capture, release, and disinfection. Nano Lett. 2015, 15, 6051–6057. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, H.; Wang, Z.; Yuan, C.; Lu, J.; Yan, X. Treatment of superbug infection through a membrane-disruption and immune-regulation cascade effect based on supramolecular peptide hydrogels. Adv. Funct. Mater. 2023, 33, 2305726. [Google Scholar] [CrossRef]
- Hou, C.; Gu, Y.; Yuan, W.; Zhang, W.; Xiu, X.; Lin, J.; Gao, Y.; Liu, P.; Chen, X.; Song, L. Application of microfluidic chips in the simulation of the urinary system microenvironment. Mater. Today Bio 2023, 19, 100553. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhao, Z.; Zhang, Q.; Qi, H.J.; Fang, D. Mechanics of shape distortion of dlp 3d printed structures during uv post-curing. Soft Matter 2019, 15, 6151–6159. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Shu, C.; Shen, Y.; Li, M.; Ma, N.; Zhao, J. 3d bioprinting of the airways and lungs for applications in tissue engineering and in vitro models. J. Tissue Eng. 2024, 15, 20417314241309183. [Google Scholar] [CrossRef]
- Jounai, N.; Kobiyama, K.; Takeshita, F. Intracellular inflammatory sensors for foreign invaders and substances of self-origin. Self Nonself 2012, 738, 60–78. [Google Scholar]
- Lee, Y.; Kim, B.; Lee, D.; Cheon, S.Y.; Lim, S.G.; Kim, Y.; Koo, H. In vivo delivery systems for crispr genome editing: Viral and non-viral carriers. Appl. Phys. Rev. 2025, 12, 021319. [Google Scholar] [CrossRef]
- Tümmler, B.; Pallenberg, S.T.; Dittrich, A.-M.; Graeber, S.Y.; Naehrlich, L.; Sommerburg, O.; Mall, M.A. Progress of personalized medicine of cystic fibrosis in the times of efficient cftr modulators. Mol. Cell. Pediatr. 2025, 12, 6. [Google Scholar] [CrossRef]
- Raguram, A.; Banskota, S.; Liu, D.R. Therapeutic in vivo delivery of gene editing agents. Cell 2022, 185, 2806–2827. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Xing, Y.; Li, J.; Deng, C.; Li, Y.; Ren, X.; Zhang, D. Rebuilding the vascular network: In vivo and in vitro approaches. Front. Cell Dev. Biol. 2021, 9, 639299. [Google Scholar] [CrossRef]
- Howell, D.W.; Duran, C.L.; Tsai, S.P.; Bondos, S.E.; Bayless, K.J. Functionalization of ultrabithorax materials with vascular endothelial growth factor enhances angiogenic activity. Biomacromolecules 2016, 17, 3558–3569. [Google Scholar] [CrossRef]
- Xie, Y.; Zhou, Y.; Guo, J.; Zhang, Z.; Zhu, Y.; Goldys, E.M.; Deng, W.; Chen, W. Illuminating gene delivery: Insights into the light-induced gene delivery systems with emphasis on mrna therapeutics. Mater. Today 2025, 88, 959–978. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, Y.; He, C.; Zhou, X.; Qi, H.; Wang, Y.; Chen, C.; Wang, D.; Li, J.; Ke, Y.; et al. Ordered packing of β-sheet nanofibrils into nanotubes: Multi-hierarchical assembly of designed short peptides. Nano Lett. 2021, 21, 10199–10207. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Kulsoom; Sun, Y.; Li, Y.; Wang, Z.; Xue, L.; Wang, F. Advancing cancer therapy: Nanomaterial-based encapsulation strategies for enhanced delivery and efficacy of curcumin. Mater. Today Bio 2025, 33, 101963. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.; Chalupska, R.; Louro, A.F.; Firth, M.; González-King Garibotti, H.; Hultin, L.; Kohl, F.; Lázaro-Ibáñez, E.; Lindgren, J.; Musa, G.; et al. Barcoded hybrids of extracellular vesicles and lipid nanoparticles for multiplexed analysis of tissue distribution. Adv. Sci. 2025, 12, 2407850. [Google Scholar] [CrossRef]
- Ferreira, M.; Carvalho, V.; Ribeiro, J.; Lima, R.A.; Teixeira, S.; Pinho, D. Advances in microfluidic systems and numerical modeling in biomedical applications: A review. Micromachines 2024, 15, 873. [Google Scholar] [CrossRef]
- Zhu, H.; Mah Jian Qiang, J.; Wang, C.G.; Chan, C.Y.; Zhu, Q.; Ye, E.; Li, Z.; Loh, X.J. Flexible polymeric patch based nanotherapeutics against non-cancer therapy. Bioact. Mater. 2022, 18, 471–491. [Google Scholar] [CrossRef]
- Gostaviceanu, A.; Gavrilaş, S.; Copolovici, L.; Copolovici, D.M. Graphene-oxide peptide-containing materials for biomedical applications. Int. J. Mol. Sci. 2024, 25, 10174. [Google Scholar] [CrossRef]
- Pandey, S.; Yadav, P. Liquid biopsy in cancer management: Integrating diagnostics and clinical applications. Pract. Lab. Med. 2025, 43, e00446. [Google Scholar] [CrossRef]
- Oliveira, C.B.P.; Gomes, V.; Ferreira, P.M.T.; Martins, J.A.; Jervis, P.J. Peptide-based supramolecular hydrogels as drug delivery agents: Recent advances. Gels 2022, 8, 706. [Google Scholar] [CrossRef]
- Busiak, R.; Masek, A.; Węgier, A.; Rylski, A. Accelerated aging of epoxy biocomposites filled with cellulose. Materials 2022, 15, 3256. [Google Scholar] [CrossRef]



| Type of Supramolecular System | Interaction Mechanism | Representative Structures | Biomedical Applications | Challenges | References |
|---|---|---|---|---|---|
| Dynamic covalent supramolecules | Reversible covalent bonds | Disulfide bond, hydrazone bond, boronate ester bond | Biodegradable drug carriers, tissue engineering scaffolds | Short half-life, instability under physiological conditions | [34,35,36,37,38,39,40,41,42] |
| Host–guest supramolecular systems | Macrocyclic hosts, mechanically interlocked molecules | Crown ethers, cyclodextrins, pillararenes, rotaxanes | Smart drug delivery, PDT/PTT, intracellular imaging | Limited targeting efficiency, non-specific binding, biological compatibility | [43,44,45,46] |
| Hydrogen-bonding network systems | Hydrogen bonding | Amine–carboxyl, HOFs, multi-hydrogen-bond arrays | Biomimetic scaffolds, drug delivery | Susceptible to physiological interference, structural homogeneity issues | [47,48,49,50,51,52,53,54,55,56,57,58,59] |
| Metal-coordinated supramolecular systems | Coordination between metals and ligands | Ru2+–bipyridine, Gd3+–DOTA, Mg-based MOFs | Antibacterial materials, MRI contrast agents | Potential metal toxicity, limited coordination stability | [60,61,62,63,64,65] |
| Host–guest supramolecular systems | Interlocked molecules | β-cyclodextrin | Fluorescence imaging | - | [66] |
| Covalent supramolecules | Covalent bond | Imine bond | Fluorescence imaging, tumor treatment | - | [67] |
| Covalent supramolecules | Covalent bond | Amide bonds | PTT | - | [68] |
| Host–guest supramolecular systems | Interlocked molecules | Cucurucide | Contrast agent | - | [31] |
| Host–guest supramolecular systems | Interlocked molecules | Rotane | Fluorescent probes, imaging agents | - | [18] |
| Host–guest supramolecular systems | Interlocked molecules | γ-cyclodextrin | Drug delivery | - | [14] |
| Metal-coordinated | Coordination between metals and ligands | Ruthenium-based complexes | Drug delivery | - | [33] |
| Metal-coordinated | Coordination between metals and ligands | Zinc ions | Drug delivery, PDT | - | [15] |
| Covalent supramolecules | Covalent bond | - | Tissue engineering | - | [69] |
| Host–guest supramolecular systems | Interlocked molecules | β-cyclodextrin | Drug delivery | - | [70] |
| Covalent supramolecules | Covalent bond | Disulfide bonds | Tissue engineering | - | [71] |
| Covalent supramolecules | Covalent bond | PEG | Drug delivery | - | [72] |
| Host–guest supramolecular systems | Interlocked molecules | Cucurucide | PTT | - | [73] |
| Host–guest supramolecular systems | Interlocked molecules | β-cyclodextrin | Drug delivery–chemotherapy | - | [74] |
| Covalent supramolecules | Covalent bond | Hydrogen bond | Drug delivery–antibacterial therapy | - | [75] |
| Host–guest supramolecular systems | Covalent bond, interlocked molecules | Disulfide bonds, paclitaxel, γ-cyclodextrin | Drug delivery–gene therapy | - | [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Xiao, Y.; Li, S.; Du, J.; Wang, J.; Peng, X. Recent Advances in Supramolecular Systems for Precision Medicine: Structural Design, Functional Integration, and Clinical Translation Challenges. Pharmaceutics 2025, 17, 1192. https://doi.org/10.3390/pharmaceutics17091192
Ma X, Xiao Y, Li S, Du J, Wang J, Peng X. Recent Advances in Supramolecular Systems for Precision Medicine: Structural Design, Functional Integration, and Clinical Translation Challenges. Pharmaceutics. 2025; 17(9):1192. https://doi.org/10.3390/pharmaceutics17091192
Chicago/Turabian StyleMa, Xiaomin, Yazhe Xiao, Shuyu Li, Jianghai Du, Junjie Wang, and Xingzhou Peng. 2025. "Recent Advances in Supramolecular Systems for Precision Medicine: Structural Design, Functional Integration, and Clinical Translation Challenges" Pharmaceutics 17, no. 9: 1192. https://doi.org/10.3390/pharmaceutics17091192
APA StyleMa, X., Xiao, Y., Li, S., Du, J., Wang, J., & Peng, X. (2025). Recent Advances in Supramolecular Systems for Precision Medicine: Structural Design, Functional Integration, and Clinical Translation Challenges. Pharmaceutics, 17(9), 1192. https://doi.org/10.3390/pharmaceutics17091192










