Improved Dual-Modality Bioequivalence Evaluation of Topical Formulations Within Human Skin Using Stimulated Raman Scattering Microscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
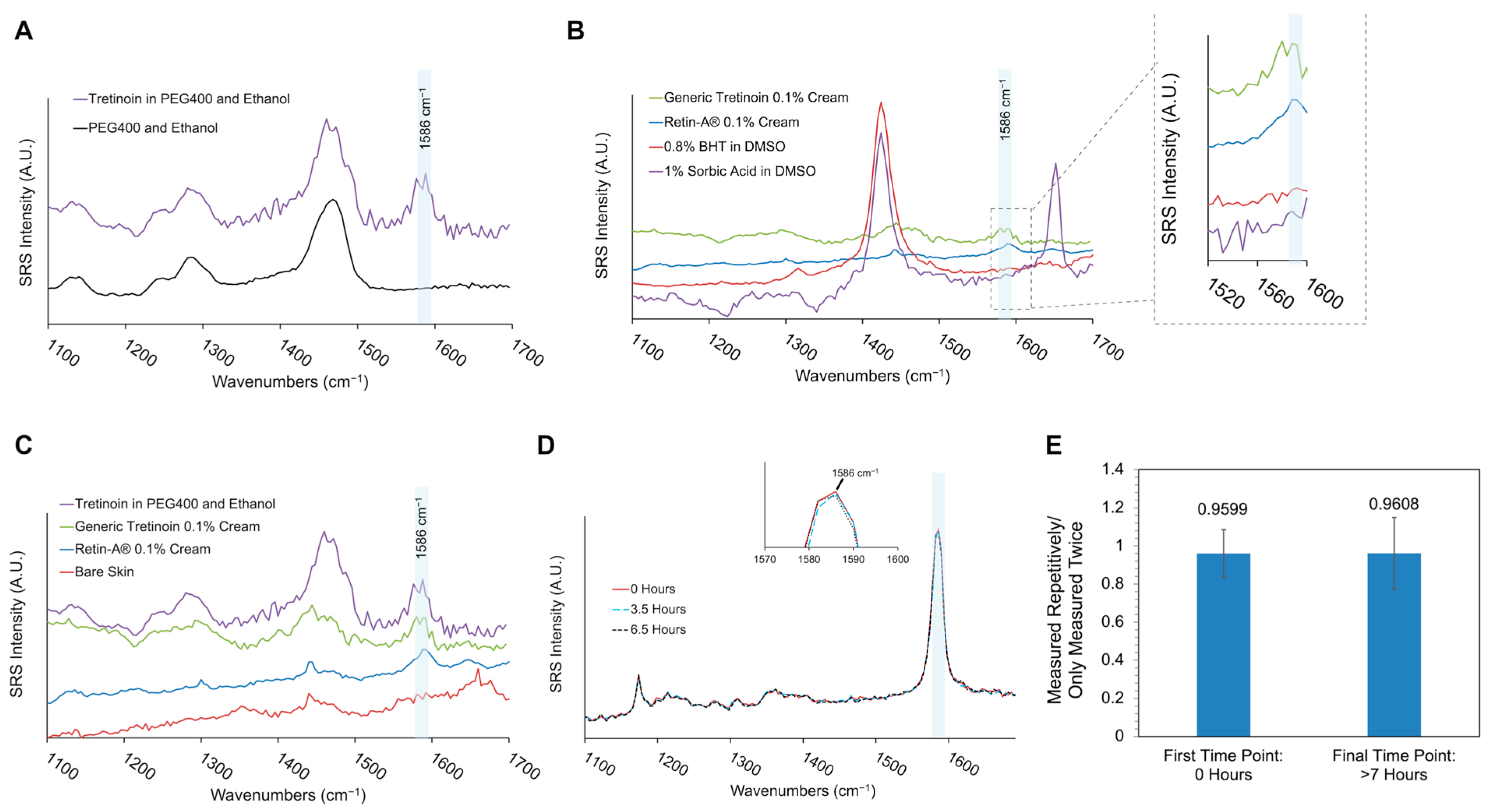
2.3. SRS Spectra Measurement and Photostability Test
2.4. Skin Preparation
2.5. Reference Polymer Film Preparation
2.6. Skin Thickness Study
2.7. Formulation Thickness Study
2.8. Bioequivalence Study
2.9. Image Processing and Statistical Analysis
3. Results and Discussion
3.1. SRS Spectra and Photostability of Tretinoin Formulations
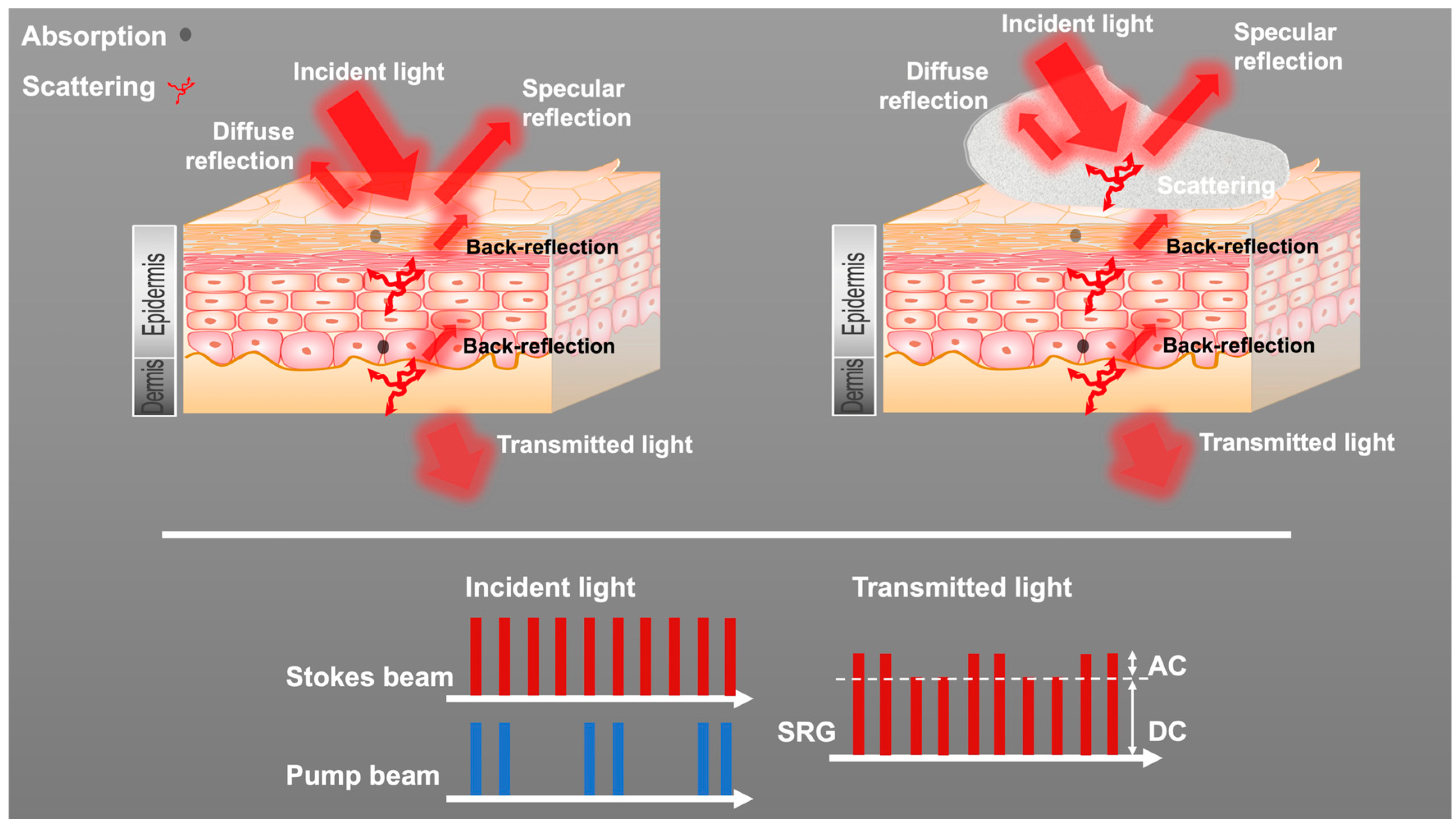
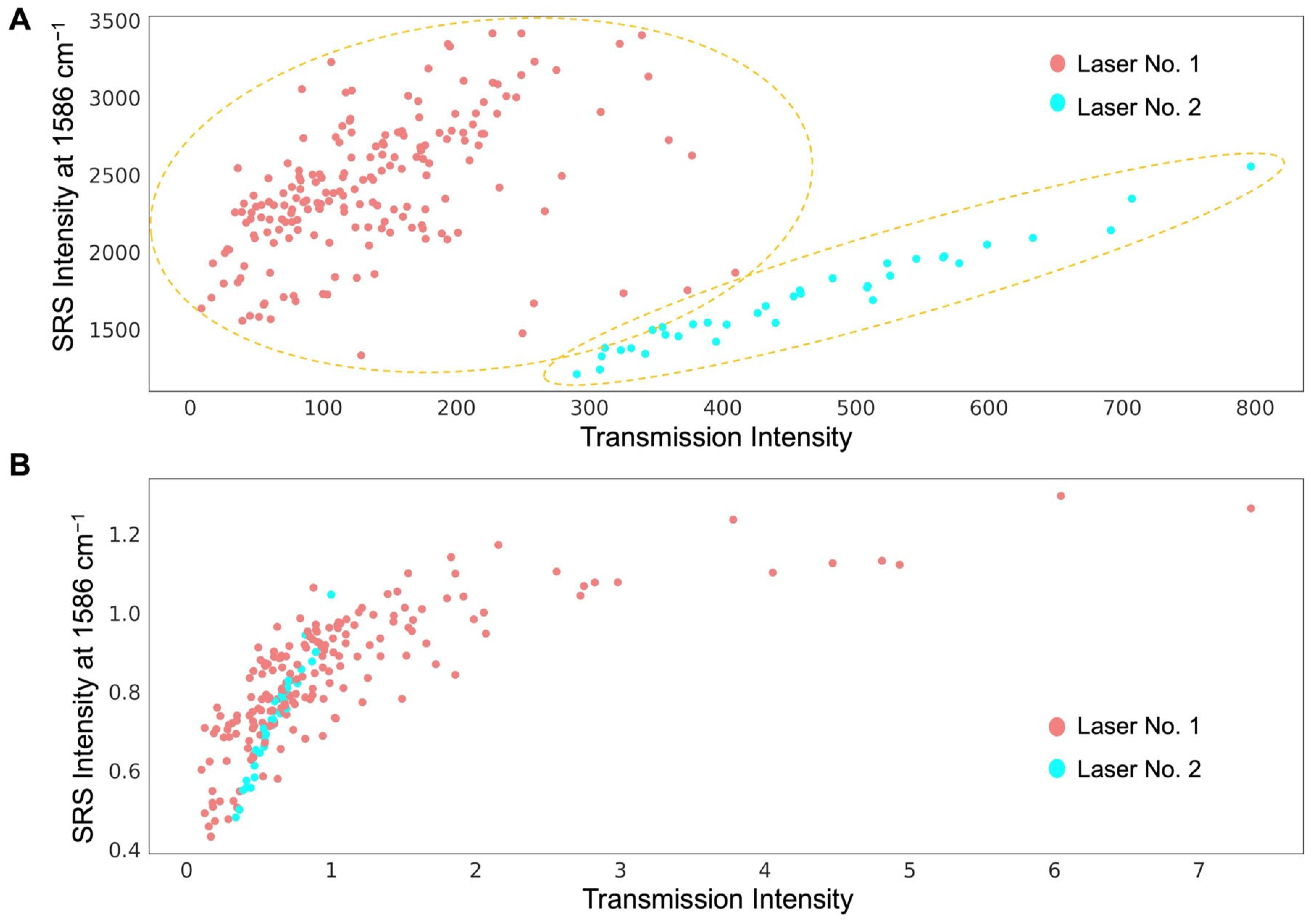
3.2. Dual-Modality Detector for SRS and Transmission Signals
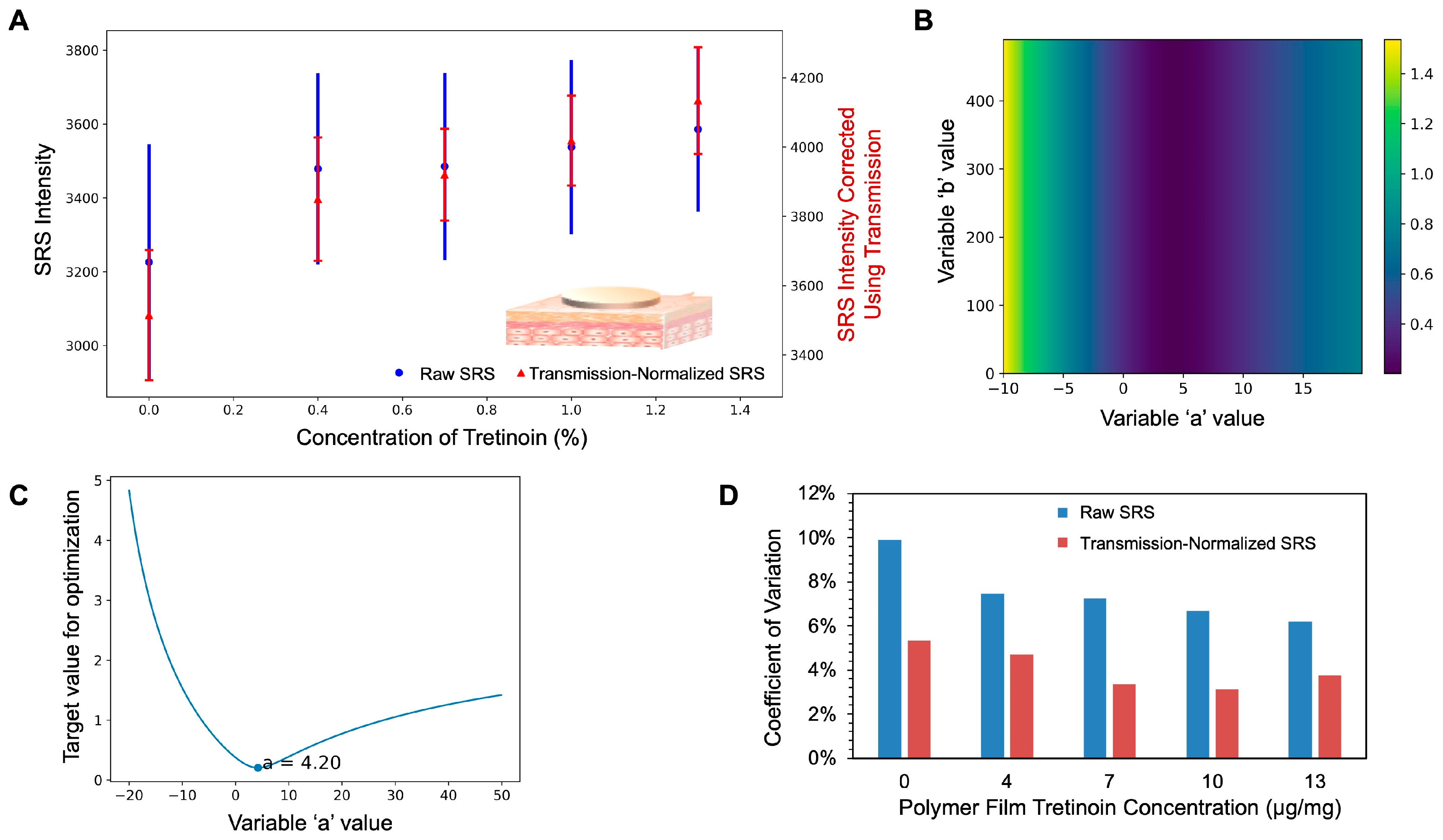
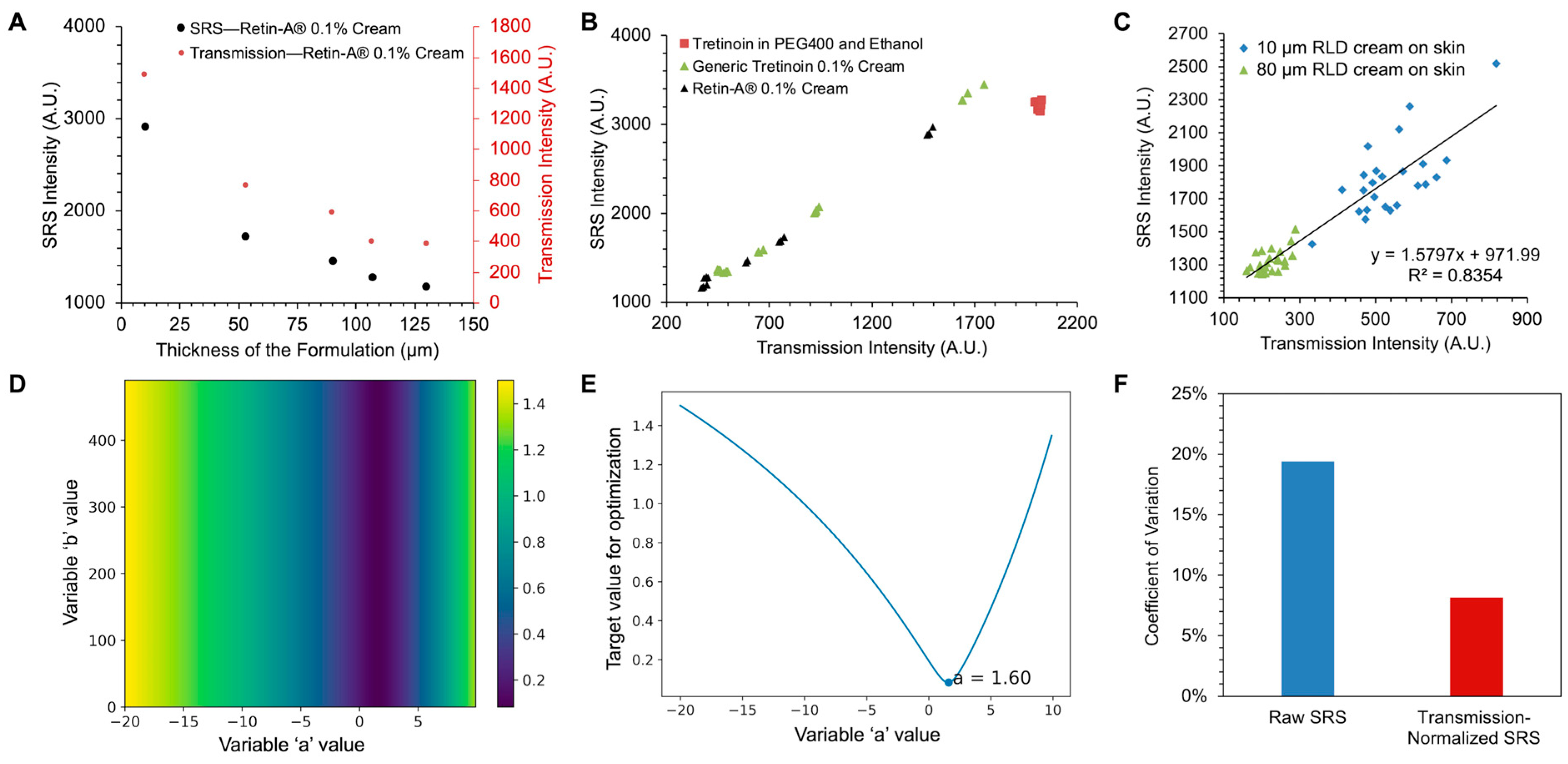
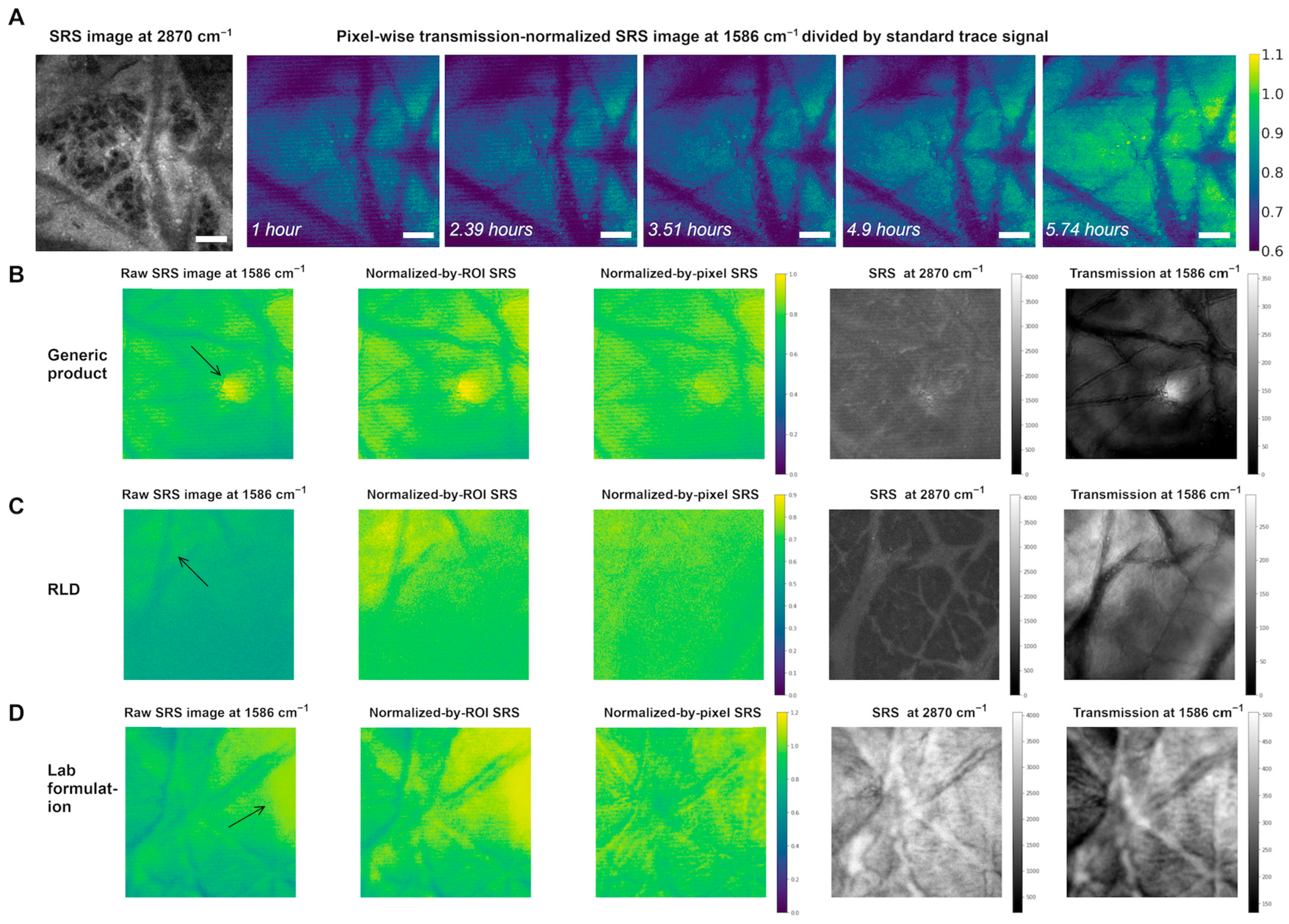
3.3. Normalization of SRS Signal Using Transmission Signal
3.4. Bioequivalence Study of Topical Tretinoin Products Using Dual-Modality Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Egawa, M. Raman microscopy for skin evaluation. Analyst 2021, 146, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.E. Optical methods for non-invasive determination of skin penetration: Current trends, advances, possibilities, prospects, and translation into in vivo human studies. Pharmaceutics 2023, 15, 2272. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration Bioequivalence. 2023. Available online: https://www.fda.gov/animal-veterinary/abbreviated-new-animal-drug-applications/bioequivalence (accessed on 1 May 2023).
- Raney, S.G.; Ghosh, P.; Ramezanli, T.; Lehman, P.A.; Franz, T.J. Cutaneous pharmacokinetic approaches to compare bioavailability and/or bioequivalence for topical drug products. Dermatol. Clin. 2022, 40, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-H.; Chon, J.; Kim, Y.-I.; Lee, H.-J.; Oh, D.-W.; Lee, H.-G.; Han, C.-S.; Kim, D.-W.; Park, C.-W. Preparation and evaluation of tacrolimus-loaded thermosensitive solid lipid nanoparticles for improved dermal distribution. Int. J. Nanomed. 2019, 14, 5381–5396. [Google Scholar] [CrossRef]
- Tabosa, M.A.M.; Vitry, P.; Zarmpi, P.; Bunge, A.L.; Belsey, N.A.; Tsikritsis, D.; Woodman, T.J.; White, K.J.; Delgado-Charro, M.B.; Guy, R.H. Quantification of Chemical Uptake into the Skin by Vibrational Spectroscopies and Stratum Corneum Sampling. Mol. Pharm. 2022, 20, 2527–2535. [Google Scholar] [CrossRef] [PubMed]
- Kocsis, D.; Kichou, H.; Döme, K.; Varga-Medveczky, Z.; Révész, Z.; Antal, I.; Erdo, F. Structural and Functional Analysis of Excised Skins and Human Reconstructed Epidermis with Confocal Raman Spectroscopy and in Microfluidic Diffusion Chambers. Pharmaceutics 2022, 14, 1689. [Google Scholar] [CrossRef]
- Kichou, H.; Munnier, E.; Dancik, Y.; Kemel, K.; Byrne, H.J.; Tfayli, A.; Bertrand, D.; Soucé, M.; Chourpa, I.; Bonnier, F. Estimating the Analytical Performance of Raman Spectroscopy for Quantification of Active Ingredients in Human Stratum Corneum. Molecules 2022, 27, 2843. [Google Scholar] [CrossRef]
- Choe, C.; Schleusener, J.; Ri, J.; Choe, S.; Kim, P.; Lademann, J.; Darvin, M.E. Quantitative determination of concentration profiles of skin components and topically applied oils by tailored multivariate curve resolution-alternating least squares using in vivo confocal Raman micro-spectroscopy. J. Biophotonics 2022, 16, e202200219. [Google Scholar] [CrossRef]
- Jung, N.; Namjoshi, S.; Mohammed, Y.; Grice, J.E.; Benson, H.A.E.; Raney, S.G.; Roberts, M.S.; Windbergs, M. Application of Confocal Raman Microscopy for the Characterization of Topical Semisolid Formulations and their Penetration into Human Skin Ex Vivo. Pharm. Res. 2022, 39, 935–948. [Google Scholar] [CrossRef]
- Feizpour, A.; Marstrand, T.; Bastholm, L.; Eirefelt, S.; Evans, C.L. Label-free quantification of pharmacokinetics in skin with stimulated raman scattering microscopy and deep learning. J. Investig. Dermatol. 2021, 141, 395–403. [Google Scholar] [CrossRef]
- Kuzma, B.A.; Tu, D.; Goss, A.; Iliopoulos, F.; Slade, J.B.; Wiatrowski, A.; Feizpour, A.; Evans, C.L. Instantaneous Topical Drug Quantification using a 3D Printed Microfluidic Device and Coherent Raman Imaging. OpenNano 2023, 12, 100151. [Google Scholar] [CrossRef]
- Saar, B.G.; Contreras-Rojas, L.R.; Xie, X.S.; Guy, R.H. Imaging drug delivery to skin with stimulated Raman scattering microscopy. Mol. Pharm. 2011, 8, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Goh, C.F.; Craig, D.Q.; Hadgraft, J.; Lane, M.E. The application of ATR-FTIR spectroscopy and multivariate data analysis to study drug crystallisation in the stratum corneum. Eur. J. Pharm. Biopharm. 2017, 111, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Pena, A.-M.; Chen, X.; Pence, I.J.; Bornschlögl, T.; Jeong, S.; Grégoire, S.; Luengo, G.S.; Hallegot, P.; Obeidy, P.; Feizpour, A.; et al. Imaging and quantifying drug delivery in skin–Part 2: Fluorescence andvibrational spectroscopic imaging methods. Adv. Drug Deliv. Rev. 2020, 153, 147–168. [Google Scholar] [CrossRef] [PubMed]
- Lunter, D.; Klang, V.; Kocsis, D.; Varga-Medveczky, Z.; Berkó, S.; Erdő, F. Novel aspects of Raman spectroscopy in skin research. Exp. Dermatol. 2022, 31, 1311–1329. [Google Scholar] [CrossRef]
- Iliopoulos, F.; Tang, C.F.; Li, Z.; Rahma, A.; Lane, M.E. Confocal Raman Spectroscopy for Assessing Bioequivalence of Topical Formulations. Pharmaceutics 2023, 15, 1075. [Google Scholar] [CrossRef] [PubMed]
- Belsey, N.A.; Garrett, N.L.; Contreras-Rojas, L.R.; Pickup-Gerlaugh, A.J.; Price, G.J.; Moger, J.; Guy, R.H. Evaluation of drug delivery to intact and porated skin by coherent Raman scattering and fluorescence microscopies. J. Control. Release 2014, 174, 37–42. [Google Scholar] [CrossRef]
- Goel, A.; Tsikritsis, D.; Belsey, N.A.; Pendlington, R.; Glavin, S.; Chen, T. Measurement of chemical penetration in skin using Stimulated Raman scattering microscopy and multivariate curve resolution-alternating least squares. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 296, 122639. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Iguchi, R.; Matsuoka, F.; Nishi, Y.; Ogihara, T.; Misawa, K. Label-free skin penetration analysis using time-resolved, phase-modulated stimulated Raman scattering microscopy. Biomed. Opt. Express 2021, 12, 6545–6557. [Google Scholar] [CrossRef]
- Saar, B.G.; Freudiger, C.W.; Reichman, J.; Stanley, C.M.; Holtom, G.R.; Xie, X.S. Video-rate molecular imaging in vivo with stimulated Raman scattering. Science 2010, 330, 1368–1370. [Google Scholar] [CrossRef]
- Kuzma, B.A.; Pence, I.J.; Ho, A.; Evans, C.L. Visualizing and Quantifying Pharmaceutical Compounds within Skin using Coherent Raman Scattering Imaging. J. Vis. Exp. Jove 2021, 177, e63264. [Google Scholar] [CrossRef]
- Pence, I.J.; Kuzma, B.A.; Brinkmann, M.; Hellwig, T.; Evans, C.L. Multi-window sparse spectral sampling stimulated Raman scattering microscopy. Biomed. Opt. Express 2021, 12, 6095–6114. [Google Scholar] [CrossRef] [PubMed]
- Zarmpi, P.; Tsikritsis, D.; Vorng, J.-L.; Belsey, N.A.; Bunge, A.L.; Woodman, T.J.; Delgado-Charro, M.B.; Guy, R.H. Evaluation of chemical disposition in skin by stimulated Raman scattering microscopy. J. Control. Release 2024, 368, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Garrett, N.L.; Singh, B.; Jones, A.; Moger, J. Imaging microscopic distribution of antifungal agents in dandruff treatments with stimulated Raman scattering microscopy. J. Biomed. Opt. 2017, 22, 066003. [Google Scholar] [CrossRef] [PubMed]
- Iliopoulos, F.; Tu, D.; Pence, I.J.; Li, X.; Ghosh, P.; Luke, M.C.; Raney, S.G.; Rantou, E.; Evans, C.L. Determining topical product bioequivalence with stimulated Raman scattering microscopy. J. Control. Release 2024, 367, 864–876. [Google Scholar] [CrossRef]
- Anderson, R.R.; Parrish, J.A. The optics of human skin. J. Investig. Dermatol. 1981, 77, 13–19. [Google Scholar] [CrossRef]
- Bashkatov, A.N.; Genina, E.A.; Tuchin, V.V. Optical properties of skin, subcutaneous, and muscle tissues: A review. J. Innov. Opt. Health Sci. 2011, 4, 9–38. [Google Scholar] [CrossRef]
- Lister, T.; Wright, P.A.; Chappell, P.H. Optical properties of human skin. J. Biomed. Opt. 2012, 17, 090901. [Google Scholar] [CrossRef]
- Heikenfeld, J.; Jajack, A.; Rogers, J.; Gutruf, P.; Tian, L.; Pan, T.; Li, R.; Khine, M.; Kim, J.; Wang, J. Wearable sensors: Modalities, challenges, and prospects. Lab. A Chip 2018, 18, 217–248. [Google Scholar] [CrossRef]
- Tsikritsis, D.; Legge, E.J.; Belsey, N.A. Practical considerations for quantitative and reproducible measurements with stimulated Raman scattering microscopy. Analyst 2022, 147, 4642–4656. [Google Scholar] [CrossRef]
- Otto, A.; Du Plessis, J.; Wiechers, J. Formulation effects of topical emulsions on transdermal and dermal delivery. Int. J. Cosmet. Sci. 2009, 31, 1–19. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Mahjub, R.; Haddadi, R.; Vafaei, S.Y. Design and Optimization of Cationic Nanocapsules for Topical Delivery of Tretinoin: Application of the Box-Behnken Design, In Vitro Evaluation, and Ex Vivo Skin Deposition Study. BioMed Res. Int. 2021, 2021, 4603545. [Google Scholar] [CrossRef]
- Monteiro e Silva, S.A.; Ricci Leonardi, G. Development and cathodic iontophoretic permeation evaluation of liquid crystalline systems provided of retinoic acid microparticles. J. Cosmet. Dermatol. 2021, 20, 2317–2327. [Google Scholar] [CrossRef]
- Sinico, C.; Manconi, M.; Peppi, M.; Lai, F.; Valenti, D.; Fadda, A.M. Liposomes as carriers for dermal delivery of tretinoin: In vitro evaluation of drug permeation and vesicle–skin interaction. J. Control. Release 2005, 103, 123–136. [Google Scholar] [CrossRef]
- Ascenso, A.; Salgado, A.; Euletério, C.; Praça, F.G.; Bentley, M.V.L.B.; Marques, H.C.; Oliveira, H.; Santos, C.; Simões, S. In vitro and in vivo topical delivery studies of tretinoin-loaded ultradeformable vesicles. Eur. J. Pharm. Biopharm. 2014, 88, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xuan, J.; Yu, Q.; Wu, W.; Lu, Y.; Zhu, Q.; Chen, Z.; Qi, J. Converting Tretinoin into Ionic Liquids for Improving Aqueous Solubility and Permeability across Skin. Pharm. Res. 2022, 39, 2421–2430. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, D.; de Andrade, D.F.; de Oliveira, E.G.; Beck, R.C.R. Liquid chromatography method to assay tretinoin in skin layers: Validation and application in skin penetration/retention studies. Heliyon 2020, 6, e03098. [Google Scholar] [CrossRef]
- Lemberger, N.S.; Wallmeier, K.; Fallnich, C. High-bandwidth noise-reduced loss-corrected autobalanced detection. Opt. Express 2025, 33, 62–74. [Google Scholar] [CrossRef]
- Bae, K. NonCompart: Noncompartmental Analysis for Pharmacokinetic Data, R package version 0.5.1; R Foundation: Vienna, Austria, 2017. [Google Scholar]
- U.S. Food and Drug Administration In Vitro Permeation Test Studies for Topical Drug Products Submitted in ANDAs Draft Guidance for Industry. 2022. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/in-vitro-permeation-test-studies-topical-drug-products-submitted-andas (accessed on 17 July 2023).
- Franzen, L.; Windbergs, M. Applications of Raman spectroscopy in skin research—From skin physiology and diagnosis up to risk assessment and dermal drug delivery. Adv. Drug Deliv. Rev. 2015, 89, 91–104. [Google Scholar] [CrossRef]
- Del Rosso, J.Q.; Harper, J.; Pillai, R.; Moore, R. Tretinoin photostability: Comparison of micronized tretinoin (0.05%) gel and tretinoin (0.025%) gel following exposure to ultraviolet a light. J. Clin. Aesthetic Dermatol. 2012, 5, 27. [Google Scholar]
- Shah, K.A.; Date, A.A.; Joshi, M.D.; Patravale, V.B. Solid lipid nanoparticles (SLN) of tretinoin: Potential in topical delivery. Int. J. Pharm. 2007, 345, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Pireddu, R.; Corrias, F.; Fadda, A.M.; Valenti, D.; Pini, E.; Sinico, C. Nanosuspension improves tretinoin photostability and delivery to the skin. Int. J. Pharm. 2013, 458, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Ourique, A.F.; Melero, A.; da Silva, C.d.B.; Schaefer, U.F.; Pohlmann, A.R.; Guterres, S.S.; Lehr, C.-M.; Kostka, K.-H.; Beck, R.C.R. Improved photostability and reduced skin permeation of tretinoin: Development of a semisolid nanomedicine. Eur. J. Pharm. Biopharm. 2011, 79, 95–101. [Google Scholar] [CrossRef]
- Berto, P.; Andresen, E.R.; Rigneault, H. Background-free stimulated Raman spectroscopy and microscopy. Phys. Rev. Lett. 2014, 112, 053905. [Google Scholar] [CrossRef]
- Crisafi, F.; Kumar, V.; Scopigno, T.; Marangoni, M.; Cerullo, G.; Polli, D. In-line balanced detection stimulated Raman scattering microscopy. Sci. Rep. 2017, 7, 10745. [Google Scholar] [CrossRef]
- Freudiger, C.W.; Yang, W.; Holtom, G.R.; Peyghambarian, N.; Xie, X.S.; Kieu, K.Q. Stimulated Raman scattering microscopy with a robust fibre laser source. Nat. Photonics 2014, 8, 153–159. [Google Scholar] [CrossRef]
- Setchfield, K.; Gorman, A.; Simpson, A.H.R.W.; Somekh, M.G.; Wright, A.J. Relevance and utility of the in-vivo and ex-vivo optical properties of the skin reported in the literature: A review. Biomed. Opt. Express 2023, 14, 3555–3583. [Google Scholar] [CrossRef] [PubMed]
| Formulation | Lab | T | R1 and R2 | All Formulations | |
|---|---|---|---|---|---|
| Donor | All Four Donors | All Four Donors | All Four Donors | All Four Donors | |
| Raw SRS signal | Mean | 2443.78 | 2323.46 | 2212.42 | 2295.71 |
| SD | 461.68 | 513.38 | 483.93 | 495.28 | |
| CV | 18.89% | 22.10% | 21.87% | 21.57% | |
| Standard film normalization: Raw SRS signal/ standard film | Mean | 0.88 | 0.79 | 0.80 | 0.82 |
| SD | 0.14 | 0.15 | 0.17 | 0.16 | |
| CV | 15.43% | 19.38% | 21.14% | 19.82% | |
| ΔCV | −3.46% | −2.72% | −0.73% | −1.75% | |
| Combined normalization: Pixel-wise transmission-normalized SRS signal/ standard film | Mean | 0.96 | 0.84 | 0.86 | 0.88 |
| SD | 0.12 | 0.11 | 0.11 | 0.12 | |
| CV | 13.06% | 13.34% | 12.41% | 13.72% | |
| ΔCV | −5.83% | −8.76% | −9.46% | −7.85% |
| Product 1 | Product 2 | 90% Confidence Interval for the Mean Difference in the Cmax Values (Product 2, Product 1) | 90% Confidence Interval for the Mean Difference in the AUC0–6h Values (Product 2, Product 1) | ||
|---|---|---|---|---|---|
| Lipid-Rich Intralamellar Region | Lipid-Poor Corneocyte Region | Lipid-Rich Intralamellar Region | Lipid-Poor Corneocyte Region | ||
| RLD (R1) | RLD (R2) | (0.91, 1.11) | (0.91, 1.12) | (0.92, 1.16) | (0.92, 1.17) |
| RLD (R1) | Generic | (0.92, 1.11) | (0.91, 1.11) | (0.91, 1.13) | (0.90, 1.12) |
| RLD (R1) | Lab (PEG/ETOH) | (1.12, 1.33) | (1.12, 1.35) | (1.11, 1.37) | (1.12, 1.38) |
| RLD (R2) | Generic | (0.93, 1.11) | (0.92, 1.10) | (0.90, 1.08) | (0.89, 1.08) |
| RLD (R2) | Lab (PEG/ETOH) | (1.12, 1.32) | (1.12, 1.32) | (1.09, 1.31) | (1.09, 1.31) |
| Product 1 | Product 2 | 90% Confidence Interval for the Mean Difference in the Cmax Values (Product 2, Product 1) | 90% Confidence Interval for the Mean Difference in the AUC0–6h Values (Product 2, Product 1) | ||
|---|---|---|---|---|---|
| Lipid-Rich Intralamellar Region | Lipid-Poor Corneocyte Region | Lipid-Rich Intralamellar Region | Lipid-Poor Corneocyte Region | ||
| RLD (R1) | RLD (R2) | (0.91, 1.02) | (0.92, 1.03) | (0.93, 1.07) | (0.93, 1.07) |
| RLD (R1) | Generic | (0.93, 1.03) | (0.92, 1.02) | (0.92, 1.04) | (0.91, 1.03) |
| RLD (R1) | Lab (PEG/ETOH) | (1.08, 1.24) | (1.09, 1.24) | (1.08, 1.27) | (1.08, 1.28) |
| RLD (R2) | Generic | (0.95, 1.08) | (0.94, 1.07) | (0.92, 1.05) | (0.91, 1.04) |
| RLD (R2) | Lab (PEG/ETOH) | (1.11, 1.29) | (1.11, 1.29) | (1.08, 1.28) | (1.08, 1.28) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, D.; Lemberger, N.-S.; Wallmeier, K.; Riseman, J.; Kuzma, B.A.; Wei, Y.; Khoo, T.C.; Rantou, E.; Ghosh, P.; Luke, M.C.; et al. Improved Dual-Modality Bioequivalence Evaluation of Topical Formulations Within Human Skin Using Stimulated Raman Scattering Microscopy. Pharmaceutics 2025, 17, 1193. https://doi.org/10.3390/pharmaceutics17091193
Tu D, Lemberger N-S, Wallmeier K, Riseman J, Kuzma BA, Wei Y, Khoo TC, Rantou E, Ghosh P, Luke MC, et al. Improved Dual-Modality Bioequivalence Evaluation of Topical Formulations Within Human Skin Using Stimulated Raman Scattering Microscopy. Pharmaceutics. 2025; 17(9):1193. https://doi.org/10.3390/pharmaceutics17091193
Chicago/Turabian StyleTu, Dandan, Nick-Sidney Lemberger, Kristin Wallmeier, Jackson Riseman, Benjamin A. Kuzma, Yuxiao Wei, Ting Chean Khoo, Elena Rantou, Priyanka Ghosh, Markham C. Luke, and et al. 2025. "Improved Dual-Modality Bioequivalence Evaluation of Topical Formulations Within Human Skin Using Stimulated Raman Scattering Microscopy" Pharmaceutics 17, no. 9: 1193. https://doi.org/10.3390/pharmaceutics17091193
APA StyleTu, D., Lemberger, N.-S., Wallmeier, K., Riseman, J., Kuzma, B. A., Wei, Y., Khoo, T. C., Rantou, E., Ghosh, P., Luke, M. C., Raney, S. G., Fallnich, C., & Evans, C. L. (2025). Improved Dual-Modality Bioequivalence Evaluation of Topical Formulations Within Human Skin Using Stimulated Raman Scattering Microscopy. Pharmaceutics, 17(9), 1193. https://doi.org/10.3390/pharmaceutics17091193







