Machine Learning for Multi-Target Drug Discovery: Challenges and Opportunities in Systems Pharmacology
Abstract
1. Introduction
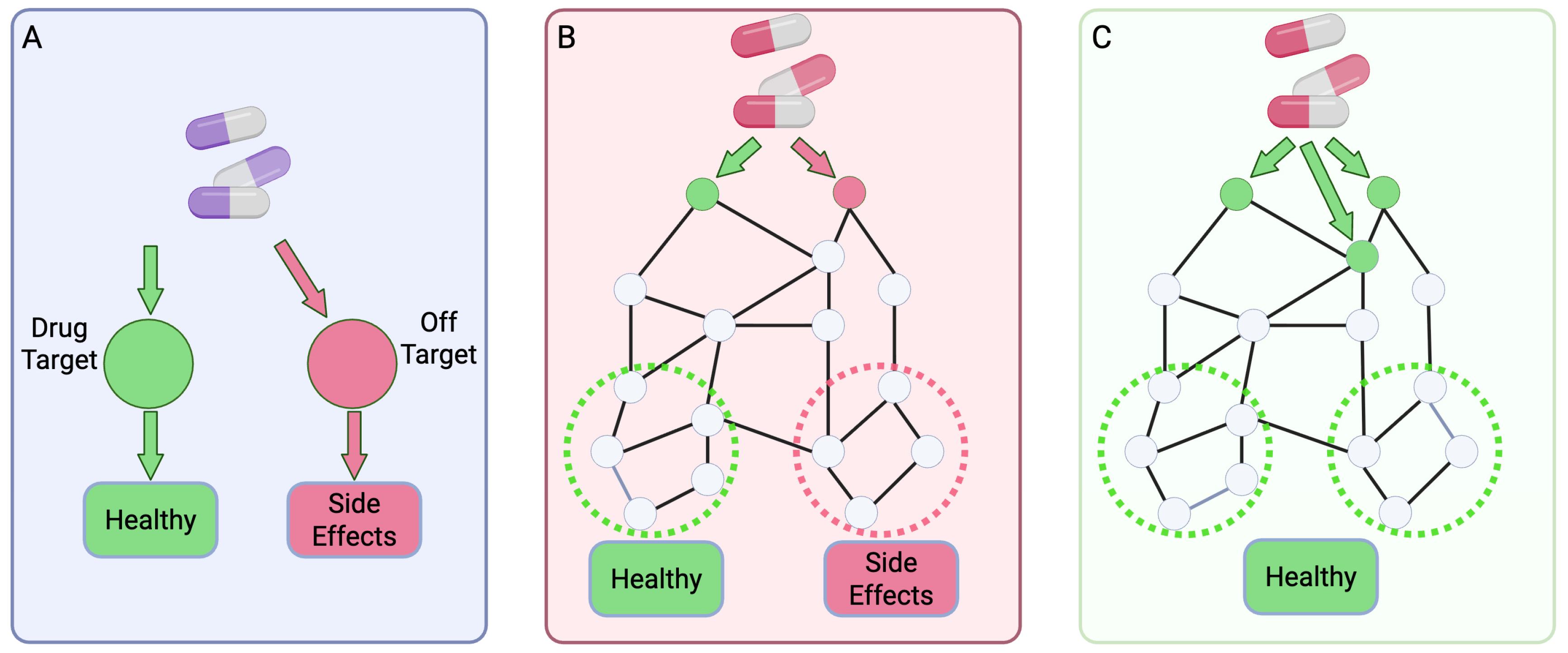
2. Fundamentals of Multi-Target Drug Discovery
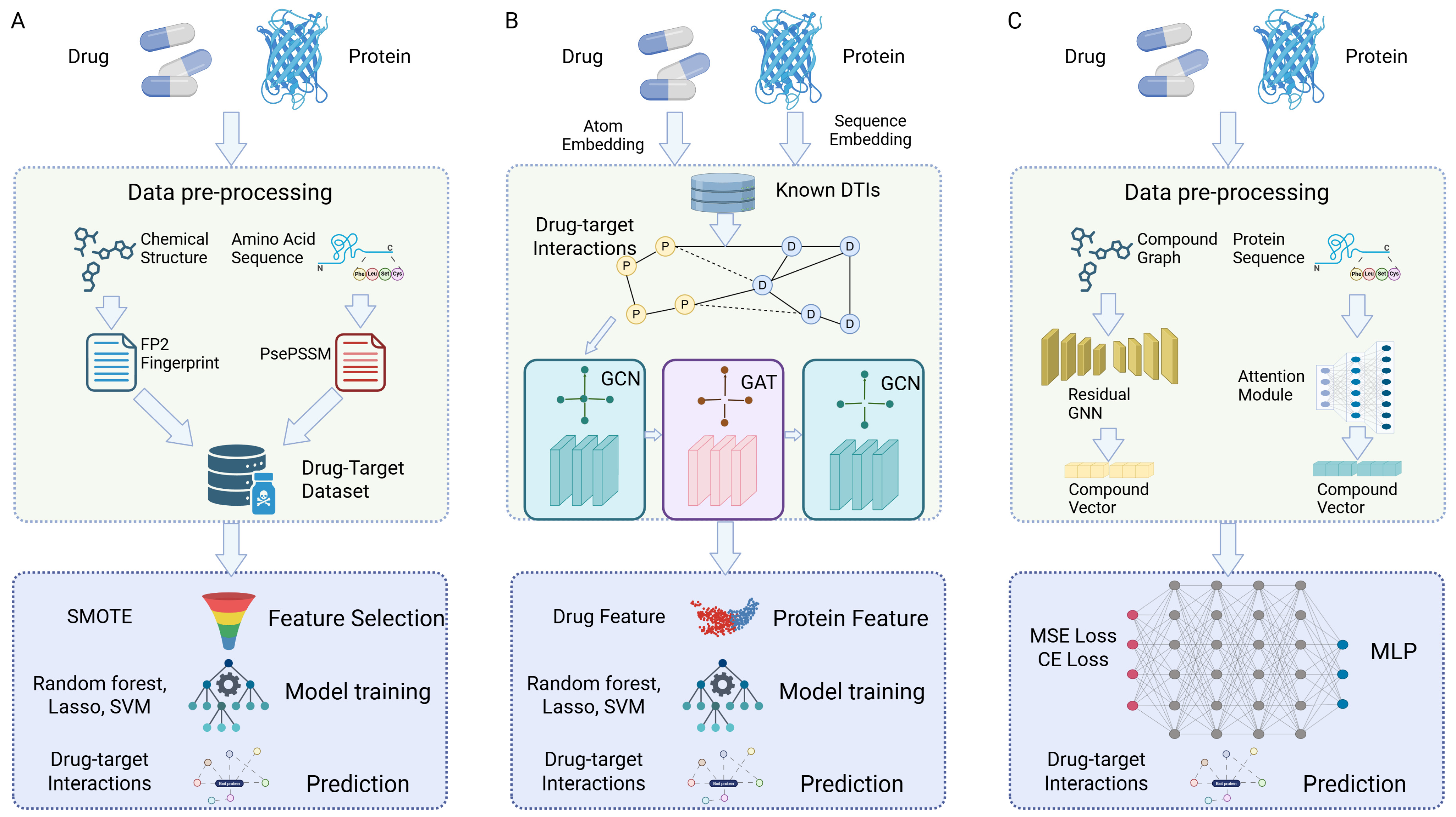
3. Machine Learning Techniques for Multi-Target Prediction
3.1. Data Sources and Feature Representations
3.2. Classical Machine Learning Models
3.3. Deep Learning and Representation Learning
3.4. Network-Based and Systems Pharmacology Approaches
4. Applications and Case Studies
4.1. Oncology: Targeting Redundant and Synergistic Pathways
4.2. Neurodegenerative Diseases: Tackling Multifactorial Pathogenesis
5. Challenges and Future Perspectives
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Lavecchia, A.; Cerchia, C. In Silico Methods to Address Polypharmacology: Current Status, Applications and Future Perspectives. Drug Discov. Today 2016, 21, 288–298. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, Y.; Lu, A.; Zhang, G. Systems Pharmacology in Small Molecular Drug Discovery. Int. J. Mol. Sci. 2016, 17, 246. [Google Scholar] [CrossRef]
- Pérez-Nueno, V.I. Using Quantitative Systems Pharmacology for Novel Drug Discovery. Expert Opin. Drug Discov. 2015, 10, 1315–1331. [Google Scholar] [CrossRef]
- Li, L.; Yang, L.; Yang, L.; He, C.; He, Y.; Chen, L.; Dong, Q.; Zhang, H.; Chen, S.; Li, P. Network Pharmacology: A Bright Guiding Light on the Way to Explore the Personalized Precise Medication of Traditional Chinese Medicine. Chin. Med. 2023, 18, 146. [Google Scholar] [CrossRef] [PubMed]
- Ryszkiewicz, P.; Malinowska, B.; Schlicker, E. Polypharmacology: New Drugs in 2023–2024. Pharmacol. Rep. 2025, 77, 543–560. [Google Scholar] [CrossRef] [PubMed]
- Stefan, S.M.; Rafehi, M. Medicinal Polypharmacology-a Scientific Glossary of Terminology and Concepts. Front. Pharmacol. 2024, 15, 1419110. [Google Scholar] [CrossRef]
- VanDongen, A.M. Drug Promiscuity: Problems and Promises. bioRxiv 2023. [Google Scholar] [CrossRef]
- Abdelsayed, M. AI-Driven Polypharmacology in Small-Molecule Drug Discovery. Int. J. Mol. Sci. 2025, 26, 6996. [Google Scholar] [CrossRef]
- Liu, K.; Chen, X.; Ren, Y.; Liu, C.; Lv, T.; Liu, Y.; Zhang, Y. Multi-Target-Based Polypharmacology Prediction (mTPP): An Approach Using Virtual Screening and Machine Learning for Multi-Target Drug Discovery. Chem.-Biol. Interact. 2022, 368, 110239. [Google Scholar] [CrossRef] [PubMed]
- Kleandrova, V.V.; Ds Cordeiro, M.N.; Speck-Planche, A. Current In Silico Methods for Multi-Target Drug Discovery in Early Anticancer Research: The Rise of the Perturbation-Theory Machine Learning Approach. Future Med. Chem. 2023, 15, 1647–1650. [Google Scholar] [CrossRef]
- Cichońska, A.; Ravikumar, B.; Rahman, R. AI for Targeted Polypharmacology: The next Frontier in Drug Discovery. Curr. Opin. Struct. Biol. 2024, 84, 102771. [Google Scholar] [CrossRef]
- Mukaidaisi, M.; Ahmed, M.; Grantham, K.; Al-Jumaily, A.; Dedhar, S.; Organ, M.; Tchagang, A.; Hou, J.; Ahmed, S.E.; Dividino, R.; et al. “Several Birds with One Stone”: Exploring the Potential of AI Methods for Multi-Target Drug Design. Mol. Divers. 2025, 29, 3023–3039. [Google Scholar] [CrossRef]
- Isigkeit, L.; Hörmann, T.; Schallmayer, E.; Scholz, K.; Lillich, F.F.; Ehrler, J.H.M.; Hufnagel, B.; Büchner, J.; Marschner, J.A.; Pabel, J.; et al. Automated Design of Multi-Target Ligands by Generative Deep Learning. Nat. Commun. 2024, 15, 7946. [Google Scholar] [CrossRef]
- Ocana, A.; Pandiella, A.; Privat, C.; Bravo, I.; Luengo-Oroz, M.; Amir, E.; Gyorffy, B. Integrating Artificial Intelligence in Drug Discovery and Early Drug Development: A Transformative Approach. Biomark. Res. 2025, 13, 45. [Google Scholar] [CrossRef]
- Ogbonna, U.E.; Ofoezie, E.F.; Babalola, O.O.; Ottu, P.O.; Ogbonna, C.A.; Olisakwe, S.; George, T.E.; Babarinde, S.; Omaba, J.O.; Chukwuemeka, C.G.; et al. Advances in Machine Learning for Optimizing Pharmaceutical Drug Discovery. Curr. Proteom. 2025, 22, 100015. [Google Scholar] [CrossRef]
- Bajorath, J. Explainable Machine Learning for Medicinal Chemistry: Exploring Multi-Target Compounds. Future Med. Chem. 2022, 14, 1171–1173. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.R.; Luciano, F.C.; Anaya, B.J.; Ongoren, B.; Kara, A.; Molina, G.; Ramirez, B.I.; Sánchez-Guirales, S.A.; Simon, J.A.; Tomietto, G.; et al. Artificial Intelligence (AI) Applications in Drug Discovery and Drug Delivery: Revolutionizing Personalized Medicine. Pharmaceutics 2024, 16, 1328. [Google Scholar] [CrossRef] [PubMed]
- Kandhare, P.; Kurlekar, M.; Deshpande, T.; Pawar, A. A Review on Revolutionizing Healthcare Technologies with AI and ML Applications in Pharmaceutical Sciences. Drugs Drug Candidates 2025, 4, 9. [Google Scholar] [CrossRef]
- Talukder, M.A.; Kazi, M.; Alazab, A. Predicting Drug-Target Interactions Using Machine Learning with Improved Data Balancing and Feature Engineering. Sci. Rep. 2025, 15, 19495. [Google Scholar] [CrossRef]
- Ali, N.; Hanif, N.; Khan, H.A.; Waseem, M.A.; Saeed, A.; Zakir, S.; Khan, A.; Aamir, M.; Ali, A.; Ali, A.; et al. Deep Learning and Artificial Intelligence for Drug Discovery, Application, Challenge, and Future Perspectives. Discov. Appl. Sci. 2025, 7, 533. [Google Scholar] [CrossRef]
- Wan, Z.; Sun, X.; Li, Y.; Chu, T.; Hao, X.; Cao, Y.; Zhang, P. Applications of Artificial Intelligence in Drug Repurposing. Adv. Sci. 2025, 12, 2411325. [Google Scholar] [CrossRef]
- Hughes, J.; Rees, S.; Kalindjian, S.; Philpott, K. Principles of Early Drug Discovery. Br. J. Pharmacol. 2011, 162, 1239–1249. [Google Scholar] [CrossRef]
- Makhoba, X.H.; Viegas, C., Jr.; Mosa, R.A.; Viegas, F.P.D.; Pooe, O.J. Potential Impact of the Multi-Target Drug Approach in the Treatment of Some Complex Diseases. Drug Des. Dev. Ther. 2020, 14, 3235–3249. [Google Scholar] [CrossRef]
- Li, Q.; Geng, S.; Luo, H.; Wang, W.; Mo, Y.-Q.; Luo, Q.; Wang, L.; Song, G.-B.; Sheng, J.-P.; Xu, B. Signaling Pathways Involved in Colorectal Cancer: Pathogenesis and Targeted Therapy. Signal Transduct. Target. Ther. 2024, 9, 266. [Google Scholar] [CrossRef]
- Chaudhari, R.; Tan, Z.; Huang, B.; Zhang, S. Computational Polypharmacology: A New Paradigm for Drug Discovery. Expert Opin. Drug Discov. 2017, 12, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Talevi, A. Multi-Target Pharmacology: Possibilities and Limitations of the “Skeleton Key Approach” from a Medicinal Chemist Perspective. Front. Pharmacol. 2015, 6, 205. [Google Scholar] [CrossRef]
- Yesilkanal, A.E.; Johnson, G.L.; Ramos, A.F.; Rosner, M.R. New strategies for targeting kinase networks in cancer. J. Biol. Chem. 2021, 297, 101128. [Google Scholar] [CrossRef]
- Leri, M.; Vasarri, M. Advancing Neuropharmacology and Neurodegenerative Disease Therapy: Bridging Gaps and Paving New Pathways. Pharmaceuticals 2025, 18, 606. [Google Scholar] [CrossRef]
- Iqbal, D.; Rehman, M.T.; Alajmi, M.F.; Alsaweed, M.; Jamal, Q.M.S.; Alasiry, S.M.; Albaker, A.B.; Hamed, M.; Kamal, M.; Albadrani, H.M. Multitargeted Virtual Screening and Molecular Simulation of Natural Product-like Compounds against GSK3β, NMDA-Receptor, and BACE-1 for the Management of Alzheimer’s Disease. Pharmaceuticals 2023, 16, 622. [Google Scholar] [CrossRef]
- Chandran, U.; Mehendale, N.; Patil, S.; Chaguturu, R.; Patwardhan, B. Network Pharmacology. In Innovative Approaches in Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2017; pp. 127–164. [Google Scholar] [CrossRef]
- Hopkins, A.L. Network Pharmacology: The next Paradigm in Drug Discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef]
- Cavasotto, C.N.; Orry, A.J.W. Ligand Docking and Structure-Based Virtual Screening in Drug Discovery. Curr. Top. Med. Chem. 2007, 7, 1006–1014. [Google Scholar] [CrossRef]
- Pal, S.; Kumar, V.; Kundu, B.; Bhattacharya, D.; Preethy, N.; Reddy, M.P.; Talukdar, A. Ligand-Based Pharmacophore Modeling, Virtual Screening and Molecular Docking Studies for Discovery of Potential Topoisomerase I Inhibitors. Comput. Struct. Biotechnol. J. 2019, 17, 291–310. [Google Scholar] [CrossRef]
- Rehman, A.U.; Li, M.; Wu, B.; Ali, Y.; Rasheed, S.; Shaheen, S.; Liu, X.; Luo, R.; Zhang, J. Role of Artificial Intelligence in Revolutionizing Drug Discovery. Fundam. Res. 2025, 5, 1273–1287. [Google Scholar] [CrossRef]
- Wigh, D.S.; Goodman, J.M.; Lapkin, A.A. A Review of Molecular Representation in the Age of Machine Learning. WIREs Comput. Mol. Sci. 2022, 12, e1603. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, M.; Wang, S.; Zhang, S. Deep Learning Methods for Molecular Representation and Property Prediction. Drug Discov. Today 2022, 27, 103373. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, S.; Zhu, F.; Huang, J. Seq2seq Fingerprint: An Unsupervised Deep Molecular Embedding for Drug Discovery. In Proceedings of the 8th ACM International Conference on Bioinformatics, Computational Biology, and Health Informatics, Boston, MA, USA, 20–23 August 2017; ACM: Boston, MA, USA, 2017; pp. 285–294. [Google Scholar]
- Xie, X.; Yu, T.; Li, X.; Zhang, N.; Foster, L.J.; Peng, C.; Huang, W.; He, G. Recent Advances in Targeting the “Undruggable” Proteins: From Drug Discovery to Clinical Trials. Signal Transduct. Target. Ther. 2023, 8, 335. [Google Scholar] [CrossRef]
- Lahti, J.L.; Tang, G.W.; Capriotti, E.; Liu, T.; Altman, R.B. Bioinformatics and Variability in Drug Response: A Protein Structural Perspective. J. R. Soc. Interface 2012, 9, 1409–1437. [Google Scholar] [CrossRef]
- Nero, T.L.; Parker, M.W.; Morton, C.J. Protein Structure and Computational Drug Discovery. Biochem. Soc. Trans. 2018, 46, 1367–1379. [Google Scholar] [CrossRef]
- Djeddi, W.E.; Hermi, K.; Ben Yahia, S.; Diallo, G. Advancing Drug–Target Interaction Prediction: A Comprehensive Graph-Based Approach Integrating Knowledge Graph Embedding and ProtBert Pretraining. BMC Bioinform. 2023, 24, 488. [Google Scholar] [CrossRef]
- Kim, M.; Baek, S.H.; Song, M. Relation Extraction for Biological Pathway Construction Using Node2vec. BMC Bioinform. 2018, 19, 206. [Google Scholar] [CrossRef]
- Ren, X.; Yan, C.-X.; Zhai, R.-X.; Xu, K.; Li, H.; Fu, X.-J. Comprehensive Survey of Target Prediction Web Servers for Traditional Chinese Medicine. Heliyon 2023, 9, e19151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, C.; Liu, M.; Liu, T.; Lin, H.; Huang, C.-B.; Ning, L. Attention Is All You Need: Utilizing Attention in AI-Enabled Drug Discovery. Brief. Bioinform. 2023, 25, bbad467. [Google Scholar] [CrossRef]
- O’Neil, J.; Benita, Y.; Feldman, I.; Chenard, M.; Roberts, B.; Liu, Y.; Li, J.; Kral, A.; Lejnine, S.; Loboda, A.; et al. An Unbiased Oncology Compound Screen to Identify Novel Combination Strategies. Mol. Cancer Ther. 2016, 15, 1155–1162. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, R.; Bajorath, J. Evolution of Support Vector Machine and Regression Modeling in Chemoinformatics and Drug Discovery. J. Comput. Aided Mol. Des. 2022, 36, 355–362. [Google Scholar] [CrossRef]
- Nayarisseri, A.; Khandelwal, R.; Tanwar, P.; Madhavi, M.; Sharma, D.; Thakur, G.; Speck-Planche, A.; Singh, S.K. Artificial Intelligence, Big Data and Machine Learning Approaches in Precision Medicine & Drug Discovery. Curr. Drug Targets 2021, 22, 631–655. [Google Scholar] [CrossRef]
- Indrajaya, D.; Setiawan, A.; Susanto, B. Comparison of K-Nearest Neighbor and Naive Bayes Methods for SNP Data Classification. MATRIK J. Manaj. Tek. Inform. Rekayasa Komput. 2022, 22, 149–164. [Google Scholar] [CrossRef]
- Bai, T.; Xie, J.; Liu, Y.; Liu, B. MMLmiRLocNet: miRNA Subcellular Localization Prediction Based on Multi-View Multi-Label Learning for Drug Design. IEEE J. Biomed. Health Inform. 2024, 1–9. [Google Scholar] [CrossRef]
- Das, P.; Sercu, T.; Wadhawan, K.; Padhi, I.; Gehrmann, S.; Cipcigan, F.; Chenthamarakshan, V.; Strobelt, H.; Dos Santos, C.; Chen, P.-Y.; et al. Accelerated Antimicrobial Discovery via Deep Generative Models and Molecular Dynamics Simulations. Nat. Biomed. Eng. 2021, 5, 613–623. [Google Scholar] [CrossRef]
- Liu, H.; Lin, X.; Hu, J.; Zhang, X. A Multi Target Drug Design Method Based on Target Protein Sequence and Feature Similarity. In Proceedings of the 2024 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Lisbon, Portugal, 3–6 December 2024; IEEE: Lisbon, Portugal, 2024; pp. 848–851. [Google Scholar]
- Born, J.; Manica, M. Trends in Deep Learning for Property-Driven Drug Design. Curr. Med. Chem. 2021, 28, 7862–7886. [Google Scholar] [CrossRef]
- Qi, G.; Zhang, A.; Xu, Z.; Li, Z.; Zeng, W.; Liu, X.; Ma, J.; Zheng, X.; Li, Z. Application of a Complex Network Modeling Approach to Explore the Material Basis and Mechanisms of Traditional Chinese Medicine: A Case Study of Xuefu Zhuyu Decoction for the Treatment of Two Types of Angina Pectoris. IEEE Access 2022, 10, 114103–114117. [Google Scholar] [CrossRef]
- Tang, X.; Lei, X.; Liu, L. A Multi-Modal Drug Target Affinity Prediction Based on Graph Features and Pre-Trained Sequence Embeddings. Interdiscip. Sci. Comput. Life Sci. 2025, 1–22. [Google Scholar] [CrossRef]
- Zeng, X.; Su, G.-P.; Du, W.-F.; Jiang, B.; Li, Y.; Yang, Z.-Z. Joint Fusion of Sequences and Structures of Drugs and Targets for Identifying Targets Based on Intra and Inter Cross-Attention Mechanisms. BMC Biol. 2025, 23, 168. [Google Scholar] [CrossRef]
- Zonyfar, C.; Njimbouom, S.N.; Mosalla, S.; Kim, J.-D. R2eGIN: Residual Reconstruction Enhanced Graph Isomorphism Network for Accurate Prediction of Poly (ADP-Ribose) Polymerase Inhibitors. Bioinform. Biol. Insights 2025, 19, 11779322251366087. [Google Scholar] [CrossRef]
- Ai, C.; Yang, H.; Liu, X.; Dong, R.; Ding, Y.; Guo, F. MTMol-GPT: De Novo Multi-Target Molecular Generation with Transformer-Based Generative Adversarial Imitation Learning. PLoS Comput. Biol. 2024, 20, e1012229. [Google Scholar] [CrossRef]
- Mao, J.; Wang, J.; Zeb, A.; Cho, K.-H.; Jin, H.; Kim, J.; Lee, O.; Wang, Y.; No, K.T. Transformer-Based Molecular Generative Model for Antiviral Drug Design. J. Chem. Inf. Model. 2024, 64, 2733–2745. [Google Scholar] [CrossRef]
- Wang, L.; Wang, S.; Yang, H.; Li, S.; Wang, X.; Zhou, Y.; Tian, S.; Liu, L.; Bai, F. Conformational Space Profiling Enhances Generic Molecular Representation for AI-Powered Ligand-Based Drug Discovery. Adv. Sci. 2024, 11, 2403998. [Google Scholar] [CrossRef]
- Zhang, Q.; Ding, K.; Lyv, T.; Wang, X.; Yin, Q.; Zhang, Y.; Yu, J.; Wang, Y.; Li, X.; Xiang, Z.; et al. Scientific Large Language Models: A Survey on Biological & Chemical Domains. arXiv 2024, arXiv:2401.14656. [Google Scholar] [CrossRef]
- Zhao, Y.; Xing, Y.; Zhang, Y.; Wang, Y.; Wan, M.; Yi, D.; Wu, C.; Li, S.; Xu, H.; Zhang, H.; et al. Evidential Deep Learning-Based Drug-Target Interaction Prediction. Nat. Commun. 2025, 16, 6915. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, N.; Santoni, D.; Nawn, D.; Ottaviani, E.; Felici, G. A Comprehensive Review of Transformer-Based Language Models for Protein Sequence Analysis and Design. arXiv 2025, arXiv:2507.13646. [Google Scholar] [CrossRef]
- Feng, X.; Ma, Z.; Yu, C.; Xin, R. MRNDR: Multihead Attention-Based Recommendation Network for Drug Repurposing. J. Chem. Inf. Model. 2024, 64, 2654–2669. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, X.; Zhou, J.; Li, F.; Wang, Y.; Liu, Q. Artificial Intelligence in Traditional Chinese Medicine: Advances in Multi-Metabolite Multi-Target Interaction Modeling. Front. Pharmacol. 2025, 16, 1541509. [Google Scholar] [CrossRef]
- Vilalta-Mor, J.; Molina, A.; Varga, L.O.; Filella-Merce, I.; Guallar, V. Active Learning-Guided Seq2Seq Variational Autoencoder for Multi-Target Inhibitor Generation. arXiv 2025, arXiv:2506.15309. [Google Scholar] [CrossRef]
- Teng, Q.; Liu, Z.; Song, Y.; Han, K.; Lu, Y. A Survey on the Interpretability of Deep Learning in Medical Diagnosis. Multimed. Syst. 2022, 28, 2335–2355. [Google Scholar] [CrossRef]
- Gangwal, A.; Ansari, A.; Ahmad, I.; Azad, A.K.; Kumarasamy, V.; Subramaniyan, V.; Wong, L.S. Generative Artificial Intelligence in Drug Discovery: Basic Framework, Recent Advances, Challenges, and Opportunities. Front. Pharmacol. 2024, 15, 1331062. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lin, T.; Peng, T.; Xing, E.; Chen, S.; Kara, L.B.; Cheng, X. TopMT-GAN: A 3D Topology-Driven Generative Model for Efficient and Diverse Structure-Based Ligand Design. Chem. Sci. 2025, 16, 2796–2809. [Google Scholar] [CrossRef]
- Zheng, J.; Yi, H.-C.; You, Z.-H. Equivariant 3D-Conditional Diffusion Model for De Novo Drug Design. IEEE J. Biomed. Health Inform. 2025, 29, 1805–1816. [Google Scholar] [CrossRef]
- Fang, Y.; Pan, X.; Shen, H.-B. De Novo Drug Design by Iterative Multiobjective Deep Reinforcement Learning with Graph-Based Molecular Quality Assessment. Bioinformatics 2023, 39, btad157. [Google Scholar] [CrossRef] [PubMed]
- Abdelbaky, I.; Tayara, H.; Chong, K.T. Prediction of Kinase Inhibitors Binding Modes with Machine Learning and Reduced Descriptor Sets. Sci. Rep. 2021, 11, 706. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, C.; Bajorath, J. Differentiating Inhibitors of Closely Related Protein Kinases with Single- or Multi-Target Activity via Explainable Machine Learning and Feature Analysis. Biomolecules 2022, 12, 557. [Google Scholar] [CrossRef]
- Kumar, S.A.; Ananda Kumar, T.D.; Beeraka, N.M.; Pujar, G.V.; Singh, M.; Narayana Akshatha, H.S.; Bhagyalalitha, M. Machine Learning and Deep Learning in Data-Driven Decision Making of Drug Discovery and Challenges in High-Quality Data Acquisition in the Pharmaceutical Industry. Future Med. Chem. 2022, 14, 245–270. [Google Scholar] [CrossRef]
- Fu, C.; Chen, Q. The Future of Pharmaceuticals: Artificial Intelligence in Drug Discovery and Development. J. Pharm. Anal. 2025, 15, 101248. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Aittokallio, T. Network Pharmacology Strategies toward Multi-Target Anticancer Therapies: From Computational Models to Experimental Design Principles. Curr. Pharm. Des. 2014, 20, 23–36. [Google Scholar] [CrossRef]
- Qiao, G.; Wang, G.; Li, Y. Causal Enhanced Drug–Target Interaction Prediction Based on Graph Generation and Multi-Source Information Fusion. Bioinformatics 2024, 40, btae570. [Google Scholar] [CrossRef] [PubMed]
- Harrold, J.M.; Ramanathan, M.; Mager, D.E. Network-Based Approaches in Drug Discovery and Early Development. Clin. Pharmacol. Ther. 2013, 94, 651. [Google Scholar] [CrossRef]
- Duan, P.; Yang, K.; Su, X.; Fan, S.; Dong, X.; Zhang, F.; Li, X.; Xing, X.; Zhu, Q.; Yu, J.; et al. HTINet2: Herb–Target Prediction via Knowledge Graph Embedding and Residual-like Graph Neural Network. Brief. Bioinform. 2024, 25, bbae414. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, S.; Pan, W.; Yao, R.; Zhang, W.; Zhang, Y.; Wang, G.; Zhang, Q.; Cheng, Y.; Dong, J.; et al. Signaling Repurposable Drug Combinations against COVID-19 by Developing the Heterogeneous Deep Herb-Graph Method. Brief. Bioinform. 2022, 23, bbac124. [Google Scholar] [CrossRef]
- Li, Y.; Qiao, G.; Wang, K.; Wang, G. Drug–Target Interaction Predication via Multi-Channel Graph Neural Networks. Brief. Bioinform. 2022, 23, bbab346. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Natarajan, M.; Wu, Z.; Gombolay, M.C. Diffusion Models for Multi-Target Adversarial Tracking. In Proceedings of the 2023 International Symposium on Multi-Robot and Multi-Agent Systems (MRS), Boston, MA, USA, 4–5 December 2023; IEEE: Boston, MA, USA, 2023; pp. 142–148. [Google Scholar]
- MacLean, F. Knowledge Graphs and Their Applications in Drug Discovery. Expert Opin. Drug Discov. 2021, 16, 1057–1069. [Google Scholar] [CrossRef]
- Gong, Z.; Zhang, N.; He, J. KGRN: Knowledge Graph Relational Path Network for Target Prediction of TCM Prescriptions. In Intelligent Computing Theories and Application; Huang, D.-S., Jo, K.-H., Li, J., Gribova, V., Premaratne, P., Eds.; Lecture Notes in Computer Science; Springer International Publishing: Cham, Switzerland, 2021; Volume 12838, pp. 148–161. ISBN 978-3-030-84531-5. [Google Scholar]
- Li, H.; Zhou, Y.; Liao, L.; Tan, H.; Li, Y.; Li, Z.; Zhou, B.; Bao, M.; He, B. Pharmacokinetics Effects of Chuanxiong Rhizoma on Warfarin in Pseudo Germ-Free Rats. Front. Pharmacol. 2022, 13, 1022567. [Google Scholar] [CrossRef]
- Huang, K.; Chandak, P.; Wang, Q.; Havaldar, S.; Vaid, A.; Leskovec, J.; Nadkarni, G.N.; Glicksberg, B.S.; Gehlenborg, N.; Zitnik, M. A Foundation Model for Clinician-Centered Drug Repurposing. Nat. Med. 2024, 30, 3601–3613. [Google Scholar] [CrossRef]
- Ung, C.Y.; Correia, C.; Li, H.; Adams, C.M.; Westendorf, J.J.; Zhu, S. Multiorgan Locked-State Model of Chronic Diseases and Systems Pharmacology Opportunities. Drug Discov. Today 2024, 29, 103825. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, F.; Ma, X.H.; Shi, Z.; Yang, S.Y.; Wei, Y.Q.; Chen, Y.Z. Predicting Targeted Polypharmacology for Drug Repositioning and Multi-Target Drug Discovery. Curr. Med. Chem. 2013, 20, 1646–1661. [Google Scholar] [CrossRef]
- Zhou, J.-B.; Tang, D.; He, L.; Lin, S.; Lei, J.H.; Sun, H.; Xu, X.; Deng, C.-X. Machine Learning Model for Anti-Cancer Drug Combinations: Analysis, Prediction, and Validation. Pharmacol. Res. 2023, 194, 106830. [Google Scholar] [CrossRef]
- Singha, M.; Pu, L.; Srivastava, G.; Ni, X.; Stanfield, B.A.; Uche, I.K.; Rider, P.J.F.; Kousoulas, K.G.; Ramanujam, J.; Brylinski, M. Unlocking the Potential of Kinase Targets in Cancer: Insights from CancerOmicsNet, an AI-Driven Approach to Drug Response Prediction in Cancer. Cancers 2023, 15, 4050. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, M.; Qin, Y. Anticancer Drug Response Prediction Integrating Multi-Omics Pathway-Based Difference Features and Multiple Deep Learning Techniques. PLoS Comput. Biol. 2025, 21, e1012905. [Google Scholar] [CrossRef] [PubMed]
- Naorem, L.D.; Muthaiyan, M.; Venkatesan, A. Integrated Network Analysis and Machine Learning Approach for the Identification of Key Genes of Triple-negative Breast Cancer. J. Cell. Biochem. 2019, 120, 6154–6167. [Google Scholar] [CrossRef] [PubMed]
- Almansour, N.M. Triple-Negative Breast Cancer: A Brief Review About Epidemiology, Risk Factors, Signaling Pathways, Treatment and Role of Artificial Intelligence. Front. Mol. Biosci. 2022, 9, 836417. [Google Scholar] [CrossRef]
- Qureshi, R.; Zou, B.; Alam, T.; Wu, J.; Lee, V.H.F.; Yan, H. Computational Methods for the Analysis and Prediction of EGFR-Mutated Lung Cancer Drug Resistance: Recent Advances in Drug Design, Challenges and Future Prospects. IEEE/ACM Trans. Comput. Biol. Bioinf. 2023, 20, 238–255. [Google Scholar] [CrossRef]
- Vatansever, S.; Schlessinger, A.; Wacker, D.; Kaniskan, H.Ü.; Jin, J.; Zhou, M.; Zhang, B. Artificial Intelligence and Machine Learning-aided Drug Discovery in Central Nervous System Diseases: State-of-the-arts and Future Directions. Med. Res. Rev. 2021, 41, 1427–1473. [Google Scholar] [CrossRef]
- Liu, M.; Srivastava, G.; Ramanujam, J.; Brylinski, M. SynerGNet: A Graph Neural Network Model to Predict Anticancer Drug Synergy. Biomolecules 2024, 14, 253. [Google Scholar] [CrossRef]
- Rafiei, F.; Zeraati, H.; Abbasi, K.; Ghasemi, J.B.; Parsaeian, M.; Masoudi-Nejad, A. DeepTraSynergy: Drug Combinations Using Multimodal Deep Learning with Transformers. Bioinformatics 2023, 39, btad438. [Google Scholar] [CrossRef]
- Hu, J.; Gao, J.; Fang, X.; Liu, Z.; Wang, F.; Huang, W.; Wu, H.; Zhao, G. DTSyn: A Dual-Transformer-Based Neural Network to Predict Synergistic Drug Combinations. Brief. Bioinform. 2022, 23, bbac302. [Google Scholar] [CrossRef]
- Sidorov, P.; Naulaerts, S.; Ariey-Bonnet, J.; Pasquier, E.; Ballester, P.J. Predicting Synergism of Cancer Drug Combinations Using NCI-ALMANAC Data. Front. Chem. 2019, 7, 509. [Google Scholar] [CrossRef] [PubMed]
- Preto, A.J.; Matos-Filipe, P.; Mourão, J.; Moreira, I.S. SYNPRED: Prediction of Drug Combination Effects in Cancer Using Different Synergy Metrics and Ensemble Learning. GigaScience 2022, 11, giac087. [Google Scholar] [CrossRef]
- Torkamannia, A.; Omidi, Y.; Ferdousi, R. SYNDEEP: A Deep Learning Approach for the Prediction of Cancer Drugs Synergy. Sci. Rep. 2023, 13, 6184. [Google Scholar] [CrossRef]
- Baptista, D.; Ferreira, P.G.; Rocha, M. A Systematic Evaluation of Deep Learning Methods for the Prediction of Drug Synergy in Cancer. PLoS Comput. Biol. 2023, 19, e1010200. [Google Scholar] [CrossRef]
- El Khili, M.R.; Memon, S.A.; Emad, A. MARSY: A Multitask Deep-Learning Framework for Prediction of Drug Combination Synergy Scores. Bioinformatics 2023, 39, btad177. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, J.; Shui, Y.; Xu, M.; Hu, J.; Liu, X.; Che, K.; Wang, J.; Liu, Y. PermuteDDS: A Permutable Feature Fusion Network for Drug-Drug Synergy Prediction. J. Cheminform. 2024, 16, 41. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Huang, X.; Guo, W.; Yan, C.; Zou, G. Predicting Synergistic Anticancer Drug Combination Based on Low-Rank Global Attention Mechanism and Bilinear Predictor. Bioinformatics 2023, 39, btad607. [Google Scholar] [CrossRef]
- Guo, Y.; Hu, H.; Chen, W.; Yin, H.; Wu, J.; Hsieh, C.-Y.; He, Q.; Cao, J. SynergyX: A Multi-Modality Mutual Attention Network for Interpretable Drug Synergy Prediction. Brief. Bioinform. 2024, 25, bbae015. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shen, B.; Feng, F.; Li, K.; Tang, Z.; Ma, L.; Li, H. Dual-View Jointly Learning Improves Personalized Drug Synergy Prediction. Bioinformatics 2024, 40, btae604. [Google Scholar] [CrossRef]
- Preuer, K.; Lewis, R.P.I.; Hochreiter, S.; Bender, A.; Bulusu, K.C.; Klambauer, G. DeepSynergy: Predicting Anti-Cancer Drug Synergy with Deep Learning. Bioinformatics 2018, 34, 1538–1546. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, L.; Payne, P.R.O.; Li, F. Synergistic Drug Combination Prediction by Integrating Multiomics Data in Deep Learning Models. In Translational Bioinformatics for Therapeutic Development; Markowitz, J., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2021; Volume 2194, pp. 223–238. ISBN 978-1-0716-0848-7. [Google Scholar]
- Kuenzi, B.M.; Park, J.; Fong, S.H.; Sanchez, K.S.; Lee, J.; Kreisberg, J.F.; Ma, J.; Ideker, T. Predicting Drug Response and Synergy Using a Deep Learning Model of Human Cancer Cells. Cancer Cell 2020, 38, 672–684.e6. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Shen, S.; Deng, L.; Liu, H. DeepDDS: Deep Graph Neural Network with Attention Mechanism to Predict Synergistic Drug Combinations. Brief. Bioinform. 2022, 23, bbab390. [Google Scholar] [CrossRef] [PubMed]
- Menden, M.P.; Wang, D.; Mason, M.J.; Szalai, B.; Bulusu, K.C.; Guan, Y.; Yu, T.; Kang, J.; Jeon, M.; Wolfinger, R.; et al. Community Assessment to Advance Computational Prediction of Cancer Drug Combinations in a Pharmacogenomic Screen. Nat. Commun. 2019, 10, 2674. [Google Scholar] [CrossRef]
- Zhang, P.; Tu, S. MGAE-DC: Predicting the Synergistic Effects of Drug Combinations through Multi-Channel Graph Autoencoders. PLoS Comput. Biol. 2023, 19, e1010951. [Google Scholar] [CrossRef]
- Jiang, P.; Huang, S.; Fu, Z.; Sun, Z.; Lakowski, T.M.; Hu, P. Deep Graph Embedding for Prioritizing Synergistic Anticancer Drug Combinations. Comput. Struct. Biotechnol. J. 2020, 18, 427–438. [Google Scholar] [CrossRef]
- Kuru, H.I.; Tastan, O.; Cicek, A.E. MatchMaker: A Deep Learning Framework for Drug Synergy Prediction. IEEE/ACM Trans. Comput. Biol. Bioinf. 2022, 19, 2334–2344. [Google Scholar] [CrossRef]
- Yang, J.; Xu, Z.; Wu, W.K.K.; Chu, Q.; Zhang, Q. GraphSynergy: A Network-Inspired Deep Learning Model for Anticancer Drug Combination Prediction. J. Am. Med. Inform. Assoc. 2021, 28, 2336–2345. [Google Scholar] [CrossRef] [PubMed]
- Ling, A.; Huang, R.S. Computationally Predicting Clinical Drug Combination Efficacy with Cancer Cell Line Screens and Independent Drug Action. Nat. Commun. 2020, 11, 5848. [Google Scholar] [CrossRef]
- Liu, Q.; Xie, L. TranSynergy: Mechanism-Driven Interpretable Deep Neural Network for the Synergistic Prediction and Pathway Deconvolution of Drug Combinations. PLoS Comput. Biol. 2021, 17, e1008653. [Google Scholar] [CrossRef]
- Kumar, V.; Banerjee, A.; Roy, K. Machine Learning-Based q-RASAR Approach for the in Silico Identification of Novel Multi-Target Inhibitors against Alzheimer’s Disease. Chemom. Intell. Lab. Syst. 2024, 245, 105049. [Google Scholar] [CrossRef]
- El Idrissi, F.; Gressier, B.; Devos, D.; Belarbi, K. A Computational Exploration of the Molecular Network Associated to Neuroinflammation in Alzheimer’s Disease. Front. Pharmacol. 2021, 12, 630003. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Xie, H.; Jacobs, T.; Gaggi, N.L.; Fortea, J.; Carlisle, N.B.; Fonzo, G.A.; Pohl, K.M.; Osorio, R.S.; Zhang, Y. Elucidating the Neuropathological and Molecular Heterogeneity of Amyloid-Beta and Tau in Alzheimer’s Disease through Machine Learning and Transcriptomic Integration. bioRxiv 2024. [Google Scholar] [CrossRef]
- Parvathy Dharshini, S.A.; Sneha, N.P.; Yesudhas, D.; Kulandaisamy, A.; Rangaswamy, U.; Shanmugam, A.; Taguchi, Y.-H.; Gromiha, M.M. Exploring Plausible Therapeutic Targets for Alzheimer’s Disease usingMulti-Omics Approach, Machine Learning and Docking. Curr. Top. Med. Chem. 2022, 22, 1868–1879. [Google Scholar] [CrossRef] [PubMed]
- Nwadiugwu, M.; Onwuekwe, I.; Ezeanolue, E.; Deng, H. Beyond Amyloid: A Machine Learning-Driven Approach Reveals Properties of Potent GSK-3β Inhibitors Targeting Neurofibrillary Tangles. Int. J. Mol. Sci. 2024, 25, 2646. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wang, Z.; Nan, J.; Li, A.; Yang, X.; Tang, X. Potential Schizophrenia Disease-Related Genes Prediction Using Metagraph Representations Based on a Protein-Protein Interaction Keyword Network: Framework Development and Validation. JMIR Form. Res. 2023, 7, e50998. [Google Scholar] [CrossRef]
- Mohamed, S.K.; Nounu, A.; Nováček, V. Biological Applications of Knowledge Graph Embedding Models. Brief. Bioinform. 2021, 22, 1679–1693. [Google Scholar] [CrossRef]
- Nian, Y.; Hu, X.; Zhang, R.; Feng, J.; Du, J.; Li, F.; Bu, L.; Zhang, Y.; Chen, Y.; Tao, C. Mining on Alzheimer’s Diseases Related Knowledge Graph to Identity Potential AD-Related Semantic Triples for Drug Repurposing. BMC Bioinform. 2022, 23, 407. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Lu, Z.; Dai, W.; Ma, C.; Xiang, Y.; Zhang, Y. Identification of Potential Therapeutic Targets and Mechanisms of COVID-19 through Network Analysis and Screening of Chemicals and Herbal Ingredients. Brief. Bioinform. 2022, 23, bbab373. [Google Scholar] [CrossRef]
- Li, V.O.K.; Han, Y.; Kaistha, T.; Zhang, Q.; Downey, J.; Gozes, I.; Lam, J.C.K. DeepDrug as an Expert Guided and AI Driven Drug Repurposing Methodology for Selecting the Lead Combination of Drugs for Alzheimer’s Disease. Sci. Rep. 2025, 15, 2093. [Google Scholar] [CrossRef]
- Wu, X.; Li, Z.; Chen, G.; Yin, Y.; Chen, C.Y.-C. Hybrid Neural Network Approaches to Predict Drug–Target Binding Affinity for Drug Repurposing: Screening for Potential Leads for Alzheimer’s Disease. Front. Mol. Biosci. 2023, 10, 1227371. [Google Scholar] [CrossRef]
- Alhassan, H.H. Integrative Machine Learning and Molecular Simulation Approaches Identify GSK3β Inhibitors for Neurodegenerative Disease Therapy. Sci. Rep. 2025, 15, 21632. [Google Scholar] [CrossRef]
- Rodriguez, S.; Hug, C.; Todorov, P.; Moret, N.; Boswell, S.A.; Evans, K.; Zhou, G.; Johnson, N.T.; Hyman, B.T.; Sorger, P.K.; et al. Machine Learning Identifies Candidates for Drug Repurposing in Alzheimer’s Disease. Nat. Commun. 2021, 12, 1033. [Google Scholar] [CrossRef] [PubMed]
- Kleandrova, V.V.; Cordeiro, M.N.D.S.; Speck-Planche, A. Perturbation-Theory Machine Learning for Multi-Objective Antibacterial Discovery: Current Status and Future Perspectives. Appl. Sci. 2025, 15, 1166. [Google Scholar] [CrossRef]
- Nava Lara, R.A.; Beltrán, J.A.; Brizuela, C.A.; Del Rio, G. Relevant Features of Polypharmacologic Human-Target Antimicrobials Discovered by Machine-Learning Techniques. Pharmaceuticals 2020, 13, 204. [Google Scholar] [CrossRef] [PubMed]
- Serafim, M.S.M.; Kronenberger, T.; Oliveira, P.R.; Poso, A.; Honório, K.M.; Mota, B.E.F.; Maltarollo, V.G. The Application of Machine Learning Techniques to Innovative Antibacterial Discovery and Development. Expert Opin. Drug Discov. 2020, 15, 1165–1180. [Google Scholar] [CrossRef]
- Speck-Planche, A.; Cordeiro, M.N.D.S. Multi-Target QSAR Approaches for Modeling Protein Inhibitors. Simultaneous Prediction of Activities Against Biomacromolecules Present in Gram-Negative Bacteria. Curr. Top. Med. Chem. 2015, 15, 1801–1813. [Google Scholar] [CrossRef]
- Abdel-Basset, M.; Hawash, H.; Elhoseny, M.; Chakrabortty, R.K.; Ryan, M. DeepH-DTA: Deep Learning for Predicting Drug-Target Interactions: A Case Study of COVID-19 Drug Repurposing. IEEE Access 2020, 8, 170433–170451. [Google Scholar] [CrossRef] [PubMed]
- Arango-Argoty, G.; Garner, E.; Pruden, A.; Heath, L.S.; Vikesland, P.; Zhang, L. DeepARG: A Deep Learning Approach for Predicting Antibiotic Resistance Genes from Metagenomic Data. Microbiome 2018, 6, 23. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Z.; Han, W.; Cao, H.; Umarov, R.; Yan, A.; Fan, M.; Chen, H.; Duarte, C.M.; Li, L.; et al. HMD-ARG: Hierarchical Multi-Task Deep Learning for Annotating Antibiotic Resistance Genes. Microbiome 2021, 9, 40. [Google Scholar] [CrossRef]
- Pei, Y.; Shum, M.H.-H.; Liao, Y.; Leung, V.W.; Gong, Y.-N.; Smith, D.K.; Yin, X.; Guan, Y.; Luo, R.; Zhang, T.; et al. ARGNet: Using Deep Neural Networks for Robust Identification and Classification of Antibiotic Resistance Genes from Sequences. Microbiome 2024, 12, 84. [Google Scholar] [CrossRef]
- López-Cortés, X.A.; Manríquez-Troncoso, J.M.; Hernández-García, R.; Peralta, D. MSDeepAMR: Antimicrobial Resistance Prediction Based on Deep Neural Networks and Transfer Learning. Front. Microbiol. 2024, 15, 1361795. [Google Scholar] [CrossRef]
- Nguyen, M.; Long, S.W.; McDermott, P.F.; Olsen, R.J.; Olson, R.; Stevens, R.L.; Tyson, G.H.; Zhao, S.; Davis, J.J. Using Machine Learning To Predict Antimicrobial MICs and Associated Genomic Features for Nontyphoidal Salmonella. J. Clin. Microbiol. 2019, 57, e01260-18. [Google Scholar] [CrossRef] [PubMed]
- Weis, C.; Cuénod, A.; Rieck, B.; Dubuis, O.; Graf, S.; Lang, C.; Oberle, M.; Brackmann, M.; Søgaard, K.K.; Osthoff, M.; et al. Direct Antimicrobial Resistance Prediction from Clinical MALDI-TOF Mass Spectra Using Machine Learning. Nat. Med. 2022, 28, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Santos-Júnior, C.D.; Torres, M.D.T.; Duan, Y.; Rodríguez Del Río, Á.; Schmidt, T.S.B.; Chong, H.; Fullam, A.; Kuhn, M.; Zhu, C.; Houseman, A.; et al. Discovery of Antimicrobial Peptides in the Global Microbiome with Machine Learning. Cell 2024, 187, 3761–3778.e16. [Google Scholar] [CrossRef]
- Wong, F.; Zheng, E.J.; Valeri, J.A.; Donghia, N.M.; Anahtar, M.N.; Omori, S.; Li, A.; Cubillos-Ruiz, A.; Krishnan, A.; Jin, W.; et al. Discovery of a Structural Class of Antibiotics with Explainable Deep Learning. Nature 2024, 626, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Pesesky, M.W.; Hussain, T.; Wallace, M.; Patel, S.; Andleeb, S.; Burnham, C.-A.D.; Dantas, G. Evaluation of Machine Learning and Rules-Based Approaches for Predicting Antimicrobial Resistance Profiles in Gram-Negative Bacilli from Whole Genome Sequence Data. Front. Microbiol. 2016, 7, 1887. [Google Scholar] [CrossRef] [PubMed]
- Macesic, N.; Bear Don’t Walk, O.J.; Pe’er, I.; Tatonetti, N.P.; Peleg, A.Y.; Uhlemann, A.-C. Predicting Phenotypic Polymyxin Resistance in Klebsiella Pneumoniae through Machine Learning Analysis of Genomic Data. mSystems 2020, 5, e00656-19. [Google Scholar] [CrossRef]
- Stokes, J.M.; Yang, K.; Swanson, K.; Jin, W.; Cubillos-Ruiz, A.; Donghia, N.M.; MacNair, C.R.; French, S.; Carfrae, L.A.; Bloom-Ackermann, Z.; et al. A Deep Learning Approach to Antibiotic Discovery. Cell 2020, 180, 688–702.e13. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Cokol-Cakmak, M.; Sahin, N.; Yilancioglu, K.; Kazan, H.; Collins, J.J.; Cokol, M. Chemogenomics and Orthology-based Design of Antibiotic Combination Therapies. Mol. Syst. Biol. 2016, 12, 872. [Google Scholar] [CrossRef] [PubMed]
- Cokol, M.; Li, C.; Chandrasekaran, S. Chemogenomic Model Identifies Synergistic Drug Combinations Robust to the Pathogen Microenvironment. PLoS Comput. Biol. 2018, 14, e1006677. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Liu, G.; Ju, Y.; Sun, Y.; Guo, W. Prediction of Synergistic Antibiotic Combinations by Graph Learning. Front. Pharmacol. 2022, 13, 849006. [Google Scholar] [CrossRef]
- Mason, D.J.; Eastman, R.T.; Lewis, R.P.I.; Stott, I.P.; Guha, R.; Bender, A. Using Machine Learning to Predict Synergistic Antimalarial Compound Combinations with Novel Structures. Front. Pharmacol. 2018, 9, 1096. [Google Scholar] [CrossRef]
- Jin, W.; Stokes, J.M.; Eastman, R.T.; Itkin, Z.; Zakharov, A.V.; Collins, J.J.; Jaakkola, T.S.; Barzilay, R. Deep Learning Identifies Synergistic Drug Combinations for Treating COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, e2105070118. [Google Scholar] [CrossRef]
- Olcay, B.; Ozdemir, G.D.; Ozdemir, M.A.; Ercan, U.K.; Guren, O.; Karaman, O. Prediction of the Synergistic Effect of Antimicrobial Peptides and Antimicrobial Agents via Supervised Machine Learning. BMC Biomed. Eng. 2024, 6, 1. [Google Scholar] [CrossRef]
- Olayo-Alarcon, R.; Amstalden, M.K.; Zannoni, A.; Bajramovic, M.; Sharma, C.M.; Brochado, A.R.; Rezaei, M.; Müller, C.L. Pre-Trained Molecular Representations Enable Antimicrobial Discovery. Nat. Commun. 2025, 16, 3420. [Google Scholar] [CrossRef]
- Zheng, E.J.; Valeri, J.A.; Andrews, I.W.; Krishnan, A.; Bandyopadhyay, P.; Anahtar, M.N.; Herneisen, A.; Schulte, F.; Linnehan, B.; Wong, F.; et al. Discovery of Antibiotics That Selectively Kill Metabolically Dormant Bacteria. Cell Chem. Biol. 2024, 31, 712–728.e9. [Google Scholar] [CrossRef]
- Rannon, E.; Shaashua, S.; Burstein, D. DRAMMA: A Multifaceted Machine Learning Approach for Novel Antimicrobial Resistance Gene Detection in Metagenomic Data. Microbiome 2025, 13, 67. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Pulkkinen, O.I.; Aittokallio, T. Target-Specific Compound Selectivity for Multi-Target Drug Discovery and Repurposing. Front. Pharmacol. 2022, 13, 1003480. [Google Scholar] [CrossRef]
- Mukherjee, A.; Abraham, S.; Singh, A.; Balaji, S.; Mukunthan, K.S. From Data to Cure: A Comprehensive Exploration of Multi-Omics Data Analysis for Targeted Therapies. Mol. Biotechnol. 2025, 67, 1269–1289. [Google Scholar] [CrossRef]
- Lim, H.; He, D.; Qiu, Y.; Krawczuk, P.; Sun, X.; Xie, L. Rational Discovery of Dual-Indication Multi-Target PDE/Kinase Inhibitor for Precision Anti-Cancer Therapy Using Structural Systems Pharmacology. PLoS Comput. Biol. 2019, 15, e1006619. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ye, K.; Van Vlijmen, H.W.T.; Emmerich, M.T.M.; IJzerman, A.P.; Van Westen, G.J.P. DrugEx v2: De Novo Design of Drug Molecules by Pareto-Based Multi-Objective Reinforcement Learning in Polypharmacology. J. Cheminform. 2021, 13, 85. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, G.; Li, J.; Li, J.; Zhang, O.; Zhang, X.; Li, L.; Hao, J.; Wang, E.; Heng, P.-A. Enabling Target-Aware Molecule Generation to Follow Multi Objectives with Pareto MCTS. Commun. Biol. 2024, 7, 1074. [Google Scholar] [CrossRef]
- Yao, L.; Jin, Z.; Mao, C.; Zhang, Y.; Luo, Y. Traditional Chinese Medicine Clinical Records Classification with BERT and Domain Specific Corpora. J. Am. Med. Inform. Assoc. 2019, 26, 1632–1636. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-C.; Ho, M.-Y.; Su, B.-H.; Wang, S.-Y.; Hsu, M.-T.; Tseng, Y.J. PanGPCR: Predictions for Multiple Targets, Repurposing and Side Effects. Bioinformatics 2021, 37, 1184–1186. [Google Scholar] [CrossRef]
- Zięba, A.; Stępnicki, P.; Matosiuk, D.; Kaczor, A.A. What Are the Challenges with Multi-Targeted Drug Design for Complex Diseases? Expert Opin. Drug Discov. 2022, 17, 673–683. [Google Scholar] [CrossRef]
- Ravikumar, B.; Aittokallio, T. Improving the Efficacy-Safety Balance of Polypharmacology in Multi-Target Drug Discovery. Expert Opin. Drug Discov. 2018, 13, 179–192. [Google Scholar] [CrossRef]
- Ramsay, R.R.; Popovic-Nikolic, M.R.; Nikolic, K.; Uliassi, E.; Bolognesi, M.L. A Perspective on Multi-target Drug Discovery and Design for Complex Diseases. Clin. Transl. Med. 2018, 7, e3. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, R.; Bajorath, J. Interpretation of Machine Learning Models Using Shapley Values: Application to Compound Potency and Multi-Target Activity Predictions. J. Comput. Aided Mol. Des. 2020, 34, 1013–1026. [Google Scholar] [CrossRef]
- Simões, R.S.; Maltarollo, V.G.; Oliveira, P.R.; Honorio, K.M. Transfer and Multi-Task Learning in QSAR Modeling: Advances and Challenges. Front. Pharmacol. 2018, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Qiang, B.; Lai, J.; Jin, H.; Zhang, L.; Liu, Z. Target Prediction Model for Natural Products Using Transfer Learning. Int. J. Mol. Sci. 2021, 22, 4632. [Google Scholar] [CrossRef] [PubMed]
- Bettanti, A.; Beccari, A.R.; Biccarino, M. Exploring the Future of Biopharmaceutical Drug Discovery: Can Advanced AI Platforms Overcome Current Challenges? Discov. Artif. Intell. 2024, 4, 102. [Google Scholar] [CrossRef]


| Database Name | Data Type | Brief Description | URL/Reference |
|---|---|---|---|
| TTD | Therapeutic targets, drugs, diseases | Provides comprehensive information on known and explored therapeutic protein and nucleic acid targets, their associated diseases, pathways, and corresponding drugs. | https://idrblab.org/ttd/ (accessed on 8 August 2025) |
| KEGG | Genomics, pathways, diseases, drugs | A knowledge base that links genomic information with higher-level functional information, such as biological pathways, diseases, and drug networks. | https://www.genome.jp/kegg/ (accessed on 8 August 2025) |
| PDB | Protein and nucleic acid 3D structures | A global archive for the experimentally determined 3D structures of biological macromolecules, including proteins and nucleic acids. | https://www.rcsb.org/ (accessed on 8 August 2025) |
| DrugBank | Drug-target, chemical, pharmacological data | A comprehensive resource that combines detailed drug data with extensive information on drug targets, mechanisms of action, and pathways. | https://go.drugbank.com/ (accessed on 8 August 2025) |
| ChEMBL | Bioactivity, chemical, genomic data | A manually curated database of bioactive drug-like small molecules, their bioactivities, and associated targets, extracted from medicinal chemistry literature. | https://www.ebi.ac.uk/chembl/ (accessed on 8 August 2025) |
| BindingDB | Protein-ligand binding affinities | A public database of experimentally measured binding affinities, focusing on interactions between small molecules and proteins. | http://www.bindingdb.org/ (accessed on 8 August 2025) |
| STITCH | Chemical-protein interactions | A database of known and predicted interactions between chemicals and proteins, integrating data from curated sources, text mining, and experiments. | http://stitch.embl.de/ (accessed on 8 August 2025) |
| PubChem | Chemical information, bioactivity | A massive repository of chemical compounds and their biological activities, including structures, bioassay results, and patents. | https://pubchem.ncbi.nlm.nih.gov/ (accessed on 8 August 2025) |
| STRING | Protein–protein interactions (PPI) | A database of known and predicted PPIs. It includes both physical and functional associations derived from experiments and genomic context. | https://string-db.org/ (accessed on 8 August 2025) |
| PharmGKB | Pharmacogenomic data | A comprehensive knowledge base that curates information on how genetic variations influence an individual’s response to drugs, linking genes, drugs, and diseases. | https://www.pharmgkb.org/ (accessed on 8 August 2025) |
| ClinicalTrials.gov | Clinical trial information | A public database maintained by the U.S. National Institutes of Health, containing a registry and results of clinical studies from around the world. | https://clinicaltrials.gov/ (accessed on 8 August 2025) |
| DisGeNET | Gene-disease associations | A discovery platform that integrates information on human gene-disease associations from various data sources and expert curations. | https://www.disgenet.org/ (accessed on 8 August 2025) |
| Reactome | Biological pathways | An open-source, curated database of biological pathways and molecular processes in humans, providing detailed information on molecular interactions and events. | https://reactome.org/ (accessed on 8 August 2025) |
| DrugCombDB | Drug combination data | A specialized database for drug combinations, featuring data on synergy, antagonism, and additivity to guide drug combination research. | https://drugcomb.org/ (accessed on 8 August 2025) |
| NCI-ALMANAC | Drug combination screening data | A large-scale cancer cell line drug combination screening dataset maintained by the National Cancer Institute (NCI). | https://dtp.cancer.gov/ncialmanac/initializePage.do (accessed on 8 August 2025) |
| O’Neil (Dataset) | Drug combination screening data | A key dataset for drug combination synergy prediction, published by O’Neil et al. in a 2016 study. | [45] |
| Data Sources | ML Approaches | Main Research Focus | Key Findings | Ref. |
|---|---|---|---|---|
| AZ-DREAM Challenges synergy data and an independent validation set from DrugCombDB | A GNN model named SynerGNet, with data augmentation | To develop a GNN model to accurately predict the synergistic effects of drug pairs against cancer cell lines. | SynerGNet achieved a balanced accuracy of 0.68, outperforming traditional ML. Data augmentation further improved accuracy to 0.73, and the model showed strong performance on unseen data from DrugCombDB. | [95] |
| DrugCombDB and Oncology-Screen datasets | DeepTraSynergy, a multimodal DL approach using transformers and a multitask learning framework | To predict drug combination synergy using a new DL model that integrates multimodal data, including drug-target, protein-protein, and cell-target interactions. | DeepTraSynergy outperformed classical and state-of-the-art models, achieving accuracies of 0.7715 and 0.8052 on the DrugCombDB and Oncology-Screen datasets, respectively. The study confirmed that integrating PPI networks significantly improved prediction. | [96] |
| DDS data and five independent datasets | DTSyn, a deep neural network (DNN) model using a multi-head attention mechanism | To identify novel drug combinations and understand the mechanisms of drug synergy from a chemical–gene–tissue interaction perspective. | DTSyn achieved the highest area under the curve (AUC) for the receiver operating characteristic curve (ROC) and best true positive rate (TPR) compared to competing methods. The ablation study confirmed the contribution of both transformer encoder blocks, and the model showed improved interpretability by extracting interactions among chemicals and cancer cell lines. | [97] |
| NCI-ALMANAC dataset | RF, XGBoost | To predict the synergy of unseen cancer drug combinations using a large-scale modeling study to reduce the need for in vitro tests. | The models predicted synergy with high accuracy. The study also found that certain drug classes—alkylating agents, tyrosine kinase inhibitors, and topoisomerase inhibitors—were better predicted, and that restricting predictions to the most reliable ones significantly decreased the error rate. | [98] |
| NCI-ALMANAC dataset | SYNPRED, an interdisciplinary approach leveraging specifically designed ensembles of AI algorithms | To develop a robust ensemble learning model for predicting anticancer drug synergy while also focusing on data interpretability. The study also aimed to determine the most appropriate synergy metric. | SYNPRED achieved state-of-the-art performance in both classification and regression models, particularly when using the Combination Sensitivity Score. The study also provided insights into which synergy metrics are most effective and achieved data interpretability using feature importance approaches. | [99] |
| NCI-ALMANAC dataset | SYNDEEP, a DNN-based binary classification model | To develop a novel DNN model for predicting synergistic drug combinations in cancer therapy. | The proposed DNN model achieved high accuracy (92.21%) and AUC (97.32%) in tenfold cross-validation. The integration of different types of physicochemical and genomic features was found to lead to more accurate predictions. | [100] |
| NCI-ALMANAC dataset | DL methods benchmarking | Systematically evaluate the impact of various methodological choices of multimodal DL models for predicting drug synergy in cancer. | Feature selection based on biological knowledge improved performance. Drug features were more predictive than a baseline model. Molecular fingerprints performed slightly better than learned representations. DL outperformed other ML methods, and an ensemble combining top DL and ML models provided further performance improvements. The models can also learn biologically meaningful associations between drug and cell line features. | [101] |
| CellMiner database and DrugComb datasets | MARSY, a multitask DL model using two encoders to capture the interplay between drug pairs and cell lines | To develop a computational model to accurately predict cancer drug-pair synergy scores by imputing missing values in sparse datasets. | MARSY outperformed state-of-the-art and traditional ML models. It successfully predicted synergy scores for new cancer drug-pair cell line combinations, and these novel predictions were validated using independent studies. | [102] |
| O’Neil and NCI-ALMANAC datasets; PubChem and GDSC databases | PermuteDDS, a Permutable feature fusion network | To develop an effective computational method for predicting drug–drug synergy (DDS) in cancer. | PermuteDDS exhibited superior performance on two benchmark datasets and showed good generalization performance on an independent test set. The permutable fusion mechanism was found to be an effective way to combine drug and cell line features. | [103] |
| DrugComb, Chembl, HURI datasets and others | DGSSynADR, a DL method using a low-rank global attention (LRGA) model and a bilinear predictor | To develop a new DL method to predict synergistic anticancer drug combinations, focusing on improving model generalization and interpretability. | DGSSynADR achieved better performance compared to seven competitive methods. The LRGA model and bilinear predictor were found to be key to improving prediction performance, and a Smooth L1 loss function helped avoid gradient explosion. | [104] |
| DrugComb, O’Neil, ALMANAC, Oncology Screen, DrugCombDB datasets and others | SynergyX, a multi-modality mutual attention network with a convolution-augmented attention structure | To develop a computational method for interpretable anti-tumor drug synergy prediction by modeling complex biological and drug interactions. | SynergyX showed superior predictive accuracy compared to other models. It also demonstrated multidimensional interpretability, identifying promising drug combinations for potential lung cancer treatment and uncovering drug-gene interactions. | [105] |
| DrugComb and CCLE databases | JointSyn, a dual-view jointly learning model | To develop a model for personalized drug synergy prediction that is accurate and robust, especially for cross-dataset predictions. | JointSyn outperformed existing models in predictive accuracy and robustness. The dual-view approach captured complementary synergy-related characteristics. The model’s generalization ability was improved with fine-tuning, and it was used to generate a drug synergy atlas for pan-cancer. | [106] |
| A large-scale oncology screen published by Merck & Co. | DeepSynergy, a DL model using conical layers | To apply DL to predict anticancer drug synergy, using chemical and genomic information. | DeepSynergy significantly outperformed other ML methods, with a 7.2% improvement over the next best method. It achieved a Pearson correlation of 0.73 and a high AUC of 0.90 for classifying novel combinations. | [107] |
| Multiomics datasets from TCGA and others | AuDNNsynergy, a DL model that uses three autoencoders to encode omics data | To develop a novel DL model for predicting the synergy of pairwise drug combinations in cancer by integrating multiomics data. | AuDNNsynergy outperformed four state-of-the-art approaches, specifically in terms of rank correlation metrics. | [108] |
| CCLE, GDSC datasets and others | DrugCell, an DL model that integrates tumor genotypes and drug structure to predict response | To develop an interpretable DL model for predicting drug response and designing synergistic drug combinations in cancer. | DrugCell accurately predicts drug responses, stratifies clinical outcomes, and successfully identifies synergistic drug combinations, which were validated through in vitro and in vivo experiments. | [109] |
| DrugBank, CCLE databases and an independent test set released by AstraZeneca | DeepDDS, a DL model based on a GNN with an attention mechanism | To develop a GNN-based DL model to identify synergistic drug combinations for specific cancer cells. | DeepDDS achieved better performance than both classical and other DL methods on a benchmark dataset. It also outperformed competitive methods by more than 16% in predictive precision on an independent test set. The model’s graph attention network provided interpretability by revealing important chemical substructures. | [110] |
| AstraZeneca’s drug combination dataset | DREAM challenge, a community-based, competitive benchmarking framework | To evaluate computational strategies for predicting synergistic drug pairs and biomarkers using a large-scale pharmacogenomic screen. | Winning methods achieved an accuracy matching biological replicates for over 60% of combinations. The study also identified a genomic rationale for some synergy predictions, but noted that 20% of combinations were poorly predicted by all methods. | [111] |
| O’Neil, ALMANAC, CLOUD and FORCINA datasets | MGAE-DC, a multi-channel graph autoencoder (MGAE) model | To develop a MGAE model that can predict the synergistic effects of drug combinations by leveraging not only synergistic data but also additive and antagonistic data. | MGAE-DC consistently outperformed state-of-the-art methods on four benchmark datasets. The model’s approach of integrating non-synergistic data and using an attention mechanism to capture invariant patterns improved its generalization performance and predictive accuracy. | [112] |
| O’Neil dataset; various online databases and published literature | A GCN model to solve a link prediction task | To develop a GCN model to predict cell line-specific synergistic anticancer drug combinations by integrating multiple biological networks. | The GCN model accurately predicted synergistic drug combinations, with a mean AUC of 0.84 across 39 cell lines. An in-depth literature survey validated many of the top predicted combinations, confirming their synergistic anti-tumor activity. | [113] |
| DrugComb dataset; cell-line omics database | MatchMaker, a DL framework that uses drug chemical structure and gene expression profiles | To develop a DL model to predict drug synergy scores with high accuracy by using the largest available dataset. | MatchMaker showed significant improvements over state-of-the-art models, with up to ~15% better correlation and ~33% lower mean squared error (MSE). The study also identified drug pairs and cell types that were harder to predict and presented novel candidate pairs. | [114] |
| DrugCombDB and Oncology-Screen datasets | GraphSynergy, a DL framework using a spatial-based GCN and an attention component | To develop an end-to-end DL framework for predicting synergistic anticancer drug combinations by encoding high-order topological relationships in a PPI network. | GraphSynergy outperformed classic and state-of-the-art models on two datasets, with accuracy values of 0.7553 and 0.7557. The study also found that the model’s attention mechanism highlighted pivotal proteins with relevant molecular functions, improving interpretability. | [115] |
| Monotherapy data from high-throughput cancer cell line screens, and efficacy data from over 5000 in vitro drug combinations and 26 clinical trials | IDACombo, a method based on the principle of independent drug action (IDA) | To develop a method to predict the clinical efficacy of cancer drug combinations using monotherapy cell line screen data. | IDACombo’s predictions showed high agreement with measured efficacies both in vitro (Pearson’s correlation = 0.93) and in clinical trials (accuracy > 84%). The method provides a framework for prioritizing new combinations. | [116] |
| Drug combination synergy score dataset; DrugBank and ChEMBL datasets; CCLE and GDSC databases | TranSynergy, a knowledge-enabled DL model using a self-attention transformer | To develop a mechanism-driven, interpretable DNN for synergistic drug combination prediction and pathway deconvolution, with a focus on cancer treatments. | TranSynergy outperformed the state-of-the-art method in performance and interpretability. It revealed novel pathways associated with synergistic combinations and predicted new high-confidence synergistic combinations for ovarian cancer. | [117] |
| Data Sources | ML Approaches | Main Research Focus | Key Findings | Ref. |
|---|---|---|---|---|
| DrugBank, DrugCentral, ChEMBL, BindingDB datasets and others | DeepDrug, a GNN model | To propose a novel AI-driven drug repurposing methodology to identify an effective combination of approved drugs for AD. | DeepDrug successfully identified a five-drug lead combination that targets multiple AD-related pathologies, demonstrating a novel expert-guided, AI-driven method for drug combination discovery in neurodegenerative diseases. | [127] |
| STRING and DrugBank databases | HNNDTA, a hybrid neural network for drug-target affinity (DTA) prediction | To propose a hybrid neural network for DTA prediction to facilitate drug repurposing for AD by identifying potential leads targeting the sigma-1 receptor. | The study identified haloperidol and bromperidol as lead compounds for AD treatment, proposing a new computer-aided drug design approach that is faster and more economical and has the potential for multi-target action. | [128] |
| BindingDB, ChEMBL, PubChem, and MPD3 datasets | ML virtual screening + molecular dynamics | To identify potential GSK3β inhibitors for treating neurodegenerative diseases, such as AD and PD, using a combined ML and molecular simulation approach. | The study identified three compounds with strong binding scores, with ZINC136900288 showing the highest affinity. These compounds feature novel chemical scaffolds and may serve as promising candidates for future experimental validation as GSK3β inhibitors. | [129] |
| ChEMBL database; Synapse portal dataset and others | DRIAD, a ML framework that links gene expression to AD pathology | To develop a ML framework for identifying drug repurposing candidates for AD. | The DRIAD method produced a ranked list of potential drug candidates for AD, providing a systematic way to nominate drugs for future clinical trials. | [130] |
| Data Sources | ML Approaches | Main Research Focus | Key Findings | Ref. |
|---|---|---|---|---|
| CARD, ARDB, and UNIPROT databeses | Two DL models, DeepARG-SS and DeepARG-LS, were developed to predict antibiotic resistance genes (ARG) | To propose a DL approach for accurately predicting antibiotic resistance genes from metagenomic data to address the limitations of traditional bioinformatics methods. | The DeepARG models demonstrated high precision and recall in predicting ARG and consistently outperformed the typical “best hit” approach by yielding lower false negative rates. | [136] |
| The HMD-ARG-DB, a curated multi-label ARG database | HMD-ARG, an end-to-end Hierarchical Multi-task DL framework for ARG annotation | To develop a multi-task DL framework for the accurate and detailed annotation of ARG from sequence data. | The HMD-ARG method demonstrates superior performance over state-of-the-art methods and can simultaneously identify an ARG’s class, resistance mechanism, and gene mobility. | [137] |
| ARGNet-DB, a dataset containing 48,615 amino acid sequences | ARGNet, a DNN that combines an unsupervised learning autoencoder with a CNN for classification | To develop a robust, alignment-free DNN to identify and classify a broad range of ARG from sequence data. | ARGNet demonstrated superior performance over other DL models. The model is flexible, efficient, and accurate, making it suitable for both targeted and metagenomic sequencing data. | [138] |
| DRIAMS database | MSDeepAMR, a DNN model | To propose a DNN model to predict AMR from raw mass spectrometry data. | The MSDeepAMR models showed good classification performance, with AUROC values above 0.83 in most cases. Additionally, adapted models improved AUROC by up to 20% compared to models trained on external data alone. | [139] |
| NARMS program for genomes and metadata | XGBoost-based models were used to predict minimum inhibitory concentration (MIC) from whole-genome sequences | To develop highly accurate ML models for predicting antimicrobial MIC and identifying associated genomic features in nontyphoidal Salmonella. | The models achieved an average accuracy of 95% for predicting MIC. The study also demonstrated that highly accurate models could be generated with a small number of genomes and that the approach is stable over time. | [140] |
| DRIAMS database | Calibrated classifiers were trained to predict AMR from MALDI-TOF mass spectra profiles | To develop a ML approach to accelerate the determination of AMR directly from clinical MALDI-TOF mass spectra. | The models showed strong predictive potential with AUROC values of 0.80, 0.74, and 0.74 for S. aureus, E. coli, and K. pneumoniae, respectively. A clinical case study showed that this approach would have been beneficial in a majority of cases by providing a faster and more accurate treatment recommendation. | [141] |
| Microbiome datasets | A ML approach that uses RF to predict and catalog antimicrobial peptides (AMPs) | To discover and catalog novel AMPs from the global microbiome to address the antibiotic-resistance crisis. | The research resulted in the AMPSphere catalog of nearly one million non-redundant peptides. Validation showed that 79 out of 100 tested peptides were active against clinically relevant drug-resistant pathogens. | [142] |
| Chemical + genomic datasets | Explainable DL using ensembles of GNN and explainable graph algorithms | To use an explainable DL approach to discover a novel structural class of antibiotics to combat antibiotic resistance. | The method successfully identified a new structural class of antibiotics that are selective against drug-resistant pathogens like MRSA and VRE and are effective in mouse models of infection. | [143] |
| Whole-genome sequence (WGS) data from Enterobacteriaceae isolates | A ML algorithm was compared against a rules-based approach for predicting resistance profiles | To evaluate and compare the performance of rules-based and ML approaches for predicting AMR from WGS data. | Both the rules-based and ML approaches achieved high agreement (89.0% and 90.3%, respectively) with standard phenotypic diagnostics. The study also identified specific sources of disagreement for each method. | [144] |
| Bacterial WGS datasets and phenotypes data | Several ML algorithms, including logistic regression, RF, SVC, and GBTC | To apply ML to WGS data to predict phenotypic polymyxin resistance in Klebsiella pneumoniae CG258. | The ML approach with a reference-based genomic data representation outperformed the rules-based approach. The models also correctly identified known resistance genes and suggested potential novel determinants. | [145] |
| Chemical libraries: Drug Repurposing Hub and ZINC15 database | A DNN model was trained to predict molecules with antibacterial activity | To use DL model to discover new, structurally divergent antibiotics to combat antibiotic resistance. | The DL model successfully identified a molecule, halicin, which is effective against multi-drug resistant pathogens. The approach also identified eight other new antibacterial compounds from a large chemical library. | [146] |
| Chemogenomics data | INDIGO, a computational approach that predicts synergistic or antagonistic interactions | To develop a computational method to predict effective antibiotic combination therapies that counter drug resistance. | The INDIGO approach significantly outperformed existing methods in predicting antibiotic interactions in E. coli and was successfully used to estimate drug-interaction outcomes in other pathogens like M. tuberculosis and S. aureus. | [147] |
| Chemogenomic profiles of drugs and metabolic perturbations | MAGENTA, a computational framework that predicts drug–drug interactions | To develop a computational framework to identify robust synergistic antibiotic combinations by predicting their efficacy in diverse microenvironments. | The framework successfully identified synergistic antibiotic combinations that are effective across different environments and accurately predicted changes in drug efficacy. | [148] |
| Antibiotic combination and PPI network data | A graph learning framework that combines network proximity with network propagation and a graph regularization model | To develop a graph learning framework to predict synergistic antibiotic combinations. | The model showed better performance and interpretability compared to existing methods for predicting synergistic antibiotic combinations. | [149] |
| Antimalarial compound libraries + in vitro assays | CoSynE: a ML model for predicting antimalarial combination synergy | To use ML to predict novel, synergistic antimalarial drug combinations with a focus on addressing drug resistance. | CoSynE successfully predicted synergistic combinations with significant enrichment over random selection, including with entirely novel compounds. | [150] |
| DTI and antiviral data, drug–drug combination datasets | A neural network architecture that jointly learns DTI and DDS | To use DL to identify synergistic drug combinations for COVID-19 with limited combination data. | The model performed significantly better than previous methods and successfully predicted two synergistic drug combinations that were validated in vitro. | [151] |
| AMP and antimicrobial agent synergy datasets | Supervised ML classifiers (RF, SVM) for AMP synergy | To predict the synergistic effects of combining AMP and antimicrobial agents using ML to reduce experimental effort and cost. | The models achieved a high accuracy of 76.92% in predicting synergistic effects. The analysis also revealed the most important features for prediction, which include the target microbial species and the MIC of the agents. | [152] |
| Large-scale chemical screening databases | MolE, a self-supervised DL framework to learn task-independent molecular representations | To develop a lightweight computational strategy for antimicrobial discovery using pre-trained molecular representations. | The model successfully identified and experimentally confirmed three human-targeted drugs as growth inhibitors of Staphylococcus aureus that were structurally distinct from existing antibiotics. | [153] |
| Broad Institute and other compound databases | DL models were used for virtual screens and toxicity filtering | To discover non-toxic antibiotics that are effective against metabolically dormant bacteria, which are associated with chronic infections and resistance. | The approach successfully identified semapimod, an anti-inflammatory drug that selectively kills stationary-phase bacteria by disrupting the outer membrane. | [154] |
| Global-scale genomic and metagenomic samples | DRAMMA, a ML method that uses protein properties, genomic context, and evolutionary patterns to detect novel genes | To develop a ML method for detecting novel ARGs in metagenomic data. | DRAMMA demonstrated robust predictive performance and holds promise for the discovery of novel ARGs that lack sequence similarity to any known resistance genes. | [155] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, X.; Wang, Y.; Wang, J.; Liu, C. Machine Learning for Multi-Target Drug Discovery: Challenges and Opportunities in Systems Pharmacology. Pharmaceutics 2025, 17, 1186. https://doi.org/10.3390/pharmaceutics17091186
Bi X, Wang Y, Wang J, Liu C. Machine Learning for Multi-Target Drug Discovery: Challenges and Opportunities in Systems Pharmacology. Pharmaceutics. 2025; 17(9):1186. https://doi.org/10.3390/pharmaceutics17091186
Chicago/Turabian StyleBi, Xueyuan, Yangyang Wang, Jihan Wang, and Cuicui Liu. 2025. "Machine Learning for Multi-Target Drug Discovery: Challenges and Opportunities in Systems Pharmacology" Pharmaceutics 17, no. 9: 1186. https://doi.org/10.3390/pharmaceutics17091186
APA StyleBi, X., Wang, Y., Wang, J., & Liu, C. (2025). Machine Learning for Multi-Target Drug Discovery: Challenges and Opportunities in Systems Pharmacology. Pharmaceutics, 17(9), 1186. https://doi.org/10.3390/pharmaceutics17091186






