Liposomal Encapsulation of Pine Green Cone Essential Oil: The Influence of the Carrier on the Enhancement of Anti-Inflammatory Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of EO
2.3. Chemical Analysis of EO
2.3.1. Gas Chromatography-GC
2.3.2. Gas Chromatography–Mass Spectrometry (GC-MS)
2.4. Protocol for the Production of Liposomal Dispersions and Nomenclature of the Samples for the In Vivo Study
2.5. Physico-Chemical Characterization of Liposomal Dispersions
2.5.1. EO Encapsulation Efficiency
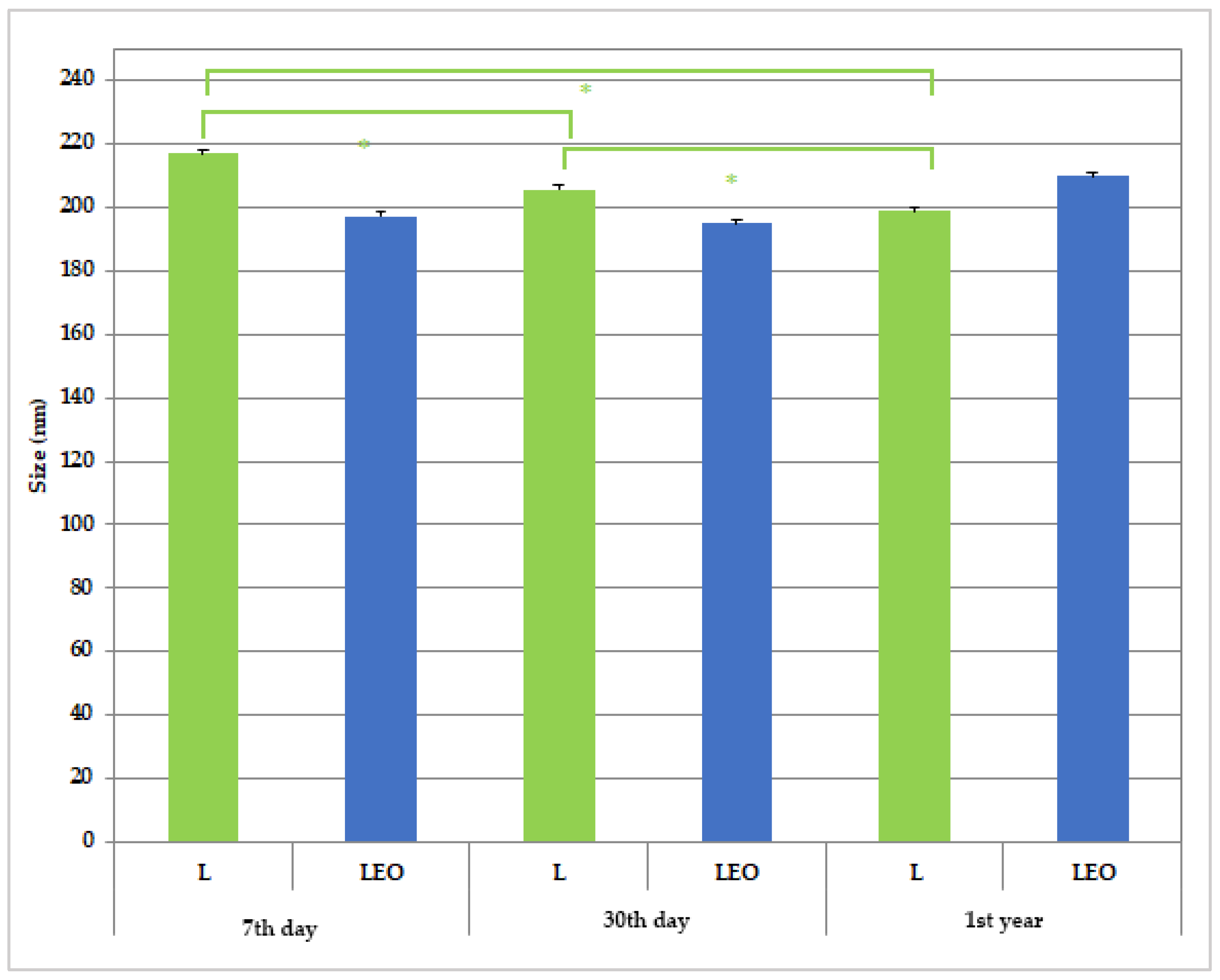
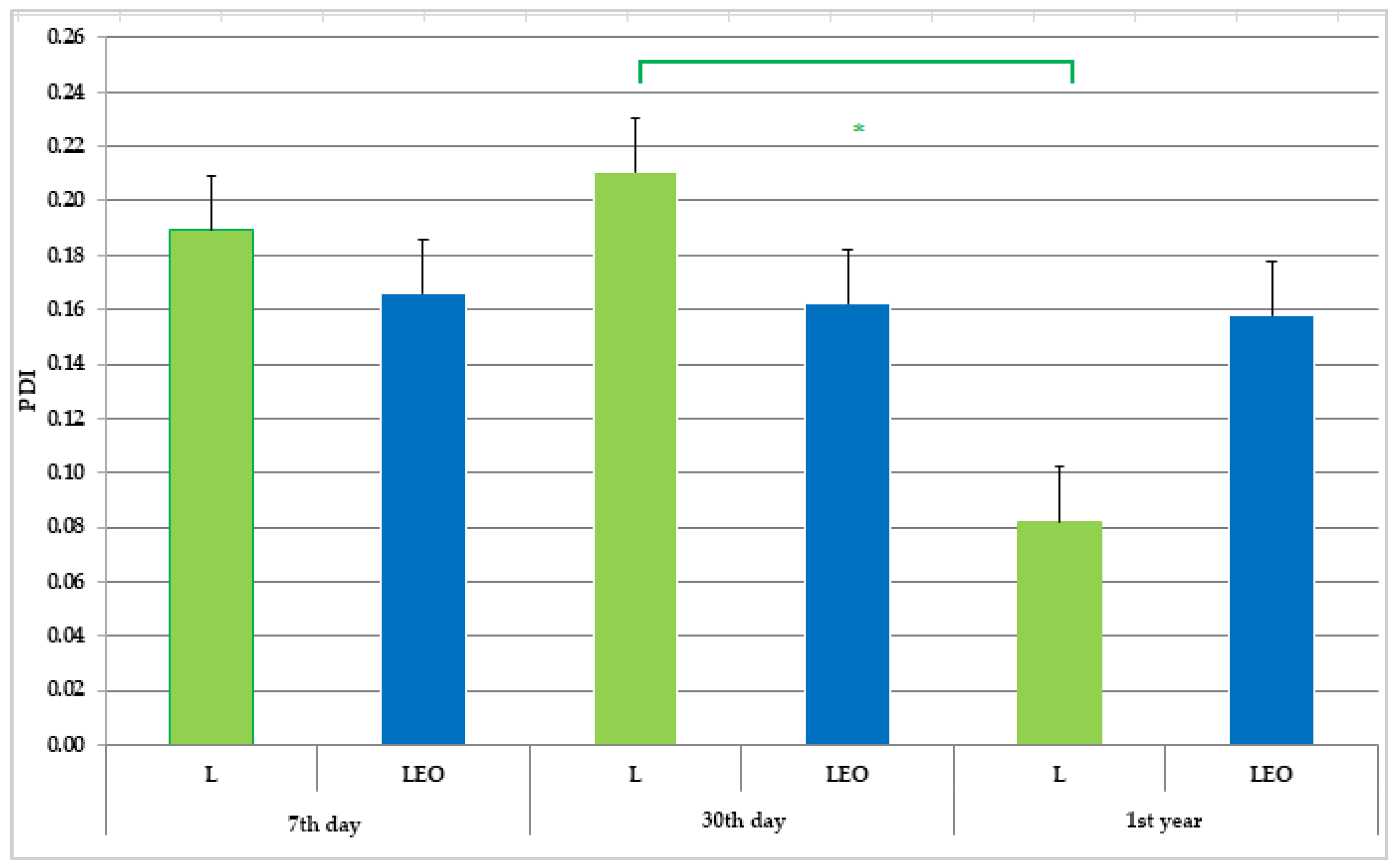
2.5.2. Determination of Mean Diameter and Polydispersity Index by Dynamic Light Scattering Method
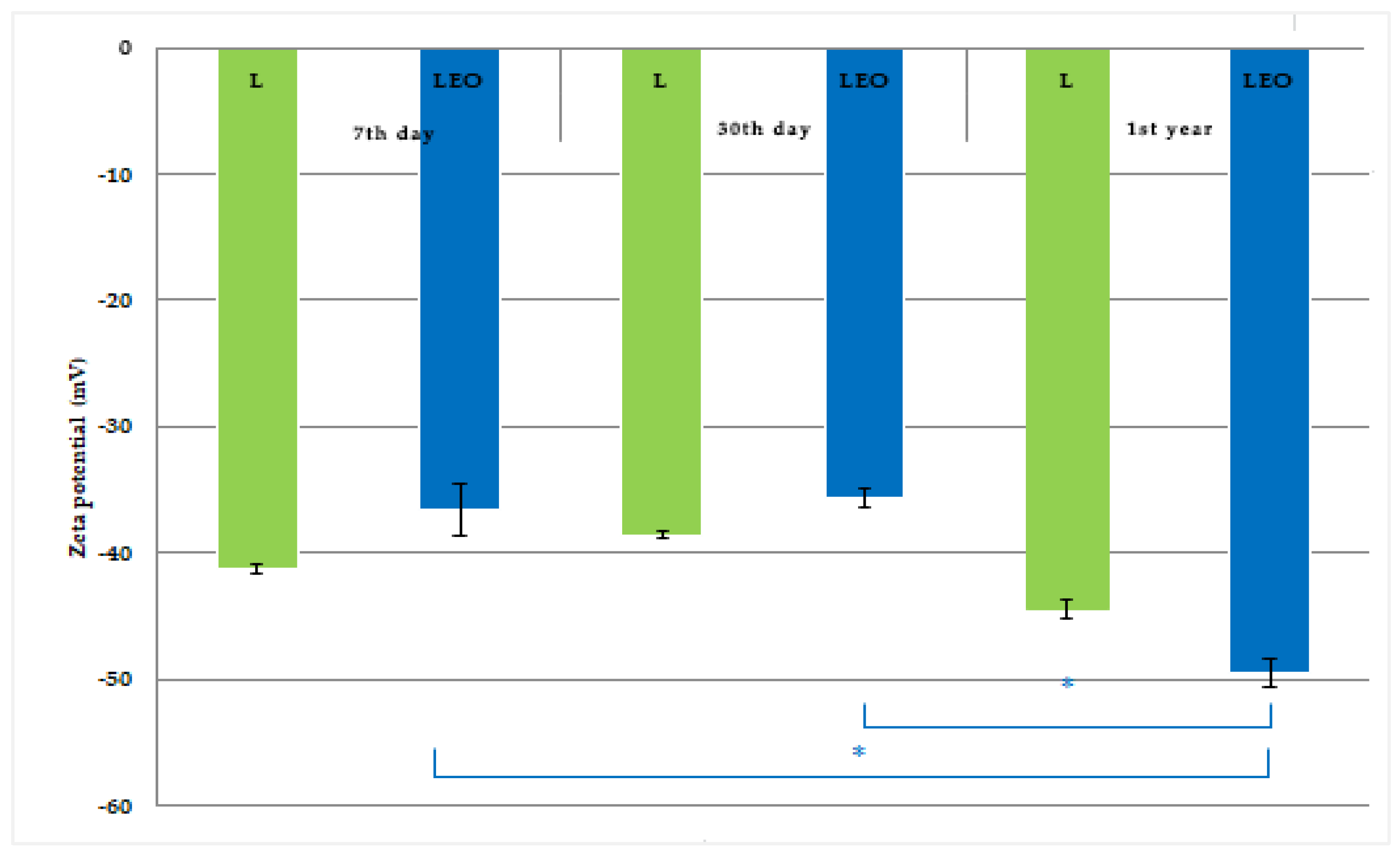
2.5.3. Determination of Surface Charge of Liposomes
2.5.4. Determination of pH Values
2.5.5. Determination of Electrical Conductivity
2.5.6. Preliminary Physico-Chemical Stability of Liposomes
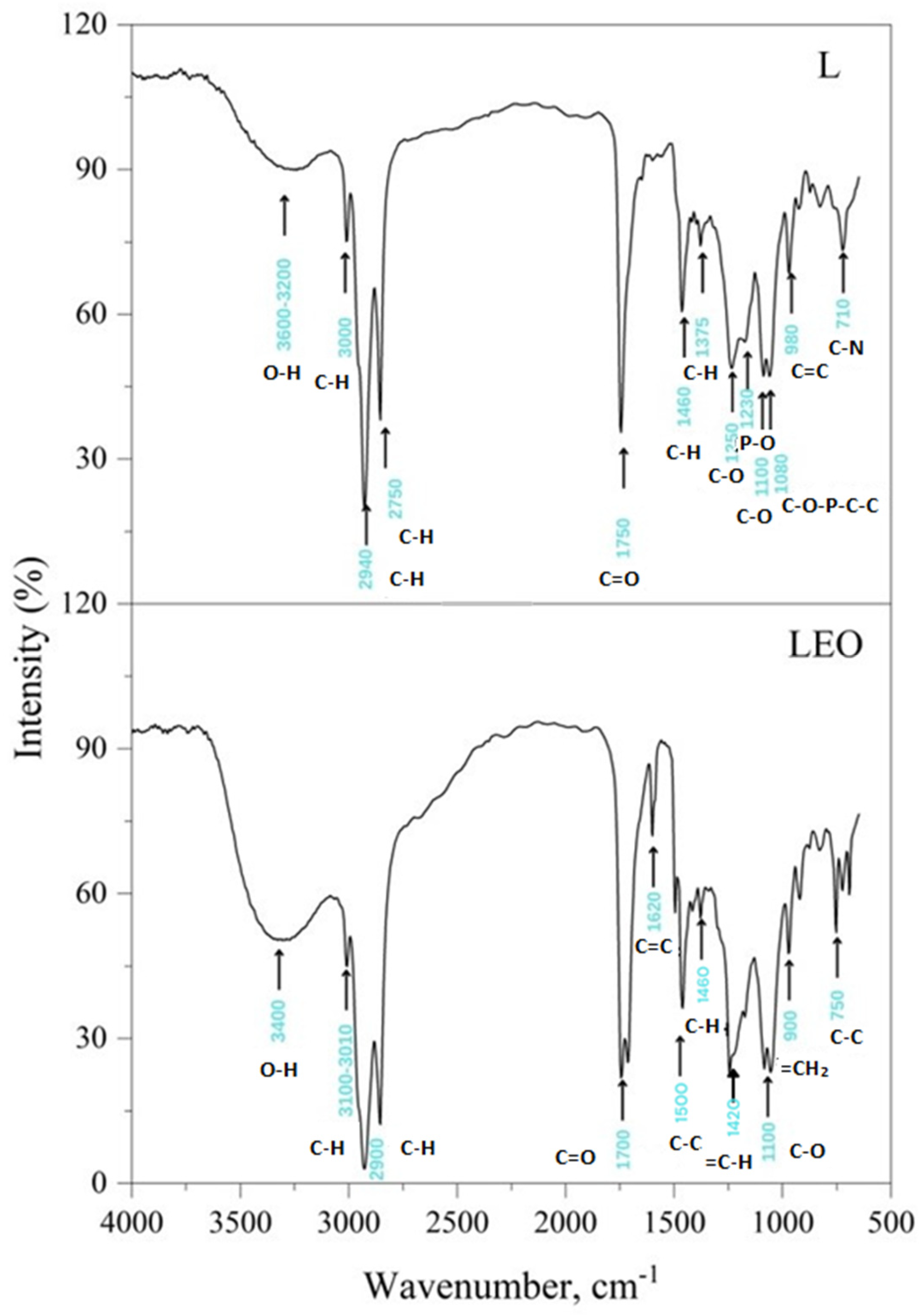
2.5.7. Fourier-Transform Infrared (FTIR) Spectral Analysis
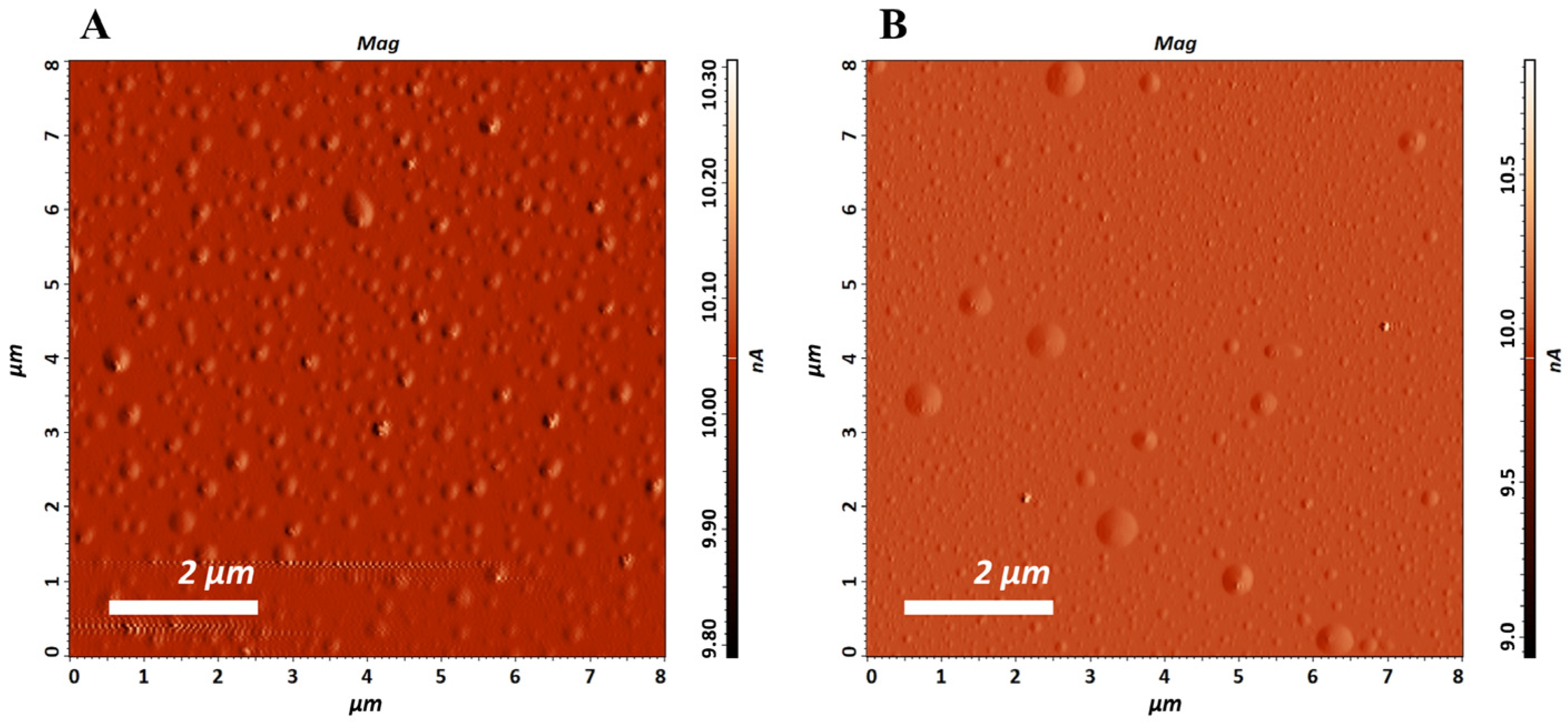
2.5.8. Atomic Force Microscopy (AFM)
2.6. In Vivo Experiments
2.6.1. Ethical Statement
2.6.2. Animals
2.6.3. Evaluation of Anti-Inflammatory Activity
2.7. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of EO
3.2. Liposomes Properties
3.3. Anti-Inflammatory Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Papp, N.; Purger, D.; Czigle, S.; Czégényi, D.; Stranczinger, S.; Tóth, M.; Dénes, T.; Kocsis, M.; Takácsi-Nagy, A.; Filep, R. The Importance of Pine Species in the Ethnomedicine of Transylvania (Romania). Plants 2022, 11, 2331. [Google Scholar] [CrossRef]
- Sharma, A.; Goyal, R.; Sharma, L. Potential Biological Efficacy of Pinus Plant Species Against Oxidative, Inflammatory and Microbial Disorders. BMC Complement. Altern. Med. 2015, 16, 35. [Google Scholar] [CrossRef]
- Kizilarslan Hancer, C.; Sevgi, E. Ethnobotanical Uses of Genus pinus L. (Pinaceae) in Turkey. Indian J. Tradit. Knowl. 2013, 12, 209–220. [Google Scholar]
- Redi, S.S. The ecological aspect of ethnobotany and ethnopharmacology of population in Bosnia and Herzegovina. Coll. Antropol. 2007, 31, 869–890. [Google Scholar]
- Kaushik, P.; Kaushik, D.; Khokra, S.L. Ethnobotany and Phytopharmacology of Pinus Roxburghii Sargent: A Plant Review. J. Integr. Med. 2013, 11, 371–376. [Google Scholar] [CrossRef]
- Tsioutsiou, E.E.; Amountzias, V.; Vontzalidou, A.; Dina, E.; Stevanović, Z.D.; Cheilari, A.; Aligiannis, N. Medicinal Plants Used Traditionally for Skin Related Problems in the South Balkan and East Mediterranean Region—A Review. Front. Pharmacol. 2022, 13, 936047. [Google Scholar] [CrossRef]
- Brazzini, B.; Pimpinelli, N. New and Established Topical Corticosteroids in Dermatology. Am. J. Clin. Dermatol. 2002, 3, 47–58. [Google Scholar] [CrossRef]
- Iravani, S.; Zolfaghari, B. Pharmaceutical and Nutraceutical Effects of Pinus Pinaster Bark Extract. Res. Pharm. Sci. 2011, 6, 1–11. [Google Scholar]
- Puente-Villegas, S.M.; Ticona, L.A.; Sánchez, Á.R.; Acebes, J.-L. Diterpenes of Pinus Pinaster Aiton with Anti-Inflammatory, Analgesic, and Antibacterial Activities. J. Ethnopharmacol. 2023, 318, 117021. [Google Scholar] [CrossRef]
- Kaushik, D.; Kumar, A.; Kaushik, P.; Rana, A.C. Analgesic and Anti-Inflammatory Activity of Pinus roxburghii Sarg. Adv. Pharmacol. Sci. 2012, 2012, 245431. [Google Scholar] [CrossRef]
- Kuo, P.-C.; Li, Y.-C.; Kusuma, A.M.; Tzen, J.T.C.; Hwang, T.-L.; Ye, G.-H.; Yang, M.-L.; Wang, S.-Y. Anti-Inflammatory Principles from the Needles of Pinus taiwanensis Hayata and In Silico Studies of Their Potential Anti-Aging Effects. Antioxidants 2021, 10, 598. [Google Scholar] [CrossRef]
- Jeong, S.-Y.; Choi, W.S.; Kwon, O.S.; Lee, J.S.; Son, S.Y.; Lee, C.H.; Lee, S.; Song, J.Y.; Lee, Y.J.; Lee, J.-Y. Extract of Pinus densiflora Needles Suppresses Acute Inflammation by Regulating Inflammatory Mediators in Raw264.7 Macrophages and Mice. Pharm. Biol. 2022, 60, 1148–1159. [Google Scholar] [CrossRef]
- Basholli-Salihu, M.; Schuster, R.; Hajdari, A.; Mulla, D.; Viernstein, H.; Mustafa, B.; Mueller, M. Phytochemical Composition, Anti-Inflammatory Activity and Cytotoxic Effects of Essential Oils from Three pinus spp. Pharm. Biol. 2017, 55, 1553–1560. [Google Scholar] [CrossRef]
- Vigo, E.; Cepeda, A.; Gualillo, O.; Perez-Fernandez, R. In-vitro Anti-Inflammatory Activity of Pinus sylvestris and Plantago lanceolata Extracts: Effect on Inducible Nos, Cox-1, Cox-2 and Their Products in J774a.1 Murine Macrophages. J. Pharm. Pharmacol. 2005, 57, 383–391. [Google Scholar] [CrossRef]
- Atmane, S.A.; Batır, M.B.; Özbek, Z.A.; Ergönül, P.G.; Balcan, E.; Eldjoudi, D.A.; Özkale, E.; Bribi, N.; Khettal, B. Cold Pressed Pinus sylvestris Mill Seed Oil for Potential Health Applications: Analgesic, Anti-Inflammatory Effects, and Assessment of Inflammatory Mediators by rt-qpcr in Skin Wound Healing. J. Ethnopharmacol. 2023, 319, 117157. [Google Scholar] [CrossRef]
- Süntar, I.; Tumen, I.; Ustün, O.; Keleş, H.; Akkol, E.K. Appraisal on the Wound Healing and Anti-Inflammatory Activities of the Essential Oils Obtained from the Cones and Needles of Pinus Species by in vivo and in vitro Experimental Models. J. Ethnopharmacol. 2012, 139, 533–540. [Google Scholar] [CrossRef]
- Kovačević, N. Osnovi Farmakognozije, 3rd ed.; Srpska Školska Knjiga: Belgrade, Serbia, 2004. [Google Scholar]
- de Lavor, É.M.; Fernandes, A.W.C.; de Andrade Teles, R.B.; Leal, A.E.B.P.; de Oliveira, R.G., Jr.; Silva, M.G.; de Oliveira, A.P.; Silva, J.C.; de Moura Fontes Araújo, M.T.; Coutinho, H.D.M.; et al. Essential Oils and Their Major Compounds in the Treatment of Chronic Inflammation: A Review of Antioxidant Potential in Preclinical Studies and Molecular Mechanisms. Oxidative Med. Cell. Longev. 2018, 2018, 6468593. [Google Scholar] [CrossRef]
- Cocero, M.J.; Martín, Á.; Mattea, F.; Varona, S. Encapsulation and Co-Precipitation Processes with Supercritical Fluids: Fundamentals and Applications. J. Supercrit. Fluids 2009, 47, 546–555. [Google Scholar] [CrossRef]
- Sherry, M.; Charcosset, C.; Fessi, H.; Greige-Gerges, H. Essential Oils Encapsulated in Liposomes: A Review. J. Liposome Res. 2013, 23, 268–275. [Google Scholar] [CrossRef]
- Swami, H.; Kataria, M.; Bilandi, A.; Kour, P.; Bala, S. Liposome: An Art for Drug Delivery. Int. J. Pharm. Sci. Lett. 2015, 5, 523–530. [Google Scholar]
- Vanic, Z.; Holaeter, A.-M.; Skalko-Basnet, N. (Phospho)lipid-Based Nanosystems for Skin Administration. Curr. Pharm. Des. 2015, 21, 4174–4192. [Google Scholar] [CrossRef]
- Filipović, M. Kozmetičke Emulzije na bazi Prirodnog Alkil Poliglukozidnog Emulgatora sa Liposominkapsuliranim Biljnim Matičnim Ćelijama: Proučavanje Fenomena na Granici Faza i Biofizička merenja na Koži (Cosmetic Emulsions Based on Natural Alkyl Polyglucoside Emulsifier with Liposome-Encapsulated Plant Stem Cells: Investigation of Interfacial Phenomena and Biophysical Skin Measurements). Ph.D. Thesis, University of Belgrade—Faculty of Pharmacy, Belgrade, Serbia, 2007. [Google Scholar]
- Farmakopeja, S.F.R.J. (Ed.) Jugoslovenska Farmakopeja IV SFRJ (Ph. Jug. IV). Pharmacopoea Jugoslavica Editio Quarta; Savezni Zavod za Zdravstvenu Zaštitu: Belgrade, Serbia, 1984. [Google Scholar]
- Sun, W.; Zhang, N.; Li, A.; Zou, W.; Xu, W. Preparation and Evaluation of N3-O-toluyl-fluorouracil-Loaded Liposomes. Int. J. Pharm. 2008, 353, 243–250. [Google Scholar] [CrossRef]
- Ćirković, J.; Radojković, A.M.; Jovanović, J.; Perać, S.; Branković, Z.M.; Milenković, I.; Milanović, S.D.; Dobrosavljević, J.N.; Tadić, V.M.; Žugić, A.R.; et al. Encapsulated Thuja Plicata Essential Oil into Biopolymer Matrix as a Potential Pesticide Against Phytophthora Root Pathogens. Int. J. Biol. Macromol. 2024, 278, 134684. [Google Scholar] [CrossRef]
- Caputo, F.; Clogston, J.; Calzolai, L.; Rösslein, M.; Prina-Mello, A. Measuring Particle Size Distribution of Nanoparticle Enabled Medicinal Products, the Joint View of EUNCL and NCI-NCL. A Step by Step Approach Combining Orthogonal Measurements with Increasing Complexity. J. Control. Release 2019, 299, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Shahrajabian, M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants—Natural Health Products for Human Health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef] [PubMed]
- Nam, A.-M.; Casanova, J.; Tomi, F.; Bighelli, A. Composition and Chemical Variability of Corsican Pinus halepensis Cone Oil. Nat. Prod. Commun. 2014, 9, 1361–1364. [Google Scholar] [CrossRef] [PubMed]
- Marzouki, H.; Falconieri, D.; Marongiu, B.; Piras, A.; Porcedda, S. Chemical Composition of Essential Oils from Needles of Pinus pinaster from Italy and Tunisia. Asian J. Chem. 2015, 27, 2662–2664. [Google Scholar] [CrossRef]
- Amri, I.; Hamrouni, L.; Hanana, M.; Gargouri, S.; Fezzani, T.; Jamoussi, B. Chemical Composition, Physico-Chemical Properties, Antifungal and Herbicidal Activities of Pinus halepensis Miller Essential Oils. Biol. Agric. Hortic 2013, 29, 91–106. [Google Scholar] [CrossRef]
- Tumen, I.; Hafizoglu, H.; Kilic, A.; Dönmez, I.E.; Sivrikaya, H.; Reunanen, M. Yields and Constituents of Essential Oil from Cones of Pinaceae spp. Natively Grown in Turkey. Molecules 2010, 15, 5797–5806. [Google Scholar] [CrossRef]
- Čutović, N.; Marković, T.; Carević, T.; Stojković, D.; Bugarski, B.; Jovanović, A.A. Liposomal and Liposomes-Film Systems as Carriers for bioactives from Paeonia tenuifolia L. Petals: Physicochemical Characterization and Biological Potential. Pharmaceutics 2023, 15, 2742. [Google Scholar] [CrossRef]
- Münster, T. PHOSAL®: Do-It-Yourself Globular Encapsulation System. Lipoid Kosmet. 2017. [Google Scholar]
- Merima, I.; Tadic, V.; Savic, S.; Đoković, J.; Arsić, I. Liposome Encapsulation of Origanum Compactum Essential Oil as the Active Ingredient: Assuring the Drug Effect. In The Lamiaceae Family: An Overview; Adler, A., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2019. [Google Scholar]
- Spyratou, E.; A Mourelatou, E.; Makropoulou, M.; Demetzos, C. Atomic Force Microscopy: A Tool to Study the Structure, Dynamics and Stability of Liposomal Drug Delivery Systems. Expert Opin. Drug Deliv. 2009, 6, 305–317. [Google Scholar] [CrossRef]
- Ge, Y.; Ge, M. Distribution of Melaleuca alternifolia Essential Oil in Liposomes with Tween 80 Addition and Enhancement of in vitro Antimicrobial Effect. J. Exp. Nanosci. 2015, 11, 345–358. [Google Scholar] [CrossRef]
- Najafi, M.N.; Arianmehr, A.; Sani, A.M. Preparation of Barije (Ferula gummosa) Essential Oil–Loaded Liposomes and Evaluation of Physical and Antibacterial Effect on Escherichia coli O157:H7. J. Food Prot. 2020, 83, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Palmas, L.; Aroffu, M.; Petretto, G.L.; Escribano-Ferrer, E.; Díez-Sales, O.; Usach, I.; Peris, J.-E.; Marongiu, F.; Ghavam, M.; Fais, S.; et al. Entrapment of Citrus limon var. pompia Essential Oil or Pure Citral in Liposomes Tailored as Mouthwash for the Treatment of Oral Cavity Diseases. Pharmaceuticals 2020, 13, 216. [Google Scholar] [CrossRef] [PubMed]
- Detoni, C.B.; Cabral-Albuquerque, E.C.M.; Hohlemweger, S.V.A.; Sampaio, C.; Barros, T.F.; Velozo, E.S. Essential Oil from Zanthoxylum Tingoassuiba Loaded into Multilamellar Liposomes Useful as Antimicrobial Agents. J. Microencapsul. 2009, 26, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Turina, A.V.; Nolan, M.; Zygadlo, J.; Perillo, M. Natural terpenes: Self-Assembly and Membrane Partitioning. Biophys. Chem. 2006, 122, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, Z.; Gharib, R.; Fourmentin, S.; Elaissari, A.; Greige-Gerges, H. New Findings on the Incorporation of Essential Oil Components into Liposomes Composed of Lipoid S100 and Cholesterol. Int. J. Pharm. 2019, 561, 161–170. [Google Scholar] [CrossRef]
- Taraj, K.; Malollari, I.; Ylli, F.; Maliqati, R.; Andoni, A.; Llupa, J. Spectroscopic Study on Chemical Composition of Essential Oil and Crude Extract from Albanian Pinus halepensis Mill. J. Agric. Inform. 2018, 41–46. [Google Scholar] [CrossRef]
- Batinić, P.M.; Đorđević, V.B.; Stevanović, S.I.; Balanč, B.D.; Marković, S.B.; Luković, N.D.; Mijin, D.Ž.; Bugarski, B.M. Formulation and Characterization of Novel Liposomes Containing Histidine for Encapsulation of A Poorly Soluble Vitamin. J. Drug Deliv. Sci. Technol. 2020, 59, 101920. [Google Scholar] [CrossRef]
- Lehman, S.E.; Benkstein, K.D.; Cleveland, T.E.; Anderson, K.W.; Carrier, M.J.; Vreeland, W.N. Particle Metrology Approach to Understanding How Storage Conditions Affect Long-Term Liposome Stability. Langmuir 2023, 39, 12313–12323. [Google Scholar] [CrossRef]
- Choi, S.; Kang, B.; Yang, E.; Kim, K.; Kwak, M.K.; Chang, P.-S.; Jung, H.-S. Precise Control of Liposome Size Using Characteristic Time Depends on Solvent Type and Membrane Properties. Sci. Rep. 2023, 13, 4728. [Google Scholar] [CrossRef] [PubMed]
- Aarabi, M.H. Preparation of Nanoliposomes Containing Rosmarinus Officinalis L Essential Oil; A Comparative Study. Biosci. Biotechnol. Res. Commun. 2017, 10, 105–110. [Google Scholar]
- Hoseini, B.; Jaafari, M.R.; Golabpour, A.; Momtazi-Borojeni, A.A.; Karimi, M.; Eslami, S. Application of Ensemble Machine Learning Approach to Assess the Factors Affecting Size and Polydispersity Index of Liposomal Nanoparticles. Sci. Rep. 2023, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Masmoudi, H.; Le Dréau, Y.; Piccerelle, P.; Kister, J. The Evaluation of Cosmetic and Pharmaceutical Emulsions Aging Process Using Classical Techniques and a New Method: FTIR. Int. J. Pharm. 2005, 289, 117–131. [Google Scholar] [CrossRef]
- Pinilla, C.M.B.; Reque, P.M.; Brandelli, A. Effect of Oleic Acid, Cholesterol, and Octadecylamine on Membrane Stability of Freeze-Dried Liposomes Encapsulating Natural Antimicrobials. Food Bioprocess Technol. 2020, 13, 599–610. [Google Scholar] [CrossRef]
- El-Aziz, E.A.E.-D.A.; Elgayar, S.F.; Mady, F.M.; Abourehab, M.A.S.; Hasan, O.A.; Reda, L.M.; Alaaeldin, E. The Potential of Optimized Liposomes in Enhancement of Cytotoxicity and Apoptosis of Encapsulated Egyptian Propolis on Hep-2 Cell Line. Pharmaceutics 2021, 13, 2184. [Google Scholar] [CrossRef]
- Ramli, N.; Ali, N.; Hamzah, S.; Yatim, N. Physicochemical Characteristics of Liposome Encapsulation of Stingless Bees’ Propolis. Heliyon 2021, 7, e06649. [Google Scholar] [CrossRef]
- Jiménez, M.; Domínguez, J.A.; Pascual-Pineda, L.A.; Azuara, E.; Beristain, C. Elaboration and Characterization of O/W Cinnamon (Cinnamomum Ceylanicum) and Black Pepper (Piper Nigrum) Emulsions. Food Hydrocoll. 2018, 77, 902–910. [Google Scholar] [CrossRef]
- Cui, H.; Li, W.; Li, C.; Vittayapadung, S.; Lin, L. Liposome Containing Cinnamon Oil with Antibacterial Activity Against Methicillin-Resistant Staphylococcus aureus Biofilm. Biofouling 2016, 32, 215–225. [Google Scholar] [CrossRef]
- Engel, J.B.; Heckler, C.; Tondo, E.C.; Daroit, D.J.; Malheiros, P.d.S. Antimicrobial Activity of Free and Liposome-Encapsulated Thymol and Carvacrol Against Salmonella and Staphylococcus Aureus Adhered to Stainless Steel. Int. J. Food Microbiol. 2017, 252, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, R.; Aliahmadi, A.; Rafati, H.; Abtahi, H.R.; Amini, S.; Feizabadi, M.M. Antibacterial and Anti-Biofilm Activity of Nanoemulsion of Thymus Daenensis Oil Against Multi-Drug Resistant Acinetobacter Baumannii. J. Mol. Liq. 2018, 265, 765–770. [Google Scholar] [CrossRef]
- Villasmil-Sánchez, S.; Drhimeur, W.; Ospino, S.C.S.; Alvarez, A.M.R.; González-Rodríguez, M. Positively and Negatively Charged Liposomes as Carriers for Transdermal Delivery of Sumatriptan: In Vitro Characterization. Drug Dev. Ind. Pharm. 2009, 36, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Jahanfar, S.; Ghavami, M.; Khosravi-Darani, K.; Jahadi, M. Liposomal Green Tea Extract: Optimization and Physicochemical Characterization. J. Appl. Biotechnol. Rep. 2021, 8, 5–12. [Google Scholar] [CrossRef]
- Lodén, M. Role of Topical Emollients and Moisturizers in the Treatment of Dry Skin Barrier Disorders. Am. J. Clin. Dermatol. 2003, 4, 771–788. [Google Scholar] [CrossRef]
- Liu, N.; Guan, T.; Zhou, W.; Li, J.; Cao, Y.; Lin, L. Preparation and Characterization of Galanga Essential Oil Liposomes. In 2016 International Conference on Advances in Energy, Environment and Chemical Science; Atlantis Press: Dordrecht, The Netherlands, 2016; pp. 99–105. [Google Scholar]
- Das, P.; Das, M.K. Physical, Chemical, and Microbiological Stability of Nanocosmetics. In Nanocosmeceuticals; Das, M.K., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 139–166. [Google Scholar]
- Kamaruzaman, N.; Yusop, S.M. Determination of Stability of Cosmetic Formulations Incorporated with Water-Soluble Elastin Isolated from Poultry. J. King Saud Univ. Sci. 2021, 33, 101519. [Google Scholar] [CrossRef]
- Ibišević, M.; Nešić, I.; Pilipović, S.; Smajlović, A.; Srabović, N.; Nikolić, V.; Cvetković, D.; Kozarević, E.C.; Horozić, E.; Karić, E.; et al. In vitro Release and Stability Assessment of Origanum Compactum Liposomal Dispersion with Antifungal Activity. J. Pharmacogn. Phytochem. 2022, 11, 192–196. [Google Scholar] [CrossRef]
- Pradhan, B.; Ki, J.-S. Biological activity of algal derived carrageenan: A Comprehensive Review in Light of Human Health and Disease. Int. J. Biol. Macromol. 2023, 238, 124085. [Google Scholar] [CrossRef]
- Pashmforosh, M.; Vardanjani, H.R.; Vardanjani, H.R.; Pashmforosh, M.; Khodayar, M.J. Topical Anti-Inflammatory and Analgesic Activities of Citrullus colocynthis Extract Cream in Rats. Medicina 2018, 54, 51. [Google Scholar] [CrossRef]
- Rautava, S.; Walker, W.A.; Lu, L. Hydrocortisone-Induced Anti-Inflammatory Effects in Immature Human Enterocytes Depend on the Timing of Exposure. Am. J. Physiol. Liver Physiol. 2016, 310, G920–G929. [Google Scholar] [CrossRef]
- Nikolic, M.; Andjic, M.; Bradic, J.; Kocovic, A.; Tomovic, M.; Samanovic, A.M.; Jakovljevic, V.; Veselinovic, M.; Capo, I.; Krstonosic, V.; et al. Topical Application of Siberian Pine Essential Oil Formulations Enhance Diabetic Wound Healing. Pharmaceutics 2023, 15, 2437. [Google Scholar] [CrossRef]
- Tümen, İ.; Akkol, E.K.; Taştan, H.; Süntar, I.; Kurtça, M. Research on the Antioxidant, Wound Healing, and Anti-Inflammatory Activities and the Phytochemical Composition of Maritime Pine (Pinus pinaster Ait). J. Ethnopharmacol. 2018, 211, 235–246. [Google Scholar] [CrossRef]
| Sample | Sample Label | Phosal 40 IP | EO from P. halepensis Green Cones | Polysorbate 20 | Euxyl PE 9010 | Purified Water | Total |
|---|---|---|---|---|---|---|---|
| “Blank” liposomes | L | 10 | - | - | 1 | 89 | 100 |
| Liposomes with EO from P. halepensis green cones | LEO | 10 | 0.5 | - | 1 | 88.5 | 100 |
| Purified water with EO from P. halepensis green cones and Polysorbate 20 | EOP | - | 0.5 | 2 | 1 | 96.5 | 100 |
| Purified water with Polysorbate 20 | P | - | - | 2 | 1 | 97 | 100 |
| Chemical Compounds | RI * | CAS | P. halepensis EO (%) ** |
|---|---|---|---|
| α-pinene | 932 | 80–56–8 | 47.47 |
| camphene | 946 | 79–92–5 | 0.65 |
| β-pinene | 974 | 127–91–3 | 3.85 |
| myrcene | 988 | 123–35–3 | 14.61 |
| δ-3-carene | 1008 | 13466–78–9 | 5.10 |
| limonene | 1024 | 138–86–3 | 5.98 |
| bornyl acetate | 1287 | 76–49–3 | 0.64 |
| (E)-caryophyllene | 1417 | 87–44–5 | 11.70 |
| α-humulene | 1452 | 6753–98–6 | 2.01 |
| δ-cadinene | 1522 | 483–76–1 | 0.45 |
| caryophyllene oxide | 1582 | 1139–30–6 | 0.80 |
| manool oxide | 1987 | 596–84–9 | 0.45 |
| abietatriene | 2055 | 19407–28–4 | 0.34 |
| dehydroabietal | 2274 | 13601–88–2 | 0.50 |
| Total | 94.55 | ||
| Monoterpene hydrocarbons | 77.66 | ||
| Oxygenated monoterpenes | 0.64 | ||
| Sesquiterpene hydrocarbons | 14.16 | ||
| Oxygenated sesquiterpenes | 0.8 | ||
| Diterpenes | 1.29 |
| Sample | pH | Conductivity (μs/cm) | ||||
|---|---|---|---|---|---|---|
| 7th day | 30th day | 1st year | 7th day | 30th day | 1st year | |
| L | 5.00 | 4.41 | 4.27 | 500 | 519 | 507 |
| LEO | 5.01 | 4.68 | 4.35 | 300 | 320 | 350 |
| Rat Paw Thickness (mm) (%Inhibition) | |||||
|---|---|---|---|---|---|
| Experimental Groups | 0 h | 1 h | 2 h | 3 h | 4 h |
| L | 4.400.00 | 5.900.16 (16.667%) | 6.970.73 (25.962%) ** | 6.500.5 (32.258%) ** | 6.070.45 (35.897%) ** |
| LEO | 4.400.29 | 5.870.05 (18.519%) | 5.930.42 (55.769%) * | 5.890.26 (51.828%) *, ** | 5.200.08 (69.231%) * |
| EOP | 4.300.08 | 5.600.08 (27.778%) | 6.830.05 (26.923%) ** | 6.370.09 (33.333%) ** | 5.730.12 (44.872%) ** |
| P | 4.000.08 | 5.630.05 (9.259%) | 7.430.12 (0.962%) ** ‡ | 7.000.22 (3.226%) ** | 6.330.12 (10.256%) ** ‡ |
| HC | 4.130.12 | 5.330.17 (33.333%) | 4.730.05 (82.692%) * | 4.600.08 (84.946%) * | 4.400.08 (89.744%) * |
| CTRL | 4.100.08 | 5.900.08 | 7.570.05 | 7.200.08 | 6.700.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirković, S.; Tadić, V.; Tomović, M.; Petrović, A.; Andjić, M.; Bradić, J.; Perać, S.; Radojković, A.; Jovanović, J.; Žugić, A. Liposomal Encapsulation of Pine Green Cone Essential Oil: The Influence of the Carrier on the Enhancement of Anti-Inflammatory Activity. Pharmaceutics 2025, 17, 1182. https://doi.org/10.3390/pharmaceutics17091182
Mirković S, Tadić V, Tomović M, Petrović A, Andjić M, Bradić J, Perać S, Radojković A, Jovanović J, Žugić A. Liposomal Encapsulation of Pine Green Cone Essential Oil: The Influence of the Carrier on the Enhancement of Anti-Inflammatory Activity. Pharmaceutics. 2025; 17(9):1182. https://doi.org/10.3390/pharmaceutics17091182
Chicago/Turabian StyleMirković, Snježana, Vanja Tadić, Marina Tomović, Anica Petrović, Marijana Andjić, Jovana Bradić, Sanja Perać, Aleksandar Radojković, Jelena Jovanović, and Ana Žugić. 2025. "Liposomal Encapsulation of Pine Green Cone Essential Oil: The Influence of the Carrier on the Enhancement of Anti-Inflammatory Activity" Pharmaceutics 17, no. 9: 1182. https://doi.org/10.3390/pharmaceutics17091182
APA StyleMirković, S., Tadić, V., Tomović, M., Petrović, A., Andjić, M., Bradić, J., Perać, S., Radojković, A., Jovanović, J., & Žugić, A. (2025). Liposomal Encapsulation of Pine Green Cone Essential Oil: The Influence of the Carrier on the Enhancement of Anti-Inflammatory Activity. Pharmaceutics, 17(9), 1182. https://doi.org/10.3390/pharmaceutics17091182






