Evaluation of Permeation Enhancers for Vaginal Delivery of Buserelin Acetate Using a Validated Chromatographic Method and Ex Vivo Porcine Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentation and Chromatographic Analysis Software
2.2. Chemicals and Solvents
Pure Samples, Reagents and Solvents
2.3. Method Development and Validations
Chromatographic Analysis
2.4. Preparation of Standard Stock Solution
2.5. Method Validation
2.6. Specificity and Peak Purity
2.7. System Suitability
2.7.1. Linearity and Range
2.7.2. Accuracy
2.7.3. Precision
2.7.4. LOD and LOQ
2.7.5. Robustness
2.7.6. Stability Test
2.8. Ex Vivo Permeation Studies
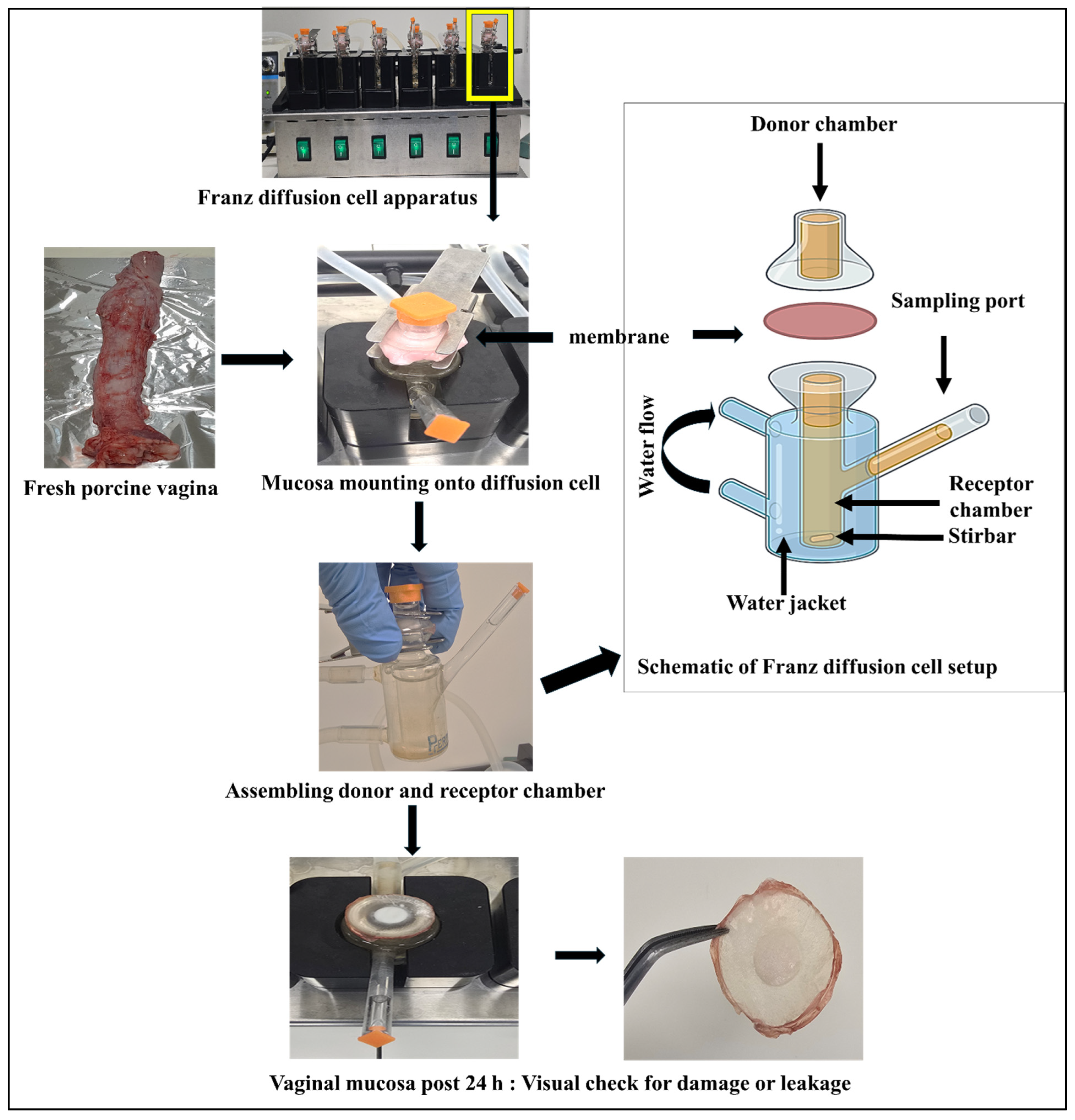
2.8.1. Tissue Preparation
2.8.2. Franz Diffusion Cell Setup
2.9. Sample Collection
2.10. Apparent Permeability Coefficient (Papp)
2.11. Kinetic Analysis
2.12. Statistical Analysis
3. Results
3.1. Chromatographic Conditions and Chromatograms
3.2. Method Validation
3.2.1. Specificity
3.2.2. System Suitability
3.2.3. Linearity and Range
3.3. Accuracy
3.4. Precision
3.4.1. LOD and LOQ
3.4.2. Robustness
3.4.3. Stability
3.5. Ex Vivo Permeation Studies
3.6. Kinetic Modelling for Permeation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bartolome, J.A.; Thatcher, W.W.; Melendez, P.; Risco, C.A.; Archbald, L.F. Strategies for the diagnosis and treatment of ovarian cysts in dairy cattle. J. Am. Vet. Med. Assoc. 2005, 227, 1409–1414. [Google Scholar] [CrossRef]
- Castagna, C.D.; Peixoto, C.H.; Bortolozzo, F.P.; Wentz, I.; Neto, G.B.; Ruschel, F. Ovarian cysts and their consequences on the reproductive performance of swine herds. Anim. Reprod. Sci. 2004, 81, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Irie, T.; Adachi, H.; Uekama, K. Combined Use of 2-Hydroxypropyl-Beta-Cyclodextrin and a Lipophilic Absorption Enhancer in Nasal Delivery of the LHRH Agonist, Buserelin Acetate, in Rats. Int. J. Pharm. 1995, 123, 103–112. [Google Scholar] [CrossRef]
- Matsubara, K.; Abe, K.; Irie, T.; Uekama, K. Improvement of nasal bioavailability of luteinizing hormone-releasing hormone agonist, buserelin, by cyclodextrin derivatives in rats. J. Pharm. Sci. 1995, 84, 1295–1300. [Google Scholar] [CrossRef]
- Rohan, L.C.; Sassi, A.B. Vaginal Drug Delivery Systems for HIV Prevention. AAPS J. 2009, 11, 78–87. [Google Scholar] [CrossRef]
- Squier, C.A.; Mantz, M.J.; Schilievert, P.M.; Davis, C.C. Porcine vagina as a model for studying permeability and pathogenesis in mucosa. J. Pharm. Sci. 2008, 97, 9–21. [Google Scholar] [CrossRef] [PubMed]
- van Eyk, A.D.; van der Bijl, P. Porcine vaginal mucosa as an in vitro permeability model for human vaginal mucosa. Int. J. Pharm. 2005, 305, 105–111. [Google Scholar] [CrossRef]
- Kong, J.Y.; Su, F.Q.; Liu, Y.; Yang, Y.X.; Cao, Y.Y.; Qiu, J.C.; Wang, Y.; Zhang, L.; Wang, J.Z.; Cao, X.Y. The pharmacokinetics of buserelin after intramuscular administration in pigs and cows. BMC Vet. Res. 2022, 18, 136. [Google Scholar] [CrossRef]
- Uddin, A.H.M.M.; Song, Y.M.; Garg, S.; Petrovski, K.R.; Kirkwood, R.N. Control of ovarian function using non-injection technologies for GnRH administration. J. Drug Deliv. Sci. Technol. 2023, 84, 104502. [Google Scholar] [CrossRef]
- Nasr, G.; Greige-Gerges, H.; Fourmentin, S.; Elaissari, A.; Khreich, N. Cyclodextrins permeabilize DPPC liposome membranes: A focus on cholesterol content, cyclodextrin type, and concentration. Beilstein J. Org. Chem. 2023, 19, 1570–1579. [Google Scholar] [CrossRef]
- Xu, B.; Lin, W.Q.; Wang, X.G.; Zeng, S.W.; Zhou, G.Q.; Chen, J.L. Molecular dynamics simulations of the effects of sodium dodecyl sulfate on lipid bilayer. Chin. Phys. B 2017, 26, 033103. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, W.; Cheng, H.B.; Zhang, M.X.; Kou, Y.Q.; Guan, J.; Liu, Q.Y.; Gao, M.Y.; Wang, X.H.; Mao, S.R. Modulating intestinal mucus barrier for nanoparticles penetration by surfactants. Asian J. Pharm. Sci. 2019, 14, 543–551. [Google Scholar] [CrossRef]
- Hirun, N.; Kraisit, P.; Tantishaiyakul, V. Thermosensitive Polymer Blend Composed of Poloxamer 407, Poloxamer 188 and Polycarbophil for the Use as Mucoadhesive In Situ Gel. Polymers 2022, 14, 1836. [Google Scholar] [CrossRef]
- Vllasaliu, D.; Exposito-Harris, R.; Heras, A.; Casettari, L.; Garnett, M.; Illum, L.; Stolnik, S. Tight junction modulation by chitosan nanoparticles: Comparison with chitosan solution. Int. J. Pharm. 2010, 400, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Kuz’mina, N.; Moiseev, S.; Krylov, V.; Deryabin, A.; Yashkir, V.; Merkulov, V. Validation of an NMR-spectroscopic method for authenticity confirmation of buserelin acetate pharmaceutical substance. Pharm. Chem. J. 2018, 52, 159–165. [Google Scholar] [CrossRef]
- Fisher, E.N.; Melnikov, E.S.; Gegeckori, V.; Potoldykova, N.; Enikeev, D.; Pavlenko, K.A.; Agatonovic-Kustrin, S.; Morton, D.W.; Ramenskaya, G. Development and Validation of an LC-MS/MS Method for Simultaneous Determination of Short Peptide-Based Drugs in Human Blood Plasma. Molecules 2022, 27, 7831. [Google Scholar] [CrossRef]
- Tambare, R.S.; Shahi, S.R.; Gurumukhi, V.C.; Kakade, S.M.; Tapadiya, G.G. Quality by design (QbD) based development and validation of RP-HPLC method for buserelin acetate in polymeric nanoparticles: Release study. Heliyon 2024, 10, e39172. [Google Scholar] [CrossRef]
- Hoitink, M.A.; Beijnen, J.H.; Boschma, M.U.S.; Bult, A.; van der Houwen, O.A.G.J.; Wiese, G.; Underberg, W.J.M. Degradation kinetics of three gonadorelin analogues: Developing a method for calculating epimerization parameters. Pharm. Res. 1998, 15, 1449–1455. [Google Scholar] [CrossRef]
- Hoitink, M.A.; Beijnen, J.H.; Boschma, M.U.S.; Bult, A.; Hop, E.; Nijholt, J.; Versluis, C.; Wiese, G.; Underberg, W.J.M. Identification of the degradation products of gonadorelin and three analogues in aqueous solution. Anal. Chem. 1997, 69, 4972–4978. [Google Scholar] [CrossRef]
- Patel, F.; Kotadiya, R.; Patel, R.; Patel, M. Development and Validation of a New Reversed Phase HPLC Method for the Quantitation of Azithromycin and Rifampicin in a Capsule Formulation. J. Chromatogr. Sci. 2024, 62, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Steyn, J.D.; Haasbroek-Pheiffer, A.; Pheiffer, W.; Weyers, M.; van Niekerk, S.E.; Hamman, J.H.; van Staden, D. Evaluation of Drug Permeation Enhancement by Using In Vitro and Ex Vivo Models. Pharmaceuticals 2025, 18, 195. [Google Scholar] [CrossRef] [PubMed]
- International Council for Harmonisation. Guideline on Validation of Analytical Procedures; International Council for Harmonisation: Geneva, Switzerland, 2022. [Google Scholar]
- Ibrahim, H.; Hamdy, A.M.; Merey, H.A.; Saad, A.S. Dual-Mode Gradient HPLC and TLC Densitometry Methods for the Simultaneous Determination of Paracetamol and Methionine in the Presence of Paracetamol Impurities. J. AOAC Int. 2021, 104, 975–982. [Google Scholar] [CrossRef]
- Bahl, D.; Daftardar, S.; Devi Bachu, R.; Boddu, S.H.; Altorok, N.; Kahaleh, B. Evaluation of topical econazole nitrate formulations with potential for treating Raynaud’s phenomenon. Pharm. Dev. Technol. 2019, 24, 689–699. [Google Scholar] [CrossRef]
- Brodin, B.; Steffansen, B.; Nielsen, C.U. Passive diffusion of drug substances: The concepts of flux and permeability. In Molecular Biopharmaceutics: Aspects of Drug Characterisation, Drug Delivery and Dosage form Evaluation; Pharmaceutical Press: London, UK, 2010; Chapter 3.2; pp. 135–151. [Google Scholar]
- Zhang, Y.; Huo, M.R.; Zhou, J.P.; Zou, A.F.; Li, W.Z.; Yao, C.L.; Xie, S.F. DDSolver: An Add-In Program for Modeling and Comparison of Drug Dissolution Profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef]
- Mant, C.T.; Chen, Y.; Yan, Z.; Popa, T.V.; Kovacs, J.M.; Mills, J.B.; Tripet, B.P.; Hodges, R.S. HPLC analysis and purification of peptides. Methods Mol. Biol. 2007, 386, 3–55. [Google Scholar]
- Maher, S.; Casettari, L.; Illum, L. Transmucosal absorption enhancers in the drug delivery field. Pharmaceutics 2019, 11, 339. [Google Scholar] [CrossRef]
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins: Effects on drug permeation through biological membranes. J. Pharm. Pharmacol. 2011, 63, 1119–1135. [Google Scholar] [CrossRef]
- Loftsson, T. Drug permeation through biomembranes: Cyclodextrins and the unstirred water layer. Pharmazie 2012, 67, 363–370. [Google Scholar]
- Anderberg, E.K.; Artursson, P. Epithelial transport of drugs in cell culture. VIII: Effects of sodium dodecyl sulfate on cell membrane and tight junction permeability in human intestinal epithelial (Caco-2) cells. J. Pharm. Sci. 1993, 82, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Umlong, I.; Ismail, K. Micellization behaviour of sodium dodecyl sulfate in different electrolyte media. Colloids Surf. A Physicochem. Eng. Asp. 2007, 299, 8–14. [Google Scholar] [CrossRef]
- Lee, R.C.; River, L.P.; Pan, F.-S.; Ji, L.; Wollmann, R.L. Surfactant-induced sealing of electropermeabilized skeletal muscle membranes in vivo. Proc. Natl. Acad. Sci. USA 1992, 89, 4524–4528. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Kabanov, A.V. Pluronic block copolymers: Evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J. Control. Release 2008, 130, 98–106. [Google Scholar] [CrossRef]
- Fischer, S.M.; Brandl, M.; Fricker, G. Effect of the non-ionic surfactant Poloxamer 188 on passive permeability of poorly soluble drugs across Caco-2 cell monolayers. Eur. J. Pharm. Biopharm. 2011, 79, 416–422. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Z.; Wang, W.; Wang, L.; Zheng, J.; Wu, S.; Pan, Y.; Li, S.; Zhao, J.; Cai, Z. Effects of Commonly used Surfactants, Poloxamer 188 and Tween 80, on the Drug Transport Capacity of Intestinal Glucose Transporters. AAPS PharmSciTech 2024, 25, 163. [Google Scholar] [CrossRef]
- Hayashi, K.; Shimanouchi, T.; Kato, K.; Miyazaki, T.; Nakamura, A.; Umakoshi, H. Span 80 vesicles have a more fluid, flexible and “wet” surface than phospholipid liposomes. Colloids Surf. B Biointerfaces 2011, 87, 28–35. [Google Scholar] [CrossRef]
- Zhang, H.; Yao, M.; Morrison, R.A.; Chong, S. Commonly used surfactant, Tween 80, improves absorption of P-glycoprotein substrate, digoxin, in rats. Arch. Pharmacal Res. 2003, 26, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnürch, A.; Dünnhaupt, S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012, 81, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Schipper, N.G.M.; Olsson, S.; Hoogstraate, J.A.; deBoer, A.G.; Varum, K.M.; Artursson, P. Chitosans as absorption enhancers for poorly absorbable drugs, 2. Mechanism of absorption enhancement. Pharm. Res. 1997, 14, 923–929. [Google Scholar] [CrossRef]
- Smith, J.; Wood, E.; Dornish, M. Effect of chitosan on epithelial cell tight junctions. Pharm. Res. 2004, 21, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Sandri, G.; Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Muzzarelli, C.; Caramella, C. Assessment of chitosan derivatives as buccal and vaginal penetration enhancers. Eur. J. Pharm. Sci. 2004, 21, 351–359. [Google Scholar] [CrossRef]
- Kimball, A.B.; Javorsky, E.; Ron, E.S.; Crowley, W.; Langer, R. A novel approach to administration of peptides in women: Systemic absorption of a GnRH agonist via transvaginal ring delivery system. J. Control. Release 2016, 233, 19–28. [Google Scholar] [CrossRef] [PubMed][Green Version]




| Group Code | Group Details |
|---|---|
| Control | BA solution (no enhancer) |
| CYL | BA + 2-hydroxypropyl-β-cyclodextrin |
| SDS | BA + sodium dodecyl sulfate |
| PLX | BA + Poloxamer 188 |
| SPN | BA + Span 80 |
| TWN | BA + Tween 80 |
| CHT | BA + Chitosan |
| Parameters | Specifications | BA Value |
|---|---|---|
| Retention time (min) | 4.77 | |
| Retention time (%RSD) | ≤2.0 | 0.05 |
| Plates | >2000 | 38,359 |
| HETP | 26.11 | |
| Areas (%RSD) | ≤2.0 | 0.78 |
| Tailing | ≤2.0 | 1.12 |
| Resolution (Rs) | >2.0 | 3.29 |
| Standard Curve | Range (µg/mL) | Slope | y-Intercept | Correlation Coefficient (R2) | Standard Error of the Slope | Standard Error of Intercept |
|---|---|---|---|---|---|---|
| Day 1 | 0.05–30 | 27.18 | −218.32 | 0.9999 | 0.06 | 867.27 |
| Day 2 | 0.05–30 | 27.43 | −259.99 | 0.9999 | 0.08 | 1153.69 |
| Day 3 | 0.05–30 | 27.40 | −217.87 | 0.9999 | 0.07 | 958.29 |
| Concentrations (µg/mL) | Accuracy (%) | Intraday Precision (%) | Interday Precision (%) |
|---|---|---|---|
| 1 | 101.89 ± 1.38 | 100.99 ± 1.13 | 99.37 ± 0.93 |
| 15 | 101.47 ± 0.10 | 100.90 ± 0.23 | 101.59 ± 0.23 |
| 30 | 100.03 ± 0.11 | 99.70 ± 0.13 | 99.82 ± 0.04 |
| Parameters | Level | Mean Recovery (%) |
|---|---|---|
| Temperature (°C) | 25 | 98.43 ± 0.37 |
| 30 | 99.58 ± 0.92 | |
| 35 | 98.05 ± 0.68 | |
| Water:Acetonitrile | 68:32 | 96.59 ± 0.21 |
| 70:30 | 99.58 ± 0.92 | |
| 72:28 | 95.65 ± 0.07 | |
| Flow rate (mL/min) | 0.70 | 98.96 ± 0.34 |
| 0.80 | 99.58 ± 0.92 | |
| 0.90 | 100.75 ± 0.13 | |
| Parameters | Day 1 | Day 7 | Day 14 | Day 28 |
|---|---|---|---|---|
| Mean recovery (%) | 100.40 | 100.17 | 97.79 | 97.23 |
| RSD | 0.39 | 0.24 | 0.18 | 0.14 |
| Permeation Parameters | BA | CYL | SDS | PLX | SPN | TWN | CHT |
|---|---|---|---|---|---|---|---|
| J (x10−2) (µg/cm2·h) | 0.47 ± 0.14 ab | 0.06 ± 0.04 d | 0.15 ± 0.02 d | 0.43 ± 0.01 abc | 0.21 ± 0.02 bcd | 0.31 ± 0.03 bcd | 0.64 ± 0.03 a |
| Papp (x10−5) (cm/h) | 9.40 ± 2.80 b | 1.25 ± 0.87 c | 3.15 ± 0.581 cd | 8.71 ± 0.26 bc | 4.25 ± 0.50 bcd | 06.21 ± 0.64 bcd | 16.20 ± 0.84 a |
| Model Name | Parameter | BA | CHT |
|---|---|---|---|
| Zero-order | R2 adjusted | 0.416 | 0.53 |
| AIC | 128.85 | 136.22 | |
| MSC | 1.21 | 1.61 | |
| Higuchi | R2 adjusted | 0.82 | 0.36 |
| AIC | 110.06 | 129.21 | |
| MSC | 0.87 | 0.735 | |
| Makoid–Banakar | R2 adjusted | 0.95 | 0.94 |
| AIC | 99.41 | 111.32 | |
| MSC | 2.05 | 1.50 | |
| Peppas–Sahlin | R2 adjusted | 0.95 | 0.92 |
| AIC | 99.65 | 114.25 | |
| MSC | 2.03 | 1.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uddin, A.M.; Kirkwood, R.N.; Petrovski, K.R.; Youssef, S.H.; Singh, B.; Mukhopadhyay, S.; Song, Y.; Garg, S. Evaluation of Permeation Enhancers for Vaginal Delivery of Buserelin Acetate Using a Validated Chromatographic Method and Ex Vivo Porcine Model. Pharmaceutics 2025, 17, 1181. https://doi.org/10.3390/pharmaceutics17091181
Uddin AM, Kirkwood RN, Petrovski KR, Youssef SH, Singh B, Mukhopadhyay S, Song Y, Garg S. Evaluation of Permeation Enhancers for Vaginal Delivery of Buserelin Acetate Using a Validated Chromatographic Method and Ex Vivo Porcine Model. Pharmaceutics. 2025; 17(9):1181. https://doi.org/10.3390/pharmaceutics17091181
Chicago/Turabian StyleUddin, AHM Musleh, Roy N. Kirkwood, Kiro R. Petrovski, Souha H. Youssef, Baljinder Singh, Songhita Mukhopadhyay, Yunmei Song, and Sanjay Garg. 2025. "Evaluation of Permeation Enhancers for Vaginal Delivery of Buserelin Acetate Using a Validated Chromatographic Method and Ex Vivo Porcine Model" Pharmaceutics 17, no. 9: 1181. https://doi.org/10.3390/pharmaceutics17091181
APA StyleUddin, A. M., Kirkwood, R. N., Petrovski, K. R., Youssef, S. H., Singh, B., Mukhopadhyay, S., Song, Y., & Garg, S. (2025). Evaluation of Permeation Enhancers for Vaginal Delivery of Buserelin Acetate Using a Validated Chromatographic Method and Ex Vivo Porcine Model. Pharmaceutics, 17(9), 1181. https://doi.org/10.3390/pharmaceutics17091181











