Formulation of Recombinant Therapeutic Proteins: Technological Innovation, Regulations, and Evolution Towards Buffer-Free Formulations
Abstract
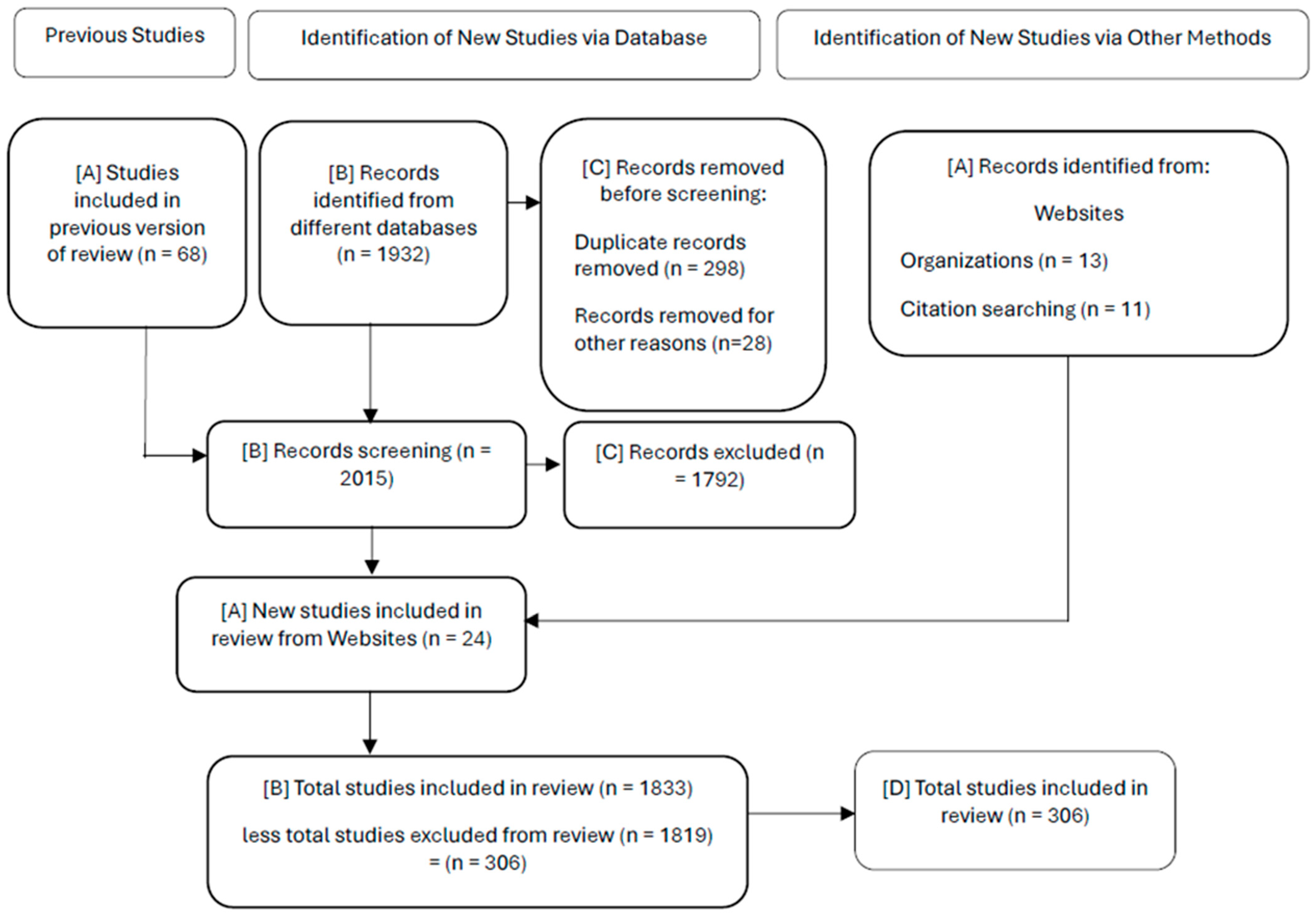
1. Introduction
2. Documentary Methodology
- ○
- Protocol Definition: Defines clear research objectives, focusing on buffer-free formulations, regulatory guidelines (FDA, EMA), safety profiles, and intellectual property challenges.
- ○
- Document Search: Systematic search of scientific databases (PubMed, Scopus, Web of Science), patent databases (USPTO, EPO, Derwent), regulatory databases (FDA Drugs@FDA, EMA EPAR) and complementary searches in Google Scholar. Coverage from 2020 to 2025.
- ○
- Critical evaluation: Selection and quality assessment of sources according to predefined inclusion and exclusion criteria, which can be seen in Table 1.
- ○
- Content Synthesis: Extraction and synthesis of relevant information in structured tables and descriptive sections, organized by topics: technological innovation, formulation strategies, excipient safety, regulatory compliance, and intellectual property challenges.
- ○
- Analytical Interpretation: Comparative analysis of buffered and unbuffered formulations, technological platforms, excipient classification, and regulatory trends.
- ○
- Report Generation: Manuscript development, including sections such as introduction, detailed methodology, results, in-depth discussion, and clearly articulated conclusions.
- Systematic Rigor: Structured and reproducible steps ensure methodological transparency and reliability.
- Holistic Integration: Combines technologies, regulatory, safety, and intellectual property perspectives into one comprehensive review.
- Analytical Depth: Allows for in-depth comparative analysis, identifying critical trends and future research needs.
- Flexibility and Applicability: Adaptable across related fields within pharmaceutical development, enabling consistent analytical standards and expanding the potential for future research.
3. Recombinant Therapeutic Proteins
3.1. Mechanisms of Expression of Recombinant Proteins
3.2. Host Organisms in Protein Production
3.3. Advances in Analytical Methodologies for Recombinant Therapeutic Proteins
3.4. Advances in Bioprocessing and Purification
3.5. Advantages of Recombinant Proteins
3.6. Innovative Applications and Platforms in the Pharmaceutical Industry
Innovative Platforms for Generating Recombinant Therapeutic Proteins with Improved PD and PK
- Protein Engineering Platforms
- Chemical formulation platforms.
- Advanced Management Systems
- Immunology-Based Platforms
- Synthetic Biology and Next-Generation Engineering Platforms
4. Immunogenicity, Excipients, and Differences Between Buffered and Unbuffered Formulations
4.1. Buffered Formulations
4.1.1. Comparative Stability of Buffered vs. Buffer-Free (Self-Buffering) Formulations
4.1.2. Immunogenicity in Buffered Formulations
4.2. Buffer-Free Formulations
4.3. Excipients and Safety Profile of Buffer-Free Formulations
4.4. Traditional Buffered Formulations vs. Innovative Unbuffered Formulations
4.5. Buffer-Free Formulations in Biosimilars
4.6. Understanding Biosimilars Through Some Examples
4.6.1. Analytical Strategies in the Development of Biosimilars. Cost-Effectiveness
4.6.2. Recombinant Proteins Approved Between 2020 and July 2025
5. IP Rights in the Protection of Recombinant and Biosimilar Therapeutic Proteins
5.1. IP Rights—Importance of Patents
Barriers to Information Exchange
6. Regulations (FDA–EMA) Involved in the Development and Approval of Recombinant and Biosimilar Therapeutic Proteins
6.1. Regulatory Challenges in the Development of Recombinant Therapeutic Protein Formulations
6.2. Regulatory Overview of Recombinant and Biosimilar Proteins—FDA and EMA
6.3. Approval Processes for Recombinant Proteins and Biosimilars
6.4. Regulation of Excipients
7. Discussion and Conclusions
7.1. Discussion
7.2. Conclusions
7.3. Tendencies
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, X.; Ma, Y.; Xiang, Q. New Frontiers in the Production of Functional Recombinant Proteins. Bioengineering 2025, 12, 351. [Google Scholar] [CrossRef]
- Wang, S.; Mao, X.; Wang, F.; Zuo, X.; Fan, C. Data Storage Using DNA. Adv. Mater. 2023, 36, e2307499. [Google Scholar] [CrossRef]
- Zheng, B.; Wang, X.; Guo, M.; Tzeng, C.-M. Therapeutic Peptides: Recent Advances in Discovery, Synthesis, and Clinical Translation. Int. J. Mol. Sci. 2025, 26, 5131. [Google Scholar] [CrossRef]
- Thakor, A.; Charles, T.C. Recombinant DNA: Unlocking Untapped Microbial Potential for Innovation in Crop Agriculture. Trends Biotechnol. 2025, 43, 533–539. [Google Scholar] [CrossRef]
- Jayakrishnan, A.; Wan Rosli, W.R.; Tahir, A.R.M.; Razak, F.S.A.; Kee, P.E.; Ng, H.S.; Chew, Y.-L.; Lee, S.-K.; Ramasamy, M.; Tan, C.S.; et al. Evolving Paradigms of Recombinant Protein Production in Pharmaceutical Industry: A Rigorous Review. Science 2024, 6, 9. [Google Scholar] [CrossRef]
- O’Flaherty, R.; Bergin, A.; Flampouri, E.; Mota, L.M.; Obaidi, I.; Quigley, A.; Xie, Y.; Butler, M. Mammalian Cell Culture for Production of Recombinant Proteins: A Review of the Critical Steps in Their Biomanufacturing. Biotechnol. Adv. 2020, 43, 107552. [Google Scholar] [CrossRef]
- Akram, M.; Jabeen, F.; Daniyal, M.; Zainab, R.; Haq, U.U.; Adetunji, C.O.; Egbuna, C.; Ephraim-Emmanuel, B.C.; Patrick-Iwuanyanwu, K.C.; Ogbo, A.B. Genetic Engineering of Novel Products of Health Significance: Recombinant DNA Technology. In Functional Foods and Nutraceuticals; Springer: Berlin/Heidelberg, Germany, 2020; pp. 595–611. [Google Scholar] [CrossRef]
- Sivamani, Y.; Hegde, S.; Bhat, A.R.; Sajal, H.; Elayaperumal, S. Recombinant DNA Technology and Gene Therapy. In Biochemical and Molecular Pharmacology in Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2024; pp. 353–376. [Google Scholar] [CrossRef]
- Kollabathula, P.; Katru, S.; Tumarada, P.; Ravuri, S.; Zainab, A.; Velagala, S.K. Harnessing Recombinant DNA Technology for Modern Innovations: A Review. Int. J. Dent. Mater. 2025, 7, 27–31. [Google Scholar] [CrossRef]
- Gupta, R.; Morten, C.; Zhu, A.; Ramachandran, R.; Shah, N.; Ross, J. Approvals and Timing of New Formulations of Novel Drugs Approved by the US Food and Drug Administration Between 1995 and 2010 and Followed Through 2021. JAMA Health Forum 2022, 3, e221096. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.H. Role of Biomolecules and Biologics in Precision Medicine, Personalized Medicine, and Emerging Therapies. Int. J. Biomed. 2022, 12, 70–81. [Google Scholar] [CrossRef]
- Geng, S.; Zhao, X.; Zhang, X.; Zhang, J.; Mi, C.; Wang, T. Recombinant Therapeutic Proteins Degradation and Overcoming Strategies in CHO Cells. Appl. Microbiol. Biotechnol. 2024, 108, 182. [Google Scholar] [CrossRef]
- Parasuraman, S.; Kumar, L.N.D.; Thanapakiam, G.; Sayem, A.S.M.; Chuah, J.J.; Krishnamoorthy, V. Biopharmaceutical Production by Recombinant DNA Technology: Future Perspectives. In Microbial Products for Health and Nutrition; Springer: Singapore, 2024; pp. 285–303. [Google Scholar] [CrossRef]
- Eadie, A.; MacGregor, A.; Wallach, J.; Ross, J.; Herder, M. US Food and Drug Administration Regulatory Reviewer Disagreements and Postmarket Safety Actions Among New Therapeutics. BMJ Evid. Based Med. 2023, 28, 151–156. [Google Scholar] [CrossRef]
- Coghlan, J.; Benet, A.; Kumaran, P.; Ford, M.; Veale, L.; Skilton, S.J.; Saveliev, S.; Schwendeman, A.A. Streamlining the Characterization of Disulfide Bond Shuffling and Protein Degradation in IgG1 Biopharmaceuticals under Native and Stressed Conditions. Front. Bioeng. Biotechnol. 2022, 10, 862456. [Google Scholar] [CrossRef]
- Le-McClain, A.; Zanelotti, C.J.; Robert, H.; Casanova, F. Analysis of Complex Mixtures with Benchtop Nuclear Magnetic Resonance: Solvent Suppression with T2 and Diffusion Filters. Magn. Reson. Chem. 2024, 62, 497–504. [Google Scholar] [CrossRef]
- Akbarian, M.; Chen, S.-H. Instability Challenges and Stabilization Strategies of Pharmaceutical Proteins. Pharmaceutics 2022, 14, 2533. [Google Scholar] [CrossRef]
- Ghosh, I.; Gutka, H.J.; Krause, M.E.; Clemens, R.; Kashi, R.S. A Systematic Review of Commercial High Concentration Antibody Drug Products Approved in the US: Formulation Composition, Dosage Form Design and Primary Packaging Considerations. mAbs 2023, 15, 2205540. [Google Scholar] [CrossRef]
- Tang, S.; Tao, J.; Li, Y. Challenges and Solutions for the Downstream Purification of Therapeutic Proteins. Antib. Ther. 2023, 7, 1–12. [Google Scholar] [CrossRef]
- Naderiyan, Z.; Shoari, A. Protein Engineering Paving the Way for Next-Generation Therapies in Cancer. Int. J. Transl. Med. 2025, 5, 28. [Google Scholar] [CrossRef]
- Ranjan, R. Development of Complex Generics and Similar Biological Products: An Industrial Perspective of Reverse Engineering. AAPS PharmSciTech 2025, 26, 95. [Google Scholar] [CrossRef]
- Niazi, S. Affordable mRNA Novel Proteins, Recombinant Protein Conversions, and Biosimilars—Advice to Developers and Regulatory Agencies. Biomedicines 2025, 13, 97. [Google Scholar] [CrossRef]
- Niazi, S.K. Biosimilars: Harmonizing the Approval Guidelines. Biologics 2022, 2, 171–195. [Google Scholar] [CrossRef]
- Bas, T.G. Biosimilars for the Next Decade in Latin America: A Window of Opportunity. Expert Opin. Biol. Ther. 2023, 23, 659–669. [Google Scholar] [CrossRef]
- Mascarenhas-Melo, F.; Diaz, M.; Gonçalves, M.B.S.; Vieira, P.; Bell, V.; Viana, S.; Nunes, S.; Paiva-Santos, A.C.; Veiga, F. An Overview of Biosimilars—Development, Quality, Regulatory Issues, and Management in Healthcare. Pharmaceuticals 2024, 17, 235. [Google Scholar] [CrossRef]
- Bas, T.; Duarte, V. Biosimilars in the Era of Artificial Intelligence—International Regulations and the Use in Oncological Treatments. Pharmaceuticals 2024, 17, 925. [Google Scholar] [CrossRef]
- Bas, T. Innovative Formulation Strategies for Biosimilars: Trends Focused on Buffer-Free Systems, Safety, Regulatory Alignment, and Intellectual Property Challenges. Pharmaceuticals 2025, 18, 908. [Google Scholar] [CrossRef]
- Niazi, S.K. Scientific Justification and Policy Recommendations to the US Food and Drug Administration for Waiving Comparative Efficacy Studies. Pharmaceuticals 2025, 18, 779. [Google Scholar] [CrossRef]
- Thacker, S.G.; Her, C.; Kelley-Baker, L.; Ireland, D.D.; Manangeeswaran, M.; Pang, E.; Verthelyi, D. Detection of Innate Immune Response Modulating Impurities (IIRMI) in Therapeutic Peptides and Proteins: Impact of Excipients. Front. Immunol. 2022, 13, 970499. [Google Scholar] [CrossRef]
- Ingle, R.G.; Fang, W. An Overview of the Stability and Delivery Challenges of Commercial Nucleic Acid Therapeutics. Pharmaceutics 2023, 15, 1158. [Google Scholar] [CrossRef]
- Žiberna, M.B.; Grabnar, P.A.; Gašperlin, M.; Matjaž, M.G. Lyophilised Protein Formulations as a Patient-Centric Dosage Form: A Contribution toward Sustainability Paradigm. Acta Pharm. 2024, 74, 289–300. [Google Scholar] [CrossRef]
- Baghban, R.; Farajnia, S.; Ghasemi, Y.; Mortazavi, M.; Samadi, N.; Zarghami, N. Assessment of E. coli Expression System for Overexpression of Active Recombinant Ocriplasmin. Adv. Pharm. Bull. 2020, 3, 564–569. [Google Scholar] [CrossRef]
- Malakar, S.; Gontor, E.; Dugbaye, M.; Shah, K.; Sinha, S.; Sutaoney, P.; Chauhan, N.S. Cancer Treatment with Biosimilar Drugs: A Review. Cancer Innov. 2024, 3, e115. [Google Scholar] [CrossRef]
- Olgenblum, G.; Carmon, N.; Harries, D. Not Always Sticky: Specificity of Protein Stabilization by Sugars Is Conferred by Protein–Water Hydrogen Bonds. J. Am. Chem. Soc. 2023, 145, 23308–23320. [Google Scholar] [CrossRef]
- Presti, K.; Jégo, M.; Frieß, W. “The More, the Better?”: The Impact of Sugar-to-Protein Molar Ratio in Freeze-Dried Monoclonal Antibody Formulations on Protein Stability. Mol. Pharm. 2024, 21, 6484–6490. [Google Scholar] [CrossRef]
- Maity, A.; Bagchi, D.; Tabassum, H.; Nath, P.; Sinha, S.; Chakraborty, A. Diverse Role of Buffer Mediums and Protein Concentrations to Mediate the Multimodal Interaction of Phenylalanine-Functionalized Gold Nanoparticle and Lysozyme Protein at Same Nominal pH. J. Phys. Chem. B 2024, 128, 10625–10635. [Google Scholar] [CrossRef]
- Rahban, M.; Ahmad, F.; Piatyszek, M.A.; Haertlé, T.; Saso, L.; Saboury, A.A. Stabilization Challenges and Aggregation in Protein-Based Therapeutics in the Pharmaceutical Industry. RSC Adv. 2023, 13, 35947–35963. [Google Scholar] [CrossRef]
- Blass, E.; Colarusso, A.; Aïd, M.; Larocca, R.; Reeves, R.; Barouch, D. Early Spatiotemporal Evolution of the Immune Response Elicited by Adenovirus Serotype 26 Vector Vaccination in Mice. J. Virol. 2025, 99, e0024725. [Google Scholar] [CrossRef]
- Burnouf, T.; Faber, J.; Radosevic, M.; Goubran, H.; Seghatchian, J. Plasma Fractionation in Countries with Limited Infrastructure and Low-/Medium Income: How to Move Forward? Transfus. Apher. Sci. 2020, 59, 102715. [Google Scholar] [CrossRef]
- Sainatham, C.; Yadav, D.; Babu, A.D.; Tallapalli, J.R.; Kanagala, S.G.; Filippov, E.; Chavez, F.M.; Ahmed, N.; Lutfi, F. The Current Socioeconomic and Regulatory Landscape of Immune Effector Cell Therapies. Front. Med. 2024, 11, 1462307. [Google Scholar] [CrossRef]
- Niazi, S.K.; Magoola, M. Advances in Escherichia coli-Based Therapeutic Protein Expression: Mammalian Conversion, Continuous Manufacturing, and Cell-Free Production. Biologics 2023, 3, 380–401. [Google Scholar] [CrossRef]
- Pathak, A.; Singh, S.P.; Tiwari, A.; Tripathi, A.M.; Jahan, T.; Singh, D.B. Biosimilar, Biobetter, and Biosuperior Therapeutic Proteins. In Protein-Based Therapeutics; Springer: Singapore, 2023; pp. 325–353. [Google Scholar] [CrossRef]
- Sridharan, G.; Kumar, S.A.S.; Kamaraj, R. The Biosimilar Revolution: Assessing the European Union’s Approach to Biosimilarity, Interchangeability, Patient Access, and Its Market Analysis. Cureus 2024, 16, e68103. [Google Scholar] [CrossRef]
- Heinemann, L.; Davies, M.J.; Home, P.; Först, T.; Vilsbøll, T.; Schnell, O. Understanding Biosimilar Insulins—Development, Manufacturing, and Clinical Trials. J. Diabetes Sci. Technol. 2022, 17, 1649–1661. [Google Scholar] [CrossRef]
- Sembiring, E.R.; Fuad, A.M.; Suryadi, H. Expression and Purification of Recombinant Human Granulocyte Colony-Stimulating Factor (rhG-CSF) from Pichia pastoris. Indones. J. Biotechnol. 2024, 29, 205. [Google Scholar] [CrossRef]
- Buzzi, R.; Owczarek, C.; Akeret, K.; Tester, A.; Pereira, N.; Butcher, R.; Brügger-Verdon, V.; Hardy, M.P.; Illi, M.; Wassmer, A.; et al. Modular Platform for the Development of Recombinant Hemoglobin Scavenger Biotherapeutics. Mol. Pharm. 2021, 18, 3158–3170. [Google Scholar] [CrossRef]
- Brader, M.L.; Kim, H.; Koo, O.; Nagapudi, K.; Su, Y. Industrial Horizons in Pharmaceutical Science. Mol. Pharm. 2024, 21, 4183–4188. [Google Scholar] [CrossRef]
- Shamsi, A.; Mohammad, T.; Anwar, S.; Alajmi, M.; Hussain, A.; Rehman, M.; Islam, A.; Hassan, I. Glecaprevir and Maraviroc Are High-Affinity Inhibitors of SARS-CoV-2 Main Protease: Possible Implication in COVID-19 Therapy. Biosci. Rep. 2020, 40, 40. [Google Scholar] [CrossRef]
- Bolleddula, J.; Brady, K.; Bruin, G.; Lee, A.; Martin, J.; Walles, M.; Xu, K.; Yang, T.-Y.; Zhu, X.; Yu, H. Absorption, Distribution, Metabolism, and Excretion of Therapeutic Proteins: Current Industry Practices and Future Perspectives. Drug Metab. Dispos. 2022, 50, 837–845. [Google Scholar] [CrossRef]
- Lexchin, J. A Comparison of the Food and Drug Administration’s and Health Canada’s Regulatory Decisions about Failed Confirmatory Trials for Oncology Drugs: An Observational Study. J. Pharm. Policy Pract. 2021, 14, 93. [Google Scholar] [CrossRef]
- Prasad, S.; Roy, I. Innovation in Stabilization of Biopharmaceuticals. In Recent Advances in Pharmaceutical Innovation and Research; Springer: Singapore, 2023; pp. 3–40. [Google Scholar] [CrossRef]
- Page, S.; Khan, T.A.; Kühl, P.; Schwach, G.; Storch, K.; Chokshi, H. Patient Centricity Driving Formulation Innovation: Improvements in Patient Care Facilitated by Novel Therapeutics and Drug Delivery Technologies. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 341–363. [Google Scholar] [CrossRef]
- Katz, J.S.; Chou, D.K.; Christian, T.R.; Das, T.K.; Patel, M.J.; Singh, S.N.; Wen, Y. Emerging Challenges and Innovations in Surfactant-Mediated Stabilization of Biologic Formulations. J. Pharm. Sci. 2022, 111, 919–932. [Google Scholar] [CrossRef]
- Kim, J.; Pritts, J.; Ngo, M.; Estoll, C.; Rao, V. Trends in Light and Temperature Sensitivity Recommendations Among Licensed Biotechnology Drug Products. Pharm. Res. 2023, 40, 1491–1505. [Google Scholar] [CrossRef]
- Eppink, M.H.M.; Wapenaar, M.; Crommelin, D.J.; Hawe, A.; Giezen, T.J.; Black, A.; Tam, A. Therapeutic Proteins and Advanced Therapy Medicinal Products. In Practical Pharmaceutics; Springer: Cham, Switzerland, 2023; pp. 551–590. [Google Scholar] [CrossRef]
- Rathore, A.S.; Bhargava, A. Biosimilars in Developed Economies: Overview, Status, and Regulatory Considerations. Regul. Toxicol. Pharmacol. 2020, 110, 104525. [Google Scholar] [CrossRef]
- Zhi, L.; Liu, D.; Shameem, M. Injection Site Reactions of Biologics and Mitigation Strategies. AAPS Open 2025, 11, 5. [Google Scholar] [CrossRef]
- Strauss, D.G.; Wang, Y.; Florian, J.; Zineh, I. Pharmacodynamic Biomarkers Evidentiary Considerations for Biosimilar Development and Approval. Clin. Pharmacol. Ther. 2022, 113, 55–61. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Wang, M.; Zhai, S.; Hou, C.; Sun, F.; Jian, L. Evaluating Biosimilars: Safety, Efficacy, and Regulatory Considerations in Clinical Studies. Int. J. Clin. Pharm. 2024, 47, 232–236. [Google Scholar] [CrossRef]
- Cherla, A.; Naci, H.; Kesselheim, A.; Gyawali, B.; Mossialos, E. Assessment of Coverage in England of Cancer Drugs Qualifying for US Food and Drug Administration Accelerated Approval. JAMA Intern. Med. 2021, 181, 490. [Google Scholar] [CrossRef]
- Alotaiq, N.; Dermawan, D. Advancements in Virtual Bioequivalence: A Systematic Review of Computational Methods and Regulatory Perspectives in the Pharmaceutical Industry. Pharmaceutics 2024, 16, 1414. [Google Scholar] [CrossRef]
- Amorim, A.; Piochi, L.; Gaspar, A.; Preto, A.; Rosário-Ferreira, N.; Moreira, I. Advancing Drug Safety in Drug Development: Bridging Computational Predictions for Enhanced Toxicity Prediction. Chem. Res. Toxicol. 2024, 37, 827–849. [Google Scholar] [CrossRef]
- Achilleos, K.; Petrou, C.; Nicolaidou, V.; Sarigiannis, Y. Beyond Efficacy: Ensuring Safety in Peptide Therapeutics through Immunogenicity Assessment. J. Pept. Sci. 2025, 31, e70016. [Google Scholar] [CrossRef]
- Qin, D.; Phung, Q.; Wu, P.; Yin, Z.; Tam, S.; Tran, P.; ElSohly, A.M.; Gober, J.; Hu, Z.; Zhou, Z.; et al. A Single Point Mutation on FLT3L-Fc Protein Increases the Risk of Immunogenicity. Front. Immunol. 2025, 16, 1519452. [Google Scholar] [CrossRef]
- Cordeiro, M.; Vitorino, C.; Sinogas, C.; Sousa, J. A Regulatory Perspective on Biosimilar Medicines. Pharmaceutics 2024, 16, 321. [Google Scholar] [CrossRef]
- Gaylis, N.; Both, C.; Lemke, L.; Richter, O.; Yamauchi, P. ‘Totality of Evidence’ Approach in the Development of GP2017, an Approved Adalimumab Biosimilar. Adv. Ther. 2024, 41, 1795–1814. [Google Scholar] [CrossRef]
- Gonçalves, J.; Caliceti, P. Optimizing Pharmacological and Immunological Properties of Therapeutic Proteins through PEGylation: Investigating Key Parameters and Their Impact. Drug Des. Dev. Ther. 2024, 18, 5041–5062. [Google Scholar] [CrossRef]
- García-Holgado, A.; Marcos-Pablos, S.; García-Peñalvo, F.J. Guidelines for performing systematic research projects reviews. Int. J. Interact. Multimed. Artif. Intell. 2020, 6, 9. [Google Scholar] [CrossRef]
- Page, M.; McKenzie, J.; Bossuyt, P.; Boutron, I.; Hoffmann, T.; Mulrow, C.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Kjeldsen, T.; Andersen, A.S.; Hubálek, F.; Johansson, E.; Kreiner, F.F.; Schluckebier, G.; Kurtzhals, P. Molecular Engineering of Insulin for Recombinant Expression in Yeast. Trends Biotechnol. 2024, 42, 464–478. [Google Scholar] [CrossRef]
- Kumar, N.; Pakalapati, A.; Chandrasekhar, K.; Durthi, C. Comparison of Analytical Method Validation Guidelines Used for Release, Stability in Biosimilar Manufacturing Process. Curr. Trends Biotechnol. Pharm. 2024, 8, 1798–1812. [Google Scholar] [CrossRef]
- Yang, H.; Qu, J.; Zou, W.; Shen, W.; Chen, X. An Overview and Future Prospects of Recombinant Protein Production in Bacillus subtilis. Appl. Microbiol. Biotechnol. 2021, 105, 6607–6626. [Google Scholar] [CrossRef]
- İncir, İ.; Kaplan, Ö. Escherichia coli as a Versatile Cell Factory: Advances and Challenges in Recombinant Protein Production. Protein Expr. Purif. 2024, 219, 106463. [Google Scholar] [CrossRef]
- Mejía-Almonte, C.; Busby, S.; Wade, J.T.; van Helden, J.; Arkin, A.P.; Stormo, G.D.; Eilbeck, K.; Palsson, B.O.; Galagan, J.E.; Collado-Vides, J. Redefining Fundamental Concepts of Transcription Initiation in Bacteria. Nat. Rev. Genet. 2020, 21, 699–714. [Google Scholar] [CrossRef]
- Terol, G.L.; Gallego-Jara, J.; Martínez, R.A.S.; Vivancos, A.M.; Cánovas, M.; Diego, T.D. Impact of the Expression System on Recombinant Protein Production in Escherichia coli BL21. Front. Microbiol. 2021, 12, 682001. [Google Scholar] [CrossRef]
- Tan, M.; Liao, C.; Liang, L.; Yi, X.; Zhou, Z.; Wei, G. Recent Advances in Recombinase Polymerase Amplification: Principle, Advantages, Disadvantages and Applications. Front. Cell. Infect. Microbiol. 2022, 12, 1019071. [Google Scholar] [CrossRef]
- Fus-Kujawa, A.; Pruś, P.; Bajdak-Rusinek, K.; Teper, P.; Gawron, K.; Kowalczuk, A.; Sieron, A.L. An Overview of Methods and Tools for Transfection of Eukaryotic Cells In Vitro. Front. Bioeng. Biotechnol. 2021, 9, 701031. [Google Scholar] [CrossRef]
- Perera, P.G.T.; Linklater, D.P.; Vilagosh, Z.; Nguyen, T.H.P.; Hanssen, E.; Rubanov, S.; Wanjara, S.; Aadum, B.; Alfred, R.; Dekiwadia, C.; et al. Genetic Transformation of Plasmid DNA into Escherichia coli Using High Frequency Electromagnetic Energy. Nano Lett. 2024, 24, 1145–1152. [Google Scholar] [CrossRef]
- Nasrollahian, S.; Graham, J.P.; Halaji, M. A Review of the Mechanisms That Confer Antibiotic Resistance in Pathotypes of E. coli. Front. Cell. Infect. Microbiol. 2024, 14, 1387497. [Google Scholar] [CrossRef]
- Dondapati, S.K.; Stech, M.; Zemella, A.; Kubick, S. Cell-Free Protein Synthesis: A Promising Option for Future Drug Development. BioDrugs 2020, 34, 327–348. [Google Scholar] [CrossRef]
- Zhang, Z.; Nong, F.; Wang, Y.; Yan, C.; Gu, Y.; Song, P.; Sun, X.-M. Strategies for Efficient Production of Recombinant Proteins in Escherichia coli: Alleviating the Host Burden and Enhancing Protein Activity. Microb. Cell Fact. 2022, 21, 191. [Google Scholar] [CrossRef]
- He, M.; Zhou, X.; Wang, X. Glycosylation: Mechanisms, Biological Functions and Clinical Implications. Signal Transduct. Target. Ther. 2024, 9, 194. [Google Scholar] [CrossRef]
- Cheng, Q.; Luo, M.; Xu, Z.; Li, F.; Zhang, Y. Developing Glycoproteomics Reveals the Role of Posttranslational Glycosylation in the Physiological and Pathological Processes of Male Reproduction. iMetaOmics 2024, 1, e10. [Google Scholar] [CrossRef]
- Zhou, Y.; Tan, C.; Zenobi, R. Rapid Profiling of the Glycosylation Effects on the Binding of SARS-CoV-2 Spike Protein to Angiotensin-Converting Enzyme 2 Using MALDI-MS with High Mass Detection. Anal. Chem. 2024, 96, 1898–1905. [Google Scholar] [CrossRef]
- Huleani, S.; Roberts, M.; Beales, L.; Papaioannou, E.H. Escherichia coli as an Antibody Expression Host for the Production of Diagnostic Proteins: Significance and Expression. Crit. Rev. Biotechnol. 2021, 42, 756–773. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Xu, Y.; Shi, T.; Ye, C.; Sun, X.; Huang, H. CRISPR-Based Construction of a BL21 (DE3)-Derived Variant Strain Library to Rapidly Improve Recombinant Protein Production. ACS Synth. Biol. 2021, 11, 343–352. [Google Scholar] [CrossRef]
- Liang, Q.; Tu, B.; Cui, L. Recombinant T7 RNA Polymerase Production Using ClearColi BL21(DE3) and Animal-Free Media for In Vitro Transcription. Appl. Microbiol. Biotechnol. 2024, 108, 41. [Google Scholar] [CrossRef]
- Bhatwa, A.; Wang, W.; Hassan, Y.I.; Abraham, N.; Li, X.; Zhou, T. Challenges Associated with the Formation of Recombinant Protein Inclusion Bodies in Escherichia coli and Strategies to Address Them for Industrial Applications. Front. Bioeng. Biotechnol. 2021, 9, 630551. [Google Scholar] [CrossRef]
- Mital, S.; Christie, G.; Dikicioǧlu, D. Recombinant Expression of Insoluble Enzymes in Escherichia coli: A Systematic Review of Experimental Design and Its Manufacturing Implications. Microb. Cell Fact. 2021, 20, 208. [Google Scholar] [CrossRef]
- Sandomenico, A.; Sivaccumar, J.; Ruvo, M. Evolution of Escherichia coli Expression System in Producing Antibody Recombinant Fragments. Int. J. Mol. Sci. 2020, 21, 6324. [Google Scholar] [CrossRef]
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-Like Particles: Preparation, Immunogenicity and Their Roles as Nanovaccines and Drug Nanocarriers. J. Nanobiotechnol. 2021, 19, 59. [Google Scholar] [CrossRef]
- Cid, R.; Bolívar, J. Platforms for Production of Protein-Based Vaccines: From Classical to Next-Generation Strategies. Biomolecules 2021, 11, 1072. [Google Scholar] [CrossRef]
- Singh, M.; Gupta, S.; Rawat, A.; Singh, S.K. Advances in Heterologous Protein Expression Strategies in Yeast and Insect Systems. In Advances in Protein Molecular and Structural Biology Methods; Elsevier: Amsterdam, The Netherlands, 2022; pp. 13–30. [Google Scholar] [CrossRef]
- Conibear, A.C. Deciphering Protein Post-Translational Modifications Using Chemical Biology Tools. Nat. Rev. Chem. 2020, 4, 674–695. [Google Scholar] [CrossRef]
- Eskandari, A.; Nezhad, N.G.; Leow, T.C.; Rahman, M.B.A.; Oslan, S.N. Current achievements, strategies, obstacles, and overcoming the challenges of the protein engineering in Pichia pastoris expression system. World J. Microbiol. Biotechnol. 2023, 40, 39. [Google Scholar] [CrossRef]
- Pouresmaeil, M.; Azizi-Dargahlou, S. Factors Involved in Heterologous Expression of Proteins in E. coli Host. Arch. Microbiol. 2023, 205, 212. [Google Scholar] [CrossRef]
- Deng, B.; Yu, Y.; Yang, J.; Yang, M.; Xing, Q.; Hang, P.; Wang, F.; Li, M.; Ma, L.; Zhai, C. Improving the Activity and Thermostability of PETase from Ideonella sakaiensis through Modulating Its Post-Translational Glycan Modification. Commun. Biol. 2023, 6, 39. [Google Scholar] [CrossRef]
- Majumdar, S.; Desai, R.; Hans, A.K.; Dandekar, P.; Jain, R. From Efficiency to Yield: Exploring Recent Advances in CHO Cell Line Development for Monoclonal Antibodies. Mol. Biotechnol. 2024, 67, 369–392. [Google Scholar] [CrossRef]
- Schmidt, S. Fusion Proteins: Current Status and Future Perspectives. In Bioprocessing, Bioengineering and Process Chemistry in the Biopharmaceutical Industry; Springer: Berlin/Heidelberg, Germany, 2024; pp. 287–343. [Google Scholar] [CrossRef]
- Allemailem, K.S.; Almatroudi, A.; Rahmani, A.H.; Alrumaihi, F.; Alradhi, A.E.; Al-Subaiyel, A.; Alsubaiyel, A.M.; Algahtani, M.; Almousa, R.M.; Mahzari, A.; et al. Recent Updates of the CRISPR/Cas9 Genome Editing System: Novel Approaches to Regulate Its Spatiotemporal Control by Genetic and Physicochemical Strategies. Int. J. Nanomed. 2024, 19, 5335–5363. [Google Scholar] [CrossRef]
- Lou, M.; Ji, S.; Wu, R.; Zhu, Y.; Wu, J.; Zhang, J. Microbial Production Systems and Optimization Strategies of Antimicrobial Peptides: A Review. World J. Microbiol. Biotechnol. 2025, 41, 66. [Google Scholar] [CrossRef]
- Sarvepalli, S.; Pasika, S.R.; Verma, V.; Thumma, A.; Bolla, S.; Nukala, P.K.; Butreddy, A.; Bolla, P.K. A Review on the Stability Challenges of Advanced Biologic Therapeutics. Pharmaceutics 2025, 17, 550. [Google Scholar] [CrossRef]
- Elsayed, Y.Y.; Kühl, T.; Imhof, D. Regulatory Guidelines for the Analysis of Therapeutic Peptides and Proteins. J. Pept. Sci. 2025, 31, e70001. [Google Scholar] [CrossRef]
- Seelig, J.; Seelig, A. Protein Stability─Analysis of Heat and Cold Denaturation without and with Unfolding Models. J. Phys. Chem. B 2023, 127, 3352–3363. [Google Scholar] [CrossRef]
- Maciel, E.; Eisert, J.; Dederer, V.; Berwanger, A.; Knapp, S.; Empting, M.; Mathea, S.; Jensen, H.; Lermyte, F. Native Flow-Induced Dispersion Analysis–Mass Spectrometry Enables Automated, Multiplexed Ligand Screening from Conventional, Nonvolatile Buffers. Anal. Chem. 2025, 97, 10388–10395. [Google Scholar] [CrossRef]
- Rajan, D.P. Extraction, Isolation, and Characterization Techniques of Structural Proteins. In Fish Structural Proteins and Its Derivatives: Functionality and Applications; Springer: Berlin/Heidelberg, Germany, 2024; pp. 37–72. [Google Scholar] [CrossRef]
- Goodarzi, M.M.; Jalalirad, R. Clear Insight into Complex Multimodal Resins and Impurities to Overcome Recombinant Protein Purification Challenges: A Review. Biotechnol. Bioeng. 2024, 122, 5–29. [Google Scholar] [CrossRef]
- Guarra, F.; Colombo, G. Computational Methods in Immunology and Vaccinology: Design and Development of Antibodies and Immunogens. J. Chem. Theory Comput. 2023, 19, 5315–5333. [Google Scholar] [CrossRef]
- Zarzar, J.; Khan, T.A.; Bhagawati, M.; Weiche, B.; Sydow-Andersen, J.; Sreedhara, A. High Concentration Formulation Developability Approaches and Considerations. mAbs 2023, 15, 2211185. [Google Scholar] [CrossRef]
- Mattei, A.E.; Gutiérrez, A.H.; Seshadri, S.; Tivin, J.; Ardito, M.; Rosenberg, A.S.; Martin, W.D.; Groot, A.S.D. In Silico Methods for Immunogenicity Risk Assessment and Human Homology Screening for Therapeutic Antibodies. mAbs 2024, 16, 2333729. [Google Scholar] [CrossRef]
- Lenders, V.; Koutsoumpou, X.; Phan, P.; Soenen, S.J.; Allegaert, K.; Vleeschouwer, S.D.; Toelen, J.; Zhao, Z.; Manshian, B.B. Modulation of Engineered Nanomaterial Interactions with Organ Barriers for Enhanced Drug Transport. Chem. Soc. Rev. 2023, 52, 4672–4724. [Google Scholar] [CrossRef]
- Tourdot, S.; Bloem, K.; Champion, L.; Groot, A.S.D.; Ducret, A.; Garidel, P.; Grudzinska-Goebel, J.; Gutknecht, M.; Hickling, T.; Horling, F.; et al. Proceedings of the 15th European Immunogenicity Platform Open Symposium on Immunogenicity of Biopharmaceuticals. mAbs 2025, 17, 2487604. [Google Scholar] [CrossRef]
- Rashid, M. Immunogenicity of an Intranasal Dual (Core and Surface)-Antigen Vaccine against Hepatitis B Virus Enhanced by Carboxyl-Vinyl Polymer Excipients. Vaccines 2025, 13, 464. [Google Scholar] [CrossRef]
- Warrick, K. Bidirectional Communication between the Innate and Adaptive Immune Systems. Annu. Rev. Immunol. 2025, 43, 489–514. [Google Scholar] [CrossRef]
- Lee, Y.; Jeong, M.; Park, J.; Jung, H.; Lee, H. Immunogenicity of Lipid Nanoparticles and Its Impact on the Efficacy of mRNA Vaccines and Therapeutics. Exp. Mol. Med. 2023, 55, 2085–2096. [Google Scholar] [CrossRef]
- Liu, F.; Stowell, S. The Role of Galectins in Immunity and Infection. Nat. Rev. Immunol. 2023, 23, 479–494. [Google Scholar] [CrossRef]
- Jarras, H.; Blais, I.; Goyer, B.; Bazié, W.; Rabezanahary, H.; Thériault, M.; Santerre, K.; Langlois, M.-A.; Masson, J.-F.; Pelletier, J.N.; et al. Impact of SARS-CoV-2 Vaccination and of Seasonal Variations on the Innate Immune Inflammatory Response. Front. Immunol. 2025, 15, 1513717. [Google Scholar] [CrossRef]
- Kachhawaha, K.; Singh, S.; Joshi, K.Y.; Nain, P.; Singh, S.K. Bioprocessing of Recombinant Proteins from Escherichia coli Inclusion Bodies: Insights from Structure-Function Relationship for Novel Applications. Prep. Biochem. Biotechnol. 2022, 53, 728–752. [Google Scholar] [CrossRef]
- Escobar, E.; Griffin, V.; Dhar, P. Correlating Surface Activity with Interface-Induced Aggregation in a High-Concentration mAb Solution. Mol. Pharm. 2024, 21, 1490–1500. [Google Scholar] [CrossRef]
- Jarvi, N.L.; Balu-Iyer, S.V. A Mechanistic Marker-Based Screening Tool to Predict Clinical Immunogenicity of Biologics. Commun. Med. 2023, 3, 174. [Google Scholar] [CrossRef]
- Ito, T.; Lutz, H.; Tan, L.; Wang, B.; Tan, J.; Patel, M.; Chen, L.; Tsunakawa, Y.; Park, B.; Banerjee, S. Host Cell Proteins in Monoclonal Antibody Processing: Control, Detection, and Removal. Biotechnol. Prog. 2024, 40, e3448. [Google Scholar] [CrossRef]
- Luitjens, A.; van Corven, E. Production and Purification of Recombinant Proteins. In Pharmaceutical Biotechnology; Springer: Cham, Switzerland, 2024; pp. 69–94. [Google Scholar] [CrossRef]
- Gote, V.; Bolla, P.K.; Kommineni, N.; Butreddy, A.; Nukala, P.K.; Palakurthi, S.S.; Khan, W. A Comprehensive Review of mRNA Vaccines. Int. J. Mol. Sci. 2023, 24, 2700. [Google Scholar] [CrossRef]
- Gholap, A.D.; Gupta, J.; Kamandar, P.; Bhowmik, D.D.; Rojekar, S.; Faiyazuddin, M.; Hatvate, N.T.; Mohanto, S.; Ahmed, M.G.; Subramaniyan, V.; et al. Harnessing Nanovaccines for Effective Immunization─A Special Concern on COVID-19: Facts, Fidelity, and Future Prospective. ACS Biomater. Sci. Eng. 2023, 10, 271–297. [Google Scholar] [CrossRef]
- Nitulescu, A.; Du, W.; Glaser, V.; Kath, J.; Aird, E.J.; Cullot, G.; Greensmith, R.; Mikkelsen, N.S.; Stein, M.; Bak, R.O.; et al. Single-Stranded HDR Templates with Truncated Cas12a-Binding Sequences Improve Knock-In Efficiencies in Primary Human T Cells. Mol. Ther. Nucleic Acids 2025, 36, 102568. [Google Scholar] [CrossRef]
- Emami, F.; Shokooh, M.K.; Yazdi, S.J.M. Recent Progress in Drying Technologies for Improving the Stability and Delivery Efficiency of Biopharmaceuticals. J. Pharm. Investig. 2022, 53, 35–57. [Google Scholar] [CrossRef]
- Boggiano-Ayo, T.; Palacios-Oliva, J.; Lozada-Chang, S.; Relova-Hernández, E.; Gómez-Pérez, J.A.; Oliva, G.; Hernandez, L.; Bueno-Soler, A.; de Oca, D.M.; Mora, O.; et al. Development of a Scalable Single Process for Producing SARS-CoV-2 RBD Monomer and Dimer Vaccine Antigens. Front. Bioeng. Biotechnol. 2023, 11, 1287551. [Google Scholar] [CrossRef]
- Lou, H.; Feng, M.; Hageman, M.J. Advanced Formulations/Drug Delivery Systems for Subcutaneous Delivery of Protein-Based Biotherapeutics. J. Pharm. Sci. 2022, 111, 2968–2982. [Google Scholar] [CrossRef]
- Wu, Y.; Gardner, R.; Schöneich, C. Near UV and Visible Light-Induced Degradation of Bovine Serum Albumin and a Monoclonal Antibody Mediated by Citrate Buffer and Fe(III): Reduction vs. Oxid. Pathways. Mol. Pharm. 2024, 21, 4060–4073. [Google Scholar] [CrossRef]
- Muralidhara, B.K.; Wong, M. Critical Considerations in the Formulation Development of Parenteral Biologic Drugs. Drug Discov. Today 2020, 25, 574–581. [Google Scholar] [CrossRef]
- Bahera, B.K.; Prasad, R.; Behera, S. Therapeutic Proteins: Production and Delivery. In New Paradigms of Living Systems; Springer: Singapore, 2021; pp. 127–207. [Google Scholar] [CrossRef]
- Zalai, D.; Kopp, J.; Kozma, B.; Küchler, M.; Herwig, C.; Kager, J. Microbial Technologies for Biotherapeutics Production: Key Tools for Advanced Biopharmaceutical Process Development and Control. Drug Discov. Today: Technol. 2020, 38, 9–24. [Google Scholar] [CrossRef]
- VandenBerg, M.A.; Dong, X.; Smith, W.C.; Tian, G.; Stephens, O.; O’Connor, T.; Xu, X. Learning from the Future: Towards Continuous Manufacturing of Nanomaterials. AAPS Open 2025, 11, 7. [Google Scholar] [CrossRef]
- Greenblatt, J.; Alberts, B.; Krogan, N.J. Discovery and Significance of Protein-Protein Interactions in Health and Disease. Cell 2024, 187, 6501–6517. [Google Scholar] [CrossRef]
- Bon, M.; Bilsland, A.; Bower, J.; McAulay, K. Fragment-Based Drug Discovery—The Importance of High-Quality Molecule Libraries. Mol. Oncol. 2022, 16, 3761–3777. [Google Scholar] [CrossRef]
- Balboni, B.; Rinaldi, F.; Previtali, V.; Ciamarone, A.; Girotto, S.; Cavalli, A. Novel Insights into RAD52′s Structure, Function, and Druggability for Synthetic Lethality and Innovative Anticancer Therapies. Cancers 2023, 15, 1817. [Google Scholar] [CrossRef]
- Favaro, M.T.P.; Atienza-Garriga, J.; Martínez-Torró, C.; Parladé, E.; Vázquez, E.; Corchero, J.L.; Villaverde, A. Recombinant Vaccines in 2022: A Perspective from the Cell Factory. Microb. Cell Fact. 2022, 21, 203. [Google Scholar] [CrossRef]
- Mohite, P.; Yadav, V.; Pandhare, R.; Maitra, S.; Saleh, F.M.; Saleem, R.M.; Uti, D.E. Revolutionizing Cancer Treatment: Unleashing the Power of Viral Vaccines, Monoclonal Antibodies, and Proteolysis-Targeting Chimeras in the New Era of Immunotherapy. ACS Omega 2024, 9, 7277–7295. [Google Scholar] [CrossRef]
- Pantaleo, G.; Correia, B.E.; Fenwick, C.; Joo, V.; Perez, L. Antibodies to Combat Viral Infections: Development Strategies and Progress. Nat. Rev. Drug Discov. 2022, 21, 676–696. [Google Scholar] [CrossRef]
- Eφpeмeнко, E.; Aslanli, A.; Lyagin, I. Advanced Situation with Recombinant Toxins: Diversity, Production and Application Purposes. Int. J. Mol. Sci. 2023, 24, 4630. [Google Scholar] [CrossRef]
- de la Fuente, M.; Lombardero, L.; Gómez-González, A.; Solari, C.; Angulo-Barturen, I.; Acera, A.; Vecino, E.; Astigarraga, E.; Barreda-Gómez, G. Enzyme Therapy: Current Challenges and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 9181. [Google Scholar] [CrossRef]
- Bharathi, J.K.; Suresh, P.R.; Prakash, M.; Muneer, S. Exploring Recent Progress of Molecular Farming for Therapeutic and Recombinant Molecules in Plant Systems. Heliyon 2024, 10, e37634. [Google Scholar] [CrossRef]
- Vickerman, B.M.; Acharya, B.R.; McGlade, C.A.; Yin, H.; Kawano, T.; Haar, L.; Mackman, N.; Sellers, R.S.; Tan, X.; Bhatt, A.P.; et al. Photothrombolytics: A Light-Driven Technology for the Targeted Lysis of Thrombi. J. Control. Release 2025, 378, 281–293. [Google Scholar] [CrossRef]
- Al-Harrasi, A.; Bhatia, S.; Khan, A. Introduction to Pharmaceutical Biotechnology, 2nd ed.; Institute of Physics: London, UK, 2024; Volume 2. [Google Scholar] [CrossRef]
- Hamada, M.; Varkoly, K.S.; Riyadh, O.; Beladi, R.; Munuswamy-Ramanujam, G.; Rawls, A.; Wilson-Rawls, J.; Chen, H.; McFadden, G.; Lucas, A.R. Urokinase-Type Plasminogen Activator Receptor (uPAR) in Inflammation and Disease: A Unique Inflammatory Pathway Activator. Biomedicines 2024, 12, 1167. [Google Scholar] [CrossRef]
- Prajapati, R.N.; Bhushan, B.; Singh, K.; Chopra, H.; Kumar, S.; Agrawal, M.C.; Pathak, D.; Chanchal, D.K.; Laxmikant. Recent Advances in Pharmaceutical Design: Unleashing the Potential of Novel Therapeutics. Curr. Pharm. Biotechnol. 2024, 25, 2060–2077. [Google Scholar] [CrossRef]
- Verma, A.K.; Singh, K.; Gupta, J.K.; Kumar, S.; Jain, D. Pharmacological Approaches and Innovative Strategies for Individualized Patient Care. Recent Pat. Biotechnol. 2025, 19, 89–107. [Google Scholar] [CrossRef]
- Aung, T.; Grubbe, W.S.; Nusbaum, R.J.; Mendoza, J.L. Recent and Future Perspectives on Engineering Interferons and Other Cytokines as Therapeutics. Trends Biochem. Sci. 2023, 48, 259–273. [Google Scholar] [CrossRef]
- Gjølberg, T.T.; Frick, R.; Mester, S.; Foss, S.; Grevys, A.; Høydahl, L.S.; Jørstad, Ø.K.; Schlothauer, T.; Sandlie, I.; Moe, M.C.; et al. Biophysical Differences in IgG1 Fc-Based Therapeutics Relate to Their Cellular Handling, Interaction with FcRn and Plasma Half-Life. Commun. Biol. 2022, 5, 832. [Google Scholar] [CrossRef]
- Pyzik, M.; Kozicky, L.K.; Gandhi, A.; Blumberg, R.S. The Therapeutic Age of the Neonatal Fc Receptor. Nat. Rev. Immunol. 2023, 23, 415–432. [Google Scholar] [CrossRef]
- Binder, U.; Skerra, A. Strategies for Extending the Half-Life of Biotherapeutics: Successes and Complications. Expert Opin. Biol. Ther. 2024, 25, 93–118. [Google Scholar] [CrossRef]
- Nilsen, J.; Aaen, K.H.; Benjakul, S.; Ruso-Julve, F.; Greiner, T.U.; Bejan, D.; Stensland, M.; Singh, S.; Schlothauer, T.; Sandlie, I.; et al. Enhanced Plasma Half-Life and Efficacy of Engineered Human Albumin-Fused GLP-1 Despite Enzymatic Cleavage of Its C-Terminal End. Commun. Biol. 2025, 8, 810. [Google Scholar] [CrossRef]
- Bennett, J.I.; Boit, M.O.; Gregorio, N.E.; Zhang, F.; Kibler, R.D.; Hoye, J.W.; Prado, O.; Rapp, P.B.; Murry, C.E.; Stevens, K.R.; et al. Genetically Encoded XTEN-Based Hydrogels with Tunable Viscoelasticity and Biodegradability for Injectable Cell Therapies. Adv. Sci. 2024, 11, e2301708. [Google Scholar] [CrossRef]
- Huang, Y.; Stankevych, M.; Gujrati, V.; Klemm, U.; Arshad, M.; Wiesner, D.; Saccomano, M.; Tost, M.; Feuchtinger, A.; Mishra, K.; et al. Photoswitching Protein-XTEN Fusions as Injectable Optoacoustic Probes. Acta Biomater. 2025, 195, 536–546. [Google Scholar] [CrossRef]
- Binder, U.; Skerra, A. PASylated Thymosin α1: A Long-Acting Immunostimulatory Peptide for Applications in Oncology and Virology. Int. J. Mol. Sci. 2021, 22, 124. [Google Scholar] [CrossRef]
- Bourayne, M.D.; Meunier, S.; Bitoun, S.; Correia, E.; Mariette, X.; Nozach, H.; Maillère, B. PEGylation Reduces the Uptake of Certolizumab Pegol by Dendritic Cells and Epitope Presentation to T-Cells. Front. Immunol. 2022, 13, 808606. [Google Scholar] [CrossRef]
- Li, C.; Li, T.; Tian, X.; An, W.; Wang, Z.; Han, B.; Tao, H.; Wang, J.; Wang, X. Research Progress on the PEGylation of Therapeutic Proteins and Peptides (TPPs). Front. Pharmacol. 2024, 15, 1353626. [Google Scholar] [CrossRef]
- Chia, S.; Tay, S.J.; Song, Z.; Yang, Y.; Walsh, I.; Pang, K.T. Enhancing Pharmacokinetic and Pharmacodynamic Properties of Recombinant Therapeutic Proteins by Manipulation of Sialic Acid Content. Biomed. Pharmacother. 2023, 163, 114757. [Google Scholar] [CrossRef]
- Rocamora, F.; Peralta, A.G.; Shin, S.; Sorrentino, J.V.; Wu, M.; Toth, E.A.; Fuerst, T.R.; Lewis, N.E. Glycosylation Shapes the Efficacy and Safety of Diverse Protein, Gene and Cell Therapies. Biotechnol. Adv. 2023, 67, 108206. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, X.; Chen, X.; Huang, J.; Wang, C.; Wang, J.; Wang, Z. Accelerating Therapeutic Protein Design with Computational Approaches Toward the Clinical Stage. Comput. Struct. Biotechnol. J. 2023, 21, 2909–2926. [Google Scholar] [CrossRef]
- Domingues, C.; Jarak, I.; Veiga, F.; Dourado, M.; Figueiras, A. Pediatric Drug Development: Reviewing Challenges and Opportunities by Tracking Innovative Therapies. Pharmaceutics 2023, 15, 2431. [Google Scholar] [CrossRef]
- Far, B.F.; Safaei, M.; Mokhtari, F.; Fallahi, M.S.; Naimi-Jamal, M.R. Fundamental Concepts of Protein Therapeutics and Spacing in Oncology: An Updated Comprehensive Review. Med. Oncol. 2023, 40, 166. [Google Scholar] [CrossRef]
- Ebrahimi, S.B.; Samanta, D. Engineering Protein-Based Therapeutics through Structural and Chemical Design. Nat. Commun. 2023, 14, 2411. [Google Scholar] [CrossRef]
- Zhao, T.; Ren, M.; Shi, J.; Wang, H.; Bai, J.; Du, W.; Bai, X. Engineering the Protein Corona: Strategies, Effects, and Future Directions in Nanoparticle Therapeutics. Biomed. Pharmacother. 2024, 175, 116627. [Google Scholar] [CrossRef]
- Syahputra, E.W.; Lee, H.; Cho, H.; Park, H.J.; Park, K.-S.; Hwang, D. PROTAC Delivery Strategies for Overcoming Physicochemical Properties and Physiological Barriers in Targeted Protein Degradation. Pharmaceutics 2025, 17, 501. [Google Scholar] [CrossRef]
- Son, A.; Park, J.; Kim, W.; Yoon, Y.; Lee, S.; Park, Y.; Kim, H. Revolutionizing molecular design for innovative therapeutic applications through artificial intelligence. Molecules 2024, 29, 4626. [Google Scholar] [CrossRef]
- Paz, M.; Moratorio, G. Deep mutational scanning and CRISPR-engineered viruses: Tools for evolutionary and functional genomics studies. mSphere 2025, 10, e0050824. [Google Scholar] [CrossRef]
- Rapp, J.; Bremer, B.; Romero, P. Self-driving laboratories to autonomously navigate the protein fitness landscape. Nat. Chem. Eng. 2024, 1, 97–107. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, P.; Wang, H.; Chen, Y.; Liu, T.; Luo, X. Genetic code expansion: Recent developments and emerging applications. Chem. Rev. 2024, 125, 523–598. [Google Scholar] [CrossRef]
- Lee, K.; Jeon, J.; Jiang, B.; Subramani, S.; Li, J.; Zhang, F. Protein-based hydrogels and their biomedical applications. Molecules 2023, 28, 4988. [Google Scholar] [CrossRef]
- Kelwick, R.; Webb, A.; Freemont, P. Opportunities for engineering outer membrane vesicles using synthetic biology approaches. Extracell. Vesicles Circ. Nucleic Acids 2023, 4, 255–261. [Google Scholar] [CrossRef]
- Caдaнoв, A.; Baimakhanova, B.; Orasymbet, S.; Paтникoвa, И.; Turlybayeva, Z.; Baimakhanova, G.; Amitova, A.A.; Omirbekova, A.A.; Aitkaliyeva, G.S.; Kossalbayev, B.D.; et al. Engineering useful microbial species for pharmaceutical applications. Microorganisms 2025, 13, 599. [Google Scholar] [CrossRef]
- Guo, P.; Wang, S.; Yue, H.; Zhang, X.; Ma, G.; Li, X.; Wei, W. Advancement of engineered bacteria for orally delivered therapeutics. Small 2023, 19, e2302702. [Google Scholar] [CrossRef]
- Kim, T.; Cho, B.; Lee, D. Synthetic biology-driven microbial therapeutics for disease treatment. J. Microbiol. Biotechnol. 2024, 34, 1947–1958. [Google Scholar] [CrossRef]
- Hoces, D.; Blanco, J.; Hernández-López, R. A synthetic biology approach to engineering circuits in immune cells. Immunol. Rev. 2023, 320, 120–137. [Google Scholar] [CrossRef]
- Zhu, B.; Yin, H.; Zhang, D.; Zhang, M.; Chao, X.; Scimeca, L.; Wu, M. Synthetic biology approaches for improving the specificity and efficacy of cancer immunotherapy. Cell. Mol. Immunol. 2024, 21, 436–447. [Google Scholar] [CrossRef]
- Gu, P.; Zhao, J.; Zhang, W.; Ruan, X.; Hu, L.; Zeng, Y.; Hou, X.; Zheng, X.; Gao, M.; Chi, J. An inducible CRISPR-dCas9-based transcriptional repression system for cancer therapy. Small Methods 2024, 8, e2301310. [Google Scholar] [CrossRef] [PubMed]
- Ono, H.; Saito, H. Sensing intracellular signatures with synthetic mRNAs. RNA Biol. 2023, 20, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Ji, X. Establishing a high-yield Bacillus subtilis-based cell-free protein synthesis system for in vitro prototyping and natural product biosynthesis. ACS Synth. Biol. 2025, 14, 1288–1297. [Google Scholar] [CrossRef]
- Goshisht, M. Machine learning and deep learning in synthetic biology: Key architectures, applications, and challenges. ACS Omega 2024, 9, 9921–9945. [Google Scholar] [CrossRef]
- Kohyama, S.; Frohn, B.; Babl, L.; Schwille, P. Machine learning-aided design and screening of an emergent protein function in synthetic cells. Nat. Commun. 2024, 15, 2010. [Google Scholar] [CrossRef]
- Lebar, B.; Zidar, M.; Mravljak, J.; Šink, R.; Žula, A.; Pajk, S. Alternative buffer systems in biopharmaceutical formulations and their effect on protein stability. Acta Pharm. 2024, 74, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Gibson, K.; Oliveira, J.C.; Ring, D. Optimisation of biopharmaceutical buffer management to maximise the benefits of inline preparation systems. Results Eng. 2025, 25, 104080. [Google Scholar] [CrossRef]
- Lakatos, D.; Idler, M.; Stibitzky, S.; Amann, J.; Schuschkewitz, J.; Krayl, D.; Liebau, J.; Grosch, J.; Gutierrez, E.A.; Kluters, S. Buffer system improves the removal of host cell protein impurities in monoclonal antibody purification. Biotechnol. Bioeng. 2024, 121, 3869–3880. [Google Scholar] [CrossRef] [PubMed]
- Aryal, B.; Lehtimaki, M.; Rao, V. Stress-mediated polysorbate 20 degradation and its potential impact on therapeutic proteins. Pharm. Res. 2024, 41, 1217–1232. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Zhang, Y.; Sun, C.; Wang, S.S.; Gong, Y.; Jia, C.; Luo, J. Fundamental properties and principal areas of focus in antibody–drug conjugates formulation development. Antib. Ther. 2025, 8, 99–110. [Google Scholar] [CrossRef]
- Xin, L.; Zhang, Z.; Shah, K.; Hashemi, V.; Li, X.; Qin, G.; Ren, S.; Chen, W.; Li, Y. Matching placebo development for injectable biologics—A practical tutorial. Antib. Ther. 2025, 8, 177–188. [Google Scholar] [CrossRef]
- Petris, P.C.; Sweere, A.J.M. Buffer screening of protein formulations using a coarse-grained protocol based on medicinal chemistry interactions. J. Phys. Chem. B 2024, 128, 9353–9362. [Google Scholar] [CrossRef] [PubMed]
- Dobrzyńska-Mizera, M.; Dodda, J.M.; Liu, X.; Knitter, M.; Oosterbeek, R.N.; Salinas, P.; Pozo, E.; Ferreira, A.M.; Sadiku, E.R. Engineering of bioresorbable polymers for tissue engineering and drug delivery applications. Adv. Health Mater. 2024, 13, 30. [Google Scholar] [CrossRef]
- Spasojević, L.; Ivanišević, I.; Dutour Sikirić, M. Stability and application of TiO2 nanomaterials in aqueous suspensions: A review. RSC Adv. 2025, 15, 21341–21368. [Google Scholar] [CrossRef]
- Saurabh, S.; Kalonia, C.; Li, Z.; Hollowell, P.; Waigh, T.A.; Li, P.; Webster, J.; Seddon, J.M.; Lu, J.R.; Bresme, F. Understanding the stabilizing effect of histidine on mAb aggregation: A molecular dynamics study. Mol. Pharm. 2022, 19, 3288–3303. [Google Scholar] [CrossRef]
- Ren, S. Effects of arginine in therapeutic protein formulations: A decade review and perspectives. Antib. Ther. 2023, 6, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Prašnikar, M.; Žiberna, M.B.; Kržišnik, N.; Roškar, R.; Grabnar, I.; Žula, A.; Grabnar, P.A. Additive effects of the new viscosity-reducing and stabilizing excipients for monoclonal antibody formulation. Int. J. Pharm. 2025, 674, 125451. [Google Scholar] [CrossRef]
- Francis, J.A.; Wright, L.; van Wegen, R.; Zhao, C.-X.; Falconer, R.J. Effects of salts, buffers and sucrose on protein–protein attractive and repulsive interactions. Int. J. Pharm. 2025, 672, 125321. [Google Scholar] [CrossRef]
- Crommelin, D.J.; Hawe, A.; Jiskoot, W. Formulation of biologics including biopharmaceutical considerations. Pharm. Biotechnol. 2024, 95, 95–117. [Google Scholar] [CrossRef]
- Lebar, B.; Orehova, M.; Japelj, B.; Šprager, E.; Podlipec, R.; Knaflič, T.; Urbančič, I.; Knez, B.; Zidar, M.; Cerar, J.; et al. A multifaceted approach to understanding protein-buffer interactions in biopharmaceuticals. Eur. J. Pharm. Biopharm. 2025, 206, 114582. [Google Scholar] [CrossRef]
- King, T.; Humphrey, J.R.; Laughton, C.A.; Thomas, N.R.; Hirst, J.D. Optimizing excipient properties to prevent aggregation in biopharmaceutical formulations. J. Chem. Inf. Model. 2023, 64, 265–275. [Google Scholar] [CrossRef]
- Rojekar, S.; Gholap, A.D.; Jadhav, K.; Shevalkar, G.; Sugandhi, V.V.; Pai, R.; Parikh, K.; Prajapati, M.K.; Desai, N.; Vora, L.K.; et al. Exploring protein aggregation in biological products: From mechanistic understanding to practical solutions. AAPS PharmSciTech 2025, 26, 189. [Google Scholar] [CrossRef]
- Sharma, K.; deSilva, B.W.; Hanauer, S.B. The role of immunogenicity in optimizing biological therapies for inflammatory bowel disease. Expert Rev. Gastroenterol. Hepatol. 2025, 19, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Swanson, S.J. What are clinically significant anti-drug antibodies and why is it important to identify them. Front. Immunol. 2024, 15, 1401178. [Google Scholar] [CrossRef] [PubMed]
- Carter, P.J.; Quarmby, V. Immunogenicity risk assessment and mitigation for engineered antibody and protein therapeutics. Nat. Rev. Drug Discov. 2024, 23, 898–913. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, B.K. Role of immunogenicity in drug development. Era’s J. Med. Res. 2024, 11, 215–224. [Google Scholar] [CrossRef]
- Davis, J.D.; Padros, M.B.; Conrado, D.J.; Ganguly, S.; Guan, X.; Hassan, H.E.; Hazra, A.; Irvin, S.C.; Jayachandran, P.; Kosloski, M.P.; et al. Subcutaneous administration of monoclonal antibodies: Pharmacology, delivery, immunogenicity, and learnings from applications to clinical development. Clin. Pharmacol. Ther. 2025, 115, 422–439. [Google Scholar] [CrossRef]
- Harris, C.T.; Cohen, S. Reducing immunogenicity by design: Approaches to minimize immunogenicity of monoclonal antibodies. BioDrugs 2024, 38, 205–226. [Google Scholar] [CrossRef]
- Jacquot, G.; Navarro, P.L.; Grange, C.; Boudali, L.; Harlepp, S.; Pivot, X.; Detappe, A. Landscape of subcutaneous administration strategies for monoclonal antibodies in oncology. Adv. Mater. 2024, 36, e2406604. [Google Scholar] [CrossRef]
- Jarvi, N.L.; Patel, M.I.; Shetty, K.A.; Nguyen, N.H.; Grasperge, B.; Mager, D.E.; Straubinger, R.M.; Balu-Iyer, S.V. Immune regulatory adjuvant approach to mitigate subcutaneous immunogenicity of monoclonal antibodies. Front. Immunol. 2024, 15, 1496169. [Google Scholar] [CrossRef]
- Jarab, A.S.; Heshmeh, S.A.; Meslamani, A.Z.A. Biosimilars and immunogenicity: A matter of concern? Expert Opin. Drug Saf. 2025, 24, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Weerarathna, I.N.; Doelakeh, E.S.; Kiwanuka, L.; Kumar, P.; Arora, S. Prophylactic and therapeutic vaccine development: Advancements and challenges. Mol. Biomed. 2024, 5, 57. [Google Scholar] [CrossRef] [PubMed]
- Pépin, X.; Arora, S.; Borges, L.; Cano-Vega, M.A.; Carducci, T.M.; Chatterjee, P.; Chen, G.; Cristofoletti, R.; Dallmann, A.; Delvadia, P.; et al. Parameterization of physiologically based biopharmaceutics models: Workshop summary report. Mol. Pharm. 2024, 21, 3697–3731. [Google Scholar] [CrossRef]
- Brouwer, B.; Della-Felice, F.; Illies, J.H.; Iglesias-Moncayo, E.; Roelfes, G.; Drienovská, I. Noncanonical amino acids: Bringing new-to-nature functionalities to biocatalysis. Chem. Rev. 2024, 124, 10877–10923. [Google Scholar] [CrossRef]
- Tien, S.L.; Kayser, V. Ionic liquids as stabilisers of therapeutic protein formulations: A review of insulin and monoclonal antibodies. Biophys. Rev. 2024, 17, 89–101. [Google Scholar] [CrossRef]
- Garg, R.; McCarthy, S.; Thompson, A.G.; Zhang, J.; Mattson, E.; Clabbers, A.; Acquah, A.; Xu, J.; Zhou, C.; Ali, A.; et al. In vitro stability study of a panel of commercial antibodies at physiological pH and temperature as a guide to screen biologic candidate molecules for the potential risk of in vivo asparagine deamidation and activity loss. Pharm. Res. 2025, 42, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Buske, J.; Mäder, K.; Garidel, P.; Diederichs, T. Oxidation of polysorbates–an underestimated degradation pathway? Int. J. Pharm. X 2023, 6, 100202. [Google Scholar] [CrossRef]
- Bhat, V.J.; Blaschke, D.; Vegesna, S.V.; Burgold-Voigt, S.; Müller, E.; Ehricht, R.; Schmidt, H. Monitoring the dilution of buffer solutions with different pH values above and below physiological pH in very small volumes. Sensors 2024, 24, 5751. [Google Scholar] [CrossRef] [PubMed]
- Pineda, S.P.; Blanco, P.M.; Staňo, R.; Košovan, P. Patchy charge distribution affects the pH in protein solutions during dialysis. Langmuir 2025, 41, 5387–5398. [Google Scholar] [CrossRef]
- Sarin, D.; Krishna, K.; Nejadnik, M.; Suryanarayanan, R.; Rathore, A. Impact of excipient extraction and buffer exchange on recombinant monoclonal antibody stability. Mol. Pharm. 2024, 21, 1872–1883. [Google Scholar] [CrossRef]
- Ranbhor, R. Advancing monoclonal antibody manufacturing: Process optimization, cost reduction strategies, and emerging technologies. Biol. Targets Ther. 2025, 19, 177–187. [Google Scholar] [CrossRef]
- Buckland, B.C.; Sanyal, G.; Ranheim, T.; Pollard, D.; Searles, J.; Behrens, S.; Pluschkell, S.; Josefsberg, J.; Roberts, C.J. Vaccine process technology—Adecade of progress. Biotechnol. Bioeng. 2024, 121, 2604–2635. [Google Scholar] [CrossRef]
- Utami, N.; Nurdiani, D.; Hariyatun, H.; Putro, E.; Patria, F.; Kusharyoto, W. Full-length versus truncated α-factor secretory signal sequences for expression of recombinant human insulin precursor in yeast Pichia pastoris: A comparison. J. Genet. Eng. Biotechnol. 2023, 21, 67. [Google Scholar] [CrossRef]
- Mofid, M.; Babaeipour, V.; Jafari, S.; Haddad, L.; Moghim, S.; Ghanavi, J. Efficient process development for high-level production, purification, formulation, and characterization of recombinant mecasermin in Escherichia coli. Biotechnol. Appl. Biochem. 2020, 68, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Jalouli, M.; Yadab, M.K.; Al-Zharani, M. Progress in drug delivery systems based on nanoparticles for improved glioblastoma therapy: Addressing challenges and investigating opportunities. Cancers 2025, 17, 701. [Google Scholar] [CrossRef]
- Abaidullah, N.; Muhammad, K.; Waheed, Y. Delving into nanoparticle systems for enhanced drug delivery technologies. AAPS PharmSciTech 2025, 26, 3. [Google Scholar] [CrossRef]
- Johnson, Y.; Shakri, A.; Pond-Tor, S.; Jnawali, A.; Najrana, T.; Wu, H.; Badhai, J.; Alameh, M.-G.; Weissman, D.; Kabyemela, E.; et al. Immunization with PfGbp130 generates antibodies that inhibit RBC invasion by P. falciparum parasites. Front. Immunol. 2024, 15, 1350560. [Google Scholar] [CrossRef] [PubMed]
- Singh, D. A short appraisal of biological macromolecules as tethering excipients for improved drug delivery: Current advances. J. Macromol. Sci. Part B 2023, 63, 604–620. [Google Scholar] [CrossRef]
- El-Tanani, M.; Satyam, S.M.; Rabbani, S.A.; El-Tanani, Y.; Aljabali, A.A.A.; Al Faouri, I.; Rehman, A. Revolutionizing drug delivery: The impact of advanced materials science and technology on precision medicine. Pharmaceutics 2025, 17, 375. [Google Scholar] [CrossRef]
- Ling, J.; Du, Y.; Wuelfing, W.P.; Buist, N.; Krishnamachari, Y.; Xi, H.; Templeton, A.C.; Su, Y. Molecular mechanisms for stabilizing biologics in the solid state. J. Pharm. Sci. 2025, 114, 736–765. [Google Scholar] [CrossRef]
- Bharmoria, P.; Tietze, A.A.; Mondal, D.; Kang, T.S.; Kumar, A.; Freire, M.G. Do ionic liquids exhibit the required characteristics to dissolve, extract, stabilize, and purify proteins? Past-present-future assessment. Chem. Rev. 2024, 124, 3037–3084. [Google Scholar] [CrossRef]
- Nguyễn, T.T.K. Stabilization and delivery of therapeutic proteins in the solid state: Toward a better shelf-life and personalized treatment with targeted delivery. Ph.D. Thesis, University of Groningen, Groningen, The Netherlands, 2024. [Google Scholar] [CrossRef]
- Xiao, E.; Mirabel, C.; Clénet, D.; Zhu, S.; James, A.; Ettorre, L.; Williams, T.; Szeto, J.; Rahman, N.; Ausar, S. Formulation development of a COVID-19 recombinant spike protein-based vaccine. Vaccines 2024, 12, 830. [Google Scholar] [CrossRef]
- Horrow, C.; Gabriele, S.; Tu, S.; Sarpatwari, A.; Kesselheim, A. Patent portfolios protecting 10 top-selling prescription drugs. JAMA Intern. Med. 2024, 184, 810. [Google Scholar] [CrossRef] [PubMed]
- Stajszczyk, M.; Batko, K.; Żuber, Z.; Kwiatkowska, B.; Krajewska–Włodarczyk, M.; Batko, B. Charting the etanercept journey: Tracing cost dynamics in Poland’s off-patent market from reference drug rivalry to biosimilar monopoly. Biodrugs 2024, 38, 557–569. [Google Scholar] [CrossRef]
- Agrawal, M.; Mishra, S.; Nayak, G.; Aggarwal, D.; Joshi, U. Knowledge, attitude and practice on biologicals and biosimilars among clinicians in radiotherapy department. J. Pharm. Care 2023, 11, 82–92. [Google Scholar] [CrossRef]
- Strickley, R.G.; Lambert, W.J. A review of formulations of commercially available antibodies. J. Pharm. Sci. 2021, 110, 2590–2608.e56. [Google Scholar] [CrossRef]
- Chen, F.; Zhong, H.; Chan, G.; Ouyang, D. A comprehensive analysis of biopharmaceutical products listed in the FDA’s Purple Book. AAPS PharmSciTech 2024, 25, 5. [Google Scholar] [CrossRef] [PubMed]
- Abitbol, V.; Benkhalifa, S.; Habauzit, C.; Marotte, H. Navigating adalimumab biosimilars: An expert opinion. J. Compar. Effect. Res. 2023, 12, 11. [Google Scholar] [CrossRef]
- Monga, A.; Gagan; Jamwal, P.; Sharma, S.; Kaur, A. Biosimilars: A Critical Review of Development, Regulatory Landscape, and Clinical Implications. AAPS PharmSciTech 2025, 26, 1. [Google Scholar] [CrossRef]
- Garg, N.; Kamal, C.M. Advancements on substitution of in vivo method(s) with in vitro method(s) for recombinant human erythropoietin potency assays. Int. J. Pept. Res. Ther. 2025, 31, 2. [Google Scholar] [CrossRef]
- Kang, H.; Wadhwa, M.; Knežević, I.; Burns, C.; Griffiths, E. The importance of World Health Organization international reference standards in the product life cycle of biosimilars. Ann. N. Y. Acad. Sci. 2024, 27, 3. [Google Scholar] [CrossRef]
- Andrade, A.M.; Girardi, J.M.; Silva, É.T.; Barbosa, J.R.; Pereira, D.C.R. Efficacy, safety, and immunogenicity of biosimilars compared with the biologic etanercept in patients with rheumatoid arthritis: A systematic review and meta-analysis. Syst. Rev. 2024, 13, 1. [Google Scholar] [CrossRef]
- Pekhenko, V.; Udovitskiy, V.; Barbukho, O. Efficacy, safety and immunogenicity of the biosimilar etanercept compared to the reference formulation original etanercept in patients with rheumatoid arthritis: An open-label, randomized, comparative, multicenter study. Medicine 2024, 103, e39060. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, H.; Tanaka, Y.; Hibino, T.; Unmesh, G.; Shah, C.; Bakhle, D.; Stefanidis, D. Lower injection-site reactions and long-term safety, immunogenicity, and efficacy of etanercept biosimilar YLB113: Results from a post-hoc analysis of a double-blind, randomized, phase III comparative study and its open-label extension in patients with rheumatoid arthritis. Int. J. Rheum. Dis. 2022, 26, 108–115. [Google Scholar] [CrossRef] [PubMed]
- McGonigle, P. How biologics have changed the drug discovery landscape. Annu. Rev. Pharmacol. Toxicol. 2025, 65, 29–46. [Google Scholar] [CrossRef]
- Alrawashdh, N.; McBride, A.; Oh, M.; Alkhatib, N.S.; Lee, C.S.; Martin, J.; MacDonald, K.; Abraham, I. Meta-analysis of same-day pegfilgrastim administration stratified by myelotoxic febrile neutropenia risk and tumor type. J. Adv. Pract. Oncol. 2022, 13, 796–811. [Google Scholar] [CrossRef] [PubMed]
- Purohit, K.; Reddy, N.; Sunna, A. Exploring the potential of bioactive peptides: From natural sources to therapeutics. Int. J. Mol. Sci. 2024, 25, 1391. [Google Scholar] [CrossRef]
- Welch, J.; Ausín, C.; Brahme, N.N.; Lacaná, E.; Ricci, S.; Schultz-DePalo, M. The mannose in the mirror: A reflection on the pharmacokinetic impact of high mannose glycans of monoclonal antibodies in biosimilar development. Clin. Pharmacol. Ther. 2022, 113, 1003–1010. [Google Scholar] [CrossRef]
- Meslamani, A.Z.A. Short and long-term economic implications of biosimilars. Expert Opin. Biol. Ther. 2024, 24, 567–570. [Google Scholar] [CrossRef]
- Woo, H.; Shin, G.; Lee, D.; Kwon, H.; Bae, S. Is the availability of biosimilar adalimumab associated with budget savings? A difference-in-difference analysis of 14 countries. Biodrugs 2023, 38, 133–144. [Google Scholar] [CrossRef]
- Ntais, C.; Kontodimopoulos, N.; Fanourgiakis, J.; Talias, Μ.A. Fostering healthcare system sustainability through efficient practices: Can adopting biosimilars ease the financial burden of rheumatoid arthritis? F1000Research 2025, 13, 1128. [Google Scholar] [CrossRef] [PubMed]
- Herrán, M.; Anaya, J.M. Mapping economic outcomes: How biosimilars enhance access to health systems in Latin America and the Caribbean. Curr. Opin. Immunol. 2025, 96, 102606. [Google Scholar] [CrossRef] [PubMed]
- Paavola, C.D.; Felippis, M.R.D.; Allen, D.P.; Garg, A.; Sabatowski, J.L.; Juneja, R.; Baldwin, D.B. Insulin. In Pharmaceutical Biotechnology; Springer: Cham, Switzerland, 2024; pp. 427–453. [Google Scholar] [CrossRef]
- Yaseen, Z.; Nandave, M.; Sharma, L. Anti-diabetic biologicals: Exploring the role of different analytical techniques. Crit. Rev. Anal. Chem. 2025, 15, 1–22. [Google Scholar] [CrossRef]
- Walsh, K.; Wallace, A.; Pavis, M.; Olszowy, N.; Griffin, J.; Hawkins, N. Intellectual property rights and access in crisis. IIC-Int. Rev. Intellect. Prop. Compet. Law 2021, 52, 379–416. [Google Scholar] [CrossRef]
- Khouilla, H.; Bastidon, C. Does increased intellectual property rights protection foster innovation in developing countries? A literature review of innovation and catch-up. J. Int. Dev. 2023, 36, 1170–1188. [Google Scholar] [CrossRef]
- Siltaloppi, J.; Ballardini, R.M. Promoting systemic collaboration for sustainable innovation through intellectual property rights. J. Co-Oper. Organ. Manag. 2023, 11, 100200. [Google Scholar] [CrossRef]
- Patil, N.; Ranjan, A.; Gaurav, G.; Mukherjee, D.; Panda, B.K.; Diksha, D.; Komal; Narang, R.K.; Singh, A. Navigating biosimilar regulatory pathways in emerging markets: Insights from BRICS nations. Appl. Drug Res. Clin. Trials Regul. Aff. 2025, 10, 18. [Google Scholar] [CrossRef]
- Ojeih, C.A.; Ogidan, O.; Oluwajobi, Y.F.; Adebayo, B.O. Biotechnology regulatory conundrum: Balancing innovation and oversight. J. Sustain. Dev. Law Policy 2024, 15, 120–144. [Google Scholar] [CrossRef]
- Williamson, R.; Munro, T.P.; Ascher, D.B.; Robertson, A.A.B.; Pregelj, L. Are manufacturing patents to blame for biosimilar market launch delays? Value Health 2024, 27, 287–293. [Google Scholar] [CrossRef]
- Yadav, P.; Singh, Y.; Chauhan, D.; Yadav, P.K.; Kedar, A.; Tiwari, A.K.; Chourasia, M.K. Development and approval of novel injectables: Enhancing therapeutic innovations. Expert Opin. Drug Deliv. 2024, 21, 639–662. [Google Scholar] [CrossRef]
- Ravindran, R.S. Role of patents in biosimilar drug development and public interest. J. Scientometr. Res. 2020, 9, 11. [Google Scholar]
- Maronero, C.; Bichlmayr, A. Flipping the paradigm of weak patent rights: From theories to evidence. J. Intellect. Prop. Law Pract. 2024, 19, 256–277. [Google Scholar] [CrossRef]
- Nupur, N.; Joshi, S.; Guillarme, D.; Rathore, A.S. Analytical similarity assessment of biosimilars: Global regulatory landscape, recent studies and major advancements in orthogonal platforms. Front. Bioeng. Biotechnol. 2022, 10, 832059. [Google Scholar] [CrossRef] [PubMed]
- Ratih, R.; Asmari, M.; Abdel-Megied, A.M.; Elbarbry, F.; Deeb, S.E. Biosimilars: Review of regulatory, manufacturing, analytical aspects and beyond. Microchem. J. 2021, 165, 106143. [Google Scholar] [CrossRef]
- Agrawal, S.; Vaidya, S.; Patel, J.; Jirvankar, P.; Gurjar, P. Challenges and pathways in regulating next-gen biological therapies. Curr. Pharm. Biotechnol. 2025, 26. [Google Scholar] [CrossRef]
- Kantaros, A.; Ganetsos, T.; Petrescu, F.I.T.; Alysandratou, E. Bioprinting and intellectual property: Challenges, opportunities, and the road ahead. Bioengineering 2025, 12, 76. [Google Scholar] [CrossRef]
- Patel, S.; Patel, Y.; Adodariya, M.; Shahiwala, A.; Mehta, P. Regulatory guidance on therapeutic proteomics and genomics. In Challenges in Delivery of Therapeutic Genomics and Proteomics; Elsevier: Amsterdam, The Netherlands, 2025; pp. 555–585. [Google Scholar] [CrossRef]
- Iqbal, Z.; Sadaf, S. Biosimilars: A comparative study of regulatory, safety and pharmacovigilance monograph in the developed and developing economies. J. Pharm. Pharm. Sci. 2022, 25, 149–182. [Google Scholar] [CrossRef]
- Amaral, C.; Rodrigues, A.; Veiga, F.; Bell, V. Biosimilar medicines: From development process to marketing authorization by the EMA and the FDA. Appl. Sci. 2024, 14, 7529. [Google Scholar] [CrossRef]
- Kurki, P.; Kang, H.; Ekman, N.; Knežević, I.; Weise, M.; Wolff-Holz, E. Regulatory evaluation of biosimilars: Refinement of principles based on the scientific evidence and clinical experience. Biodrugs 2022, 36, 359–371. [Google Scholar] [CrossRef]
- Gherghescu, I.; Delgado-Charro, M.B. The biosimilar landscape: An overview of regulatory approvals by the EMA and FDA. Pharmaceutics 2021, 13, 48. [Google Scholar] [CrossRef]
- Geaghan-Breiner, C. The patent trap: The struggle for competition and affordability in the field of biologic drugs. Columbia J. Law. Soc. Probl. 2020, 54, 589. [Google Scholar]
- Song, C.H. How non-product-specific manufacturing patents block biosimilars. Duke Law. J. 2022, 71, 1923–1973. [Google Scholar]
- Heled, Y. The Biologics Price Competition and Innovation Act 10--a stocktaking. Tex. A&M. J. Prop. Law 2021, 7, 81–109. [Google Scholar] [CrossRef]
- Jayeoba, D. The U.S. pharmaceutical market maze: How exploitative practices and legal loopholes trap competition and delay affordable medicines. SSRN Electron. J. 2025, 17, 581. [Google Scholar] [CrossRef]
- Karas, L.; Sachs, R.; Anderson, G. Legal obstacles to biosimilar market entry. HPHR 2021, 28. [Google Scholar] [CrossRef]
- Dong, L.; Shi, S.; Qu, X.; Ding, L.; Wang, B. Ligand binding affinity prediction with fusion of graph neural networks and 3D structure-based complex graph. Phys. Chem. Chem. Phys. 2023, 25, 24110–24120. [Google Scholar] [CrossRef]
- Abraham, I.; MacDonald, K. The evolving landscape of biologics—Biosimilars, biobetters, and bioparallels. JAMA Dermatol. 2025, 161, 355. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, C.; Dattilo, M.; Patitucci, F.; Prete, S.; Scopelliti, G.; Parisi, O.I.; Puoci, F. Exploring protein-based carriers in drug delivery: A review. Pharmaceutics 2024, 16, 1172. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Huerta, I.; Gómez-Verduzco, G.; Téllez-Isaías, G.; Ayora-Talavera, G.; Bañuelos-Hernández, B.; Petrone-García, V.; Velázquez-Juárez, G.; Fernández-Siurob, I. Transformation of Dunaliella salina by Agrobacterium tumefaciens for the expression of the hemagglutinin of avian influenza virus H5. Microorganisms 2022, 10, 361. [Google Scholar] [CrossRef]
- Gandhi, S.; Patankar, D.; Kashiramka, S.; Rathore, A. The economics of translating a biosimilar from lab to market in India. Ann. N. Y. Acad. Sci. 2024, 1541, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Laššánová, M.; Líšková, S.; Tisonová, J.; Fundarkova, S.; Laššán, Š. Biopharmaceuticals safety perception in Slovakia: Considerations and real-life pharmacovigilance data. Bratisl. Med. J. 2021, 122, 443–448. [Google Scholar] [CrossRef]
- Yousefi, N.; Ahmadi, R.; Tayeba, H.; Taheri, S.; Mahboudi, F.; Peiravian, F. Biosimilar medicines in the Iranian market: A way to more affordable medicines. Indian J. Pharm. Sci. 2020, 82, 483–490. [Google Scholar] [CrossRef]
- Rahalkar, H.; Sheppard, A.; López-Morales, C.; Lobo, L.; Salek, S. Challenges faced by the biopharmaceutical industry in the development and marketing authorization of biosimilar medicines in BRICS-TM countries: An exploratory study. Pharm. Med. 2021, 35, 235–251. [Google Scholar] [CrossRef]
- Abe, T.; Sagara, A.; Okada, D.; Matsuzaka, K. Safety survey on infusion reaction and cardiac dysfunction when switching from reference trastuzumab (Herceptin®) to biosimilar trastuzumab (trastuzumab NK) in the treatment of HER2 positive breast cancer. Mol. Clin. Oncol. 2023, 18, 41. [Google Scholar] [CrossRef]
- Nick, C. Streamlining biosimilar development based on 20 years’ experience. Expert Opin. Biol. Ther. 2024, 24, 571–581. [Google Scholar] [CrossRef]
- Raposo, M.C.; Feiteira, C.; Ribeiro, M.H. Regulatory and clinical aspects in biosimilar medicines: Comparability, extrapolation, interchangeability, and safety. Drugs Ther. Perspect. 2025, 41, 111–125. [Google Scholar] [CrossRef]
- Ruiz, A.; Boushehri, M.A.S.; Phan, T.; Carle, S.; Garidel, P.; Buske, J.; Lamprecht, A. Alternative excipients for protein stabilization in protein therapeutics: Overcoming the limitations of polysorbates. Pharmaceutics 2022, 14, 2575. [Google Scholar] [CrossRef]
- Kaur, S.; Yadav, S.; Sahu, V.; Sharma, N.; Shukla, V.K. Biosimilar regulations: Current framework and future prospects. Curr. Drug Saf. 2025, 20. [Google Scholar] [CrossRef]
- Mali, A.; Kuvar, V.; Bharadwaj, S. Bridging the gap: A comparative investigation of pharmaceutical excipient regulations. Ther. Innov. Regul. Sci. 2023, 58, 258–272. [Google Scholar] [CrossRef]
- Kang, H.; Wadhwa, M.; Knežević, I.; Ondari, C.; Simão, M. WHO guidelines on biosimilars: Toward improved access to safe and effective products. Ann. N. Y. Acad. Sci. 2023, 1521, 96–103. [Google Scholar] [CrossRef]
- Myrzagulova, S.; N, Z.A.; Kumar, M.; Kumar, D.; Kumar, A. Foam-based drug delivery systems for skin disorders: A comprehensive review. AAPS PharmSciTech 2025, 26, 102. [Google Scholar] [CrossRef] [PubMed]
- Halawa, M.; ElSayed, R.; Aderibigbe, T.; Newman, P.; Reid, B.; Carabetta, V. Biosimilars targeting pathogens: A comprehensive review of their role in bacterial, fungal, parasitic, and viral infections. Pharmaceutics 2025, 17, 581. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Luong, A.; Doan, A.; Lin, T.; Duong, P.; Villa, M.; Parekh, C. T-cell receptor signaling and dysfunction during blinatumomab therapy in pediatric acute lymphoblastic leukemia. Blood 2024, 144, 1435. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.; Park, C.; Kim, J.; Lee, D. Data-driven prediction models for forecasting multistep ahead profiles of mammalian cell culture toward bioprocess digital twins. Biotechnol. Bioeng. 2023, 120, 2494–2508. [Google Scholar] [CrossRef]
- Menon, N.; Lee, J.; Tang, T.; Lim, C. Microfluidics for morpholomics and spatial omics applications. Lab. Chip 2025, 25, 752–763. [Google Scholar] [CrossRef]
- Machado, F.; Cañás, M.; Doubova, S.; Urtasun, M.; Marín, G.; Osorio-de-Castro, C.; Albuquerque, F.C.; Ribeiro, T.B.; Pont, L.; Landeros, J.C.; et al. Biosimilars approvals by thirteen regulatory authorities: A cross-national comparison. Regul. Toxicol. Pharmacol. 2023, 144, 105485. [Google Scholar] [CrossRef] [PubMed]
- Jarab, A.; Heshmeh, S.; Meslamani, A. Bridging the gap: The future of biosimilars regulations. Hum. Vaccin. Immunother. 2024, 20, 2362450. [Google Scholar] [CrossRef]
- Jha, L. The influence of regulatory changes on market entry strategies for biosimilars. Int. J. Res. Med. Pharm. 2025, 14, 1–9. [Google Scholar] [CrossRef]
- Sathyan, A.; Mohanapriya, M.; Madhanraja, R.; Vadana, K.; Indhuja, K.; Sumathi, P.; Varshini, G. A comprehensive review of evidence and challenges in switching from originator drugs to biosimilars of monoclonal antibodies: Focus on rituximab and trastuzumab. J. Drug Deliv. Ther. 2025, 15, 149–155. [Google Scholar] [CrossRef]
- Katte, J.; McDonald, T.; Sobngwi, E.; Jones, A. The phenotype of type 1 diabetes in sub-Saharan Africa. Front. Public Heal. 2023, 11, 1014626. [Google Scholar] [CrossRef]
- Lixia, F.; Jia, G.; Liu, Z.; Pang, X.; Cui, Y. The applications and advances of artificial intelligence in drug regulation: A global perspective. Acta Pharm. Sin. B 2025, 15, 1–14. [Google Scholar] [CrossRef]
- Palaparthi, E.; Padala, T.; Singamaneni, R.; Manaswini, R.; Kantula, A.; Reddy, P.; Chandini, P.; Eliana, A.S.; Samhita, P.S.; Patnaik, P. Emerging therapeutic strategies for heart failure: A comprehensive review of novel pharmacological and molecular targets. Cureus 2025, 17, e81573. [Google Scholar] [CrossRef] [PubMed]
- Lacosta, T.; Vulto, A.; Huys, I.; Simoens, S. An exploration of biosimilar TNF-alpha inhibitors uptake determinants in hospital environments in Italy, Portugal, and Spain. Front. Med. 2023, 9, 1029040. [Google Scholar] [CrossRef]
- Abitbol, A.; Chu, L. What do the guidelines say about use of biosimilar insulin therapy? Simple practical considerations to guide clinicians in different patient subgroups—Sharing Canadian perspectives. Diabetes Obes. Metab. 2025, 27 (Suppl. S5), 36–44. [Google Scholar] [CrossRef]
- Prabhash, K.; Deshmukh, C.; Malhotra, H.; Sharma, A.; Jain, M.; Dhamne, N.; Nagarakar, R.; Ganesan, P.; Mahobia, V.K.; Das, C.K.; et al. Efficacy and safety of biosimilar cetuximab versus innovator cetuximab in Indian patients with head and neck cancer: A multicenter, randomized, double-blind, phase III trial. JCO Glob. Oncol. 2024, 10, e2400059. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, H.; Li, J.; Du, X.; Li, Z.; Liu, J.; Lv, X.; Li, H.; Guo, Q.; Wang, C.; et al. Clinical benefit, price, and uptake for cancer biosimilars vs reference drugs in China. JAMA Netw. Open 2023, 6, e2337348. [Google Scholar] [CrossRef]
- Sagi, S.; Kalsekar, S.; Kottke, A.; Cohen, H. Long-Term Real-World Post-Approval Safety Data of Multiple Biosimilars from One Marketing-Authorization Holder after More Than 18 Years since Their First Biosimilar Launch. Drug Saf. 2023, 46, 1391–1404. [Google Scholar] [CrossRef]
- Kirsch-Stefan, N.; Guillén, E.; Ekman, N.; Barry, S.; Knippel, V.; Killalea, S.; Wolff-Holz, E. Do the Outcomes of Clinical Efficacy Trials Matter in Regulatory Decision-Making for Biosimilars? Biodrugs 2023, 37, 855–871. [Google Scholar] [CrossRef] [PubMed]
- Heise, T.; DeVries, J. Biosimilar Insulins: Narrative Review of the Regulatory Framework and Registration Studies. Diabetes Obes. Metab. 2025, 27, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.; Turner, M.; McCabe, D.; Woollett, G. Future Evolution of Biosimilar Development by Application of Current Science and Available Evidence: The Developer’s Perspective. Biodrugs 2023, 37, 583–593. [Google Scholar] [CrossRef] [PubMed]
| Criteria | Inclusion | Exclusion |
|---|---|---|
| Publication Date | 2020–2025 | Pre-2020 |
| Document Type | Peer-reviewed articles, patents, regulatory approvals | Abstracts, editorials, non-peer-reviewed |
| Language | English, Spanish | Other languages |
| Scope | Buffer-free formulations, recombinant proteins | Non-recombinant proteins, irrelevant topics |
| Technical Focus | Formulation strategies, safety, regulatory, patents | Unrelated clinical studies, basic research |
| Phase | Activity Description | Resources/Databases | Outcome/Deliverable |
|---|---|---|---|
| Protocol Definition | Establish research objectives focusing on buffer-free formulations, regulatory guidelines (FDA, EMA), safety profiles, and IP | Research proposal, previous studies | Defined research questions, scope, and specific objectives |
| Document Search | Systematic search across scientific, patent, and regulatory databases from 2020 to 2025 | PubMed, Scopus, Web of Science, USPTO, EPO, Derwent, FDA, EMA | Comprehensive list of relevant publications and documents |
| Critical Appraisal | Apply inclusion/exclusion criteria to evaluate the quality and relevance of selected documents | Inclusion/Exclusion Criteria Table, Assessment matrices | high-quality curated documents (~150 documents) |
| Content Synthesis | Extract and synthesize key information structured by themes (technological innovation, formulation strategies, regulatory, safety) | Selected documents, extraction forms | Structured tables, thematic descriptive summaries |
| Analytical Interpretation | Perform comparative analysis of buffered vs. buffer-free formulations, technological platforms, excipients, regulatory trends | Comparative analysis tools, thematic summaries | Comparison of analytical tables, identification of key trends |
| Report Generation | Draft manuscript including introduction, detailed methodology, results, discussion, and conclusions | Analysis results, comparative tables, thematic summaries | Completed manuscript prepared for peer-review submission |
| Thematic Area | Subcategory | Description | Documents (n) |
|---|---|---|---|
| Technological Innovation | Protein engineering platforms | Fc fusion, PASylation, XTENylation, PEGylation | 28 |
| Analytical/formulation techniques | DSC, Native MS, stability assessments | 25 | |
| Bioprocessing/purification | Advanced bioprocessing methods | 19 | |
| Innovative delivery strategies | Novel formulation/delivery approaches | 13 | |
| Regulatory Framework | FDA guidelines/approvals | Regulatory submissions and guidance | 22 |
| EMA regulatory perspectives | EMA regulations and evaluation processes | 18 | |
| WHO/ICH harmonization | International Council for Harmonization (ICH) and global harmonization efforts | 12 | |
| Immunogenicity and Safety | Excipient safety/classification | Safety profiles and classifications | 23 |
| Immunogenicity mitigation | Mechanisms and strategies | 21 | |
| Buffer-free tolerability | Safety/tolerability evaluations | 20 | |
| Intellectual Property Challenges | Patent database analyses | USPTO, EPO, Derwent analysis | 45 |
| Patent expiration issues | Transparency, replication challenges | 32 | |
| Biosimilar IP case studies | Disputes and legal examples | 28 |
| Platform | Mechanism | Advantages | Examples | Synergy with Buffer-Free Formulations | Reference |
|---|---|---|---|---|---|
| Fc-Fusion | Fusion with the IgG Fc domain enables FcRn recycling | Extended half-life, enhanced stability, reduced dosing | Etanercept, Aflibercept | It provides intrinsic ionizable residues (His, Asp, Glu) that act as buffer groups at high concentrations (>50–100 mg/mL), stabilizing the pH without the need for exogenous buffer salts. | [149,150] |
| Albumin Fusion/Binding | Fusion or binding to albumin extends the half-life via FcRn | Prolonged half-life, reduced renal clearance | Albiglutide, Idelvion | Albumin has ionizable groups capable of buffering pH changes, reducing the dependence on buffers to control solubility and aggregation in concentrated formulations. | [151,152] |
| XTEN Technology | Fusion with synthetic unstructured polypeptide (XTEN) | Customizable half-life, low immunogenicity | VRS-859, Somavaratan | The high density of polar residues acts as a protein buffer, absorbing or releasing protons to maintain stable pH in the absence of conventional buffers. | [153,154] |
| PASylation | Fusion with Pro-Ala-Ser (PAS) repeats to increase hydrodynamic radius | Improved half-life, biodegradable and non-immunogenic | PASylated IFN-α, PASylated Factor VIII | PAS bands increase the intrinsic buffering capacity as a result of the presence of carboxyl and amino groups, stabilizing pH, and reducing viscosity without the need for buffering agents. | [155] |
| PEGylation | Chemical conjugation with polyethylene glycol (PEG) | Increased half-life, reduced immunogenicity | Pegfilgrastim, Certolizumab pegol | The hydrophilic, non-ionic mantle of PEG limits ionic interactions and CO2 uptake, reducing pH-dependent degradation pathways and the need for buffers to control the protein’s environment. | [67,156,157] |
| Glycoengineering | Engineering glycosylation patterns to optimize PK/PD | Enhanced PK/PD, modulated effector function | Darbepoetin alfa, Obinutuzumab | Glycans modify the surface charge distribution, providing local cushioning and creating a protective microenvironment that reduces aggregation and maintains pH without traditional buffers. | [158,159] |
| Platform | Mechanism | Examples |
|---|---|---|
| PEGylation | Covalent attachment of polyethylene glycol (PEG) chains to proteins to reduce renal clearance and immunogenicity. | Pegfilgrastim, Peginterferon alfa |
| Fc Fusion Proteins | Fusion of therapeutic protein with the Fc domain of IgG to utilize the FcRn recycling pathway and prolong the half-life. | Etanercept, Epoetin alfa-Fc (darbepoetin-alfa, Aranesp) |
| Albumin Fusion Proteins | Fusion with albumin or albumin-binding domains for extended half-life via albumin recycling. | Albiglutide, Tanzeum |
| Glycoengineering | Modification of glycosylation patterns to enhance serum half-life, reduce immunogenicity, or alter receptor binding. | Obinutuzumab (glycoengineered antibody), Darbepoetin alfa |
| Amino Acid Mutagenesis | Site-specific mutations to increase stability, reduce degradation, or improve receptor interaction. | Insulin analogs (glargine, degludec), Modified interferons |
| XTENylation | Genetic fusion to XTEN polypeptides, and unstructured hydrophilic sequences, to increase the hydrodynamic radius and half-life. | VRS-317 (insulin-like growth factor fusion) |
| PASylation | Fusion to Proline, Alanine, and serine (PAS) sequences to mimic PEG-like effects without PEG-related toxicity. | PASylated growth hormone analogs |
| Multimerization | Generation of multimeric proteins (eg tandem scFvs, bispecific antibodies) to improve avidity, selectivity, and PK properties. | Blinatumomab (BiTEs) |
| Platform | Mechanism | Examples |
|---|---|---|
| Buffer-free formulations | Traditional buffers to reduce aggregation and improve stability. | Newer botulinum toxin and monoclonal antibody formulations |
| Lyophilized or spray-dried formulations | Improve stability and allow alternative routes of administration. | Inhaled insulin (Afrezza), freeze-dried vaccines |
| Stabilizing excipients (e.g., trehalose, polysorbates, sugars) | Prevent aggregation, maintain conformational stability. | Monoclonal antibody formulations |
| High-concentration formulations (HCF) | For subcutaneous delivery of large protein doses. | Subcutaneous immunoglobulin therapies |
| Microenvironment modulating formulations | pH-shifting and osmolarity control for local absorption. | SubQ monoclonal antibodies |
| Platform | Mechanism | Examples |
|---|---|---|
| Encapsulation technologies (microspheres, nanoparticles) | Slow release or targeted delivery of proteins. | Lupron Depot (leuprolide) acetate microspheres) |
| Hydrogel-based delivery systems | Controlled release over time. | Injectable depot formulations |
| Lipid nanoparticles (LNPs) | Deliver mRNA coding for therapeutic proteins (indirect protein delivery). | mRNA-based therapeutics (e.g., Moderna’s mRNA-encoded antibodies in development) |
| Transdermal delivery systems | Protein administration via skin with micro-needles or patches. | Still mostly experimental for proteins |
| Platform | Mechanism | Examples |
|---|---|---|
| Tolerization protocols | Co-administration of immune modulators to reduce anti-drug antibodies (ADAs). | Treg-inducing co-therapies |
| De-immunization by epitope removal | Removal or alteration of T-cell epitopes in protein sequences. | De-immunized coagulation factors |
| Humanization and fully human antibodies | Reduces immunogenicity by using human sequences. | Adalimumab, Pembrolizumab |
| Nanobody and VHH platforms | Use of single-domain antibodies with distinct PK/PD profiles. | Caplacizumab (Cablivi), ALX-0171 (anti-RSV nanobody) |
| Platform | Mechanism | Examples |
|---|---|---|
| Gene editing-based delivery (CRISPR/AAV) | Direct in vivo production of therapeutic proteins through gene therapy. | Hemophilia gene therapies |
| Cell-based delivery (CAR-T, engineered T cells) | Live cells are engineered to secrete or display therapeutic proteins. | CAR-T therapies, engineered MSCs |
| Self-amplifying RNA platforms | Replication of RNA inside cells to express proteins over prolonged periods. | Preclinical vaccine work |
| Artificial protein scaffolds (DARPins, afilibodies) | Non-antibody protein scaffolds engineered for high-affinity binding and novel PK properties. | Abicipar pegol (anti-VEGF) DARPin) |
| Platform | Mechanism | Impact on PK/PD |
|---|---|---|
| PEGylation | Covalent PEG attachment | ↑ Half-life, ↓ renal clearance, ↓ immunogenicity |
| Glycoengineering | Modify glycan patterns (branching, sialylation, M6P) | ↑ Stability, ↓ clearance (ASGPR), ↑ ADCC/CDC |
| GlycoPEGylation | Site-selective PEGylation via glycans | Combines PEG benefits with controlled glycosylation |
| Fc-fusion/Albumin-fusion | Genetic fusion to Fc or albumin | ↑ half-life through IgG recycling or albumin pathway |
| XTEN/PASylation | Fusion to hydrophilic polypeptides (XTEN/PAS) | PEG-like sterics without PEG safety issues |
| Protein scaffolds (DARPins, etc.) | Engineered non-Ig scaffolds | Tailorable binding, novel PK/PD |
| Nanoparticle encapsulation | Protein-loaded microspheres, hydrogels, LNPs | Controlled release, targeted delivery |
| Immunological editing | Epitope removal, humanization, and tolerization strategies | ↓ ADA, ↑ efficacy |
| Category | Description |
|---|---|
| Buffers and pH Control | Phosphate, histidine, citrate and acetate buffers with molarities of 0.01 to 0.10 M are the most common and provide robust pH control in the range of 4.8 to 8.0. |
| Stabilizers | Sugars/Polyols (sucrose, trehalose, glycerol) to protect against freeze–thaw and dehydration stress. |
| Surfactants | Polysorbate 20/80 to prevent interfacial aggregation. |
| Amino acids | Arginine and glycine were used to improve solubility and inhibit aggregation. |
| Presentation Formats | Liquid: Immediate use is usually refrigerated. Lyophilized: Reconstituted prior to administration; allows for more extreme pH/excipient options. |
| Advantage | Description |
|---|---|
| Simplicity | Fewer excipients in the formulation simplifies development and eliminates potential incompatibilities. This aligns with a minimalist formulation philosophy that uses only what is necessary to stabilize the protein. |
| Reduced Injection Site Pain | Eliminating acidic solutions such as citrate can significantly reduce local pain after subcutaneous injection. Patients often tolerate injections better when foreign ionic ingredients are minimized. |
| Improved Stability in Some Cases | Eliminating a buffer can prevent certain instability issues. For example, buffer salts can sometimes promote protein aggregation or opalescence; one study found that citrate-buffered mAb solutions exhibited higher levels of opalescence and aggregation than unbuffered solutions. Buffer-free formulations also avoid pH disturbances caused by crystallization or buffer degradation (e.g., phosphate precipitation during freezing). |
| Lower Risk of Excipient Interactions | Without multivalent anions or other buffer components, there is a lower risk of interactions with diluents or leachable from the packaging. For intravenous drugs, a self-buffering concentrate can be diluted in saline without the risk of buffer dilution altering the pH or causing precipitation. |
| Regulatory Flexibility | The use of fewer excipients means fewer variables to control in the manufacturing process and, potentially, fewer regulatory concerns about the purity or origin of the excipients. A buffer-free formulation could simplify regulatory review, as all components (except the protein) are inert stabilizers. |
| Excipient Type | Description/Safety Profile |
|---|---|
| Amino Acids (e.g., histidine, arginine, glycine, aspartic acid, proline) | Natural amino acids are generally safe for injectable use. Histidine and arginine are widely used in approved biologics (~82% of high potency mAb formulations). Typically, non-toxic at 1–50 mM. Glycine serves as a stabilizer/tonic in immunoglobulin products. Arginine in high concentrations may have osmotic effects orally but is well tolerated in moderate injectable doses. Recognized as safe by FDA/EMA. |
| Sugars and Polyols (e.g., sucrose, trehalose, sorbitol, mannitol, glycerol) | Serve as stabilizers (especially in lyophilized or liquid forms to prevent aggregation) and tonicity adjusters. The antiviral agent has been well established as safe for parenteral use. Trehalose and sucrose protect against freeze/thaw or heat stress. Sorbitol and mannitol ensure isotonicity (~5% w/v) and may stabilize by preferential exclusion. Sorbitol in SC mAbs is well tolerated. Mannitol may crystallize when frozen; amorphous stabilizers are preferred in such cases. All are FDA-listed for injectables. |
| Salts (e.g., NaCl) | NaCl is commonly added for isotonicity, widely considered safe in small doses (e.g., 9 mg per 1 mL SC injection). Some buffer-free products such as Humira (where mannitol is used) are omitted. Acetate or succinate is used as buffers and not in strict buffer-free formulations. The salts used in biologics are chosen from food/pharma-grade sources. |
| Surfactants (e.g., polysorbate 80, polysorbate 20, poloxamer 188) | Prevent protein adsorption and interface-induced aggregation. PS-80 appears in >90% of antibody products. Safe at ~0.01–0.1%, although rare hypersensitivity reactions have been noted. Can degrade into particles or peroxides. PS-20 is used in interferon-beta, poloxamer 188 in Hemlibra and Enspryng. These surfactants are pharmacopeia-listed and approved for injectables. |
| Chelators and Antioxidants (e.g., EDTA, methionine) | Chelators like EDTA (used in ppm levels) bind to trace metals, methionine acts as a sacrificial antioxidant. Rare in minimalist buffer-free designs, but used in specific cases like Cyltezo®. EDTA is safe in small quantities (0.1 mg/mL) and used in pediatric vaccines. Methionine is a benign amino acid at ~1–5 mM. Considered GRAS and accepted when justified. |
| Feature | Description |
|---|---|
| Protein Self-Buffering | Studies demonstrate that intrinsic titratable groups of antibodies can maintain pH within a therapeutic window, even under stress conditions. |
| Reduced Aggregation and Opalescence | Buffer-free preparations show significantly less aggregation and opalescence compared to their phosphate-buffered counterparts during the shaking and freeze–thaw cycles. |
| High-concentration Formats | The transition to formulations of ≥100 mg/mL (essential for subcutaneous administration) has been facilitated by eliminating high-ionic-strength buffers, which can promote viscosity and protein–protein interactions. |
| Patient Comfort | Citrate buffers are known to cause injection site pain; Replacement with self-buffering proteins or minimal excipients has significantly reduced discomfort. |
| Aspect | Traditional Buffered Formulations | Buffer-Free (Self-Buffering) Formulations | Practical Implications |
|---|---|---|---|
| pH control | The external buffer controls pH within its effective range (e.g., phosphate, citrate, histidine). | Initial pH is set during manufacturing; intrinsic ionizable residues provide limited extrinsic buffering. | Buffers are reliable across concentrations, and self-buffering works best at high protein concentrations or for inherently stable proteins. |
| Stability | Excellent when pH is optimized; some buffers can cause issues (e.g., phosphate crystallization on freeze/thaw; citrate interactions). | Equal or better for high-concentration proteins; often less aggregation/opalescence compared to buffered counterparts. | Choose buffer-free to reduce the aggregation pathways linked to buffer salts; monitor storage tightly to avoid pH drift. |
| Injection tolerability (SC) | Depending on the type/level of buffer; acidic citrate may sting and increase pain. | Often superior; removal of citrate reduces injection pain and enables smaller volumes at high concentration. | Patient-centric advantage for SC biologics; “citrate-free” positioning supports comfort claims. |
| Immunogenicity | Driven more by aggregates/impurities than by “buffer vs. no buffer”; good control can reduce risk. | Comparable to buffered; reduced aggregation may further reduce anti-drug antibody risk; no regulator-attributed issues to absence of buffers to date. | Focus analytical control on aggregate minimization in either approach. |
| Formulation complexity | More components (buffer + stabilizers/surfactants/salts) increase interaction space but leverage mature platform know-how. | Fewer excipients simplify supply chain/QC; may require deeper protein-focused stability work. | Buffer-free can streamline CMC and messaging; buffered can speed development via established platforms. |
| Typical use cases | IV products at low–moderate concentration; lyophilized products; proteins with narrow pH stability windows. | High-concentration SC biologics (≥100 mg/mL); IV concentrates intended for immediate dilution; excipient-minimizing designs. | Match approach to the route, concentration, and pH tolerance of API. |
| Representative buffer-free products (regulatory status) | Adalimumab biosimilars: Cyltezo® (adalimumab-adbm), US 2017; interchangeable 2021; high-conc. citrate-free 2024; EU 2017. Hadlima® (adalimumab-bwwd), US 2019 (50 mg/mL), US 2022 (100 mg/mL citrate-free), EU 2017 (Imraldi®). Yuflyma® (adalimumab-aaty), EU 2021 (first citrate-free high-conc. in the EU); US 2023. Simlandi™ (adalimumab-ryvk), US 2024; high-conc., citrate-free, interchangeable. Insulins/GH: Semglee® (insulin glargine-yfgn), unbuffered acid (pH ≈ 4); US 2021 (first biosimilar interchangeable insulin); EU 2018. Omnitrope® (somatropin), lyophilized; minimal/no traditional buffer salts in powder (pH set on reconstitution); EU 2006. | The Buffer-free approach has already been approved in major markets and therapy areas; examples support comfort and simplicity narratives for SC use while maintaining regulatory acceptance. |
| Product (Brand) | International Non-Property Name (INN) | FDA Status (BLA #; Approval Date) | EMA Status (EPAR #; Authorization Date) | Year |
|---|---|---|---|---|
| Emrelis | Telisotuzumab Vedotin | BLA 761384; 14 May 2025 | Not yet centrally authorized (MAA submitted Q2 2025) | 2025 |
| Andembry | Garadacimab | BLA 761367; 19 June 2025 | EPAR XXXX; authorized February 2025 | 2025 |
| Enflonsia | Clesrovimab | BLA 761395; 20 June 2025 | MAA under evaluation (no EPAR yet) | 2025 |
| Encelto | Revakinagene Tarot | BLA 761402; 21 March 2025 | EPAR 1054; authorized March 2025 | 2025 |
| Alhemo | Concizumab | BLA 761315 s000; 20 December 2024 | EU/1/24/1881; authorized 13 December 2024 | 2024 |
| Anktiva | Nogapendekin Alfa Inbakicept-pmln | BLA 761336; 22 April 2024 | MAA accepted 27 January 2025 (under review) | 2024 |
| Ebglyss | Lebrikizumab | BLA 761306; 13 September 2024 | EPAR 0765; authorized 21 November 2023 | 2024 |
| Hympavzi | Marstacimab | BLA 761283; 11 October 2024 (label) | EPAR 0976; authorized December 2024 | 2024 |
| Imdelltra | Tarlatamab | BLA 761259; 16 May 2024 | EPAR 0893; authorized July 2024 | 2024 |
| Kisunla | Donanemab | BLA 761227; 2 July 2024 | EPAR 0850; authorized April 2024 | 2024 |
| Winrevair | Sotatercept | BLA 761201; 26 March 2024 | EPAR 0802; authorized March 2024 | 2024 |
| Omvoh | Mirikizumab | BLA 761287; 31 October 2023 | EPAR decision; authorized 8 June 2023 | 2023 |
| Udenyca On-Body | Pegfilgrastim-cbqv | BLA 761161; 22 December 2023 | EPAR 0684; authorized December 2023 | 2023 |
| Darzalex Faspro | Daratumumab + Hyaluronidase-fihj | BLA 761174; 27 January 2023 | EPAR 0642; authorized January 2023 | 2023 |
| Opdualag | Nivolumab + Relatlimab | BLA 761150; 18 March 2023 | EPAR 0453; authorized 15 September 2022 | 2023 |
| Evkeeza | Evinacumab | BLA 761168; 17 February 2023 | EPAR 0560; authorized February 2023 | 2023 |
| Omvoh | Mirikizumab-mrkz | BLA 761279; 26 October 2023 | EPAR; authorized 8 June 2023 | 2023 |
| Izervay | Avacincaptad pegol | NDA 217225; 4 August 2023 | Not central EPAR yet (MAA under evaluation) | 2023 |
| Udenyca OnBody | Pegfilgrastim-cbqv | BLA 761039 s 015; 26 December 2023 | EPAR 0684; authorized 17 June 2021 | 2023 |
| Opdualag | Nivolumab + Relatlimab | BLA 761150; 18 March 2023 | EPAR 0453; authorized 16 September 2022 | 2023 |
| Vabysmo | Faricimab-svoa | BLA 761235; 28 January 2022 | CHMP positive opinion 21 July 2022; EU conditional authorisation 28 September 2022 | 2022 |
| Beyfortus | Nirsevimab-alip | STN 125730; 17 July 2023 | Conditional authorisation EU (CHMP 24 Mar 2022; EC 25 March 2022) | 2023 |
| Spevigo | Spesolimab | BLA 761244; 2 September 2022 | CHMP positive opinion 12 October 2022; EU conditional authorisation 12 December 2022 | 2022 |
| Saphnelo | Anifrolumab | BLA 761056; 16 July 2021 | EPAR 0742; authorized 27 January 2022 | 2021 |
| Lupkynis | Voclosporin | BLA 761048; 22 January 2021 | EPAR 0739; authorized 24 February 2021 | 2021 |
| Tezepelumab-ekko | Tezspire | FDA approved 17 December 2021 | CHMP positive opinion 21 July 2022; authorized EU 21 September 2022 | 2021 |
| Efgartigimod alfa-fcab | Vyvgart | BLA 761195; FDA approved 17 December 2021 | MAA submitted Q1 2022 (under evaluation) | 2021 |
| Ultomiris | Ravulizumab-cwvz | BLA 761205; 21 December 2021 | EPAR 0567; authorized 14 January 2022 | 2021 |
| Teprotumumab-trbw | Tepezza | BLA 761143; FDA approved 21 January 2020 | MAA submitted Q2 2024 (under evaluation) | 2020 |
| Empaveli | Pegcetacoplan | BLA 761202; 14 May 2021 | EPAR 0578; authorized 24 July 2021 | 2021 |
| Evusheld | Tixagevimab + Cilgavimab | USA (not full BLA)—Authorized for PrEP on 8 December 2021 | EPAR: pre-exposure clearance 25 March 2022 | 2021 |
| Blenrep | Belantamab mafodotin-blmf | Accelerated approval BLA 761052; 5 August 2020 | EPAR 0618; authorized 21 July 2020 | 2020 |
| Vyepti | Eptinezumab-jjmr | BLA 761017; 21 February 2020 | EPAR 0620; authorized 1 April 2021 | 2020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bas, T.G. Formulation of Recombinant Therapeutic Proteins: Technological Innovation, Regulations, and Evolution Towards Buffer-Free Formulations. Pharmaceutics 2025, 17, 1183. https://doi.org/10.3390/pharmaceutics17091183
Bas TG. Formulation of Recombinant Therapeutic Proteins: Technological Innovation, Regulations, and Evolution Towards Buffer-Free Formulations. Pharmaceutics. 2025; 17(9):1183. https://doi.org/10.3390/pharmaceutics17091183
Chicago/Turabian StyleBas, Tomas Gabriel. 2025. "Formulation of Recombinant Therapeutic Proteins: Technological Innovation, Regulations, and Evolution Towards Buffer-Free Formulations" Pharmaceutics 17, no. 9: 1183. https://doi.org/10.3390/pharmaceutics17091183
APA StyleBas, T. G. (2025). Formulation of Recombinant Therapeutic Proteins: Technological Innovation, Regulations, and Evolution Towards Buffer-Free Formulations. Pharmaceutics, 17(9), 1183. https://doi.org/10.3390/pharmaceutics17091183








