Nanoencapsulation of Biotics: Feasibility to Enhance Stability and Delivery for Improved Gut Health
Abstract
1. Introduction
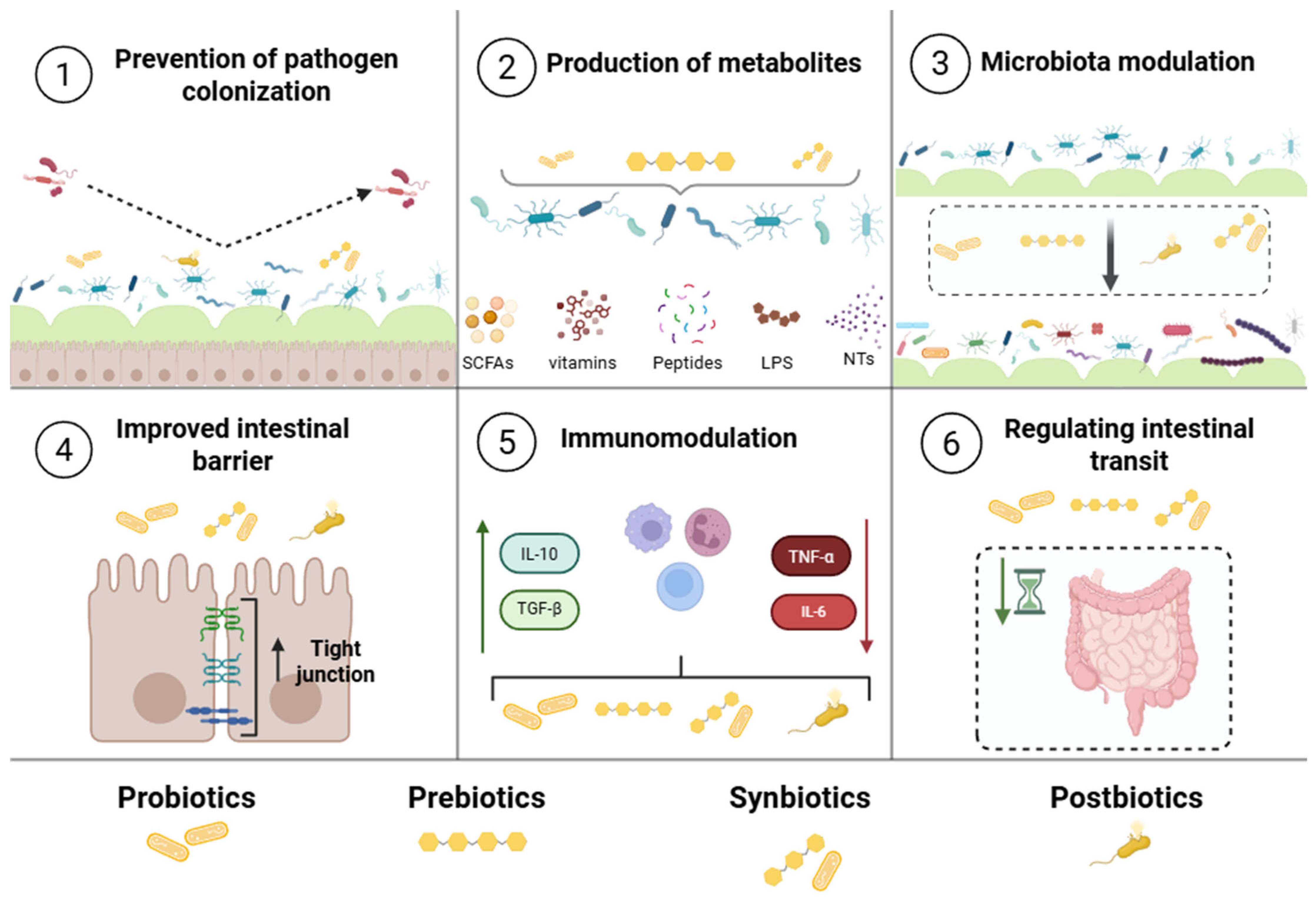
2. Biotics: Main Characteristics and Bioactivity
3. Nanoencapsulation
4. Nanoencapsulation of Biotics
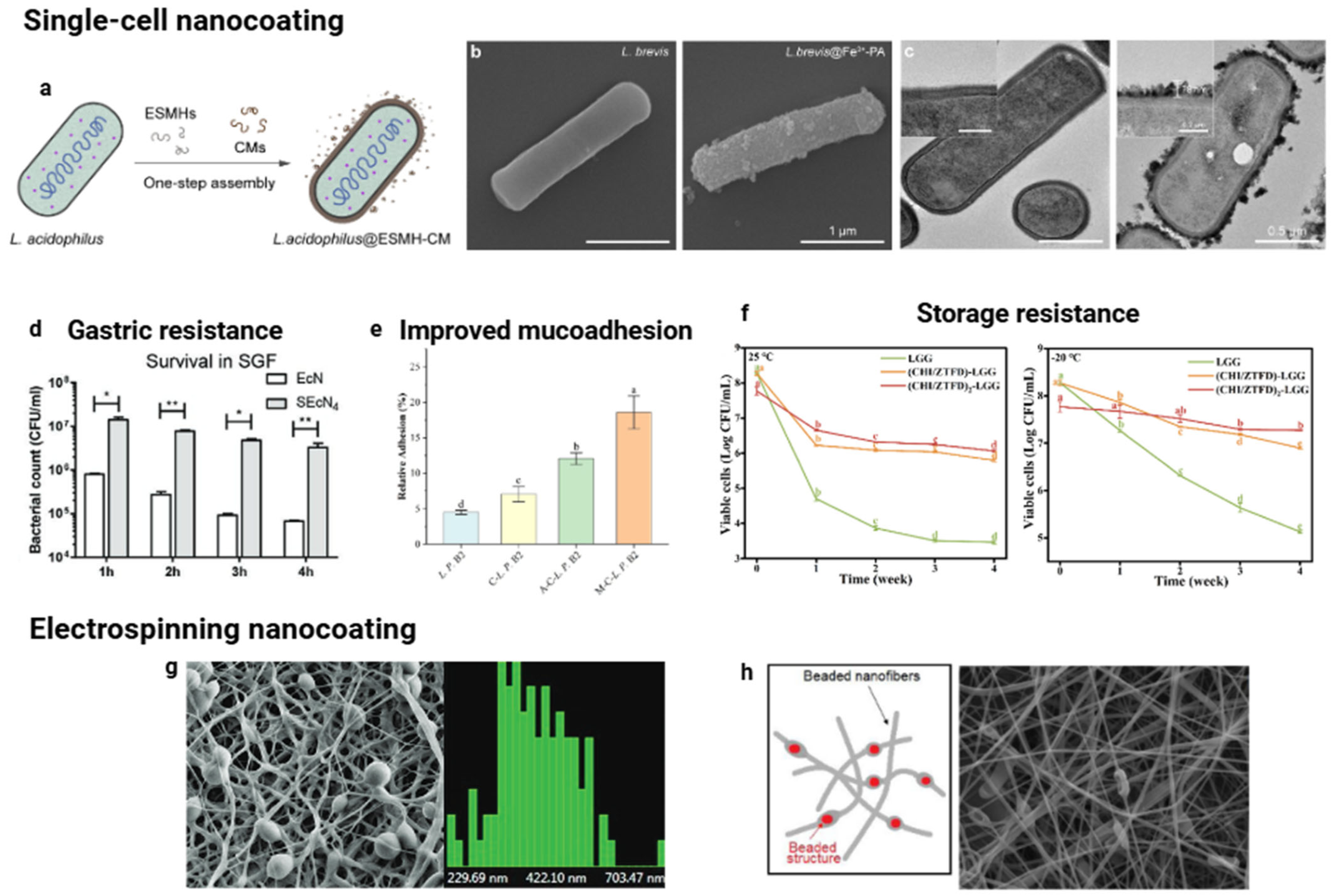
4.1. Single-Cell Nanocoating of Probiotics
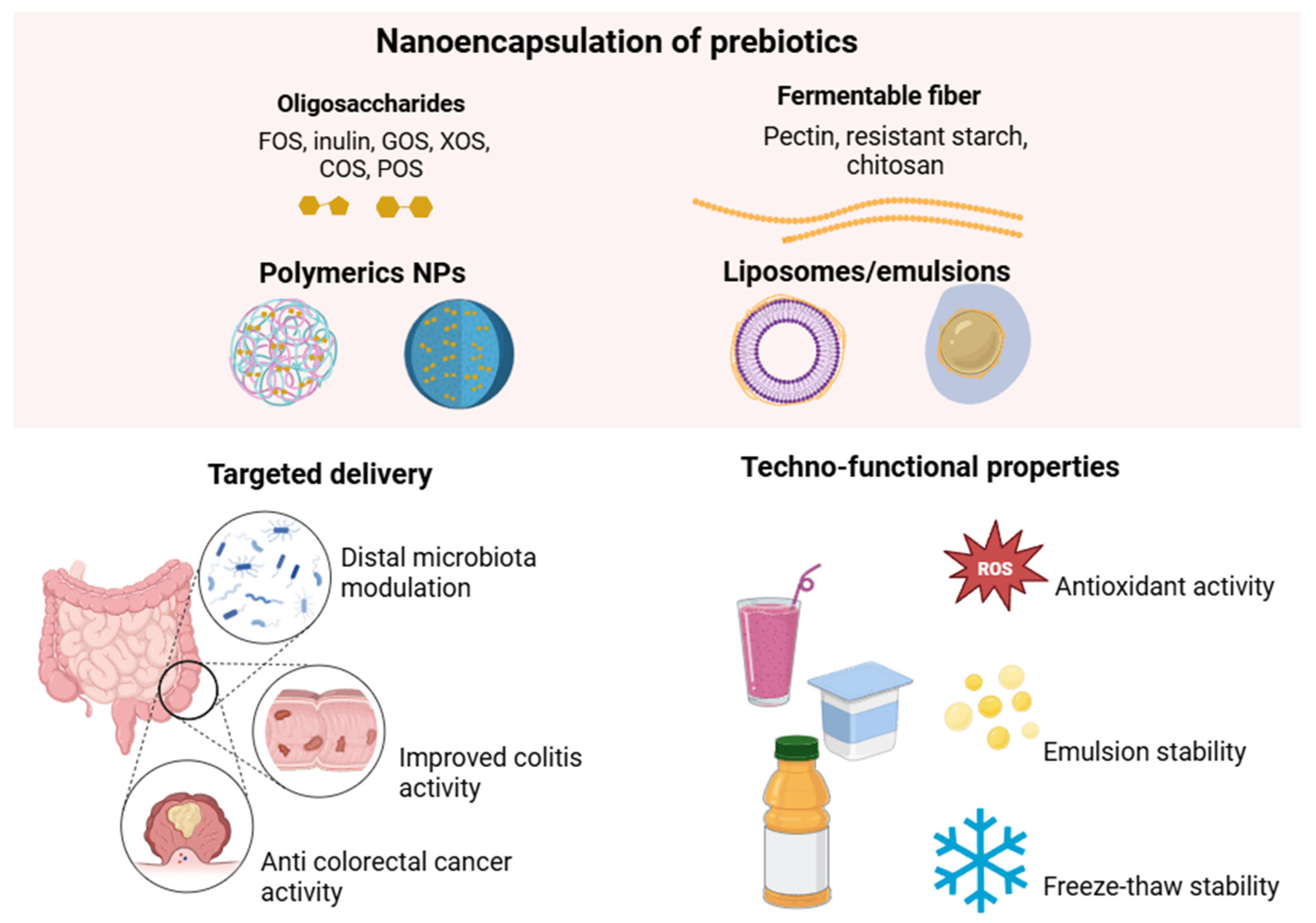
4.2. Nanoencapsulation of Prebiotics
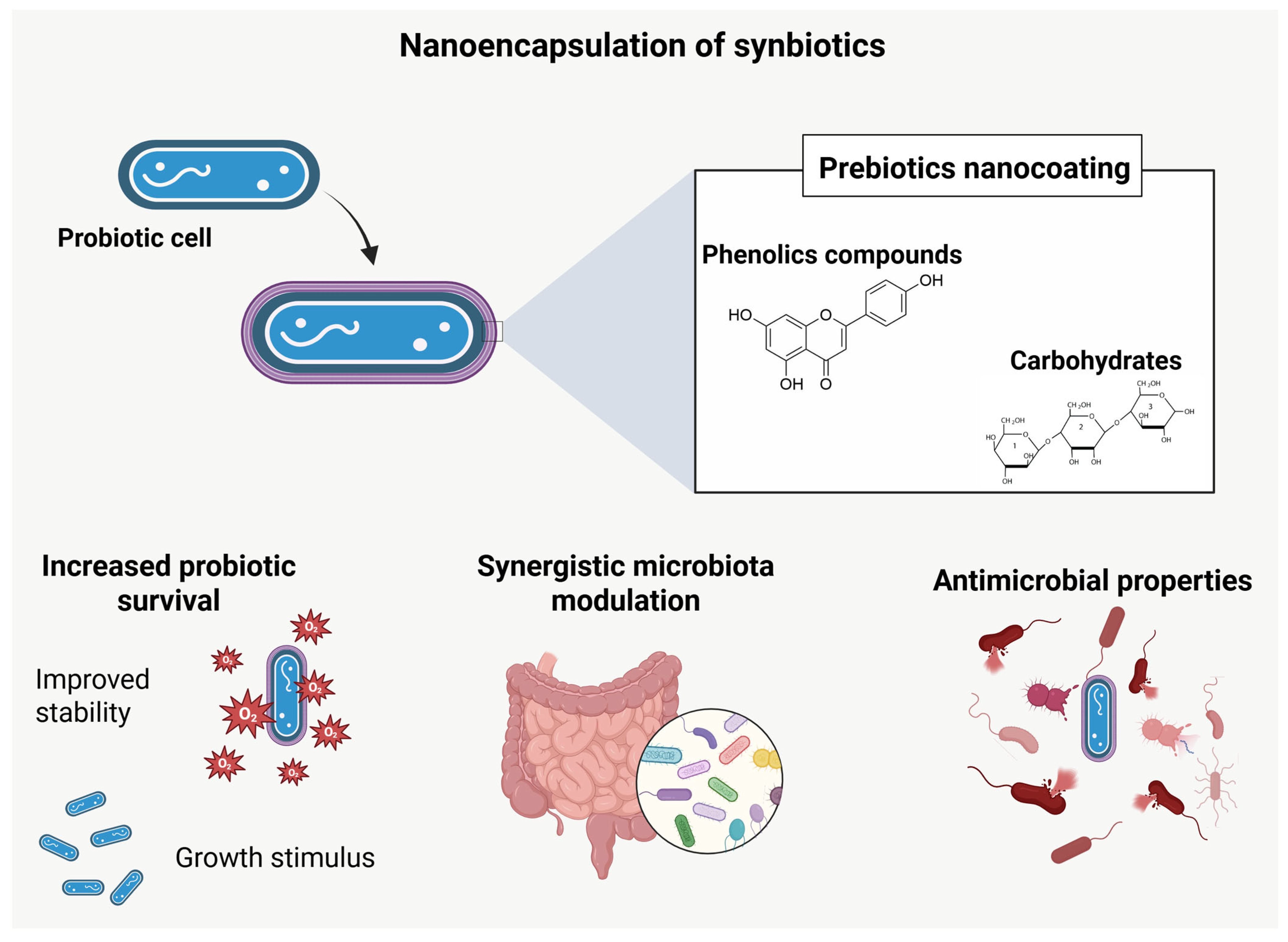
4.3. Nanoencapsulation of Synbiotics
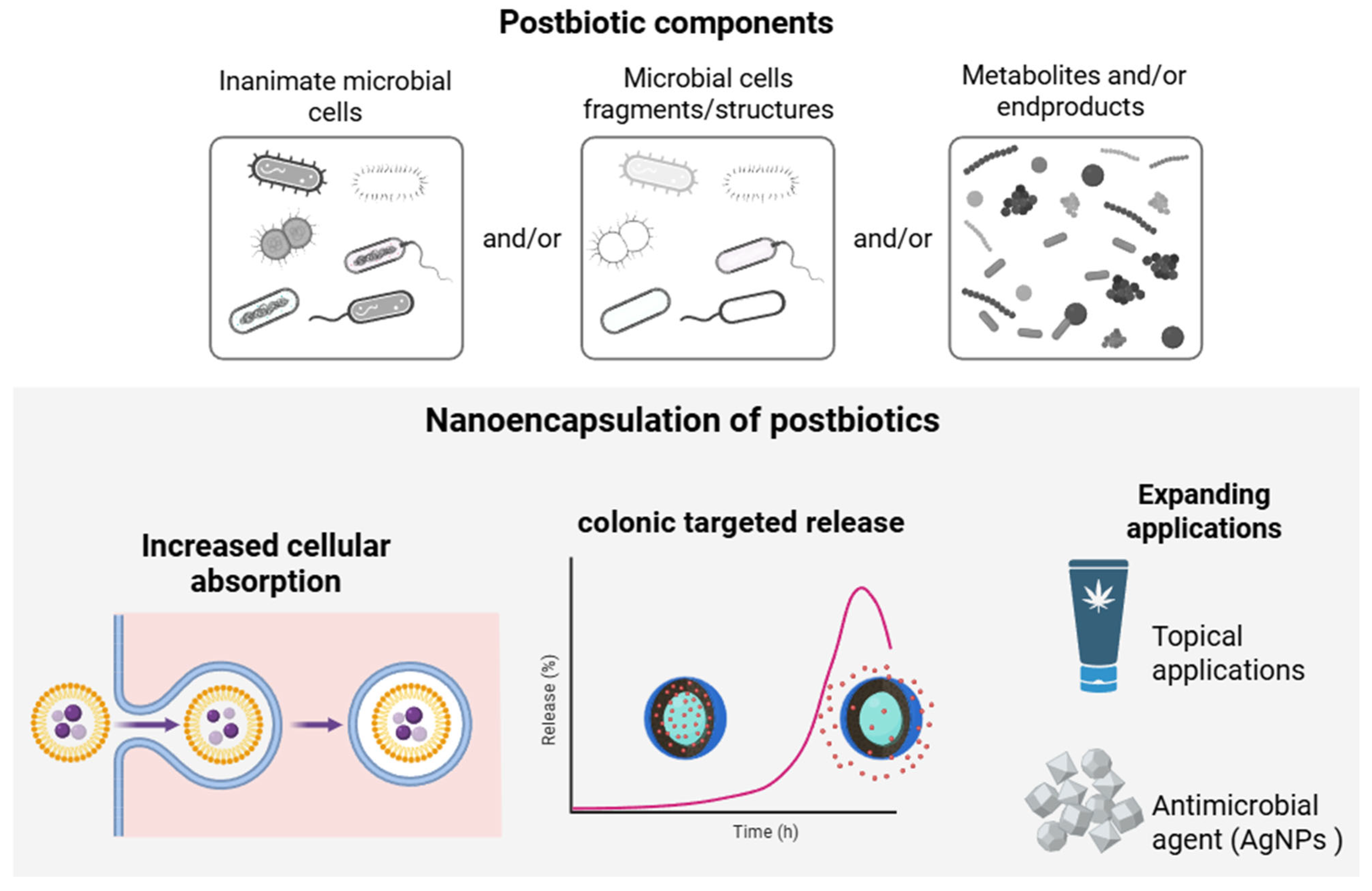
4.4. Nanoencapsulation of Postbiotics
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut Microbiome and Health: Mechanistic Insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- López-Otín, C.; Kroemer, G. Hallmarks of Health. Cell 2021, 184, 33–63. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut Microbiota in Human Metabolic Health and Disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Van Hul, M.; Cani, P.D.; Petitfils, C.; De Vos, W.M.; Tilg, H.; El-Omar, E.M. What Defines a Healthy Gut Microbiome? Gut 2024, 73, 1893–1908. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The Healthy Human Microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Bäckhed, F.; Landmesser, U.; Hazen, S.L. Intestinal Microbiota in Cardiovascular Health and Disease. J. Am. Coll. Cardiol. 2019, 73, 2089–2105. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of Aging: An Expanding Universe. Cell 2023, 183, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.; Round, J.L. Defining Dysbiosis and Its Influence on Host Immunity and Disease. Cell Microbiol. 2014, 16, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Valentina, C.; Fabio, P. Gut Microbiota, Dysbiosis and Colon Lavage. Dig. Liver Dis. 2019, 51, 1209–1213. [Google Scholar] [CrossRef] [PubMed]
- Haneishi, Y.; Furuya, Y.; Hasegawa, M.; Picarelli, A.; Rossi, M.; Miyamoto, J. Inflammatory Bowel Diseases and Gut Microbiota. Int. J. Mol. Sci. 2023, 24, 3817. [Google Scholar] [CrossRef]
- Cani, P.D.; Van Hul, M. Gut Microbiota in Overweight and Obesity: Crosstalk with Adipose Tissue. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 164–183. [Google Scholar] [CrossRef]
- Brown, J.A.; Bashir, H.; Zeng, M.Y. Lifelong Partners: Gut Microbiota-Immune Cell Interactions from Infancy to Old Age. Mucosal Immunol. 2025, 18, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, B.; Zheng, Q.; Li, H.; Meng, X.; Zhou, F.; Zhang, L. A Review of Gut Microbiota-Derived Metabolites in Tumor Progression and Cancer Therapy. Adv. Sci. 2023, 10, e2207366. [Google Scholar] [CrossRef]
- Altamura, F.; Maurice, C.F.; Castagner, B. Drugging the Gut Microbiota: Toward Rational Modulation of Bacterial Composition in the Gut. Curr. Opin. Chem. Biol. 2020, 56, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Hill, C. The Microbiome: An Actor or Stage for the Beneficial Action of Probiotics, Prebiotics, Synbiotics, and Postbiotics? Cell Host Microbe 2025, 33, 777–789. [Google Scholar] [CrossRef]
- Xu, C.; Ban, Q.; Wang, W.; Hou, J.; Jiang, Z. Novel Nano-Encapsulated Probiotic Agents: Encapsulate Materials, Delivery, and Encapsulation Systems. J. Control Release 2022, 349, 184–205. [Google Scholar] [CrossRef]
- Arratia-Quijada, J.; Nuño, K.; Ruíz-Santoyo, V.; Andrade-Espinoza, B.A. Nano-Encapsulation of Probiotics: Need and Critical Considerations to Design New Non-Dairy Probiotic Products. J. Funct. Foods. 2024, 116, 106192. [Google Scholar] [CrossRef]
- Cui, J.; Zhao, C.; Zhao, S.; Tian, G.; Wang, F.; Li, C.; Wang, F.; Zheng, J. Alkali + Cellulase-Extracted Citrus Pectins Exhibit Compact Conformation and Good Fermentation Properties. Food Hydrocoll. 2020, 108, 106079. [Google Scholar] [CrossRef]
- Yin, Y.; Li, Z.; Gao, H.; Zhou, D.; Zhu, Z.; Tao, L.; Guan, W.; Gao, Y.; Song, Y.; Wang, M. Microfluidics-Derived Microparticles with Prebiotics and Probiotics for Enhanced In Situ Colonization and Immunoregulation of Colitis. Nano Lett. 2024, 24, 1081–1089. [Google Scholar] [CrossRef]
- Bu, W.; McClements, D.J.; Zhang, Z.; Zhang, R.; Jin, Z.; Chen, L. Encapsulation Method of Probiotic Embedded Delivery System and Its Application in Food. Food Hydrocoll. 2025, 159, 110625. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Fentie, E.G.; Lim, K.; Andargie, Y.E.; Azizoglu, U.; Shin, J.-H. Globalizing the Health-Promoting Potential of Fermented Foods: A Culturomics Pathway to Probiotics. Trends Food Sci. Technol. 2025, 163, 105119. [Google Scholar] [CrossRef]
- Yan, S.; Gao, J.; Sun, Q. Research Progress on Colonization Ability of Probiotics. J. Future Foods 2025, 6, 195–204. [Google Scholar] [CrossRef]
- Yadav, M.; Mandeep; Shukla, P. Probiotics of Diverse Origin and Their Therapeutic Applications: A Review. J. Am. Coll. Nutr. 2020, 39, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Al-Fakhrany, O.M.; Elekhnawy, E. Next-Generation Probiotics: The Upcoming Biotherapeutics. Mol. Biol. Rep. 2024, 51, 505. [Google Scholar] [CrossRef]
- Abouelela, M.E.; Helmy, Y.A. Next-Generation Probiotics as Novel Therapeutics for Improving Human Health: Current Trends and Future Perspectives. Microorganisms 2024, 12, 430. [Google Scholar] [CrossRef] [PubMed]
- De Jesus, L.C.L.; Freitas, A.D.S.; Dutra, J.D.C.F.; Campos, G.M.; Américo, M.F.; Laguna, J.G.; Dornelas, E.G.; Carvalho, R.D.D.O.; Vital, K.D.; Fernandes, S.O.A.; et al. Lactobacillus delbrueckii CIDCA 133 Fermented Milk Modulates Inflammation and Gut Microbiota to Alleviate Acute Colitis. Food Res. Int. 2024, 186, 114322. [Google Scholar] [CrossRef]
- Di Salvo, C.; D’Antongiovanni, V.; Benvenuti, L.; d’Amati, A.; Ippolito, C.; Segnani, C.; Pierucci, C.; Bellini, G.; Annese, T.; Virgintino, D.; et al. Lactiplantibacillus plantarum HEAL9 Attenuates Cognitive Impairment and Progression of Alzheimer’s Disease and Related Bowel Symptoms in SAMP8 Mice by Modulating Microbiota-Gut-Inflammasome-Brain Axis. Food Funct. 2024, 15, 10323–10338. [Google Scholar] [CrossRef]
- Kim, W.; Jang, Y.J.; Park, S.; Min, S.; Kwon, H.; Jo, M.J.; Ko, G. Lactobacillus acidophilus KBL409 Ameliorates Atopic Dermatitis in a Mouse Model. J. Microbiol. 2024, 62, 91–99. [Google Scholar] [CrossRef]
- Calvanese, C.M.; Villani, F.; Ercolini, D.; De Filippis, F. Postbiotics versus Probiotics: Possible New Allies for Human Health. Food Res. Int. 2025, 217, 116869. [Google Scholar] [CrossRef]
- Meena, K.K.; Joshi, M.; Gupta, L.; Meena, S. Comprehensive Insights into Postbiotics: Bridging the Gap to Real-World Application. Food Nutr. 2025, 1, 100024. [Google Scholar] [CrossRef]
- Srivastava, S.; Basak, U.; Naghibi, M.; Vijayakumar, V.; Parihar, R.; Patel, J.; Jadon, P.; Pandit, A.; Dargad, R.; Khanna, S.; et al. A Randomized Double-Blind, Placebo-Controlled Trial to Evaluate the Safety and Efficacy of Live Bifidobacterium longum CECT 7347 (ES1) and Heat-Treated Bifidobacterium longum CECT 7347 (HT-ES1) in Participants with Diarrhea-Predominant Irritable Bowel Syndrome. Gut Microbes 2024, 16, 2338322. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Rosales, T.K.O.; Fabi, J.P. Valorization of Polyphenolic Compounds from Food Industry By-Products for Application in Polysaccharide-Based Nanoparticles. Front. Nutr. 2023, 10, 1144677. [Google Scholar] [CrossRef]
- Ali, S.; Hamayun, M.; Siraj, M.; Khan, S.A.; Kim, H.-Y.; Lee, B. Recent Advances in Prebiotics: Classification, Mechanisms, and Health Applications. Future Foods 2025, 12, 100680. [Google Scholar] [CrossRef]
- Jurić, S.; Jurić, M.; Siddique, M.A.B.; Fathi, M. Vegetable Oils Rich in Polyunsaturated Fatty Acids: Nanoencapsulation Methods and Stability Enhancement. Food Rev. Int. 2022, 38, 32–69. [Google Scholar] [CrossRef]
- You, S.; Ma, Y.; Yan, B.; Pei, W.; Wu, Q.; Ding, C.; Huang, C. The Promotion Mechanism of Prebiotics for Probiotics: A Review. Front. Nutr. 2022, 9, 1000517. [Google Scholar] [CrossRef]
- Huang, J.; Liu, D.; Wang, Y.; Liu, L.; Li, J.; Yuan, J.; Jiang, Z.; Jiang, Z.; Hsiao, W.W.; Liu, H.; et al. Ginseng Polysaccharides Alter the Gut Microbiota and Kynurenine/Tryptophan Ratio, Potentiating the Antitumour Effect of Antiprogrammed Cell Death 1/Programmed Cell Death Ligand 1 (Anti-PD-1/PD-L1) Immunotherapy. Gut 2022, 71, 734–745. [Google Scholar] [CrossRef]
- Li, H.; Dong, T.; Tao, M.; Zhao, H.; Lan, T.; Yan, S.; Gong, X.; Hou, Q.; Ma, X.; Song, Y. Fucoidan Enhances the Anti-Tumor Effect of Anti-PD-1 Immunotherapy by Regulating Gut Microbiota. Food Funct. 2024, 15, 3463–3478. [Google Scholar] [CrossRef]
- Boucher, E.; Plazy, C.; Richard, M.L.; Suau, A.; Mangin, I.; Cornet, M.; Aldebert, D.; Toussaint, B.; Hannani, D. Inulin Prebiotic Reinforces Host Cancer Immunosurveillance via Ɣδ T Cell Activation. Front. Immunol. 2023, 14, 1104224. [Google Scholar] [CrossRef]
- Bhutto, R.A.; Bhutto, N.U.A.H.; Mahar, H.; Khanal, S.; Wang, M.; Iqbal, S.; Fan, Y.; Yi, J. Recent Trends in Co-Encapsulation of Probiotics with Prebiotics and Their Applications in the Food Industry. Trends Food Sci. Technol. 2025, 156, 104829. [Google Scholar] [CrossRef]
- Lee, S.; Choi, S.-P.; Choi, H.-J.; Jeong, H.; Park, Y.-S. A Comprehensive Review of Synbiotics: An Emerging Paradigm in Health Promotion and Disease Management. World J. Microbiol. Biotechnol. 2024, 40, 280. [Google Scholar] [CrossRef]
- Yavorov-Dayliev, D.; Milagro, F.I.; Ayo, J.; Oneca, M.; Goyache, I.; López-Yoldi, M.; Aranaz, P. Glucose-Lowering Effects of a Synbiotic Combination Containing Pediococcus acidilactici in C. elegans and Mice. Diabetologia 2023, 66, 2117–2138. [Google Scholar] [CrossRef]
- European Union. Regulation No N 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers. Available online: https://eur-lex.europa.eu/eli/reg/2011/1169/oj/eng (accessed on 1 August 2025).
- Assadpour, E.; Mahdi Jafari, S. A Systematic Review on Nanoencapsulation of Food Bioactive Ingredients and Nutraceuticals by Various Nanocarriers. Crit. Rev. Food Sci. Nutr. 2019, 59, 3129–3151. [Google Scholar] [CrossRef]
- Wasilewska, A.; Bielicka, M.; Klekotka, U.; Kalska-Szostko, B. Nanoparticle Applications in Food—A Review. Food Funct. 2023, 14, 2544–2567. [Google Scholar] [CrossRef]
- Joudeh, N.; Linke, D. Nanoparticle Classification, Physicochemical Properties, Characterization, and Applications: A Comprehensive Review for Biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef]
- Bazana, M.T.; Codevilla, C.F.; De Menezes, C.R. Nanoencapsulation of bioactive compounds: Challenges and perspectives. Curr. Opin. Food Sci. 2019, 26, 47–56. [Google Scholar] [CrossRef]
- Nsairat, H.; Lafi, Z.; Al-Sulaibi, M.; Gharaibeh, L.; Alshaer, W. Impact of nanotechnology on the oral delivery of phyto-bioactive compounds. Food Chem. 2023, 424, 136438. [Google Scholar] [CrossRef]
- Bodbodak, S.; Nejatian, M.; Ghandehari Yazdi, A.P.; Kamali Rousta, L.; Rafiee, Z.; Jalali-Jivan, M.; Kharazmi, M.S.; Jafari, S.M. Improving the thermal stability of natural bioactive ingredients via encapsulation technology. Crit. Rev. Food Sci. Nutr. 2024, 64, 2824–2846. [Google Scholar] [CrossRef]
- Taouzinet, L.; Djaoudene, O.; Fatmi, S.; Bouiche, C.; Amrane-Abider, M.; Bougherra, H.; Rezgui, F.; Madani, K. Trends of Nanoencapsulation Strategy for Natural Compounds in the Food Industry. Processes 2023, 11, 1459. [Google Scholar] [CrossRef]
- Jain, K.; Takuli, A.; Gupta, T.K.; Gupta, D. Rethinking Nanoparticle Synthesis: A Sustainable Approach vs. Traditional Methods. Chem. Asian J. 2024, 19, e202400701. [Google Scholar] [CrossRef]
- Liu, Z.; Fontana, F.; Python, A.; Hirvonen, J.T.; Santos, H.A. Microfluidics for Production of Particles: Mechanism, Methodology, and Applications. Small 2020, 16, 1904673. [Google Scholar] [CrossRef]
- Dangi, P.; Chaudhary, N.; Chaudhary, V.; Virdi, A.S.; Kajla, P.; Khanna, P.; Jha, S.K.; Jha, N.K.; Alkhanani, M.F.; Singh, V.; et al. Nanotechnology Impacting Probiotics and Prebiotics: A Paradigm Shift in Nutraceuticals Technology. Int. J. Food Microbiol. 2023, 388, 110083. [Google Scholar] [CrossRef]
- Centurion, F.; Basit, A.W.; Liu, J.; Gaisford, S.; Rahim, M.A.; Kalantar-Zadeh, K. Nanoencapsulation for Probiotic Delivery. ACS Nano 2021, 15, 18653–18660. [Google Scholar] [CrossRef]
- Han, J.; McClements, D.J.; Liu, X.; Liu, F. Oral Delivery of Probiotics Using Single-cell Encapsulation. Comp. Rev. Food Sci. Food Saf. 2024, 23, e13322. [Google Scholar] [CrossRef]
- Han, S.Y.; Nguyen, D.T.; Kim, B.J.; Kim, N.; Kang, E.K.; Park, J.H.; Choi, I.S. Cytoprotection of Probiotic Lactobacillus acidophilus with Artificial Nanoshells of Nature-Derived Eggshell Membrane Hydrolysates and Coffee Melanoidins in Single-Cell Nanoencapsulation. Polymers 2023, 15, 1104. [Google Scholar] [CrossRef]
- Hou, W.; Li, J.; Cao, Z.; Lin, S.; Pan, C.; Pang, Y.; Liu, J. Decorating Bacteria with a Therapeutic Nanocoating for Synergistically Enhanced Biotherapy. Small 2021, 17, 2101810. [Google Scholar] [CrossRef]
- Lei, Y.; Xie, Z.; Zhao, A.; Colarelli, J.P.; Miller, M.J.; Lee, Y. Layer-by-Layer Coating of Lacticaseibacillus rhamnosus GG (LGG) Using Chitosan and Zein/Tween-80/Fucoidan Nanoparticles to Enhance LGG’s Survival under Adverse Conditions. Food Hydrocoll. 2024, 154, 110039. [Google Scholar] [CrossRef]
- Zhu, Y.-Y.; Ma, R.-H.; Thakur, K.; Zhang, W.-W.; Zhang, J.-G.; Khan, M.R.; Liao, C.; Wei, Z.-J. Layer-by-Layer Nanoencapsulation Strategies for Enhanced Oral Delivery and Function of Lactobacillus plantarum B2. Food Hydrocoll. 2025, 160, 110865. [Google Scholar] [CrossRef]
- Han, S.Y.; Kim, N.; Nguyen, D.T.; Yang, S.; Kang, E.K.; Baskoro, G.A.; Lee, S.-H.; Lee, K.-B.; Kim, B.J.; Choi, I.S. Cytoprotective Nanoencapsulation of Probiotic Cells within Fe3+-Phytic Acid Nanoshells. Langmuir 2025, 41, 10625–10631. [Google Scholar] [CrossRef]
- Fung, W.-Y.; Yuen, K.-H.; Liong, M.-T. Agrowaste-Based Nanofibers as a Probiotic Encapsulant: Fabrication and Characterization. J. Agric. Food Chem. 2011, 59, 8140–8147. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; Taylan, O.; Karakas, C.Y.; Dertli, E. An Alternative Way to Encapsulate Probiotics within Electrospun Alginate Nanofibers as Monitored under Simulated Gastrointestinal Conditions and in Kefir. Carbohydr. Polym. 2020, 244, 116447. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.T.; Han, S.Y.; Kozlowski, F.; Seisenbaeva, G.A.; Kessler, V.G.; Kim, B.J.; Choi, I.S. Biphasic Water–Oil Systems for Functional Augmentation of Probiotic Lactobacillus acidophilus Nanoencapsulated in Luteolin-Fe3+ Shells. Chem. Commun. 2024, 60, 5330–5333. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Gong, G.; Wang, Q.; Shang, J.; He, Y.; Catania, C.; Birnbaum, D.; Li, Y.; Jia, Z.; Zhang, Y.; et al. A Single-Cell Nanocoating of Probiotics for Enhanced Amelioration of Antibiotic-Associated Diarrhea. Nat. Commun. 2022, 13, 2117. [Google Scholar] [CrossRef]
- Cheng, Q.; Xie, M.; Ying, H.; Jin, C.; Yang, L.; Ma, D.; Cui, S.; Shi, L. A Spatiotemporal Probiotic Spore-Loaded Oxygen Generator Resumes Gut Microbiome Balance and Improves Hypoxia for Treating Viral Pneumonia. J. Chem. Eng. 2025, 505, 159706. [Google Scholar] [CrossRef]
- Peng, P.; Feng, T.; Yang, X.; Nie, C.; Yu, L.; Ding, R.; Zhou, Q.; Jiang, X.; Li, P. Gastrointestinal Microenvironment Responsive Nanoencapsulation of Probiotics and Drugs for Synergistic Therapy of Intestinal Diseases. ACS Nano 2023, 17, 14718–14730. [Google Scholar] [CrossRef] [PubMed]
- Jurić, M.; Goksen, G.; Donsì, F.; Jurić, S. Innovative Applications of Electrospun Nanofibers Loaded with Bacterial Cells Towards Sustainable Agri-Food Systems and Regulatory Compliance. Food. Eng. Rev. 2024, 16, 270–303. [Google Scholar] [CrossRef]
- Feng, K.; Huangfu, L.; Liu, C.; Bonfili, L.; Xiang, Q.; Wu, H.; Bai, Y. Electrospinning and Electrospraying: Emerging Techniques for Probiotic Stabilization and Application. Polymers 2023, 15, 2402. [Google Scholar] [CrossRef]
- Feng, K.; Huang, R.; Wu, R.; Wei, Y.; Zong, M.; Linhardt, R.J.; Wu, H. A Novel Route for Double-Layered Encapsulation of Probiotics with Improved Viability under Adverse Conditions. Food Chem. 2020, 310, 125977. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, H.; Yang, Y.; Yang, X.; Li, X.; Zhong, W.; Wen, B.; He, F.; Li, J. Reinventing Gut Health: Leveraging Dietary Bioactive Compounds for the Prevention and Treatment of Diseases. Front. Nutr. 2024, 11, 1491821. [Google Scholar] [CrossRef]
- Yoo, S.; Jung, S.-C.; Kwak, K.; Kim, J.-S. The Role of Prebiotics in Modulating Gut Microbiota: Implications for Human Health. Int. J. Mol. Sci. 2024, 25, 4834. [Google Scholar] [CrossRef]
- Krishnan, M.; Babu, S.; Bari, A.B.A. Recent Innovations in Probiotics and Prebiotics and Gut Health. In Microbiota and Dietary Mediators in Colon Cancer Prevention and Treatment; Pathak, S., Banerjee, A., Duttaroy, A.K., Eds.; Springer Nature: Singapore, 2024; pp. 21–35. [Google Scholar]
- Plamada, D.; Vodnar, D.C. Polyphenols—Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2021, 14, 137. [Google Scholar] [CrossRef] [PubMed]
- Smolinska, S.; Popescu, F.-D.; Zemelka-Wiacek, M. A Review of the Influence of Prebiotics, Probiotics, Synbiotics, and Postbiotics on the Human Gut Microbiome and Intestinal Integrity. J. Clin. Med. 2025, 14, 3673. [Google Scholar] [CrossRef]
- Verspreet, J.; Damen, B.; Broekaert, W.F.; Verbeke, K.; Delcour, J.A.; Courtin, C.M. A Critical Look at Prebiotics Within the Dietary Fiber Concept. Annu. Rev. Food Sci. Technol. 2016, 7, 167–190. [Google Scholar] [CrossRef] [PubMed]
- Hashem, N.M.; Hosny, N.S.; El-Desoky, N.I.; Shehata, M.G. Effect of Nanoencapsulated Alginate-Synbiotic on Gut Microflora Balance, Immunity, and Growth Performance of Growing Rabbits. Polymers 2021, 13, 4191. [Google Scholar] [CrossRef]
- Ren, Y.; Nie, L.; Luo, C.; Zhu, S.; Zhang, X. Advancement in Therapeutic Intervention of Prebiotic-Based Nanoparticles for Colonic Diseases. Int. J. Nanomed. 2022, 17, 6639–6654. [Google Scholar] [CrossRef]
- Rosales, T.K.O.; Fabi, J.P. Polysaccharides as Natural Nanoencapsulants for Controlled Release of Compounds. In Smart Nanomaterials for Bioencapsulation; Castro, G., Nadda, A., Nguyen, T., Sharma, S., Gupta, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 23–39. [Google Scholar]
- da Silva, M.P.; Rosales, T.K.O.; Pedrosa, L.d.F.; Fabi, J.P. Creation of a New Proof-of-Concept Pectin/Lysozyme Nanocomplex as Potential β-Lactose Delivery Matrix: Structure and Thermal Stability Analyses. Food Hydrocoll. 2023, 134, 108011. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, L.; Wen, Q.; Cheng, X.; Zhou, L.; Chen, M.; Ke, D.; Tu, Z. Prebiotic Saccharides Polymerization Improves the Encapsulation Efficiency, Stability, Bioaccessibility and Gut Microbiota Modulation of Urolithin A Liposomes. Int. J. Biol. Macromol. 2024, 273, 133045. [Google Scholar] [CrossRef]
- Feng, Z.; Peng, S.; Wu, Z.; Jiao, L.; Xu, S.; Wu, Y.; Liu, Z.; Hu, Y.; Liu, J.; Wu, Y.; et al. Ramulus Mori Polysaccharide-Loaded PLGA Nanoparticles and Their Anti-Inflammatory Effects In Vivo. Int. J. Biol. Macromol. 2021, 182, 2024–2036. [Google Scholar] [CrossRef]
- Ismail, S.A.; El-Sayed, H.S.; Fayed, B. Production of Prebiotic Chitooligosaccharide and Its Nano/Microencapsulation for the Production of Functional Yoghurt. Carbohydr. Polym. 2020, 234, 115941. [Google Scholar] [CrossRef] [PubMed]
- Afinjuomo, F.; Abdella, S.; Youssef, S.H.; Song, Y.; Garg, S. Inulin and Its Application in Drug Delivery. Pharmaceuticals 2021, 14, 855. [Google Scholar] [CrossRef]
- Rosales, T.K.O.; Fabi, J.P. Pectin-Based Nanoencapsulation Strategy to Improve the Bioavailability of Bioactive Compounds. Int. J. Biol. Macromol. 2023, 229, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.-H.; Na, G.; Kang, J.-W. A Green Nanocoating Approach to Lactobacillus plantarum Using Tea Residue-Derived Phenolic Compounds and Cellulose Nanocrystals. Food Hydrocoll. 2025, 167, 111469. [Google Scholar] [CrossRef]
- Hu, Q.; Li, J.; Wang, T.; Xu, X.; Duan, Y.; Jin, Y. Polyphenolic Nanoparticle-Modified Probiotics for Microenvironment Remodeling and Targeted Therapy of Inflammatory Bowel Disease. ACS Nano 2024, 18, 12917–12932. [Google Scholar] [CrossRef]
- Fayed, B.; Abood, A.; El-Sayed, H.S.; Hashem, A.M.; Mehanna, N.S.H. A Synbiotic Multiparticulate Microcapsule for Enhancing Inulin Intestinal Release and Bifidobacterium Gastro-Intestinal Survivability. Carbohydr. Polym. 2018, 193, 137–143. [Google Scholar] [CrossRef]
- Noman, M.; Afzaal, M.; Saeed, F.; Ahmad, A.; Imran, A.; Akram, N.; Asghar, A.; Shah, Y.A.; Ateeq, H.; Khan, M.R.; et al. Effect of Starch-Based Nanoparticles on the Viability and Stability of Probiotics under Adverse Conditions. Int. J. Food Prop. 2023, 26, 1841–1854. [Google Scholar] [CrossRef]
- Hashem, N.M.; Hosny, N.S.; El-Desoky, N.; Soltan, Y.A.; Elolimy, A.A.; Sallam, S.M.A.; Abu-Tor, E.-S.M. Alginate Nanoencapsulated Synbiotic Composite of Pomegranate Peel Phytogenics and Multi-Probiotic Species as a Potential Feed Additive: Physicochemical, Antioxidant, and Antimicrobial Activities. Animals 2023, 13, 2432. [Google Scholar] [CrossRef] [PubMed]
- Senthil Kumar, S.; Sheik Mohideen, S. Chitosan-Coated Probiotic Nanoparticles Mitigate Acrylamide-Induced Toxicity in the Drosophila Model. Sci. Rep. 2024, 14, 21182. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.; Çon, A.H. Postbiotic Nanoparticles (Postbiotics-NPs): A Novel Strategy for Providing Probiotics’ Health Advantages through Food Consumption. Food Sci. Biotechnol. 2024, 33, 2729–2736. [Google Scholar] [CrossRef]
- Hosny, N.S.; Morsy, A.S.; Abo-elezz, Z.R.; Hashem, N.M. Physiological Responses and Reproductive Performance of Naturally Heat-Stressed Rabbit Does Treated with Postbiotic of Bacillus Subtilis and Saccharomyces Cerevisiae in Free and Nano-Encapsulated Forms. BMC Vet. Res. 2025, 21, 288. [Google Scholar] [CrossRef]
- Huang, H.-L.; Lai, C.-H.; Tsai, W.-H.; Chen, K.-W.; Peng, S.-L.; Lin, J.-H.; Lin, Y.-H. Nanoparticle-Enhanced Postbiotics: Revolutionizing Cancer Therapy through Effective Delivery. Life Sci. 2024, 337, 122379. [Google Scholar] [CrossRef]
- Yu, T.; Bai, R.; Wang, Z.; Qin, Y.; Wang, J.; Wei, Y.; Zhao, R.; Nie, G.; Han, B. Colon-Targeted Engineered Postbiotics Nanoparticles Alleviate Osteoporosis through the Gut-Bone Axis. Nat. Commun. 2024, 15, 10893. [Google Scholar] [CrossRef] [PubMed]
- Gökçe, H.B.; Aslan, İ. Novel Liposome–Gel Formulations Containing a Next Generation Postbiotic: Characterization, Rheological, Stability, Release Kinetic, and In Vitro Antimicrobial Activity Studies. Gels 2024, 10, 746. [Google Scholar] [CrossRef]
- Youssef, H.; Azmy, A.F.; Eid, H.M.; Sayed, O.M.; Eldomany, E.B.; Farghali, A.A.; Molham, F. The Enterococcus Secretome Inhibits the Growth of Vancomycin-Resistant Enterococcus faecalis V853 with Their Antiproliferative Properties and Nanoencapsulation Effects. Int. Microbiol. 2024, 28, 227–239. [Google Scholar] [CrossRef]
- Qiu, M.; Tian, Y.; Qu, W.; Ma, Y.; Zhao, F.; Jiang, Y.; Zhao, Q.; Man, C. Postbiotic-Biosynthesized Silver Nanoparticles Anchored on Covalent Organic Frameworks Integrated into Carboxymethyl Chitosan-Based Film for Enhancing Antibacterial Packaging. Int. J. Biol. Macromol. 2025, 291, 139143. [Google Scholar] [CrossRef]
- Yuksekdag, Z.; Kilickaya, R.; Kara, F.; Acar, B.C. Biogenic-Synthesized Silver Nanoparticles Using the Ligilactobacillus salivarius KC27L Postbiotic: Antimicrobial, Anti-Biofilm, and Antioxidant Activity and Cytotoxic Effects. Probiotics Antimicrob. Prot. 2025. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, P.B.V.d.; Rosales, T.K.O.; Fabi, J.P. Nanoencapsulation of Biotics: Feasibility to Enhance Stability and Delivery for Improved Gut Health. Pharmaceutics 2025, 17, 1180. https://doi.org/10.3390/pharmaceutics17091180
Silva PBVd, Rosales TKO, Fabi JP. Nanoencapsulation of Biotics: Feasibility to Enhance Stability and Delivery for Improved Gut Health. Pharmaceutics. 2025; 17(9):1180. https://doi.org/10.3390/pharmaceutics17091180
Chicago/Turabian StyleSilva, Pedro Brivaldo Viana da, Thiécla Katiane Osvaldt Rosales, and João Paulo Fabi. 2025. "Nanoencapsulation of Biotics: Feasibility to Enhance Stability and Delivery for Improved Gut Health" Pharmaceutics 17, no. 9: 1180. https://doi.org/10.3390/pharmaceutics17091180
APA StyleSilva, P. B. V. d., Rosales, T. K. O., & Fabi, J. P. (2025). Nanoencapsulation of Biotics: Feasibility to Enhance Stability and Delivery for Improved Gut Health. Pharmaceutics, 17(9), 1180. https://doi.org/10.3390/pharmaceutics17091180







