Identification of Variable Lymphocyte Receptors That Target the Human Blood–Brain Barrier
Abstract
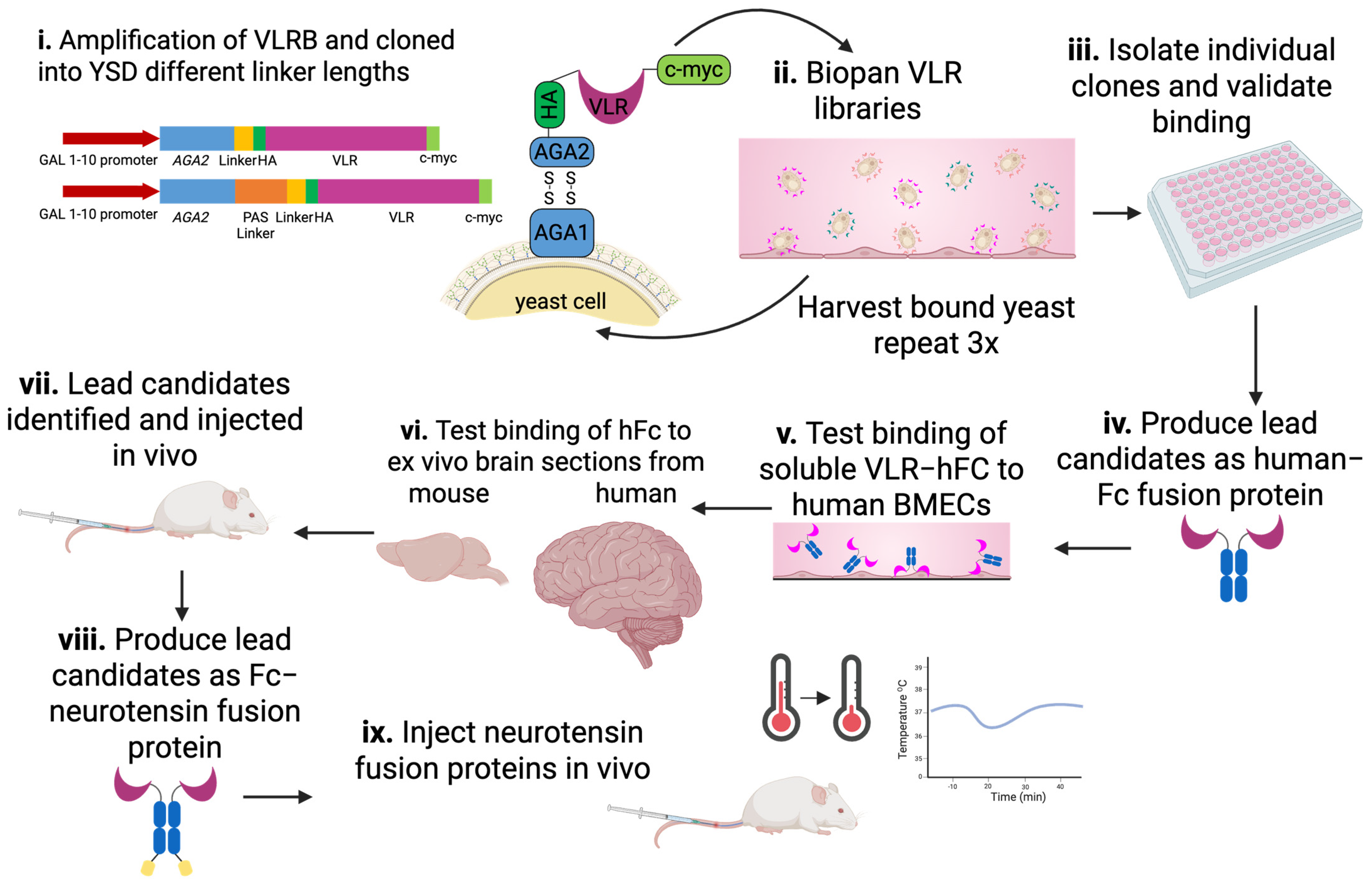
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Lamprey Immunization
2.3. VLR Library Cloning
2.4. YSD Library Screening–Biopanning
2.5. Immunolabeling of Tissue and Cells with VLRs
2.6. Fc-Fusion Protein Production
2.7. Cell-Based Assays
2.8. Animal Experiments
2.9. Imaging
2.10. Statistical Analysis
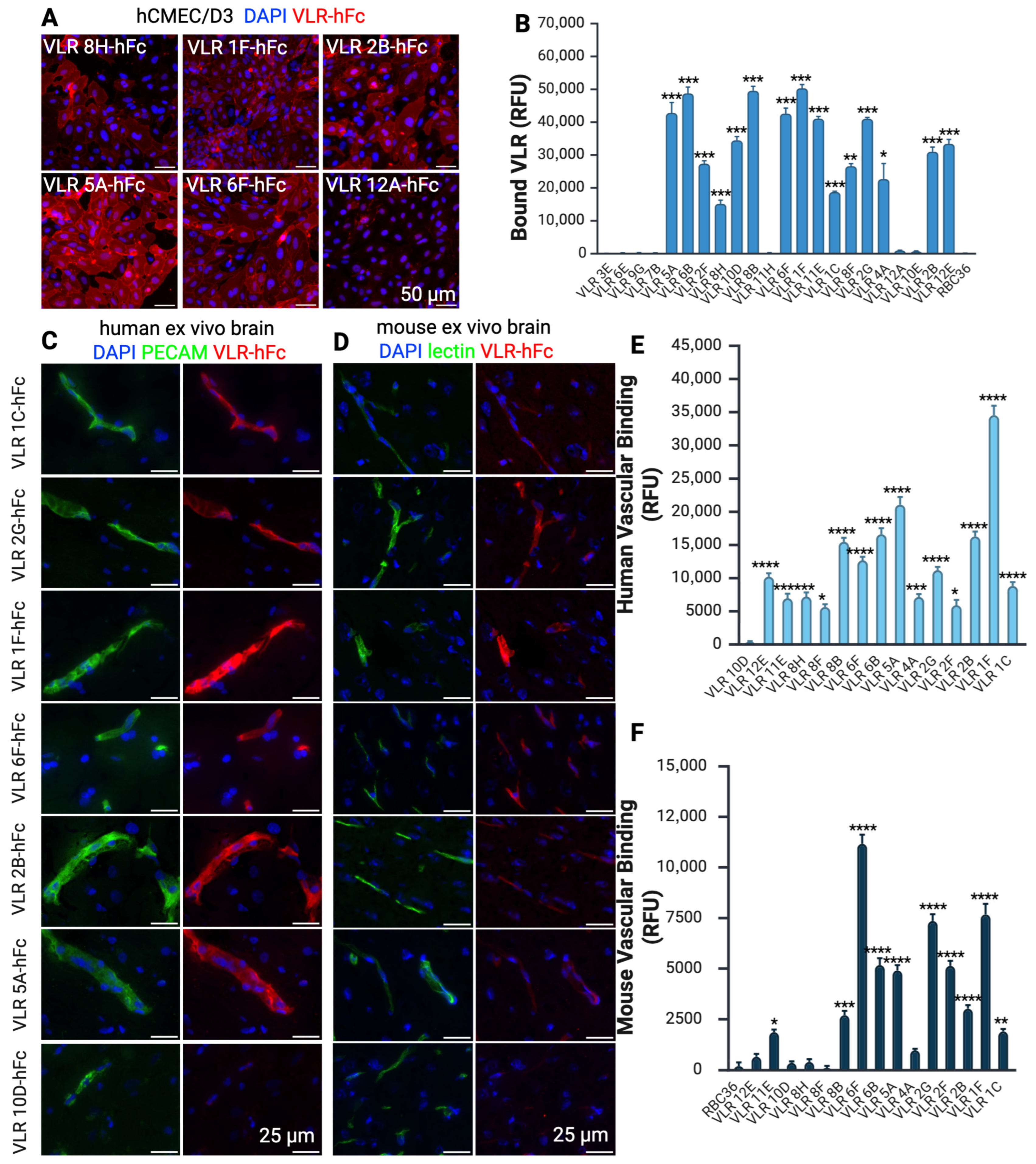
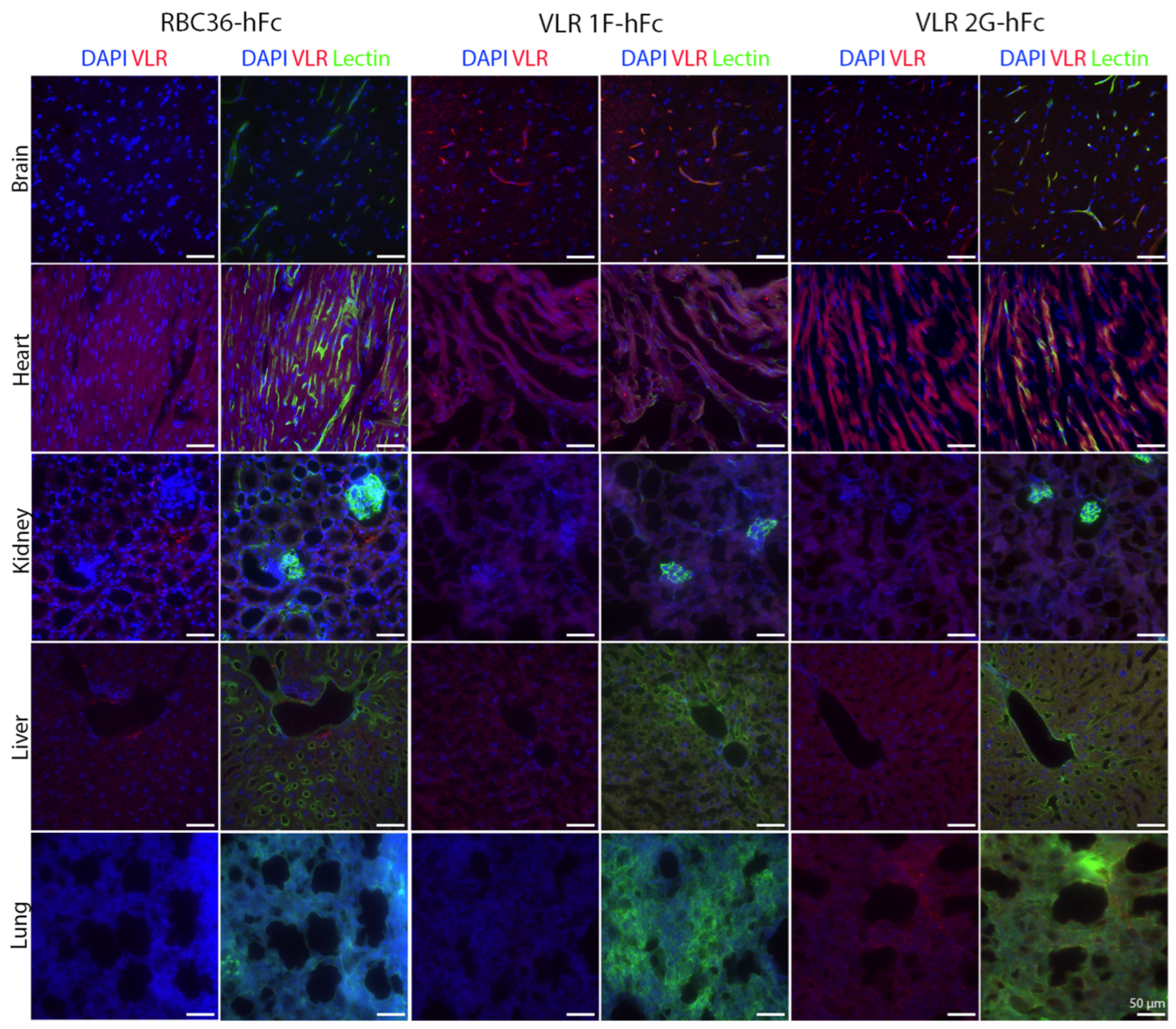
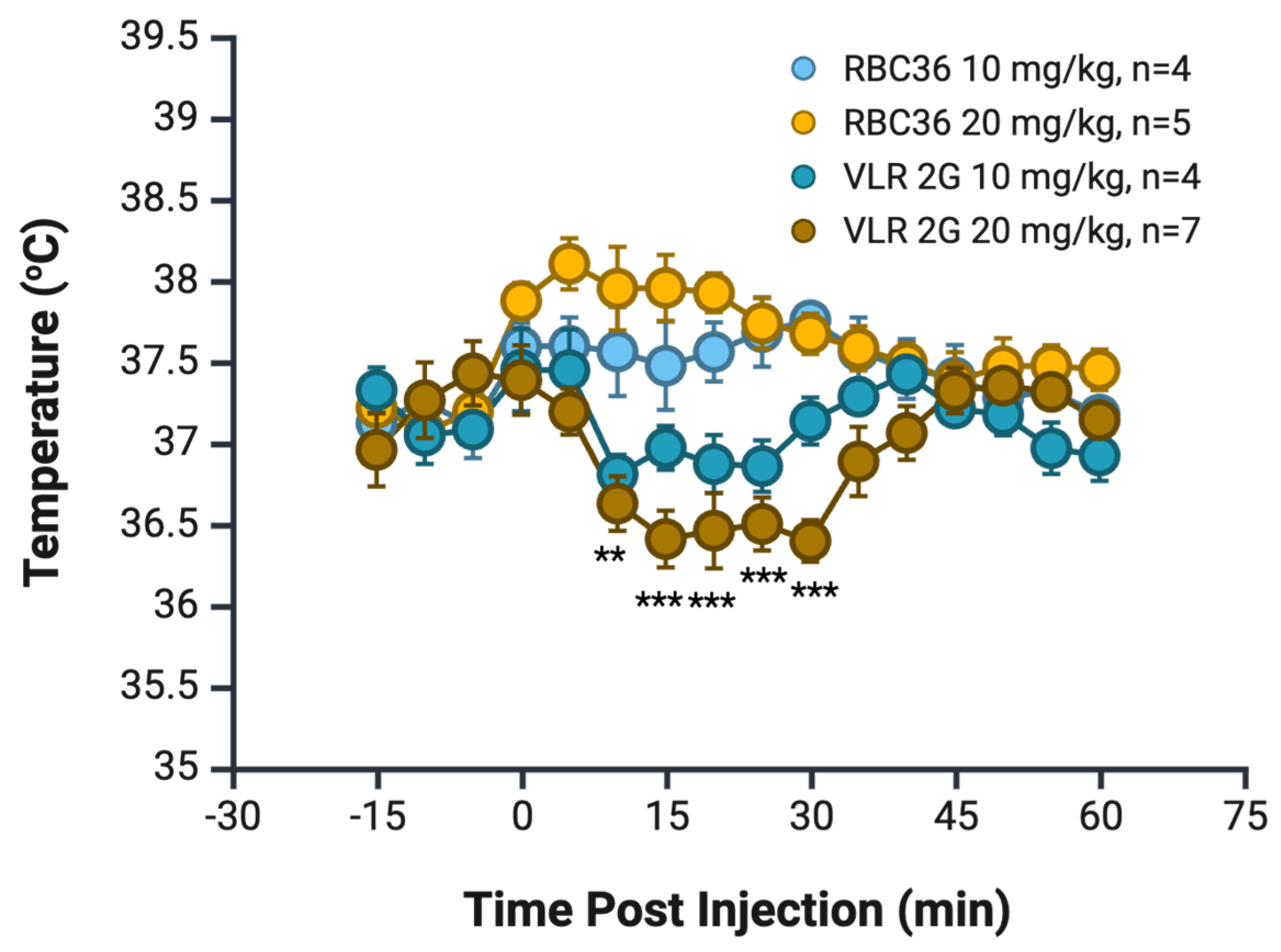
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BBB | Blood–brain barrier |
| hiPSC | Human-induced pluripotent stem cells |
| BMEC | Brain microvascular endothelial cell |
| VLR | Variable lymphocyte receptor |
| RMT | Receptor mediate transcytosis |
| RNAseq | Ribonucleic acid sequencing |
| scFv | Single chain variable fragments |
| IgG | Immunoglobulin G |
| LRR | Leucine-rich repeat |
| LRRV | Leucine-rich repeat variable |
| FGF-2 | Fibroblast growth factor 2 |
| YSD | Yeast surface display |
| BSA | Bovine serum albumin |
| PBSGA | Phosphate-buffered solution with goat serum and bovine serum albumin |
| IRB | Institutional review board |
| LL | Longer linker |
| CHO | Chinese hamster ovary cells |
| NT | Neurotensin |
| SDS | Sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| Fc | Fragment crystallizable |
| PFA | Paraformaldehyde |
| IACUC | Institutional animal care and use committee |
| hFC | Human Fc |
| rbFc | Rabbit Fc |
| CNS | Central nervous system |
| LDLR | Low-density lipoprotein receptor |
References
- Marin, B.-M.; Porath, K.A.; Jain, S.; Kim, M.; Conage-Pough, J.E.; Oh, J.-H.; Miller, C.L.; Talele, S.; Kitange, G.J.; Tian, S.; et al. Heterogeneous Delivery across the Blood-Brain Barrier Limits the Efficacy of an EGFR-Targeting Antibody Drug Conjugate in Glioblastoma. Neuro-Oncology 2021, 23, 2042–2053. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Ye, M.; Levy, A.; Rothstein, J.; Bergles, D.; Searson, P. The Blood-Brain Barrier: An Engineering Perspective. Front. Neuroeng. 2013, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Hajal, C.; Le Roi, B.; Kamm, R.D.; Maoz, B.M. Biology and Models of the Blood–Brain Barrier. Annu. Rev. Biomed. Eng. 2021, 23, 359–384. [Google Scholar] [CrossRef]
- Pandit, R.; Chen, L.; Götz, J. The Blood-Brain Barrier: Physiology and Strategies for Drug Delivery. Adv. Drug Deliv. Rev. 2020, 165–166, 1–14. [Google Scholar] [CrossRef]
- Pardridge, W.M. A Historical Review of Brain Drug Delivery. Pharmaceutics 2022, 14, 1283. [Google Scholar] [CrossRef]
- Bors, L.A.; Erdő, F. Overcoming the Blood–Brain Barrier. Challenges and Tricks for CNS Drug Delivery. Sci. Pharm. 2019, 87, 6. [Google Scholar] [CrossRef]
- Haumann, R.; Videira, J.C.; Kaspers, G.J.L.; van Vuurden, D.G.; Hulleman, E. Overview of Current Drug Delivery Methods Across the Blood–Brain Barrier for the Treatment of Primary Brain Tumors. CNS Drugs 2020, 34, 1121–1131. [Google Scholar] [CrossRef]
- Lajoie, J.M.; Shusta, E.V. Targeting Receptor-Mediated Transport for Delivery of Biologics across the Blood-Brain Barrier. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 613–631. [Google Scholar] [CrossRef]
- Pardridge, W.M. Receptor-Mediated Peptide Transport through the Blood-Brain Barrier. Endocr. Rev. 1986, 7, 314–330. [Google Scholar] [CrossRef]
- Anthony, D.P.; Hegde, M.; Shetty, S.S.; Rafic, T.; Mutalik, S.; Rao, B.S.S. Targeting Receptor-Ligand Chemistry for Drug Delivery across Blood-Brain Barrier in Brain Diseases. Life Sci. 2021, 274, 119326. [Google Scholar] [CrossRef]
- Chew, K.S.; Wells, R.C.; Moshkforoush, A.; Chan, D.; Lechtenberg, K.J.; Tran, H.L.; Chow, J.; Kim, D.J.; Robles-Colmenares, Y.; Srivastava, D.B.; et al. CD98hc Is a Target for Brain Delivery of Biotherapeutics. Nat. Commun. 2023, 14, 5053. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Burkhart, A.; Thomsen, L.B.; Andresen, T.L.; Moos, T. Targeting the Transferrin Receptor for Brain Drug Delivery. Prog. Neurobiol. 2019, 181, 101665. [Google Scholar] [CrossRef]
- Pardridge, W.M. Kinetics of Blood–Brain Barrier Transport of Monoclonal Antibodies Targeting the Insulin Receptor and the Transferrin Receptor. Pharmaceuticals 2022, 15, 3. [Google Scholar] [CrossRef]
- Okuyama, T.; Eto, Y.; Sakai, N.; Nakamura, K.; Yamamoto, T.; Yamaoka, M.; Ikeda, T.; So, S.; Tanizawa, K.; Sonoda, H.; et al. A Phase 2/3 Trial of Pabinafusp Alfa, IDS Fused with Anti-Human Transferrin Receptor Antibody, Targeting Neurodegeneration in MPS-II. Mol. Ther. 2021, 29, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Grimm, H.P.; Schumacher, V.; Schäfer, M.; Imhof-Jung, S.; Freskgård, P.-O.; Brady, K.; Hofmann, C.; Rüger, P.; Schlothauer, T.; Göpfert, U.; et al. Delivery of the BrainshuttleTM Amyloid-Beta Antibody Fusion Trontinemab to Non-Human Primate Brain and Projected Efficacious Dose Regimens in Humans. mAbs 2023, 15, 2261509. [Google Scholar] [CrossRef]
- Pornnoppadol, G.; Bond, L.G.; Lucas, M.J.; Zupancic, J.M.; Kuo, Y.-H.; Zhang, B.; Greineder, C.F.; Tessier, P.M. Bispecific Antibody Shuttles Targeting CD98hc Mediate Efficient and Long-Lived Brain Delivery of IgGs. Cell Chem. Biol. 2024, 31, 361–372.e8. [Google Scholar] [CrossRef] [PubMed]
- Khoury, N.; Pizzo, M.E.; Discenza, C.B.; Joy, D.; Tatarakis, D.; Todorov, M.I.; Negwer, M.; Ha, C.; De Melo, G.L.; Sarrafha, L.; et al. Fc-Engineered Large Molecules Targeting Blood-Brain Barrier Transferrin Receptor and CD98hc Have Distinct Central Nervous System and Peripheral Biodistribution. Nat. Commun. 2025, 16, 1822. [Google Scholar] [CrossRef]
- Uchida, Y. Quantitative Proteomics-Based Blood–Brain Barrier Study. Biol. Pharm. Bull. 2021, 44, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, H.-J. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS)-Based Proteomics of Drug-Metabolizing Enzymes and Transporters. Molecules 2020, 25, 2718. [Google Scholar] [CrossRef]
- Al-Majdoub, Z.M.; Al Feteisi, H.; Achour, B.; Warwood, S.; Neuhoff, S.; Rostami-Hodjegan, A.; Barber, J. Proteomic Quantification of Human Blood–Brain Barrier SLC and ABC Transporters in Healthy Individuals and Dementia Patients. Mol. Pharm. 2019, 16, 1220–1233. [Google Scholar] [CrossRef]
- Song, H.W.; Foreman, K.L.; Gastfriend, B.D.; Kuo, J.S.; Palecek, S.P.; Shusta, E.V. Transcriptomic Comparison of Human and Mouse Brain Microvessels. Sci. Rep. 2020, 10, 12358. [Google Scholar] [CrossRef]
- Zuchero, Y.J.Y.; Chen, X.; Bien-Ly, N.; Bumbaca, D.; Tong, R.K.; Gao, X.; Zhang, S.; Hoyte, K.; Luk, W.; Huntley, M.A.; et al. Discovery of Novel Blood-Brain Barrier Targets to Enhance Brain Uptake of Therapeutic Antibodies. Neuron 2016, 89, 70–82. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Q.Y.; Haqqani, A.S.; Leclerc, S.; Liu, Z.; Fauteux, F.; Baumann, E.; Delaney, C.E.; Ly, D.; Star, A.T.; et al. Differential Expression of Receptors Mediating Receptor-Mediated Transcytosis (RMT) in Brain Microvessels, Brain Parenchyma and Peripheral Tissues of the Mouse and the Human. Fluids Barriers CNS 2020, 17, 47. [Google Scholar] [CrossRef]
- Lippmann, E.S.; Al-Ahmad, A.; Azarin, S.M.; Palecek, S.P.; Shusta, E.V. A Retinoic Acid-Enhanced, Multicellular Human Blood-Brain Barrier Model Derived from Stem Cell Sources. Sci. Rep. 2014, 4, 4160. [Google Scholar] [CrossRef]
- Lippmann, E.S.; Azarin, S.M.; Palecek, S.P.; Shusta, E.V. Commentary on Human Pluripotent Stem Cell-Based Blood–Brain Barrier Models. Fluids Barriers CNS 2020, 17, 64. [Google Scholar] [CrossRef]
- Katt, M.E.; Xu, Z.S.; Gerecht, S.; Searson, P.C. Human Brain Microvascular Endothelial Cells Derived from the BC1 iPS Cell Line Exhibit a Blood-Brain Barrier Phenotype. PLoS ONE 2016, 11, e0152105. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, X.; Liu, H.; Huang, L.; Meng, G.; Ding, Y.; Su, W.; Lu, J.; Gong, S.; Terstappen, G.C.; et al. Development of Human in Vitro Brain-Blood Barrier Model from Induced Pluripotent Stem Cell-Derived Endothelial Cells to Predict the in Vivo Permeability of Drugs. Neurosci. Bull. 2019, 35, 996–1010. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, J.V.; Goulatis, L.I.; Stutz, C.C.; Canfield, S.G.; Song, H.W.; Gastfriend, B.D.; Shusta, E.V. Antibody Screening Using a Human iPSC-Based Blood-Brain Barrier Model Identifies Antibodies That Accumulate in the CNS. FASEB J. 2020, 34, 12549–12564. [Google Scholar] [CrossRef] [PubMed]
- Burgio, F.; Gaiser, C.; Brady, K.; Gatta, V.; Class, R.; Schrage, R.; Suter-Dick, L. A Perfused In Vitro Human iPSC-Derived Blood–Brain Barrier Faithfully Mimics Transferrin Receptor-Mediated Transcytosis of Therapeutic Antibodies. Cell. Mol. Neurobiol. 2023, 43, 4173–4187. [Google Scholar] [CrossRef]
- Georgieva, J.V.; Katt, M.; Ye, Z.; Umlauf, B.J.; Wenthur, C.J.; Shusta, E.V. The 46.1 Antibody Mediates Neurotensin Uptake into the CNS and the Effects Depend on the Route of Intravenous Administration. Pharmaceutics 2022, 14, 1706. [Google Scholar] [CrossRef]
- Jovčevska, I.; Muyldermans, S. The Therapeutic Potential of Nanobodies. BioDrugs 2020, 34, 11–26. [Google Scholar] [CrossRef]
- Sanjanwala, D.; Patravale, V. Aptamers and Nanobodies as Alternatives to Antibodies for Ligand-Targeted Drug Delivery in Cancer. Drug Discov. Today 2023, 28, 103550. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-López, E.; Schuhmacher, A.J. Transportation of Single-Domain Antibodies through the Blood–Brain Barrier. Biomolecules 2021, 11, 1131. [Google Scholar] [CrossRef] [PubMed]
- Lajoie, J.M.; Katt, M.E.; Waters, E.A.; Herrin, B.R.; Shusta, E.V. Identification of Lamprey Variable Lymphocyte Receptors That Target the Brain Vasculature. Sci. Rep. 2022, 12, 6044. [Google Scholar] [CrossRef] [PubMed]
- Umlauf, B.J.; Clark, P.A.; Lajoie, J.M.; Georgieva, J.V.; Bremner, S.; Herrin, B.R.; Kuo, J.S.; Shusta, E.V. Identification of Variable Lymphocyte Receptors That Can Target Therapeutics to Pathologically Exposed Brain Extracellular Matrix. Sci. Adv. 2019, 5, eaau4245. [Google Scholar] [CrossRef]
- Alder, M.N.; Herrin, B.R.; Sadlonova, A.; Stockard, C.R.; Grizzle, W.E.; Gartland, L.A.; Gartland, G.L.; Boydston, J.A.; Turnbough, C.L.; Cooper, M.D. Antibody Responses of Variable Lymphocyte Receptors in the Lamprey. Nat. Immunol. 2008, 9, 319–327. [Google Scholar] [CrossRef]
- Han, B.W.; Herrin, B.R.; Cooper, M.D.; Wilson, I.A. Antigen Recognition by Variable Lymphocyte Receptors. Science 2008, 321, 1834–1837. [Google Scholar] [CrossRef]
- Pancer, Z.; Saha, N.R.; Kasamatsu, J.; Suzuki, T.; Amemiya, C.T.; Kasahara, M.; Cooper, M.D. Variable Lymphocyte Receptors in Hagfish. Proc. Natl. Acad. Sci. USA 2005, 102, 9224–9229. [Google Scholar] [CrossRef]
- Kim, H.M.; Oh, S.C.; Lim, K.J.; Kasamatsu, J.; Heo, J.Y.; Park, B.S.; Lee, H.; Yoo, O.J.; Kasahara, M.; Lee, J.-O. Structural Diversity of the Hagfish Variable Lymphocyte Receptors *. J. Biol. Chem. 2007, 282, 6726–6732. [Google Scholar] [CrossRef]
- Waters, E.A.; Shusta, E.V. The Variable Lymphocyte Receptor as an Antibody Alternative. Curr. Opin. Biotechnol. 2018, 52, 74–79. [Google Scholar] [CrossRef]
- Chan, T.H.J. Discovery of Novel Human Biomarkers Using Variable Lymphocyte Receptor Antibodies of the Sea Lamprey. Ph.D. Thesis, University of Toronto, Ontario, CA, Canada, 2019. [Google Scholar]
- McKitrick, T.R.; Goth, C.K.; Rosenberg, C.S.; Nakahara, H.; Heimburg-Molinaro, J.; McQuillan, A.M.; Falco, R.; Rivers, N.J.; Herrin, B.R.; Cooper, M.D.; et al. Development of Smart Anti-Glycan Reagents Using Immunized Lampreys. Commun. Biol. 2020, 3, 91. [Google Scholar] [CrossRef] [PubMed]
- McKitrick, T.R.; Bernard, S.M.; Noll, A.J.; Collins, B.C.; Goth, C.K.; McQuillan, A.M.; Heimburg-Molinaro, J.; Herrin, B.R.; Wilson, I.A.; Cooper, M.D.; et al. Novel Lamprey Antibody Recognizes Terminal Sulfated Galactose Epitopes on Mammalian Glycoproteins. Commun. Biol. 2021, 4, 674. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Song, D.H.; Kim, H.M.; Choi, B.-S.; Lee, H.; Lee, J.-O. The Structural Basis of Lipopolysaccharide Recognition by the TLR4–MD-2 Complex. Nature 2009, 458, 1191–1195. [Google Scholar] [CrossRef]
- Appelt, E.A.; Thoden, J.B.; Gehrke, S.A.; Bachmeier, H.D.; Rayment, I.; Shusta, E.V.; Holden, H.M. The High-Resolution Structure of a Variable Lymphocyte Receptor From Petromyzon Marinus Capable of Binding to the Brain Extracellular Matrix. Proteins Struct. Funct. Bioinform. 2025, 93, 801–811. [Google Scholar] [CrossRef]
- Collins, B.C.; Gunn, R.J.; McKitrick, T.R.; Cummings, R.D.; Cooper, M.D.; Herrin, B.R.; Wilson, I.A. Structural Insights into VLR Fine Specificity for Blood Group Carbohydrates. Structure 2017, 25, 1667–1678.e4. [Google Scholar] [CrossRef]
- Herrin, B.R.; Cooper, M.D. Alternative Adaptive Immunity in Jawless Vertebrates. J. Immunol. 2010, 185, 1367–1374. [Google Scholar] [CrossRef]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef]
- Ding, Y.; Palecek, S.P.; Shusta, E.V. iPSC-Derived Blood-Brain Barrier Modeling Reveals APOE Isoform-Dependent Interactions with Amyloid Beta. Fluids Barriers CNS 2024, 21, 79. [Google Scholar] [CrossRef]
- Altman, M.O.; Bennink, J.R.; Yewdell, J.W.; Herrin, B.R. Lamprey VLRB Response to Influenza Virus Supports Universal Rules of Immunogenicity and Antigenicity. eLife 2015, 4, e07467. [Google Scholar] [CrossRef]
- Burns, M.L.; Malott, T.M.; Metcalf, K.J.; Hackel, B.J.; Chan, J.R.; Shusta, E.V. Directed Evolution of Brain-Derived Neurotrophic Factor for Improved Folding and Expression in Saccharomyces Cerevisiae. Appl. Environ. Microbiol. 2014, 80, 5732–5742. [Google Scholar] [CrossRef] [PubMed]
- Lown, P.S.; Cai, J.J.; Ritter, S.C.; Otolski, J.J.; Wong, R.; Hackel, B.J. Extended Yeast Surface Display Linkers Enhance the Enrichment of Ligands in Direct Mammalian Cell Selections. Protein Eng. Des. Sel. 2021, 34, gzab004. [Google Scholar] [CrossRef]
- Tillotson, B.J.; Cho, Y.K.; Shusta, E.V. Cells and Cell Lysates: A Direct Approach for Engineering Antibodies against Membrane Proteins Using Yeast Surface Display. Methods 2013, 60, 27–37. [Google Scholar] [CrossRef]
- VanAntwerp, J.J.; Wittrup, K.D. Fine Affinity Discrimination by Yeast Surface Display and Flow Cytometry. Biotechnol. Prog. 2000, 16, 31–37. [Google Scholar] [CrossRef]
- Huang, D.; Shusta, E.V. A Yeast Platform for the Production of Single-Chain Antibody-Green Fluorescent Protein Fusions. Appl. Environ. Microbiol. 2006, 72, 7748–7759. [Google Scholar] [CrossRef] [PubMed]
- Weksler, B.; Romero, I.A.; Couraud, P.-O. The hCMEC/D3 Cell Line as a Model of the Human Blood Brain Barrier. Fluids Barriers CNS 2013, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Esparza, T.J.; Brody, D.L. Selection of Single Domain Anti-Transferrin Receptor Antibodies for Blood-Brain Barrier Transcytosis Using a Neurotensin Based Assay and Histological Assessment of Target Engagement in a Mouse Model of Alzheimer’s Related Amyloid-Beta Pathology. PLoS ONE 2022, 17, e0276107. [Google Scholar] [CrossRef]
- Demeule, M.; Beaudet, N.; Régina, A.; Besserer-Offroy, É.; Murza, A.; Tétreault, P.; Belleville, K.; Ché, C.; Larocque, A.; Thiot, C.; et al. Conjugation of a Brain-Penetrant Peptide with Neurotensin Provides Antinociceptive Properties. J. Clin. Investig. 2014, 124, 1199–1213. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.M.; Houghton, S.; Magdeldin, T.; Durán, J.G.B.; Minotti, A.P.; Snead, A.; Sproul, A.; Nguyen, D.-H.T.; Xiang, J.; Fine, H.A.; et al. Pluripotent Stem Cell-Derived Epithelium Misidentified as Brain Microvascular Endothelium Requires ETS Factors to Acquire Vascular Fate. Proc. Natl. Acad. Sci. USA 2021, 118, e2016950118. [Google Scholar] [CrossRef]
- Wang, Y.; Gallagher, E.; Jorgensen, C.; Troendle, E.P.; Hu, D.; Searson, P.C.; Ulmschneider, M.B. An Experimentally Validated Approach to Calculate the Blood-Brain Barrier Permeability of Small Molecules. Sci. Rep. 2019, 9, 6117. [Google Scholar] [CrossRef]
- Zorniak, M.; Clark, P.A.; Umlauf, B.J.; Cho, Y.; Shusta, E.V.; Kuo, J.S. Yeast Display Biopanning Identifies Human Antibodies Targeting Glioblastoma Stem-like Cells. Sci. Rep. 2017, 7, 15840. [Google Scholar] [CrossRef]
- Kalivas, P.W.; Jennes, L.; Nemeroff, C.B.; Prange, A.J., Jr. Neurotensin: Topographical Distribution of Brain Sites Involoved in Hypothermia and Antinociception. J. Comp. Neurol. 1982, 210, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Esparza, T.J.; Su, S.; Francescutti, C.M.; Rodionova, E.; Kim, J.H.; Brody, D.L. Enhanced in Vivo Blood Brain Barrier Transcytosis of Macromolecular Cargo Using an Engineered pH-Sensitive Mouse Transferrin Receptor Binding Nanobody. Fluids Barriers CNS 2023, 20, 64. [Google Scholar] [CrossRef] [PubMed]
- Wouters, Y.; Jaspers, T.; De Strooper, B.; Dewilde, M. Identification and in Vivo Characterization of a Brain-Penetrating Nanobody. Fluids Barriers CNS 2020, 17, 62. [Google Scholar] [CrossRef]
- Ferhat, L.; Soussi, R.; Masse, M.; Kyriatzis, G.; Girard, S.; Gassiot, F.; Gaudin, N.; Laurencin, M.; Bernard, A.; Bôle, A.; et al. A Peptide-Neurotensin Conjugate That Crosses the Blood-Brain Barrier Induces Pharmacological Hypothermia Associated with Anticonvulsant, Neuroprotective, and Anti-Inflammatory Properties Following Status Epilepticus in Mice. eLife 2025, 13, RP100527. [Google Scholar] [CrossRef]
- Yu, Y.J.; Zhang, Y.; Kenrick, M.; Hoyte, K.; Luk, W.; Lu, Y.; Atwal, J.; Elliott, J.M.; Prabhu, S.; Watts, R.J.; et al. Boosting Brain Uptake of a Therapeutic Antibody by Reducing Its Affinity for a Transcytosis Target. Sci. Transl. Med. 2011, 3, 84ra44. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.K.; Chen, I.; Wei, X.; Li, L.; Shusta, E.V. A Yeast Display Immunoprecipitation Method for Efficient Isolation and Characterization of Antigens. J. Immunol. Methods 2009, 341, 117–126. [Google Scholar] [CrossRef] [PubMed][Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katt, M.E.; Waters, E.A.; Gastfriend, B.D.; Herrin, B.R.; Cooper, M.D.; Shusta, E.V. Identification of Variable Lymphocyte Receptors That Target the Human Blood–Brain Barrier. Pharmaceutics 2025, 17, 1179. https://doi.org/10.3390/pharmaceutics17091179
Katt ME, Waters EA, Gastfriend BD, Herrin BR, Cooper MD, Shusta EV. Identification of Variable Lymphocyte Receptors That Target the Human Blood–Brain Barrier. Pharmaceutics. 2025; 17(9):1179. https://doi.org/10.3390/pharmaceutics17091179
Chicago/Turabian StyleKatt, Moriah E., Elizabeth A. Waters, Benjamin D. Gastfriend, Brantley R. Herrin, Max D. Cooper, and Eric V. Shusta. 2025. "Identification of Variable Lymphocyte Receptors That Target the Human Blood–Brain Barrier" Pharmaceutics 17, no. 9: 1179. https://doi.org/10.3390/pharmaceutics17091179
APA StyleKatt, M. E., Waters, E. A., Gastfriend, B. D., Herrin, B. R., Cooper, M. D., & Shusta, E. V. (2025). Identification of Variable Lymphocyte Receptors That Target the Human Blood–Brain Barrier. Pharmaceutics, 17(9), 1179. https://doi.org/10.3390/pharmaceutics17091179





