Advances in Second Near-Infrared Window Photothermal Agents and Photothermal Therapy for Tumors in Interdisciplinary Medical Research
Abstract
1. Introduction
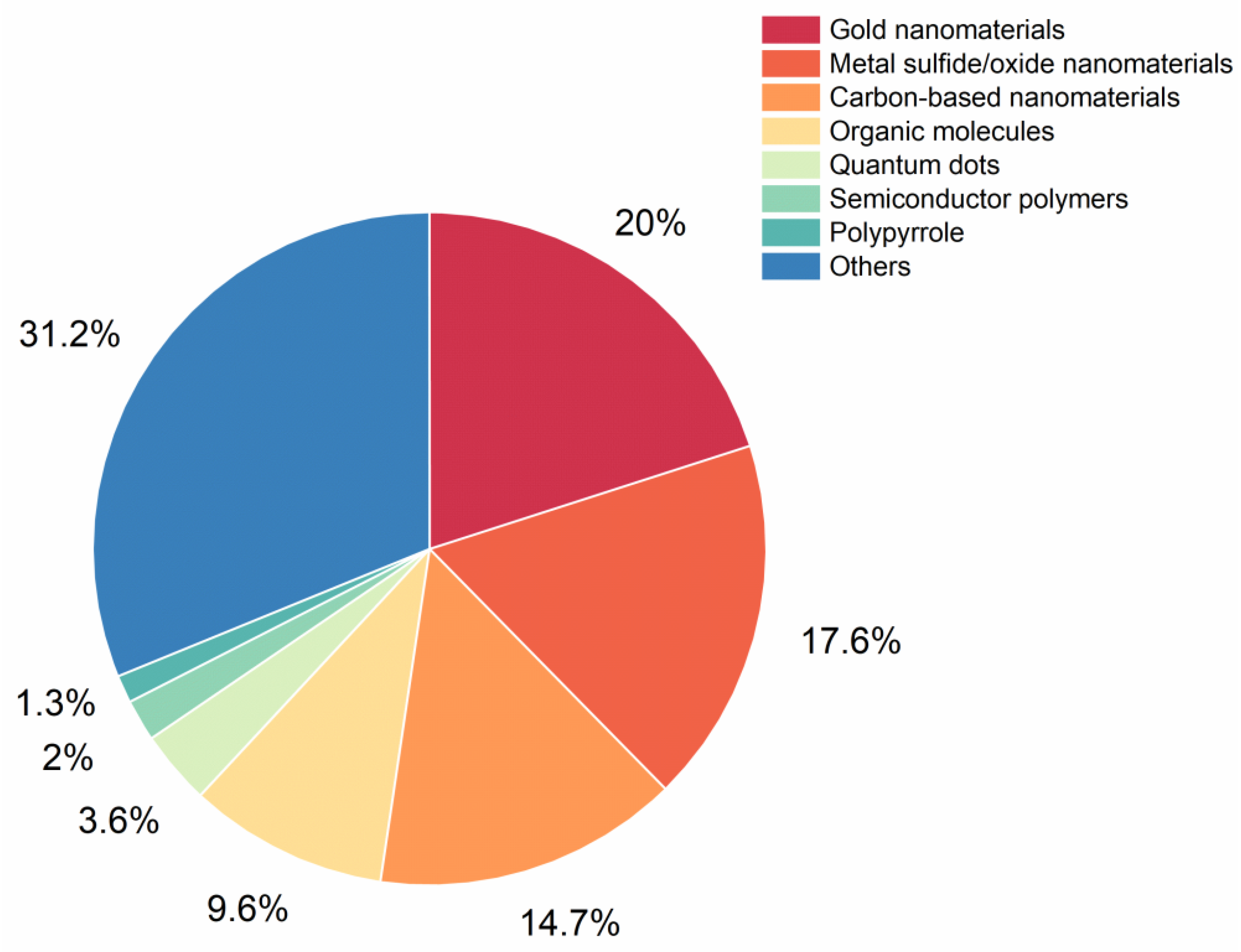
2. NIR-II Photothermal Agents
2.1. Inorganic Agents
2.1.1. Metal Nanomaterials
2.1.2. Metal Sulfide/Oxide Nanomaterials
2.1.3. Carbon-Based Nanomaterials
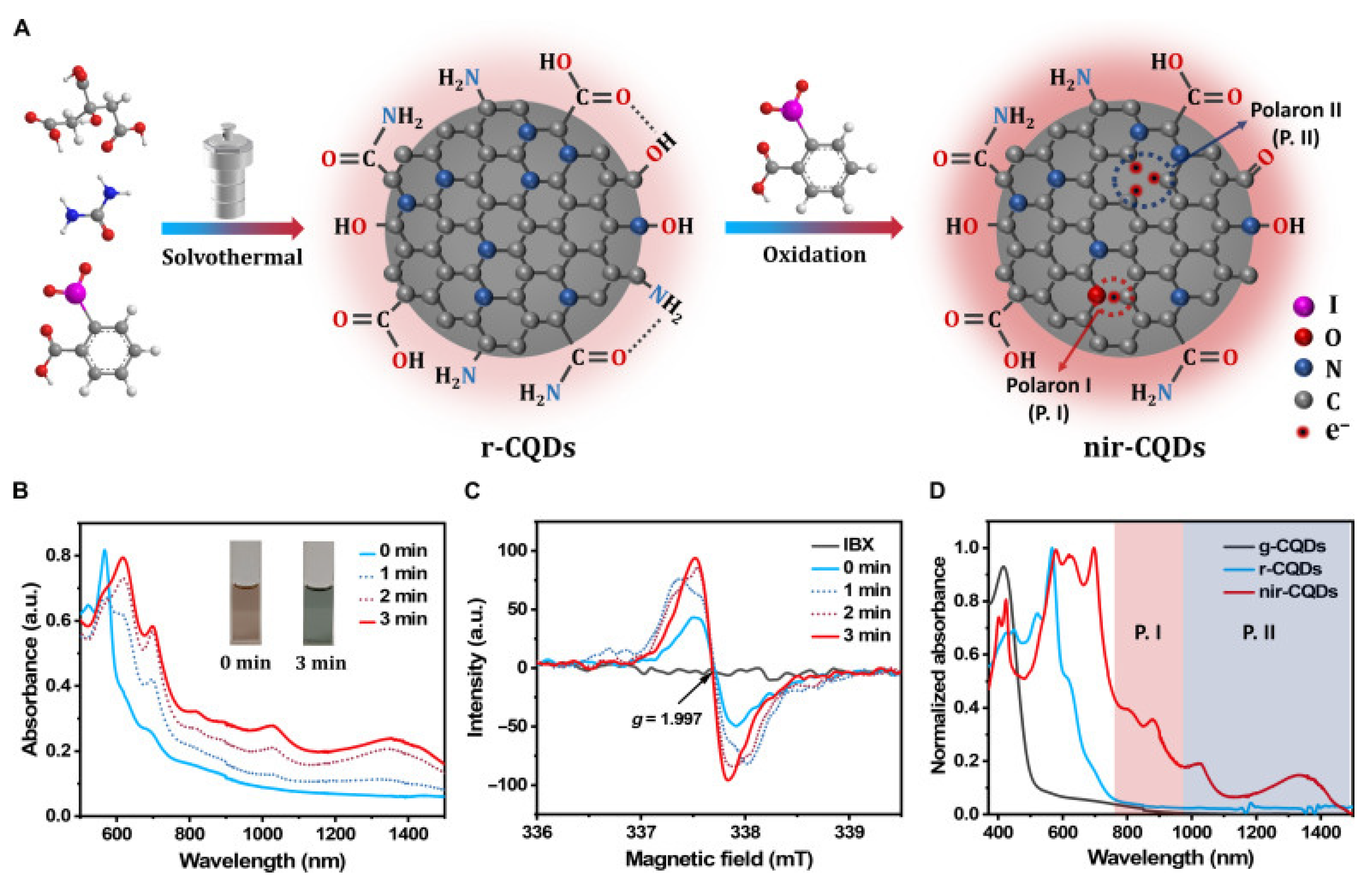
2.1.4. Quantum Dots
2.2. Organic Agents
2.2.1. Semiconductor Polymers
2.2.2. Organic Molecules
| Type | Nanoparticles /Nano-Conjugates | Ex | PCE | Application | Ref. |
|---|---|---|---|---|---|
| Metal nanomaterials | Au-on-AuNR hybrid structures: structure 2a–2d and nanocorals 3b–3d | 1060 | 26.1, 26.7, 25.6, 26.6, 56.9, 67.2 and 59.8 | / | [28] |
| Au@Cu2−xS core@shell NCs | 1064 | 43.25 | HeLa cells | [29] | |
| GNR@SiO2@MnO2 | 1064 | 27.47 | U87MG cells, U87MG-tumor-bearing mice | [30] | |
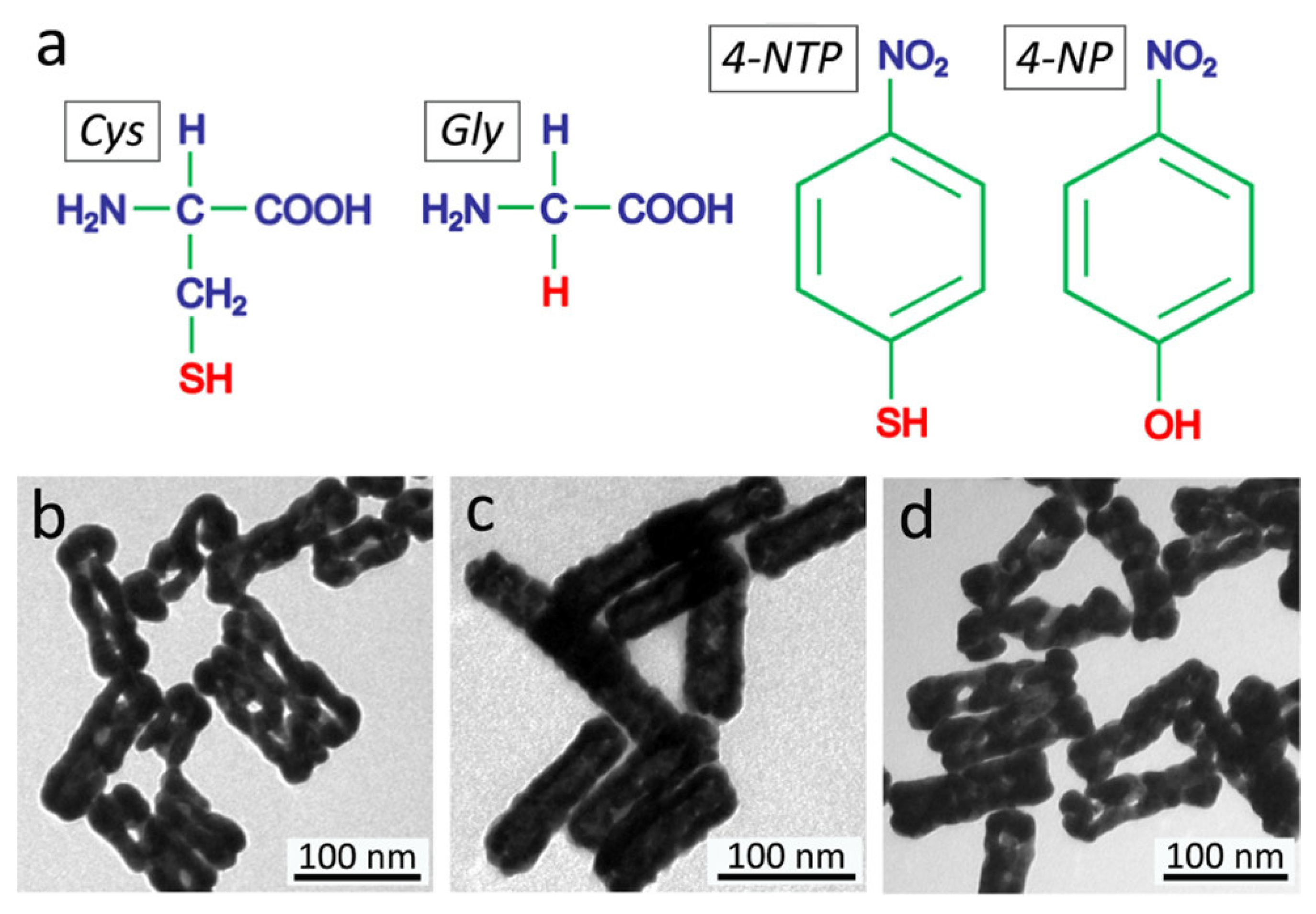
| AuHNRs | 1064 | 33 | SCC-7 cells, SCC-7 tumor-bearing nude mice | [36] | |
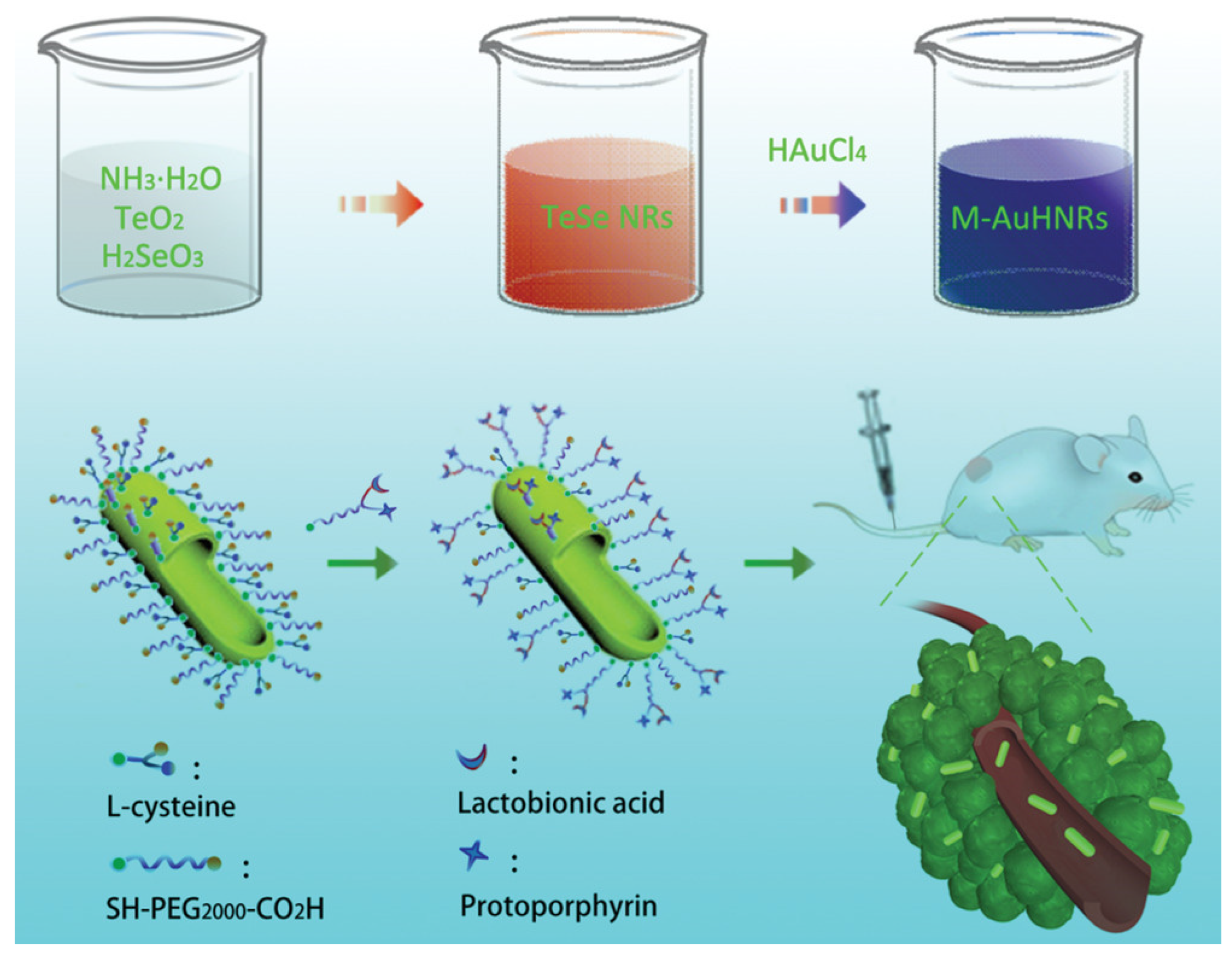
| M-AuHNRs | 1064 | 34 | HeLa, murine breast cancer 4T1, HepG-2, and COS-7 cells, HepG-2 tumor-bearing nude mice | [37] | |
| Metal sulfide/oxide nanomaterials | Ni9S8 | 400–1100 | 46 (1064) | HeLa cells, HeLa tumor-bearing mice | [41] |
| Cu2MnS2 | 800–1300 | 49.38 (1064) | MCF-7 and HeLa cells, S180 tumors-bearing BALB/c nude mice | [42] | |
| CuS@PDA/Pd | 1064 | 50.6 | MCF-7, 4T1, MDA-MB-231, HepG2, and B16F10 cells, 4T1 tumor-bearing mice | [43] | |
| CuCo2S4-Pt-PEG | 1064 | 78.46 | 4T1 cells, 4T1 tumor-bearing mice | [46] | |
| RCuS@tMCP | 1064 | 69.6 | RAW264.7 and 4T1 cells, 4T1 tumor-bearing mice | [48] | |
| BCS NPs | 1064 | 29.8 | 4T1 cells, 4T1 tumor-bearing mice | [50] | |
| AuDAg2S | 1064 | 67.1 | HUVEC, hepatic and CT26 colon tumor cells, CT26 tumor-bearing nude mice | [51] | |
| AT-CuS NCs | 1064 | 94.3 | U87 cells | [52] | |
| HMNC | 1064 | 36.3 | HeLa cells, HeLa tumor-bearing mice | [57] | |
| AuNCs@SiO2 | 1064 | 82.2 | 4T1 and A549 cells, 4T1 tumor-bearing BALB/c nude mice | [58] | |
| H-SiOx | 1064 | 48.6 | 4T1 cells, 4T1 tumor-bearing mice | [59] | |
| Carbon-based nanomaterials | CNPs | 1064 | 50.6 | MCF-7 cells and Jurkat cells, MCF-7 tumor-bearing mice | [64] |
| Water-dispersible nanoparticles containing two nanographene-porphyrin hybrids (NGP-1-NPs and NGP-2-NPs) | 808, 1064 | 60, 69 | 4T1 and MCF-7 cells, 4T1 tumor-bearing mice | [65] | |
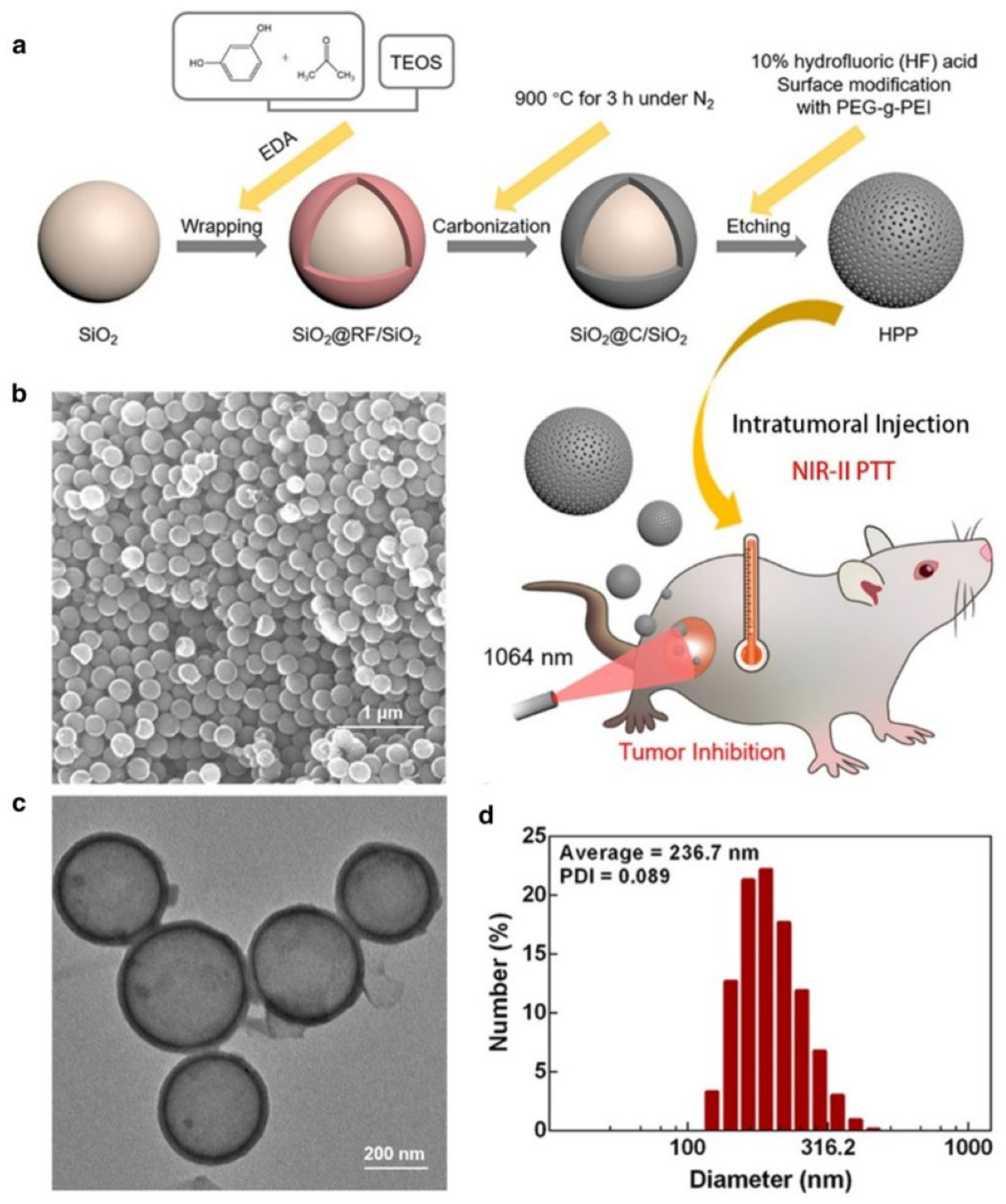
| HPP | 1064 | 45.1 | 4T1 and MCF-7 cells, 4T1 tumor-bearing mice | [66] | |
| Quantum dots | 9T-GQDs | 1064 | 33.45 | 4T1, HeLa and NCI–H196 cells, 4T1 tumor-bearing mice | [71] |
| nir-CQD | 1064 | 40 | 4T1 cells, 4T1 tumor-bearing mice | [83] | |
| Cet-CDs-SNO | 1064 | 31.8 | HCT-116 cells, HCT-116 xenograft tumor-bearing nude mice | [84] | |
| Semiconductor polymers | NPPBTPBF-BT | 1064 | 66.4 | MDA-MB-231 cells, MDA-MB-231 tumor-bearing mice | [90] |
| DPP-IID-FA | 1064 | 49.5 | HeLa cells, tumor xenografts in nude mice | [91] | |
| SPNI–II | 808, 1064 | 44.9, 43.4 | 4T1 cells, 4T1 xenograft tumor-bearing nude mice | [92] | |
| Small organic molecules | IC-790, IC-830, IC-1030, IC-1060, IC-1080 and IC-1224 | 1064 | 83.2 (IC-1224) | 4T1 cells, 4T1 tumor-bearing mice | [96] |
| Ultrathin PPy nanosheets | 1064 | 64.6 | MDA-MB-231 cells, MDA-MB-231 xenograft-bearing mice | [98] | |
| IR-TT, IR-TS, and IR-SS | 1064 | 61, 73, and 77 | A549 and 4T1 cells, 4T1-tumor-bearing mice | [102] |
| Molecular Name | Chemical Structural Formula | Constitutional Formula |
|---|---|---|
| ICG | C43H47N2NaO6S2 | 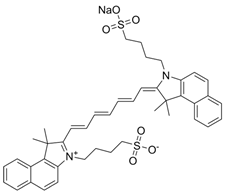 |
| Polypyrrole | C4H5N | 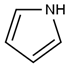 |
| Conjugated small molecules (IR-SS) | C74H100N2S2Se2 | 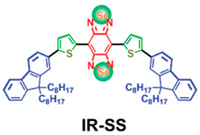 |
3. PTT-Based Synergy Therapy
3.1. Combination of PTT and PDT
3.2. Combined PTT and Immunotherapy
3.3. Combined PTT and Chemotherapy
4. Clinical Progress of PTT
4.1. Skin Cancer
4.2. Prostate Cancer
4.3. Breast Cancer
4.4. Liver Cancer
4.5. Lung Cancer
| Type | Treatment | Nanoparticles/Nano-Conjugates | Number of Patient Enrolled | Ref. |
|---|---|---|---|---|
| Skin cancer | Combined photothermal therapy and immunotherapy | Topical imiquimod and indocyanine green | 11 | [174] |
| Prostate cancer | Photothermal therapy | Laser-driven gold silica nanoshells | 16 | [179] |
| Breast cancer | Combined photothermal therapy and immunotherapy | Indocyanine green combined with glycated chitosan | 10 | [188] |
| Laser treatment | - | 61 | [189] | |
| Liver cancer | Laser interstitial thermal therapy (LITT) | - | 603 | [195] |
| Laser interstitial thermal therapy (LITT) | - | 148 | [196] | |
| Laser interstitial thermal therapy (LITT) | - | 74 | [197] | |
| Lung cancer | Laser interstitial thermal therapy (LITT) | - | - | [162] |
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| WHO | World Health Organization |
| PTT | Photothermal therapy |
| PTAs | Photothermal agents |
| NIR | Near-infrared |
| MPE | Maximum permissible exposure |
| ANSI | American National Standards Institute |
| NIR-II | Second near-infrared |
| PCE | Photothermal conversion efficiency |
| AuNPs | Gold nanoparticles |
| SPR | Surface plasmon resonance |
| LSPR | localized surface plasmon resonance |
| GSM | GNR@SiO2@MnO2 |
| AuHNRs | Hollow gold nanorods |
| M-AuHNRs | Microscale hollow gold nanorods |
| CuS | Copper sulfide |
| Ag2S | Silver sulfide |
| PbS | Lead sulfide |
| FeS | Iron sulfide |
| 5FU | 5-fluorouracil |
| CuAAC | Copper-catalyzed azide-alkyne cycloaddition |
| CuS NPs | Copper sulfide nanoparticles |
| RBCm | Red blood cell membrane |
| tMCP | CpG/protamine |
| NCS | Non-stoichiometric copper sulfide |
| BCS NPs | Biomineralized copper sulfide nanoparticles |
| SERS | Surface-enhanced Raman scattering |
| 2D | two-dimensional |
| AT-CuS NCs | Atomic-thin 2D CuS nanocrystals |
| HMNC | Hollow magnetite nanocluster |
| AuNCs@SiO2 | Silica-encapsulated self-assembled gold nanochains |
| H-SiOx-PEG NPs | Hollow silicon oxide nanoparticles |
| CNPs | carbon materials |
| COFs | covalent organic frameworks |
| HPP | Hollow carbon nanosphere modified with polyethylene glycol-graft-polyethylenimine |
| QDs | Quantum dots |
| GQDs | Graphene quantum dots |
| EPR | Enhanced permeability and retention |
| CQDs | Carbon quantum dots |
| CCS | Cet-CDs-SNO |
| OPTAs | Organic photothermal agents |
| IPTAs | Inorganic photothermal agents |
| PPTAs | polymer-based photothermal agents |
| MPTAs | molecule photothermal agents |
| D-A | donor-acceptor |
| PBTPBF-BT | Thieno-isoindigo derivative-based D-A polymer |
| DPP-IID-FA | Diketopyrrole polymer |
| PDCDT | Semiconducting copolymer poly [(diketopyrrolopyrrole-cyclopentadithiophene)-ran-(diketopyrrolopyrrole-thiadiazoquinoline)] |
| ICG | Indocyanine green |
| FDA | Food and Drug Administration |
| PPy | Polypyrrole |
| Se | Selenium |
| Te | Tellurium |
| PDT | Photodynamic therapy |
| O2 | Molecular oxygen |
| PS | Photosensitizer |
| ROS | Reactive oxygen species |
| CN-NPs | Carbon nitride nanoparticles |
| PTCDA | Perylene-3,4,9,10-tetracarboxylic dianhydride |
| SnO2−x@SiO2-HA | Oxygen-deficient black tin oxide nanoparticles |
| HA | Hyaluronic acid |
| ICB | Immune checkpoint blockade |
| CAR-T | Chimeric antigen receptor T-cell |
| TAAs | Tumor-associated antigens |
| DCs | Dendritic cells |
| APC | Antigen-presenting cell |
| bmNPs | biomimetic nanoplatforms |
| DAMPs | Damage-associated molecular patterns |
| ICD | Immunogenic cell death |
| GSH | Glutathione |
| CBI | checkpoint blockade immunotherapy |
| NK | Natural killer |
| CTLs | Cytotoxic T lymphocytes |
| ITME | Immunosuppressive tumor microenvironment |
| TAMs | Tumor-associated macrophages |
| CRT | Calreticulin |
| HMGB1 | High-mobility group box 1 protein |
| MDR | Multidrug resistance |
| DOX | Doxorubicin |
| PCM | Phase change materials |
| H2O2 | Hydrogen peroxide |
| AuMC | AuHNR@MnO2@CS |
| CS | Chitosan |
| TME | Tumor microenvironment |
| CDT | Chemodynamic therapy |
| BCC | Basal cell carcinoma |
| SCC | Squamous cell carcinoma |
| ISPI | In situ photoimmunotherapy |
| CLR | Complete local response |
| GSN | Gold silica nanoshells |
| LITT | laser interstitial thermal therapy |
| SCLC | Small cell lung cancer |
| NSCLC | Non-small cell lung cancer |
| LUAD | Lung adenocarcinoma |
References
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Chang, C.A.; Jen, J.; Jiang, S.; Sayad, A.; Mer, A.S.; Brown, K.R.; Nixon, A.M.L.; Dhabaria, A.; Tang, K.H.; Venet, D.; et al. Ontogeny and Vulnerabilities of Drug-Tolerant Persisters in HER2+ Breast Cancer. Cancer Discov. 2022, 12, 1022–1045. [Google Scholar] [CrossRef]
- Hanker, A.B.; Sudhan, D.R.; Arteaga, C.L. Overcoming Endocrine Resistance in Breast Cancer. Cancer Cell 2020, 37, 496–513. [Google Scholar] [CrossRef] [PubMed]
- Franzoi, M.A.; Agostinetto, E.; Perachino, M.; Del Mastro, L.; de Azambuja, E.; Vaz-Luis, I.; Partridge, A.H.; Lambertini, M. Evidence-based approaches for the management of side-effects of adjuvant endocrine therapy in patients with breast cancer. Lancet Oncol. 2021, 22, e303–e313. [Google Scholar] [CrossRef] [PubMed]
- Rafn, B.S.; Christensen, J.; Larsen, A.; Bloomquist, K. Prospective Surveillance for Breast Cancer-Related Arm Lymphedema: A Systematic Review and Meta-Analysis. J. Clin. Oncol. 2022, 40, 1009–1026. [Google Scholar] [CrossRef]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2008, 23, 217–228. [Google Scholar] [CrossRef]
- Liang, P.; Mao, L.; Dong, Y.; Zhao, Z.; Sun, Q.; Mazhar, M.; Ma, Y.; Yang, S.; Ren, W. Design and Application of Near-Infrared Nanomaterial-Liposome Hybrid Nanocarriers for Cancer Photothermal Therapy. Pharmaceutics 2021, 13, 2070. [Google Scholar] [CrossRef]
- de Melo-Diogo, D.; Pais-Silva, C.; Dias, D.R.; Moreira, A.F.; Correia, I.J. Strategies to Improve Cancer Photothermal Therapy Mediated by Nanomaterials. Adv. Healthc. Mater. 2017, 6, 1700073. [Google Scholar] [CrossRef]
- American National Standard Institute. American National Standard for Safe Use of Lasers in Health Care; Laser Institute of America: Orlando, FL, USA, 2018. [Google Scholar]
- Wang, S.; Fan, Y.; Li, D.; Sun, C.; Lei, Z.; Lu, L.; Wang, T.; Zhang, F. Anti-quenching NIR-II molecular fluorophores for in vivo high-contrast imaging and pH sensing. Nat. Commun. 2019, 10, 1058. [Google Scholar] [CrossRef]
- Gong, B.; Shen, Y.; Li, H.; Li, X.; Huan, X.; Zhou, J.; Chen, Y.; Wu, J.; Li, W. Thermo-responsive polymer encapsulated gold nanorods for single continuous wave laser-induced photodynamic/photothermal tumour therapy. J. Nanobiotechnol. 2021, 19, 41. [Google Scholar] [CrossRef]
- Yin, F.; Yang, C.; Wang, Q.; Zeng, S.; Hu, R.; Lin, G.; Tian, J.; Hu, S.; Lan, R.F.; Yoon, H.S.; et al. A Light-Driven Therapy of Pancreatic Adenocarcinoma Using Gold Nanorods-Based Nanocarriers for Co-Delivery of Doxorubicin and siRNA. Theranostics 2015, 5, 818–833. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, W.; Kang, M.; Wen, H.; Guo, H.; Zhang, P.; Xi, L.; Li, K.; Wang, L.; Wang, D.; et al. An All-Round Athlete on the Track of Phototheranostics: Subtly Regulating the Balance between Radiative and Nonradiative Decays for Multimodal Imaging-Guided Synergistic Therapy. Adv. Mater. 2020, 32, e2003210. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gao, Y.; Chen, Y.; Liu, L.; Mo, A.; Peng, Q. Nanomaterials-based photothermal therapy and its potentials in antibacterial treatment. J. Control. Release 2020, 328, 251–262. [Google Scholar] [CrossRef]
- Gupta, N.; Malviya, R. Understanding and advancement in gold nanoparticle targeted photothermal therapy of cancer. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188532. [Google Scholar] [CrossRef]
- Liu, T.; Shi, S.; Liang, C.; Shen, S.; Cheng, L.; Wang, C.; Song, X.; Goel, S.; Barnhart, T.E.; Cai, W.; et al. Iron oxide decorated MoS2 nanosheets with double PEGylation for chelator-free radiolabeling and multimodal imaging guided photothermal therapy. ACS Nano 2015, 9, 950–960. [Google Scholar] [CrossRef]
- Miao, W.; Shim, G.; Lee, S.; Oh, Y.K. Structure-dependent photothermal anticancer effects of carbon-based photoresponsive nanomaterials. Biomaterials 2014, 35, 4058–4065. [Google Scholar] [CrossRef]
- Chu, M.; Pan, X.; Zhang, D.; Wu, Q.; Peng, J.; Hai, W. The therapeutic efficacy of CdTe and CdSe quantum dots for photothermal cancer therapy. Biomaterials 2012, 33, 7071–7083. [Google Scholar] [CrossRef]
- Li, J.; Yu, X.; Jiang, Y.; He, S.; Zhang, Y.; Luo, Y.; Pu, K. Second Near-Infrared Photothermal Semiconducting Polymer Nanoadjuvant for Enhanced Cancer Immunotherapy. Adv. Mater. 2021, 33, e2003458. [Google Scholar] [CrossRef]
- Guo, S.; Gu, D.; Yang, Y.; Tian, J.; Chen, X. Near-infrared photodynamic and photothermal co-therapy based on organic small molecular dyes. J. Nanobiotechnol. 2023, 21, 348. [Google Scholar] [CrossRef]
- Alamdari, S.G.; Amini, M.; Jalilzadeh, N.; Baradaran, B.; Mohammadzadeh, R.; Mokhtarzadeh, A.; Oroojalian, F. Recent advances in nanoparticle-based photothermal therapy for breast cancer. J. Control. Release 2022, 349, 269–303. [Google Scholar] [CrossRef]
- Pramanik, S.; Mohanto, S.; Manne, R.; Rajendran, R.R.; Deepak, A.; Edapully, S.J.; Patil, T.; Katari, O. Nanoparticle-Based Drug Delivery System: The Magic Bullet for the Treatment of Chronic Pulmonary Diseases. Mol. Pharm. 2021, 18, 3671–3718. [Google Scholar] [CrossRef]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef]
- Niculescu, A.G.; Grumezescu, A.M. Novel Tumor-Targeting Nanoparticles for Cancer Treatment-A Review. Int. J. Mol. Sci. 2022, 23, 5253. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Gowda, B.H.J.; Ahmed, M.G.; Abourehab, M.A.S.; Chen, Z.S.; Zhang, C.; Li, J.; Kesharwani, P. Advancements in nanoparticle-based treatment approaches for skin cancer therapy. Mol. Cancer 2023, 22, 10. [Google Scholar] [CrossRef]
- Masuda, H.; Tanaka, H.; Baba, N. Preparation of porous material by replacing microstructure of anodic alumina film with metal. Chem. Lett. 1990, 19, 621–622. [Google Scholar] [CrossRef]
- Jia, J.; Liu, G.; Xu, W.; Tian, X.; Li, S.; Han, F.; Feng, Y.; Dong, X.; Chen, H. Fine-Tuning the Homometallic Interface of Au-on-Au Nanorods and Their Photothermal Therapy in the NIR-II Window. Angew. Chem. Int. Ed. Engl. 2020, 59, 14443–14448. [Google Scholar] [CrossRef]
- Ji, M.; Xu, M.; Zhang, W.; Yang, Z.; Huang, L.; Liu, J.; Zhang, Y.; Gu, L.; Yu, Y.; Hao, W.; et al. Structurally Well-Defined Au@Cu2- x S Core-Shell Nanocrystals for Improved Cancer Treatment Based on Enhanced Photothermal Efficiency. Adv. Mater. 2016, 28, 3094–3101. [Google Scholar] [CrossRef]
- He, T.; Jiang, C.; He, J.; Zhang, Y.; He, G.; Wu, J.; Lin, J.; Zhou, X.; Huang, P. Manganese-Dioxide-Coating-Instructed Plasmonic Modulation of Gold Nanorods for Activatable Duplex-Imaging-Guided NIR-II Photothermal-Chemodynamic Therapy. Adv. Mater. 2021, 33, e2008540. [Google Scholar] [CrossRef]
- Genç, A.; Patarroyo, J.; Sancho-Parramon, J.; Bastús, N.G.; Puntes, V.; Arbiol, J. Hollow metal nanostructures for enhanced plasmonics: Synthesis, local plasmonic properties and applications. Nanophotonics 2017, 6, 193–213. [Google Scholar] [CrossRef]
- Park, J.E.; Kim, M.; Hwang, J.H.; Nam, J.M. Golden opportunities: Plasmonic gold nanostructures for biomedical applications based on the second near-infrared window. Small Methods 2017, 1, 1600032. [Google Scholar] [CrossRef]
- Abadeer, N.S.; Murphy, C.J. Recent progress in cancer thermal therapy using gold nanoparticles. In Nanomaterials and Neoplasms; Jenny Stanford Publishing: New York, NY, USA, 2021; pp. 143–217. [Google Scholar]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef]
- Yang, X.; Yang, M.; Pang, B.; Vara, M.; Xia, Y. Gold Nanomaterials at Work in Biomedicine. Chem. Rev. 2015, 115, 10410–10488. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Zhang, W.; Zhang, J.; Li, H.; Han, H.; Zhai, T. Design of Gold Hollow Nanorods with Controllable Aspect Ratio for Multimodal Imaging and Combined Chemo-Photothermal Therapy in the Second Near-Infrared Window. ACS Appl. Mater. Interfaces 2018, 10, 36703–36710. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Zhang, W.; Foda, M.F.; Li, X.; Zhang, J.; Zhong, Y.; Liang, H.; Li, H.; Han, H.; Zhai, T. Miniature Hollow Gold Nanorods with Enhanced Effect for In Vivo Photoacoustic Imaging in the NIR-II Window. Small 2020, 16, e2002748. [Google Scholar] [CrossRef] [PubMed]
- Dahoumane, S.A.; Wujcik, E.K.; Jeffryes, C. Noble metal, oxide and chalcogenide-based nanomaterials from scalable phototrophic culture systems. Enzym. Microb. Technol. 2016, 95, 13–27. [Google Scholar] [CrossRef]
- Vena, M.P.; Jobbagy, M.; Bilmes, S.A. Microorganism mediated biosynthesis of metal chalcogenides; a powerful tool to transform toxic effluents into functional nanomaterials. Sci. Total Environ. 2016, 565, 804–810. [Google Scholar] [CrossRef]
- Yang, T.; Tang, Y.; Liu, L.; Lv, X.; Wang, Q.; Ke, H.; Deng, Y.; Yang, H.; Yang, X.; Liu, G.; et al. Size-Dependent Ag(2)S Nanodots for Second Near-Infrared Fluorescence/Photoacoustics Imaging and Simultaneous Photothermal Therapy. ACS Nano 2017, 11, 1848–1857. [Google Scholar] [CrossRef]
- Lei, Z.; Zhang, W.; Li, B.; Guan, G.; Huang, X.; Peng, X.; Zou, R.; Hu, J. A full-spectrum-absorption from nickel sulphide nanoparticles for efficient NIR-II window photothermal therapy. Nanoscale 2019, 11, 20161–20170. [Google Scholar] [CrossRef]
- Ke, K.; Yang, W.; Xie, X.; Liu, R.; Wang, L.L.; Lin, W.W.; Huang, G.; Lu, C.H.; Yang, H.H. Copper Manganese Sulfide Nanoplates: A New Two-Dimensional Theranostic Nanoplatform for MRI/MSOT Dual-Modal Imaging-Guided Photothermal Therapy in the Second Near-Infrared Window. Theranostics 2017, 7, 4763–4776. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, Z.; Wei, Y.; Zhang, L.; Wang, Z.; Ren, J.; Qu, X. NIR-II Light Leveraged Dual Drug Synthesis for Orthotopic Combination Therapy. ACS Nano 2022, 16, 20353–20363. [Google Scholar] [CrossRef]
- Bhattacharyya, B.; Balischewski, C.; Pacholski, C.; Pandey, A.; Bald, I.; Taubert, A. Copper iron chalcogenide semiconductor nanocrystals in energy and optoelectronics applications—State of the art, challenges, and future potential. Adv. Opt. Mater. 2023, 11, 2202411. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, S.; Ren, F.; Chen, L.; Zeng, J.; Zhu, M.; Cheng, Z.; Gao, M.; Li, Z. Ultrasmall magnetic CuFeSe2 ternary nanocrystals for multimodal imaging guided photothermal therapy of cancer. ACS Nano 2017, 11, 5633–5645. [Google Scholar] [CrossRef]
- Xu, M.; Zhao, R.; Liu, B.; Geng, F.; Wu, X.; Zhang, F.; Shen, R.; Lin, H.; Feng, L.; Yang, P. Ultrasmall copper-based nanoplatforms for NIR-II light-triggered photothermal/photodynamic and amplified nanozyme catalytic therapy of hypoxic tumor. Chem. Eng. J. 2024, 491, 151776. [Google Scholar] [CrossRef]
- Chen, F.; Geng, Z.; Wang, L.; Zhou, Y.; Liu, J. Biomimetic nanoparticles enabled by cascade cell membrane coating for direct cross-priming of T cells. Small 2022, 18, 2104402. [Google Scholar] [CrossRef]
- Li, N.; Wei, X.; Huang, H.; Guo, J.; Li, Q.; Yang, H.; Cai, L.; Liu, Y.; Wu, C. Activatable biomimetic raspberry-like nanoplatform enabled robust cascade therapy via spatiotemporal regulation of tumor immunogenicity and immunosuppression. Chem. Eng. J. 2024, 479, 147563. [Google Scholar] [CrossRef]
- Zhao, Y.; Pan, H.; Lou, Y.; Qiu, X.; Zhu, J.; Burda, C. Plasmonic Cu2− x S nanocrystals: Optical and structural properties of copper-deficient copper (I) sulfides. J. Am. Chem. Soc. 2009, 131, 4253–4261. [Google Scholar] [CrossRef]
- Liu, M.; Tang, Y.; Yan, M.; Zhang, J.; Chen, H.; Zhang, Q. Self-regulating immunosuppressive tumor microenvironment by NIR-II photothermal agent with anti-inflammatory activity for self-reinforcing immunotherapy synergy with cancer photothermal ablation. Biomaterials 2025, 318, 123187. [Google Scholar] [CrossRef]
- He, J.; Hua, S.; Zhang, D.; Wang, K.; Chen, X.; Zhou, M. SERS/NIR-II optical nanoprobes for multidimensional tumor imaging from living subjects, pathology, and single cells and guided NIR-II photothermal therapy. Adv. Funct. Mater. 2022, 32, 2208028. [Google Scholar] [CrossRef]
- Su, M.; Wu, Z.; Yan, T.; Li, N.; Li, X.; Hou, T.; Liu, J.; Zhang, C.; Zhu, C.; Wang, Z. Atomic-Thin 2D Copper Sulfide Nanocrystals with over 94% Photothermal Conversion Efficiency as Superior NIR-II Photoacoustic Agents. Adv. Funct. Mater. 2024, 34, 2409580. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Xia, Q.; Shang, J.; He, Y.; Li, Z.; Chen, Y.; Gao, F.; Yu, X.; Yuan, Z.; et al. Photothermal Fe(3)O(4) nanoparticles induced immunogenic ferroptosis for synergistic colorectal cancer therapy. J. Nanobiotechnol. 2024, 22, 630. [Google Scholar] [CrossRef]
- Zhao, S.; Yu, X.; Qian, Y.; Chen, W.; Shen, J. Multifunctional magnetic iron oxide nanoparticles: An advanced platform for cancer theranostics. Theranostics 2020, 10, 6278–6309. [Google Scholar] [CrossRef]
- Wu, K.; Mohsin, A.; Zaman, W.Q.; Zhang, Z.; Guan, W.; Chu, M.; Zhuang, Y.; Guo, M. Urchin-like magnetic microspheres for cancer therapy through synergistic effect of mechanical force, photothermal and photodynamic effects. J. Nanobiotechnol. 2022, 20, 224. [Google Scholar] [CrossRef]
- Wan, X.; Zhang, Y.; Wan, Y.; Xiong, M.; Xie, A.; Liang, Y.; Wan, H. A Multifunctional Biomimetic Nanoplatform for Dual Tumor Targeting-Assisted Multimodal Therapy of Colon Cancer. ACS Nano 2024, 18, 26666–26689. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Qian, J.; Lv, X.; Li, H.; Zou, J.; Zhang, J.; Meng, X.; Liu, H.; Qian, Y.; et al. NIR-II Responsive Hollow Magnetite Nanoclusters for Targeted Magnetic Resonance Imaging-Guided Photothermal/Chemo-Therapy and Chemodynamic Therapy. Small 2021, 17, e2100794. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, L.; Sun, T.; Zhang, Y.; Liu, Y.; Gong, M.; Xu, Z.; Du, M.; Liu, Y.; Liu, G.; et al. Activatable NIR-II Plasmonic Nanotheranostics for Efficient Photoacoustic Imaging and Photothermal Cancer Therapy. Adv. Mater. 2021, 33, e2006532. [Google Scholar] [CrossRef]
- Yu, X.; Yang, K.; Chen, X.; Li, W. Black hollow silicon oxide nanoparticles as highly efficient photothermal agents in the second near-infrared window for in vivo cancer therapy. Biomaterials 2017, 143, 120–129. [Google Scholar] [CrossRef]
- Sharma, A.; Das, J. Small molecules derived carbon dots: Synthesis and applications in sensing, catalysis, imaging, and biomedicine. J. Nanobiotechnol. 2019, 17, 92. [Google Scholar] [CrossRef]
- Panwar, N.; Soehartono, A.M.; Chan, K.K.; Zeng, S.; Xu, G.; Qu, J.; Coquet, P.; Yong, K.T.; Chen, X. Nanocarbons for Biology and Medicine: Sensing, Imaging, and Drug Delivery. Chem. Rev. 2019, 119, 9559–9656. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Tian, Y.; Men, Y.; Guo, R.; Peng, H.; Jiang, Q.; Yang, W. Metal-Organic Frameworks-Derived Carbon Nanoparticles for Photoacoustic Imaging-Guided Photothermal/Photodynamic Combined Therapy. ACS Appl. Mater. Interfaces 2018, 10, 42039–42049. [Google Scholar] [CrossRef]
- Peng, X.; Wang, R.; Wang, T.; Yang, W.; Wang, H.; Gu, W.; Ye, L. Carbon Dots/Prussian Blue Satellite/Core Nanocomposites for Optical Imaging and Photothermal Therapy. ACS Appl. Mater. Interfaces 2018, 10, 1084–1092. [Google Scholar] [CrossRef]
- Guan, Q.; Zhou, L.L.; Zhou, L.N.; Li, M.; Qin, G.X.; Li, W.Y.; Li, Y.A.; Dong, Y.B. A carbon nanomaterial derived from a nanoscale covalent organic framework for photothermal therapy in the NIR-II biowindow. Chem. Commun. 2020, 56, 7793–7796. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Y.; Chen, Q.; Liu, Y.; Gao, Y.; Mullen, K.; Li, S.; Narita, A. A Nanographene-Porphyrin Hybrid for Near-Infrared-Ii Phototheranostics. Adv. Sci. 2024, 11, e2309131. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, Y.; Zhou, W.; Wang, L.; Xu, G.; Ma, M.; Liu, F.; Wang, Z.; Wang, Y.; Kong, T.; et al. NIR-II-activated biocompatible hollow nanocarbons for cancer photothermal therapy. J. Nanobiotechnol. 2021, 19, 137. [Google Scholar] [CrossRef] [PubMed]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef]
- Li, A.; Wang, Y.; Chen, T.; Zhao, W.; Zhang, A.; Feng, S.; Liu, J. NIR-laser switched ICG/DOX loaded thermo-responsive polymeric capsule for chemo-photothermal targeted therapy. Eur. Polym. J. 2017, 92, 51–60. [Google Scholar] [CrossRef]
- Das, S.K.; Luk, C.M.; Martin, W.E.; Tang, L.; Kim, D.Y.; Lau, S.P.; Richards, C.I. Size and dopant dependent single particle fluorescence properties of graphene quantum dots. J. Phys. Chem. C 2015, 119, 17988–17994. [Google Scholar] [CrossRef]
- Alaghmandfard, A.; Sedighi, O.; Tabatabaei Rezaei, N.; Abedini, A.A.; Malek Khachatourian, A.; Toprak, M.S.; Seifalian, A. Recent advances in the modification of carbon-based quantum dots for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111756. [Google Scholar] [CrossRef]
- Liu, H.; Li, C.; Qian, Y.; Hu, L.; Fang, J.; Tong, W.; Nie, R.; Chen, Q.; Wang, H. Magnetic-induced graphene quantum dots for imaging-guided photothermal therapy in the second near-infrared window. Biomaterials 2020, 232, 119700. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Nichols, J.W.; Toh, K.; Nomoto, T.; Cabral, H.; Miura, Y.; Christie, R.J.; Yamada, N.; Ogura, T.; Kano, M.R.; et al. Vascular bursts enhance permeability of tumour blood vessels and improve nanoparticle delivery. Nat. Nanotechnol. 2016, 11, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Setyawati, M.I.; Tay, C.Y.; Docter, D.; Stauber, R.H.; Leong, D.T. Understanding and exploiting nanoparticles’ intimacy with the blood vessel and blood. Chem. Soc. Rev. 2015, 44, 8174–8199. [Google Scholar] [CrossRef]
- Setyawati, M.I.; Tay, C.Y.; Chia, S.L.; Goh, S.L.; Fang, W.; Neo, M.J.; Chong, H.C.; Tan, S.M.; Loo, S.C.; Ng, K.W.; et al. Titanium dioxide nanomaterials cause endothelial cell leakiness by disrupting the homophilic interaction of VE-cadherin. Nat. Commun. 2013, 4, 1673. [Google Scholar] [CrossRef]
- Tee, J.K.; Yip, L.X.; Tan, E.S.; Santitewagun, S.; Prasath, A.; Ke, P.C.; Ho, H.K.; Leong, D.T. Nanoparticles’ interactions with vasculature in diseases. Chem. Soc. Rev. 2019, 48, 5381–5407. [Google Scholar] [CrossRef]
- Tee, J.K.; Setyawati, M.I.; Peng, F.; Leong, D.T.; Ho, H.K. Angiopoietin-1 accelerates restoration of endothelial cell barrier integrity from nanoparticle-induced leakiness. Nanotoxicology 2019, 13, 682–700. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Setyawati, M.I.; Tee, J.K.; Ding, X.; Wang, J.; Nga, M.E.; Ho, H.K.; Leong, D.T. Nanoparticles promote in vivo breast cancer cell intravasation and extravasation by inducing endothelial leakiness. Nat. Nanotechnol. 2019, 14, 279–286. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, J.; Tang, Z.; Qu, S. Tuning the photothermal properties of carbon dots in the deep-red to near-infrared wavelength regions for tumor therapy. Mater. Chem. Front. 2023, 7, 2359–2372. [Google Scholar] [CrossRef]
- Guo, D.; Lei, J.H.; Rong, D.; Zhang, T.; Zhang, B.; Tang, Z.; Shen, H.M.; Deng, C.X.; Qu, S. Photocatalytic Pt(IV)-Coordinated Carbon Dots for Precision Tumor Therapy. Adv. Sci. 2022, 9, e2205106. [Google Scholar] [CrossRef]
- Li, S.; Li, L.; Tu, H.; Zhang, H.; Silvester, D.S.; Banks, C.E.; Zou, G.; Hou, H.; Ji, X. The development of carbon dots: From the perspective of materials chemistry. Mater. Today 2021, 51, 188–207. [Google Scholar] [CrossRef]
- Ethordevic, L.; Arcudi, F.; Cacioppo, M.; Prato, M. A multifunctional chemical toolbox to engineer carbon dots for biomedical and energy applications. Nat. Nanotechnol. 2022, 17, 112–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lu, S. The light of carbon dots: From mechanism to applications. Matter 2022, 5, 110–149. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, B.; Cheng, Q.; Wang, Q.; Zhou, Q.; Li, L.; Qu, S.; Sun, H.; Deng, C.; Tang, Z. Polaron engineering promotes NIR-II absorption of carbon quantum dots for bioimaging and cancer therapy. Sci. Adv. 2024, 10, eadn7896. [Google Scholar] [CrossRef]
- Ren, G.; Wang, X.; Cao, J.; Pu, H.; Li, J.; Yan, L.; Ma, S.; Li, L.; Guo, L.; Zhang, B.; et al. NIR II Laser-Triggered Photothermal Nanoplatform for Multimodal Imaging-Guided Synergistic Therapy toward Colon Cancer. ACS Appl. Mater. Interfaces 2025, 17, 5984–5994. [Google Scholar] [CrossRef] [PubMed]
- Obiweluozor, F.O.; Emechebe, G.A.; Tiwari, A.P.; Kim, J.Y.; Park, C.H.; Kim, C.S. Short duration cancer treatment: Inspired by a fast bio-resorbable smart nano-fiber device containing NIR lethal polydopamine nanospheres for effective chemo–photothermal cancer therapy. Int. J. Nanomed. 2018, 13, 6375–6390. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pu, K. Semiconducting Polymer Nanomaterials as Near-Infrared Photoactivatable Protherapeutics for Cancer. Acc. Chem. Res. 2020, 53, 752–762. [Google Scholar] [CrossRef]
- Yue, X.; Zhang, Q.; Dai, Z. Near-infrared light-activatable polymeric nanoformulations for combined therapy and imaging of cancer. Adv. Drug Deliv. Rev. 2017, 115, 155–170. [Google Scholar] [CrossRef]
- Li, D.D.; Wang, J.X.; Ma, Y.; Qian, H.S.; Wang, D.; Wang, L.; Zhang, G.; Qiu, L.; Wang, Y.C.; Yang, X.Z. A Donor-Acceptor Conjugated Polymer with Alternating Isoindigo Derivative and Bithiophene Units for Near-Infrared Modulated Cancer Thermo-Chemotherapy. ACS Appl. Mater. Interfaces 2016, 8, 19312–19320. [Google Scholar] [CrossRef]
- Guo, B.; Feng, G.; Manghnani, P.N.; Cai, X.; Liu, J.; Wu, W.; Xu, S.; Cheng, X.; Teh, C.; Liu, B. A Porphyrin-Based Conjugated Polymer for Highly Efficient In Vitro and In Vivo Photothermal Therapy. Small 2016, 12, 6243–6254. [Google Scholar] [CrossRef]
- Cao, Z.; Feng, L.; Zhang, G.; Wang, J.; Shen, S.; Li, D.; Yang, X. Semiconducting polymer-based nanoparticles with strong absorbance in NIR-II window for in vivo photothermal therapy and photoacoustic imaging. Biomaterials 2018, 155, 103–111. [Google Scholar] [CrossRef]
- Wei, Z.; Wu, M.; Lan, S.; Li, J.; Zhang, X.; Zhang, D.; Liu, X.; Liu, J. Semiconducting polymer-based nanoparticles for photothermal therapy at the second near-infrared window. Chem. Commun. 2018, 54, 13599–13602. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, J.; Zhen, X.; Xie, C.; Pu, K. Dual-Peak Absorbing Semiconducting Copolymer Nanoparticles for First and Second Near-Infrared Window Photothermal Therapy: A Comparative Study. Adv. Mater. 2018, 30, e1705980. [Google Scholar] [CrossRef]
- Sun, W.; Guo, S.; Hu, C.; Fan, J.; Peng, X. Recent Development of Chemosensors Based on Cyanine Platforms. Chem. Rev. 2016, 116, 7768–7817. [Google Scholar] [CrossRef]
- Kwon, N.; Jasinevicius, G.O.; Kassab, G.; Ding, L.; Bu, J.; Martinelli, L.P.; Ferreira, V.G.; Dhaliwal, A.; Chan, H.H.L.; Mo, Y.; et al. Nanostructure-Driven Indocyanine Green Dimerization Generates Ultra-Stable Phototheranostics Nanoparticles. Angew. Chem. Int. Ed. Engl. 2023, 62, e202305564. [Google Scholar] [CrossRef]
- Qing, W.; Xing, X.; Feng, D.; Chen, R.; Liu, Z. Indocyanine green loaded pH-responsive bortezomib supramolecular hydrogel for synergistic chemo-photothermal/photodynamic colorectal cancer therapy. Photodiagn. Photodyn. Ther. 2021, 36, 102521. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhu, H.; Duo, Y.Y.; Wang, Z.G.; Pang, D.W.; Liu, S.L. A Cyanine with 83.2% Photothermal Conversion Efficiency and Absorption Wavelengths over 1200 nm for Photothermal Therapy. Adv. Healthc. Mater. 2024, 13, 2304421. [Google Scholar] [CrossRef]
- Ramanaviciene, A.; Kausaite, A.; Tautkus, S.; Ramanavicius, A. Biocompatibility of polypyrrole particles: An in-vivo study in mice. J. Pharm. Pharmacol. 2007, 59, 311–315. [Google Scholar] [CrossRef]
- Wang, X.; Ma, Y.; Sheng, X.; Wang, Y.; Xu, H. Ultrathin polypyrrole nanosheets via space-confined synthesis for efficient photothermal therapy in the second near-infrared window. Nano Lett. 2018, 18, 2217–2225. [Google Scholar] [CrossRef]
- Yang, R.; Smyrl, W.; Evans, D.; Hendrickson, W. Evolution of polypyrrole band structure: A scanning tunneling spectroscopy study. J. Phys. Chem. 1992, 96, 1428–1430. [Google Scholar] [CrossRef]
- Čabala, R.; Škarda, J.; Potje-Kamloth, K. Spectroscopic investigation of thermal treatment of doped polypyrrole. Phys. Chem. Chem. Phys. 2000, 2, 3283–3291. [Google Scholar] [CrossRef]
- Bredas, J.; Scott, J.; Yakushi, K.; Street, G. Polarons and bipolarons in polypyrrole: Evolution of the band structure and optical spectrum upon doing. Phys. Rev. B 1984, 30, 1023. [Google Scholar] [CrossRef]
- Li, S.; Deng, Q.; Zhang, Y.; Li, X.; Wen, G.; Cui, X.; Wan, Y.; Huang, Y.; Chen, J.; Liu, Z.; et al. Rational Design of Conjugated Small Molecules for Superior Photothermal Theranostics in the NIR-II Biowindow. Adv. Mater. 2020, 32, e2001146. [Google Scholar] [CrossRef]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Juarranz, A.; Jaen, P.; Sanz-Rodriguez, F.; Cuevas, J.; Gonzalez, S. Photodynamic therapy of cancer. Basic principles and applications. Clin. Transl. Oncol. 2008, 10, 148–154. [Google Scholar] [CrossRef]
- Celli, J.P.; Spring, B.Q.; Rizvi, I.; Evans, C.L.; Samkoe, K.S.; Verma, S.; Pogue, B.W.; Hasan, T. Imaging and photodynamic therapy: Mechanisms, monitoring, and optimization. Chem. Rev. 2010, 110, 2795–2838. [Google Scholar] [CrossRef]
- Wang, S.; Huang, P.; Nie, L.; Xing, R.; Liu, D.; Wang, Z.; Lin, J.; Chen, S.; Niu, G.; Lu, G.; et al. Single continuous wave laser induced photodynamic/plasmonic photothermal therapy using photosensitizer-functionalized gold nanostars. Adv. Mater. 2013, 25, 3055–3061. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Min, K.; Jeon, J.; Yang, H.S.; Tae, G. Catalytic nanographene oxide with hemin for enhanced photodynamic therapy. J. Control. Release 2020, 326, 442–454. [Google Scholar] [CrossRef]
- Zhang, W.; Cai, K.; Li, X.; Zhang, J.; Ma, Z.; Foda, M.F.; Mu, Y.; Dai, X.; Han, H. Au Hollow Nanorods-Chimeric Peptide Nanocarrier for NIR-II Photothermal Therapy and Real-time Apoptosis Imaging for Tumor Theranostics. Theranostics 2019, 9, 4971–4981. [Google Scholar] [CrossRef]
- Tavakkoli Yaraki, M.; Wu, M.; Middha, E.; Wu, W.; Daqiqeh Rezaei, S.; Liu, B.; Tan, Y.N. Gold Nanostars-AIE Theranostic Nanodots with Enhanced Fluorescence and Photosensitization Towards Effective Image-Guided Photodynamic Therapy. Nano Micro Lett. 2021, 13, 58. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, Y.; Yang, Z.; Yang, M.; Wong, C.Y. NIR-II-driven and glutathione depletion-enhanced hypoxia-irrelevant free radical nanogenerator for combined cancer therapy. J. Nanobiotechnol. 2021, 19, 265. [Google Scholar] [CrossRef]
- Tian, Y.; Younis, M.R.; Tang, Y.; Liao, X.; He, G.; Wang, S.; Teng, Z.; Huang, P.; Zhang, L.; Lu, G. Dye-loaded mesoporous polydopamine nanoparticles for multimodal tumor theranostics with enhanced immunogenic cell death. J. Nanobiotechnol. 2021, 19, 365. [Google Scholar] [CrossRef]
- Bian, H.; Ma, D.; Zhang, X.; Xin, K.; Yang, Y.; Peng, X.; Xiao, Y. Tailored Engineering of Novel Xanthonium Polymethine Dyes for Synergetic PDT and PTT Triggered by 1064 nm Laser toward Deep-Seated Tumors. Small 2021, 17, e2100398. [Google Scholar] [CrossRef]
- Tian, Y.; Zhao, D.; Huang, X.; Guan, X.; Wang, F.; Wei, X. Extended pi-Conjugative Carbon Nitride for Single 1064 nm Laser-Activated Photodynamic/Photothermal Synergistic Therapy and Photoacoustic Imaging. ACS Appl. Mater. Interfaces 2022, 14, 7626–7635. [Google Scholar] [CrossRef]
- Gao, C.; Guo, W.; Guo, X.; Ding, Z.; Ding, Y.; Shen, X.C. Black SnO(2-x) based nanotheranostic for imaging-guided photodynamic/photothermal synergistic therapy in the second near-infrared window. Acta Biomater. 2021, 129, 220–234. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Keenan, T.E.; Burke, K.P.; Van Allen, E.M. Genomic correlates of response to immune checkpoint blockade. Nat. Med. 2019, 25, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Wieder, T.; Eigentler, T.; Brenner, E.; Rocken, M. Immune checkpoint blockade therapy. J. Allergy Clin. Immunol. 2018, 142, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yu, J.; Sui, L.; Zhu, S.; Tang, Z.; Yang, B.; Lu, S. Rational Design of Multi-Color-Emissive Carbon Dots in a Single Reaction System by Hydrothermal. Adv. Sci. 2020, 8, 2001453. [Google Scholar] [CrossRef]
- Chang, Z.L.; Hou, A.J.; Chen, Y.Y. Engineering primary T cells with chimeric antigen receptors for rewired responses to soluble ligands. Nat. Protoc. 2020, 15, 1507–1524. [Google Scholar] [CrossRef]
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F.; et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014, 371, 1507–1517. [Google Scholar] [CrossRef]
- Teachey, D.T.; Bishop, M.R.; Maloney, D.G.; Grupp, S.A. Toxicity management after chimeric antigen receptor T cell therapy: One size does not fit ‘ALL’. Nat. Rev. Clin. Oncol. 2018, 15, 218. [Google Scholar] [CrossRef]
- Kacherovsky, N.; Cardle, I.I.; Cheng, E.L.; Yu, J.L.; Baldwin, M.L.; Salipante, S.J.; Jensen, M.C.; Pun, S.H. Traceless aptamer-mediated isolation of CD8(+) T cells for chimeric antigen receptor T-cell therapy. Nat. Biomed. Eng. 2019, 3, 783–795. [Google Scholar] [CrossRef]
- Kono, K.; Iinuma, H.; Akutsu, Y.; Tanaka, H.; Hayashi, N.; Uchikado, Y.; Noguchi, T.; Fujii, H.; Okinaka, K.; Fukushima, R.; et al. Multicenter, phase II clinical trial of cancer vaccination for advanced esophageal cancer with three peptides derived from novel cancer-testis antigens. J. Transl. Med. 2012, 10, 141. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brahmer, J.R.; Callahan, M.K.; Flores-Chavez, A.; Keegan, N.; Khamashta, M.A.; Lambotte, O.; Mariette, X.; Prat, A.; Suarez-Almazor, M.E. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Primers 2020, 6, 38. [Google Scholar] [CrossRef]
- Ishizuka, J.J.; Manguso, R.T.; Cheruiyot, C.K.; Bi, K.; Panda, A.; Iracheta-Vellve, A.; Miller, B.C.; Du, P.P.; Yates, K.B.; Dubrot, J.; et al. Loss of ADAR1 in tumours overcomes resistance to immune checkpoint blockade. Nature 2019, 565, 43–48. [Google Scholar] [CrossRef]
- Kalbasi, A.; Ribas, A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat. Rev. Immunol. 2020, 20, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.M.; Pan, W.Y.; Wu, C.Y.; Yeh, C.Y.; Korupalli, C.; Luo, P.K.; Chou, C.J.; Chia, W.T.; Sung, H.W. Modulation of tumor microenvironment using a TLR-7/8 agonist-loaded nanoparticle system that exerts low-temperature hyperthermia and immunotherapy for in situ cancer vaccination. Biomaterials 2020, 230, 119629. [Google Scholar] [CrossRef] [PubMed]
- Moy, A.J.; Tunnell, J.W. Combinatorial immunotherapy and nanoparticle mediated hyperthermia. Adv. Drug Deliv. Rev. 2017, 114, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Kuwano, H.; Araki, K.; Egashira, A.; Kawaguchi, H.; Saeki, H.; Kitamura, K.; Ohno, S.; Sugimachi, K. Prognostic significance of lymphocyte infiltration following preoperative chemoradiotherapy and hyperthermia for esophageal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2001, 49, 1259–1266. [Google Scholar] [CrossRef]
- Duan, X.; Chan, C.; Lin, W. Nanoparticle-Mediated Immunogenic Cell Death Enables and Potentiates Cancer Immunotherapy. Angew. Chem. Int. Ed. 2019, 58, 670–680. [Google Scholar] [CrossRef]
- Kobayashi, H.; Choyke, P.L. Near-Infrared Photoimmunotherapy of Cancer. Acc. Chem. Res. 2019, 52, 2332–2339. [Google Scholar] [CrossRef]
- Irvine, D.J.; Dane, E.L. Enhancing cancer immunotherapy with nanomedicine. Nat. Rev. Immunol. 2020, 20, 321–334. [Google Scholar] [CrossRef]
- Pribila, J.T.; Quale, A.C.; Mueller, K.L.; Shimizu, Y. Integrins and T cell-mediated immunity. Annu. Rev. Immunol. 2004, 22, 157–180. [Google Scholar] [CrossRef]
- Kurup, S.P.; Butler, N.S.; Harty, J.T. T cell-mediated immunity to malaria. Nat. Rev. Immunol. 2019, 19, 457–471. [Google Scholar] [CrossRef]
- Vaeth, M.; Yang, J.; Yamashita, M.; Zee, I.; Eckstein, M.; Knosp, C.; Kaufmann, U.; Karoly Jani, P.; Lacruz, R.S.; Flockerzi, V.; et al. ORAI2 modulates store-operated calcium entry and T cell-mediated immunity. Nat. Commun. 2017, 8, 14714. [Google Scholar] [CrossRef]
- Liu, G.; Burns, S.; Huang, G.; Boyd, K.; Proia, R.L.; Flavell, R.A.; Chi, H. The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat. Immunol. 2009, 10, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Kooreman, N.G.; Kim, Y.; de Almeida, P.E.; Termglinchan, V.; Diecke, S.; Shao, N.Y.; Wei, T.T.; Yi, H.; Dey, D.; Nelakanti, R.; et al. Autologous iPSC-Based Vaccines Elicit Anti-tumor Responses In Vivo. Cell Stem Cell 2018, 22, 501–513.e7. [Google Scholar] [CrossRef] [PubMed]
- Nemunaitis, J.; Sterman, D.; Jablons, D.; Smith, J.W., II; Fox, B.; Maples, P.; Hamilton, S.; Borellini, F.; Lin, A.; Morali, S.; et al. Granulocyte-macrophage colony-stimulating factor gene-modified autologous tumor vaccines in non-small-cell lung cancer. J. Natl. Cancer Inst. 2004, 96, 326–331. [Google Scholar] [CrossRef]
- Hirschowitz, E.A.; Foody, T.; Kryscio, R.; Dickson, L.; Sturgill, J.; Yannelli, J. Autologous dendritic cell vaccines for non-small-cell lung cancer. J. Clin. Oncol. 2004, 22, 2808–2815. [Google Scholar] [CrossRef]
- Berd, D.; Sato, T.; Maguire, H.C., Jr.; Kairys, J.; Mastrangelo, M.J. Immunopharmacologic analysis of an autologous, hapten-modified human melanoma vaccine. J. Clin. Oncol. 2004, 22, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Kuai, R.; Xu, Y.; Ochyl, L.J.; Irvine, D.J.; Moon, J.J. Immunogenic Cell Death Amplified by Co-localized Adjuvant Delivery for Cancer Immunotherapy. Nano Lett. 2017, 17, 7387–7393. [Google Scholar] [CrossRef]
- Galluzzi, L.; Buque, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 2017, 17, 97–111. [Google Scholar] [CrossRef]
- Xiong, X.; Zhang, Y.; Huang, X.; Zhang, S.; Li, Q. Generating Immunological Memory Against Cancer by Camouflaging Gold-Based Photothermal Nanoparticles in NIR-II Biowindow for Mimicking T-Cells. Small 2024, 20, e2407038. [Google Scholar] [CrossRef]
- Huang, Z.; Song, J.; Huang, S.; Wang, S.; Shen, C.; Song, S.; Lian, J.; Ding, Y.; Gong, Y.; Zhang, Y.; et al. Phase and Defect Engineering of MoSe(2) Nanosheets for Enhanced NIR-II Photothermal Immunotherapy. Nano Lett. 2024, 24, 7764–7773. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.; Lin, M.; Hou, Y.; Ding, M.; Kong, D.; Sun, H.; Zhang, Q.; Li, J.; Zhou, Q. Liposome-based nanocomplexes with pH-sensitive second near-infrared photothermal property for combinational immunotherapy. Appl. Mater. Today 2021, 25, 101258. [Google Scholar] [CrossRef]
- Shi, C.; Li, M.; Zhang, Z.; Yao, Q.; Shao, K.; Xu, F.; Xu, N.; Li, H.; Fan, J.; Sun, W.; et al. Catalase-based liposomal for reversing immunosuppressive tumor microenvironment and enhanced cancer chemo-photodynamic therapy. Biomaterials 2020, 233, 119755. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, C.; Zhang, X.; Chen, G.; Hu, Q.; Li, H.; Wang, J.; Wen, D.; Zhang, Y.; Lu, Y.; et al. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat. Nanotechnol. 2019, 14, 89–97. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Ji, Y.; Fan, L.; Ding, B.; Lin, J.; Wang, L. Mitochondrial targeted melanin@ mSiO2 yolk-shell nanostructures for NIR-II-driven photo-thermal-dynamic/immunotherapy. Chem. Eng. J. 2022, 435, 134869. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Y.; Li, X.; Zhao, Y.; Li, M.; Jiang, W.; Tang, X.; Dou, J.; Lu, L.; Wang, F.; et al. Near-Infrared II Phototherapy Induces Deep Tissue Immunogenic Cell Death and Potentiates Cancer Immunotherapy. ACS Nano 2019, 13, 11967–11980. [Google Scholar] [CrossRef]
- Lin, H.; Chen, Y.; Shi, J. Nanoparticle-triggered in situ catalytic chemical reactions for tumour-specific therapy. Chem. Soc. Rev. 2018, 47, 1938–1958. [Google Scholar] [CrossRef] [PubMed]
- Matera, C.; Gomila, A.M.J.; Camarero, N.; Libergoli, M.; Soler, C.; Gorostiza, P. Photoswitchable Antimetabolite for Targeted Photoactivated Chemotherapy. J. Am. Chem. Soc. 2018, 140, 15764–15773. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Pu, Y.; Shi, J. Nanomedicine-enabled chemotherapy-based synergetic cancer treatments. J. Nanobiotechnol. 2022, 20, 4. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, H.; Xu, W.; Jiang, G.Q. Recent advances in photothermal therapy-based multifunctional nanoplatforms for breast cancer. Front. Chem. 2022, 10, 1024177. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Yu, D.; Wang, Y.; Chen, J.; Liu, S.; Deng, S.; Liu, C.; McCulloch, I.; Yue, W.; Cheng, D. Co-delivery of NIR-II semiconducting polymer and pH-sensitive doxorubicin-conjugated prodrug for photothermal/chemotherapy. Acta Biomater. 2022, 137, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, J.; Hu, W.; Wang, Y.; Zhang, Q.; Hu, X.; Chou, T.; Zhang, B.; Gallaro, C.; Halloran, M.; et al. A Porous Bimetallic Au@Pt Core-Shell Oxygen Generator to Enhance Hypoxia-Dampened Tumor Chemotherapy Synergized with NIR-II Photothermal Therapy. ACS Nano 2022, 16, 10711–10728. [Google Scholar] [CrossRef]
- Zhang, W.; Cai, K.; Sun, Z.; Xiang, Q.; Yuan, L.; Fu, M.; Liu, X.; Foda, M.F.F.; Ye, Z.; Huang, J.; et al. Elevating Second Near-Infrared Photothermal Conversion Efficiency of Hollow Gold Nanorod for a Precise Theranostic of Orthotopic Bladder Cancer. ACS Nano 2023, 17, 18932–18941. [Google Scholar] [CrossRef]
- Perez-Herrero, E.; Fernandez-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.; Krause, M.; Hill, R. Exploring the role of cancer stem cells in radioresistance. Nat. Rev. Cancer 2008, 8, 545–554. [Google Scholar] [CrossRef]
- Wu, X.; Suo, Y.; Shi, H.; Liu, R.; Wu, F.; Wang, T.; Ma, L.; Liu, H.; Cheng, Z. Deep-Tissue Photothermal Therapy Using Laser Illumination at NIR-IIa Window. Nano Micro Lett. 2020, 12, 38. [Google Scholar] [CrossRef]
- Chen, J.; Ning, C.; Zhou, Z.; Yu, P.; Zhu, Y.; Tan, G.; Mao, C. Nanomaterials as photothermal therapeutic agents. Prog. Mater. Sci. 2019, 99, 1–26. [Google Scholar] [CrossRef]
- Hansen, G.; Sundset, A. Transbronchial laser ablation of benign and malignant tumors. Minim. Invasive Ther. Allied Technol. 2006, 15, 4–8. [Google Scholar] [CrossRef]
- Chen, Y.W.; Su, Y.L.; Hu, S.H.; Chen, S.Y. Functionalized graphene nanocomposites for enhancing photothermal therapy in tumor treatment. Adv. Drug Deliv. Rev. 2016, 105, 190–204. [Google Scholar] [CrossRef]
- Shao, W.; Yang, C.; Li, F.; Wu, J.; Wang, N.; Ding, Q.; Gao, J.; Ling, D. Molecular Design of Conjugated Small Molecule Nanoparticles for Synergistically Enhanced PTT/PDT. Nano Micro Lett. 2020, 12, 147. [Google Scholar] [CrossRef]
- Noh, I.; Son, Y.; Jung, W.; Kim, M.; Kim, D.; Shin, H.; Kim, Y.C.; Jon, S. Targeting the tumor microenvironment with amphiphilic near-infrared cyanine nanoparticles for potentiated photothermal immunotherapy. Biomaterials 2021, 275, 120926. [Google Scholar] [CrossRef]
- Zhao, L.; Yuan, W.; Tham, H.P.; Chen, H.; Xing, P.; Xiang, H.; Yao, X.; Qiu, X.; Dai, Y.; Zhu, L.; et al. Fast-Clearable Nanocarriers Conducting Chemo/Photothermal Combination Therapy to Inhibit Recurrence of Malignant Tumors. Small 2017, 13, 1700963. [Google Scholar] [CrossRef]
- Linares, M.A.; Zakaria, A.; Nizran, P. Skin Cancer. Prim. Care 2015, 42, 645–659. [Google Scholar] [CrossRef]
- Smith, A.W. Incidence and Profile of Uveal Melanoma in the United States, 2001–2017; Idaho State University: Pocatello, ID, USA, 2021. [Google Scholar]
- Quazi, S.J.; Aslam, N.; Saleem, H.; Rahman, J.; Khan, S. Surgical Margin of Excision in Basal Cell Carcinoma: A Systematic Review of Literature. Cureus 2020, 12, e9211. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.T.; Lehrer, E.J.; Aphale, A.; Lango, M.; Galloway, T.J.; Zaorsky, N.G. Surgical excision, Mohs micrographic surgery, external-beam radiotherapy, or brachytherapy for indolent skin cancer: An international meta-analysis of 58 studies with 21,000 patients. Cancer 2019, 125, 3582–3594. [Google Scholar] [CrossRef] [PubMed]
- Pashazadeh, A.; Boese, A.; Friebe, M. Radiation therapy techniques in the treatment of skin cancer: An overview of the current status and outlook. J. Dermatol. Treat. 2019, 30, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Kuflik, E.G. Cryosurgery for skin cancer: 30-year experience and cure rates. Dermatol. Surg. 2004, 30, 297–300. [Google Scholar] [CrossRef]
- Yanovsky, R.L.; Bartenstein, D.W.; Rogers, G.S.; Isakoff, S.J.; Chen, S.T. Photodynamic therapy for solid tumors: A review of the literature. Photodermatol. Photoimmunol. Photomed. 2019, 35, 295–303. [Google Scholar] [CrossRef]
- Li, X.; Naylor, M.F.; Le, H.; Nordquist, R.E.; Teague, T.K.; Howard, C.A.; Murray, C.; Chen, W.R. Clinical effects of in situ photoimmunotherapy on late-stage melanoma patients: A preliminary study. Cancer Biol. Ther. 2010, 10, 1081–1087. [Google Scholar] [CrossRef]
- Rajaee, Z.; Khoei, S.; Mahdavian, A.; Shirvalilou, S.; Mahdavi, S.R.; Ebrahimi, M. Radio-thermo-sensitivity Induced by Gold Magnetic Nanoparticles in the Monolayer Culture of Human Prostate Carcinoma Cell Line DU145. Anticancer Agents Med. Chem. 2020, 20, 315–324. [Google Scholar] [CrossRef]
- Evans, A.J. Treatment effects in prostate cancer. Mod. Pathol. 2018, 31, S110–S121. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Zhang, J.; Jiao, J.; Qin, W.; Yang, X. Photodynamic therapy for prostate cancer: Recent advances, challenges and opportunities. Front. Oncol. 2022, 12, 980239. [Google Scholar] [CrossRef]
- Pellegrino, A.; Cirulli, G.O.; Mazzone, E.; Barletta, F.; Scuderi, S.; de Angelis, M.; Rosiello, G.; Gandaglia, G.; Montorsi, F.; Briganti, A.; et al. Focal therapy for prostate cancer: What is really needed to move from investigational to valid therapeutic alternative? A narrative review. Ann. Transl. Med. 2022, 10, 755. [Google Scholar] [CrossRef]
- Rastinehad, A.R.; Anastos, H.; Wajswol, E.; Winoker, J.S.; Sfakianos, J.P.; Doppalapudi, S.K.; Carrick, M.R.; Knauer, C.J.; Taouli, B.; Lewis, S.C.; et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc. Natl. Acad. Sci. USA 2019, 116, 18590–18596. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Schnitt, S.J.; Moran, M.S.; Giuliano, A.E. Lumpectomy Margins for Invasive Breast Cancer and Ductal Carcinoma in Situ: Current Guideline Recommendations, Their Implications, and Impact. J. Clin. Oncol. 2020, 38, 2240–2245. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Bryant, J.; Wolmark, N.; Mamounas, E.; Brown, A.; Fisher, E.R.; Wickerham, D.L.; Begovic, M.; DeCillis, A.; Robidoux, A.; et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J. Clin. Oncol. 2023, 41, 1795–1808. [Google Scholar] [CrossRef]
- Chua, B.H.; Link, E.K.; Kunkler, I.H.; Whelan, T.J.; Westenberg, A.H.; Gruber, G.; Bryant, G.; Ahern, V.; Purohit, K.; Graham, P.H.; et al. Radiation doses and fractionation schedules in non-low-risk ductal carcinoma in situ in the breast (BIG 3-07/TROG 07.01): A randomised, factorial, multicentre, open-label, phase 3 study. Lancet 2022, 400, 431–440. [Google Scholar] [CrossRef]
- Andre, F.; Ismaila, N.; Allison, K.H.; Barlow, W.E.; Collyar, D.E.; Damodaran, S.; Henry, N.L.; Jhaveri, K.; Kalinsky, K.; Kuderer, N.M.; et al. Biomarkers for Adjuvant Endocrine and Chemotherapy in Early-Stage Breast Cancer: ASCO Guideline Update. J. Clin. Oncol. 2022, 40, 1816–1837. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Shastry, M.; Hamilton, E. Targeting HER2-positive breast cancer: Advances and future directions. Nat. Rev. Drug Discov. 2023, 22, 101–126. [Google Scholar] [CrossRef] [PubMed]
- Franzoi, M.A.; Romano, E.; Piccart, M. Immunotherapy for early breast cancer: Too soon, too superficial, or just right? Ann. Oncol. 2021, 32, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Korde, L.A.; Somerfield, M.R.; Carey, L.A.; Crews, J.R.; Denduluri, N.; Hwang, E.S.; Khan, S.A.; Loibl, S.; Morris, E.A.; Perez, A.; et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J. Clin. Oncol. 2021, 39, 1485–1505. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ferrel, G.L.; Guerra, M.C.; Hode, T.; Lunn, J.A.; Adalsteinsson, O.; Nordquist, R.E.; Liu, H.; Chen, W.R. Preliminary safety and efficacy results of laser immunotherapy for the treatment of metastatic breast cancer patients. Photochem. Photobiol. Sci. 2011, 10, 817–821. [Google Scholar] [CrossRef]
- Schwartzberg, B.; Lewin, J.; Abdelatif, O.; Bernard, J.; Bu-Ali, H.; Cawthorn, S.; Chen-Seetoo, M.; Feldman, S.; Govindarajulu, S.; Jones, L.; et al. Phase 2 Open-Label Trial Investigating Percutaneous Laser Ablation for Treatment of Early-Stage Breast Cancer: MRI, Pathology, and Outcome Correlations. Ann. Surg. Oncol. 2018, 25, 2958–2964. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, X.; Peng, Y.; Zhou, K.; Hu, J.; Yu, L.; Chen, F.; Qiu, S.; Zhou, J.; Fan, J.; et al. Surgical Resection plus Radiofrequency Ablation versus Radical Surgery for Hepatocellular Carcinoma: A Propensity Score Matching Analysis. J. Cancer 2019, 10, 3933–3940. [Google Scholar] [CrossRef]
- Wang, H.; Hou, W.; Perera, A.; Bettler, C.; Beach, J.R.; Ding, X.; Li, J.; Denning, M.F.; Dhanarajan, A.; Cotler, S.J.; et al. Targeting EphA2 suppresses hepatocellular carcinoma initiation and progression by dual inhibition of JAK1/STAT3 and AKT signaling. Cell Rep. 2021, 34, 108765. [Google Scholar] [CrossRef]
- Pinato, D.J.; Cortellini, A.; Sukumaran, A.; Cole, T.; Pai, M.; Habib, N.; Spalding, D.; Sodergren, M.H.; Martinez, M.; Dhillon, T.; et al. PRIME-HCC: Phase Ib study of neoadjuvant ipilimumab and nivolumab prior to liver resection for hepatocellular carcinoma. BMC Cancer 2021, 21, 301. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Vogl, T.J.; Straub, R.; Eichler, K.; Sollner, O.; Mack, M.G. Colorectal carcinoma metastases in liver: Laser-induced interstitial thermotherapy--local tumor control rate and survival data. Radiology 2004, 230, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Pacella, C.M.; Bizzarri, G.; Francica, G.; Forlini, G.; Petrolati, A.; Valle, D.; Anelli, V.; Bianchini, A.; Nuntis, S.D.; Pacella, S.; et al. Analysis of factors predicting survival in patients with hepatocellular carcinoma treated with percutaneous laser ablation. J. Hepatol. 2006, 44, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Pacella, C.M.; Bizzarri, G.; Magnolfi, F.; Cecconi, P.; Caspani, B.; Anelli, V.; Bianchini, A.; Valle, D.; Pacella, S.; Manenti, G.; et al. Laser thermal ablation in the treatment of small hepatocellular carcinoma: Results in 74 patients. Radiology 2001, 221, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Abdelaziz, H.M.; Gaber, M.; Abd-Elwakil, M.M.; Mabrouk, M.T.; Elgohary, M.M.; Kamel, N.M.; Kabary, D.M.; Freag, M.S.; Samaha, M.W.; Mortada, S.M.; et al. Inhalable particulate drug delivery systems for lung cancer therapy: Nanoparticles, microparticles, nanocomposites and nanoaggregates. J. Control. Release 2018, 269, 374–392. [Google Scholar] [CrossRef]
- Wathoni, N.; Puluhulawa, L.E.; Joni, I.M.; Muchtaridi, M.; Mohammed, A.F.A.; Elamin, K.M.; Milanda, T.; Gozali, D. Monoclonal antibody as a targeting mediator for nanoparticle targeted delivery system for lung cancer. Drug Deliv. 2022, 29, 2959–2970. [Google Scholar] [CrossRef]
- Mathieu, L.N.; Larkins, E.; Sinha, A.K.; Mishra-Kalyani, P.S.; Jafri, S.; Kalavar, S.; Ghosh, S.; Goldberg, K.B.; Pazdur, R.; Beaver, J.A.; et al. FDA Approval Summary: Atezolizumab as Adjuvant Treatment following Surgical Resection and Platinum-Based Chemotherapy for Stage II to IIIA NSCLC. Clin. Cancer Res. 2023, 29, 2973–2978. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, R.; Chen, Y.; Yao, S.; Zhang, W.; Ye, D. Advances in Second Near-Infrared Window Photothermal Agents and Photothermal Therapy for Tumors in Interdisciplinary Medical Research. Pharmaceutics 2025, 17, 1178. https://doi.org/10.3390/pharmaceutics17091178
Zhou R, Chen Y, Yao S, Zhang W, Ye D. Advances in Second Near-Infrared Window Photothermal Agents and Photothermal Therapy for Tumors in Interdisciplinary Medical Research. Pharmaceutics. 2025; 17(9):1178. https://doi.org/10.3390/pharmaceutics17091178
Chicago/Turabian StyleZhou, Runxuan, Yufei Chen, Shuxi Yao, Weiyun Zhang, and Dawei Ye. 2025. "Advances in Second Near-Infrared Window Photothermal Agents and Photothermal Therapy for Tumors in Interdisciplinary Medical Research" Pharmaceutics 17, no. 9: 1178. https://doi.org/10.3390/pharmaceutics17091178
APA StyleZhou, R., Chen, Y., Yao, S., Zhang, W., & Ye, D. (2025). Advances in Second Near-Infrared Window Photothermal Agents and Photothermal Therapy for Tumors in Interdisciplinary Medical Research. Pharmaceutics, 17(9), 1178. https://doi.org/10.3390/pharmaceutics17091178








