Oral Tablet Formulations with Lactoferrin, a Cohesive Biomacromolecule
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Powder Physical Properties
2.3. Schulze Ring Shear Testing
2.4. Dry Granulation (Roller Compaction)
2.5. Tablet Formulations
2.6. Tableting
2.7. Tablet Physical Attributes
2.8. Tablet Content Uniformity
2.9. Drug Dissolution
2.10. Disintegration
2.11. Enzyme-Linked Immunosorbent Assay (ELISA)
2.12. Determination of Intact Lactoferrin Content in Tablet Formulations
2.13. Stability
3. Results
3.1. Powder Physical Attributes
3.1.1. Bulk and Tapped Densities
3.1.2. Particle Size
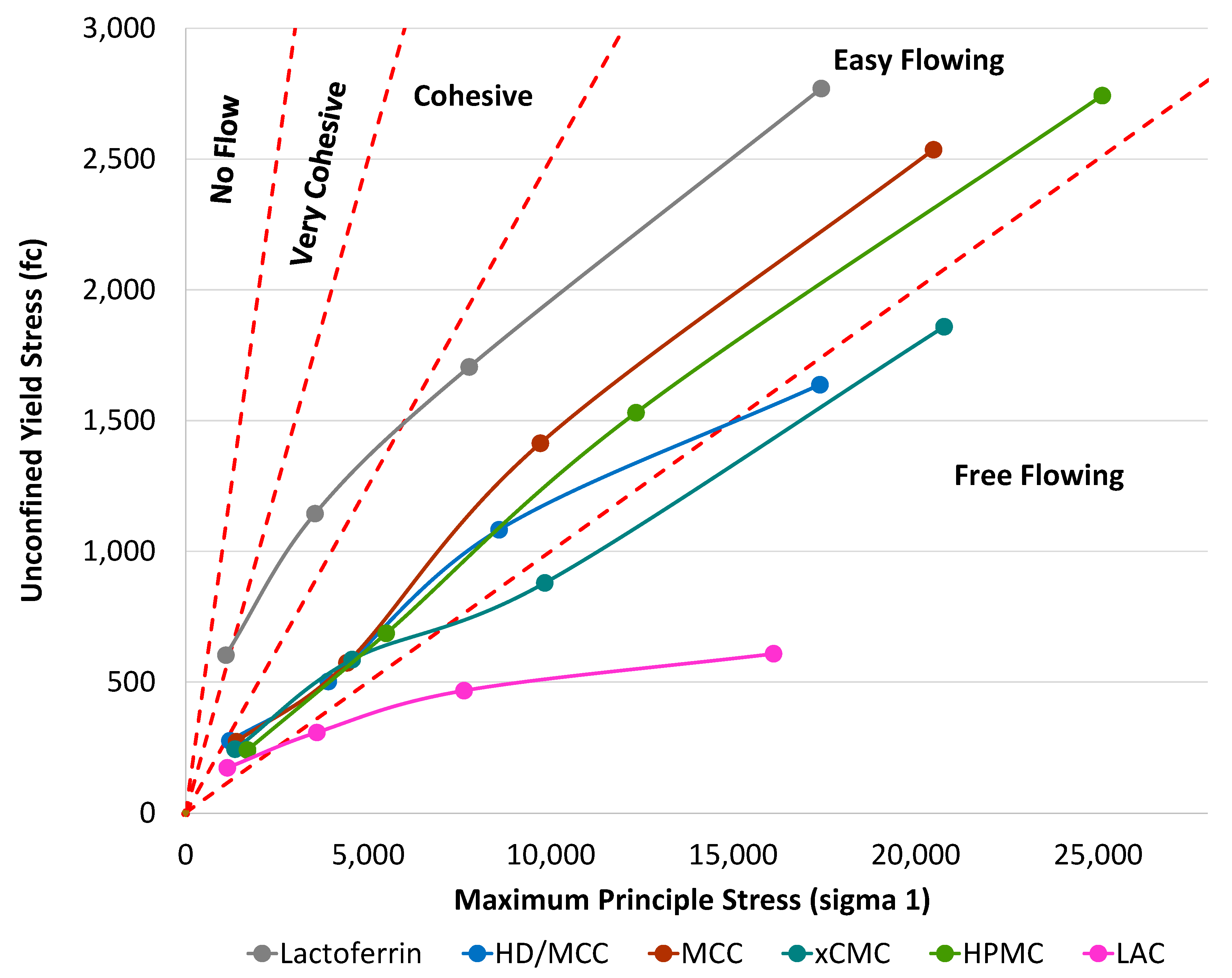
3.1.3. Schulze Ring Shear
3.2. Tablet Physical Attributes and Performance
3.2.1. Tensile Strength
3.2.2. Friability
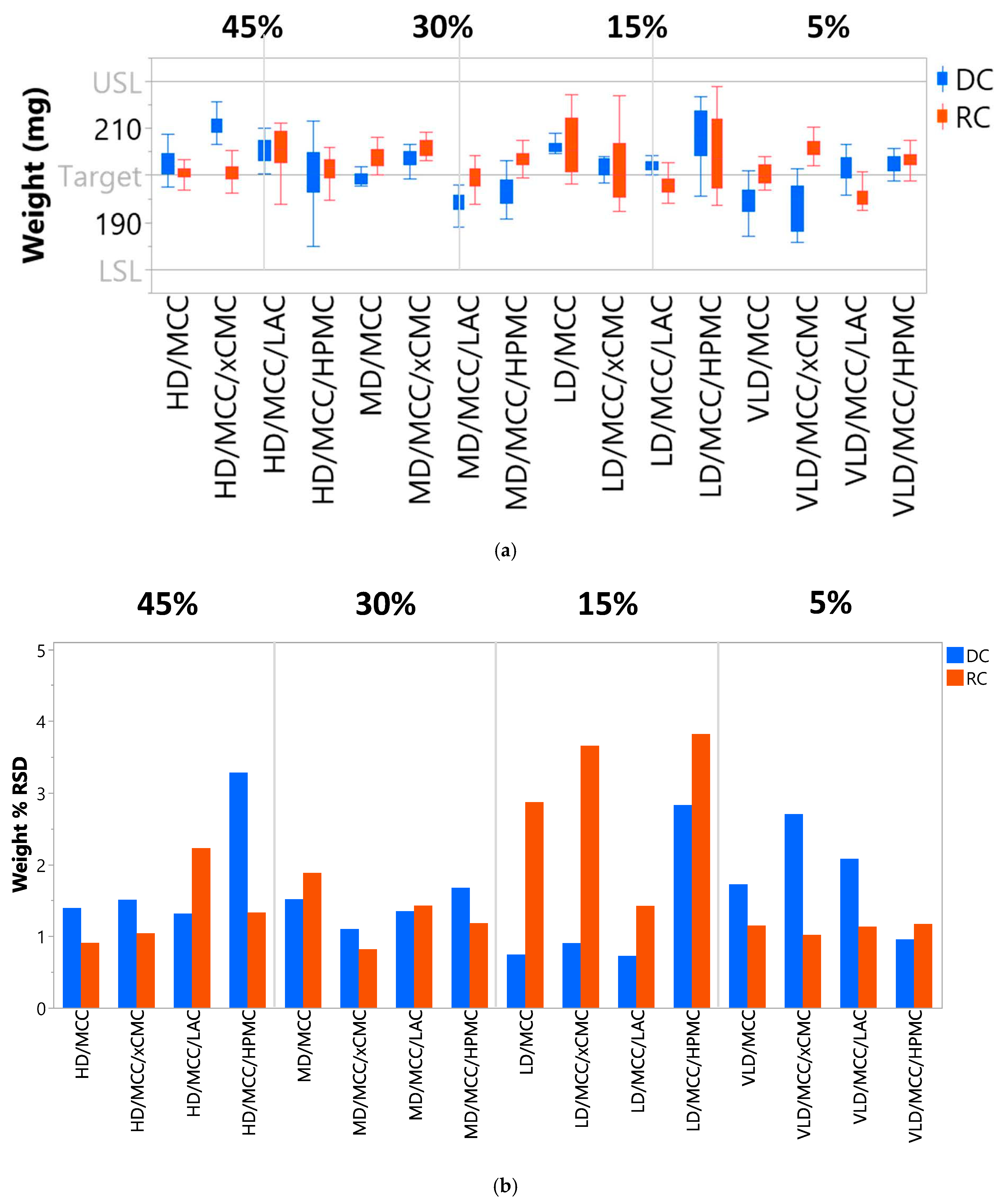
3.2.3. Tablet Weight Reproducibility
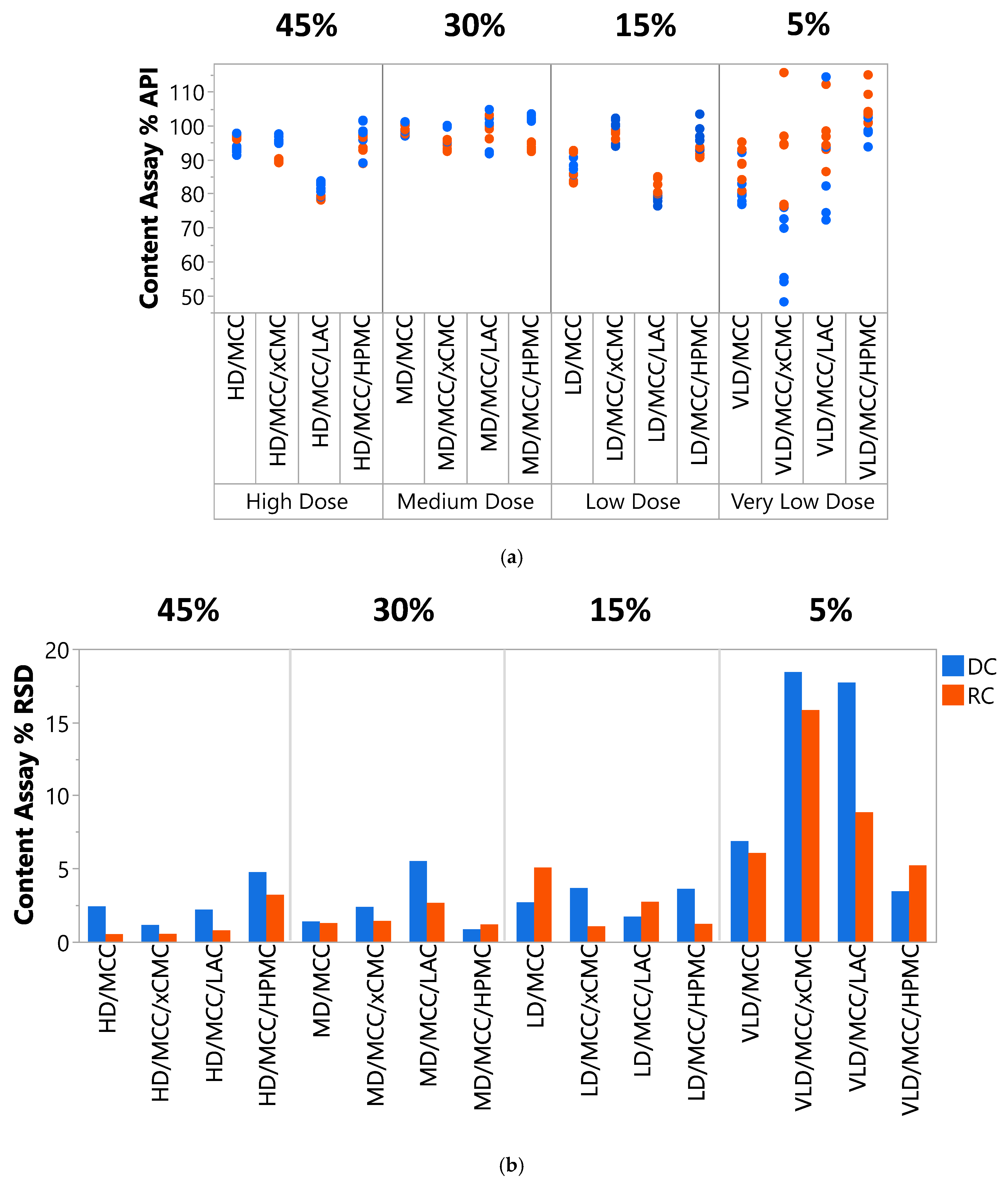
3.2.4. Content Uniformity
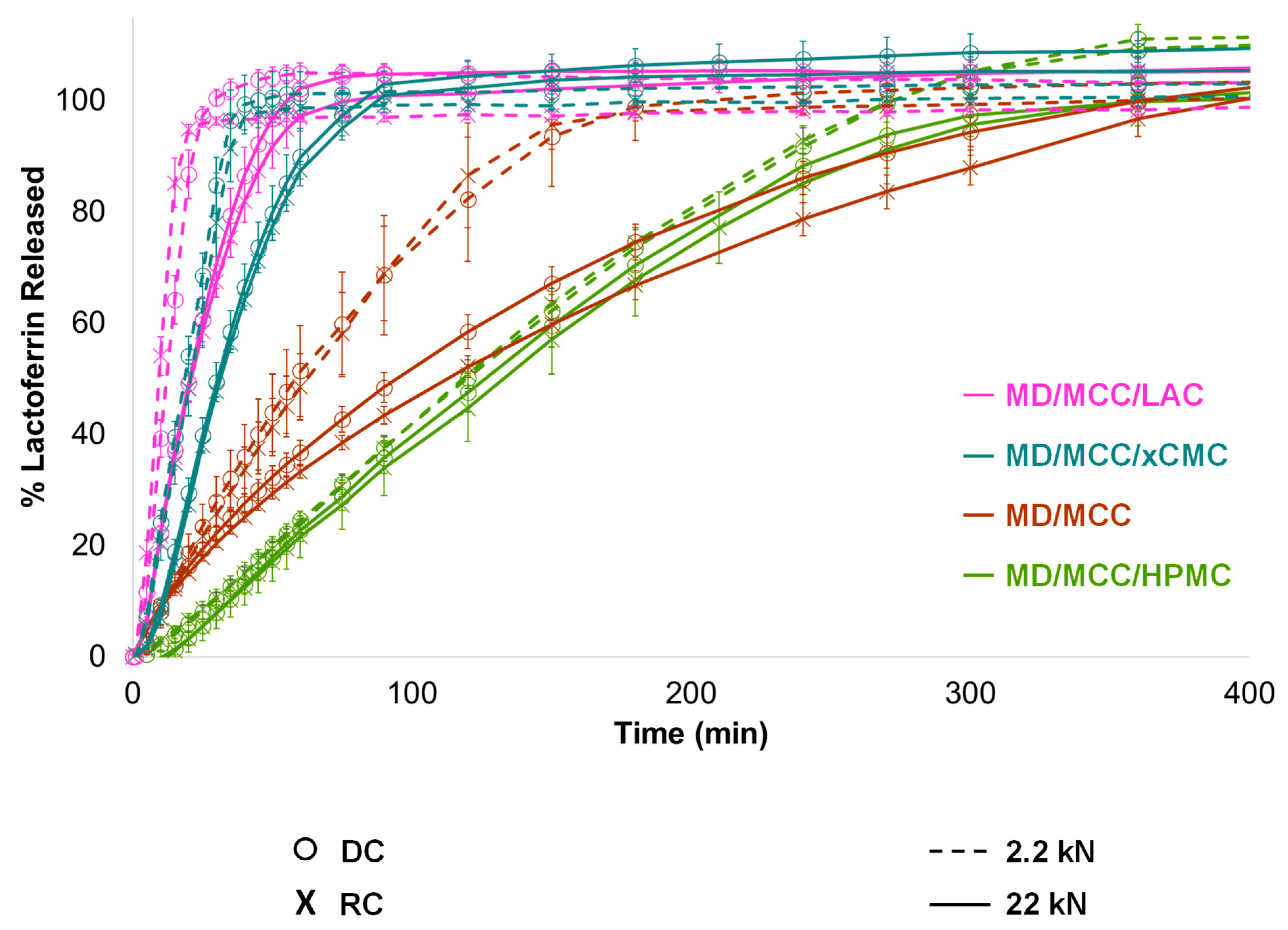
3.2.5. Lactoferrin Release
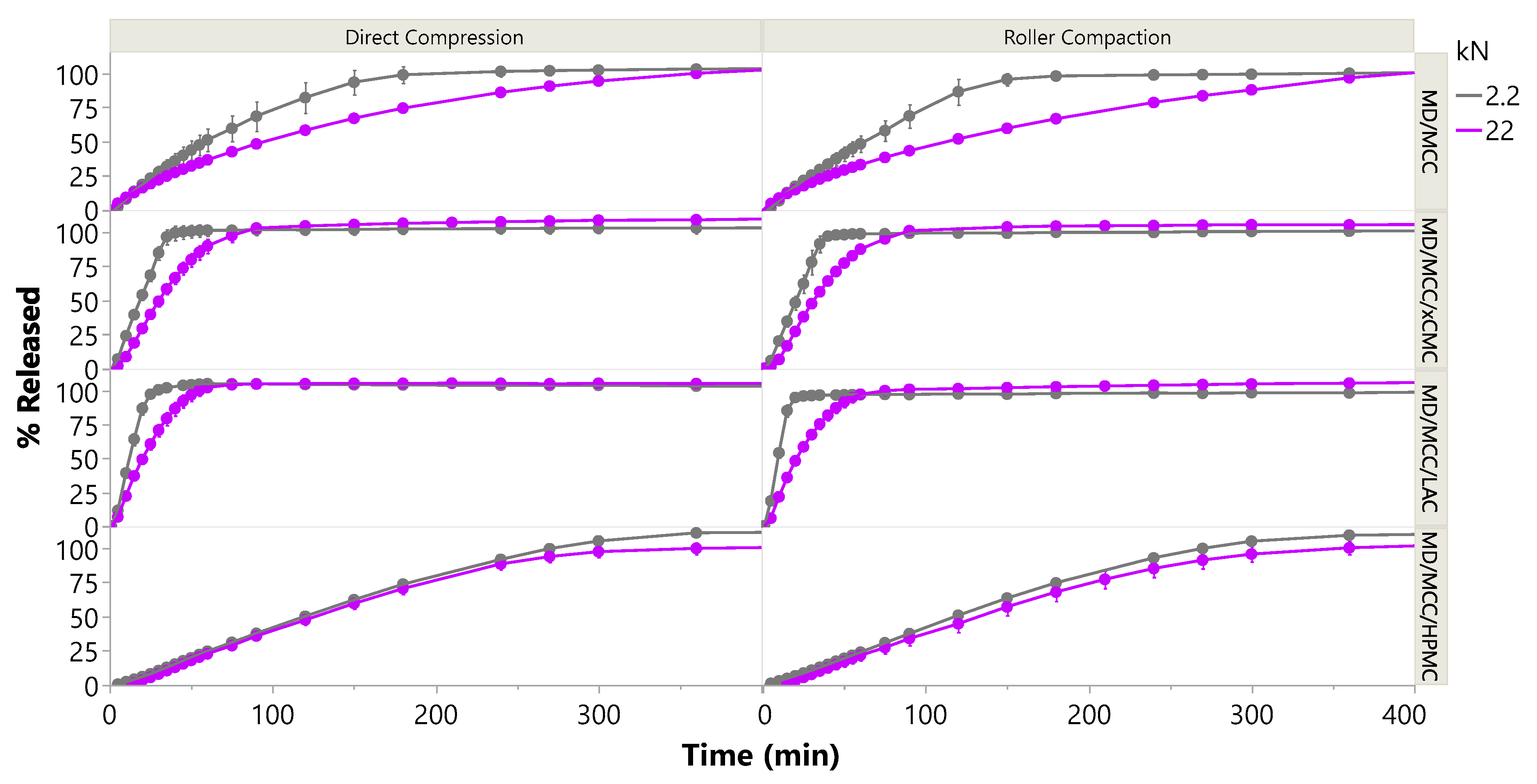
3.2.6. Impact of Compression Force
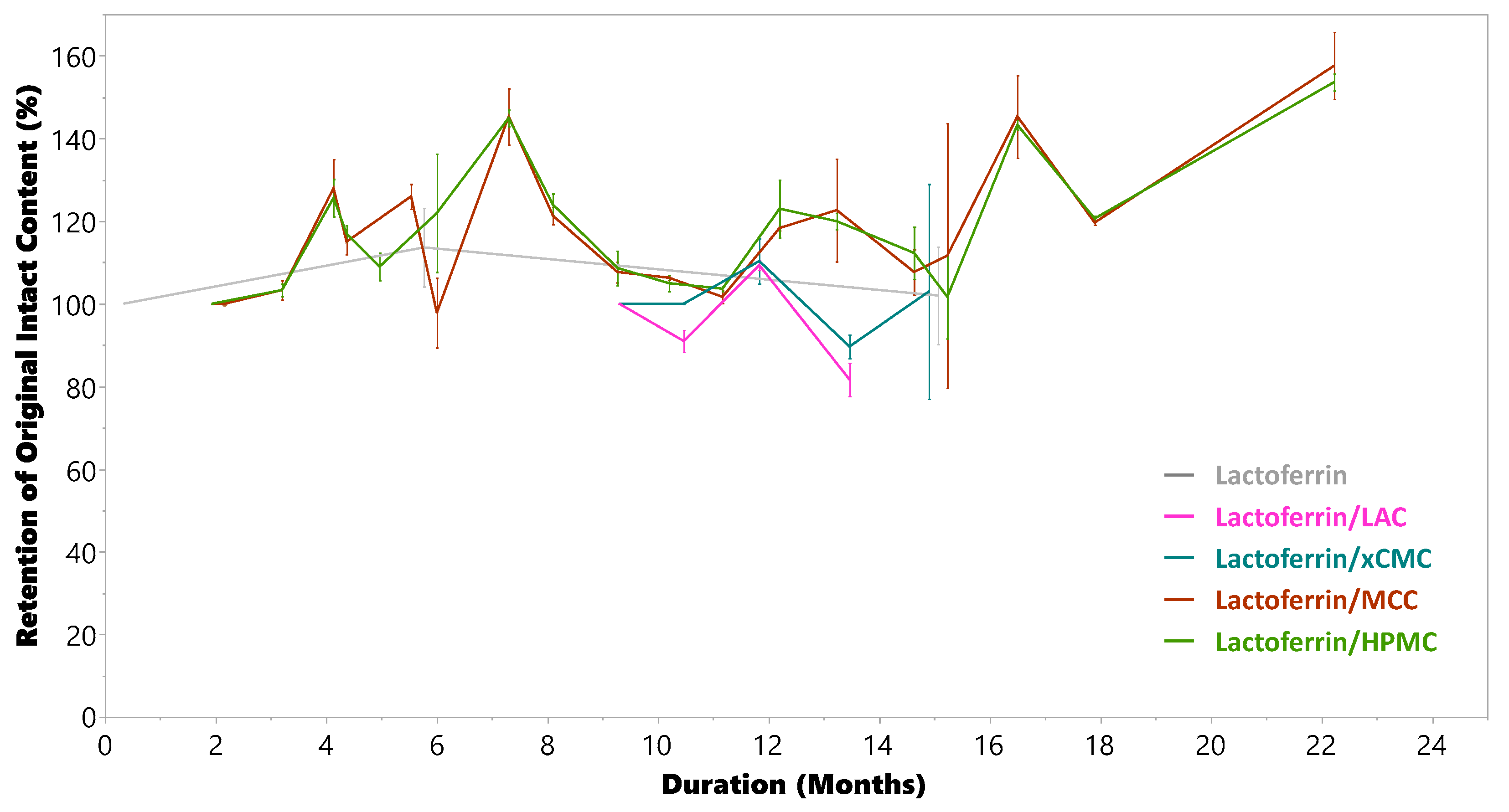
3.2.7. Compatibility and Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| API | Active Pharmaceutical Ingredient |
| OSDF | Oral Solid Dosage Form |
| MCC | Microcrystalline Cellulose |
| xCMC | Croscarmellose |
| LAC | Lactose |
| HPMC | Hydroxypropyl Methylcellulose |
| SiO2 | Silicon Dioxide |
| SSF | Sodium Stearyl Fumarate |
| BSA | Bovine Serum Albumin |
| PSD | Particle Size Distribution |
| HD | High Dose |
| MD | Medium Dose |
| LD | Low Dose |
| VLD | Very Low Dose |
| DC | Direct Compression |
| RC | Roller Compaction |
| IR | Immediate Release |
| CR | Controlled Release |
| USP | United States Pharmacopeia |
| ASTM | American Society for Testing and Materials |
| SEM | Scanning Electron Microscopy |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| min | Minutes |
| h | Hours |
| Mean | |
| σ | Standard Deviation |
| RSD | Relative Standard Deviation |
Appendix A. ELISA Methodology
Appendix B. Methodology for Preparing Dissipated Tablet Samples for ELISA Analysis
Appendix C. Pictures of the Ingredients Used in This Study
Appendix D. f2 Similarity Factors
| Delivery Mode | Abbreviation | f2, DC (Ref) vs. RC (Test) 2.2 kN | f2, DC (Ref) vs. RC (Test) 22 kN |
|---|---|---|---|
| IR | MD/MCC | 81 | 67 |
| IR | MD/MCC/xCMC | 71 | 79 |
| IR | MD/MCC/LAC | 53 | 74 |
| CR | MD/MCC/HPMC | 95 | 87 |
References
- Sharma, A.; Arora, S. Commercial challenges and emerging trends in oral delivery of peptide and protein drugs: A review. Res. J. Pharm. Biol. Chem. Sci. 2011, 2, 778–790. [Google Scholar]
- PharmaCircle. Search: Molecule/API Group (Peptides OR Biologics OR Nucleic Acids) and Route (Oral). 2024. [Google Scholar]
- Auerbach, M. Drug delivery challenges: Issues and opportunities for solid dosage products. Tablets Capsul. Mag. 2024, 22, 46. [Google Scholar]
- Andersen, A.; Knop, F.K.; Vilsbøll, T. A Pharmacological and Clinical Overview of Oral Semaglutide for the Treatment of Type 2 Diabetes. Drugs 2021, 81, 1003–1030. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, S. Rybelsus: An oral formulation of the GLP-1 agonist semaglutide. Prescriber 2020, 31, 32–33. [Google Scholar] [CrossRef]
- Moroz, E.; Matoori, S.; Leroux, J.-C. Oral delivery of macromolecular drugs: Where we are after almost 100 years of attempts. Adv. Drug Deliv. Rev. 2016, 101, 108–121. [Google Scholar] [CrossRef]
- Smart, A.L.; Gaisford, S.; Basit, A.W. Oral peptide and protein delivery; intestinal obstacles and commercial prospects. Expert Opin. Drug Deliv. 2014, 11, 1323–1335. [Google Scholar] [CrossRef]
- Aungst, B.J. Absorption Enhancers: Applications and Advances. AAPS J. 2012, 14, 10–18. [Google Scholar] [CrossRef]
- Choonara, B.F.; Choonara, Y.E.; Kumar, P.; Bijukumar, D.; du Toit, L.C.; Pillay, V. A review of advanced oral drug delivery technologies facilitating the protection and absorption of protein and peptide molecules. Biotechnol. Adv. 2014, 32, 1269–1282. [Google Scholar] [CrossRef]
- Rekha, M.R.; Sharma, C.P. Oral delivery of therapeutic protein/peptide for diabetes—Future perspectives. Int. J. Pharm. 2013, 440, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Renukuntla, J.; Vadlapudi, A.D.; Patel, A.; Boddu, S.H.S.; Mitra, A.K. Approaches for enhancing oral bioavailability of peptides and proteins. Int. J. Pharm. 2013, 447, 75–93. [Google Scholar] [CrossRef]
- Hazlett, R.; Schmidmeier, C.; O’MAhony, J. Approaches for improving the flowability of high-protein dairy powders post spray drying—A review. Powder Technol. 2021, 388, 26–40. [Google Scholar] [CrossRef]
- Holmfred, E.; Hirschberg, C.; Rantanen, J. Compaction properties of particulate proteins in binary powder mixtures with common excipients. Pharmaceutics 2024, 16, 19. [Google Scholar] [CrossRef] [PubMed]
- Dudhat, S.M.; Kettler, C.N.; Dave, R.H. To study capping or lamination tendency of tablets through evaluation of powder rheological properties and tablet mechanical properties of directly compressible blends. AAPS PharmSciTech 2017, 18, 1177–1189. [Google Scholar] [CrossRef]
- Pedersen, M.D.; Megarry, A.; Naelapää, K.; Rades, T.; Pessi, J. Enhancing tabletability of high-dose tablets by tailoring properties of spray-dried insulin particles. Int. J. Pharm. 2023, 631, 122526. [Google Scholar] [CrossRef]
- Chen, L.; He, Z.; Kunnath, K.T.; Fan, S.; Wei, Y.; Ding, X.; Zheng, K.; Davé, R.N. Surface engineered excipients: III. Facilitating direct compaction tableting of binary blends containing fine cohesive poorly-compactable APIs. Int. J. Pharm. 2019, 557, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Macho, O.; Gabrišová, Ľ.; Brokešová, J.; Svačinová, P.; Mužíková, J.; Galbavá, P.; Blaško, J.; Šklubalová, Z. Systematic study of paracetamol powder mixtures and granules tabletability: Key role of rheological properties and dynamic image analysis. Int. J. Pharm. 2021, 608, 121110. [Google Scholar] [CrossRef]
- Lin, Z.; Cabello, B.; Kossor, C.; Davé, R. Facilitating direct compaction tableting of fine cohesive APIs using dry coated fine excipients: Effect of the excipient size and amount of coated silica. Int. J. Pharm. 2024, 660, 124359. [Google Scholar] [CrossRef]
- Wei, G.; Mangal, S.; Denman, J.; Gengenbach, T.; Bonar, K.L.; Khan, R.I.; Qu, L.; Li, T.; Zhou, Q. Effects of coating materials and processing conditions on flow enhancement of cohesive acetaminophen powders by high-shear processing with pharmaceutical lubricants. J. Pharm. Sci. 2017, 106, 3022–3032. [Google Scholar] [CrossRef]
- Qu, L.; Zhou, Q.; Gengenback, T.; Denman, J.A.; Stewart, P.J.; Hapgood, K.P.; Gamlen, M.; Morton, D.A.V. Investigation of the potential for direct compaction of a fine ibuprofen powder dry-coated with magnesium stearate. Drug Dev. Ind. Pharm. 2015, 41, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Kunnath, K.; Huang, Z.; Chen, L.; Zheng, K.; Davé, R. Improved properties of fine active pharmaceutical ingredient powder blends and tablets at high drug loading via dry particle coating. Int. J. Pharm. 2018, 543, 288–299. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, C.; Jiang, B.; Sun, C.C.; Middaugh, C.R. Developing Biologics Tablets: The Effects of Compression on the Structure and Stability of Bovine Serum Albumin and Lysozyme. Mol. Pharm. 2019, 16, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Chen, G. Advances in the Oral Delivery of Protein and Peptide Drugs. Pharmaceutics 2025, 17, 616. [Google Scholar] [CrossRef]
- Vidakovic, I.; Kornmueller, K.; Fiedler, D.; Khinast, J.; Fröhlich, E.; Leitinger, G.; Horn, C.; Quehenberger, J.; Spadiut, O.; Prassl, R. Archaeosomes for oral drug delivery: From continuous microfluidics production to powdered formulations. Pharmaceutics 2024, 16, 694. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Svirskis, D.; Proft, T.; Loh, J.; Huang, Y.; Wen, J. Cellular uptake and transport mechanism investigations of pegylated niosomes for improving the oral delivery of thymopentin. Pharmaceutics 2024, 16, 397. [Google Scholar] [CrossRef]
- Masloh, S.; Chevrel, A.; Culot, M.; Perrocheau, A.; Kalia, Y.N.; Frehel, S.; Gaussin, R.; Gosselet, F.; Huet, S.; Labouebe, M.Z.; et al. Enhancing oral delivery of biologics: A non-competitive and cross-reactive anti-leptin receptor nanofitin demonstrates a gut-crossing capacity in an ex vivo porcine intestinal model. Pharmaceutics 2024, 16, 116. [Google Scholar] [CrossRef]
- Yang, X.; Lin, R.; Feng, C.; Kang, Q.; Yu, P.; Deng, Y.; Jin, Y. Research progress on peptide drugs for type 2 diabetes and the possibility of oral administration. Pharmaceutics 2024, 16, 1353. [Google Scholar] [CrossRef] [PubMed]
- United States Pharmacopeia 47—National Formulary 42 (USP 47—NF 42) Chapter <616>. 1 May 2024. Available online: www.usp.org (accessed on 10 January 2025).
- ASTM D6773-16; Standard Test Method for Bulk Solids Using Schulze Ring Shear Tester. ASTM International: West Conshohocken, PA, USA, 2016.
- United States Pharmacopeia 47—National Formulary 42 (USP 47—NF 42) Felodipine Extended Release Tablets Monograph. 1 May 2022. Available online: www.usp.org (accessed on 21 August 2025).
- Moore, J.; Flanner, H. Mathematical comparison of dissolution profiles. Pharm. Technol. 1996, 6, 64. [Google Scholar]
- United States Pharmacopeia 47—National Formulary 42 (USP 47—NF 42) Chapter <1092>. 1 May 2024. Available online: www.usp.org (accessed on 10 January 2025).
- RE, H.H.R. Effect of particle size distribution on the friction in a powder mass. Int. J. Powder Metall. 1970, 6, 17–22. [Google Scholar]
- Ban, S.v.D.; Goodwin, D.J. The impact of granule density on tabletting and pharmaceutical product performance. Pharm. Res. 2017, 34, 1002–1011. [Google Scholar] [CrossRef]
- Liu, L.; Marziano, I.; Bentham, A.; Litster, J.; White, E.; Howes, T. Effect of particle properties on the flowability of ibuprofen powders. Int. J. Pharm. 2008, 362, 109–117. [Google Scholar] [CrossRef]
- Paul, S.C. Dependence of friability on tablet mechanical properties and a predictive approach for binary mixtures. Pharm. Res. 2017, 34, 2901–2909. [Google Scholar] [CrossRef] [PubMed]
- United States Pharmacopeia 47—National Formulary 42 (USP 47—NF 42) Chapter <1216>. 1 May 2024. Available online: www.usp.org (accessed on 3 January 2025).
- Okoye, E.; Onyekweli, A.; Kunle, O.; Arhewoh, M. Brittle fracture index (BFI) as a tool in the classification, grouping and ranking of some binders used in tablet formulation: Lactose tablets. Sci. Res. Essays 2010, 5, 500–506. [Google Scholar]
- Tofiq, M.; Nordström, J.; Persson, A.-S.; Alderborn, G. Effect of excipient properties and blend ratio on the compression properties of dry granulated particles prepared from microcrystalline cellulose and lactose. Powder Technol. 2022, 399, 117207–117221. [Google Scholar] [CrossRef]
- Pitt, K.G.; Heasley, M.G. Determination of the tensile strength of elongated tablets. Powder Technol. 2013, 238, 169–175. [Google Scholar] [CrossRef]
- Natoli, D. The Art of Tablet Compression. December 2015. Available online: https://natoli.com/wp-content/uploads/2015/12/The-Art-of-Tablet-Compression.pdf (accessed on 4 January 2025).
- United States Pharmacopeia 47—National Formulary 42 (USP 47—NF 42) Chapter <905>. 1 May 2024. Available online: www.usp.org (accessed on 10 January 2025).
- Krukowski, S.; Karasiewicz, M.; Kolodziejski, W. Convenient UV-spectrophotometric determination of citrates in aqueous solutions with applications in the pharmaceutical analysis of oral electrolyte formulations. J. Food Drug Anal. 2017, 25, 717–722. [Google Scholar] [CrossRef]
- Rahman, M.; Roy, S.; Das, S.; Jha, M.; Begum, T.; Ahsan, M.; Islam, M.; Reza, M. Evaluation of various grades of hydroxypropylmethylcellulose matrix systems as oral sustained release drug delivery systems. Int. J. Pharm. Sci. Res. 2011, 3, 930–938. [Google Scholar]





| Material | Name/Grade | Manufacturer/Supplier | Lot # |
|---|---|---|---|
| Bovine Lactoferrin (Lactoferrin) | 95% | Parchem | B20352248 |
| Microcrystalline Cellulose (MCC) | Avicel® PH-102 NF | Roquette | P218832253 |
| Croscarmellose Sodium (xCMC) | Spectrum Chemical | WQ0223 | |
| Lactose (LAC) | Flo Lac® 100 | Molkerei Meggle Wasserburg | L1015 |
| Hypromellose (HPMC) | METHOCEL™ K100 LV | Roquette | D180K5E022 |
| Silicon Dioxide (SiO2) | CAB-O-SIL® | CABOT | 3869248 |
| Sodium Stearyl Fumarate (SSF) | Alubra® PG-100 | Roquette | SF13106308 |
| Delivery Mode | Formulation | Abbreviation | ||||||
|---|---|---|---|---|---|---|---|---|
| Lactoferrin, wt.% | MCC, wt.% | xCMC, wt.% | LAC, wt.% | HPMC, wt.% | SiO2, wt.% | SSF, wt.% | ||
| IR | 45 | 54 | 0.5 | 0.5 | HD/MCC | |||
| IR | 30 | 69 | 0.5 | 0.5 | MD/MCC | |||
| IR | 15 | 84 | 0.5 | 0.5 | LD/MCC | |||
| IR | 5 | 94 | 0.5 | 0.5 | VLD/MCC | |||
| IR | 45 | 51.5 | 2.5 | 0.5 | 0.5 | HD/MCC/xCMC | ||
| IR | 30 | 66.5 | 2.5 | 0.5 | 0.5 | MD/MCC/xCMC | ||
| IR | 15 | 81.5 | 2.5 | 0.5 | 0.5 | LD/MCC/xCMC | ||
| IR | 5 | 91.5 | 2.5 | 0.5 | 0.5 | VLD/MCC/xCMC | ||
| IR | 45 | 27 | 27 | 0.5 | 0.5 | HD/MCC/LAC | ||
| IR | 30 | 34.5 | 34.5 | 0.5 | 0.5 | MD/MCC/LAC | ||
| IR | 15 | 42 | 42 | 0.5 | 0.5 | LD/MCC/LAC | ||
| IR | 5 | 47 | 47 | 0.5 | 0.5 | VLD/MCC/LAC | ||
| CR | 45 | 24 | 30 | 0.5 | 0.5 | HD/MCC/HPMC | ||
| CR | 30 | 39 | 30 | 0.5 | 0.5 | MD/MCC/HPMC | ||
| CR | 15 | 54 | 30 | 0.5 | 0.5 | LD/MCC/HPMC | ||
| CR | 5 | 64 | 30 | 0.5 | 0.5 | VLD/MCC/HPMC | ||
| Tablet Press | 16-Station Manesty Beta Rotary Press with Small Baffle Feeder System |
|---|---|
| Tooling | 0.3125 in (7.94 mm) round concave, tooling positioned at every other station in the turret |
| Tablet target weight | 200 mg total weight per tablet |
| Compression force | 500 and 5000 lb (2.2 & 22 kN) |
| Turret speed | 15 RPM |
| Equipment configuration | USP II paddle method with tablet placed in suspended hanging basket |
| Replicates | 6 tablets per formulation (n = 6) |
| Dissolution media | 900 mL pH 7.4 phosphate buffer |
| Temperature | 37 ± 0.5 °C |
| Paddle speed | 100 RPM |
| UV absorbance | 226 nm |
| UV cell path length | 10 mm |
| Tablet placement | In suspended basket hanging 2 cm above paddle |
| Material | Bulk Density (g/cc) | Tapped Density (g/cc) | Hausner Ratio |
|---|---|---|---|
| Lactoferrin | 0.267 ± 0.005 | 0.440 ± 0.001 | 1.647 ± 0.030 |
| MCC | 0.320 ± 0.002 | 0.476 ± 0.003 | 1.486 ± 0.010 |
| xCMC | 0.494 ± 0.006 | 0.759 ± 0.001 | 1.536 ± 0.016 |
| LAC | 0.597 ± 0.006 | 0.730 ± 0.000 | 1.224 ± 0.012 |
| HPMC | 0.281 ± 0.001 | 0.505 ± 0.002 | 1.796 ± 0.011 |
| HD/MCC | 0.362 ± 0.002 | 0.541 ± 0.004 | 1.493 ± 0.004 |
| Sample Name | D10 (µm) | D50 (µm) | D90 (µm) | D[4,3] (µm) |
|---|---|---|---|---|
| Lactoferrin | 4.9 | 15.4 | 30.6 | 16.9 |
| MCC | 42.1 | 127 | 263 | 174 |
| xCMC | 25.2 | 59.5 | 119 | 66.8 |
| LAC | 21.4 | 68.9 | 157.7 | 80.9 |
| HPMC | 28.8 | 79.1 | 199 | 99.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogers, T.L.; Horton, A.J.; Watson, T.; Robart, S.; DeFrancesco, B.; Bishop, H.; Tocce, E. Oral Tablet Formulations with Lactoferrin, a Cohesive Biomacromolecule. Pharmaceutics 2025, 17, 1151. https://doi.org/10.3390/pharmaceutics17091151
Rogers TL, Horton AJ, Watson T, Robart S, DeFrancesco B, Bishop H, Tocce E. Oral Tablet Formulations with Lactoferrin, a Cohesive Biomacromolecule. Pharmaceutics. 2025; 17(9):1151. https://doi.org/10.3390/pharmaceutics17091151
Chicago/Turabian StyleRogers, True L., Andrew J. Horton, Thomas Watson, Stephanie Robart, Brooklynn DeFrancesco, Hannah Bishop, and Elizabeth Tocce. 2025. "Oral Tablet Formulations with Lactoferrin, a Cohesive Biomacromolecule" Pharmaceutics 17, no. 9: 1151. https://doi.org/10.3390/pharmaceutics17091151
APA StyleRogers, T. L., Horton, A. J., Watson, T., Robart, S., DeFrancesco, B., Bishop, H., & Tocce, E. (2025). Oral Tablet Formulations with Lactoferrin, a Cohesive Biomacromolecule. Pharmaceutics, 17(9), 1151. https://doi.org/10.3390/pharmaceutics17091151






