Selective Laser Sintering of Atomoxetine Tablets: An Innovative Approach for Small-Scale, Personalized Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- Mannitol Parteck® M 200 (Merck, Darmstadt, Germany);
- Kollidon® VA 64 Fine (vinylpyrrolidone-vinyl acetate copolymer, BASF, Ludwigshafen, Germany);
- Hydroxypropyl methylcellulose (HPMC, Vivapharm® E3, JRS Pharma, Rosenberg, Germany);
- Sodium stearyl fumarate (Merck, Darmstadt, Germany);
- Croscarmellose sodium (Sigma-Aldrich, Darmstadt, Germany);and directly compressible, co-processed excipients:
- Retalac® (Meggle, Wasserburg am Inn, Germany), consisting of 50% lactose monohydrate and 50% HPMC;
- Starlac® (Meggle, Wasserburg am Inn, Germany), consisting of 85% lactose monohydrate and 15% maize starch.
2.2. Methods
2.2.1. Preparation of the Mixture for Tablet Production
2.2.2. Tablet Production Using the SLS Technique
2.2.3. Tablet Production Using a Compaction Simulator
2.2.4. Tablet Characterization Methods
3. Results and Discussion
3.1. SLS Tablet Production
3.2. Tablet Production Using the Compaction Simulator
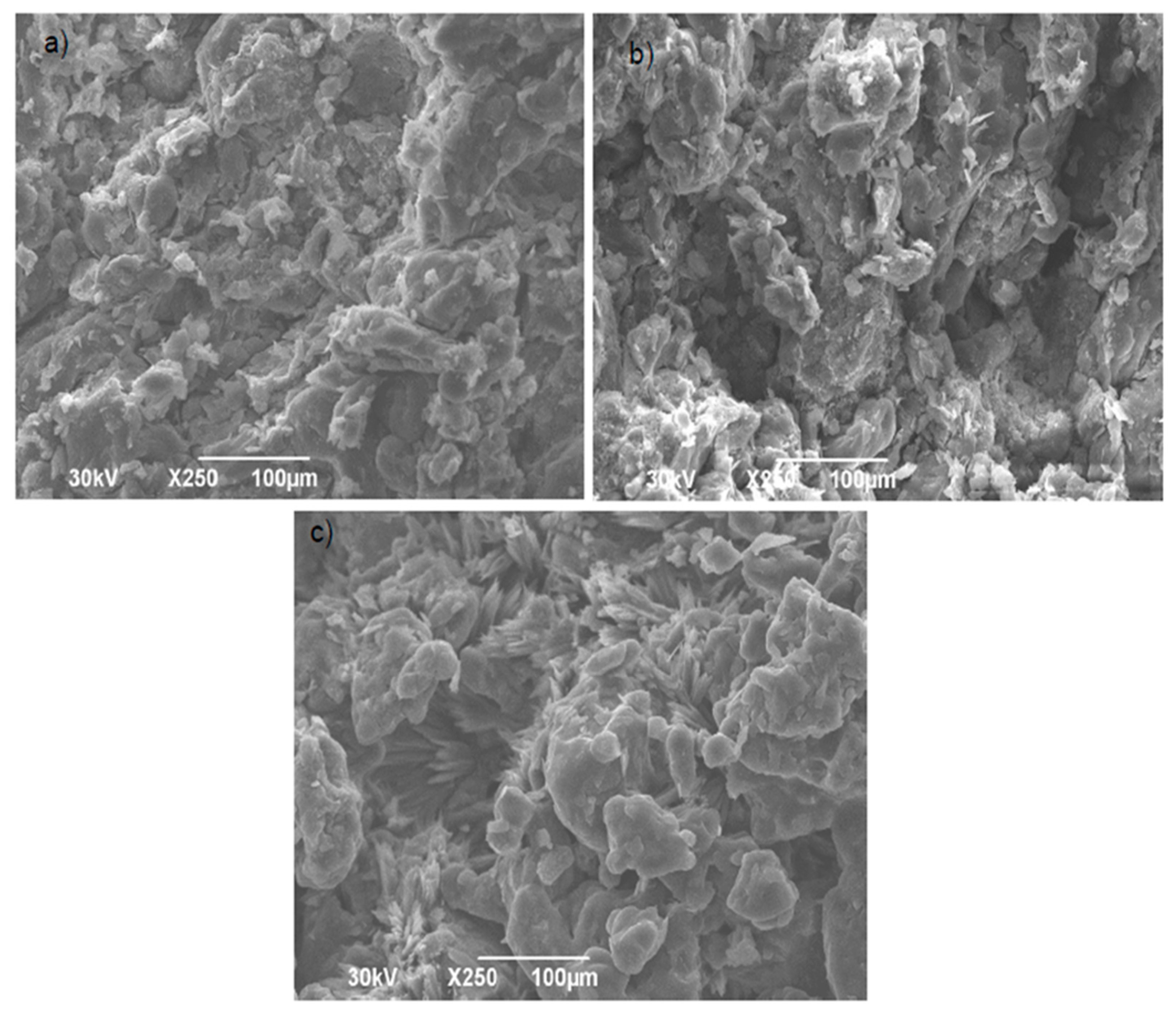
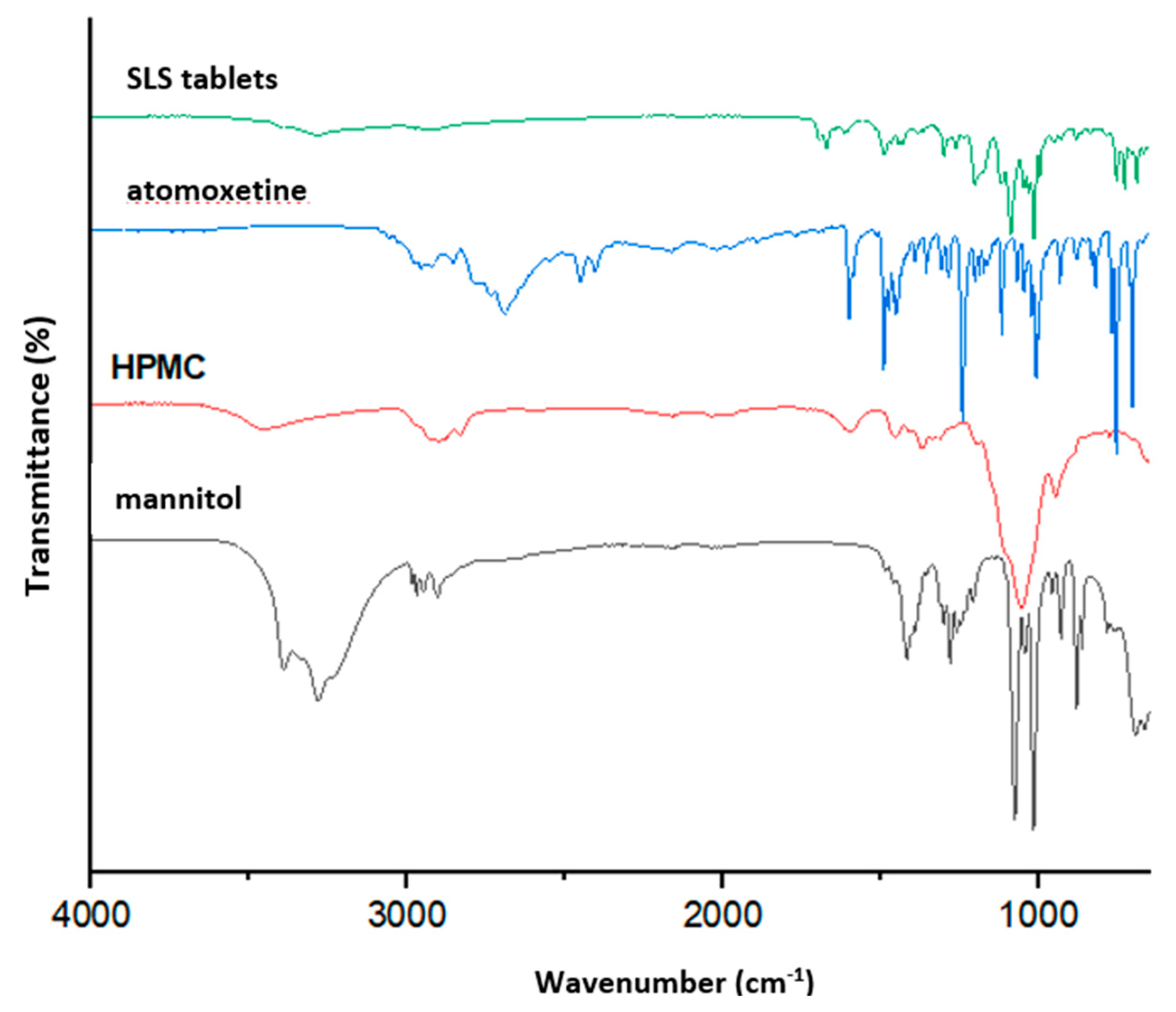
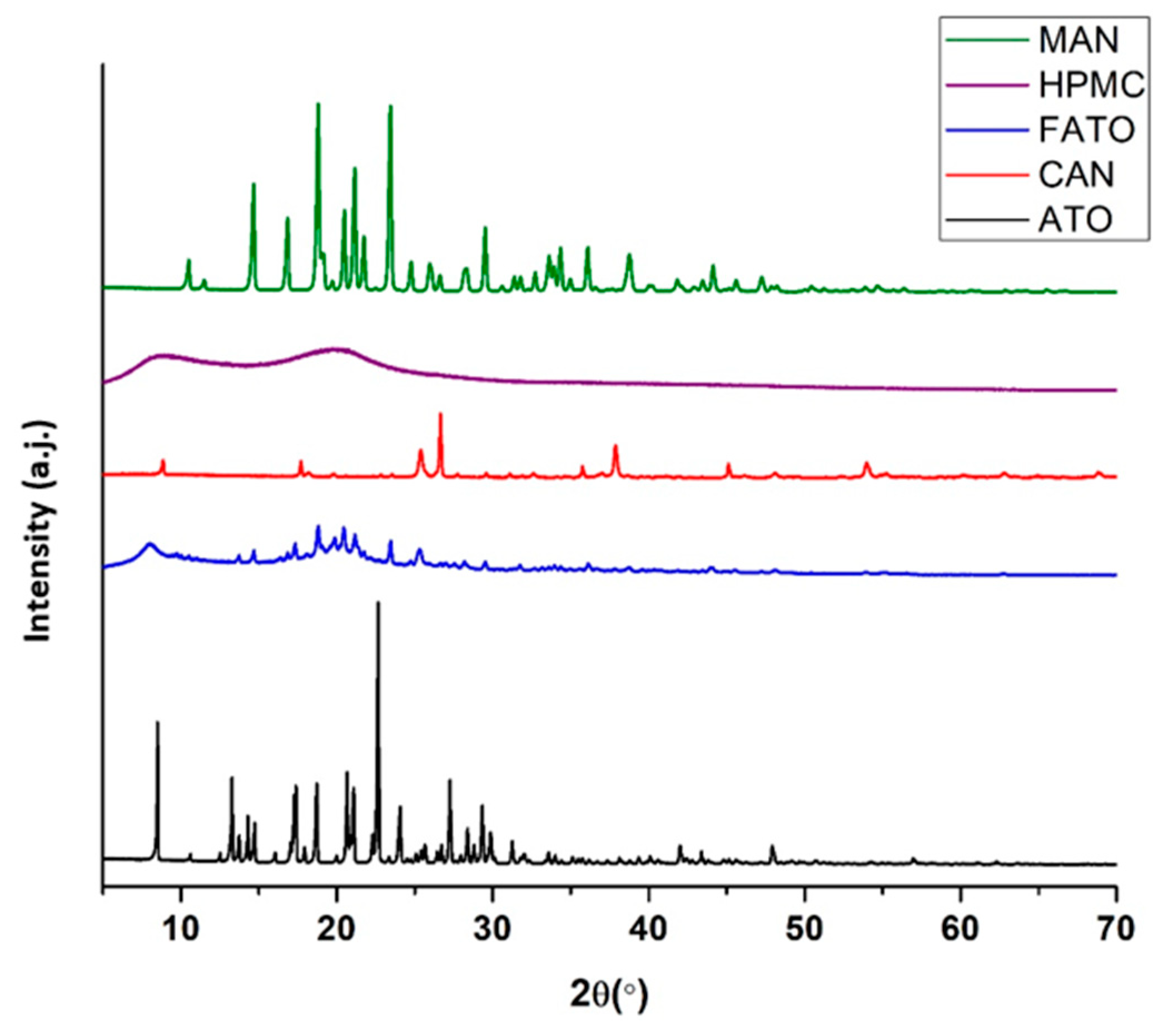
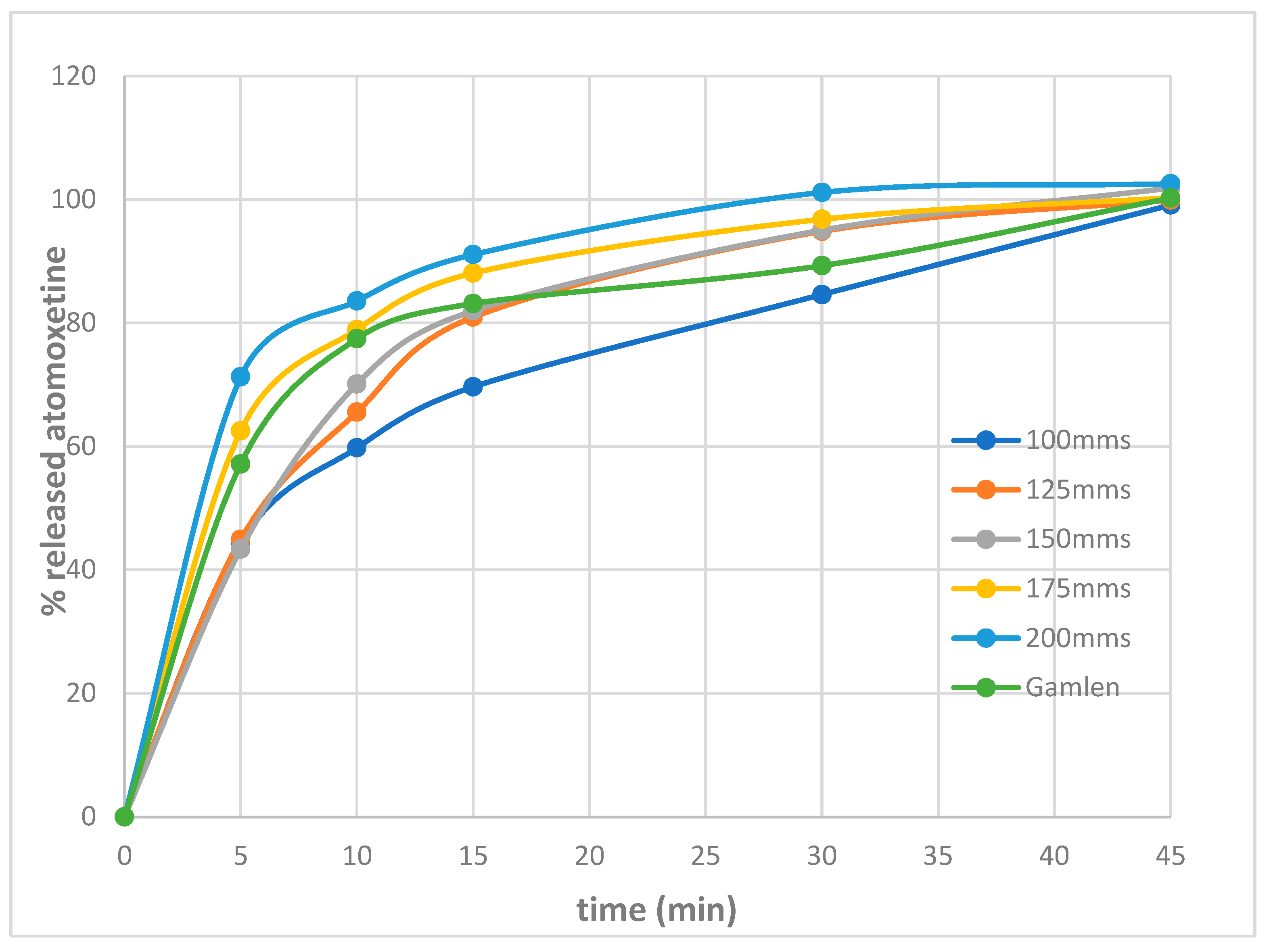
3.3. Tablet Characterization Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nørfeldt, L.; Bøtker, J.; Edinger, M.; Genina, N.; Rantanen, J. Cryptopharmaceuticals: Increasing the safety of medication by a blockchain of pharmaceutical products. J. Pharm. Sci. 2019, 108, 2838–2841. [Google Scholar] [CrossRef] [PubMed]
- Seoane-Viaño, I.; Trenfield, S.J.; Basit, A.W.; Goyanes, A. Translating 3D printed pharmaceuticals: From hype to real-world clinical applications. Adv. Drug Deliv. Rev. 2021, 174, 553–575. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.; Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D printing: Principles and pharmaceutical applications of selective laser sintering. Int. J. Pharm. 2020, 586, 119594. [Google Scholar] [CrossRef]
- Medarevic, D.; Krstic, M.; Ibric, S. Fundamentals of 3D printing of pharmaceuticals. In From Current to Future Trends in Pharmaceutical Technology; Elsevier: Amsterdam, The Netherlands, 2024; pp. 1–65. [Google Scholar] [CrossRef]
- Chen, G.; Xu, Y.; Kwok, P.C.L.; Kang, L. Pharmaceutical applications of 3D printing. Addit. Manuf. 2020, 34, 101209. [Google Scholar] [CrossRef]
- Pandey, M.; Choudhury, H.; Fern, J.L.C.; Kee, A.T.K.; Kou, J.; Jing, J.L.J.; Her, H.C.; Yong, H.S.; Ming, H.C.; Bhattamisra, S.K.; et al. 3D printing for oral drug delivery: A new tool to customize drug delivery. Drug Deliv. Transl. Res. 2020, 10, 986–1001. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, X.; Han, X.; Hong, X.; Li, X.; Zhang, H.; Li, M.; Wang, Z.; Zheng, A. A review of 3D printing technology in pharmaceutics: Technology and applications, now and future. Pharmaceutics 2023, 15, 416. [Google Scholar] [CrossRef]
- Alhijjaj, M.; Belton, P.; Qi, S. An investigation into the use of polymer blends to improve the printability of and regulate drug release from pharmaceutical solid dispersions prepared via fused deposition modeling (FDM) 3D printing. Pharmaceutics 2016, 8, 15. [Google Scholar] [CrossRef]
- Mora-Castaño, L.; Domínguez-Robles, J.; Himawan, A.; Millán-Jiménez, M.; Caraballo, I. Current trends in 3D printed gastroretentive floating drug delivery systems: A comprehensive review. Int. J. Pharm. 2024, 663, 124543. [Google Scholar] [CrossRef]
- Gueche, Y.A.; Sanchez-Ballester, N.M.; Cailleaux, S.; Bataille, B.; Soulairol, I. Selective Laser Sintering (SLS), a New Chapter in the Production of Solid Oral Forms (SOFs) by 3D Printing. Pharmaceutics 2021, 13, 1212. [Google Scholar] [CrossRef]
- Maroni, A.; Melocchi, A.; Parolin, C.; Foppoli, A.; Zema, L.; Gazzaniga, A. 3D printing by stereolithography and digital light processing: A guide for the pharmaceutical and biomedical applications. Drug Discov. Today 2020, 25, 1126–1137. [Google Scholar] [CrossRef]
- Norman, J.; Madurawe, R.D.; Moore, C.M.V.; Khan, M.A.; Khairuzzaman, A. A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Adv. Drug Deliv. Rev. 2017, 108, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Chai, X.; Tan, K.B.; Tan, M.J.; Ruan, S. 3D printing in drug delivery and biomedical applications: A comprehensive review of recent advances. Mater. Sci. Eng. C 2022, 127, 112215. [Google Scholar] [CrossRef]
- Hettesheimer, T.; Hirzel, S.; Roß, H.B. Energy savings through additive manufacturing: An analysis of selective laser sintering for automotive and aircraft components. Energy Effic. 2018, 11, 1227–1245. [Google Scholar] [CrossRef]
- Seoane-Viaño, I.; Pérez-Ramos, T.; Liu, J.; Januskaite, P.; Guerra-Baamonde, E.; González-Ramírez, J.; Vázquez-Caruncho, M.; Basit, A.W.; Goyanes, A. Visualizing disintegration of 3D printed tablets in humans using MRI and comparison with in vitro data. J. Control. Release 2024, 365, 348–357. [Google Scholar] [CrossRef]
- Charoo, N.A.; Barakh Ali, S.F.; Mohamed, E.M.; Kuttolamadom, M.A.; Ozkan, T.; Khan, M.A.; Rahman, Z. Selective laser sintering 3D printing—An overview of the technology and pharmaceutical applications. Drug Dev. Ind. Pharm. 2020, 46, 869–877. [Google Scholar] [CrossRef]
- Adamov, I.; Stanojevic, G.; Pavlovic, S.M.; Medarevic, D.; Ivkovic, B.; Kocovic, D.; Ibric, S. Powder bed fusion–laser beam (PBF-LB) three-dimensional (3D) printing: Influence of laser hatching distance on the properties of zolpidem tartrate tablets. Int. J. Pharm. 2024, 657, 124161. [Google Scholar] [CrossRef]
- Goyanes, A.; Fina, F.; Martorana, A.; Sedough, D.; Gaisford, S.; Basit, A.W. Development of modified release 3D printed tablets (printlets) with pharmaceutical excipients using additive manufacturing. Int. J. Pharm. 2017, 527, 21–30. [Google Scholar] [CrossRef]
- Januskaite, P.; Xu, X.; Ranmal, S.R.; Gaisford, S.; Basit, A.W.; Tuleu, C.; Goyanes, A. I spy with my little eye: A paediatric visual preferences survey of 3D printed tablets. Pharmaceutics 2020, 12, 1100. [Google Scholar] [CrossRef]
- Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Selective laser sintering (SLS) 3D printing of medicines. Int. J. Pharm. 2017, 529, 285–293. [Google Scholar] [CrossRef]
- Fina, F.; Goyanes, A.; Madla, C.M.; Awad, A.; Trenfield, S.J.; Kuek, J.M.; Patel, P.; Gaisford, S.; Basit, A.W. 3D printing of drug-loaded gyroid lattices using selective laser sintering. Int. J. Pharm. 2018, 547, 44–52. [Google Scholar] [CrossRef]
- Barakh Ali, S.F.; Mohamed, E.M.; Ozkan, T.; Kuttolamadom, M.A.; Khan, M.A.; Asadi, A.; Rahman, Z. Understanding the effects of formulation and process variables on the printlets quality manufactured by selective laser sintering 3D printing. Int. J. Pharm. 2019, 570, 118651. [Google Scholar] [CrossRef]
- Tikhomirov, E.; Åhlén, M.; Di Gallo, N.; Strømme, M.; Kipping, T.; Quodbach, J.; Lindh, J. Selective laser sintering additive manufacturing of dosage forms: Effect of powder formulation and process parameters on the physical properties of printed tablets. Int. J. Pharm. 2023, 635, 122780. [Google Scholar] [CrossRef] [PubMed]
- Carou-Senra, P.; Rodríguez-Pombo, L.; Awad, A.; Basit, A.W.; Alvarez-Lorenzo, C.; Goyanes, A. Inkjet printing of pharmaceuticals. Adv. Mater. 2024, 36, 2309164. [Google Scholar] [CrossRef] [PubMed]
- Stanojević, G.; Medarević, D.; Adamov, I.; Pešić, N.; Kovačević, J.; Ibrić, S. Tailoring Atomoxetine Release Rate from DLP 3D-Printed Tablets Using Artificial Neural Networks: Influence of Tablet Thickness and Drug Loading. Molecules 2021, 26, 111. [Google Scholar] [CrossRef]
- Davis, D.A.; Thakkar, R.; Su, Y.; Williams, R.O.; Maniruzzaman, M. Selective laser sintering 3-dimensional printing as a single step process to prepare amorphous solid dispersion dosage forms for improved solubility and dissolution rate. J. Pharm. Sci. 2021, 110, 1432–1443. [Google Scholar] [CrossRef]
- Basit, A.W.; Gaisford, S. 3D Printing of Pharmaceuticals; AAPS Advances in the Pharmaceutical Sciences Series 31; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Fina, F.; Madla, C.M.; Goyanes, A.; Zhang, J.; Gaisford, S.; Basit, A.W. Fabricating 3D printed orally disintegrating printlets using selective laser sintering. Int. J. Pharm. 2018, 541, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.M.; Barakh Ali, S.F.; Rahman, Z.; Dharani, S.; Ozkan, T.; Kuttolamadom, M.A.; Khan, M.A. Formulation optimization of selective laser sintering 3D-printed tablets of clindamycin palmitate hydrochloride by response surface methodology. AAPS PharmSciTech 2020, 21, 232–247. [Google Scholar] [CrossRef]
- Trenfield, S.J.; Xu, X.; Goyanes, A.; Rowland, M.; Wilsdon, D.; Gaisford, S.; Basit, A.W. Releasing fast and slow: Non-destructive prediction of density and drug release from SLS 3D printed tablets using NIR spectroscopy. Int. J. Pharm. 2023, 5, 100148. [Google Scholar] [CrossRef]
- Podczeck, F.; Newton, J.M. Powder compaction. In Encyclopedia of Pharmaceutical Technology, 3rd ed.; Swarbrick, J., Ed.; Marcel Dekker: New York, NY, USA, 2001; Volume 3, pp. 45–66. [Google Scholar]
- Franca, C.A.; Etcheverry, S.B.; Diez, R.P.; Williams, P.A.M. Irbesartan: FTIR and Raman spectra. Density functional study on vibrational and NMR spectra. J. Raman Spectrosc. 2006, 40, 1296–1300. [Google Scholar] [CrossRef]
- Akinosho, H.; Hawkins, S.; Wicker, L. Hydroxypropyl methylcellulose substituent analysis and rheological properties. Carbohydr. Polym. 2013, 98, 276–281. [Google Scholar] [CrossRef]
- Bashir, S.; Zafar, N.; Lebaz, N.; Mahmood, A.; Elaissari, A. Hydroxypropyl methylcellulose-based hydrogel copolymeric for controlled delivery of galantamine hydrobromide in dementia. Processes 2020, 8, 1350. [Google Scholar] [CrossRef]
- Rajbanshi, K.; Bajracharya, R.; Shrestha, A.; Thapa, P. Dissolution enhancement of aceclofenac tablet by solid dispersion technique. Int. J. Pharma Sci. Res. 2014, 5, 127–139. [Google Scholar]
- Stephenson, G.A.; Liang, C. Structural determination of the stable and meta-stable forms of atomoxetine HCl using single crystal and powder X-ray diffraction methods. J. Pharm. Sci. 2006, 95, 1677–1683. [Google Scholar] [CrossRef] [PubMed]




| Composition of the Mixtures | Chamber/Surface Temperature (°C) | Laser Scanning Speed (mm/s) |
|---|---|---|
| ATH 10% Candurin 3% Sodium stearyl fumarate 2% Croscarmellose sodium 2% Kollidon® 6% Starlac® 76% | 120/130 120/130 120/135 130/140 140/150 140/150 | 100 150 100 100 150 100 150 |
| ATH 10% Candurin 3% Sodium stearyl fumarate 2% Croscarmellose sodium 2% Kollidon® 6% Retalac® 76% | 120/130 120/130 120/135 130/140 140/150 140/150 | 100 150 100 100 150 100 150 |
| ATH 10% Candurin 3% Sodium stearyl fumarate 2% Mannitol Partec 25% HPMC 60% | 120/130 | 100 |
| 130/140 | 100 | |
| 140/150 | 100 | |
| ATH 10% Candurin 3% Mannitol Partec 27% HPMC 60% | 130/140 130/140 140/150 140/150 140/150 140/150 | 100 150 100 150 175 200 |
| Formulation | Weight (mg) | Diameter (mm) | Thickness (mm) | Hardness (N) | Disintegration Time (s) | Drug Content (%) | Drug Amount (mg) |
|---|---|---|---|---|---|---|---|
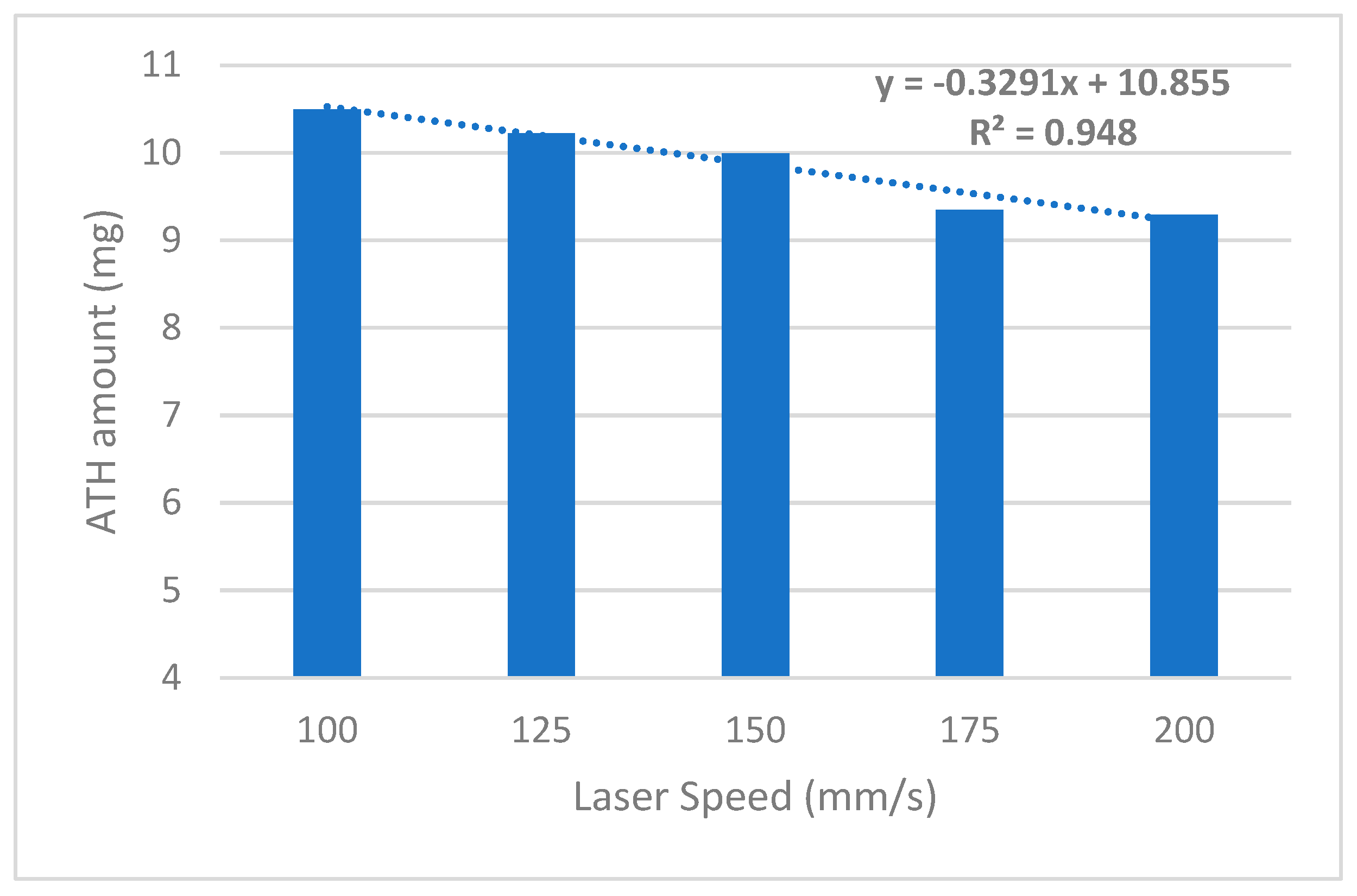
| Fsls-100 | 103 ± 0.91 | 7.97 ± 0.1 | 3.16 ± 0.1 | 45.5 ± 4.8 | 420 | 10.19 | 10.49 |
| Fsls-125 | 101 ± 0.25 | 7.99 ± 0.1 | 3.15 ± 0.1 | 38.7 ± 5.6 | 380 | 10.12 | 10.22 |
| Fsls-150 | 98.9 ± 0.74 | 7.99 ± 0.1 | 3.09 ± 0.1 | 22.6 ± 7.9 | 320 | 10.10 | 9.98 |
| Fsls-175 | 94.5 ± 0.15 | 7.91 ± 0.1 | 3.16 ± 0.1 | 18.3 ± 2.5 | 200 | 9.89 | 9.35 |
| Fsls-200 | 92.6 ± 0.11 | 7.93 ± 0.1 | 3.15 ± 0.1 | 14.5 ± 3.2 | 120 | 10.03 | 9.28 |
| F-Gamlen | 99.8 ± 0.4 | 6.00 | 3.00 | 24 | 360 | 10.00 | 9.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanojević, G.; Adamov, I.; Mugoša, S.; Vukićević, V.; Ibrić, S. Selective Laser Sintering of Atomoxetine Tablets: An Innovative Approach for Small-Scale, Personalized Production. Pharmaceutics 2025, 17, 794. https://doi.org/10.3390/pharmaceutics17060794
Stanojević G, Adamov I, Mugoša S, Vukićević V, Ibrić S. Selective Laser Sintering of Atomoxetine Tablets: An Innovative Approach for Small-Scale, Personalized Production. Pharmaceutics. 2025; 17(6):794. https://doi.org/10.3390/pharmaceutics17060794
Chicago/Turabian StyleStanojević, Gordana, Ivana Adamov, Snežana Mugoša, Veselinka Vukićević, and Svetlana Ibrić. 2025. "Selective Laser Sintering of Atomoxetine Tablets: An Innovative Approach for Small-Scale, Personalized Production" Pharmaceutics 17, no. 6: 794. https://doi.org/10.3390/pharmaceutics17060794
APA StyleStanojević, G., Adamov, I., Mugoša, S., Vukićević, V., & Ibrić, S. (2025). Selective Laser Sintering of Atomoxetine Tablets: An Innovative Approach for Small-Scale, Personalized Production. Pharmaceutics, 17(6), 794. https://doi.org/10.3390/pharmaceutics17060794









