Microfluidic and Turbulent Mixing for mRNA LNP Vaccines
Abstract
1. Introduction
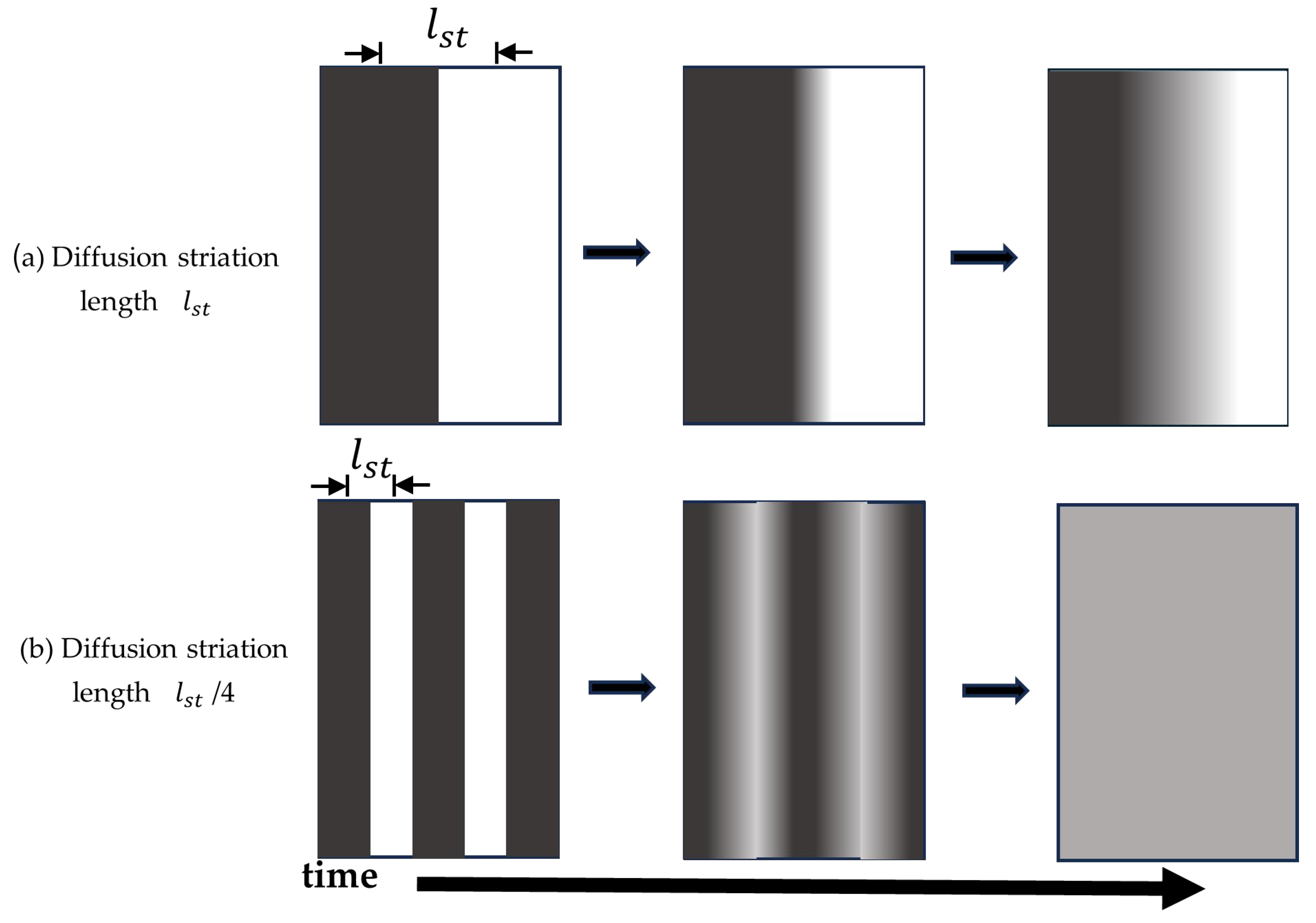
2. Fundamentals of Diffusion-Mediated Mixing Distances
- Average displacement distance
- dimension
- diffusivity time
3. Microfluidic Mixer Fundamentals
- fluid density (
- fluid flow ();
- characteristic length (which is typically the microfluidic channel height);
- dynamic viscosity ();
- kinematic viscosity ().
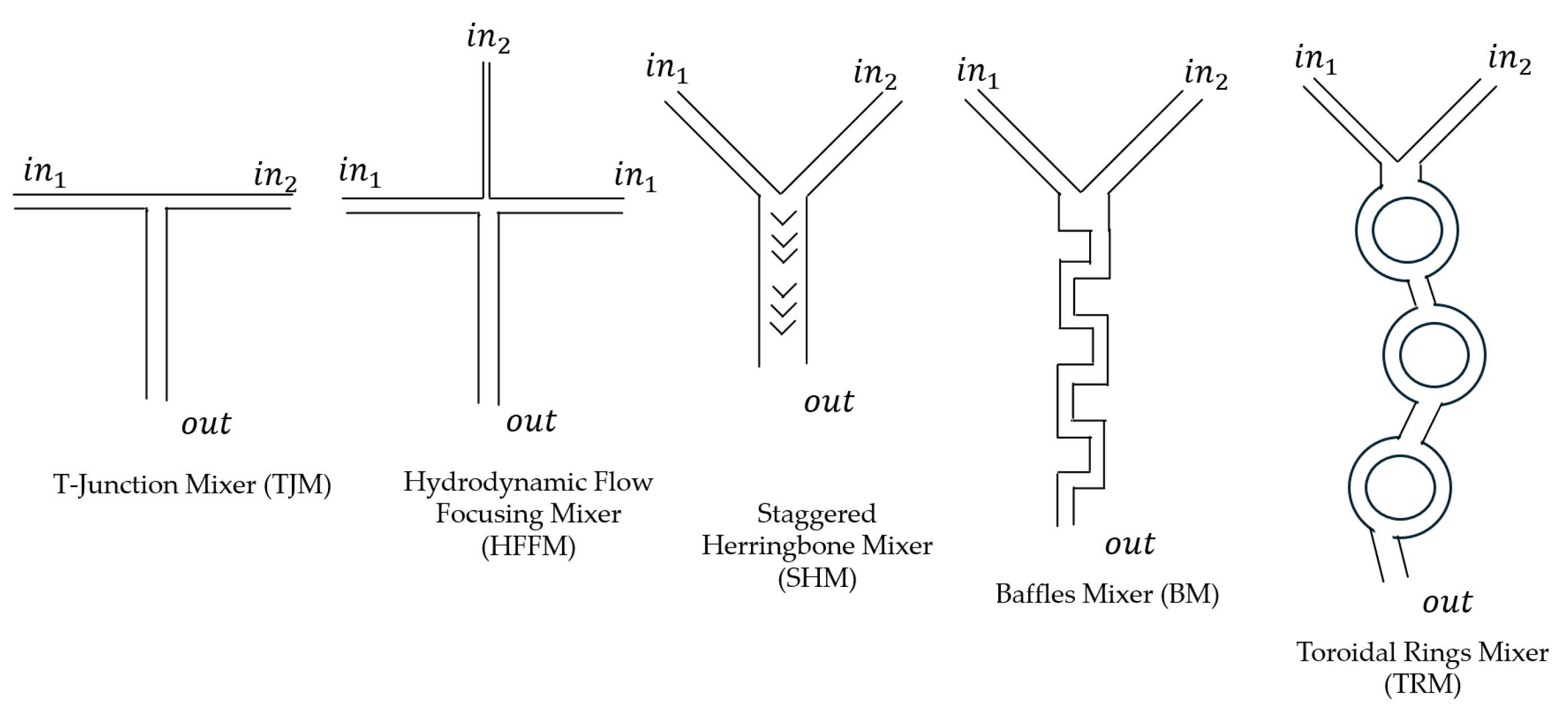
3.1. T-Junction Mixers (TJMs)
3.2. Hydrodynamic Flow Focusing Mixers (HFFMs)
3.3. Staggered Herringbone Mixers (SHMs)
3.4. Baffles Mixers (BMs)
3.5. Toroidal Ring Mixers (TRMs)
- = Reynold number of the fluid flow;
- channel diameter;
- radius of ring.
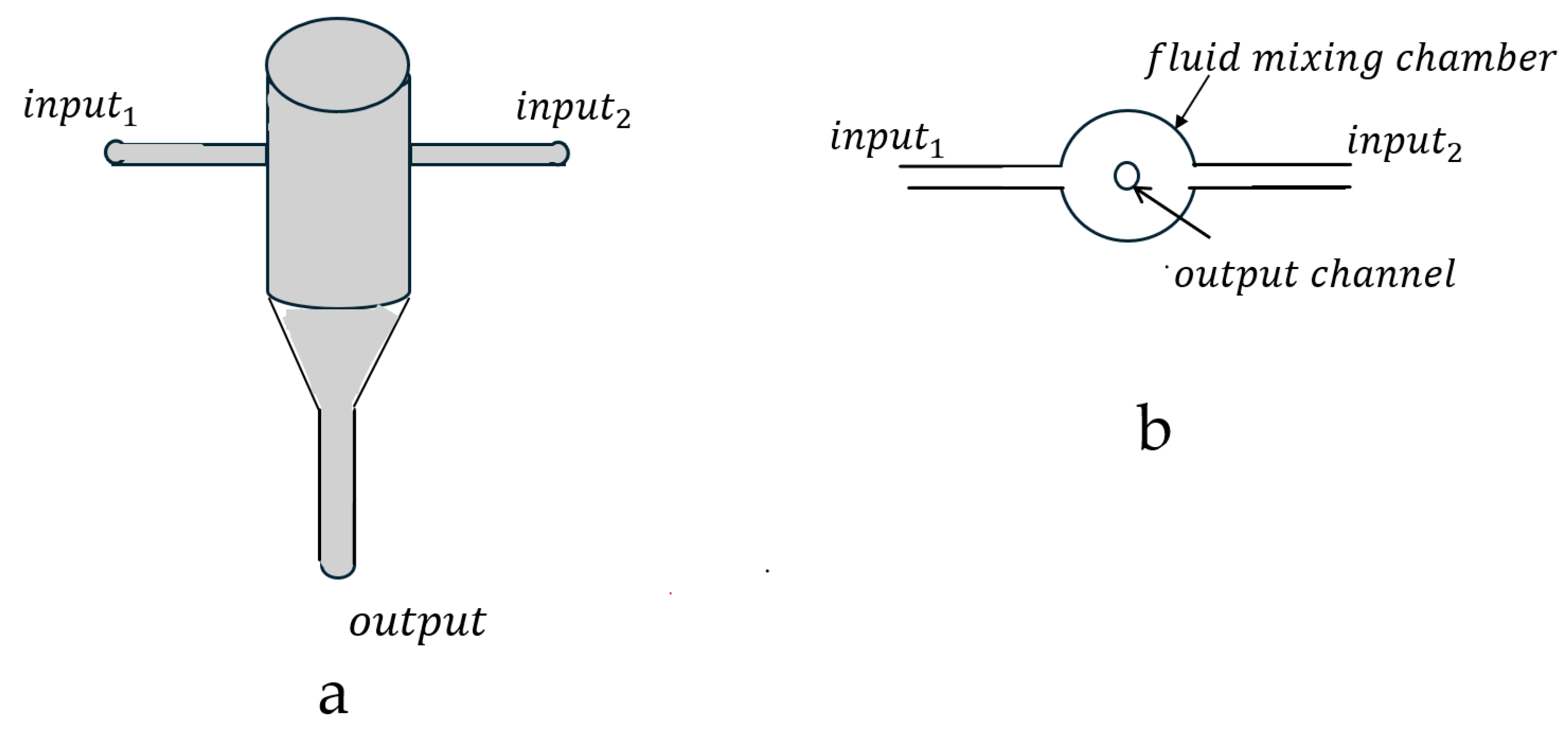
4. Turbulent Flow Mixer Fundamentals
- = Kolmogorov Length (;
- = kinematic viscosity ();
- energy dissipation rate .
- P = input energy );
- = fluid density ();
- mixing volume ().
- fluid flow rate (;
- = distance between the two CIJ fluid injection ports (;
- = fluid injection stream diameter at the two input injection ports ().
- = fluid velocity at input , ();
- = kinematic viscosity at input ();
- = mixing chamber diameter ().
5. Flash Nanoprecipitation, Nanocomplexation, and mRNA Incorporation
- = particle surface tension;
- = hydrophobic molecule molecular weight;
- = density hydrophobic molecules;
- = gas constant;
- = temperature.
- (1)
- Lipophilic complexes which contained some mRNA;
- (2)
- Positively charged lipophilic particles with no mRNA (empty LNPs);
- (3)
- Non-lipophilic complexes which contained mRNA.
6. Formulation of Empty and mRNA LNPs with Microfluidic Mixers
- (1)
- Z-average intensity DLS diameter near 100 nm;
- (2)
- DLS polydispersity index (PDI) < 0.2;
- (3)
- LNP nucleic acid encapsulation efficiency (EE) > 80%;
- (4)
- Consistent cryo-electron microscopy (Cryo-EM) structures;
- (5)
- Average LNP ζ-potential between 0 and −5 mV.
- (1)
- mRNA-loaded LNP;
- (2)
- Empty LNP;
- (3)
- Free mRNA nanoparticles.
7. Formulation of mRNA by Turbulent Mixing
8. Micromixing Practical Information and Future Directions
- (1)
- Flow rate ratio (FRR);
- (2)
- Ratio of ionizable amines to nucleic acid phosphates, i.e., the N/P ratio;
- (3)
- Ionizable lipid (MC3) formulation mole percentage.
- (1)
- LNP particle diameter;
- (2)
- LNP encapsulation efficiency;
- (3)
- RNA integrity;
- (4)
- In vitro protein expression;
- (5)
- In vitro IL-6 release [67].
9. Summary
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BM | Microfluidic Baffles Mixer Design |
| CICS | Cylindrical illumination confocal spectroscopy |
| CIJ | Confined Impinging Jet Turbulent Flow Mixer |
| CFD | Computational fluid dynamics |
| DMP | Dimethyloxypropane |
| FNC | Flash nanocomplexation (of nanoparticles) |
| FNP | Flash nanoprecipitation (of nanoparticles) |
| FRR | Flow rate ratio |
| HFFM | Hydrodynamic Flow-Focusing Mixer Design |
| LNP | Lipid nanoparticle |
| MIVM | Multi-inlet vortex turbulent flow mixer |
| SHM | Staggered Herringbone Mixer Design |
| TFR | Total flow rate |
| TJM | T-junction mixer design |
| TRM | Toroidal ring mixer design |
References
- Chaurasiya, A.; Gorajiya, A.; Panchal, K.; Katke, S.; Singh, A.K. A review on multivesicular liposomes for pharmaceutical applications: Preparation, characterization, and translational challenges. Drug Deliv. Transl. Res. 2022, 12, 1569–1587. [Google Scholar] [CrossRef] [PubMed]
- Kumari, L.; Choudhari, Y.; Patel, P.; Gupta, G.D.; Singh, D.; Rosenholm, J.M.; Bansal, K.K.; Kurmi, B.D. Advancement in Solubilization Approaches: A Step towards Bioavailability Enhancement of Poorly Soluble Drugs. Life 2023, 13, 1099. [Google Scholar] [CrossRef] [PubMed]
- Wikipedia. Lyotropic Liquid Crystal. 2025. Available online: https://en.wikipedia.org/wiki/Lyotropic_liquid_crystal (accessed on 23 April 2025).
- Wikipedia. Self-Assembly. 2025. Available online: https://en.wikipedia.org/wiki/Self-assembly (accessed on 23 April 2025).
- Liu, J.; Tang, J.; Tong, Z.; Teng, G.; Yang, D. DNA-guided self-assembly in living cells. Science 2023, 26, 106620. [Google Scholar] [CrossRef]
- Saad, S.; Jarosz, D.F. Protein self-assembly: A new frontier in cell signaling. Curr. Opin. Cell Biol. 2021, 69, 62–69. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Pallares, R.M.; Mottaghy, F.M.; Schulz, V.; Kiessling, F.; Lammers, T. Nanoparticle Diagnostics and Theranostics in the Clinic. J. Nucl. Med. 2022, 63, 1802–1808. [Google Scholar] [CrossRef]
- Mohsen, M.O.; Zha, L.; Cabral-Miranda, G.; Bachmann, M.F. Major findings and recent advances in virus-like particle (VLP)-based vaccines. Semin. Immunol. 2017, 34, 123–132. [Google Scholar] [CrossRef]
- Chatzikleanthous, D.; O’Hagan, D.T.; Adamo, R. Lipid-Based Nanoparticles for Delivery of Vaccine Adjuvants and Antigens: Toward Multicomponent Vaccines. Mol. Pharm. 2021, 18, 2867–2888. [Google Scholar] [CrossRef]
- Wikipedia. Self-Assembly of Nanoparticles. 2025. Available online: https://en.wikipedia.org/wiki/Self-assembly_of_nanoparticAles (accessed on 25 April 2025).
- FDA. COMIRNATY® (COVID-19 Vaccine, mRNA) Package Insert. Available online: https://www.fda.gov/vaccines-blood-biologics/comirnaty (accessed on 27 August 2025).
- FDA. SPIKEVAX (COVID-19 Vaccine, mRNA) Package Insert. Available online: https://www.fda.gov/vaccines-blood-biologics/spikevax (accessed on 27 August 2025).
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines-a new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Igyártó, B.Z.; Qin, Z. The mRNA-LNP vaccines-the good, the bad and the ugly? Front. Immunol. 2024, 15, 1336906. [Google Scholar] [CrossRef]
- Chong, S.H.; Burn, L.A.; Cheng, T.K.M.; Warr, I.S.; Kenyon, J.C. A review of COVID vaccines: Success against a moving target. Br. Med. Bull. 2022, 144, 12–44. [Google Scholar] [CrossRef]
- Friedrichs, S.; Bowman, D.M. COVID-19 may become nanomedicine’s finest hour yet. Nat. Nanotechnol. 2021, 16, 362–364. [Google Scholar] [CrossRef]
- Hald Albertsen, C.; Kulkarni, J.A.; Witzigmann, D.; Lind, M.; Petersson, K.; Simonsen, J.B. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv. Drug Deliv. Rev. 2022, 188, 114416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, C.; Wang, C.; Jankovic, K.E.; Dong, Y. Lipids and Lipid Derivatives for RNA Delivery. Chem. Rev. 2021, 121, 12181–12277. [Google Scholar] [CrossRef] [PubMed]
- Wikipedia. Fick’s Laws of Diffusion. 2025. Available online: https://en.wikipedia.org/wiki/Fick%27s_laws_of_diffusion (accessed on 25 April 2025).
- Price, H.C.; Mattsson, J.; Murray, B.J. Sucrose diffusion in aqueous solution. Phys. Chem. Chem. Phys. 2016, 18, 19207–19216. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.K.; Prud’homme, R.K. Mechanism for rapid self-assembly of block copolymer nanoparticles. Phys. Rev. Lett. 2003, 91, 118302. [Google Scholar] [CrossRef]
- Stroock, A.D.; Dertinger, S.K.; Ajdari, A.; Mezic, I.; Stone, H.A.; Whitesides, G.M. Chaotic mixer for microchannels. Science 2002, 295, 647–651. [Google Scholar] [CrossRef]
- Lee, C.Y.; Chang, C.L.; Wang, Y.N.; Fu, L.M. Microfluidic mixing: A review. Int. J. Mol. Sci. 2011, 12, 3263–3287. [Google Scholar] [CrossRef]
- Cheng, Y.; Hay, C.D.; Mahuttanatan, S.M.; Hindley, J.W.; Ces, O.; Elani, Y. Microfluidic technologies for lipid vesicle generation. Lab. Chip 2024, 24, 4679–4716. [Google Scholar] [CrossRef]
- Dawson, H.; Elias, J.; Etienne, P.; Calas-Etienne, S. The Rise of the OM-LoC: Opto-Microfluidic Enabled Lab-on-Chip. Micromachines 2021, 12, 1467. [Google Scholar] [CrossRef]
- Narayanamurthy, V.; Jeroish, Z.E.; Bhuvaneshwari, K.S.; Bayat, P.; Premkumar, R.; Samsuri, F.; Yusoff, M.M. Advances in passively driven microfluidics and lab-on-chip devices: A comprehensive literature review and patent analysis. RSC Adv. 2020, 10, 11652–11680. [Google Scholar] [CrossRef] [PubMed]
- Cytiva. Nanoparticle Formulation Systems. 2025. Available online: https://www.cytivalifesciences.com/en/us/shop/lipid-nanoparticle-instruments-and-reagents/nanoparticle-formulation-systems?sort=NameAsc&chunk=1 (accessed on 27 April 2025).
- Li, S.; Hu, Y.; Li, A.; Lin, J.; Hsieh, K.; Schneiderman, Z.; Zhang, P.; Zhu, Y.; Qiu, C.; Kokkoli, E.; et al. Payload distribution and capacity of mRNA lipid nanoparticles. Nat. Commun. 2022, 13, 5561. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, M.; Martin, E.; Enot, M.; Robbe, O.; Rapisarda, C.; Nicolai, M.C.; Deliot, A.; Tabeling, P.; Authelin, J.R.; Nakach, M.; et al. Optimal self-assembly of lipid nanoparticles (LNP) in a ring micromixer. Sci. Rep. 2022, 12, 9483. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Gwak, H.; Han, B. Advanced manufacturing of nanoparticle formulations of drugs and biologics using microfluidics. Analyst 2024, 149, 614–637. [Google Scholar] [CrossRef]
- Diant Pharma, I. DIANT’s Nanoparticle Technology. 2025. Available online: https://diantpharma.com/diants-nanoparticle-technology/ (accessed on 5 May 2025).
- Liu, R.H.; Stremler, M.A.; Sharp, K.V.; Olse, M.G.; Santiag, J.G.; Adrian, R.J. Passive mixing in a three-dimensional serpentine microchannel. J. Microelectromech. Syst. 2000, 9, 192–197. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Kim, B.J.; Sung, H.J. Two-fluid mixing in a microchannel. Int. J. Heat. Fluid. Flow. 2004, 25, 986–995. [Google Scholar] [CrossRef]
- Amiri, M.M. Tubulance: An Introduction. 2020. Available online: https://www.youtube.com/watch?v=sUqfatUVUPM&list=PLLYH8CQiuCNUS0o1dzgmvPK1mjt5BXVc7 (accessed on 5 April 2025).
- Joseph, H. New ‘Superdiffusion’ Proof Probes the Mysterious Math of Turbulence. 2025. Available online: https://www.quantamagazine.org/new-superdiffusion-proof-probes-the-mysterious-math-of-turbulence-20250516/?print=1 (accessed on 4 June 2025).
- Johnson, B.K.; Prud’homme, R.K. Chemical Processing and Micromixing in Confined Impinging Jets. AIcE J. 2003, 49, 2264–2281. [Google Scholar] [CrossRef]
- Baldyga, J.; Bourne, J.R.; Walker, B. Non-isothermal Micromixing in Liquids: Theory and Experiment. Can. J. Chem. Eng. 1998, 76, 641–649. [Google Scholar] [CrossRef]
- Baldyga, J.; Bourne, J.R. Time Constants for Kenics Mixer. In Turbulent Mixing and Chemical Reaction; Baldyga, J., Bourne, J.R., Eds.; John Wiley & Sons: New York, NY, USA, 1999; pp. 733–763. [Google Scholar]
- Taylor, R.A.; Penney, W.R.; Hanh, X.V. Scale-up Methods for Fast Competitive Chemical Reactions in Pipeline Mixers. Ind. Eng. Chem. Res. 2005, 44, 6095–6102. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, C.; Prud’homme, R.K.; Fox, R.O. Mixing in a multi-inlet vortex mixer (MIVM) for flash nano-precipitation. Chem. Eng. Sci. 2008, 63, 2829–2842. [Google Scholar] [CrossRef]
- Hu, H.; Yang, C.; Li, M.; Shao, D.; Mao, H.Q.; Leong, K.W. Flash Technology-Based Self-Assembly in Nanoformulation: From Fabrication to Biomedical Applications. Mater. Today 2021, 42, 99–116. [Google Scholar] [CrossRef]
- Markwalter, C.E.; Prud’homme, R.K. Design of a Small-Scale Multi-Inlet Vortex Mixer for Scalable Nanoparticle Production and Application to the Encapsulation of Biologics by Inverse Flash NanoPrecipitation. J. Pharm. Sci. 2018, 107, 2465–2471. [Google Scholar] [CrossRef]
- D’Addio, S.M.; Prud’homme, R.K. Controlling drug nanoparticle formation by rapid precipitation. Adv. Drug Deliv. Rev. 2011, 63, 417–426. [Google Scholar] [CrossRef]
- Misra, B.; Hughes, K.A.; Pentz, W.H.; Samart, P.; Geldenhuys, W.J.; Bobbala, S. Flash nanoprecipitation assisted self-assembly of ionizable lipid nanoparticles for nucleic acid delivery. Nanoscale 2024, 16, 6939–6948. [Google Scholar] [CrossRef]
- Wikipedia. Kelvin Equation. 2025. Available online: https://en.wikipedia.org/wiki/Kelvin_equation (accessed on 5 April 2025).
- Lin, P.J.; Tam, Y.Y.; Hafez, I.; Sandhu, A.; Chen, S.; Ciufolini, M.A.; Nabi, I.R.; Cullis, P.R. Influence of cationic lipid composition on uptake and intracellular processing of lipid nanoparticle formulations of siRNA. Nanomedicine 2013, 9, 233–246. [Google Scholar] [CrossRef] [PubMed]
- O’Brien Laramy, M.N.; Costa, A.P.; Cebrero, Y.M.; Joseph, J.; Sarode, A.; Zang, N.; Kim, L.J.; Hofmann, K.; Wang, S.; Goyon, A.; et al. Process Robustness in Lipid Nanoparticle Production: A Comparison of Microfluidic and Turbulent Jet Mixing. Mol. Pharm. 2023, 20, 4285–4296. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, J.A.; Witzigmann, D.; Leung, J.; Tam, Y.Y.C.; Cullis, P.R. On the role of helper lipids in lipid nanoparticle formulations of siRNA. Nanoscale 2019, 11, 21733–21739. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.; Nguyen, N.; Renkes, S.; Nguyen, K.T.; Nguyen, T.; Alexandrakis, G. Current and Near-Future Technologies to Quantify Nanoparticle Therapeutic Loading Efficiency and Surface Coating Efficiency with Targeted Moieties. Bioengineering 2025, 12, 362. [Google Scholar] [CrossRef]
- Leung, A.K.; Tam, Y.Y.; Chen, S.; Hafez, I.M.; Cullis, P.R. Microfluidic Mixing: A General Method for Encapsulating Macromolecules in Lipid Nanoparticle Systems. J. Phys. Chem. B 2015, 119, 8698–8706. [Google Scholar] [CrossRef]
- Semple, S.C.; Klimuk, S.K.; Harasym, T.O.; Dos Santos, N.; Ansell, S.M.; Wong, K.F.; Maurer, N.; Stark, H.; Cullis, P.R.; Hope, M.J.; et al. Efficient encapsulation of antisense oligonucleotides in lipid vesicles using ionizable aminolipids: Formation of novel small multilamellar vesicle structures. Biochim. Biophys. Acta 2001, 1510, 152–166. [Google Scholar] [CrossRef]
- Maurer, N.; Wong, K.F.; Stark, H.; Louie, L.; McIntosh, D.; Wong, T.; Scherrer, P.; Semple, S.C.; Cullis, P.R. Spontaneous entrapment of polynucleotides upon electrostatic interaction with ethanol-destabilized cationic liposomes. Biophys. J. 2001, 80, 2310–2326. [Google Scholar] [CrossRef]
- Pagels, R.F.; Prud’homme, R.K. Polymeric nanoparticles and microparticles for the delivery of peptides, biologics, and soluble therapeutics. J. Control Release 2015, 219, 519–535. [Google Scholar] [CrossRef]
- Markwalter, C.E.; Pagels, R.F.; Hejazi, A.N.; Gordon, A.G.R.; Thompson, A.L.; Prud’homme, R.K. Polymeric Nanocarrier Formulations of Biologics Using Inverse Flash NanoPrecipitation. AAPS J. 2020, 22, 18. [Google Scholar] [CrossRef] [PubMed]
- Ristroph, K.D.; Prud’homme, R.K. Hydrophobic ion pairing: Encapsulating small molecules, peptides, and proteins into nanocarriers. Nanoscale Adv. 2019, 1, 4207–4237. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Markwalter, C.E.; Tian, C.; Armstrong, M.; Prud’homme, R.K. Translational formulation of nanoparticle therapeutics from laboratory discovery to clinical scale. J. Transl. Med. 2019, 17, 200. [Google Scholar] [CrossRef] [PubMed]
- Gavi, E.; Marchisio, D.L.; Barresi, A.A. CFD modelling and scale-up of Confined Impinging Jet Reactors. Chem. Eng. Sci. 2007, 62, 2228–2241. [Google Scholar] [CrossRef]
- Marchisio, D. Large Eddy Simulation of mixing and reaction in a Confined Impinging Jets Reactor. Comput. Chem. Eng. 2009, 33, 408–420. [Google Scholar] [CrossRef]
- Zhigaltsev, I.V.; Belliveau, N.; Hafez, I.; Leung, A.K.; Huft, J.; Hansen, C.; Cullis, P.R. Bottom-up design and synthesis of limit size lipid nanoparticle systems with aqueous and triglyceride cores using millisecond microfluidic mixing. Langmuir 2012, 28, 3633–3640. [Google Scholar] [CrossRef]
- Maeki, M.; Fujishima, Y.; Sato, Y.; Yasui, T.; Kaji, N.; Ishida, A.; Tani, H.; Baba, Y.; Harashima, H.; Tokeshi, M. Understanding the formation mechanism of lipid nanoparticles in microfluidic devices with chaotic micromixers. PLoS ONE 2017, 12, e0187962. [Google Scholar] [CrossRef]
- Lasic, D.D. The mechanism of vesicle formation. Biochem. J. 1988, 256, 1–11. [Google Scholar] [CrossRef]
- Webb, C.; Forbes, N.; Roces, C.B.; Anderluzzi, G.; Lou, G.; Abraham, S.; Ingalls, L.; Marshall, K.; Leaver, T.J.; Watts, J.A.; et al. Using microfluidics for scalable manufacturing of nanomedicines from bench to GMP: A case study using protein-loaded liposomes. Int. J. Pharm. 2020, 582, 119266. [Google Scholar] [CrossRef] [PubMed]
- Loughney, J.W.; Minsker, K.; Ha, S.; Rustandi, R.R. Development of an imaged capillary isoelectric focusing method for characterizing the surface charge of mRNA lipid nanoparticle vaccines. Electrophoresis 2019, 40, 2602–2609. [Google Scholar] [CrossRef] [PubMed]
- Roces, C.B.; Lou, G.; Jain, N.; Abraham, S.; Thomas, A.; Halbert, G.W.; Perrie, Y. Manufacturing Considerations for the Development of Lipid Nanoparticles Using Microfluidics. Pharmaceutics 2020, 12, 1095. [Google Scholar] [CrossRef] [PubMed]
- Terada, T.; Kulkarni, J.A.; Huynh, A.; Chen, S.; van der Meel, R.; Tam, Y.Y.C.; Cullis, P.R. Characterization of Lipid Nanoparticles Containing Ionizable Cationic Lipids Using Design-of-Experiments Approach. Langmuir 2021, 37, 1120–1128. [Google Scholar] [CrossRef]
- Ly, H.H.; Daniel, S.; Soriano, S.K.V.; Kis, Z.; Blakney, A.K. Optimization of Lipid Nanoparticles for saRNA Expression and Cellular Activation Using a Design-of-Experiment Approach. Mol. Pharm. 2022, 19, 1892–1905. [Google Scholar] [CrossRef]
- Maier, M.A.; Jayaraman, M.; Matsuda, S.; Liu, J.; Barros, S.; Querbes, W.; Tam, Y.K.; Ansell, S.M.; Kumar, V.; Qin, J.; et al. Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics. Mol. Ther. 2013, 21, 1570–1578. [Google Scholar] [CrossRef]
- Pardi, N.; Tuyishime, S.; Muramatsu, H.; Kariko, K.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; Hope, M.J.; Weissman, D. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J. Control Release 2015, 217, 345–351. [Google Scholar] [CrossRef]
- Cullis, P.R.; Hope, M.J. Lipid Nanoparticle Systems for Enabling Gene Therapies. Mol. Ther. 2017, 25, 1467–1475. [Google Scholar] [CrossRef]
- Cullis, P.R.; Felgner, P.L. The 60-year evolution of lipid nanoparticles for nucleic acid delivery. Nat. Rev. Drug Discov. 2024, 23, 709–722. [Google Scholar] [CrossRef]
- Chen, S.; Tam, Y.Y.C.; Lin, P.J.C.; Sung, M.M.H.; Tam, Y.K.; Cullis, P.R. Influence of particle size on the in vivo potency of lipid nanoparticle formulations of siRNA. J. Control Release 2016, 235, 236–244. [Google Scholar] [CrossRef]
- Jayaraman, M.; Ansell, S.M.; Mui, B.L.; Tam, Y.K.; Chen, J.; Du, X.; Butler, D.; Eltepu, L.; Matsuda, S.; Narayanannair, J.K.; et al. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew. Chem. Int. Ed. Engl. 2012, 51, 8529–8533. [Google Scholar] [CrossRef]
- Belliveau, N.M.; Huft, J.; Lin, P.J.; Chen, S.; Leung, A.K.; Leaver, T.J.; Wild, A.W.; Lee, J.B.; Taylor, R.J.; Tam, Y.K.; et al. Microfluidic Synthesis of Highly Potent Limit-size Lipid Nanoparticles for In Vivo Delivery of siRNA. Mol. Ther. Nucleic Acids 2012, 1, e37. [Google Scholar] [CrossRef]
- Yanez Arteta, M.; Kjellman, T.; Bartesaghi, S.; Wallin, S.; Wu, X.; Kvist, A.J.; Dabkowska, A.; Székely, N.; Radulescu, A.; Bergenholtz, J.; et al. Successful reprogramming of cellular protein production through mRNA delivered by functionalized lipid nanoparticles. Proc. Natl. Acad. Sci. USA 2018, 115, E3351–E3360. [Google Scholar] [CrossRef]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef]
- Brader, M.L.; Williams, S.J.; Banks, J.M.; Hui, W.H.; Zhou, Z.H.; Jin, L. Encapsulation state of messenger RNA inside lipid nanoparticles. Biophys. J. 2021, 120, 2766–2770. [Google Scholar] [CrossRef]
- Subraveti, S.N.; Wilson, B.K.; Bizmark, N.; Liu, J.; Prud’homme, R.K. Synthesizing Lipid Nanoparticles by Turbulent Flow in Confined Impinging Jet Mixers. J. Vis. Exp. 2024, 210, e67047. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Raffaele, J.; Feller, K.; Dornadula, G.; Devlin, J.; Boyd, D.; Loughney, J.W.; Shanter, J.; Rustandi, R.R. Correlating Stability-Indicating Biochemical and Biophysical Characteristics with In Vitro Cell Potency in mRNA LNP Vaccine. Vaccines 2024, 12, 169. [Google Scholar] [CrossRef] [PubMed]
- Abrams, M.T.; Koser, M.L.; Seitzer, J.; Williams, S.C.; DiPietro, M.A.; Wang, W.; Shaw, A.W.; Mao, X.; Jadhav, V.; Davide, J.P.; et al. Evaluation of efficacy, biodistribution, and inflammation for a potent siRNA nanoparticle: Effect of dexamethasone co-treatment. Mol. Ther. 2010, 18, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Davis, Z.; Hofmann, C.; Vlasak, J.; Loughney, J.W.; DePhillips, P.; Mukherjee, M. Development and Characterization of an In Vitro Cell-Based Assay to Predict Potency of mRNA-LNP-Based Vaccines. Vaccines 2023, 11, 1224. [Google Scholar] [CrossRef]
- Offit, P.A. Tell Me When It’s Over; National Geographic Partners, LLC: Washington, DC, USA, 2024. [Google Scholar]
- Pardi, N.; Parkhouse, K.; Kirkpatrick, E.; McMahon, M.; Zost, S.J.; Mui, B.L.; Tam, Y.K.; Karikó, K.; Barbosa, C.J.; Madden, T.D.; et al. Nucleoside-modified mRNA immunization elicits influenza virus hemagglutinin stalk-specific antibodies. Nat. Commun. 2018, 9, 3361. [Google Scholar] [CrossRef]
- Liang, F.; Lindgren, G.; Lin, A.; Thompson, E.A.; Ols, S.; Röhss, J.; John, S.; Hassett, K.; Yuzhakov, O.; Bahl, K.; et al. Efficient Targeting and Activation of Antigen-Presenting Cells In Vivo after Modified mRNA Vaccine Administration in Rhesus Macaques. Mol. Ther. 2017, 25, 2635–2647. [Google Scholar] [CrossRef] [PubMed]
- Richner, J.M.; Himansu, S.; Dowd, K.A.; Butler, S.L.; Salazar, V.; Fox, J.M.; Julander, J.G.; Tang, W.W.; Shresta, S.; Pierson, T.C.; et al. Modified mRNA Vaccines Protect against Zika Virus Infection. Cell 2017, 169, 176. [Google Scholar] [CrossRef] [PubMed]
- Bahl, K.; Senn, J.J.; Yuzhakov, O.; Bulychev, A.; Brito, L.A.; Hassett, K.J.; Laska, M.E.; Smith, M.; Almarsson, Ö.; Thompson, J.; et al. Preclinical and Clinical Demonstration of Immunogenicity by mRNA Vaccines against H10N8 and H7N9 Influenza Viruses. Mol. Ther. 2017, 25, 1316–1327. [Google Scholar] [CrossRef] [PubMed]
- Lederer, K.; Castaño, D.; Gómez Atria, D.; Oguin, T.H., 3rd; Wang, S.; Manzoni, T.B.; Muramatsu, H.; Hogan, M.J.; Amanat, F.; Cherubin, P.; et al. SARS-CoV-2 mRNA Vaccines Foster Potent Antigen-Specific Germinal Center Responses Associated with Neutralizing Antibody Generation. Immunity 2020, 53, 1281–1295.e5. [Google Scholar] [CrossRef]
- Laczkó, D.; Hogan, M.J.; Toulmin, S.A.; Hicks, P.; Lederer, K.; Gaudette, B.T.; Castaño, D.; Amanat, F.; Muramatsu, H.; Oguin, T.H., 3rd; et al. A Single Immunization with Nucleoside-Modified mRNA Vaccines Elicits Strong Cellular and Humoral Immune Responses against SARS-CoV-2 in Mice. Immunity 2020, 53, 724–732.e7. [Google Scholar] [CrossRef]
- Corbett, K.S.; Flynn, B.; Foulds, K.E.; Francica, J.R.; Boyoglu-Barnum, S.; Werner, A.P.; Flach, B.; O’Connell, S.; Bock, K.W.; Minai, M.; et al. Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates. N. Engl. J. Med. 2020, 383, 1544–1555. [Google Scholar] [CrossRef]
- Lopes, C.; Cristóvão, J.; Silvério, V.; Lino, P.R.; Fonte, P. Microfluidic production of mRNA-loaded lipid nanoparticles for vaccine applications. Expert. Opin. Drug Deliv. 2022, 19, 1381–1395. [Google Scholar] [CrossRef]
- Egorov, E.; Pieters, C.; Korach-Rechtman, H.; Shklover, J.; Schroeder, A. Robotics, microfluidics, nanotechnology and AI in the synthesis and evaluation of liposomes and polymeric drug delivery systems. Drug Deliv. Transl. Res. 2021, 11, 345–352. [Google Scholar] [CrossRef]
- Rapalli, V.K.; Khosa, A.; Singhvi, G.; Girdhar, V.; Jain, R.; Dubey, S.K. Application of QbQ Principles in Nanocarrier-Based Drug Delivery Systems. In Pharmaceutical Quality by Design; Beg, S., Hasnain, M.S., Eds.; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar] [CrossRef]
- Gindy, M.E.; DiFelice, K.; Kumar, V.; Prud’homme, R.K.; Celano, R.; Haas, R.M.; Smith, J.S.; Boardman, D. Mechanism of macromolecular structure evolution in self-assembled lipid nanoparticles for siRNA delivery. Langmuir 2014, 30, 4613–4622. [Google Scholar] [CrossRef]
- Gindy, M.E.; Feuston, B.; Glass, A.; Arrington, L.; Haas, R.M.; Schariter, J.; Stirdivant, S.M. Stabilization of Ostwald ripening in low molecular weight amino lipid nanoparticles for systemic delivery of siRNA therapeutics. Mol. Pharm. 2014, 11, 4143–4153. [Google Scholar] [CrossRef]
- Espeseth, A.S.; Cejas, P.J.; Citron, M.P.; Wang, D.; DiStefano, D.J.; Callahan, C.; Donnell, G.O.; Galli, J.D.; Swoyer, R.; Touch, S.; et al. Modified mRNA/lipid nanoparticle-based vaccines expressing respiratory syncytial virus F protein variants are immunogenic and protective in rodent models of RSV infection. NPJ Vaccines 2020, 5, 16. [Google Scholar] [CrossRef]
- Shepherd, S.J.; Warzecha, C.C.; Yadavali, S.; El-Mayta, R.; Alameh, M.G.; Wang, L.; Weissman, D.; Wilson, J.M.; Issadore, D.; Mitchell, M.J. Scalable mRNA and siRNA Lipid Nanoparticle Production Using a Parallelized Microfluidic Device. Nano Lett. 2021, 21, 5671–5680. [Google Scholar] [CrossRef] [PubMed]
- Warne, N.; Ruesch, M.; Siwik, P.; Mensah, P.; Ludwig, J.; Hripcsak, M.; Godavarti, R.; Prigodich, A.; Dolsten, M. Delivering 3 billion doses of Comirnaty in 2021. Nat. Biotechnol. 2023, 41, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S. CNN Video on Comirnaty Manufacture. 2021. Available online: https://www.cnn.com/2021/03/31/health/pfizer-vaccine-manufacturing/index.html (accessed on 4 May 2025).
- Dorsey, P.J.; Lau, C.L.; Chang, T.C.; Doerschuk, P.C.; D’Addio, S.M. Review of machine learning for lipid nanoparticle formulation and process development. J. Pharm. Sci. 2024, 113, 3413–3433. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zhu, Y.; Ma, J.; Aggarwal, A.; Toh, W.H.; Shin, C.; Sangpachatanaruk, W.; Weng, G.; Kumar, R.; Mao, H.Q. Machine Learning Elucidates Design Features of Plasmid DNA Lipid Nanoparticles for Cell Type-Preferential Transfection. bioRxiv 2023. [Google Scholar] [CrossRef]
- Carvalho, B.G.; Ceccato, B.T.; Michelon, M.; Han, S.W.; de la Torre, L.G. Advanced Microfluidic Technologies for Lipid Nano-Microsystems from Synthesis to Biological Application. Pharmaceutics 2022, 14, 141. [Google Scholar] [CrossRef]
- Zhu, Y.; Shen, R.; Vuong, I.; Reynolds, R.A.; Shears, M.J.; Yao, Z.C.; Hu, Y.; Cho, W.J.; Kong, J.; Reddy, S.K.; et al. Multi-step screening of DNA/lipid nanoparticles and co-delivery with siRNA to enhance and prolong gene expression. Nat. Commun. 2022, 13, 4282. [Google Scholar] [CrossRef]
- Hanna, A.R.; Shepherd, S.J.; Datto, G.A.; Navarro, I.B.; Ricciardi, A.S.; Padilla, M.S.; Srikumar, N.; Zhang, S.; Yamagata, H.M.; Meng, N.Y.; et al. Automated and parallelized microfluidic generation of large and precisely-defined lipid nanoparticle libraries. bioRxiv 2025. [Google Scholar] [CrossRef]
- Fan, Y.; Yen, C.W.; Lin, H.C.; Hou, W.; Estevez, A.; Sarode, A.; Goyon, A.; Bian, J.; Lin, J.; Koenig, S.G.; et al. Automated high-throughput preparation and characterization of oligonucleotide-loaded lipid nanoparticles. Int. J. Pharm. 2021, 599, 120392. [Google Scholar] [CrossRef]
- Precigenome. NanoGenerator® Synthesis System Nanoparticle Formulation & Preparation. 2025. Available online: https://www.precigenome.com/ (accessed on 10 April 2025).
- Unchained. Lipid Nanoparticles. 2025. Available online: https://www.unchainedlabs.com/lipid-nanoparticles/ (accessed on 10 April 2025).
- Ottonelli, I.; Duskey, J.T.; Rinaldi, A.; Grazioli, M.V.; Parmeggiani, I.; Vandelli, M.A.; Wang, L.Z.; Prud’homme, R.K.; Tosi, G.; Ruozi, B. Microfluidic Technology for the Production of Hybrid Nanomedicines. Pharmaceutics 2021, 13, 1495. [Google Scholar] [CrossRef]
- Holland. The Confined Impinging Jet (CIJ) and the Multi Inlet Vortex Mixer (MIVM). 2019. Available online: https://hollandapt.com/products/fittings-components/cij-mivm-mixers/ (accessed on 12 April 2025).
- Hermosilla, J.; Alonso-García, A.; Salmerón-García, A.; Cabeza-Barrera, J.; Medina-Castillo, A.L.; Pérez-Robles, R.; Navas, N. Analysing the In-Use Stability of mRNA-LNP COVID-19 Vaccines Comirnaty™ (Pfizer) and Spikevax™ (Moderna): A Comparative Study of the Particulate. Vaccines 2023, 11, 1635. [Google Scholar] [CrossRef]
- Fongaro, B.; Campara, B.; Moscatiello, G.Y.; De Luigi, A.; Panzeri, D.; Sironi, L.; Bigini, P.; Carretta, G.; Miolo, G.; Pasut, G.; et al. Assessing the physicochemical stability and intracellular trafficking of mRNA-based COVID-19 vaccines. Int. J. Pharm. 2023, 644, 123319. [Google Scholar] [CrossRef]



| Process Condition | Fluorescently Identified Particle | Population by Number | mRNA Copy per Particle | Average mRNA EE % | Average DLS Diameter, nm | Average ζ-Potential, mV |
|---|---|---|---|---|---|---|
| Initial LNP, post-T-mixing 3:1, aqueous/ethanol (v/v) mixed solution, pH 4.0 | Lipophilic mRNA complex (LNC) | 34 ± 8% | 3.4 ± 0.4 | N/A | 106 ± 13 | +45 ± 1 |
| Non-lipophilic mRNA complex (NLNC) | 25 ± 4% | 1.3 ± 0.2 | ||||
| mRNA lipid nanoparticle (LNP) | 0% | N/A | ||||
| Empty particles, i.e., no mRNA (ENP) | 41 ± 10% | 0 | ||||
| Final-process LNP, post-dialysis aqueous pH 7.4 buffer | Lipophilic mRNA complex (LNC) | 0 | N/A | 94.2 ± 0.4% | 120 ± 6 | −6.3 ± 1.3 |
| Non-lipophilic mRNA complex (NLNC) | 0 | N/A | ||||
| mRNA lipid nanoparticle (LNP) | 23 ± 8% | 2.8 ± 0.4 | ||||
| Empty particles, i.e., no mRNA (ENP) | 77 ± 8% | N/A |
| Mixing Method | DLS Diameter, nm | ζ-Potential, mV | mRNA EE % * | HeLa Cell In Vitro Transfection, RLU/1000 Cells | HeLa Cell Viability |
|---|---|---|---|---|---|
| Manual pipette | ≈140 | ≈0.5 | >95% | ≈15 | ≈100% |
| Two-jet CIJ mixer | ≈90 | ≈2.0 | >95% | ≈100 | ≈100% |
| Four-jet MIVM | ≈90 | ≈1.0 | >95% | ≈100 | ≈100% |
| Reference | Mixing Technology | Lipids (Total mg/mL) | Lipid Solvent | RNA Type (mg/mL) | mRNA or si RNA Buffer | Dialysis Buffer | Diameter, nm | LNP EE% | Comments |
|---|---|---|---|---|---|---|---|---|---|
| 2013, Maier, M.A., et al. [68] | TJM | L13:DSP:chol:PEG-DMG 55:10:32:2 mole ratio | Ethanol | Si RNA 1 mg/mL | 10 mM Citrate pH 4.0 | PBS pH 7.5 | ≈60 | >90% | Demonstrate in vitro and in vivo potency |
| 2015, Leung, A.K., et al. [51] | SHM | DLIN-KC2-DMA:DSPC:Chol:PEG-lipid 40:11:47:1 mol ratio (xxx) | Ethanol | 1.7 kbp siRNA (yyy) | 25 mM Na Acetate pH 4.0 | 50 mM MES/50 mM Na Citrate pH 6.7 -> PBS pH 7.4 | ≈50 | 90% | Evaluated many mixing conditions using Cryo-TM |
| 2017, Richner, J. M., et al. [85] | SHM | ionizable lipid:DSPC:cholesterol:PEG-lipid 50:10:38:2 mole ratio | Ethanol | preM-E Zika mRNA | 50 mM citrate buffer pH 4.0 | PBS pH 7.5 | 80–100 | >90% | Induced protective antibodies in mice |
| 2017, Liang, F., et al. [84] | SHM | ionizable lipid:DSPC:cholesterol:PEG-lipid:GLA 50:10:38:2:0.2 mole ratio | Ethanol | HA influenza mRNA | 50 mM citrate buffer pH 4.0 | PBS pH 7.4 | 80–100 | >95% | NHP Exaluation of mRNA vaccine |
| 2017, Bahl, K., et al. [86] | SHM | ionizable lipid:DSPC: cholesterol: PEG-lipid 50:10:38.5:1.5 mole ratio | Ethanol | HA influenza mRNA | 50 mM citrate buffer pH 4.0 | PBS pH 7.4 | 80–100 | >90% | Clinical Exaluation of mRNA vaccine |
| 2018, Pardi, N., et al. [83] | SHM | ionizable cationic lipid/ phosphatidylcholine/cholesterol/PEG-lipid 50:10:38.5:1.5 mole ratio | Ethanol | HA influenza mRNA | 50 mM citrate buffer pH 4.0 | PBS pH 7.4 | 80 | >90% | Induces HA influenza anti-stalk antibodies in mice |
| 2020, Ripoll, M., et al. [30] | TRM | MC3:DOPC: cholesterol:PEG-lipid 50:10:38.5:1.5 mole ratio | Ethanol | pDNA | 50 mM citrate pH 4.0 | PBS pH 7.4 | 100 | ≈ 80 | Evaluated many mixing conditions using Cryo-TM |
| 2924, Subraveti, S. N., et al. [78] | CJI | MC3:DSPC: cholesterol:DMG-PEG2000 50:10:38.5:1.5 mole ratio | Ethanol | Yeast mRNA | 20 mM Acetate pH 4 | 10 mM Hepes pH 7.5 | ≈90 | >95% | Input jet 1 -> lipids Input jet 2 -> RNA |
| 2924, Subraveti, S. N., et al. [78] | MIVM | MC3:DSPC: cholesterol:DMG-PEG2000 50:10:38.5:1.5 mole ratio | Ethanol | Yeast mRNA | 20 mM Acetate pH 4 | 10 mM Hepes pH 7.5 | ≈90 | >95% | Input jets 1 & 3 -> lipids Input jets 2 & 4 -> RNA |
| (a) | |||
|---|---|---|---|
| Product Trade Name | General Comments | Vendor | Website |
| NanoAssemblr™ Spark™ | Small-scale rapid formulation screening 25 to 250 µL batch volume | Cytiva | https://www.cytivalifesciences.com/en/us/shop/lipid-nanoparticle-instruments-and-reagents/nanoparticle-formulation-systems?sort=NameAsc&chunk=1 (accessed on 13 July 2025) |
| NanoAssemblr™ Ignite™ | Pre-clinical formulation screening, easy to use TRM, 5 to 60 mL batch | ||
| NanoAssemblr™ Blaze™ | Large-scale for process scale-up, includes TFF 0.2 to 10 L batch | ||
| NanoAssemblr™ GMP System | GMP system for clinical supplies 1 to 50 L batch size | ||
| NanoAssemblr™ commercial formulation system | GMP system for large-scale commercial manufacturing | ||
| Tamara | Easy to use, reusable microchips 0.2 to 30 mL batch size | Inside Therapeutics | https://insidetx.com/product/tamara/ (accessed on 13 July 2025) |
| Lipid nanoparticle synthesis pack | Pressure controlled SHM, easy to use 0.5 mL to 5 L batch size | Elve Flow | https://www.elveflow.com/microfluidics-application-packs/lipid-nanoparticle-synthesis/ (accessed on 13 July 2025) |
| NanoGenerator™ Flex-S | Small-scale discovery screening, multi-sample 1 to 4, 0.1 to 0.5 mL per sample | PreciGenome LLC | https://www.precigenome.com/ (accessed on 13 July 2025) |
| NanoGenerator™ Flex-S Plus | Early discovery, fully automated HT, multi-sample 1 to 96, 0.1 to 0. mL per sample | ||
| NanoGenerator™ Flex-M | Pre-clinical formulation, in-line ethanol dilution 1 to 12 mL batch | ||
| NanoGenerator™ MAX + | cGMP system for clinical manufacturing, product throughput > 10 L/h. | ||
| Sunscreen | Discovery, microfluid chip options, automated HT, 1 to 96 samples, 0.2 to 2.0 mL per sample | Unchained Labs | https://www.unchainedlabs.com/lipid-nanoparticles/ (accessed on 13 July 2025) |
| Sunshine | Pre-clinical, microfluid chip options, automated 1 to 10 samples, continuous flow up to 30 mL/min | ||
| Sunbather | GMP Clinical ready, microfluid chip options, continuous flow up to 1.8 L/h. | ||
| (b) | |||
| Product Trade Name | General Comments | Vendor | Website |
| DIANT® LARU Discovery | Discovery-scale continuous turbulent jet mixing 2 mL minimum output volume | Diant Pharma Inc. | https://diantpharma.com/ (accessed on 13 July 2025) |
| DIANT® LARU—Benchtop | Pilot-scale continuous turbulent jet mixing with TFF and PAT max output 0.4 L/min | ||
| DIANT® LiFT—HT | Commercial-scale GMP continuous turbulent jet mixing with TFF and PAT max output 20 L/min | ||
| Nova™ Benchtop | Discovery-scale CIJ mixer system in-line dilution TFR 0.1 to 100 mL/min | Helix Biotech Inc. | https://www.helixbiotech.com/ (accessed on 13 July 2025) |
| Platform for Intracelluar Delivery of DNA & RNA | Discovery-scale turbulent mixing technology for intracellular RNA and DNA delivery | Optimeos Life Sciences Inc. | https://optimeos.com/ (accessed on 13 July 2025) |
| CIJ & MIVM Mixers Design by Dr. Prud’homme’s Princeton Lab | Manufactures CIJ and MIVM turbulent mixer units for lab-scale formulation development | Holland Applied Technologies | https://hollandapt.com/products/fittings-components/cij-mivm-mixers/ (accessed on 13 July 2025) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahl, P.L. Microfluidic and Turbulent Mixing for mRNA LNP Vaccines. Pharmaceutics 2025, 17, 1148. https://doi.org/10.3390/pharmaceutics17091148
Ahl PL. Microfluidic and Turbulent Mixing for mRNA LNP Vaccines. Pharmaceutics. 2025; 17(9):1148. https://doi.org/10.3390/pharmaceutics17091148
Chicago/Turabian StyleAhl, Patrick L. 2025. "Microfluidic and Turbulent Mixing for mRNA LNP Vaccines" Pharmaceutics 17, no. 9: 1148. https://doi.org/10.3390/pharmaceutics17091148
APA StyleAhl, P. L. (2025). Microfluidic and Turbulent Mixing for mRNA LNP Vaccines. Pharmaceutics, 17(9), 1148. https://doi.org/10.3390/pharmaceutics17091148






