Marine Macroalgae in Topical Formulations: Bioactive Compounds, Variability, Analytical Challenges and Skin Benefits
Abstract
1. Introduction
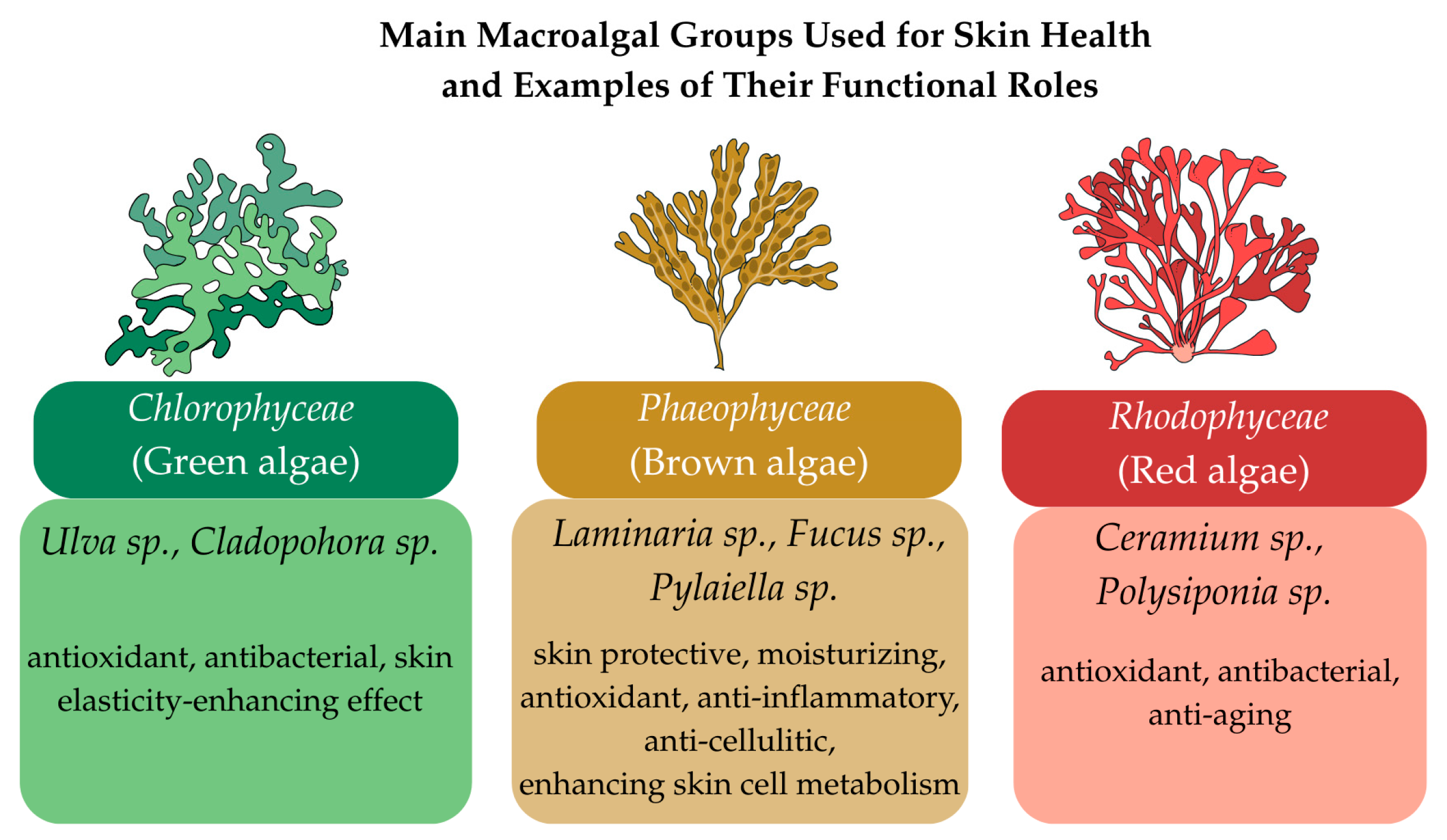
2. Sources of Macroalgal Bioactives
2.1. Phaeophyceae (Brown Algae) as Sources of Bioactive Compounds
2.2. Chlorophyceae (Green Algae) as Sources of Bioactive Compounds
2.3. Rhodophyceae (Red Algae) as Sources of Bioactive Compounds
3. Macroalgal Bioactives
3.1. Polysaccharides
3.1.1. Alginates
3.1.2. Laminarin
3.1.3. Fucoidans
3.1.4. Ulvan
3.1.5. Agar
3.1.6. Carrageenan
3.2. Proteins, Peptides and Amino Acids
Mycosporine-like Amino Acids
3.3. Pigments
3.3.1. Phycobillins
3.3.2. Chlorophylls
3.3.3. Carotenoids
3.4. Phenolic Compounds
3.4.1. Phlorotannins
3.4.2. Phenolic Terpenoids
3.5. Lipids
3.6. Vitamins and Minerals
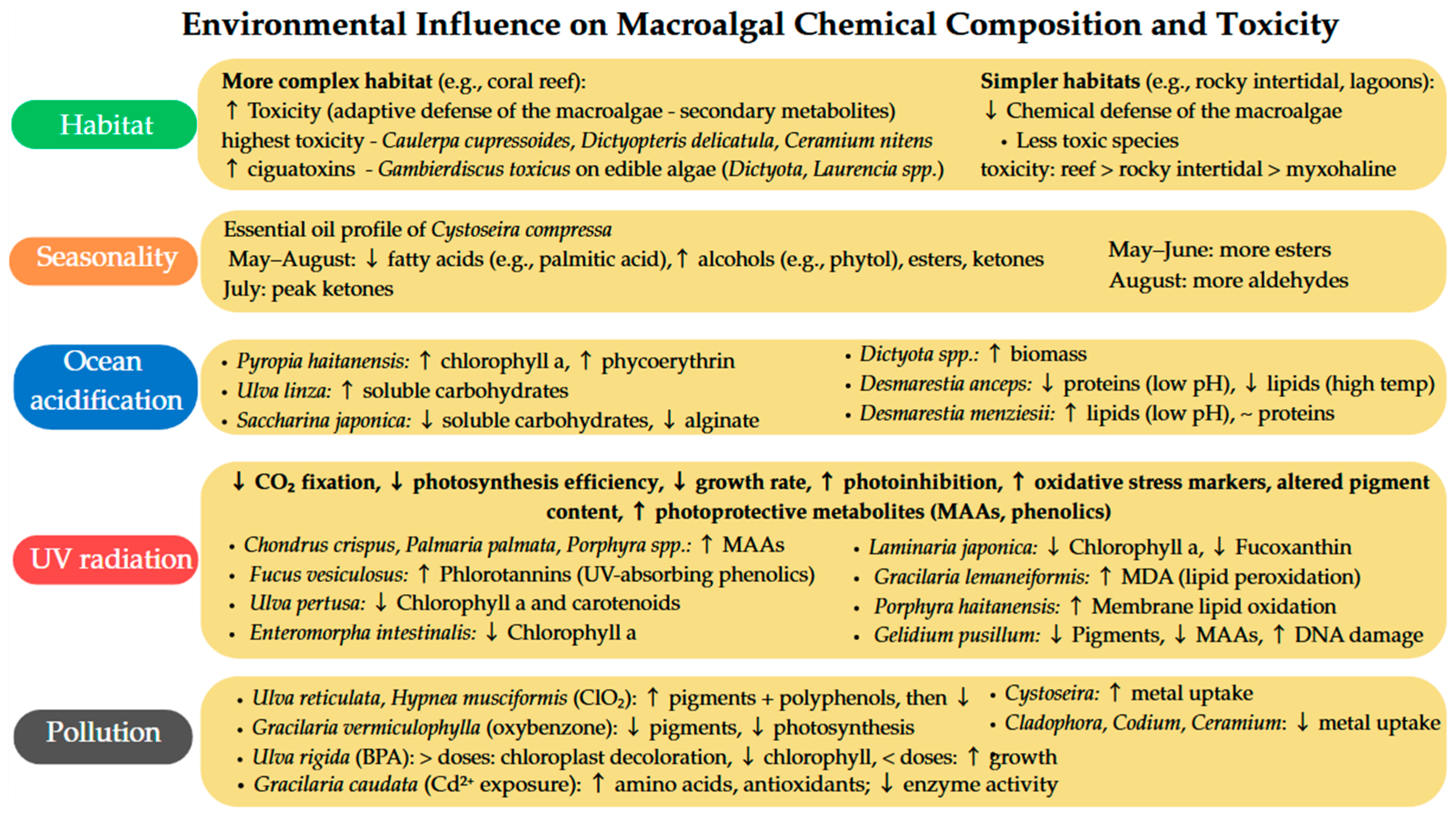
4. Environmental Influence on Macroalgal Chemical Composition and Toxicity
4.1. Habitat
4.2. Season
4.3. CO2 Concentration and Ocean Acidification
4.4. UV Radiation
4.5. Pollution
5. Regulatory and Safety Considerations of Key Compounds
6. Extraction Methods of Bioactive Compounds from Macroalgae
6.1. Classical Extraction Methods
6.1.1. Maceration
6.1.2. Percolation
6.1.3. Soxhlet Extraction
6.1.4. Hot-Water Extraction
6.2. Modern Extraction Methods
6.2.1. Microwave-Assisted Extraction
6.2.2. Ultrasound-Assisted Extraction
6.2.3. Enzymatic-Assisted Extraction
6.2.4. Pressurized Liquid Extraction
6.2.5. Supercritical Fluid Extraction
7. Quantitative Analysis of Bioactive Compounds in Macroalgae
7.1. Liquid Chromatography
7.2. Gas Chromatography
8. Evaluation of Biological Activities of Algae Through in Vitro and in Vivo Studies
8.1. Prevention of Age-Related Skin Changes
8.1.1. Inhibition of Collagenase and Elastase and Implications for the Prevention of Skin Aging
8.1.2. Hyaluronidase Inhibition Activity
8.2. Antioxidant Activity
8.3. Anti-Melanogenic Activity
8.4. Moisturizing Activity
| Active Ingredients from Algae | Beneficial Effects in Skin Health | Reference |
|---|---|---|
| Main active ingredients from algae and their skin health benefits | ||
| Phlorotannins, carotenoids, photolyase, mycosporine-like amino acids | UV protection | [117] |
| Tocopherols, polyphenols, β-carotenoids (vitamin A), other carotenoids | Skin protection (radical scavenging and immune system stimulation) | |
| (Sulfated) polysaccharides, glycosaminoglycans | Skin moisturizing | |
| Essential and nonproteinogenic amino acids, highly unsaturated fatty acids | Skin smoothing and regeneration | |
| Phlorotannins, phloroglucinol and its oligomers | Skin whitening | |
| Low-molecular-weight fucoidans | Stimulation of collagen synthesis | |
| Bioactive extracts from specific algae species and their in vitro/in vivo demonstrated effects | ||
| Alginates (Sargassum vulgare) | ↗ antimicrobial properties (on Pseudomonas aeruginosa, Staphylococcus aureus, Candida albicans, Escherichia coli, Aspergillus brasiliensis strains) | [116] |
| Brown algae polysaccharides (Hizikia fusiforme) | UVB protection (protection collagen synthesis and ↘ MMPs expression in UVB-irradiated human dermal fibroblasts | [112] |
| Fucoidans (Sargassum tenerrimum, Laminaria japonica, Fucus vesiculosus) | Antioxidant activity (superoxide radical scavenging properties) | [113] |
| Fucoidans (Ascophyllum nodosum) | Anti-aging, UV protection, wrinkle reduction, antioxidant, collagen synthesis | [13] |
| Fucoidan (Laminaria cichorioides) | Beneficial effect in atopic dermatitis (inhibits production of IgE) | [118] |
| Laminarin | UVB protection (↘ ROS production and ↗endogenous antioxidant levels against UVB irradiation | [119] |
| Phlorotannins from Cystoseira nodicaulis, Cystoseira tamariscifolia, Cystoseira usneoides Fucus spiralis | Hyaluronidase inhibition, antioxidant activity (superoxide radical scavenging properties, lipid peroxidation inhibition) | [120] |
| Polysaccharides from Saccharina japonica | Skin moisturizing (moisture-absorption and moisture-retention abilities) | [121] |
| Porphyran from Porphyra haitanensis | Antioxidant capacity (↗ antioxidant enzyme activity-aging mice) | [122] |
| Porphyran from Pyropiaa yezoensis | Anti-inflammatory activity in LPS-stimulated macrophages | [123] |
| HMW ulvans | ↗ hyaluronan biosynthesis in dermal fibroblasts | [124] |
| Ecklonia cava, E. kurome, Ishige sinicola extracts | Anti-acne activity (on Cutibacterium acnes) | [125] |
| Fucus vesiculosus extract | ↗ skin elasticity | [106] |
| Marine complex including Ulva lactuca, Lola implexa extracts | Skin moisturizing, skin firming and tightening | [109] |
| Pyropia yezoensis extract | ↗ collagen synthesis, prevents its degradation (human skin fibroblasts) ↘ melanin content (inhibits tyrosinase activity) | [115] |
| Sargassum muticum, Osmundea pinnatifida, Codium tomentosum water-based extracts | In vitro antioxidant activity (hydroxyl-radical scavenging properties) | [111] |
| Undaria pinnatifida extract (85% fucoidan) and Fucus vesiculosus co-extract, (60% fucoidan, 30% polyphenol) | Soothing, UV protection, wrinkle depth reduction; Fucus vesiculosus extract—antioxidant effect | [108] |
| Algae Species (Type) | Formulation | Study Design (Subjects, Duration) | Outcomes | Main Cosmetic Effect | Reference |
|---|---|---|---|---|---|
| Codium tomentosun (green) | Moisturizing cream (5%) | 7 days, 10–12 women | Hydration ↑ 2–3× vs. placebo; improved barrier | Hydration/barrier support | [126] |
| Laminaria japonica (brown) | Cream (0–15%) | Single use, 10 women | Dose-dependent hydration; 10% ↓ TEWL | Hydration/barrier support | [126] |
| Rhizoclonium hieroglyphicum (green) | Cream (0.3%) | 30 subjects, 1 week | Immediate + sustained hydration; superior to glycerin | Hydration | [126] |
| Sargassum hornieri (brown, fucoidan) | Lotion (1%) | 3 weeks, forearm study | ↓ TEWL; ↑ hydration; restored barrier | Barrier function/hydration | [127] |
| Fucus vesiculosus + Ulva lactuca (brown + green) +ectoin | Serum mist (split-face) | 28 days, 33 adults | +17% hydration; pH normalized | Hydration/wrinkle reduction | [128] |
| Brown algal alginate + calcium | Sheet mask | Single use | ↑ hydration, pH regulation; ↓ wrinkles, ↑ smoothness; maintained microbiome diversity; ↓ Corinebacterium | Hydration/microbiome/anti-wrinkle | [129] |
| Fucus vesiculosus (brown) | Cream (1%) | 5 weeks, 10 women | ↑ elasticity, 7–8% ↓ skin thickness | Firming/anti-aging | [126] |
| Undaria pinnatifida + Fucus vesiculosus (brown) | Gel (0.3%) | UVB test, 25 subjects | ↓ UV erythema, ↓ TEWL | Photoprotection/anti-aging | [126] |
| Fucus vesiculosus (brown, fucoidan/polyphenols) | Gel (0.3%) | 60 days, 30 subjects | ↑ brightness, ↓ wrinkles, ↓ age spots | Brightening/anti-aging | [126] |
| Porphyra umbilicalis (red, MAAs) | Cream (0.005%) vs. suncreen | 4 weeks, 20 women | UVA protection; ↑ firmness, ↑ smoothness | Photoprotection/anti-aging | [130] |
| Ascophyllum nodosum (brown) + herbal extract | Botanical regiment | 18 weeks, 56 women; 1 year extension | Comparable to hydroquinone/tretinoin; no rebound pigmentation | Anti-pigmentation | [126] |
| Laminaria digitata + Gelidium cartilagineum + Pelvetia canaliculata (brown + red) | Anti-cellulite cream | 4 weeks, 90 women | ↓ thigh/waist/hip circumference; ↑ firmness | Slimming/firming | [126] |
| Fucus vesiculosus + Furcellaria lumbricalis (brown + red) | Anti-cellulite cream | 12 weeks, 35 women | ↓ cellulite grade; ↓ adipose thickness | Anti-cellulite/firming | [126] |
| Laminaria digitata (brown, oligosaccharide-zinc complex) | Gel | 8 weeks (acne vulgaris | ↓ lesion severity; ↓ sebum; ↓ P.acnes | Anti-acne | [131] |
8.5. Skin Regeneration Activity
9. Formulation Strategies and Challenges
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- El-Chaghaby, G.A.; Rashad, S. An overview of algae prospects in cosmeceuticals. J. Egypt. Women’s Dermatol. Soc. 2021, 18, 161–166. [Google Scholar] [CrossRef]
- Kelman, D.; Posner, E.K.; McDermid, K.J.; Tabandera, N.K.; Wright, P.R.; Wright, A.D. Antioxidant activity of Hawaiian marine algae. Mar. Drugs 2012, 10, 403–416. [Google Scholar] [CrossRef]
- Wang, H.D.; Chen, C.C.; Huynh, P.; Chang, J.S. Exploring the potential of using algae in cosmetics. Bioresour. Technol. 2015, 184, 355–362. [Google Scholar] [CrossRef]
- Thiyagarasaiyar, K.; Goh, B.H.; Jeon, Y.J.; Yow, Y.Y. Algae Metabolites in Cosmeceutical: An Overview of Current Applications and Challenges. Mar. Drugs 2020, 18, 323. [Google Scholar] [CrossRef]
- Lee, R. Phycology, 4th ed.; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Khan, M.; Cheng, J.; Bankole, P. The Algae World—Classification of Algae; IntechOpen: London, UK, 2015. [Google Scholar]
- Łęska, B.; Messyasz, B.; Schroeder, G. Application of Algae Biomass and Algae Extracts in Cosmetic Formulations. In Algae Biomass: Characteristics and Applications: Towards Algae-Based Products; Chojnacka, K., Wieczorek, P.P., Schroeder, G., Michalak, I., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 89–101. [Google Scholar]
- Cho, S.; Lee, D.H.; Won, C.H.; Kim, S.M.; Lee, S.; Lee, M.J.; Chung, J.H. Differential effects of low-dose and high-dose beta-carotene supplementation on the signs of photoaging and type I procollagen gene expression in human skin in vivo. Dermatology 2010, 221, 160–171. [Google Scholar] [CrossRef]
- Commission, E. CosIng—Cosmetics Ingredients. Available online: https://ec.europa.eu/growth/tools-databases/cosing/ (accessed on 18 August 2025).
- Gupta, A.; Singh, A.P.; Singh, V.K.; Singh, P.R.; Jaiswal, J.; Kumari, N.; Upadhye, V.; Singh, S.C.; Sinha, R.P. Natural Sun-Screening Compounds and DNA-Repair Enzymes: Photoprotection and Photoaging. Catalysts 2023, 13, 745. [Google Scholar] [CrossRef]
- Castro, V.; Oliveira, R.; Dias, A.C.P. Microalgae and cyanobacteria as sources of bioactive compounds for cosmetic applications: A systematic review. Algal Res. 2023, 76, 103287. [Google Scholar] [CrossRef]
- Resende, D.I.S.P. Trends in the use of marine ingredients in anti-aging cosmetics. Algal Res. 2021, 55, 102273. [Google Scholar] [CrossRef]
- López-Hortas, L.; Flórez-Fernández, N.; Torres, M.D.; Ferreira-Anta, T.; Casas, M.P.; Balboa, E.M.; Falqué, E.; Domínguez, H. Applying Seaweed Compounds in Cosmetics, Cosmeceuticals and Nutricosmetics. Mar. Drugs 2021, 19, 552. [Google Scholar] [CrossRef] [PubMed]
- Santos, J. Extraction and Formulation of Macroalgal Phenolic Compounds for Cosmetic Application. Master’s Thesis, Technical University of Lisbon, Lisbon, Portugal, 2021. [Google Scholar]
- Shafie, M.H.; Kamal, M.L.; Zulkiflee, F.F.; Hasan, S.; Uyup, N.H.; Abdullah, S.; Hussin, N.A.M.; Tan, Y.C.; Zafarina, Z. Application of Carrageenan extract from red seaweed (Rhodophyta) in cosmetic products: A review. J. Indian Chem. Soc. 2022, 99, 100613. [Google Scholar] [CrossRef]
- Robinson, M.; Visscher, M.; Laruffa, A.; Wickett, R. Natural moisturizing factors (NMF) in the stratum corneum (SC). I. Effects of lipid extraction and soaking. J. Cosmet. Sci. 2010, 61, 13–22. [Google Scholar]
- Ozanne, H.; Toumi, H.; Roubinet, B.; Landemarre, L.; Lespessailles, E.; Daniellou, R.; Cesaro, A. Laminarin Effects, a β-(1,3)-Glucan, on Skin Cell Inflammation and Oxidation. Cosmetics 2020, 7, 66. [Google Scholar] [CrossRef]
- Wang, L.; Oh, J.Y.; Kim, Y.S.; Lee, H.G.; Lee, J.S.; Jeon, Y.J. Anti-Photoaging and Anti-Melanogenesis Effects of Fucoidan Isolated from. Mar. Drugs 2020, 18, 427. [Google Scholar] [CrossRef] [PubMed]
- Manandhar, B.; Wagle, A.; Seong, S.H.; Paudel, P.; Kim, H.R.; Jung, H.A.; Choi, J.S. Phlorotannins with Potential Anti-tyrosinase and Antioxidant Activity Isolated from the Marine Seaweed. Antioxidants 2019, 8, 240. [Google Scholar] [CrossRef] [PubMed]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.M.M.; da Silva, G.J.; Pereira, L. Seaweed Phenolics: From Extraction to Applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef]
- Thomas, N.V.; Kim, S.K. Beneficial effects of marine algal compounds in cosmeceuticals. Mar. Drugs 2013, 11, 146–164. [Google Scholar] [CrossRef]
- Kumar, L.R.G.; Paul, P.T.; Anas, K.K.; Tejpal, C.S.; Chatterjee, N.S.; Anupama, T.K.; Mathew, S.; Ravishankar, C.N. Phlorotannins-bioactivity and extraction perspectives. J. Appl. Phycol. 2022, 34, 2173–2185. [Google Scholar] [CrossRef]
- Ziboh, V.A.; Miller, C.C.; Cho, Y. Metabolism of polyunsaturated fatty acids by skin epidermal enzymes: Generation of antiinflammatory and antiproliferative metabolites. Am. J. Clin. Nutr. 2000, 71, 361S–366S. [Google Scholar] [CrossRef]
- Aslam, A.; Bahadar, A.; Liaquat, R.; Saleem, M.; Waqas, A.; Zwawi, M. Algae as an attractive source for cosmetics to counter environmental stress. Sci. Total Environ. 2021, 772, 144905. [Google Scholar] [CrossRef]
- Fabrowska, J.; Łęska, B.; Schroeder, G.; Messyasz, B.; Pikosz, M. Biomass and Extracts of Algae as Material for Cosmetics. In Marine Algae Extracts; Wiley: Hoboken, NJ, USA, 2015; pp. 681–706. [Google Scholar]
- Fujimura, T.; Tsukahara, K.; Moriwaki, S.; Kitahara, T.; Sano, T.; Takema, Y. Treatment of human skin with an extract of Fucus vesiculosus changes its thickness and mechanical properties. J. Cosmet. Sci. 2002, 53, 1–9. [Google Scholar]
- Alvarez-Hernández, S.; Lozano-Ramírez, C.; Rodríguez-Palacio, M. Influence of the Habitat on Marine Macroalgae Toxicity. Annu. Res. Rev. Biol. 2019, 33, 1–9. [Google Scholar] [CrossRef]
- Cruz-Rivera, E.; Villareal, T.A. Macroalgal palatability and the flux of ciguatera toxins through marine food webs. Harmful Algae 2006, 5, 497–525. [Google Scholar] [CrossRef]
- Dromard, C.R.; Bouchon-Navaro, Y.; Harmelin-Vivien, M.; Bouchon, C. The nutritional quality of non-calcified macroalgae in Guadeloupe (Lesser Antilles) evaluated by their biochemical composition. Gulf Caribb. Res. 2017, 28, GCFI1-GCGI6. [Google Scholar] [CrossRef]
- Cresson, P.; Ruitton, S.; Noisette, F.; Harmelin-Vivien, M. Isotopic and biochemical composition of Western Mediterranean macroalgae. Eur. J. Phycol. 2023, 59, 21–37. [Google Scholar] [CrossRef]
- Generalić Mekinić, I.; Čagalj, M.; Tabanelli, G.; Montanari, C.; Barbieri, F.; Skroza, D.; Šimat, V. Seasonal Changes in Essential Oil Constituents of Cystoseira compressa: First Report. Molecules 2021, 26, 6649. [Google Scholar] [CrossRef]
- Ji, Y.; Xu, Z.; Zou, D.; Gao, K. Ecophysiological responses of marine macroalgae to climate change factors. J. Appl. Phycol. 2016, 28, 2953–2967. [Google Scholar] [CrossRef]
- Burnham, K.A.; Nowicki, R.J.; Hall, E.R.; Pi, J.; Page, H.N. Effects of ocean acidification on the performance and interaction of fleshy macroalgae and a grazing sea urchin. J. Exp. Mar. Biol. Ecol. 2022, 547, 151662. [Google Scholar] [CrossRef]
- Schram, J.B.; Schoenrock, K.M.; McClintock, J.B.; Amsler, C.D.; Angus, R.A. Ocean warming and acidification alter Antarctic macroalgal biochemical composition but not amphipod grazer feeding preferences. Mar. Ecol. Prog. Ser. 2017, 581, 45–56. [Google Scholar] [CrossRef][Green Version]
- Zakharikhina, L.V.; Gorbunova, T.L.; Ryndin, A.V.; Lesnikova, P.S.; Rogozhina, E.V. Chemical composition and growth responses of brown, green, and red macroalgae to pollution in the Russian black sea. Mar. Pollut. Bull. 2024, 207, 116831. [Google Scholar] [CrossRef]
- Araujo-Motta, L.; Alves-Lima, C.; Zambotti-Vilella, L.; Colepicolo, P. Metabolome of Cadmium Stressed Gracilaria caudata (Rhodophyta). Phycology 2023, 3, 255–269. [Google Scholar] [CrossRef]
- El-Sherbiny, M.M.; Satheesh, S.; Ba-Akdah, M.A. Physiological Responses of Marine Macroalgae to Chlorine Dioxide Treatment. Thalassas 2021, 37, 291–302. [Google Scholar] [CrossRef]
- Xing, Q.; Kim, Y.W.; Kim, D.; Park, J.-S.; Yoo, H.I.; Yarish, C.; Kim, J.K. Effects of the ultraviolet filter oxybenzone on physiological responses in a red macroalga, Gracilaria vermiculophylla. Aquat. Bot. 2022, 179, 103514. [Google Scholar] [CrossRef]
- Malakhova, L.; Lobko, V.; Murashova, A.; Malakhova, T.; Zheleznova, S.; Egorov, V. Morphological changes and biochemical reaction of Ulva rigida in response to the toxic effect of bisphenol A under experimental conditions. Aquat. Bot. 2023, 184, 103579. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Rajauria, G.; Miranda, M.; Sweeney, T.; Lopez-Alonso, M.; O’Doherty, J. Seasonal Variation of the Proximate Composition, Mineral Content, Fatty Acid Profiles and Other Phytochemical Constituents of Selected Brown Macroalgae. Mar. Drugs 2021, 19, 204. [Google Scholar] [CrossRef] [PubMed]
- Miguel, S.P.; D’Angelo, C.; Ribeiro, M.P.; Simões, R.; Coutinho, P. Chemical Composition of Macroalgae Polysaccharides from Galician and Portugal Coasts: Seasonal Variations and Biological Properties. Mar. Drugs 2023, 21, 589. [Google Scholar] [CrossRef]
- Pistollato, F.; Madia, F.; Corvi, R.; Munn, S.; Grignard, E.; Paini, A.; Worth, A.; Bal-Price, A.; Prieto, P.; Casati, S.; et al. Current EU regulatory requirements for the assessment of chemicals and cosmetic products: Challenges and opportunities for introducing new approach methodologies. Arch. Toxicol. 2021, 95, 1867–1897. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Hermund, D.B.; Torsteinsen, H.; Vega, J.; Figueroa, F.L.; Jacobsen, C. Screening for New Cosmeceuticals from Brown Algae. Mar. Drugs 2022, 20, 687. [Google Scholar] [CrossRef]
- Zheng, H.; Zhao, Y.; Guo, L. A Bioactive Substance Derived from Brown Seaweeds: Phlorotannins. Mar. Drugs 2022, 20, 742. [Google Scholar] [CrossRef]
- Knight, J.; Rovida, C.; Kreiling, R.; Zhu, C.; Knudsen, M.; Hartung, T. Continuing Animal Tests on Cosmetic Ingredients for REACH in the EU. ALTEX 2021, 38, 653–668. [Google Scholar] [CrossRef]
- Soleimani, S.; Yousefzadi, M.; Nezhad, S.B.M.; Pozharitskaya, O.N.; Shikov, A.N. Evaluation of fractions extracted from Polycladia myrica: Biological activities, UVR protective effect, and stability of cream formulation based on it. J. Appl. Phycol. 2022, 34, 1763–1777. [Google Scholar] [CrossRef]
- Nishida, Y.; Kumagai, Y.; Michiba, S.; Yasui, H.; Kishimura, H. Efficient Extraction and Antioxidant Capacity of Mycosporine-Like Amino Acids from Red Alga Dulse Palmaria palmata in Japan. Mar. Drugs 2020, 18, 502. [Google Scholar] [CrossRef] [PubMed]
- Pirotta, G. Sunscreen Regulation in the World. In Sunscreens in Coastal Ecosystems: Occurrence, Behavior, Effect and Risk; Tovar-Sánchez, A., Sánchez-Quiles, D., Blasco, J., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 15–35. [Google Scholar]
- The European Parliament and The Council of the European Union. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products; The European Parliament and The Council of the European Union: Brussels, Belgium, 2009. [Google Scholar]
- Bitwell, C.; Indra, S.S.; Luke, C.; Kakoma, M.K. A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- Belyagoubi, L.; Belyagoubi-Benhammou, N.; Atik-Bekkara, F.; Abdelouahid, D.E. Influence of harvest season and different polarity solvents on biological activities, phenolic compounds and lipid-soluble pigment contents of Spirogyra sp. from Algeria. Adv. Tradit. Med. 2022, 22, 359–369. [Google Scholar] [CrossRef]
- Gager, L.; Connan, S.; Molla, M.; Couteau, C.; Arbona, J.-F.; Coiffard, L.; Cérantola, S.; Stiger-Pouvreau, V. Active phlorotannins from seven brown seaweeds commercially harvested in Brittany (France) detected by 1H NMR and in vitro assays: Temporal variation and potential valorization in cosmetic applications. J. Appl. Phycol. 2020, 32, 2375–2386. [Google Scholar] [CrossRef]
- Bhuyar, P.; Rahim, M.H.; Sundararaju, S.; Maniam, G.P.; Govindan, N. Antioxidant and antibacterial activity of red seaweed Kappaphycus alvarezii against pathogenic bacteria. Glob. J. Environ. Sci. Manag. 2020, 6, 47–58. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Rajauria, G.; Tiwari, B. Chapter 7—Conventional extraction techniques: Solvent extraction. In Advances in Green and Sustainable Chemistry; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Wassie, T.; Niu, K.; Xie, C.; Wang, H.; Xin, W. Extraction Techniques, Biological Activities and Health Benefits of Marine Algae. Front. Nutr. 2021, 8, 747928. [Google Scholar] [CrossRef]
- Phomkaivon, N.; Pongponpai, P.; Kosawatpat, P.; Thongdang, B.; Pan-utai, W. Extraction, Characterisation and Evaluation of Antioxidant and Probiotic Growth Potential of Water-Soluble Polysaccharides from Ulva rigida Macroalgae. Foods 2024, 13, 1630. [Google Scholar] [CrossRef]
- Sosa-Hernández, J.E.; Escobedo-Avellaneda, Z.; Iqbal, H.M.N.; Welti-Chanes, J. State-of-the-Art Extraction Methodologies for Bioactive Compounds from Algal Biome to Meet Bio-Economy Challenges and Opportunities. Molecules 2018, 23, 2953. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, J.; Fan, J.; Clark, J.; Shen, P.; Li, Y.; Zhang, C. Microwave assisted extraction of phenolic compounds from four economic brown macroalgae species and evaluation of their antioxidant activities and inhibitory effects on α-amylase, α-glucosidase, pancreatic lipase and tyrosinase. Food Res. Int. 2018, 113, 288–297. [Google Scholar] [CrossRef]
- Amarante, S.; Catarino, M.; Marçal, C.; Silva, A.; Ferreira, R.; Cardoso, S. Microwave-Assisted Extraction of Phlorotannins from Fucus vesiculosus. Mar. Drugs 2020, 18, 559. [Google Scholar] [CrossRef]
- Lopes, A.; Correia-Sá, L.; Vieira, M.; Delerue-Matos, C.; Soares, C.; Grosso, C. Sustainable Carotenoid Extraction from Macroalgae: Optimizing Microwave-Assisted Extraction Using Response Surface Methodology. Life 2024, 14, 1573. [Google Scholar] [CrossRef]
- Torabi, P.; Hamdami, N.; Keramat, J. Microwave-assisted extraction of sodium alginate from brown macroalgae Nizimuddinia zanardini, optimization and physicochemical properties. Sep. Sci. Technol. 2021, 57, 872–885. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Ummat, V.; Tiwari, B.; Rajauria, G. Exploring Ultrasound, Microwave and Ultrasound–Microwave Assisted Extraction Technologies to Increase the Extraction of Bioactive Compounds and Antioxidants from Brown Macroalgae. Mar. Drugs 2020, 18, 172. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, D.; Genovese, G. Comparative polysaccharides extraction methods ulvans from Ulva ohnoi (Chlorophyta): Advantages of microwave-assisted techniques. Algologia 2025, 35, 73–83. [Google Scholar] [CrossRef]
- Song, Y.; Lee, J.; Kwon, H.K.; Kim, M.; Shin, S.; Kim, S.; Son, H.; Park, C.; Yoo, H.Y. Efficient Ultrasound-Assisted Extraction of Bioactive Molecules from Brown Macroalga Sargassum horneri: Optimal Extraction, Antioxidant and Cytotoxicity Evaluation. Int. J. Mol. Sci. 2025, 26, 2749. [Google Scholar] [CrossRef]
- Goksen, G. Elucidation and quantification health-promoting phenolic compounds, antioxidant properties and sugar levels of ultrasound assisted extraction, aroma compositions and amino acids profiles of macroalgae, Laurencia papillosa. Ultrason. Sonochem. 2023, 98, 106527. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Ravindran, R.; Walsh, O.; O’Doherty, J.; Tiwari, B.; Rajauria, G. Evaluation of Ultrasound, Microwave, Ultrasound–Microwave, Hydrothermal and High Pressure Assisted Extraction Technologies for the Recovery of Phytochemicals and Antioxidants from Brown Macroalgae. Mar. Drugs 2021, 19, 309. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Rajauria, G.; Tiwari, B.; Sweeney, T.; O’Doherty, J. Extraction and Yield Optimisation of Fucose, Glucans and Associated Antioxidant Activities from Laminaria digitata by Applying Response Surface Methodology to High Intensity Ultrasound-Assisted Extraction. Mar. Drugs 2018, 16, 257. [Google Scholar] [CrossRef]
- Cikoš, A.-M.; Aladić, K.; Velić, D.; Tomas, S.; Lončarić, P.; Jerković, I. Evaluation of ultrasound-assisted extraction of fucoxanthin and total pigments from three croatian macroalgal species. Chem. Pap. 2022, 77, 1545–1559. [Google Scholar] [CrossRef]
- Mittal, R.; Tavanandi, H.; Mantri, V.; Raghavarao, K. Ultrasound assisted methods for enhanced extraction of phycobiliproteins from marine macro-algae, Gelidium pusillum (Rhodophyta). Ultrason. Sonochem. 2017, 38, 92–103. [Google Scholar] [CrossRef]
- Kumar, Y.; Singhal, S.; Tarafdar, A.; Pharande, A.; Ganesan, M.; Badgujar, P. Ultrasound assisted extraction of selected edible macroalgae: Effect on antioxidant activity and quantitative assessment of polyphenols by liquid chromatography with tandem mass spectrometry (LC-MS/MS). Algal Res.-Biomass Biofuels Bioprod. 2020, 52, 102114. [Google Scholar] [CrossRef]
- Cikoš, A.M.; Jokić, S.; Šubarić, D.; Jerković, I. Overview on the Application of Modern Methods for the Extraction of Bioactive Compounds from Marine Macroalgae. Mar. Drugs 2018, 16, 348. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Mikkelsen, M.; Tran, V.; Trang, V.T.D.; Rhein-Knudsen, N.; Holck, J.; Rasin, A.; Cao, H.; Vân, T.; Meyer, A. Enzyme-Assisted Fucoidan Extraction from Brown Macroalgae Fucus distichus subsp. evanescens and Saccharina latissima. Mar. Drugs 2020, 18, 296. [Google Scholar] [CrossRef]
- Romero, A.M.; Morales, J.J.P.; Klose, L.; Liese, A. Enzyme-Assisted Extraction of Ulvan from the Green Macroalgae Ulva fenestrata. Molecules 2023, 28, 6781. [Google Scholar] [CrossRef]
- Quitério, E.; Grosso, C.; Ferraz, R.; Delerue-Matos, C.; Soares, C. A Critical Comparison of the Advanced Extraction Techniques Applied to Obtain Health-Promoting Compounds from Seaweeds. Mar. Drugs 2022, 20, 677. [Google Scholar] [CrossRef]
- Yücetepe, A.; Aydar, E.F.; Dogu-Baykut, E.; Dinç, H.; Onat, İ.A.; Demircan, E.; Şensu, E.; Okudan, E.; Özçelik, B. Optimization of Protein Extraction from Halopteris scoparia Macroalgae by Ultrasonic-Assisted Enzymatic Extraction (UAEE): Bioactive, Chemical, and Technological Properties. ACS Food Sci. Technol. 2024, 4, 1375–1387. [Google Scholar] [CrossRef]
- Maribu, I.; Blikra, M.; Eilertsen, K.-E.; Elvevold, K. Protein enrichment of the red macroalga Palmaria palmata using pulsed electric field and enzymatic processing. J. Appl. Phycol. 2024, 36, 3665–3673. [Google Scholar] [CrossRef]
- Steinbruch, E.; Wise, J.; Levkov, K.; Chemodnov, A.; Israel, Á.; Livney, Y.; Golberg, A. Enzymatic cell wall degradation combined with pulsed electric fields increases yields of water-soluble-protein extraction from the green marine macroalga Ulva sp. Innov. Food Sci. Emerg. Technol. 2023, 84, 103231. [Google Scholar] [CrossRef]
- Gordalina, M.; Pinheiro, H.; Mateus, M.; Da Fonseca, M.; Cesário, M. Macroalgae as Protein Sources—A Review on Protein Bioactivity, Extraction, Purification and Characterization. Appl. Sci. 2021, 11, 7969. [Google Scholar] [CrossRef]
- Otero, P.; Quintana, S.; Reglero, G.; Fornari, T.; García-Risco, M. Pressurized Liquid Extraction (PLE) as an Innovative Green Technology for the Effective Enrichment of Galician Algae Extracts with High Quality Fatty Acids and Antimicrobial and Antioxidant Properties. Mar. Drugs 2018, 16, 156. [Google Scholar] [CrossRef]
- Sáez-González, L.; Carreño-Díaz, M.; Blázquez-Abellán, G.; Santander-Ortega, M.; Martínez-García, R.; Martínez, L.; Carbajal, J.; Castro-Vázquez, L. Antioxidant Valorization of PLE Extracts from Macroalgae (Cladophora glomerata): In Vitro Assessment of Nanoemulsions Against Oxidative Stress. Antioxidants 2024, 13, 1370. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, A.C.S.P.; Alves, A.A.A.; Martins, J.R.P.; Feitosa, F.X.; De Sant’Ana, H.; Da Silva Júnior, I.J. Enhanced recovery of r-phycoerythrin using recombination cell-assisted pressurized fluid extraction: Yield, characterization, and antioxidant evaluation. Sep. Purif. Technol. 2024, 354, 128896. [Google Scholar] [CrossRef]
- De Sousa, A.C.S.P.; Martins, J.R.P.; Alves, A.; Maranhão, S.S.A.; Pessoa, C.; Feitosa, F.; De Sant’Ana, H.; Da Silva, I.J. Extraction and characterization of R-phycoerythrin from wet and lyophilized macroalgae Solieria filiformis by pressurized water method. Algal Res. 2024, 80, 103493. [Google Scholar] [CrossRef]
- Messyasz, B.; Michalak, I.; Łęska, B.; Schroeder, G.; Górka, B.; Korzeniowska, K.; Lipok, J.; Wieczorek, P.; Rój, E.; Wilk, R.; et al. Valuable natural products from marine and freshwater macroalgae obtained from supercritical fluid extracts. J. Appl. Phycol. 2017, 30, 591–603. [Google Scholar] [CrossRef]
- Singh, S.; Verma, D.K.; Thakur, M.; Tripathy, S.; Patel, A.; Shah, N.; Utama, G.L.; Srivastav, P.P.; Benavente-Valdés, J.R.; Chávez-González, M.; et al. Supercritical fluid extraction (SCFE) as green extraction technology for high-value metabolites of algae, its potential trends in food and human health. Food Res. Int. 2021, 150 Pt A, 110746. [Google Scholar] [CrossRef]
- Sarkar, S.; Gayen, K.; Bhowmick, T. Green extraction of biomolecules from algae using subcritical and supercritical fluids. Biomass Convers. Biorefinery 2022, 1–23. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K.; Dmytryk, A.; Wilk, R.; Gramza, M.; Rój, E. Evaluation of Supercritical Extracts of Algae as Biostimulants of Plant Growth in Field Trials. Front. Plant Sci. 2016, 7, 1591. [Google Scholar] [CrossRef]
- Michalak, I.; Górka, B.; Wieczorek, P.; Rój, E.; Lipok, J.; Łęska, B.; Messyasz, B.; Wilk, R.; Schroeder, G.; Dobrzyńska-Inger, A.; et al. Supercritical fluid extraction of algae enhances levels of biologically active compounds promoting plant growth. Eur. J. Phycol. 2016, 51, 243–252. [Google Scholar] [CrossRef]
- Kalasariya, H.S.; Pereira, L.; Patel, N.B. Comprehensive Phytochemical Analysis and Bioactivity Evaluation of Padina boergesenii: Unveiling Its Prospects as a Promising Cosmetic Component. Mar. Drugs 2023, 21, 358. [Google Scholar] [CrossRef]
- Sharma, G.; John, J. Identification of polyphenols using UPLC-QTOF MS/MS, in-vitro photoprotective and antiaging activities of brown macroalga Padina tetrastromatica. Algal Res. 2023, 75, 103255. [Google Scholar] [CrossRef]
- Kiani, H.; Aznar, R.; Poojary, M.M.; Tiwari, B.K.; Halim, R. Chromatographic Techniques to Separate and Identify Bioactive Compounds in Microalgae. Front. Energy Res. 2022, 10, 904014. [Google Scholar] [CrossRef]
- Zwerger, M.; Ganzera, M. Fast and Efficient Separation of Eleven Mycosporine-like Amino Acids by UHPLC-DAD and Their Quantification in Diverse Red Algae. Mar. Drugs 2022, 20, 395. [Google Scholar] [CrossRef] [PubMed]
- Terriente-Palacios, C.; Diaz, I.; Castellari, M. A validated ultra-performance liquid chromatography with diode array detection coupled to electrospray ionization and triple quadrupole mass spectrometry method to simultaneously quantify taurine, homotaurine, hypotaurine and amino acids in macro- and microalgae. J. Chromatogr. A 2019, 1589, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Steevensz, A.; Mackinnon, S.; Hankinson, R.; Craft, C.; Connan, S.; Stengel, D.; Melanson, J. Profiling phlorotannins in brown macroalgae by liquid chromatography-high resolution mass spectrometry. Phytochem. Anal. PCA 2012, 23, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Klejdus, B.; Lojková, L.; Plaza, M.; Šnóblová, M.; Štěrbová, D. Hyphenated technique for the extraction and determination of isoflavones in algae: Ultrasound-assisted supercritical fluid extraction followed by fast chromatography with tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 7956–7965. [Google Scholar] [CrossRef]
- Lawrence, J.F.; Niedzwiadek, B.; Menard, C.; Lau, B.P.Y.; Lewis, D.; Kuper-Goodman, T.; Carbone, S.; Holmes, C. Comparison of Liquid Chromatography/Mass Spectrometry, ELISA, and Phosphatase Assay for the Determination of Microcystins in Blue-Green Algae Products. J. AOAC Int. 2001, 84, 1035–1044. [Google Scholar] [CrossRef]
- Hartmann, A.; Becker, K.; Karsten, U.; Remias, D.; Ganzera, M. Analysis of Mycosporine-Like Amino Acids in Selected Algae and Cyanobacteria by Hydrophilic Interaction Liquid Chromatography and a Novel MAA from the Red Alga Catenella repens. Mar. Drugs 2015, 13, 6291–6305. [Google Scholar] [CrossRef]
- Vega, J.; Bárcenas-Pérez, D.; Fuentes-Ríos, D.; López-Romero, J.M.; Hrouzek, P.; Figueroa, F.L.; Cheel, J. Isolation of Mycosporine-like Amino Acids from Red Macroalgae and a Marine Lichen by High-Performance Countercurrent Chromatography: A Strategy to Obtain Biological UV-Filters. Mar. Drugs 2023, 21, 357. [Google Scholar] [CrossRef]
- Pérez-Míguez, R.; Plaza, M.; Castro-Puyana, M.; Marina, M.L. Separation and identification of peptides in hydrolysed protein extracts from edible macroalgae by HPLC-ESI-QTOF/MS. Algal Res. 2019, 39, 101465. [Google Scholar] [CrossRef]
- Sardari, R.R.R.; Prothmann, J.; Gregersen, O.; Turner, C.; Nordberg Karlsson, E. Identification of Phlorotannins in the Brown Algae, Saccharina latissima and Ascophyllum nodosum by Ultra-High-Performance Liquid Chromatography Coupled to High-Resolution Tandem Mass Spectrometry. Molecules 2021, 26, 43. [Google Scholar] [CrossRef] [PubMed]
- Yum, T.; Kim, E.-Y.; Kim, Y.; Choi, S.; Paeng, K.-J. The Development of an Extraction Method for Simultaneously Analyzing Fatty Acids in Macroalgae Using SPE with Derivatization for LC–MS/MS. Molecules 2024, 29, 430. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; David, F.; Lynen, F.; Vanormelingen, P.; Vyverman, W.; Rumpel, K.; Xu, G.; Sandra, P. Evaluation of ionic liquid stationary phases for one dimensional gas chromatography–mass spectrometry and comprehensive two dimensional gas chromatographic analyses of fatty acids in marine biota. J. Chromatogr. A 2011, 1218, 3056–3063. [Google Scholar] [CrossRef] [PubMed]
- Shobier, A.H.; Abdel Ghani, S.A.; Barakat, K.M. GC/MS spectroscopic approach and antifungal potential of bioactive extracts produced by marine macroalgae. Egypt. J. Aquat. Res. 2016, 42, 289–299. [Google Scholar] [CrossRef]
- Bajwa, B.; Xing, X.; Serin, S.C.; Hayes, M.; Terry, S.A.; Gruninger, R.J.; Abbott, D.W. Characterization of Unfractionated Polysaccharides in Brown Seaweed by Methylation-GC-MS-Based Linkage Analysis. Mar. Drugs 2024, 22, 464. [Google Scholar] [CrossRef]
- Palaniyappan, S.; Sridhar, A.; Kari, Z.A.; Téllez-Isaías, G.; Ramasamy, T. Evaluation of Phytochemical Screening, Pigment Content, In Vitro Antioxidant, Antibacterial Potential and GC-MS Metabolite Profiling of Green Seaweed Caulerpa racemosa. Mar. Drugs 2023, 21, 278. [Google Scholar] [CrossRef]
- Fitton, J.H.; Dell’Acqua, G.; Gardiner, V.-A.; Karpiniec, S.S.; Stringer, D.N.; Davis, E. Topical Benefits of Two Fucoidan-Rich Extracts from Marine Macroalgae. Cosmetics 2015, 2, 66–81. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Martínez-Sanz, M.; Martínez-López, R.; Martínez-Abad, A.; Panikuttira, B.; López-Rubio, A.; Tuohy, M.G.; Hogan, S.A.; Brodkorb, A. Characterization and gelling properties of a bioactive extract from. Curr. Res. Food Sci. 2021, 4, 354–364. [Google Scholar] [CrossRef]
- Xhauflaire-Uhoda, E.; Fontaine, K.; Piérard, G.E. Kinetics of moisturizing and firming effects of cosmetic formulations. Int. J. Cosmet. Sci. 2008, 30, 131–138. [Google Scholar] [CrossRef]
- Cha, S.H.; Ko, S.C.; Kim, D.; Jeon, Y.J. Screening of marine algae for potential tyrosinase inhibitor: Those inhibitors reduced tyrosinase activity and melanin synthesis in zebrafish. J. Dermatol. 2011, 38, 354–363. [Google Scholar] [CrossRef]
- Clarys, P.; Barel, A. Handbook of Cosmetic Science and Technology—New Trends in Antiaging Cosmetic Ingredients and Treatments, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Jesumani, V.; Du, H.; Aslam, M.; Pei, P.; Huang, N. Potential Use of Seaweed Bioactive Compounds in Skincare-A Review. Mar. Drugs 2019, 17, 688. [Google Scholar] [CrossRef]
- Sayin, S.; Depci, T.; Naz, M.; Sezer, S.; Karaaslan, M.G.; Aras, A.; Uğur, S.; Çetïn, Z.; Saygili, E.I.; Ateş, B. Characterization and evaluation of the antimicrobial properties of algal alginate; a potential natural protective for cosmetics. J. Res. Pharm. 2022, 26, 198–209. [Google Scholar] [CrossRef]
- Park, J.; Lee, H.; Choi, S.; Pandey, L.K.; Depuydt, S.; De Saeger, J.; Park, J.-T.; Han, T. Extracts of red seaweed, Pyropia yezoensis, inhibit melanogenesis but stimulate collagen synthesis. J. Appl. Phycol. 2021, 33, 653–662. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.E.; Kim, K.H.; Kang, N.J. Beneficial Effects of Marine Algae-Derived Carbohydrates for Skin Health. Mar. Drugs 2018, 16, 459. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lee, W.; Oh, J.Y.; Cui, Y.R.; Ryu, B.; Jeon, Y.J. Protective effect of sulfated polysaccharides from celluclast-assisted extract of Hizikia fusiforme against ultraviolet B-induced skin damage by regulating NF-κB, AP-1, and MAPKs signaling pathways in vitro in human dermal fibroblasts. Mar. Drugs 2018, 16, 239. [Google Scholar] [CrossRef] [PubMed]
- Adamiak, K.; Sionkowska, A. State of Innovation in Alginate-Based Materials. Mar. Drugs 2023, 21, 353. [Google Scholar] [CrossRef] [PubMed]
- Rahse, W. Proven Active Ingredients for Various Categories of Skin Creams. In Cosmetic Creams; Rahse, W., Ed.; Wiley: Hoboken, NJ, USA, 2019; pp. 175–230. [Google Scholar]
- Iwamoto, K.; Hiragun, T.; Takahagi, S.; Yanase, Y.; Morioke, S.; Mihara, S.; Kameyoshi, Y.; Hide, M. Fucoidan suppresses IgE production in peripheral blood mononuclear cells from patients with atopic dermatitis. Arch. Dermatol. Res. 2011, 303, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Kim, D.W.; Park, C.W.; Kim, B.; Sim, H.; Kim, H.S.; Lee, T.K.; Lee, J.C.; Yang, G.E.; Her, Y.; et al. Laminarin Attenuates Ultraviolet-Induced Skin Damage by Reducing Superoxide Anion Levels and Increasing Endogenous Antioxidants in the Dorsal Skin of Mice. Mar. Drugs 2020, 18, 345. [Google Scholar] [CrossRef]
- Ferreres, F.; Lopes, G.; Gil-Izquierdo, A.; Andrade, P.B.; Sousa, C.; Mouga, T.; Valentão, P. Phlorotannin extracts from fucales characterized by HPLC-DAD-ESI-MSn: Approaches to hyaluronidase inhibitory capacity and antioxidant properties. Mar. Drugs 2012, 10, 2766–2781. [Google Scholar] [CrossRef]
- Wang, J.; Jin, W.; Hou, Y.; Niu, X.; Zhang, H.; Zhang, Q. Chemical composition and moisture-absorption/retention ability of polysaccharides extracted from five algae. Int. J. Biol. Macromol. 2013, 57, 26–29. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, N.; Liu, X.; Zhao, Z.; Li, Z.; Xu, Z. The structure of a sulfated galactan from Porphyra haitanensis and its in vivo antioxidant activity. Carbohydr. Res. 2004, 339, 105–111. [Google Scholar] [CrossRef]
- Jiang, Z.; Hama, Y.; Yamaguchi, K.; Oda, T. Inhibitory effect of sulphated polysaccharide porphyran on nitric oxide production in lipopolysaccharide-stimulated RAW264.7 macrophages. J. Biochem. 2012, 151, 65–74. [Google Scholar] [CrossRef]
- Adrien, A.; Bonnet, A.; Dufour, D.; Baudouin, S.; Maugard, T.; Bridiau, N. Pilot production of ulvans from Ulva sp. and their effects on hyaluronan and collagen production in cultured dermal fibroblasts. Carbohydr. Polym. 2017, 157, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Bae, H.J.; Kim, S.J.; Choi, I.S. In vitro antibacterial and anti-inflammatory properties of seaweed extracts against acne inducing bacteria, Propionibacterium acnes. J. Environ. Biol. 2011, 32, 313–318. [Google Scholar] [PubMed]
- Murphy, M.J.; Dow, A.A. Clinical Studies of the Safety and Efficacy of Macroalgae Extracts in Cosmeceuticals. J. Clin. Aesthet. Dermatol. 2021, 14, 37–41. [Google Scholar]
- Kang, J.W.; Hyun, S.H.; Kim, H.M.; Park, S.Y.; Lee, J.A.; Lee, I.C.; Bae, J.S. The effects of fucoidan-rich polysaccharides extracted from Sargassum horneri on enhancing collagen-related skin barrier function as a potential cosmetic product. J. Cosmet. Dermatol. 2024, 23, 1365–1373. [Google Scholar] [CrossRef]
- Janssens-Böcker, C.; Wiesweg, K.; Doberenz, C. The Tolerability and Effectiveness of Marine-Based Ingredients in Cosmetics: A Split-Face Clinical Study of a Serum Spray Containing Fucus vesiculosus Extract, Ulva lactuca Extract, and Ectoin. Cosmetics 2023, 10, 93. [Google Scholar] [CrossRef]
- Janssens-Böcker, C.; Wiesweg, K.; Doberenz, C. Exploring the Therapeutic Potential of Algae-Based Sheet Masks in Skincare: A Comprehensive Study of Cosmetological Benefits and Microbiome Balanced Interactions. J. Cosmet. Dermatol. Sci. Appl. 2023, 13, 277–301. [Google Scholar] [CrossRef]
- Schmid, D.; Schürch, C.; Zülli, F. UVA-screening compounds from red algae protect against photoageing. Personal Care 2024, 1, 29–31. [Google Scholar]
- Capitanio, B.; Sinagra, J.L.; Weller, R.B.; Brown, C.; Berardesca, E. Randomized controlled study of a cosmetic treatment for mild acne. Clin. Exp. Dermatol. 2012, 37, 346–349. [Google Scholar] [CrossRef]
- Jeong, J.W.; Park, D.J.; Kim, S.C.; Kang, H.W.; Lee, B.; Kim, H.W.; Kim, Y.M.; Linh, N.V.; Jung, W.K. Wound healing effect of fucoidan-loaded gelatin/oxidized carboxymethyl cellulose hydrogel. Int. J. Biol. Macromol. 2025, 286, 138254. [Google Scholar] [CrossRef] [PubMed]
- Kikionis, S.; Koromvoki, M.; Tagka, A.; Polichronaki, E.; Stratigos, A.; Panagiotopoulos, A.; Kyritsi, A.; Karalis, V.; Vitsos, A.; Rallis, M.; et al. Ulvan-Based Nanofibrous Patches Enhance Wound Healing of Skin Trauma Resulting from Cryosurgical Treatment of Keloids. Mar. Drugs 2022, 20, 551. [Google Scholar] [CrossRef] [PubMed]
- Sulastri, E.; Lesmana, R.; Zubair, M.S.; Abdelwahab Mohammed, A.F.; Elamin, K.M.; Wathoni, N. Ulvan/Silver nanoparticle hydrogel films for burn wound dressing. Heliyon 2023, 9, e18044. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Alle, M.; Son, H.K.; Kim, J.C. Dialdehyde modification of laminarin for facile synthesis of ultrafine silver nanoparticles with excellent antibacterial and wound healing properties. Int. J. Biol. Macromol. 2022, 222, 1364–1375. [Google Scholar] [CrossRef]
- Foroughi, J.; Ruhparwar, A.; Aloko, S.; Wang, C.H. Manufacturing Ulvan Biopolymer for Wound Dressings. Macromol. Mater. Eng. 2024, 309, 2300268. [Google Scholar] [CrossRef]
- Hwang, P.A.; Chen, H.Y.; Chang, J.S.; Hsu, F.Y. Electrospun nanofiber composite mat based on ulvan for wound dressing applications. Int. J. Biol. Macromol. 2023, 253, 126646. [Google Scholar] [CrossRef]
- Terezaki, A.; Kikionis, S.; Ioannou, E.; Sfiniadakis, I.; Tziveleka, L.-A.; Vitsos, A.; Roussis, V.; Rallis, M. Ulvan/gelatin-based nanofibrous patches as a promising treatment for burn wounds. J. Drug Deliv. Sci. Technol. 2022, 74, 103535. [Google Scholar] [CrossRef]
- Mariia, K.; Arif, M.; Shi, J.; Song, F.; Chi, Z.; Liu, C. Novel chitosan-ulvan hydrogel reinforcement by cellulose nanocrystals with epidermal growth factor for enhanced wound healing: In vitro and in vivo analysis. Int. J. Biol. Macromol. 2021, 183, 435–446. [Google Scholar] [CrossRef]
- Ren, Y.; Aierken, A.; Zhao, L.; Lin, Z.; Jiang, J.; Li, B.; Wang, J.; Hua, J.; Tu, Q. hUC-MSCs lyophilized powder loaded polysaccharide ulvan driven functional hydrogel for chronic diabetic wound healing. Carbohydr. Polym. 2022, 288, 119404. [Google Scholar] [CrossRef]
- Don, T.M.; Ma, C.H.; Huang, Y.C. In Situ Release of Ulvan from Crosslinked Ulvan/Chitosan Complex Films and Their Evaluation as Wound Dressings. Polymers 2022, 14, 5382. [Google Scholar] [CrossRef]
- Sellimi, S.; Maalej, H.; Rekik, D.M.; Benslima, A.; Ksouda, G.; Hamdi, M.; Sahnoun, Z.; Li, S.; Nasri, M.; Hajji, M. Antioxidant, antibacterial and in vivo wound healing properties of laminaran purified from Cystoseira barbata seaweed. Int. J. Biol. Macromol. 2018, 119, 633–644. [Google Scholar] [CrossRef]
- Amaral, K.R.; Silva, A.S.; Santos, L.F.; Castanheira, E.J.; Mendes, M.C.; Costa, D.C.S.; Rodrigues, J.M.M.; Marto, J.; Mano, J.F. Biomimetic Adhesive Micropatterned Hydrogel Patches for Drug Release. Adv. Healthc. Mater. 2023, 12, e2301513. [Google Scholar] [CrossRef]
- Feng, L.; Hao, Y.; Zhu, M.; Zhai, Y.; Yang, L.; Liu, Y.; Cheng, G. Incorporation of Laminarin-Based Hydrogel with Graphene Foam to Enhance the Toughness of Scaffold and Regulate the Stem Cell Behavior. ACS Biomater. Sci. Eng. 2019, 5, 5295–5304. [Google Scholar] [CrossRef]
- Hao, J.; Liu, X.; Du, Y. Fabrication of gelatine/fucoidan nanogel-coated silver nanoparticles for the treatment of wound healing therapy and nursing care. Regen. Ther. 2025, 29, 282–291. [Google Scholar] [CrossRef]
- Phulmogare, G.; Rani, S.; Lodhi, S.; Patil, U.K.; Sinha, S.; Ajazuddin; Gupta, U. Fucoidan loaded PVA/Dextran blend electrospun nanofibers for the effective wound healing. Int. J. Pharm. 2024, 650, 123722. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Li, Z.; Kim, Y.; Park, S.; Keon, K.; Lee, C.M.; Ahn, G.; Cho, N. Fucoidan-coated cotton dressing functionalized with biomolecules capped silver nanoparticles (LB-Ag NPs-FN-OCG) for rapid healing therapy of infected wounds. Environ. Res. 2024, 246, 118004. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, J.; Zhang, Z.; Pan, Y.; Jiang, Z.; Hu, Y.; Wang, L. Fucoidan-loaded, neutrophil membrane-coated nanoparticles facilitate MRSA-accompanied wound healing. Mater. Des. 2023, 227, 111758. [Google Scholar] [CrossRef]
- Younas, A.; Dong, Z.; Hou, Z.; Asad, M.; Li, M.; Zhang, N. A chitosan/fucoidan nanoparticle-loaded pullulan microneedle patch for differential drug release to promote wound healing. Carbohydr. Polym. 2023, 306, 120593. [Google Scholar] [CrossRef]
- Peng, J.; Liu, C.; Mo, M.; Huang, Y.; Lu, Y.; Xiao, M.; Zhao, X.; Ruan, Q.; Ti, H. Construction of multifunctional hydrogel containing pH-responsive gold nanozyme for bacteria-infected wound healing. Int. J. Biol. Macromol. 2024, 283, 137746. [Google Scholar] [CrossRef]
- Hernández Muñoz, A.C.; Rodríguez Martínez, I.A.; Serafini, M.R.; Aragón, D.M. Innovative applications of marine-derived algae in cosmetics: A patent review (2010−2023). Algal Res. 2024, 84, 103806. [Google Scholar] [CrossRef]
- Michalak, I.; Dmytryk, A.; Chojnacka, K. Algae Cosmetics. In Encyclopedia of Marine Biotechnology; Wiley: Hoboken, NJ, USA, 2020; pp. 65–85. [Google Scholar]
- Lourenço-Lopes, C.; Garcia-Oliveira, P.; Carpena, M.; Fraga-Corral, M.; Jimenez-Lopez, C.; Pereira, A.G.; Prieto, M.A.; Simal-Gandara, J. Scientific Approaches on Extraction, Purification and Stability for the Commercialization of Fucoxanthin Recovered from Brown Algae. Foods 2020, 9, 1113. [Google Scholar] [CrossRef]
- Robal, M.; Truus, K.; Volobujeva, O.; Mellikov, E.; Tuvikene, R. Thermal stability of red algal galactans: Effect of molecular structure and counterions. Int. J. Biol. Macromol. 2017, 104, 213–223. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Pereira, A.G.; Lourenço-Lopes, C.; Garcia-Oliveira, P.; Cassani, L.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Main bioactive phenolic compounds in marine algae and their mechanisms of action supporting potential health benefits. Food Chem. 2021, 341, 128262. [Google Scholar] [CrossRef]
- Costa, R.; Santos, L. Delivery systems for cosmetics—From manufacturing to the skin of natural antioxidants. Powder Technol. 2017, 322, 402–416. [Google Scholar] [CrossRef]
- Evoluderm. Detox Serum. Available online: https://www.evoluderm.com/en/products/serum-detox?srsltid=AfmBOor_OwLMq3KGtuZBOGg1yIdTau3bxRVpwiudgng5mZ6_yPUwcnqs (accessed on 21 August 2025).
- heimish. Marine Care Deep Moisture Nourishing Melting Cream. Available online: https://heimish.us/products/renewal-marine-care-deep-moisture-nourishing-melting-cream-60ml-2-03fl-oz-copy (accessed on 21 August 2025).
- Farmona. Moisturizing and Regenerating Night Bio-Cream. Available online: https://farmona.pl/en/products/moisturizing-and-regenerating-night-bio-cream/ (accessed on 21 August 2025).
- NALA. Antipollution Face Cream—Combination Skin—Seaweed & Cucumber. Available online: https://nala.eu/products/antipollution-face-cream-combination-skin-seaweed-cucumber?_pos=4&_psq=algae&_ss=e&_v=1.0 (accessed on 21 August 2025).
- NALA. Blonde Hair Purple Shampoo—Violet Absolute Extract, Algae Extract, Lime Extract. Available online: https://nala.eu/products/blonde-hair-purple-shampoo-violet-absolute-extract-algae-extract-lime-extract?_pos=1&_psq=algae&_ss=e&_v=1.0 (accessed on 21 August 2025).
- NALA. Natural Soap—Seaweed. Available online: https://nala.eu/products/natural-soap-seaweed?_pos=2&_psq=algae&_ss=e&_v=1.0 (accessed on 21 August 2025).
- evanhealy. Sea Algae Serum. Available online: https://www.evanhealy.com/products/sea-algae-serum?variant=32645363040349 (accessed on 21 August 2025).
- Algologie. Algologie Natural Marine Based Skincare. Available online: https://www.algologie.com/gb/ (accessed on 21 August 2025).
- Thalgo. Available online: https://www.thalgo.fr/ (accessed on 21 August 2025).

| INCI Name/Description | Function |
|---|---|
| Fucoidan (Sulfated polysaccharide) | Antioxidant Skin conditioning |
| Fucoxanthin (Carotenoid from Sargassum siliquastrum) | Skin conditioning |
| Sargachromanol F (Chromene from Sargassum siliquastrum) | Skin conditioning |
| Sargachromanol D (Chromene from Sargassum siliquastrum) | Skin conditioning |
| Sargachromanol E (Chromene from Sargassum siliquastrum) | Skin conditioning |
| Sargassum filipendula extract | Skin protecting |
| Sargassum fusiforme extract | Skin protecting |
| Pylaiella littoralis extract (Extract of the whole plant) | Skin protecting |
| Laminaria longissima extract | Humectant |
| Laminaria digitata extract | Skin protecting |
| Laminaria diabolica extract | Humectant Skin conditioning |
| Laminaria hyperborea extract | Skin protecting |
| Laminaria ochroleuca extract | Skin conditioning |
| Laminaria cloustoni extract (Kelp extract) | Skin protecting |
| Laminaria saccharina extract (Extract of the thallus of Laminaria saccharina) | Skin protecting |
| Laminaria japonica extract (Extract of the Japan kelp/seaweed, Laminaria japonica) | Skin protecting |
| Deaminated Laminaria japonica extract (Extract of Laminaria japonica extract enzymatically deaminated) | Skin conditioning |
| Laminaria digitata powder (Powder from the dried and ground thallus of Laminaria digitata) | Skin conditioning |
| Laminaria japonica powder (Powder from the dried and ground alga of Laminaria japonica) | Skin conditioning |
| Laminaria digitata water (Aqueous solution of the steam distillate obtained from Laminaria digitata) | Skin protecting Skin conditioning |
| Fucus vesiculosus (The algae Fucus vesiculosus, Fucaceae) | Skin conditioning |
| Fucus spiralis extract | Skin conditioning—emollient |
| Fucus serratus extract | Skin protecting |
| Fucus vesiculosus extract (Extract of the dried thallus of the Bladderwrack, Fucus vesiculosus L., Fucaceae) | Skin conditioning—emollient Smoothing Soothing |
| Fucus vesiculosus powder (Powder obtained from Bladderwrack, Fucus vesiculosus L., Fucaceae) | Skin conditioning |
| Fucus crispus thalle extract (Extract from the dried thallus of Fucus crispus, Fucaceae) | Perfuming |
| Hydrolyzed Fucus vesiculosus extract (Hydrolyzed iodinated extract from the Bladderwrack, Fucus vesiculosus L., Fucaceae) | Skin conditioning Smoothing Soothing |
| Fucus crispus thalle oil/(Essential oil from the dried thallus of Fucus crispus, Fucaceae) | Perfuming |
| Fucus vesiculosus thale oil (Essential oil obtained from the dried thallus of the Bladderwrack, Fucus vesiculosus L., Fucaceae) | Perfuming |
| Hydrolyzed Fucus vesiculosus protein(Hydrolysate of the protein obtained from the Bladderwrack, Fucus vesiculosus L., Furaceae, derived by acid, enzyme or other methods of hydrolysis) | Skin conditioning |
| Macrocystis pyrifera extract | Skin conditioning |
| Macrocystis pyrifera juice(Juice expressed from the whole plant) | Skin conditioning |
| Macrocystis pyrifera protein (Protein from the Giant Kelp, Macrocystis pyrifera L., Lessoniaceae) | Skin conditioning |
| Macrocystis pyrifera | Viscosity controlling |
| Kelp sulfated oligosaccharides (Obtained from the seaweed kelp (Macrocystis pyrifera, Lessoniaceae) hydrolysate) | Hair conditioning |
| Sargassum horneri powder (Powder from the dried and ground Sargassum horneri) | Humectant Skin conditioning—humectant |
| Sargassum fulvellum extract | Skin conditioning |
| Sargassum macrocarpum extract | Skin conditioning—emollient |
| Sargassum aquifolium extract | Skin conditioning—miscellaneous |
| Sargassum ilicifolium extract | Skin conditioning—miscellaneous |
| Sargassum oligocystum extract | Skin conditioning—humectant |
| Sargassum hemiphyllum extract | Antioxidant Skin protecting Skin conditioning—miscellaneous |
| Sargassum yezoense extract | Antimicrobial |
| Sargassum serratifolium extract | Antioxidant |
| Sargassum vulgare extract | Skin conditioning |
| Sargassum muticum extract | Skin protecting |
| Sargassum glaucescens extract | Antioxidant |
| Sargassum thunbergii extract | Antimicrobial |
| Sargassum siliquastrum extract | Skin conditioning |
| Sargassum fusiforme extract | Skin protecting |
| Sargassum pacificum thallus extract | Skin conditioning—emollient |
| Sargassum fluitans/Natans extract | Emulsion stabilizing |
| Hydrolyzed Sargassum horneri extract/Hydrolysate of Sargassum horneri extract derived by acid, enzyme, or other methods of hydrolysis) | Skin conditioning—miscellaneous |
| Hydrolyzed Sargassum thunbergii extract (Hydrolysate of Sargassum thunbergii extract derived by acid, enzyme or other methods of hydrolysis) | Antimicrobial Hair conditioning Humectant Skin protecting Skin conditioning—humectant |
| Ecklonia/Laminaria extract (Extract of mixture of Ecklonia and Laminaria algae) | Skin conditioning |
| Ascophyllum nodosum/Fucus vesiculosus extract | Humectant Skin conditioning |
| Ascophyllum nodosum/Fucus vesiculosus/Laminaria cloustoni/Laminaria digitata extract | Skin conditioning—emollient |
| Ascophyllum nodosum/Fucus vesiculosus/Hizikia fusiforme/Kjellmaniella gyrata/Lessonia nigrescens/Saccharina angustata/Saccharina japonica/Undaria pinnatifida extract | Skin conditioning |
| INCI Name/Description | Function |
|---|---|
| Cladophora wrightiana extract | Skin conditioning |
| Ulva australis extract | Skin conditioning—emollient |
| Ulva rigida extract | Skin conditioning |
| Ulva linza extract | Skin conditioning—emollient |
| Ulva lactuca extract | Skin conditioning Skin protecting |
| Ulva ramulosa callus culture extract | Skin conditioning |
| Ulva lactuca powder (Powder obtained from dried and ground Ulva lactuca, Cyperaceae) | Absorbent Binding Viscosity controlling |
| Hydrolyzed Ulva pertusa extract (Hydrolysate of Ulva pertusa extract derived by acid, enzyme or other methods of hydrolysis) | Skin conditioning |
| Hydrolyzed Ulva lactuca extract (Hydrolysate of Ulva lactuca extract derived by acid, enzyme or other methods of hydrolysis) | Skin conditioning |
| Hydrolyzed Ulva linza leaf extract (Hydrolysate of Ulva linza extract derived by acid, enzyme or other methods of hydrolysis) | Skin conditioning—emollient Skin conditioning—humectant |
| INCI Name/Description | Function |
|---|---|
| Ceramium kondoi extract | Humectant Skin conditioning |
| Ceramium rubrum extract | Skin conditioning—emollient Humectant Skin conditioning |
| Polysiphonia brodiei extract | Skin conditioning |
| Polysiphonia lanosa extract | Skin conditioning |
| Polysiphonia elongata extract | Humectant Skin conditioning |
| Polysiphonia morrowii extract | Antioxidant |
| Phytocompound-Based Topical Systems | Biological Activity Beneficial in Wound Healing | Reference |
|---|---|---|
| Ulvan | ||
| Nanofibrous patches (ulvan, polyethylene oxide) | In vivo study: anti-inflammatory, antioxidant effects, ↘ inflammation, restoring biophysical parameters of skin | [133] |
| Wet-spun fibers, 3D printed hydrogel | In vitro cell studies: higher cell viability than alginate and chitosan in biocompatibility tests | [136] |
| Polycaprolactone-ulvan fibrous composite mats | In vitro cell studies-NIH3T3 fibroblasts: ↗ cellular proliferation, ↗ expression α-SMA and MMP-9 genes | [137] |
| Ulvan/gelatin-based nanofibrous patches | In vivo study with burn wound mouse model: faster wound contraction during early stages of healing, ↘ inflammation, uniform wound closure | [138] |
| Chitosan-ulvan hydrogels with cellulose nanocrystals and epidermal growth factor | In vitro cytocompatibility studies, in vivo wound-healing study on mice: epithelial regeneration and collagen deposition | [139] |
| Ulvan-based hydrogel matrix loaded with silver nanoparticles and human umbilical cord mesenchymal stem cell lyophilized powder | In vitro antibacterial activity, cytocompatibility, ↗ cell proliferation and cell migration, In vivo study with type II diabetes mellitus mouse model, ↗ wound-healing effect | [140] |
| Ulvan/Silver nanoparticle hydrogel films | In vitro antimicrobial activity, In vivo study with Wistar rats with second-degree burns accelerated wound healing, modulating inflammation, ↗ re-epithelialization, ↗ vascularization | [134] |
| Crosslinked ulvan/Chitosan complex films | In vitro studies: biocompatibility, scratch assay—↗ HaCaT cell migration and proliferation In vivo study with Sprague Dawley rats: regeneration of dermis, collagen production | [141] |
| Laminarin | ||
| Cystoseira barbata laminaran based cream | In vitro antibacterial and antioxidant activity In vivo study with Wistar rats: ↗ collagen deposition, ↗ fibroblast and vascular density | [142] |
| Hydrogel patch of methacrylated laminarin loaded with ciprofloxacin | In vitro antibacterial activity, biocompatibility human dermal fibroblasts | [143] |
| 3D graphene foam/laminarin hydrogel composite scaffold | In vitro: biocompatibility ↗ cell migration | [144] |
| Dialdehyde-modified laminarin- silver nanoparticles | In vitro antibacterial activity and inhibition of biofilm formation Cell viability and scratch assay: ↗ migration of human HaCaT | [135] |
| Fucoidan | ||
| Gelatine/fucoidan nanogel-coated silver nanoparticles | In vitro antibacterial activity and inhibition of biofilm formation Cell viability and scratch assay: ↗cell regeneration | [145] |
| Fucoidan-loaded gelatin/oxidized carboxymethyl cellulose hydrogel | In vitro: cytocompatibility—RAW 264.7 macrophages, human dermal fibroblasts; protection against oxidative stress, ↘ NO production in LPS-stimulated RAW 264.7 macrophages, ↗ collagen synthesis and cell migration in HDF cells In vivo wound-healing model- mice: ↗ full-thickness wound healing | [132] |
| Fucoidan loaded PVA/Dextran blend electrospun nanofibers | In vivo studies-Sprague Dawley rats: ↗ wound-healing rate: ↘inflammatory response through antioxidant effects, ↗ epidermal regeneration and collagen deposition | [146] |
| Fucoidan-coated cotton dressing loaded with silver nanoparticles | In vitro antibacterial activity, cytotoxicity fibroblast cell line, scratch assay In vivo infected wound mouse model: inhibition of bacterial infection, tissue proliferation and collagen deposition | [147] |
| Fucoidan-loaded neutrophil membrane-coated nanoparticles | In vitro antibacterial activity, intracellular uptake ability of RAW264.7 cells In vivo MRSA-infected trauma mouse mode: long term antibacterial effect, accumulation in the infection site, ↗ infected wound closure | [148] |
| Moxifloxacin chitosan/fucoidan nanoparticle-loaded pullulan microneedle patch | In vitro antibacterial activity Ex vivo permeation study, cytotoxicity In vivo biocompatibility, BALB/c mice infectious wound model: accelerated wound healing, rapid wound closure | [149] |
| Fucoidan confined gold nanoparticles hydrogel | In vitro antibacterial activity, blood compatibility, cytocompatibility, anti-inflammatory assay on LPS-induced Raw 264.7 macrophage model, S. aureus-infected full-thickness wound-healing test: adherence to wound site, ↗ tissue regeneration, ↘ bacterial growth, ↘ inflammatory responses | [150] |
| Algae Species | Product Type | Claimed Effects | Ref. |
|---|---|---|---|
| Jania rubens extract | Serum | Moisturizing, smoothing, antioxidant, plumping, detoxing | [157] |
| Undaria pinnatifida, Ecklonia cava, Laminaria japonica, Hizikia fusiforme, Porphyra yezoensis, Laminaria digitata, Laminaria cloustoni, Enteromorpha compressa, Codium tomentosum, Codium fragile, Agarum cribosum, Ulva lactuca extracts | Cream | Moisturizing, smoothing, skin barrier-repairing, protecting, nourishing | [158] |
| Ascophyllum nodosum, Fucus vesiculosus, Crithmum maritimum, Corallina officinalis extracts | Cream | Moisturizing, regenerating | [159] |
| Prasinococcus capsulatus exopolysaccharides | Cream | Protecting, hydrating, anti-aging | [160] |
| Fucus serratus extract | Shampoo | Moisturizing, UV-protecting, long-lasting skin protection | [161] |
| Laminaria japonica powder | Solid soap | Moisturizing, smoothing, | [162] |
| Ascophyllum nodosum, Fucus vesiculosus extracts | Serum | Energizing, antioxidants | [163] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogdan, C.; Molnar, M.; Dima, E.I.; Olteanu, A.A.; Safta, D.A.; Moldovan, M.-L. Marine Macroalgae in Topical Formulations: Bioactive Compounds, Variability, Analytical Challenges and Skin Benefits. Pharmaceutics 2025, 17, 1143. https://doi.org/10.3390/pharmaceutics17091143
Bogdan C, Molnar M, Dima EI, Olteanu AA, Safta DA, Moldovan M-L. Marine Macroalgae in Topical Formulations: Bioactive Compounds, Variability, Analytical Challenges and Skin Benefits. Pharmaceutics. 2025; 17(9):1143. https://doi.org/10.3390/pharmaceutics17091143
Chicago/Turabian StyleBogdan, Cătălina, Mara Molnar, Elena Ines Dima, Andreea Alexandra Olteanu, Diana Antonia Safta, and Mirela-Liliana Moldovan. 2025. "Marine Macroalgae in Topical Formulations: Bioactive Compounds, Variability, Analytical Challenges and Skin Benefits" Pharmaceutics 17, no. 9: 1143. https://doi.org/10.3390/pharmaceutics17091143
APA StyleBogdan, C., Molnar, M., Dima, E. I., Olteanu, A. A., Safta, D. A., & Moldovan, M.-L. (2025). Marine Macroalgae in Topical Formulations: Bioactive Compounds, Variability, Analytical Challenges and Skin Benefits. Pharmaceutics, 17(9), 1143. https://doi.org/10.3390/pharmaceutics17091143









