Chitosan Nanoparticles Loaded with Polyphenols for Cosmeceutical Applications: A State-of-the-Art Review
Abstract
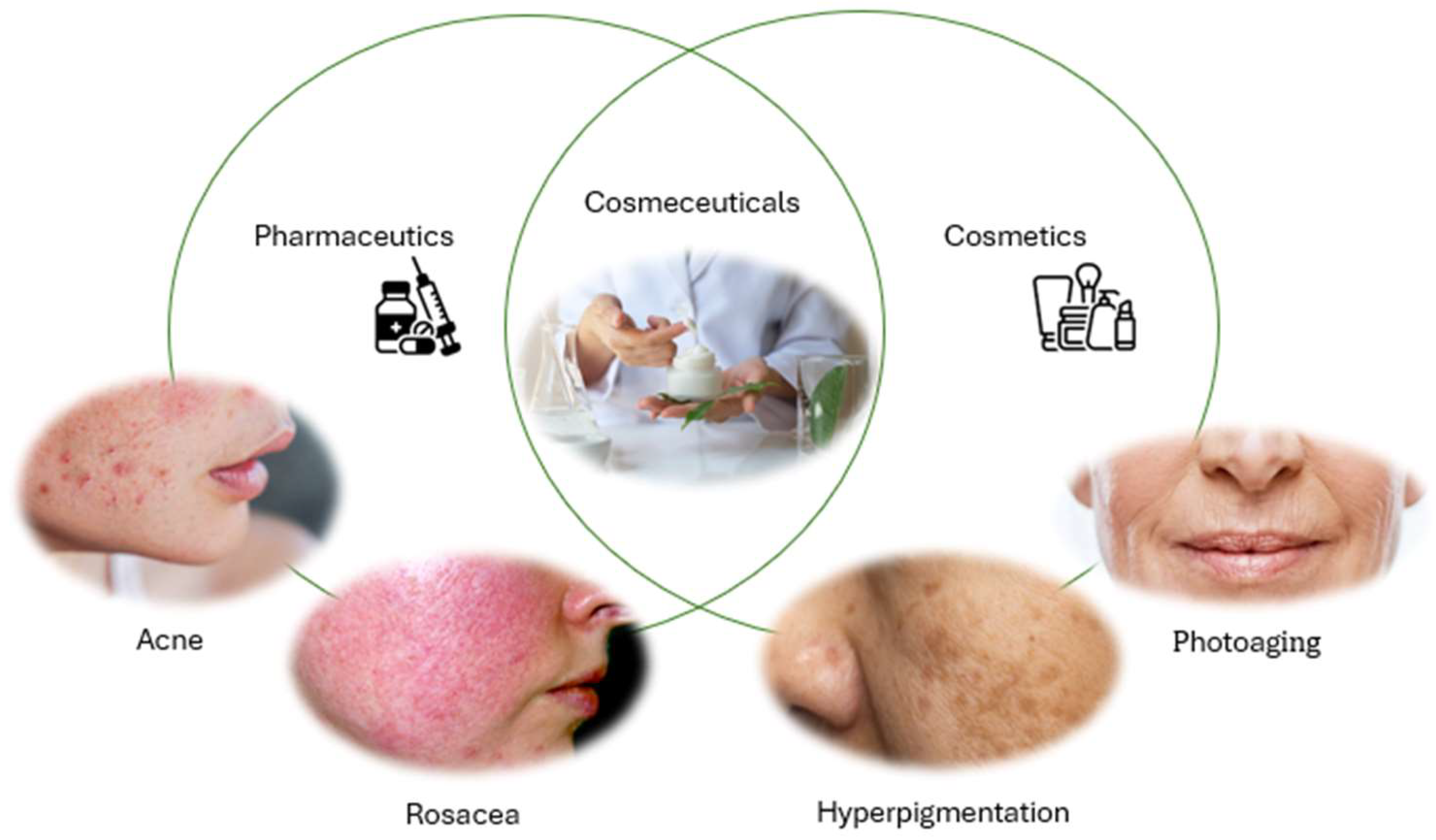
1. Introduction
2. Skin as a Biobarrier
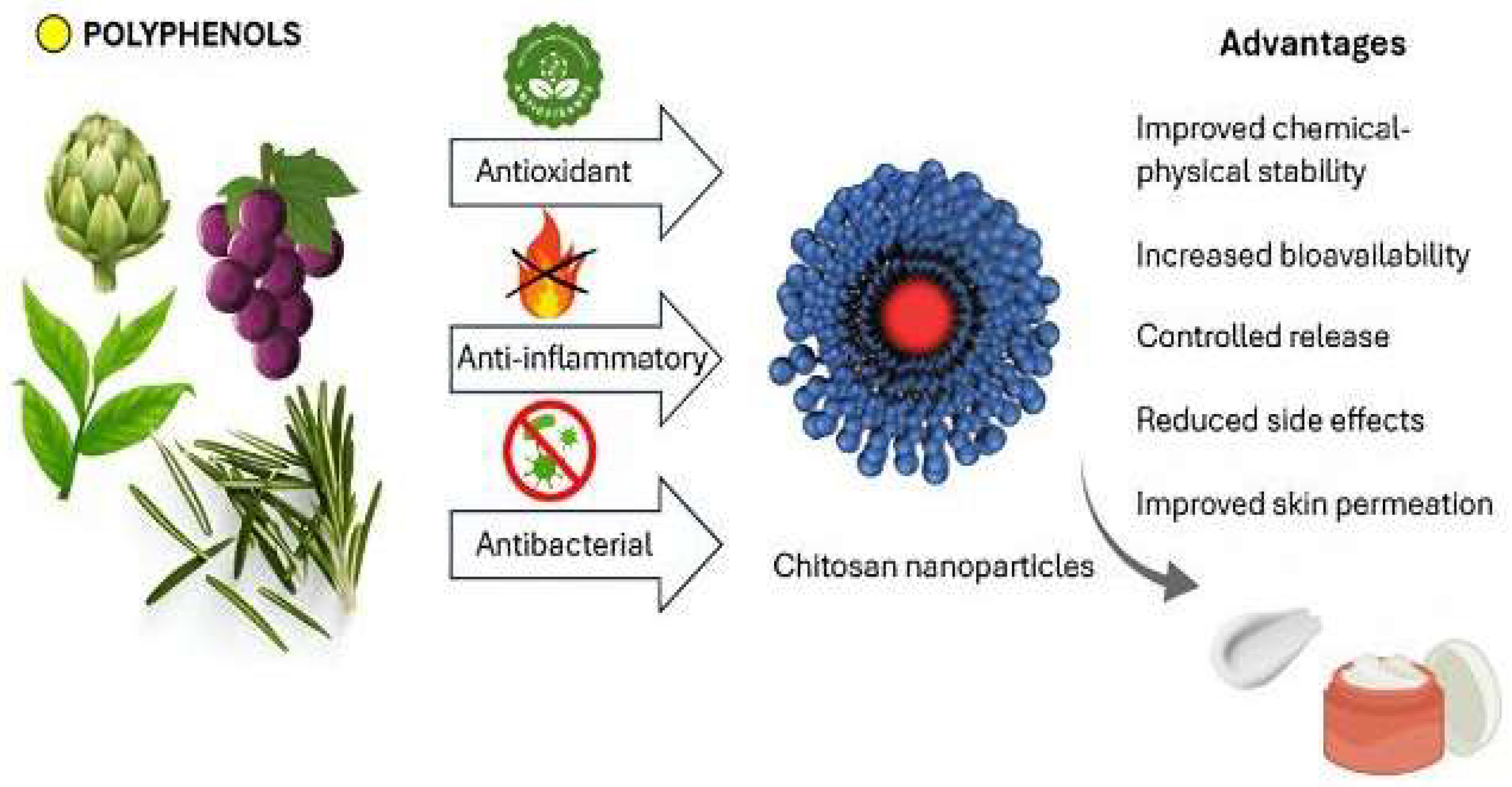
3. The Role of Polyphenols in Skin Health
3.1. Antioxidant Activity
3.2. Anti-Inflammatory Activity
3.3. Antimicrobial Activity
4. Limits of Polyphenols and Innovative Strategies of Administration
5. Chitosan in Cosmetics and Cosmeceuticals
6. Chitosan Nanoparticles as Carriers of Polyphenols for Topical Applications
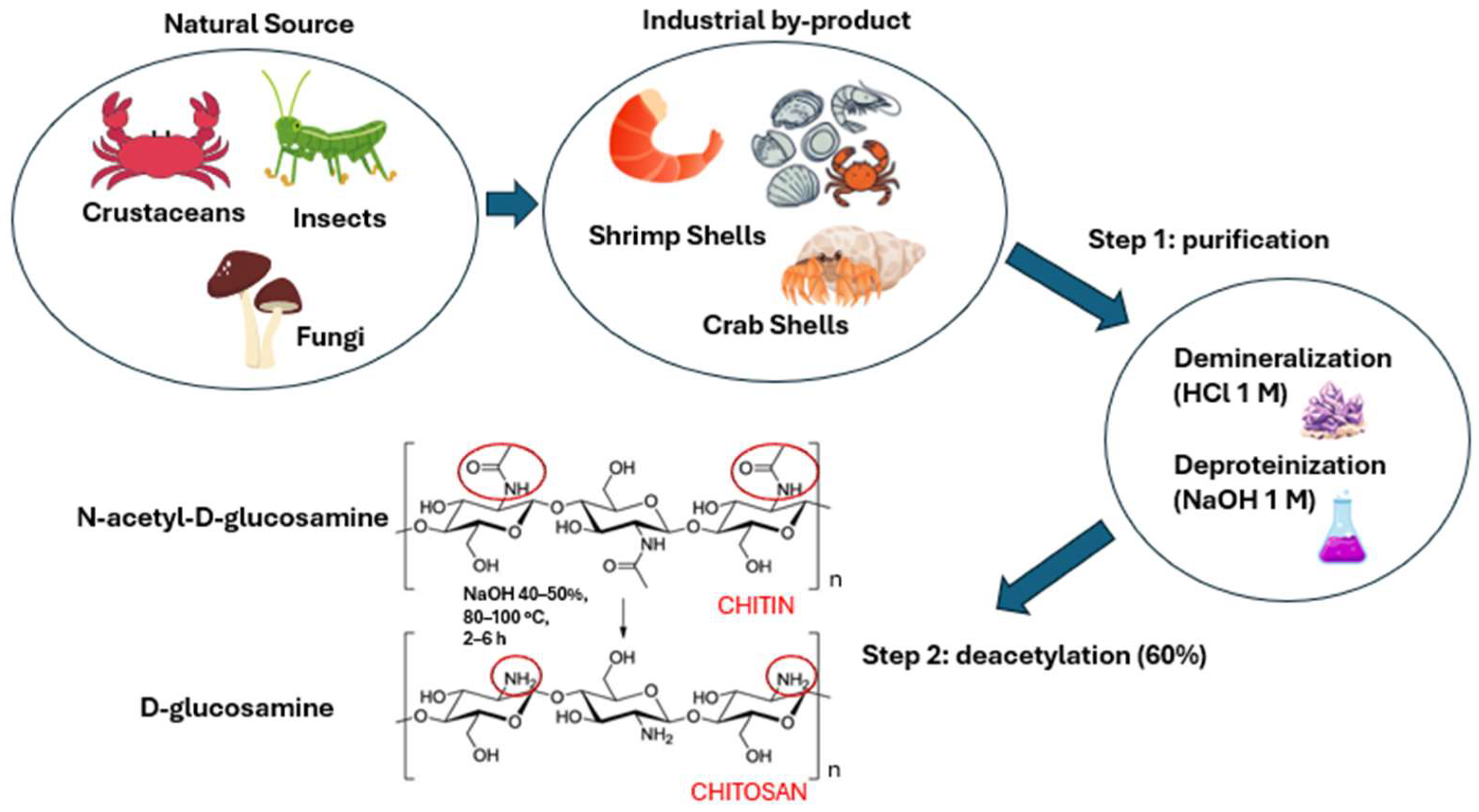
6.1. Physico-Chemical Properties of Chitosan
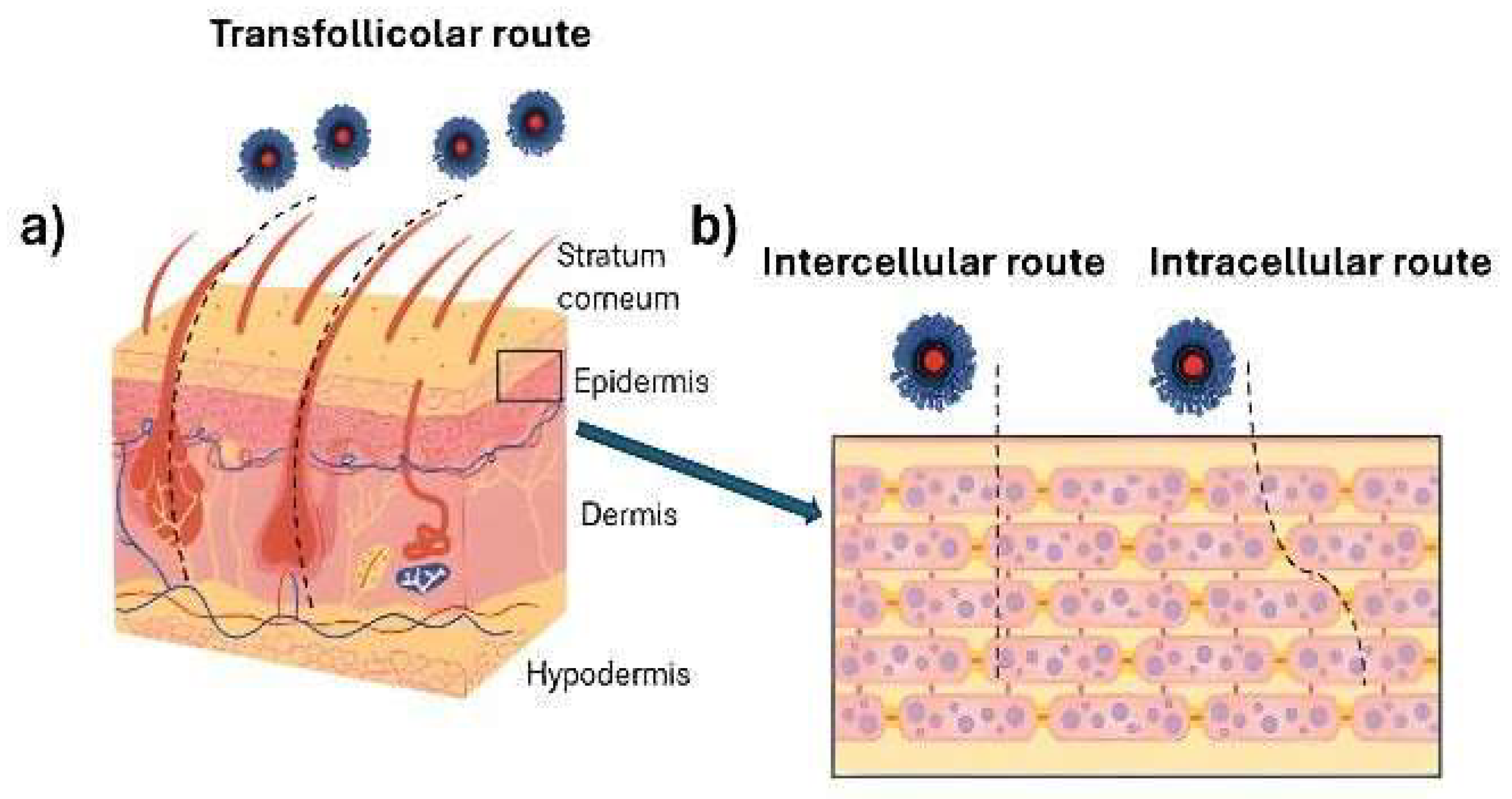
6.2. Enhancement of Bioavailability and Skin Permeability by Chitosan Nanoparticles
6.3. Preparation Methods of Chitosan Nanoparticles
6.4. Applications of Chitosan Nanoparticles for Specific Polyphenol Classes
6.4.1. Flavonoids
6.4.2. Phenolic Acids
6.4.3. Stilbenes
| Polyphenol | Composition and Characteristics | Biological Properties | Applications | Study Type | Results | Reference |
|---|---|---|---|---|---|---|
| Curcumin | Chitosan–TPP Mean diameter: ~30 to ~200 nm PdI: 0.129–0.536 ZP: / | Antioxidant and anti-inflammatory activity | Potential topical treatment for inflammatory skin conditions | Ex vivo/ in vitro | Enhanced skin penetration by transdermal pathways demonstrated via confocal microscopy; targeted follicular localization acting as drug reservoirs sustained drug release | [165] |
| Curcumin | Chitosan–TPP Mean diameter: 167.3 nm (1:3) to 251.5 nm (1:5) PdI: ~0.45 ZP: 18–20 mV | Antioxidant, anti-inflammatory, and antimicrobial activity | Cancer, psoriasis, hypertrophic scars, vitiligo, wound healing | In vitro | Uniform size, enhanced permeation, controlled release, good cell compatibility | [169] |
| Quercetin | Chitosan–lecithin Mean diameter: ~95 nm PdI: ~0.45 ZP: ~11 mV | Antioxidant and anti- inflammatory activity | Acne, rosacea | Ex vivo/ in vitro | Enhanced skin permeation and higher epidermal accumulation compared to the free quercetin solution. | [171] |
| Quercetin | Chitosan Mean diameter: ~184 nm PdI: / ZP: 37 mV | Antioxidant, UVB radiation damage protection | Skin aging, skin damage | In vitro/ in vivo | Reduced inflammation and oxidative stress, UVB protection | [174] |
| Epigallocatechin gallate (EGCG) | Chitosan Mean diameter: ~200 nm PdI: 0.13 ZP: ~38 mV | Antioxidant, anti-inflammatory, and antimicrobial activity | Psoriasis | In vitro/ in vivo | Anti-inflammatory effects, improved psoriatic symptoms | [180] |
| Green Tea Extract (GTE) | Chitosan Mean diameter: ~300 nm PdI: ~0.25 ZP: ~41 mV | Antioxidant, anti-inflammatory, and antimicrobial activity | Cellulitis | Ex vivo/ in vitro | Reduced adipocyte size and skin thickness; effective anti-cellulite action | [181] |
| Gallic acid/Rutin | Chitosan Mean diameter: 300–350 nm PdI: <1.0 ZP: / | Antioxidant, anti-inflammatory, and antimicrobial activity | Psoriasis | In vitro | Inhibition of hyperproliferation, oxidative stress, and inflammation | [187] |
| Ellagic acid | Chitosan-coated Niosomes Mean diameter: ~53 nm PdI: ~0.87 ZP: / | UVB radiation damage protection | Skin wrinkles and photoaging | In vitro | Anti-aging activity, protection against UV damage | [188] |
| Resveratrol | Chitosan Mean diameter: ~120 nm PdI: ~0.24 ZP: ~19 mV | Antioxidant and anti-inflammatory activity | Atopic dermatitis | In vitro | Decreased oxidative stress and inflammation, potential coadjuvant treatment | [189] |
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, M.; Deng, Y.; Cao, X.; Xiao, L.; Ding, Q.; Luo, F.; Huang, P.; Gao, Y.; Liu, M.; Zhao, H. Effects of Natural Polyphenols on Skin and Hair Health: A Review. Molecules 2022, 27, 7832. [Google Scholar] [CrossRef] [PubMed]
- Searle, T.; Al-Niaimi, F.; Ali, F.R. The Top 10 Cosmeceuticals for Facial Hyperpigmentation. Dermatol. Ther. 2020, 33, e14095. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Jatana, G.K.; Sonthalia, S. Cosmeceuticals; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Dubey, S.K.; Dey, A.; Singhvi, G.; Pandey, M.M.; Singh, V.; Kesharwani, P. Emerging Trends of Nanotechnology in Advanced Cosmetics. Colloids Surf. B Biointerfaces 2022, 214, 112440. [Google Scholar] [CrossRef]
- Baumann, L. How to Use Oral and Topical Cosmeceuticals to Prevent and Treat Skin Aging. Facial Plast. Surg. Clin. N. Am. 2018, 26, 407–413. [Google Scholar] [CrossRef]
- Liang, Y.; Su, W.; Wang, F. Skin Ageing: A Progressive, Multi-Factorial Condition Demanding an Integrated, Multilayer-Targeted Remedy. Clin. Cosmet. Investig. Dermatol. 2023, 16, 1215–1229. [Google Scholar] [CrossRef]
- Jesus, A.; Mota, S.; Torres, A.; Cruz, M.T.; Sousa, E.; Almeida, I.F.; Cidade, H. Antioxidants in Sunscreens: Which and What For? Antioxidants 2023, 12, 138. [Google Scholar] [CrossRef]
- Weihermann, A.C.; Lorencini, M.; Brohem, C.A.; de Carvalho, C.M. Elastin Structure and Its Involvement in Skin Photoageing. Int. J. Cosmet. Sci. 2017, 39, 241–247. [Google Scholar] [CrossRef]
- Gromkowska-Kępka, K.J.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Socha, K. The Impact of Ultraviolet Radiation on Skin Photoaging—Review of in Vitro Studies. J. Cosmet. Dermatol. 2021, 20, 3427–3431. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, C.; Xiang, L.F. Application of integrated skincare in medical aesthetics: A literature review. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Russell-Goldman, E.; Murphy, G.F. The Pathobiology of Skin Aging: New Insights into an Old Dilemma. Am. J. Pathol. 2020, 190, 1356–1369. [Google Scholar] [CrossRef]
- Makrantonaki, E.; Zouboulis, C.C. The Skin as a Mirror of the Aging Process in the Human Organism—State of the Art and Results of the Aging Research in the German National Genome Research Network 2 (NGFN-2). Exp. Gerontol. 2007, 42, 879–886. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.A.; Robinson, M.K.; Binder, R.L. Skin differences based on age and chronicity of ultraviolet exposure: Results from a gene expression profiling study. Br. J. Dermatol. 2012, 166, 9–15. [Google Scholar] [CrossRef]
- Zhou, H.; Luo, D.; Chen, D.; Tan, X.; Bai, X.; Liu, Z.; Yang, X.; Liu, W. Current Advances of Nanocarrier Technology-Based Active Cosmetic Ingredients for Beauty Applications. Clin. Cosmet. Investig. Dermatol. 2021, 14, 867–887. [Google Scholar] [CrossRef]
- Rakotondrabe, T.F.; Fan, M.X.; Muema, F.W.; Guo, M.Q. Modulating Inflammation-Mediated Diseases via Natural Phenolic Compounds Loaded in Nanocarrier Systems. Pharmaceutics 2023, 15, 699. [Google Scholar] [CrossRef]
- Fargnoli, M.C.; De Simone, C.; Gisondi, P.; Pellacani, G.; Calzavara-Pinton, P. Topical Treatment for the Management of Mild-to-Moderate Psoriasis: A Critical Appraisal of the Current Literature. Dermatol. Ther. 2023, 13, 2527–2547. [Google Scholar] [CrossRef] [PubMed]
- Althwanay, A.; AlEdani, E.M.; Kaur, H.; Kasapoglu, M.; Yadavalli, R.; Nawaz, S.; Nath, T.S. Efficacy of Topical Treatments in the Management of Mild-to-Moderate Acne Vulgaris: A Systematic Review. Cureus 2024, 16, e57909. [Google Scholar] [CrossRef]
- Jeskey, J.; Kurien, C.; Blunk, H.; Sehmi, K.; Areti, S.; Nguyen, D.; Hostoffer, R. Atopic Dermatitis: A Review of Diagnosis and Treatment. J. Pediatr. Pharmacol. Ther. 2024, 29, 587–603. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, M.; Alexander, A.; Singh, M.R.; Singh, D.; Saraf, S.; Saraf, S.; Ajazuddin. Understanding the Prospective of Nano-Formulations towards the Treatment of Psoriasis. Biomed. Pharmacother. 2018, 107, 447–463. [Google Scholar] [CrossRef]
- Souto, E.B.; Fernandes, A.R.; Martins-Gomes, C.; Coutinho, T.E.; Durazzo, A.; Lucarini, M.; Souto, S.B.; Silva, A.M.; Santini, A. Nanomaterials for Skin Delivery of Cosmeceuticals and Pharmaceuticals. Appl. Sci. 2020, 10, 1594. [Google Scholar] [CrossRef]
- Dhapte-Pawar, V.; Kadam, S.; Saptarsi, S.; Kenjale, P.P. Nanocosmeceuticals: Facets and Aspects. Future Sci. OA 2020, 6, FSO613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tsai, P.C.; Ramezanli, T.; Michniak-Kohn, B.B. Polymeric Nanoparticles-Based Topical Delivery Systems for the Treatment of Dermatological Diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 205–218. [Google Scholar] [CrossRef]
- Sachdeva, B.; Sachdeva, P.; Negi, A.; Ghosh, S.; Han, S.; Dewanjee, S.; Jha, S.K.; Bhaskar, R.; Sinha, J.K.; Paiva-Santos, A.C.; et al. Chitosan Nanoparticles-Based Cancer Drug Delivery: Application and Challenges. Mar. Drugs 2023, 21, 211. [Google Scholar] [CrossRef]
- Abd-Allah, H.; Youshia, J.; Abdel Jaleel, G.A.; Hassan, A.; El Madani, M.; Nasr, M. Gastroprotective Chitosan Nanoparticles Loaded with Oleuropein: An In Vivo Proof of Concept. Pharmaceutics 2024, 16, 153. [Google Scholar] [CrossRef] [PubMed]
- Elbehairi, S.E.I.; Ismail, L.A.; Alfaifi, M.Y.; Elshaarawy, R.F.M.; Hafez, S.H. Chitosan nano-vehicles as biocompatible delivering tools for a new Ag(I) curcuminoid-Gboxin analog complex in cancer and inflammation therapy. Int. J. Biol. Macromol. 2020, 165, 2750–2764. [Google Scholar] [CrossRef]
- Shati, A.A.; Alkabli, J.; Alfaifi, M.Y.; Elbehairi, S.E.I.; Elshaarawy, R.F.M.; Serag, W.M.; Hassan, Y.A. Comparison of the ameliorative roles of crab chitosan nanoparticles and mesenchymal stem cells against cisplatin-triggered nephrotoxicity. Int. J. Biol. Macromol. 2023, 242, 124985. [Google Scholar] [CrossRef]
- Ta, Q.; Ting, J.; Harwood, S.; Browning, N.; Simm, A.; Ross, K.; Olier, I.; Al-Kassas, R. Chitosan Nanoparticles for Enhancing Drugs and Cosmetic Components Penetration through the Skin. Eur. J. Pharm. Sci. 2021, 160, 105765. [Google Scholar] [CrossRef]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef]
- Asghar, B.H.; Hassan, R.K.A.; Barakat, L.A.A.; Alharbi, A.; El Behery, M.; Elshaarawy, R.F.M.; Hassan, Y.A. Cross-linked quaternized chitosan nanoparticles for effective delivery and controllable release of Olea europaea phenolic extract targeting cancer therapy. J. Drug Deliv. Sci. Technol. 2023, 83, 104388. [Google Scholar] [CrossRef]
- Hendawy, O.M.; Al-Sanea, M.M.; Elbargisy, R.M.; Rahman, H.U.; Mohamed, A.A.B.; Kamal, I.; Elshaarawy, R.F.M.; Khedr, A.I.M.; El-Fattah, W.A. Phenylboronic acid-grafted chitosan nanocapsules for effective delivery and controlled release of natural antioxidants: Olive oil and hydroxytyrosol. Pharmaceutics 2023, 15, 81. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, W.N.; Elshaarawy, R.F.M. Carboxymethyl chitosan polyelectrolyte nanoparticles loaded with Origanum syriacum oil: Box-Behnken design optimization of nanoencapsulation, physicochemical, and therapeutic properties. Polym. Bull. 2025, 82, 3321–3351. [Google Scholar] [CrossRef]
- Nasr, A.M.; Mortagi, Y.I.; Elwahab, N.H.A.; Alfaifi, M.Y.; Shati, A.A.; Elbehairi, S.E.I.; Elshaarawy, R.F.M.; Kamal, I. Enhancement of Transdermal Biomedical Capabilities of Thyme Essential Oil Nanoemulsions Using Amphiphilic Oligochitosan Carriers. Pharmaceutics 2022, 14, 1350. [Google Scholar] [CrossRef]
- Lankanayaka, A.; Lakshan, N.D.; Jayathunge, L.; Bandara, P.; Manatunga, D.C.; Senanayake, C.M. A review of sustainable strategies for encapsulating antioxidant-rich plant polyphenolic extracts using nanoemulsification to enhance the oxidative stability of edible oils. Discov. Food 2025, 5, 65. [Google Scholar] [CrossRef]
- Edo, G.I.; Yousif, E.; Al-Mashhadani, M.H. Chitosan: An overview of biological activities, derivatives, properties, and current advancements in biomedical applications. Carbohydr. Res. 2024, 542, 109199. [Google Scholar] [CrossRef]
- Nabil, Y.; Atta, A.H.; Abd El-Fattah, W.; Hafez, H.S.; Elshaarawy, R.F.M. Progress in chitosan/essential oil/ZnO nanobiocomposites fabrication for wound healing applications: A review. Int. J. Biol. Macromol. 2025, 318, 145123. [Google Scholar] [CrossRef] [PubMed]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; De la Caba, K. Chitosan as a Bioactive Polymer: Processing, Properties and Applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Heenatigala Palliyage, G.; Singh, S.; Ashby, C.R.; Tiwari, A.K.; Chauhan, H. Pharmaceutical Topical Delivery of Poorly Soluble Polyphenols: Potential Role in Prevention and Treatment of Melanoma. AAPS PharmSciTech 2019, 20, 250. [Google Scholar] [CrossRef] [PubMed]
- Di Santo, M.C.; D’ Antoni, C.L.; Domínguez Rubio, A.P.; Alaimo, A.; Pérez, O.E. Chitosan-tripolyphosphate nanoparticles designed to encapsulate polyphenolic compounds for biomedical and pharmaceutical applications A review. Biomed. Pharmacother. 2021, 142, 111970. [Google Scholar] [CrossRef]
- Charles, A.P.R.; Rajasekaran, B.; Awasti, N.; Choudhary, P.; Khanashyam, A.C.; Majumder, K.; Wu, Y.; Pandiselvam, R.; Jin, T.Z. Emerging chitosan systems incorporated with polyphenols: Their applications in intelligent packaging, active packaging, and nutraceutical systems–A comprehensive review. Int. J. Biol. Macromol. 2025, 308, 142714. [Google Scholar] [CrossRef]
- Patzelt, A.; Lademann, J. Recent advances in follicular drug delivery of nanoparticles. Expert Opin. Drug Deliv. 2020, 17, 49–60. [Google Scholar] [CrossRef]
- Blume-Peytavi, U.; Vogt, A. Human hair follicle: Reservoir function and selective targeting. Br. J. Dermatol. 2011, 165, 13–17. [Google Scholar] [CrossRef]
- Wosicka, H.; Cal, K. Targeting to the hair follicles: Current status and potential. J. Dermatol. Sci. 2010, 57, 83–89. [Google Scholar] [CrossRef]
- Svenskaya, Y.I.; Verkhovskii, R.A.; Zaytsev, S.M.; Lademann, J.; Genina, E.A. Current issues in optical monitoring of drug delivery via hair follicles. Adv. Drug Deliv. Rev. 2025, 217, 115477. [Google Scholar] [CrossRef]
- Matos, B.N.; Lima, A.L.; Cardoso, C.O.; Cunha-Filho, M.; Gratieri, T.; Gelfuso, G.M. Follicle-Targeted Delivery of Betamethasone and Minoxidil Co-Entrapped in Polymeric and Lipid Nanoparticles for Topical Alopecia Areata Treatment. Pharmaceuticals 2023, 16, 1322. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.; Lehr, C.M. Transfollicular delivery takes root: The future for vaccine design? Expert Rev. Vaccines 2014, 13, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Jain, A.; Hurkat, P.; Jain, S.K. Transfollicular drug delivery: Current perspectives. Res. Res. Rep. Transderm. Drug Deliv. 2016, 5, 1–17. [Google Scholar]
- Lademann, J.; Richter, H.; Teichmann, A.; Otberg, N.; Blume-Peytavi, U.; Luengo, J.; Weiß, B.; Schaefer, U.F.; Lehr, C.M.; Wepf, R.; et al. Nanoparticles—An Efficient Carrier for Drug Delivery into the Hair Follicles. Eur. J. Pharm. Biopharm. 2007, 66, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Cosco, D.; Celia, C.; Cilurzo, F.; Trapasso, E.; Paolino, D. Colloidal carriers for the enhanced delivery through the skin. Expert Opin. Drug Deliv. 2008, 5, 737–755. [Google Scholar] [CrossRef]
- Chen, X. Current and future technological advances in transdermal gene delivery. Adv. Drug Deliv. Rev. 2018, 127, 85–105. [Google Scholar] [CrossRef]
- Schneider, M.; Stracke, F.; Hansen, S.; Schaefer, U.F. Nanoparticles and their interactions with the dermal barrier. Dermatoendocrinology 2009, 1, 197–206. [Google Scholar] [CrossRef]
- Tiwari, N.; Osorio-Blanco, E.R.; Sonzogni, A.S.; Esporrín-Ubieto, D.; Wang, H.; Calderón, M. Nanocarriers for skin applications: Where do we stand? Angew. Chem. Int. Ed. 2022, 61, e202107960. [Google Scholar] [CrossRef]
- Raszewska-Famielec, M.; Flieger, J. Nanoparticles for Topical Application in the Treatment of Skin Dysfunctions-An Overview of Dermo-Cosmetic and Dermatological Products. Int. J. Mol. Sci. 2022, 23, 15980. [Google Scholar] [CrossRef]
- Mohammed, E.S.I.; Madkour, F.A.; Zayed, M.; Radey, R.; Ghallab, A.; Hassan, R. Comparative histological analysis of the skin for forensic investigation of some animal species. EXCLI J. 2022, 21, 1286–1298. [Google Scholar]
- Abd, E.; Yousef, S.A.; Pastore, M.N.; Telaprolu, K.; Mohammed, Y.H.; Namjoshi, S.; Grice, J.E.; Roberts, M.S. Skin Models for the Testing of Transdermal Drugs. Clin. Pharmacol. 2016, 8, 163–176. [Google Scholar] [CrossRef]
- Dzyhovskyi, V.; Romani, A.; Pula, W.; Bondi, A.; Ferrara, F.; Melloni, E.; Gonelli, A.; Pozza, E.; Voltan, R.; Sguizzato, M.; et al. Characterization Methods for Nanoparticle-Skin Interactions: An Overview. Life 2024, 14, 599. [Google Scholar] [CrossRef]
- Sathyaseelan, S.; Rao, B.H.; Anushmati, S. Cosmeceuticals: A Transit State from Synthetic to Natural. Indian J. Pharmacol. 2024, 56, 42–51. [Google Scholar] [CrossRef]
- Oliveira, A.L.S.; Valente, D.; Moreira, H.R.; Pintado, M.; Costa, P. Effect of Squalane-Based Emulsion on Polyphenols Skin Penetration: Ex Vivo Skin Study. Colloids Surf. B 2022, 218, 112779. [Google Scholar] [CrossRef]
- De Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; da Silva de Lucca, R.A. Polyphenols as Natural Antioxidants in Cosmetics Applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef]
- Gomez-Molina, M.; Albaladejo-Marico, L.; Yepes-Molina, L.; Nicolas-Espinosa, J.; Navarro-León, E.; Garcia-Ibañez, P.; Carvajal, M. Exploring Phenolic Compounds in Crop By-Products for Cosmetic Efficacy. Int. J. Mol. Sci. 2024, 25, 5884. [Google Scholar] [CrossRef]
- Rispo, F.; De Negri Atanasio, G.; Demori, I.; Costa, G.; Marchese, E.; Perera-del-Rosario, S.; Serrano-Candelas, E.; Palomino-Schätzlein, M.; Perata, E.; Robino, F.; et al. An Extensive Review on Phenolic Compounds and Their Potential Estrogenic Properties on Skin Physiology. Front. Cell Dev. Biol. 2023, 11, 362. [Google Scholar] [CrossRef]
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Cortina, J.L.; Saurina, J.; Granados, M. Recovery of Polyphenols from Agri-Food By-Products: The Olive Oil and Winery Industries Cases. Foods 2022, 11, 362. [Google Scholar] [CrossRef]
- Kołton, A.; Długosz-Grochowska, O.; Wojciechowska, R.; Czaja, M. Biosynthesis Regulation of Folates and Phenols in Plants. Sci. Hortic. 2022, 291, 110561. [Google Scholar] [CrossRef]
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Kazantseva, V.V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in Plants: Structure, Biosynthesis, Abiotic Stress Regulation, and Practical Applications. Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef]
- Bié, J.; Sepodes, B.; Fernandes, P.C.B.; Ribeiro, M.H.L. Polyphenols in Health and Disease: Gut Microbiota, Bioaccessibility, and Bioavailability. Compounds 2023, 3, 40–72. [Google Scholar] [CrossRef]
- Aljuffali, I.A.; Lin, C.H.; Yang, S.C.; Alalaiwe, A.; Fang, J.Y. Nanoencapsulation of Tea Catechins for Enhancing Skin Absorption and Therapeutic Efficacy. AAPS PharmSciTech 2022, 23, 187. [Google Scholar] [CrossRef]
- Jalali, O.; Best, M.; Wong, A.; Schaeffer, B.; Bauer, B.; Johnson, L. Protocatechuic Acid as a Topical Antimicrobial for Surgical Skin Antisepsis Preclinical Investigations. JBJS Open Access 2020, 5, e19.00079. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary Polyphenols, Oxidative Stress and Antioxidant and Anti-Inflammatory Effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Salazar, J.; Ortega, Á.; Pérez, J.L.; Garrido, B.; Santeliz, R.; Galbán, N.; Díaz, M.P.; Cano, R.; Cano, G.; Contreras-Velasquez, J.C.; et al. Role of Polyphenols in Dermatological Diseases: Exploring Pharmacotherapeutic Mechanisms and Clinical Implications. Pharmaceuticals 2025, 18, 247. [Google Scholar] [CrossRef]
- Ferreira, M.S.; Magalhães, M.C.; Oliveira, R.; Sousa-Lobo, J.M.; Almeida, I.F. Trends in the Use of Botanicals in Anti-Aging Cosmetics. Molecules 2021, 26, 3584. [Google Scholar] [CrossRef]
- Fernandes, A.; Rodrigues, P.M.; Pintado, M.; Tavaria, F.K. A Systematic Review of Natural Products for Skin Applications: Targeting Inflammation, Wound Healing, and Photo-Aging. Phytomedicine 2023, 115, 154824. [Google Scholar] [CrossRef]
- De Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet Light Induced Generation of Reactive Oxygen Species. Adv. Exp. Med. Biol. 2017, 996, 15–23. [Google Scholar]
- Gupta, A.; Singh, A.P.; Singh, V.K.; Singh, P.R.; Jaiswal, J.; Kumari, N.; Upadhye, V.; Singh, S.C.; Sinha, R.P. Natural Sun-Screening Compounds and DNA-Repair Enzymes: Photoprotection and Photoaging. Catalysts 2023, 13, 745. [Google Scholar] [CrossRef]
- Farhan, M. The Promising Role of Polyphenols in Skin Disorders. Molecules 2024, 29, 865. [Google Scholar] [CrossRef]
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as active ingredients for cosmetic products. Int. J. Cosmet. Sci. 2015, 37, 455–464. [Google Scholar] [CrossRef]
- Marko, M.; Pawliczak, R. Resveratrol and Its Derivatives in Inflammatory Skin Disorders—Atopic Dermatitis and Psoriasis: A Review. Antioxidants 2023, 12, 1954. [Google Scholar] [CrossRef]
- Mascarenhas-Melo, F.; Araújo, A.R.T.S.; Rodrigues, M.; Mathur, A.; Gonçalves, M.B.S.; Tanwar, K.; Heidarizadeh, F.; Nejaddehbashi, F.; Rahdar, A.; Mazzola, P.G.; et al. Dermatological Bioactivities of Resveratrol and Nanotechnology Strategies to Boost Its Efficacy—An Updated Review. Cosmetics 2023, 10, 68. [Google Scholar] [CrossRef]
- Lin, M.H.; Hung, C.F.; Sung, H.C.; Yang, S.C.; Yu, H.P.; Fang, J.Y. The Bioactivities of Resveratrol and Its Naturally Occurring Derivatives on Skin. J. Food Drug Anal. 2021, 29, 15–38. [Google Scholar] [CrossRef]
- Ratz-Łyko, A.; Arct, J. Resveratrol as an Active Ingredient for Cosmetic and Dermatological Applications: A Review. J. Cosmet. Laser Ther. 2019, 21, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Gu, S.; Cheng, Y. Resveratrol inhibits adventitial fibroblast proliferation and induces cell apoptosis through the SIRT1 pathway. Mol. Med. Rep. 2017, 15, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Farris, P.; Yatskayer, M.; Chen, N.; Krol, Y.; Oresajo, C. Evaluation of efficacy and tolerance of a nighttime topical antioxidant containing resveratrol, baicalin, and vitamin e for treatment of mild to moderately photodamaged skin. J. Drugs Dermatol. 2014, 13, 1467–1472. [Google Scholar] [PubMed]
- Hecker, A.; Schellnegger, M.; Hofmann, E.; Luze, H.; Nischwitz, S.P.; Kamolz, L.P.; Kotzbeck, P. The Impact of Resveratrol on Skin Wound Healing, Scarring, and Aging. Int. Wound J. 2022, 19, 9–28. [Google Scholar] [CrossRef]
- Igielska-Kalwat, J.; Firlej, M.; Lewandowska, A.; Biedziak, B. In Vivo Studies of Resveratrol Contained in Cosmetic Emulsions. Acta Biochim. Pol. 2019, 66, 371–374. [Google Scholar] [CrossRef]
- Punzo, A.; Porru, E.; Silla, A.; Simoni, P.; Galletti, P.; Roda, A.; Tagliavini, E.; Samorì, C.; Caliceti, C. Grape Pomace for Topical Application: Green Nades Sustainable Extraction, Skin Permeation Studies, Antioxidant and Anti-Inflammatory Activities Characterization in 3D Human Keratinocytes. Biomolecules 2021, 11, 1181. [Google Scholar] [CrossRef]
- Alves-Silva, J.M.; Zuzarte, M.; Salgueiro, L.; Cocco, E.; Ghiani, V.; Falconieri, D.; Maccioni, D.; Maxia, A. Agroprospecting of Biowastes: Globe Artichoke (Cynara scolymus L. Cultivar Tema, Asteraceae) as Potential Source of Bioactive Compounds. Molecules 2024, 29, 3960. [Google Scholar] [CrossRef] [PubMed]
- El Assar, M.; Angulo, J.; Rodríguez-Mañas, L. Oxidative Stress and Vascular Inflammation in Aging. Free Radic. Biol. Med. 2013, 65, 380–401. [Google Scholar] [CrossRef] [PubMed]
- Feiden, T.; Valduga, E.; Zeni, J.; Steffens, J. Bioactive Compounds from Artichoke and Application Potential. Food Technol. Biotechnol. 2023, 61, 312–327. [Google Scholar] [CrossRef] [PubMed]
- D’Antuono, I.; Carola, A.; Sena, L.M.; Linsalata, V.; Cardinali, A.; Logrieco, A.F.; Colucci, M.G.; Apone, F. Artichoke Polyphenols Produce Skin Anti-Age Effects by Improving Endothelial Cell Integrity and Functionality. Molecules 2018, 23, 2729. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic Composition, Antioxidant Potential and Health Benefits of Citrus Peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef]
- Zheng, X.Q.; Zhang, X.H.; Gao, H.Q.; Huang, L.Y.; Ye, J.J.; Ye, J.H.; Lu, J.L.; Ma, S.C.; Liang, Y.R. Green Tea Catechins and Skin Health. Antioxidants 2024, 13, 1506. [Google Scholar] [CrossRef]
- Frasheri, L.; Schielein, M.C.; Tizek, L.; Mikschl, P.; Biedermann, T.; Zink, A. Great Green Tea Ingredient? A Narrative Literature Review on Epigallocatechin Gallate and Its Biophysical Properties for Topical Use in Dermatology. Phytother. Res. 2020, 34, 2170–2179. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, W.; Chen, J.; Fu, R.; Lin, X.; Zhou, S.; Wang, L. Investigating the Inhibition of Xanthine Oxidase by Five Catechins: Kinetic Studies, Spectroscopy, Molecular Docking, and Dynamics Simulations. Int. J. Biol. Macromol. 2024, 281, 136231. [Google Scholar] [CrossRef]
- Bae, J.; Kim, N.; Shin, Y.; Kim, S.-Y.; Kim, Y.-J. Activity of Catechins and Their Applications. Biomed. Dermatol. 2020, 4, 8. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Y.; Ruan, X.; Zhang, T.; Zi, M.; Zhang, Q. Photoprotective Effects of Epigallocatechin Gallate on Ultraviolet-Induced Zebrafish and Human Skin Fibroblasts Cells. Mediat. Inflamm. 2024, 24, 7887678. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Wu, Y.; Wei, H.C.; Xu, Y.Y.; Jia, L.L.; Chen, J.; Yang, X.S.; Dong, G.H.; Gao, X.H.; Chen, H.D. Protective Effects of Green Tea Extracts on Photoaging and Photommunosuppression. Skin. Res. 2009, 15, 338–345. [Google Scholar] [CrossRef]
- Di Salvo, E.; Gangemi, S.; Genovese, C.; Cicero, N.; Casciaro, M. Polyphenols from Mediterranean Plants: Biological Activities for Skin Photoprotection in Atopic Dermatitis, Psoriasis, and Chronic Urticaria. Plants 2023, 12, 3579. [Google Scholar] [CrossRef]
- Hong, Y.-H.; Jung, E.Y.; Shin, K.-S.; Yu, K.-W.; Chang, U.J.; Hyung, S.J. Tannase-Converted Green Tea Catechins and Their Anti-Wrinkle Activity in Humans. J. Cosmet. Dermatol. 2013, 12, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.Z.; Desai, R.M.; Olsen, R.; Davis, M. The Pathophysiology, Diagnosis and Management of Chronic Inflammatory Skin Diseases. Discov. Med. 2024, 36, 1933. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Chen, S.C.; Hsu, S.Y.; Lin, Y.A.; Shih, C.M.; Huang, C.Y.; Wang, K.H.; Lee, A.W. Annoying Psoriasis and Atopic Dermatitis: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 4898. [Google Scholar] [CrossRef]
- Harvey, J.; Lax, S.J.; Lowe, A.; Santer, M.; Lawton, S.; Langan, S.M.; Roberts, A.; Stuart, B.; Williams, H.C.; Thomas, K.S. The long-term safety of topical corticosteroids in atopic dermatitis: A systematic review. Skin. Health Dis. 2023, 3, e268. [Google Scholar] [CrossRef]
- Yoshizaki, N.; Fujii, T.; Masaki, H.; Okubo, T.; Shimada, K.; Hashizume, R. Orange Peel Extract, Containing High Levels of Polymethoxyflavonoid, Suppressed UVB-Induced COX-2 Expression and PGE2 Production in HaCaT Cells through PPAR-γ Activation. Exp. Dermatol. 2014, 23, 18–22. [Google Scholar] [CrossRef]
- Choi, K.S.; Kundu, J.K.; Chun, K.S.; Na, H.K.; Surh, Y.J. Rutin Inhibits UVB Radiation-Induced Expression of COX-2 and INOS in Hairless Mouse Skin: P38 MAP Kinase and JNK as Potential Targets. Arch. Biochem. Biophys. 2014, 559, 38–45. [Google Scholar] [CrossRef]
- Menicacci, B.; Cipriani, C.; Margheri, F.; Mocali, A.; Giovannelli, L. Modulation of the Senescence-Associated Inflammatory Phenotype in Human Fibroblasts by Olive Phenols. Int. J. Mol. Sci. 2017, 18, 2275. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Tsatalas, P.; Galanakis, I.M. Implementation of Phenols Recovered from Olive Mill Wastewater as UV Booster in Cosmetics. Ind. Crops Prod. 2018, 111, 30–37. [Google Scholar] [CrossRef]
- Nisticò, S.P.; Bottoni, U.; Gliozzi, M.; Ehrlich, J.; Fini, M. Bergamot Polyphenolic Fraction Counteracts Photoageing in Human Keratinocytes. PharmaNutrition 2016, 4, S32–S34. [Google Scholar] [CrossRef]
- Csekes, E.; Račková, L. Skin Aging, Cellular Senescence and Natural Polyphenols. Int. J. Mol. Sci. 2021, 22, 12641. [Google Scholar] [CrossRef]
- Veenstra, J.P.; Vemu, B.; Tocmo, R.; Nauman, M.C.; Johnson, J.J. Pharmacokinetic Analysis of Carnosic Acid and Carnosol in Standardized Rosemary Extract and the Effect on the Disease Activity Index of Dss-Induced Colitis. Nutrients 2021, 13, 773. [Google Scholar] [CrossRef]
- Guedes, B.N.; Krambeck, K.; Durazzo, A.; Lucarini, M.; Santini, A.; Oliveira, M.B.P.P.; Fathi, F.; Souto, E.B. Natural Antibiotics against Antimicrobial Resistance: Sources and Bioinspired Delivery Systems. Braz. J. Microbiol. 2024, 55, 2753–2766. [Google Scholar] [CrossRef]
- Luo, J.; Luo, J.; Sheng, Z.; Fang, Z.; Fu, Y.; Wang, N.; Yang, B.; Xu, B. Latest Research Progress on Anti-Microbial Effects, Mechanisms of Action, and Product Developments of Dietary Flavonoids: A Systematic Literature Review. Trends Food Sci. Technol. 2025, 156, 104839. [Google Scholar] [CrossRef]
- Fik-Jaskółka, M.; Mittova, V.; Motsonelidze, C.; Vakhania, M.; Vicidomini, C.; Roviello, G.N. Antimicrobial Metabolites of Caucasian Medicinal Plants as Alternatives to Antibiotics. Antibiotics 2024, 13, 487. [Google Scholar] [CrossRef]
- Biharee, A.; Sharma, A.; Kumar, A.; Jaitak, V. Antimicrobial Flavonoids as a Potential Substitute for Overcoming Antimicrobial Resistance. Fitoterapia 2020, 146, 104720. [Google Scholar] [CrossRef]
- McGillick, B.E.; Kumaran, D.; Vieni, C.; Swaminathan, S. β-Hydroxyacyl-Acyl Carrier Protein Dehydratase (FabZ) from Francisella Tularensis and Yersinia Pestis: Structure Determination, Enzymatic Characterization, and Cross-Inhibition Studies. Biochemistry 2016, 55, 1091–1099. [Google Scholar] [CrossRef]
- Shevelev, A.B.; La Porta, N.; Isakova, E.P.; Martens, S.; Biryukova, Y.K.; Belous, A.S.; Sivokhin, D.A.; Trubnikova, E.V.; Zylkova, M.V.; Belyakova, A.V.; et al. In Vivo Antimicrobial and Wound-Healing Activity of Resveratrol, Dihydroquercetin, and Dihydromyricetin against Staphylococcus Aureus, Pseudomonas Aeruginosa, and Candida Albicans. Pathogens 2020, 9, 296. [Google Scholar] [CrossRef]
- Lobiuc, A.; Pavăl, N.E.; Mangalagiu, I.I.; Gheorghiță, R.; Teliban, G.C.; Amăriucăi-Mantu, D.; Stoleru, V. Future Antimicrobials: Natural and Functionalized Phenolics. Molecules 2023, 28, 1114. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, F.; He, L. Skin Barrier Dysfunction in Acne Vulgaris: Pathogenesis and Therapeutic Approaches. Med. Sci. Monit. 2024, 30, e945336. [Google Scholar] [CrossRef]
- Proença, A.C.; Luís, Â.; Duarte, A.P. The Role of Herbal Medicine in the Treatment of Acne Vulgaris: A Systematic Review of Clinical Trials. Evid.-Based Complement. Altern. Med. 2022, 15, 2011945. [Google Scholar] [CrossRef]
- Koch, W.; Zagórska, J.; Marzec, Z.; Kukula-Koch, W. Applications of Tea (Camellia Sinensis) and Its Active Constituents in Cosmetics. Molecules 2019, 24, 4277. [Google Scholar] [CrossRef]
- Mahmood, T.; Akhtar, A. Comparison of the Effects of Topical Green Tea and Lotus on Facial Sebum Control in Healthy. Humans 2013, 17, 64–67. [Google Scholar]
- Butkeviciute, A.; Ramanauskiene, K.; Kurapkiene, V.; Janulis, V. Dermal Penetration Studies of Potential Phenolic Compounds Ex Vivo and Their Antioxidant Activity In Vitro. Plants 2022, 11, 1901. [Google Scholar] [CrossRef]
- Aguiar, B.; Carmo, H.; Garrido, J.; Sousa Lobo, J.M.; Almeida, I.F. In Vitro Evaluation of the Photoreactivity and Phototoxicity of Natural Polyphenol Antioxidants. Molecules 2022, 27, 189. [Google Scholar] [CrossRef]
- Shivgotra, R.; Soni, B.; Kaur, P.; Sharma, A.; Singh, V.; Partap, N.; Bakrey, H.; Jain, S.K. An Updated Perspective on Skin Disease Therapy: From Conventional Methods to Nanocarrier Innovations. AAPS PharmSciTech 2025, 26, 209. [Google Scholar] [CrossRef]
- Yang, B.; Dong, Y.; Wang, F.; Zhang, Y. Nanoformulations to Enhance the Bioavailability and Physiological Functions of Polyphenols. Molecules 2020, 25, 4613. [Google Scholar] [CrossRef]
- Szumała, P.; Macierzanka, A. Topical Delivery of Pharmaceutical and Cosmetic Macromolecules Using Microemulsion Systems. Int. J. Pharm. 2022, 615, 121488. [Google Scholar] [CrossRef]
- Gagliardi, A.; Giuliano, E.; Venkateswararao, E.; Fresta, M.; Bulotta, S.; Awasthi, V.; Cosco, D. Biodegradable Polymeric Nanoparticles for Drug Delivery to Solid Tumors. Front. Pharmacol. 2021, 12, 601626. [Google Scholar] [CrossRef]
- Thandapani, G.; Supriya Prasad, P.; Sudha, P.N.; Sukumaran, A. Size Optimization and in Vitro Biocompatibility Studies of Chitosan Nanoparticles. Int. J. Biol. Macromol. 2017, 104, 1794–1806. [Google Scholar] [CrossRef]
- Raina, N.; Rani, R.; Thakur, V.K.; Gupta, M. New Insights in Topical Drug Delivery for Skin Disorders: From a Nanotechnological Perspective. ACS Omega 2023, 8, 19145–19167. [Google Scholar] [CrossRef]
- Qi, C.; Liu, G.; Ping, Y.; Yang, K.; Tan, Q.; Zhang, Y.; Chen, G.; Huang, X.; Xu, D. A Comprehensive Review of Nano-Delivery System for Tea Polyphenols: Construction, Applications, and Challenges. Food Chem. X 2023, 17, 100571. [Google Scholar] [CrossRef]
- Lewicka, K.; Smola-Dmochowska, A.; Dobrzyński, P.; Śmigiel-Gac, N.; Jelonek, K.; Musiał-Kulik, M.; Rychter, P. Microspheres Based on Blends of Chitosan Derivatives with Carrageenan as Vitamin Carriers in Cosmeceuticals. Polymers 2024, 16, 1815. [Google Scholar] [CrossRef]
- Bektas, N.; Şenel, B.; Yenilmez, E.; Özatik, O.; Arslan, R. Evaluation of Wound Healing Effect of Chitosan-Based Gel Formulation Containing Vitexin. Saudi Pharm. J. 2020, 28, 87–94. [Google Scholar] [CrossRef]
- Baek, J.; Ramasamy, M.; Willis, N.C.; Kim, D.S.; Anderson, W.A.; Tam, K.C. Encapsulation and Controlled Release of Vitamin C in Modified Cellulose Nanocrystal/Chitosan Nanocapsules. Curr. Res. Food Sci. 2021, 4, 215–223. [Google Scholar] [CrossRef]
- Kulka, K.; Sionkowska, A. Chitosan Based Materials in Cosmetic Applications: A Review. Molecules 2023, 28, 1817. [Google Scholar] [CrossRef]
- Afonso, C.R.; Hirano, R.S.; Gaspar, A.L.; Chagas, E.G.L.; Carvalho, R.A.; Silva, F.V.; Leonardi, G.R.; Lopes, P.S.; Silva, C.F.; Yoshida, C.M.P. Biodegradable Antioxidant Chitosan Films Useful as an Anti-Aging Skin Mask. Int. J. Biol. Macromol. 2019, 132, 1262–1273. [Google Scholar] [CrossRef]
- Raza, Z.A.; Khalil, S.; Ayub, A.; Banat, I.M. Recent Developments in Chitosan Encapsulation of Various Active Ingredients for Multifunctional Applications. Carbohydr. Res. 2020, 492, 108004. [Google Scholar] [CrossRef]
- Cosco, D.; Federico, C.; Maiuolo, J.; Bulotta, S.; Molinaro, R.; Paolino, D.; Tassone, P.; Fresta, M. Physicochemical Features and Transfection Properties of Chitosan/Poloxamer 188/Poly(D,L-Lactide-Co-Glycolide) Nanoplexes. Int. J. Nanomed. 2014, 9, 2359–2372. [Google Scholar] [CrossRef]
- Iannone, M.; Mare, R.; Paolino, D.; Gagliardi, A.; Froiio, F.; Cosco, D.; Fresta, M. Characterization and in Vitro Anticancer Properties of Chitosan-Microencapsulated Flavan-3-Ols-Rich Grape Seed Extracts. Int. J. Biol. Macromol. 2017, 104, 1039–1045. [Google Scholar] [CrossRef]
- Cheng, G.; Zhang, X.; Zhang, H.; Feng, Z.; Cai, J.; Li, J.; Du, L.; Liu, K. Gentiopicrin-Loaded Chitosan Nanoparticles as a Topical Agent for the Treatment of Psoriasis. Nanomaterials 2024, 14, 610. [Google Scholar] [CrossRef]
- Frigaard, J.; Jensen, J.L.; Galtung, H.K.; Hiorth, M. The Potential of Chitosan in Nanomedicine: An Overview of the Cytotoxicity of Chitosan Based Nanoparticles. Front. Pharmacol. 2022, 13, 880377. [Google Scholar] [CrossRef]
- Crini, G.; Grégorio, C. Historical review on chitin and chitosan biopolymers. Environ. Chem. Lett. 2019, 17, 123–152. [Google Scholar] [CrossRef]
- Mathaba, M.; Daramola, M.O. Effect of Chitosan’s Degree of Deacetylation on the Performance of PES Membrane Infused with Chitosan during AMD Treatment. Membranes 2020, 10, 52. [Google Scholar] [CrossRef]
- Berezina, N. Production and application of chitin. Phyl. Sci. Rev. 2016, 1, 20160048. [Google Scholar]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A Versatile Semi-Synthetic Polymer in Biomedical Applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Yuan, Y.; Chesnutt, B.M.; Haggard, W.O.; Bumgardner, J.D. Deacetylation of Chitosan: Material Characterization and in vitro Evaluation via Albumin Adsorption and Pre-Osteoblastic Cell Cultures. Materials 2011, 4, 1399–1416. [Google Scholar] [CrossRef]
- Desai, N.; Rana, D.; Salave, S.; Gupta, R.; Patel, P.; Karunakaran, B.; Sharma, A.; Giri, J.; Benival, D.; Kommineni, N. Chitosan: A Potential Biopolymer in Drug Delivery and Biomedical Applications. Pharmaceutics 2023, 15, 1313. [Google Scholar] [CrossRef]
- Geng, F.; Zhang, M.; Sun, T.; Xie, J.; Gan, J.; Li, X.; Xue, B. Effect of Molecular Weight of Chitosan on Quercetin-Loaded Chitosan Nanoparticles. J. Sci. Food Agric. 2024, 104, 9531–9539. [Google Scholar] [CrossRef]
- Azmana, M.; Mahmood, S.; Hilles, A.R.; Rahman, A.; Arifin, M.A.B.; Ahmed, S. A Review on Chitosan and Chitosan-Based Bionanocomposites: Promising Material for Combatting Global Issues and Its Applications. Int. J. Biol. Macromol. 2021, 185, 832–848. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Lei, Y.; Jiang, W.; Peng, C.; Wu, D.; Wu, J.; Xu, Y.; Yan, H.; Xia, X. Advances in polymeric nano-delivery systems targeting hair follicles for the treatment of acne. Drug Deliv. 2024, 31, 2372269. [Google Scholar] [CrossRef]
- Ali, S.M.; Yosipovitch, G. Skin pH: From basic science to basic skin care. Acta Derm. Venereol. 2013, 93, 261–267. [Google Scholar] [CrossRef]
- Lakshmi, A.S. Chitosan nanoparticles in topical drug delivery systems for skin: A review of enhanced penetration and bioavailability. Int. J. Pharm. Res. Dev. 2025, 7, 100–110. [Google Scholar] [CrossRef]
- Sheir, M.F.; Abdel-Moneim, A.A.; El-Sayed, M.A. Repaglinide–Solid Lipid Nanoparticles in Chitosan Patches: Enhanced Transdermal Delivery via Modulation of Tight Junctions and Lipid Barrier. Int. J. Nanomed. 2024, 19, 209–225. [Google Scholar]
- Ma, J.; Wang, Y.; Lu, R. Mechanism and Application of Chitosan and Its Derivatives in Promoting Permeation in Transdermal Drug Delivery Systems: A Review. Pharmaceuticals 2022, 15, 459. [Google Scholar] [CrossRef]
- Sahudin, S.; Sahrum Ayumi, N.; Kaharudin, N. Enhancement of Skin Permeation and Penetration of β-Arbutin Fabricated in Chitosan Nanoparticles as the Delivery System. Cosmetics 2022, 9, 114. [Google Scholar] [CrossRef]
- Aydın, T.R.S.; Pulat, M. 5-Fluorouracil encapsulated chitosan nanoparticles for pH-stimulated drug delivery: Evaluation of controlled release kinetics. J. Nanomater. 2012, 2012, 313961. [Google Scholar] [CrossRef]
- Sangnim, T.; Dheer, D.; Jangra, N.; Huanbutta, K.; Puri, V.; Sharma, A. Chitosan in oral drug delivery formulations: A review. Pharmaceutics 2023, 15, 2361. [Google Scholar] [CrossRef]
- Santana Gomes, A.; Silva Simplicio, S.; Marinheiro da Cunha Gonsalves, J.K. Chitosan Nanoparticles as a Potential Drug Delivery System in the Skin: A Systematic Review Based on In Vivo Studies. ChemistrySelect 2024, 9, e202402058. [Google Scholar] [CrossRef]
- Nagpal, K.; Singh, S.K.; Mishra, D.N. Chitosan nanoparticles: A promising system in novel drug delivery. Chem. Pharm. Bull. 2010, 58, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Desai, K.G. Chitosan Nanoparticles Prepared by Ionotropic Gelation: An Overview of Recent Advances. Crit. Rev. Ther. Drug Carrier Syst. 2016, 33, 107–158. [Google Scholar] [CrossRef] [PubMed]
- Mirnejad, R.; Mofazzal Jahromi, M.A.; Al-Musawi, S.; Pirestani, M.; Fasihi Ramandi, M.; Ahmadi, K.; Rajayi, H.; Mohammad Hassan, Z.; Kamali, M. Curcumin-loaded Chitosan Tripolyphosphate Nanoparticles as a safe, natural and effective antibiotic inhibits the infection of Staphylococcus aureus and Pseudomonas aeruginosa in vivo. Iran. J. Biotechnol. 2014, 12, 1–8. [Google Scholar]
- Bavel, V.N.; Issler, T.; Pang, L.; Anikovskiy, M.; Prenner, E.J. A Simple Method for Synthesis of Chitosan Nanoparticles with Ionic Gelation and Homogenization. Molecules 2023, 28, 4328. [Google Scholar] [CrossRef]
- Jung, E.; Hui, X.; Zhu, H.; Zhang, A.; Wang, W.; Buchholz, B.; Maibach, H. Effect of iron and silica nanoparticles’ size on in vitro human skin binding and penetration. Toxicol. Res. Appl. 2019, 3, 2397847319893054. [Google Scholar] [CrossRef]
- Wu, D.; Zhu, L.; Li, Y.; Zhang, X.; Ma, H. Chitosan-based colloidal polyelectrolyte complexes for drug delivery: A review. Carbohydr. Polym. 2020, 238, 116126. [Google Scholar] [CrossRef]
- Jha, R.; Mayanovic, R.A. A Review of the Preparation, Characterization, and Applications of Chitosan Nanoparticles in Nanomedicine. Nanomaterials 2023, 13, 1302. [Google Scholar] [CrossRef]
- Sethi, A.; Ahmad, M.; Huma, T.; Ahmad, W. Pharmacokinetic variables of medium molecular weight cross linked chitosan nanoparticles to enhance the bioavailability of 5-fluorouracil and reduce the acute oral toxicity. Drug Deliv. 2021, 28, 1569–1584. [Google Scholar] [CrossRef]
- Plucinski, A.; Lyu, Z.; Schmidt, B. Polysaccharide nanoparticles: From fabrication to applications. J. Mater. Chem. B 2021, 9, 7030–7062. [Google Scholar] [CrossRef]
- Abdel-Hafez, S.M.; Hathout, R.M.; Sammour, O.A. Tracking the Transdermal Penetration Pathways of Optimized Curcumin-Loaded Chitosan Nanoparticles via Confocal Laser Scanning Microscopy. Int. J. Biol. Macromol. 2018, 108, 753–764. [Google Scholar] [CrossRef]
- Pelikh, O.; Eckert, R.W.; Pinnapireddy, S.R.; Keck, C.M. Hair Follicle Targeting with Curcumin Nanocrystals: Influence of the Formulation Properties on the Penetration Efficacy. J. Control. Release 2021, 329, 598–613. [Google Scholar] [CrossRef]
- Di Lorenzo, R.; Forgione, F.; Bernardi, A.; Sacchi, A.; Laneri, S.; Greco, G. Clinical Studies on Topical Curcumin. Skin. Pharmacol. Physiol. 2024, 36, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Rezaii, M.; Oryan, S.; Javeri, A. Curcumin nanoparticles incorporated collagen-chitosan scaffold promotes cutaneous wound healing through regulation of TGF-β1/Smad7 gene expression. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.S.; Morris, A.; Billa, N.; Leong, C.O. An Evaluation of Curcumin-Encapsulated Chitosan Nanoparticles for Transdermal Delivery. AAPS PharmSciTech 2019, 20, 69. [Google Scholar] [CrossRef]
- Niu, J.; Yuan, M.; Gao, P.; Wang, L.; Qi, Y.; Chen, J.; Bai, K.; Fan, Y.; Liu, X. Microemulsion-Based Keratin–Chitosan Gel for Improvement of Skin Permeation/Retention and Activity of Curcumin. Gels 2023, 9, 587. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Liu, W.; Guo, C.; Zhai, G. Preparation and Evaluation of Quercetin-Loaded Lecithin-Chitosan Nanoparticles for Topical Delivery. Int. J. Nanomed. 2011, 6, 1621–1630. [Google Scholar]
- Şenyiǧit, T.; Sonvico, F.; Barbieri, S.; Özer, Ö.; Santi, P.; Colombo, P. Lecithin/Chitosan Nanoparticles of Clobetasol-17-Propionate Capable of Accumulation in Pig Skin. J. Control. Release 2010, 142, 368–373. [Google Scholar] [CrossRef]
- Suwannateep, N.; Wanichwecharungruang, S.; Haag, S.F.; Devahastin, S.; Groth, N.; Fluhr, J.W.; Lademann, J.; Meinke, M.C. Encapsulated curcumin results in prolonged curcumin activity in vitro and radical scavenging activity ex vivo on skin after UVB-irradiation. Eur. J. Pharm. Biopharm. 2012, 82, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Nan, W.; Ding, L.; Chen, H.; Khan, F.U.; Yu, L.; Sui, X.; Shi, X. Topical Use of Quercetin-Loaded Chitosan Nanoparticles against Ultraviolet B Radiation. Front. Pharmacol. 2018, 9, 826. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant Flavonoids: Classification, Distribution, Biosynthesis, and Antioxidant Activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Wisuitiprot, W.; Somsiri, A.; Ingkaninan, K.; Waranuch, N. In Vitro Human Skin Permeation and Cutaneous Metabolism of Catechins from Green Tea Extract and Green Tea Extract-Loaded Chitosan Microparticles. Int. J. Cosmet. Sci. 2011, 33, 572–579. [Google Scholar] [CrossRef]
- Mehta, G.; Hsiao, A.Y.; Ingram, M.; Luker, G.D.; Takayama, S. Opportunities and Challenges for Use of Tumor Spheroids as Models to Test Drug Delivery and Efficacy. J. Control. Release 2012, 164, 192–204. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, W.; Wang, Z.; Lu, J. Lipid-Based Nanotechnology: Liposome. Pharmaceutics 2024, 16, 34. [Google Scholar] [CrossRef]
- Fereig, S.A.; El-Zaafarany, G.M.; Arafa, M.G.; Abdel-Mottaleb, M.M.A. Tackling the Various Classes of Nano-Therapeutics Employed in Topical Therapy of Psoriasis. Drug Deliv. 2020, 27, 662–680. [Google Scholar] [CrossRef]
- Chamcheu, J.C.; Siddiqui, I.A.; Adhami, V.M.; Esnault, S.; Bharali, D.J.; Babatunde, A.S.; Adame, S.; Massey, R.J.; Wood, G.S.; Longley, B.J.; et al. Chitosan-Based Nanoformulated (-)-Epigallocatechin-3-Gallate (EGCG) Modulates Human Keratinocyte-Induced Responses and Alleviates Imiquimod-Induced Murine Psoriasiform Dermatitis. Int. J. Nanomed. 2018, 13, 4189–4206. [Google Scholar] [CrossRef]
- Elmowafy, M.; Shalaby, K.; Elkomy, M.H.; Alsaidan, O.A.; Gomaa, H.A.M.; Abdelgawad, M.A.; Mostafa, E.M. Polymeric Nanoparticles for Delivery of Natural Bioactive Agents: Recent Advances and Challenges. Polymers 2023, 15, 1123. [Google Scholar] [CrossRef] [PubMed]
- Abosabaa, S.A.; Arafa, M.G.; ElMeshad, A.N. Hybrid Chitosan-Lipid Nanoparticles of Green Tea Extract as Natural Anti-Cellulite Agent with Superior in Vivo Potency: Full Synthesis and Analysis. Drug Deliv. 2021, 28, 2160–2176. [Google Scholar] [CrossRef]
- Khan, M.M.; Madni, A.; Torchilin, V.; Filipczak, N.; Pan, J.; Tahir, N.; Shah, H. Lipid-Chitosan Hybrid Nanoparticles for Controlled Delivery of Cisplatin. Drug Deliv. 2019, 26, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Valanciene, E.; Jonuskiene, I.; Syrpas, M.; Augustiniene, E.; Matulis, P.; Simonavicius, A.; Malys, N. Advances and Prospects of Phenolic Acids Production, Biorefinery and Analysis. Biomolecules 2020, 10, 874. [Google Scholar] [CrossRef]
- Chaikul, P.; Khat-domkiri, N.; Iangthanarat, K.; Manosroi, J.; Manosroi, A. Characteristics and in Vitro Anti-Skin Aging Activity of Gallic Acid Loaded in Cationic CTAB Niosome. Eur. J. Pharm. Sci. 2019, 131, 39–49. [Google Scholar] [CrossRef]
- Shandil, A.; Yadav, M.; Sharma, N.; Nagpal, K.; Jindal, D.K.; Deep, A.; Kumar, S. Targeting Keratinocyte Hyperproliferation, Inflammation, Oxidative Species and Microbial Infection by Biological Macromolecule-Based Chitosan Nanoparticle-Mediated Gallic Acid–Rutin Combination for the Treatment of Psoriasis. Polym. Bull. 2020, 77, 4713–4738. [Google Scholar] [CrossRef]
- Abd-Elghany, A.A.; Mohamad, E.A. Chitosan-Coated Niosomes Loaded with Ellagic Acid Present Antiaging Activity in a Skin Cell Line. ACS Omega 2023, 8, 16620–16629. [Google Scholar] [CrossRef]
- Conte, R.; De Luca, I.; Valentino, A.; Cerruti, P.; Pedram, P.; Cabrera-Barjas, G.; Moeini, A.; Calarco, A. Hyaluronic Acid Hydrogel Containing Resveratrol-Loaded Chitosan Nanoparticles as an Adjuvant in Atopic Dermatitis Treatment. J. Funct. Biomater. 2023, 14, 82. [Google Scholar] [CrossRef]
- Sarma, S.; Agarwal, S.; Bhuyan, P.; Hazarika, J.; Ganguly, M. Resveratrol-Loaded Chitosan-Pectin Core-Shell Nanoparticles as Novel Drug Delivery Vehicle for Sustained Release and Improved Antioxidant Activities. R. Soc. Open Sci. 2022, 9, 210784. [Google Scholar] [CrossRef]
- Moeini, A.; Cimmino, A.; Dal Poggetto, G.; Di Biase, M.; Evidente, A.; Masi, M.; Lavermicocca, P.; Valerio, F.; Leone, A.; Santagata, G.; et al. Effect of PH and TPP Concentration on Chemico-Physical Properties, Release Kinetics and Antifungal Activity of Chitosan-TPP-Ungeremine Microbeads. Carbohydr. Polym. 2018, 195, 631–641. [Google Scholar] [CrossRef]
- Hoang, H.T.; Moon, J.Y.; Lee, Y.C. Natural Antioxidants from Plant Extracts in Skincare Cosmetics: Recent Applications, Challenges and Perspectives. Cosmetics 2021, 8, 106. [Google Scholar] [CrossRef]
- Alves, P.L.M.; Nieri, V.; de Campos Moreli, F.; Constantino, E.; de Souza, J.; Oshima-Franco, Y.; Grotto, D. Unveiling New Horizons: Advancing Technologies in Cosmeceuticals for Anti-Aging Solutions. Molecules 2024, 29, 4890. [Google Scholar] [CrossRef] [PubMed]
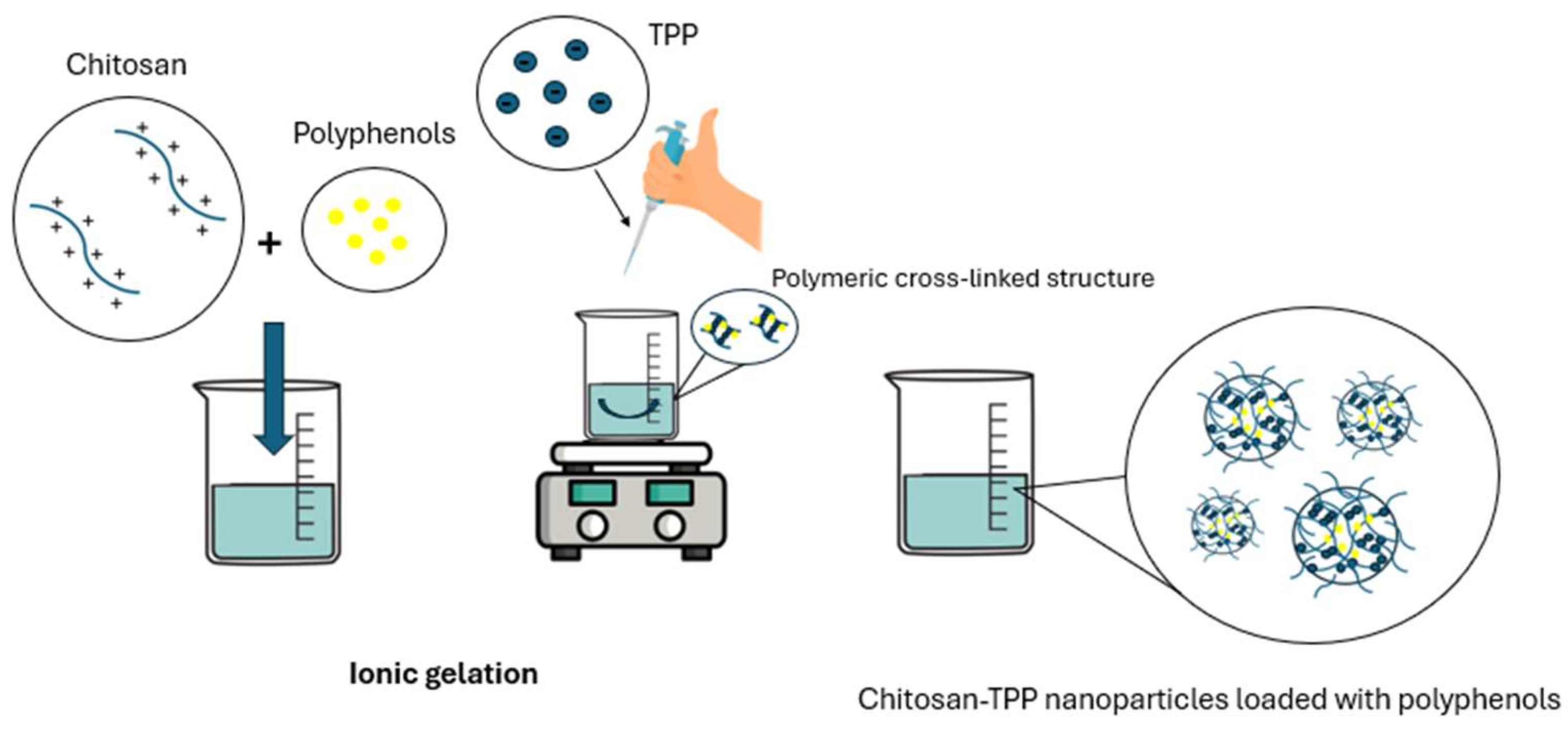
| Polyphenol Class | Subclasses | Description | Common Examples | Main Properties |
|---|---|---|---|---|
| Flavonoids | Flavonols | Flavonoids with a 3-hydroxyflavone structure | Quercetin, curcumin | Antioxidant, anti-inflammatory, UV protection, antimicrobial activity |
| Flavones | Flavonoids lacking the 3-hydroxy group | Apigenin, luteolin | Antioxidant, anti-inflammatory, antimicrobial activity | |
| Flavanones | Saturated flavonoids | Naringenin, hesperetin | Antioxidant, anti-inflammatory | |
| Flavanols (Catechins) | Flavonoids with hydroxyl groups | Epigallocatechin gallate (EGCG) | Antioxidant, antimicrobial | |
| Anthocyanidins | Pigmented flavonoids | Cyanidin | Antioxidant, pigment properties | |
| Isoflavones | Flavonoids with B-ring attached at position 3 | Genistein | Phytoestrogenic, antioxidant | |
| Phenolic Acids | Hydroxybenzoic acids | Phenolic acids with a C6–C1 structure | Gallic acid, | Antioxidant, anti-inflammatory |
| Hydroxycinnamic acids | Phenolic acids with a C6–C3 structure | Ferulic acid, caffeic acid | Antioxidant, anti-inflammatory | |
| Stilbenes | Compounds with two aromatic rings linked by ethylene bridge | Resveratrol | Antioxidant, anti-inflammatory, cardioprotective | |
| Lignans | Dimers of phenylpropanoids | Secoisolariciresinol | Antioxidant, estrogen-like effects |
| Method | Principle | Advantages | Disadvantage | Cosmetic/Pharmaceutical Applications | References |
|---|---|---|---|---|---|
| Ionic gelation | Crosslinking with polyanionic agents (e.g., TPP) | Simple, fast, no organic solvents, room temperature | Limited stability if not optimized, particle size > 100 nm | Encapsulation of polyphenols and essential oils (anti-aging, antioxidant) | [156,157] |
| Polyelectrolyte complexation | Electrostatic complex formation between oppositely charged polymers without organic solvents | Free of organic solvents | Less reliable particle size and stability control depending on materials | Usable but with limitations on quality control | [161] |
| Emulsification and solvent evaporation | Formation of emulsions with organic solvents, followed by solvent evaporation to obtain nanoparticles | Production of nanoparticles | Use of organic solvents and surfactants that may be irritating or toxic; less precise size control | Less suitable for delicate applications such as cosmetics | [156] |
| Microemulsion | Formation of thermodynamically stable emulsions containing organic solvents to produce very small nanoparticles | Very small nanoparticles (<100 nm) | Use of organic solvents; longer processing times | Produces very small nanoparticles but less practical for cosmetics | [156,162] |
| Chemical crosslinking | Chemical crosslinking of chitosan using agents like glutaraldehyde | Stable nanoparticles | Use of agents such as glutaraldehyde that may leave undesirable residues; compromises biological properties | Unsuitable for cosmetic use | [163] |
| Complex coacervation | Phase separation of a polymer-rich phase in aqueous environment at low temperature | Aqueous environment at low temperature | Sensitive to pH variations, causing destabilization or ineffective encapsulation | Limited by the need to maintain pH compatible with skin | [164] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaetano, V.; Gagliardi, A.; Giuliano, E.; Longo, E.; Cosco, D. Chitosan Nanoparticles Loaded with Polyphenols for Cosmeceutical Applications: A State-of-the-Art Review. Pharmaceutics 2025, 17, 1068. https://doi.org/10.3390/pharmaceutics17081068
Gaetano V, Gagliardi A, Giuliano E, Longo E, Cosco D. Chitosan Nanoparticles Loaded with Polyphenols for Cosmeceutical Applications: A State-of-the-Art Review. Pharmaceutics. 2025; 17(8):1068. https://doi.org/10.3390/pharmaceutics17081068
Chicago/Turabian StyleGaetano, Valeria, Agnese Gagliardi, Elena Giuliano, Emanuela Longo, and Donato Cosco. 2025. "Chitosan Nanoparticles Loaded with Polyphenols for Cosmeceutical Applications: A State-of-the-Art Review" Pharmaceutics 17, no. 8: 1068. https://doi.org/10.3390/pharmaceutics17081068
APA StyleGaetano, V., Gagliardi, A., Giuliano, E., Longo, E., & Cosco, D. (2025). Chitosan Nanoparticles Loaded with Polyphenols for Cosmeceutical Applications: A State-of-the-Art Review. Pharmaceutics, 17(8), 1068. https://doi.org/10.3390/pharmaceutics17081068










