Triple-Loaded Nanoemulsions Incorporating Coffee Extract for the Photoprotection of Curcumin and Capsaicin: Experimental and Computational Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemical and Reagents
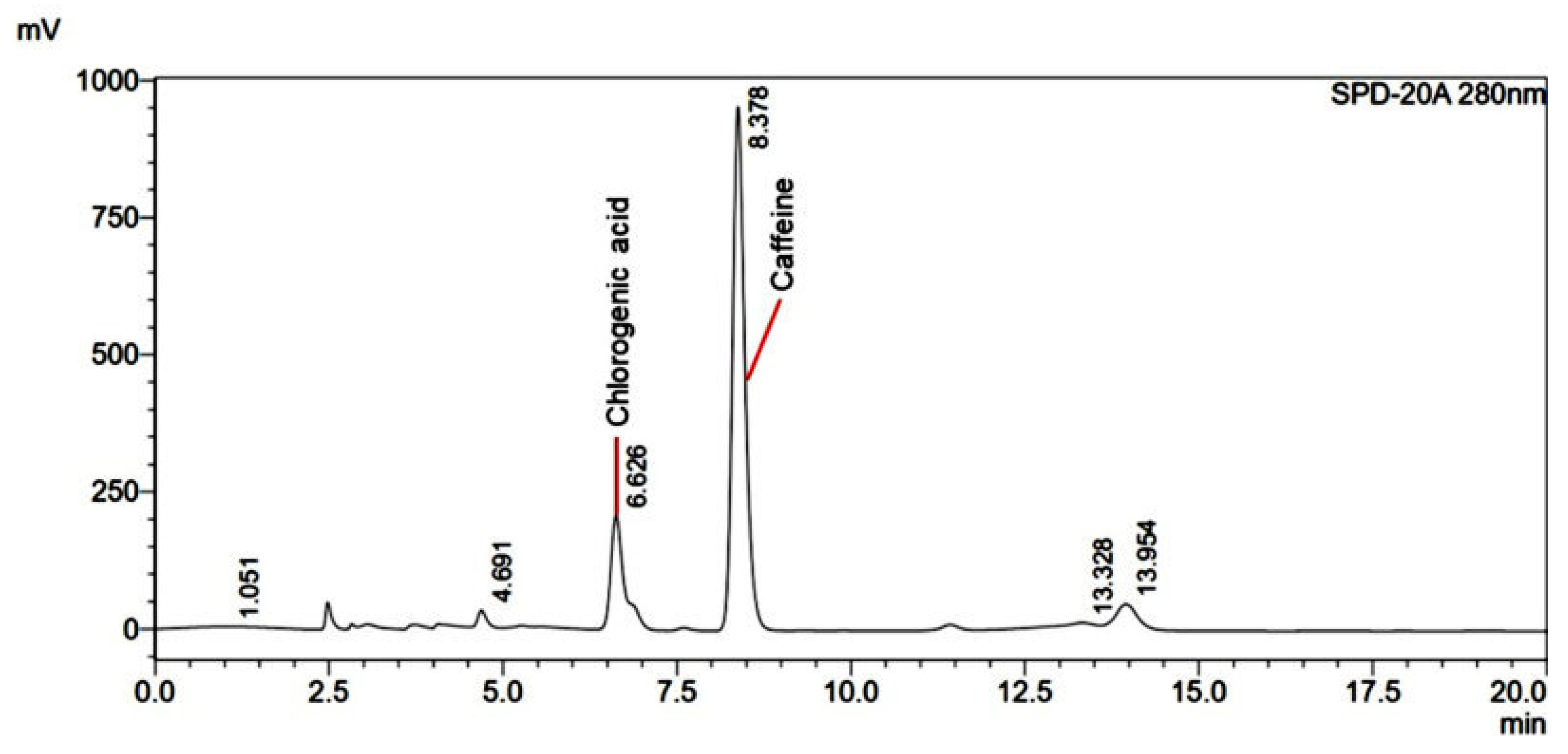
2.3. Identification of Active Compound in Coffee Extract
2.4. Mixtures of Extract Solutions for Photo- and Antioxidative Stability Tests
2.5. Preparation of Extract-Loaded Nanoemulsions
2.5.1. Solubility Study of the Extracts in Various Vehicles
2.5.2. Development of Turmeric, Chili, and Coffee Extract-Loaded Nanoemulsions
2.5.3. Characteristics and Stability of Extract-Loaded Nanoemulsions
2.5.4. Determination of Percentage Entrapment Efficiency
2.6. Determination of Major Markers in Turmeric, Chili, and Coffee Extracts
Chromatographic System
2.7. Photostability Evaluation
2.7.1. Chemical Stability of Active Markers in the Extract Mixtures
2.7.2. Chemical Stability of Active Markers in Nanoemulsion
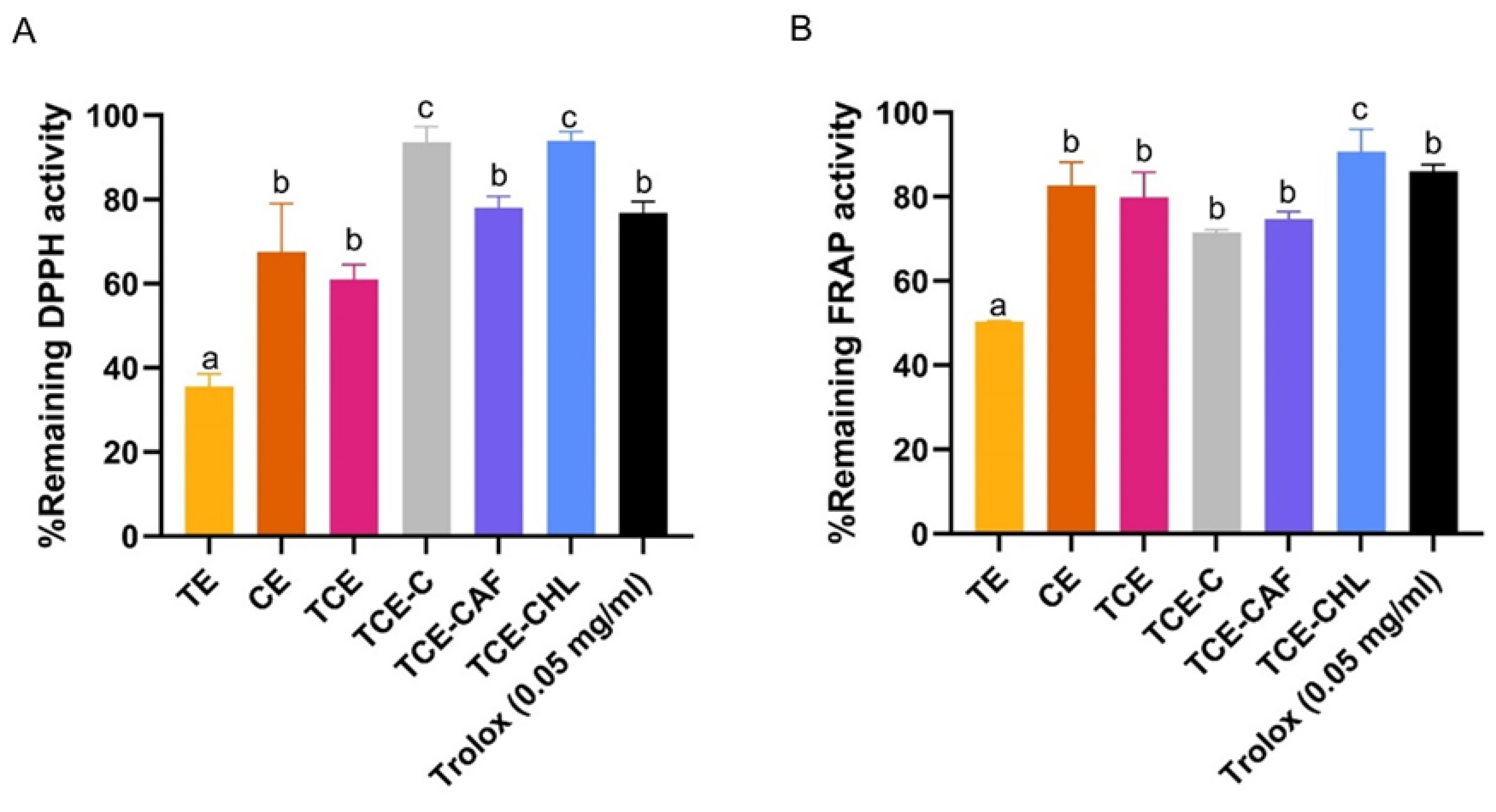
2.7.3. Antioxidative Stability of the Extract Mixture
DPPH Assay
FRAP Assay
2.8. Density Functional Theory (DFT) Calculation
2.8.1. Interaction Energy (Eint)
2.8.2. HOMO-LUMO Energy Gap (HLG) Analysis
2.8.3. Global Reactive Descriptors
2.9. Statistical Analysis
3. Results and Discussion
3.1. RP-HPLC Method Validation for Determination of Active Markers
3.2. Nanoemulsion Formulation
3.2.1. Solubility Study of the Extracts in Various Vehicles
3.2.2. Preparation of Extract-Loaded Nanoemulsions
3.2.3. Entrapment Efficiency of Extract-Loaded Nanoemulsions
3.3. Effects of Antioxidants on Photostability of Curcumin and Capsaicin in the Extract Mixture
3.3.1. Photostability on Turmeric and Chili Extract Solutions and Nanoemulsions
3.3.2. Photostabilizing Efficiency of Natural Stabilizers
3.4. Quantification of Active Compounds in Crude Coffee Extract
3.5. Photostabilizing Efficiency of Coffee Extracts and Their Isolated Components
3.6. Chemical and Structural Features Influencing the Stability of Curcumin and Capsaicin
3.6.1. Interaction Energy (Eint)
3.6.2. HOMO-LUMO Energy Gap (HLG) Analysis and Global Reactive Descriptors
3.6.3. The Proposed Interaction of Compounds in Roasted Arabica Coffee Bean Extract with Capsaicin and Curcumin
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HPLC | High-performance liquid chromatography |
| FRAP | Ferric reducing antioxidant power |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| T1/2 | Half-lives |
| Eint | Interaction energy |
| HLG | HOMO-LUMO energy gap |
| NLC | Nanostructure lipid carriers |
| DFT | Density functional theory |
References
- Fuloria, S.; Mehta, J.; Chandel, A.; Sekar, M.; Rani, N.N.I.M.; Begum, M.Y.; Subramaniyan, V.; Chidambaram, K.; Thangavelu, L.; Nordin, R. A comprehensive review on the therapeutic potential of Curcuma longa Linn. in relation to its major active constituent curcumin. Front. Pharmacol. 2022, 13, 820806. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Chen, C.; Lan, Y.; Xiao, J.; Li, R.; Huang, J.; Huang, Q.; Cao, Y.; Ho, C.T. Capsaicin—The major bioactive ingredient of chili peppers: Bio-efficacy and delivery systems. Food Funct. 2020, 11, 2848–2860. [Google Scholar] [CrossRef] [PubMed]
- Bieng, M.A.N.; Barrey, S.; Bertheau, Y.; Boy, L.; Coutellec, L.; Doussan, I.; Mambrini-Doudet, M.; Mariojouls, C.; Pucheu, E.; Schmid, A.F. Presentation of DOGMATIS, an inter and multidisciplinary programme for the assessment of the impacts of Genetically Modified Fish, and results about risk of fortuitous import on French market. In Proceedings of the 15th Biennial Conference of the International Institute for Fisheries Economics and Trade (IIFET), Montpellier, France, 13–16 July 2010; p. 365. [Google Scholar]
- Hernández-Pérez, T.; Gómez-García, M.d.R.; Valverde, M.E.; Paredes-López, O. Capsicum annuum (hot pepper): An ancient Latin-American crop with outstanding bioactive compounds and nutraceutical potential. A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2972–2993. [Google Scholar] [CrossRef] [PubMed]
- Koop, B.L.; da Silva, M.N.; da Silva, F.D.; dos Santos Lima, K.T.; Soares, L.S.; de Andrade, C.J.; Valencia, G.A.; Monteiro, A.R. Flavonoids, anthocyanins, betalains, curcumin, and carotenoids: Sources, classification and enhanced stabilization by encapsulation and adsorption. Food Res. Int. 2022, 153, 110929. [Google Scholar] [CrossRef]
- Jobin, C.; Bradham, C.A.; Russo, M.P.; Juma, B.; Narula, A.S.; Brenner, D.A.; Sartor, R.B. Curcumin blocks cytokine-mediated NF-κB activation and proinflammatory gene expression by inhibiting inhibitory factor I-κB kinase activity. J. Immunol. 1999, 163, 3474–3483. [Google Scholar] [CrossRef]
- Kim, C.-S.; Kawada, T.; Kim, B.-S.; Han, I.-S.; Choe, S.-Y.; Kurata, T.; Yu, R. Capsaicin exhibits anti-inflammatory property by inhibiting IκB-α degradation in LPS-stimulated peritoneal macrophages. Cell. Signal. 2003, 15, 299–306. [Google Scholar] [CrossRef]
- Handler, N.; Jaeger, W.; Puschacher, H.; Leisser, K.; Erker, T. Synthesis of novel curcumin analogues and their evaluation as selective cyclooxygenase-1 (COX-1) inhibitors. Chem. Pharm. Bull. 2007, 55, 64–71. [Google Scholar] [CrossRef]
- Suresh, K.; Nangia, A. Curcumin: Pharmaceutical solids as a platform to improve solubility and bioavailability. CrystEngComm. 2018, 20, 3277–3296. [Google Scholar] [CrossRef]
- Feng, Y.; Zhu, Y.; Wan, J.; Yang, X.; Firempong, C.K.; Yu, J.; Xu, X. Enhanced oral bioavailability, reduced irritation and increased hypolipidemic activity of self-assembled capsaicin prodrug nanoparticles. J. Funct. Foods 2018, 44, 137–145. [Google Scholar] [CrossRef]
- Wang, Y.J.; Pan, M.H.; Cheng, A.L.; Lin, L.I.; Ho, Y.S.; Hsieh, C.Y.; Lin, J.K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Gordon, O.N.; Luis, P.B.; Sintim, H.O.; Schneider, C. Unraveling curcumin degradation: Autoxidation proceeds through spiroepoxide and vinylether intermediates en route to the main bicyclopentadione. J. Biol. Chem. 2015, 290, 4817–4828. [Google Scholar] [CrossRef] [PubMed]
- Griesser, M.; Pistis, V.; Suzuki, T.; Tejera, N.; Pratt, D.A.; Schneider, C. Autoxidative and cyclooxygenase-2 catalyzed transformation of the dietary chemopreventive agent curcumin. J. Biol. Chem. 2011, 286, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, K.T.; Kim, M.R. Effect of gamma-irradiated red pepper powder on the chemical and volatile characteristics of kakdugi, a Korean traditional fermented radish kimchi. J. Food. Sci. 2005, 70, C441–C447. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, M.H.; Kwon, J.H. Effects of electron beam irradiation on physicochemical qualities of red pepper powder. Korean J. Food Sci. Technol. 2000, 32, 271–276. [Google Scholar]
- Afonso, S.; Horita, K.; Silva, J.S.; Almeida, I.; Amaral, M.; Lobão, P.; Costa, P.; Miranda, M.S.; da Silva, J.C.E.; Lobo, J.S. Photodegradation of avobenzone: Stabilization effect of antioxidants. J. Photochem. Photobiol. B 2014, 140, 36–40. [Google Scholar] [CrossRef]
- Ahmad, I.; Arsalan, A.; Ali, S.A.; Sheraz, M.A.; Ahmed, S.; Anwar, Z.; Munir, I.; Shah, M.R. Formulation and stabilization of riboflavin in liposomal preparations. J. Photochem. Photobiol. B 2015, 153, 358–366. [Google Scholar] [CrossRef]
- Jamrógiewicz, M.; Wielgomas, B.; Strankowski, M. Evaluation of the photoprotective effect of β-cyclodextrin on the emission of volatile degradation products of ranitidine. J. Pharm. Biomed. Anal. 2014, 98, 113–119. [Google Scholar] [CrossRef]
- Jaiswal, M.; Dudhe, R.; Sharma, P. Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech. 2015, 5, 123–127. [Google Scholar] [CrossRef]
- Jandang, W.; Ampasavate, C.; Kiattisin, K. Natural stabilizers and nanostructured lipid carrier entrapment for photosensitive compounds, curcumin and capsaicin. Pharmaceutics 2024, 16, 412. [Google Scholar] [CrossRef]
- Yukuyama, M.; Ghisleni, D.D.M.; Pinto, T.d.J.A.; Bou-Chacra, N.A. Nanoemulsion: Process selection and application in cosmetics–a review. Int. J. Cosmet. Sci. 2016, 38, 13–24. [Google Scholar] [CrossRef]
- Hong, S.J.; Garcia, C.V.; Park, S.J.; Shin, G.H.; Kim, J.T. Retardation of curcumin degradation under various storage conditions via turmeric extract-loaded nanoemulsion system. LWT 2019, 100, 175–182. [Google Scholar] [CrossRef]
- Clapham, D. Stability Testing: Photostability Testing of New Drug Substances and Products ICH Q1B. In ICH Quality Guidelines: An Implementation Guide; Ermer, J., Nethercote, P., Eds.; Wiley-VCH: Weinheim, Germany, 2017; pp. 45–72. [Google Scholar]
- Sarfraz, R.M.; Bashir, S.; Mahmood, A.; Ahsan, H.; Riaz, H.; Raza, H.; Rashid, Z.; Raza, S.A.; Abrar, M.A.; Abbas, K. Application of various polymers and polymers-based techniques used to improve solubility of poorly water-soluble drugs: A review. Acta Pol. Pharm. 2017, 74, 347–356. [Google Scholar]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef]
- Nitthikan, N.; Leelapornpisid, P.; Natakankitkul, S.; Chaiyana, W.; Mueller, M.; Viernstein, H.; Kiattisin, K. Improvement of stability and transdermal delivery of bioactive compounds in green robusta coffee beans extract loaded nanostructured lipid carriers. J. Nanotechnol. 2018, 2018, 7865024. [Google Scholar] [CrossRef]
- Nuttapol, B.; Kanokwan, K.; Chadarat, A. Effects of antioxidants and sun-screening agent on the photostability of curcumin. In Proceedings of the 10th International Conference on Nutrition and Physical Activity in Ageing, Obesity, and Cancer, Chonburi, Thailand, 18–20 December 2019; pp. 18–20. [Google Scholar]
- Jayaprakasha, G.K.; Jagan Mohan Rao, L.; Sakariah, K.K. Improved HPLC method for the determination of curcumin, demethoxycurcumin, and bisdemethoxycurcumin. J. Agric. Food Chem. 2002, 50, 3668–3672. [Google Scholar] [CrossRef]
- Juangsamoot, J.; Ruangviriyachai, C.; Techawongstien, S.; Chanthai, S. Determination of capsaicin and dihydrocapsaicin in some hot chilli varieties by RP-HPLC-PDA after magnetic stirring extraction and clean up with C18 cartridge. Int. Food Res. J. 2012, 19, 1117–1123. [Google Scholar]
- Huck, C.; Guggenbichler, W.; Bonn, G. Analysis of caffeine, theobromine and theophylline in coffee by near infrared spectroscopy (NIRS) compared to high-performance liquid chromatography (HPLC) coupled to mass spectrometry. Anal. Chim. Acta 2005, 538, 195–203. [Google Scholar] [CrossRef]
- Walfish, S. Analytical methods: A statistical perspective on the ICH Q2A and Q2B guidelines for validation of analytical methods. Bio Pharm. Int. 2006, 19, 1–6. [Google Scholar]
- Horwitz, W. AOAC Requirements for Single Laboratory Validation of Chemical Methods. AOAC Int. 2002, Draft 2002-11-07. Available online: http://www.aoac.org/ (accessed on 30 December 2005).
- Tønnesen, H.H.; Másson, M.; Loftsson, T. Studies of curcumin and curcuminoids. XXVII. Cyclodextrin complexation: Solubility, chemical and photochemical stability. Int. J. Pharm. 2002, 244, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Baertschi, S.W.; Alsante, K.M.; Tønnesen, H.H. A critical assessment of the ICH guideline on photostability testing of new drug substances and products (Q1B): Recommendation for revision. J. Pharm. Sci. 2010, 99, 2934–2940. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Maciel, E.N.; Soares, I.N.; da Silva, S.C.; de Souza, G.L. A computational study on the reaction between fisetin and 2,2-diphenyl-1-picrylhydrazyl (DPPH). J. Mol. Model. 2019, 25, 164. [Google Scholar] [CrossRef]
- Kaur, J.; Singla, P.; Goel, N. Adsorption of oxazole and isoxazole on BNNT surface: A DFT study. Appl. Surf. Sci. 2015, 328, 632–640. [Google Scholar] [CrossRef]
- Rajan, V.K.; Ragi, C.; Muraleedharan, K. A computational exploration into the structure, antioxidant capacity, toxicity and drug-like activity of the anthocyanidin “Petunidin”. Heliyon 2019, 5, e02115. [Google Scholar] [CrossRef] [PubMed]
- Kar, R.; Chandrakumar, K.; Pal, S. The influence of electric field on the global and local reactivity descriptors: Reactivity and stability of weakly bonded complexes. J. Phys. Chem. A. 2007, 111, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Rajan, V.K.; Ahamed, T.S.; Muraleedharan, K. Studies on the UV filtering and radical scavenging capacity of the bitter masking flavanone Eriodictyol. J. Photochem. Photobiol. B. 2018, 185, 254–261. [Google Scholar] [CrossRef]
- Isravel, A.D.; Jeyaraj, J.K.; Thangasamy, S.; John, W.J. DFT, NBO, HOMO-LUMO, NCI, stability, Fukui function and hole–electron analyses of tolcapone. Comput. Theor. Chem. 2021, 1202, 113296. [Google Scholar] [CrossRef]
- Tabanelli, R.; Brogi, S.; Calderone, V. Improving curcumin bioavailability: Current strategies and future perspectives. Pharmaceutics 2021, 13, 1715. [Google Scholar] [CrossRef]
- Teeranachaideekul, V.; Chantaburanan, T.; Junyaprasert, V.B. Influence of state and crystallinity of lipid matrix on physicochemical properties and permeation of capsaicin-loaded lipid nanoparticles for topical delivery. J. Drug. Deliv. Sci. Technol. 2017, 39, 300–307. [Google Scholar] [CrossRef]
- Alsabri, S.G.; Mari, W.O.; Younes, S.; Elsadawi, M.A.; Oroszi, T.L. Kinetic and dynamic description of caffeine. J. Caffeine Adenosine Res. 2018, 8, 3–9. [Google Scholar] [CrossRef]
- Van Nong, H.; Hung, L.X.; Thang, P.N.; Chinh, V.D.; Vu, L.V.; Dung, P.T.; Van Trung, T.; Nga, P.T. Fabrication and vibration characterization of curcumin extracted from turmeric (Curcuma longa) rhizomes of the northern Vietnam. SpringerPlus 2016, 5, 1422. [Google Scholar] [CrossRef]
- Ahmad Bhawani, S.; Fong, S.S.; Mohamad Ibrahim, M.N. Spectrophotometric analysis of caffeine. Int. J. Anal. Chem. 2015, 2015, 162504. [Google Scholar] [CrossRef]
- González-Zamora, A.; Sierra-Campos, E.; Pérez-Morales, R.; Vázquez-Vázquez, C.; Gallegos-Robles, M.A.; López-Martínez, J.D.; García-Hernández, J.L. Measurement of capsaicinoids in chiltepin hot pepper: A comparison study between spectrophotometric method and high-performance liquid chromatography analysis. J. Chem. 2015, 2015, 143505. [Google Scholar] [CrossRef]
- Bergamante, V.; Ceschel, G.C.; Marazzita, S.; Ronchi, C.; Fini, A. Effect of vehicles on topical application of Aloe vera and Arnica montana components. Drug. Deliv. 2007, 14, 427–432. [Google Scholar] [CrossRef][Green Version]
- Hanno, I.; Centini, M.; Anselmi, C.; Bibiani, C. Green cosmetic surfactant from rice: Characterization and application. Cosmetics 2015, 2, 322–341. [Google Scholar] [CrossRef]
- Magrode, N.; Poomanee, W.; Kiattisin, K.; Ampasavate, C. Microemulsions and nanoemulsions for topical delivery of tripeptide-3: From design of experiment to anti-sebum efficacy on facial skin. Pharmaceutics 2024, 16, 554. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Qin, D.; Vu, G.; McClements, D.J. Impact of operating parameters on the production of nanoemulsions using a high-pressure homogenizer with flow pattern and back pressure control. Colloids Interfaces 2023, 7, 21. [Google Scholar] [CrossRef]
- Ruiz-Montañez, G.; Ragazzo-Sanchez, J.A.; Picart-Palmade, L.; Calderón-Santoyo, M.; Chevalier-Lucia, D. Optimization of nanoemulsions processed by high-pressure homogenization to protect a bioactive extract of jackfruit (Artocarpus heterophyllus Lam). Innov. Food Sci. Emerg. Technol. 2017, 40, 35–41. [Google Scholar] [CrossRef]
- Che Marzuki, N.H.; Wahab, R.A.; Abdul Hamid, M. An overview of nanoemulsion: Concepts of development and cosmeceutical applications. Biotechnol. Biotechnol. Equip. 2019, 33, 779–797. [Google Scholar] [CrossRef]
- Kumar, M.; Pathak, K.; Misra, A. Formulation and characterization of nanoemulsion-based drug delivery system of risperidone. Drug. Dev. Ind. Pharm. 2009, 35, 387–395. [Google Scholar] [CrossRef]
- Yin, L.-J.; Chu, B.-S.; Kobayashi, I.; Nakajima, M. Performance of selected emulsifiers and their combinations in the preparation of β-carotene nanodispersions. Food Hydrocoll. 2009, 23, 1617–1622. [Google Scholar] [CrossRef]
- Mohammed, N.K.; Muhialdin, B.J.; Meor Hussin, A.S. Characterization of nanoemulsion of Nigella sativa oil and its application in ice cream. Food Sci. Nutr. 2020, 8, 2608–2618. [Google Scholar] [CrossRef] [PubMed]
- Anantaworasakul, P.; Chaiyana, W.; Michniak-Kohn, B.B.; Rungseevijitprapa, W.; Ampasavate, C. Enhanced transdermal delivery of concentrated capsaicin from chili extract-loaded lipid nanoparticles with reduced skin irritation. Pharmaceutics 2020, 12, 463. [Google Scholar] [CrossRef] [PubMed]
- Nazarzadeh, E.; Anthonypillai, T.; Sajjadi, S. On the growth mechanisms of nanoemulsions. J. Colloid Interface Sci. 2013, 397, 154–162. [Google Scholar] [CrossRef]
- Shalaby, K.S.; Soliman, M.E.; Casettari, L.; Bonacucina, G.; Cespi, M.; Palmieri, G.F.; Sammour, O.A.; El Shamy, A.A. Determination of factors controlling the particle size and entrapment efficiency of noscapine in PEG/PLA nanoparticles using artificial neural networks. Int. J. Nanomed. 2014, 9, 4953–4964. [Google Scholar] [CrossRef]
- Abd, E.; Namjoshi, S.; Mohammed, Y.H.; Roberts, M.S.; Grice, J.E. Synergistic skin penetration enhancer and nanoemulsion formulations promote the human epidermal permeation of caffeine and naproxen. J. Pharm. Sci. 2016, 105, 212–220. [Google Scholar] [CrossRef]
- Sintov, A.C.; Greenberg, I. Comparative percutaneous permeation study using caffeine-loaded microemulsion showing low reliability of the frozen/thawed skin models. Int. J. Pharm. 2014, 471, 516–524. [Google Scholar] [CrossRef]
- Dias, L.D.; Blanco, K.C.; Mfouo-Tynga, I.S.; Inada, N.M.; Bagnato, V.S. Curcumin as a photosensitizer: From molecular structure to recent advances in antimicrobial photodynamic therapy. J. Photochem. Photobiol. C 2020, 45, 100384. [Google Scholar] [CrossRef]
- Nimiya, Y.; Wang, W.; Du, Z.; Sukamtoh, E.; Zhu, J.; Decker, E.; Zhang, G. Redox modulation of curcumin stability: Redox active antioxidants increase chemical stability of curcumin. Mol. Nutr. Food Res. 2016, 60, 487–494. [Google Scholar] [CrossRef]
- Indira Priyadarsini, K. Chemical and structural features influencing the biological activity of curcumin. Curr. Pharm. Des. 2013, 19, 2093–2100. [Google Scholar] [CrossRef]
- Gonçalves, M.V.S.; Chandran, D.; Nelliyaparambath, L.; Gokul, A.K.; da Silva, L.E. Applications of Capsaicin in the Food Industry. In Capsaicinoids: From Natural Sources to Biosynthesis and Their Clinical Applications; Springer: Berlin/Heidelberg, Germany, 2024; pp. 293–320. [Google Scholar] [CrossRef]
- Villanueva-Bermejo, D.; de las Nieves Siles-Sánchez, M.; Hernández, D.M.; García-Risco, M.R.; Jaime, L.; Santoyo, S.; Fornari, T. Theoretical framework to evaluate antioxidant synergistic effects from the coextraction of marjoram, rosemary and parsley. Food Chem. 2024, 437, 137919. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Choi, H.A.; Kim, M.-R.; Hong, J. Changes in chemical stability and bioactivities of curcumin by ultraviolet radiation. Food Sci. Biotechnol. 2013, 22, 279–282. [Google Scholar] [CrossRef]
- Jeszka-Skowron, M.; Sentkowska, A.; Pyrzyńska, K.; De Peña, M.P. Chlorogenic acids, caffeine content and antioxidant properties of green coffee extracts: Influence of green coffee bean preparation. Eur. Food Res. Technol. 2016, 242, 1403–1409. [Google Scholar] [CrossRef]
- Kongor, A.; Panchal, M.; Athar, M.; Makwana, B.; Sindhav, G.; Jha, P.; Jain, V. Synthesis and modeling of calix[4]pyrrole wrapped Au nanoprobe for specific detection of Pb (II): Antioxidant and radical scavenging efficiencies. J. Photochem. Photobiol. A Chem. 2018, 364, 801–810. [Google Scholar] [CrossRef]
- Adindu, E.A.; Godfrey, O.C.; Agwupuye, E.I.; Ekpong, B.O.; Agurokpon, D.C.; Ogbodo, S.E.; Benjamin, I.; Louis, H. Structural analysis, reactivity descriptors (HOMO-LUMO, ELF, NBO), effect of polar (DMSO, EtOH, H2O) solvation, and libido-enhancing potential of resveratrol by molecular docking. Chem. Phys. Impact. 2023, 7, 100296. [Google Scholar] [CrossRef]
- Boulmokh, Y.; Belguidoum, K.; Meddour, F.; Amira-Guebailia, H. Enhanced antioxidant properties of novel curcumin derivatives: A comprehensive DFT computational study. Struct. Chem. 2024, 35, 825–839. [Google Scholar] [CrossRef]
- Schrödinger, L. The PyMOL Molecular Graphics System; Version 1.8; Schrödinger LLC: New York, NY, USA, 2015. [Google Scholar]
- Jeffrey, G.A. Hydrogen-bonding: An update. Crystallogr. Rev. 2003, 9, 135–176. [Google Scholar] [CrossRef]
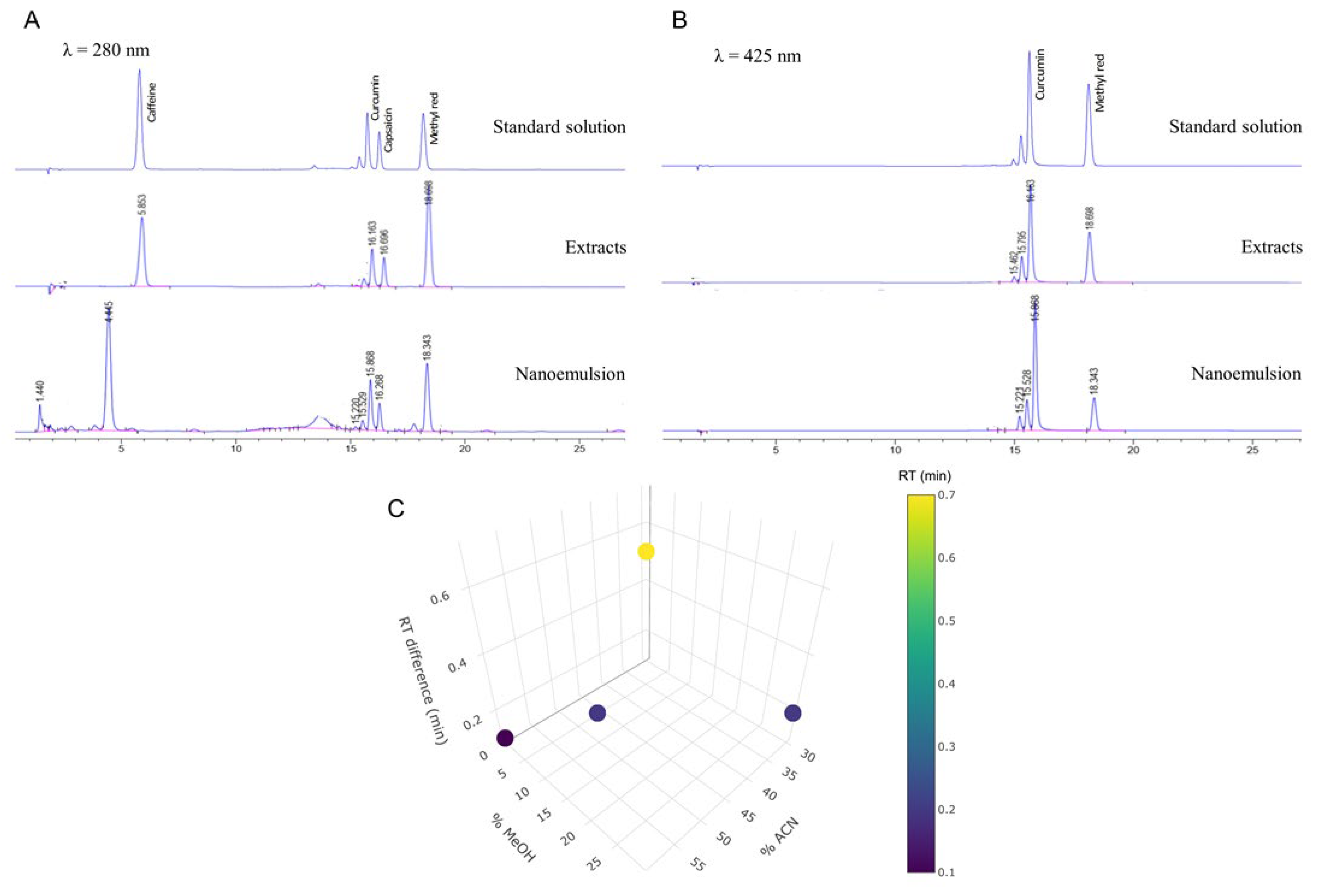
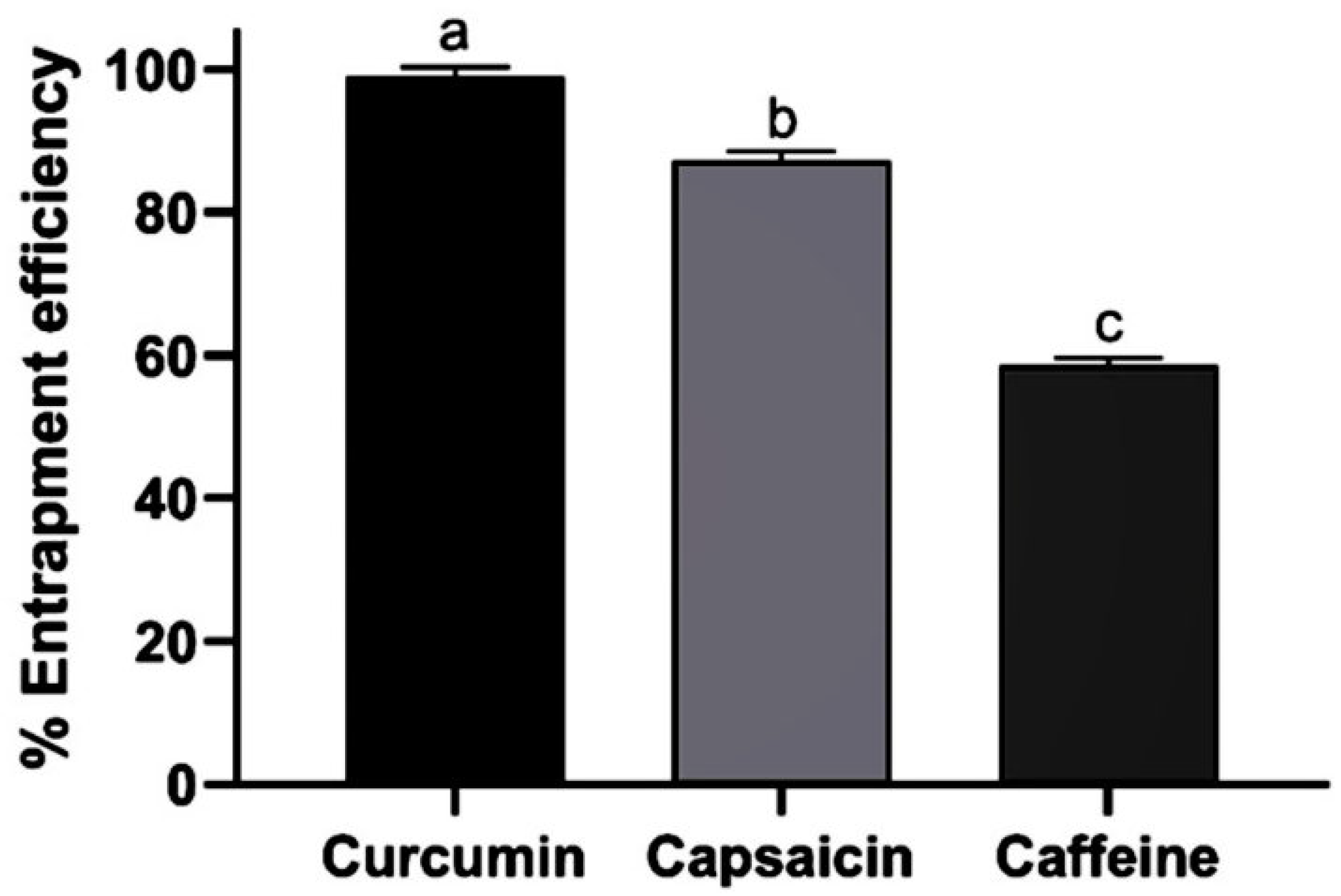
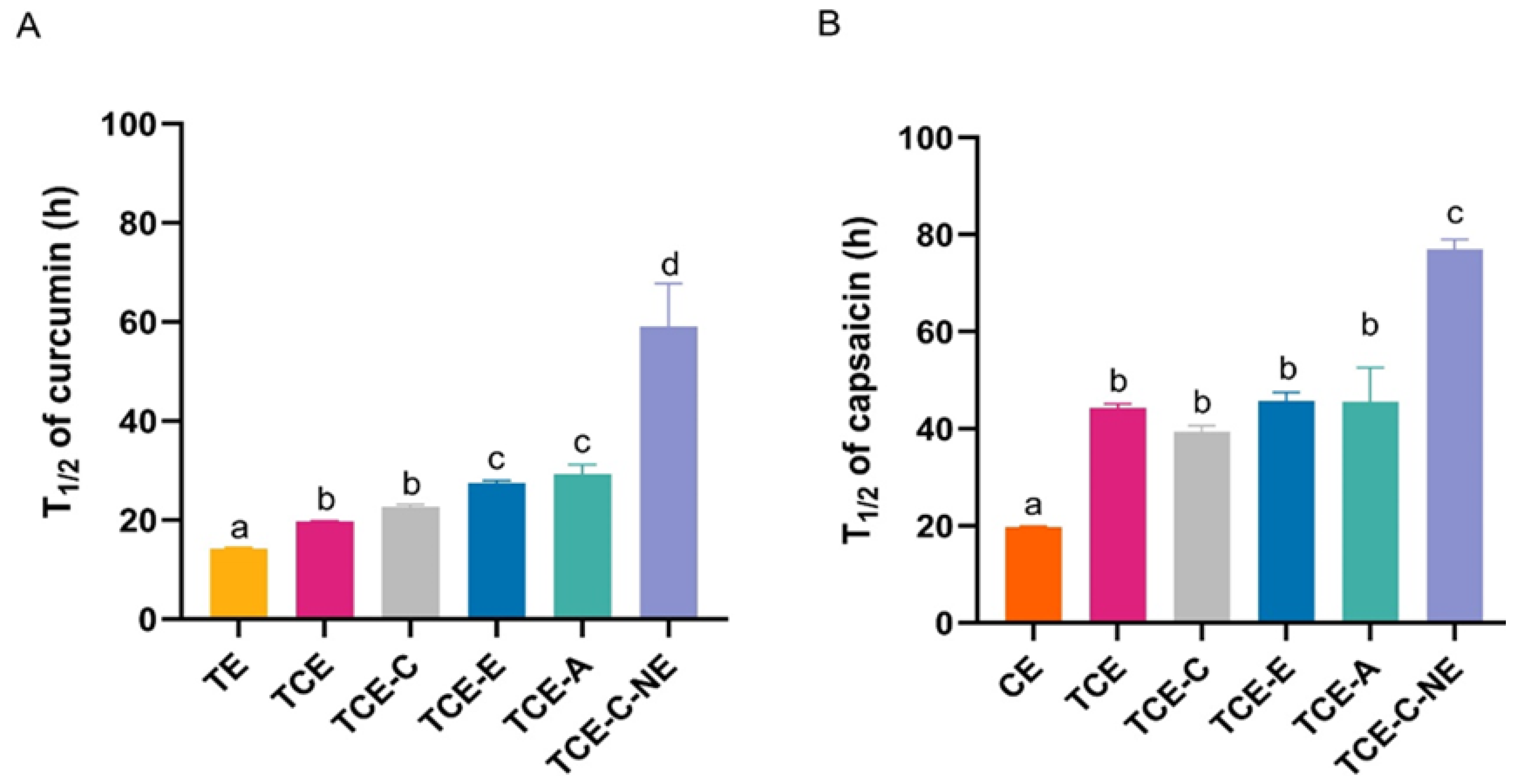
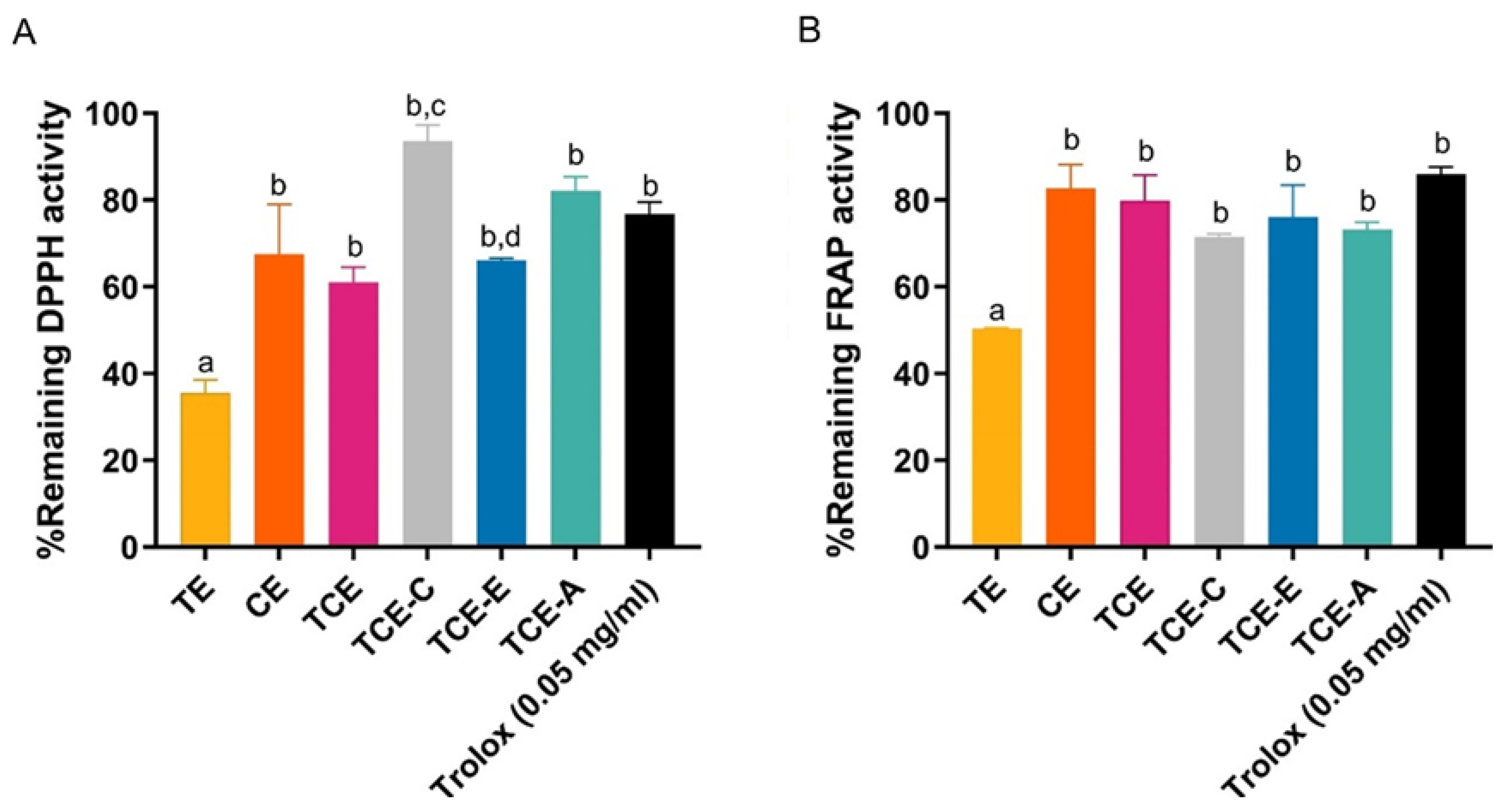


| Ingredients | F1-12 |
|---|---|
| 1. Oil mixture | 21 |
| 2. Emulsifier | 1–6 |
| 3. Polyethylene glycol 400 | 5 |
| 4. Phenoxyethanol | 1 |
| 5. Water | q.s.100 |
| Ingredients | TCE-C-NE-1 | TCE-C-NE-2 | TCE-C-NE-3 |
|---|---|---|---|
| 1. Turmeric extract | 0.025 | 0.050 | 0.075 |
| 2. Chili extract | 0.050 | 0.050 | 0.050 |
| 3. Coffee extract | 0.025 | 0.050 | 0.075 |
| 4. Oil mixture | 21 | 21 | 21 |
| 5. Emulsifier (selected) | 4 | 4 | 4 |
| 6. Polyethylene glycol 400 | 5 | 5 | 5 |
| 7. Phenoxyethanol | 1 | 1 | 1 |
| 8. Tween 80 | 10 | 10 | 10 |
| 9. Water | q.s.100 | q.s.100 | q.s.100 |
| Record | Unloaded Nanoemulsions | ||
|---|---|---|---|
| Homogenization Time (min) | 5 | 10 | 15 |
| Average particle size (nm) | 303.12 ± 1.83 a | 251.74 ± 1.9 b | 179.52 ± 2.43 c |
| Average PDI | 0.35 ± 0.01 a | 0.32 ± 0.05 a | 0.12 ± 0.02 b |
| Average zeta potential (mV) | −48.21 ± 0.46 a | −45.35 ± 2.83 a | −46.38 ± 2.48 a |
| TCE-C-NE-1 | |||||
|---|---|---|---|---|---|
| Parameters | Day 0 | 30 °C | 4 °C | ||
| 30 Days | 60 Days | 30 Days | 60 Days | ||
| Particle size (nm) | 170.2 ± 1.32 a | 246.03 ± 0.45 b | 248.86 ± 1.96 b | 180.1 ± 1.75 a | 182.7 ± 1.75 a |
| PDI | 0.11 ± 0.03 a | 0.12 ± 0.02 a | 0.12 ± 0.02 a | 0.14 ± 0.03 a | 0.11 ± 0.08 a |
| Zeta potential (mV) | −39.6 ± 2.25 a | −38.7 ± 0.79 a | −37.76 ± 1.62 a | −39.20 ± 0.55 a | −39.60 ± 1.02 a |
| Compounds | E(RB3LYP) (Hartree *) | E(RB3LYP) (kcal/mol) |
|---|---|---|
| Caffeine | −680.3906 | −426,951.5527 |
| Capsaicin | −982.6340 | −616,612.1449 |
| Caffeine–Capsaicin | −1663.0421 | −1,043,574.7166 |
| Eint of Caffeine–Capsaicin | −0.0176 | −11.0191 |
| Chlorogenic acid | −1297.5884 | −814,249.0481 |
| Capsaicin | −982.6340 | −616,612.1449 |
| Chlorogenic acid–Capsaicin | −2280.2347 | −1,430,868.9365 |
| Eint of Chlorogenic acid–Capsaicin | −0.0123 | −7.7435 |
| Curcumin | −1263.5968 | −792,918.9962 |
| Capsaicin | −982.6340 | −616,612.1449 |
| Curcumin–Capsaicin | −2246.2430 | −1,409,538.8218 |
| Eint of Curcumin–Capsaicin | −0.0122 | −7.6807 |
| Chlorogenic acid | −1297.5884 | −814,249.0481 |
| Curcumin | −1263.5968 | −792,918.9962 |
| Chlorogenic acid–Curcumin | −2561.1939 | −1,607,173.5036 |
| Eint Chlorogenic acid–Curcumin | −0.0087 | −5.4593 |
| Caffeine | −680.3906 | −426,951.5527 |
| Curcumin | −1263.5968 | −792,918.9962 |
| Caffeine–Curcumin | −1943.9909 | −1,219,872.7577 |
| Eint of Caffeine–Curcumin | −0.0035 | −2.2088 |
| System | HOMO | LUMO | HLG |
|---|---|---|---|
| Curcumin | −0.20480 | −0.07210 | 0.13270 |
| Capsaicin | −0.20221 | 0.00413 | 0.20634 |
| Chlorogenic acid | −0.21483 | −0.06417 | 0.15066 |
| Caffeine | −0.21953 | −0.03152 | 0.18801 |
| Curcumin–Capsaicin | −0.19566 | −0.06302 | 0.13264 |
| Chlorogenic acid–Capsaicin | −0.20658 | −0.05509 | 0.15149 |
| Chlorogenic acid–Curcumin | −0.20999 | −0.07817 | 0.13182 |
| Caffeine–Capsaicin | −0.19328 | −0.04704 | 0.14624 |
| Caffeine–Curcumin | −0.20168 | −0.06132 | 0.14036 |
| System | Ionization Potential | Electron Affinity | Electronegativity | Hardness | Softness | Chemical Potential |
|---|---|---|---|---|---|---|
| Curcumin | 0.20480 | 0.07210 | 0.13845 | 0.06635 | 7.53580 | −0.13845 |
| Capsaicin | 0.20221 | −0.00413 | 0.09904 | 0.10317 | 4.84637 | −0.09904 |
| Chlorogenic acid | 0.21483 | 0.06417 | 0.13950 | 0.07533 | 6.63746 | −0.13950 |
| Caffeine | 0.21953 | 0.03152 | 0.12553 | 0.09401 | 5.31887 | −0.12553 |
| Curcumin–Capsaicin | 0.19566 | 0.06302 | 0.12934 | 0.06632 | 7.53920 | −0.12934 |
| Chlorogenic acid–Capsaicin | 0.20658 | 0.05509 | 0.13084 | 0.07575 | 6.60110 | −0.13084 |
| Chlorogenic acid–Curcumin | 0.20999 | 0.07817 | 0.14408 | 0.06591 | 7.58610 | −0.14408 |
| Caffeine–Capsaicin | 0.19328 | 0.04704 | 0.12016 | 0.07312 | 6.83807 | −0.12016 |
| Caffeine–Curcumin | 0.20168 | 0.06132 | 0.13150 | 0.07018 | 7.12454 | −0.13150 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boonrueang, N.; Chaichit, S.; Yooin, W.; Okonogi, S.; Kiattisin, K.; Ampasavate, C. Triple-Loaded Nanoemulsions Incorporating Coffee Extract for the Photoprotection of Curcumin and Capsaicin: Experimental and Computational Evaluation. Pharmaceutics 2025, 17, 926. https://doi.org/10.3390/pharmaceutics17070926
Boonrueang N, Chaichit S, Yooin W, Okonogi S, Kiattisin K, Ampasavate C. Triple-Loaded Nanoemulsions Incorporating Coffee Extract for the Photoprotection of Curcumin and Capsaicin: Experimental and Computational Evaluation. Pharmaceutics. 2025; 17(7):926. https://doi.org/10.3390/pharmaceutics17070926
Chicago/Turabian StyleBoonrueang, Nuttapol, Siripat Chaichit, Wipawadee Yooin, Siriporn Okonogi, Kanokwan Kiattisin, and Chadarat Ampasavate. 2025. "Triple-Loaded Nanoemulsions Incorporating Coffee Extract for the Photoprotection of Curcumin and Capsaicin: Experimental and Computational Evaluation" Pharmaceutics 17, no. 7: 926. https://doi.org/10.3390/pharmaceutics17070926
APA StyleBoonrueang, N., Chaichit, S., Yooin, W., Okonogi, S., Kiattisin, K., & Ampasavate, C. (2025). Triple-Loaded Nanoemulsions Incorporating Coffee Extract for the Photoprotection of Curcumin and Capsaicin: Experimental and Computational Evaluation. Pharmaceutics, 17(7), 926. https://doi.org/10.3390/pharmaceutics17070926









