Toward Natural Wound Healing Therapy: Honey and Calendula officinalis Loaded κ-Carrageenan Films with Promising Hemostatic Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Films
2.3. Characterization of the Films
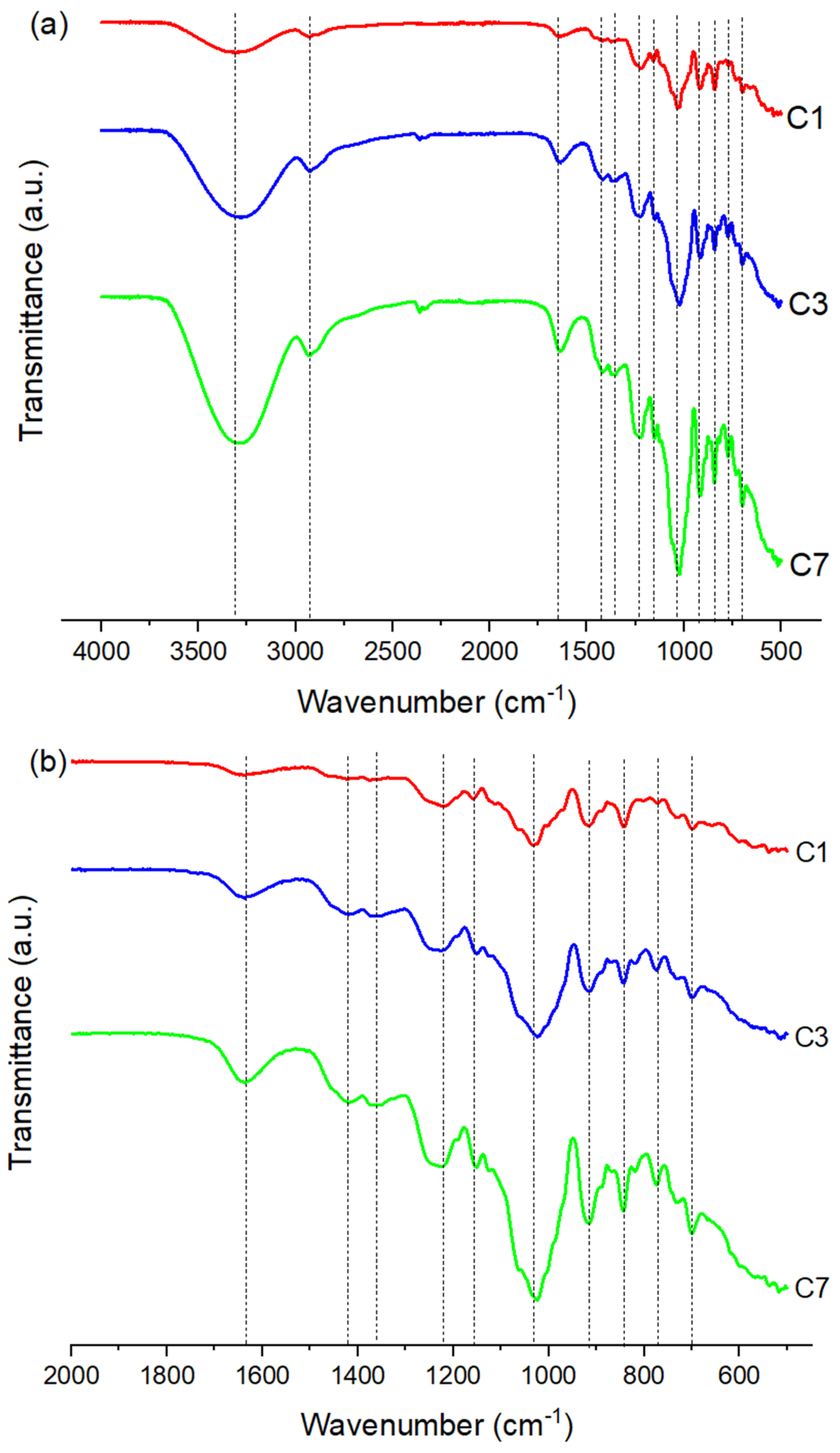
2.3.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.2. Thickness, Weight Variation and Folding Endurance
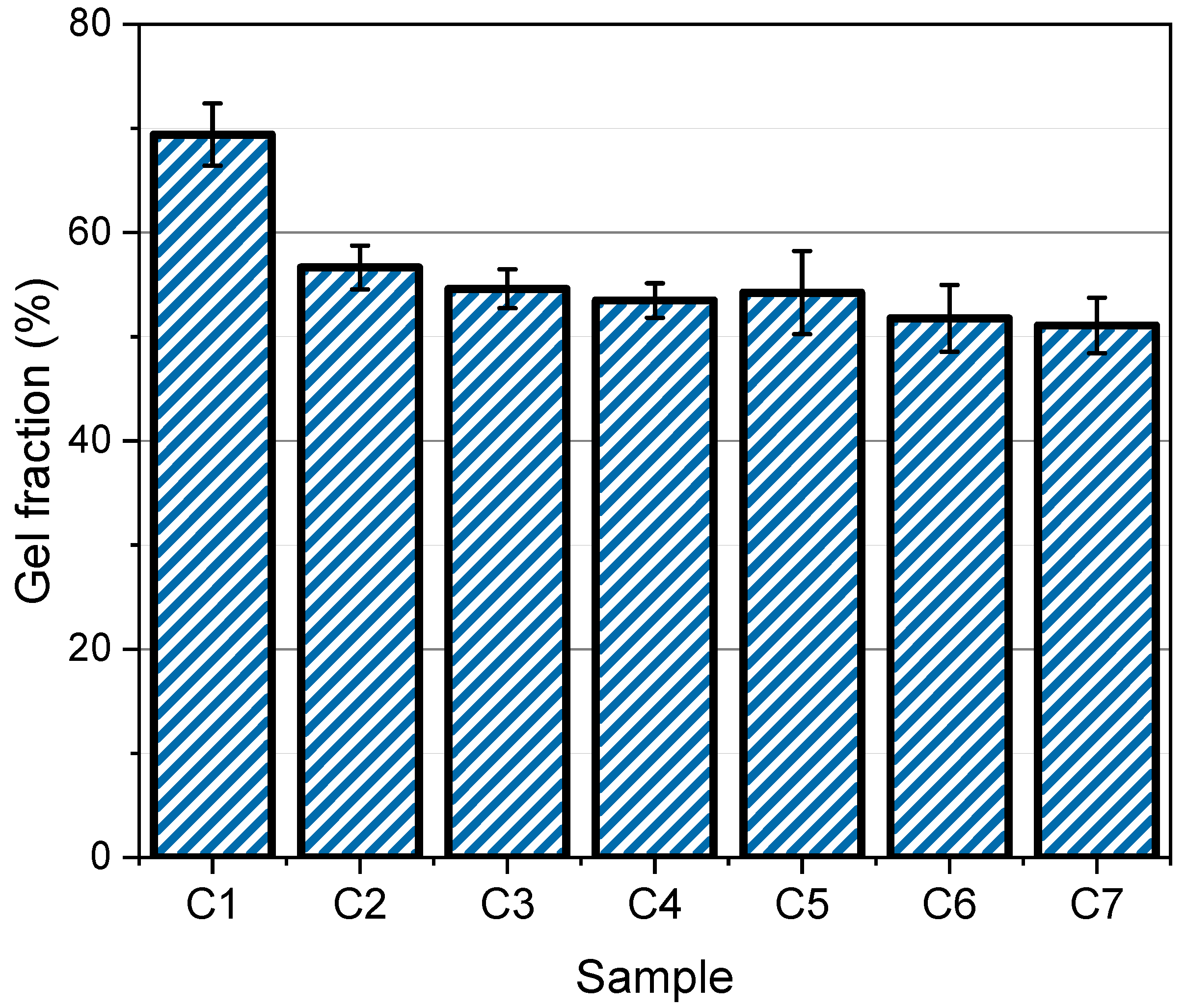
2.3.3. Gel Fraction Measurement
2.3.4. Swelling Study
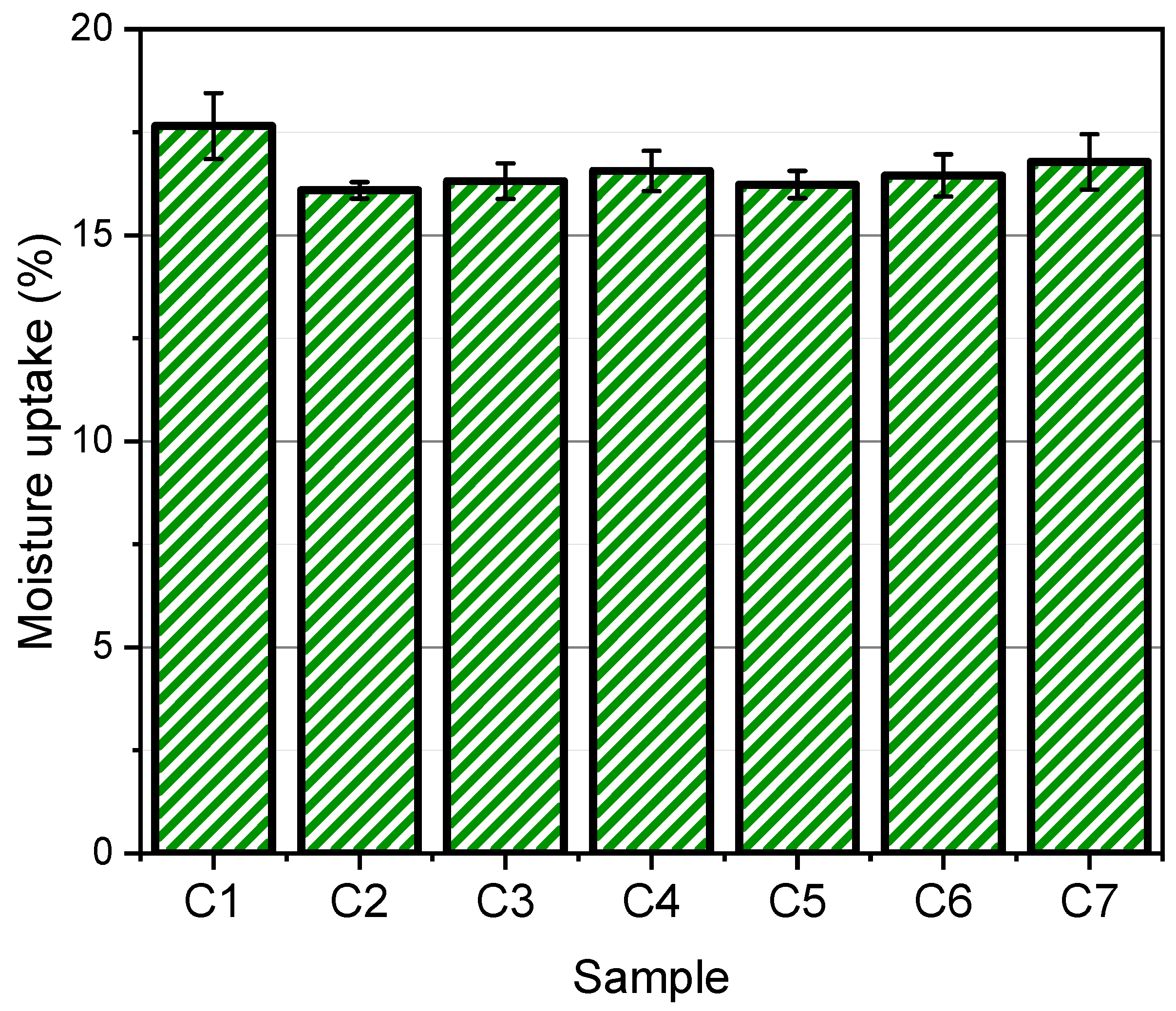
2.3.5. Moisture Uptake
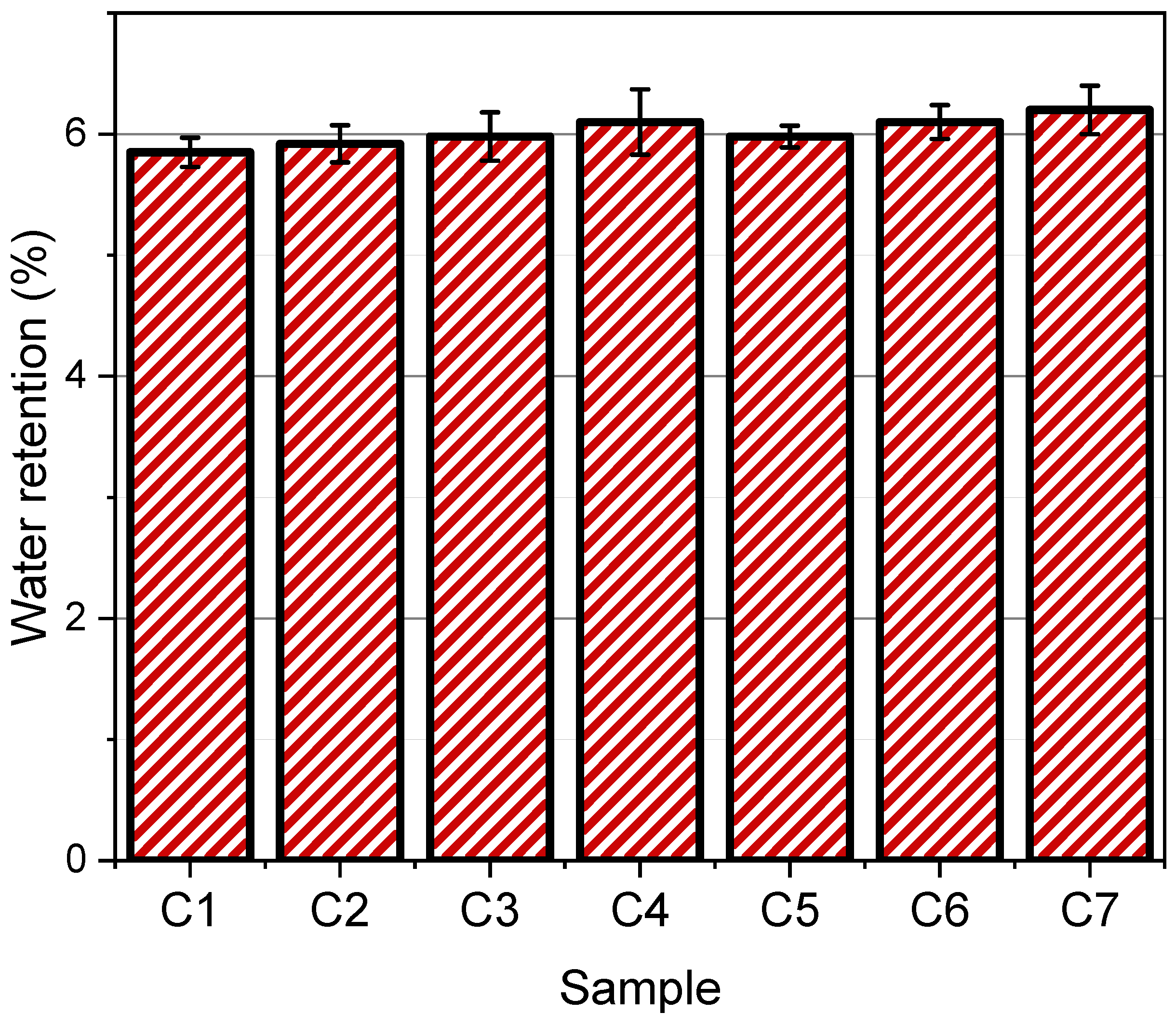
2.3.6. Water Retention Capacity
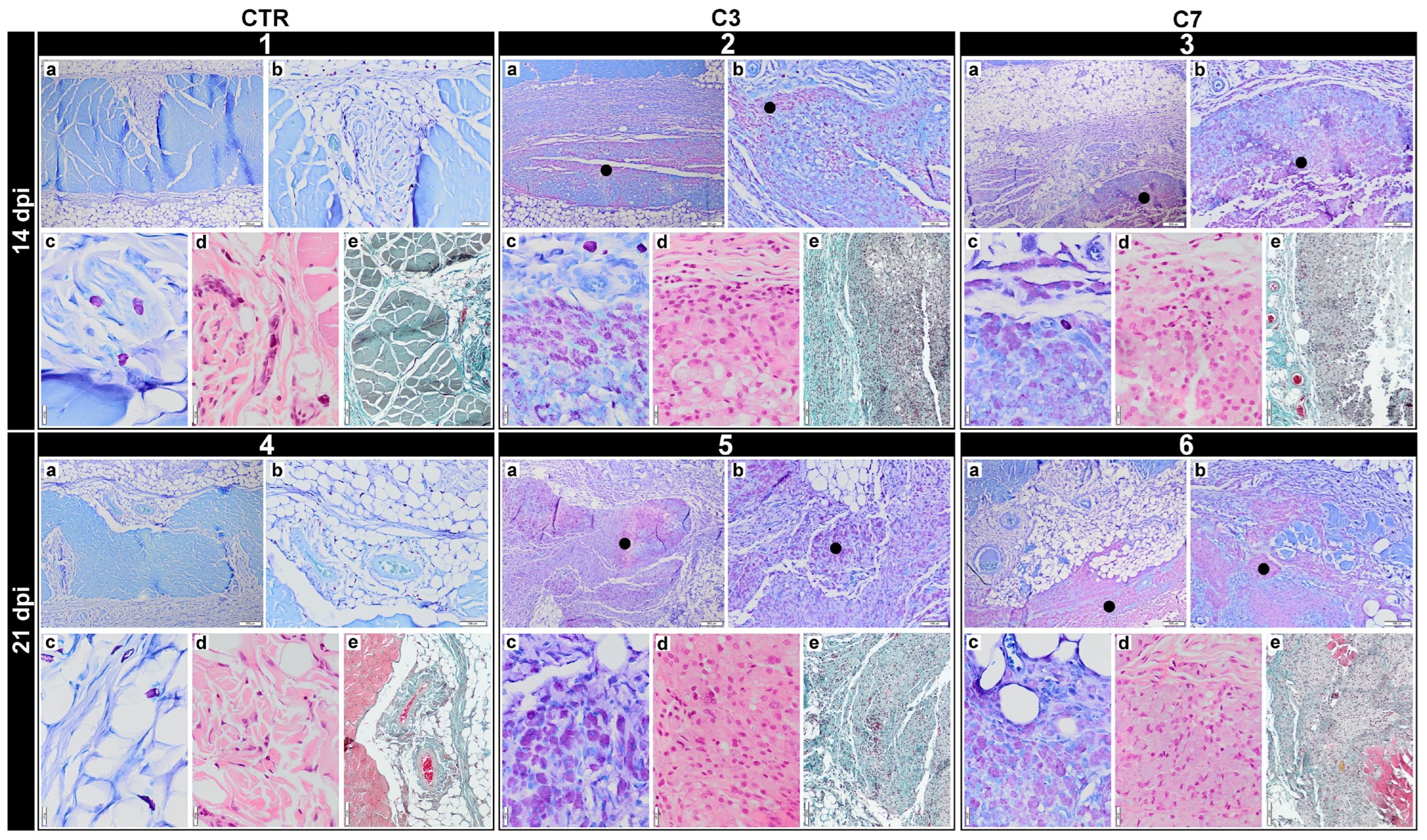
2.3.7. In Vivo Biocompatibility Assay
Animals
Experimental Design
Histological Analyses
Statistical Analysis
3. Results and Discussion
3.1. Structural Characterization of the Films
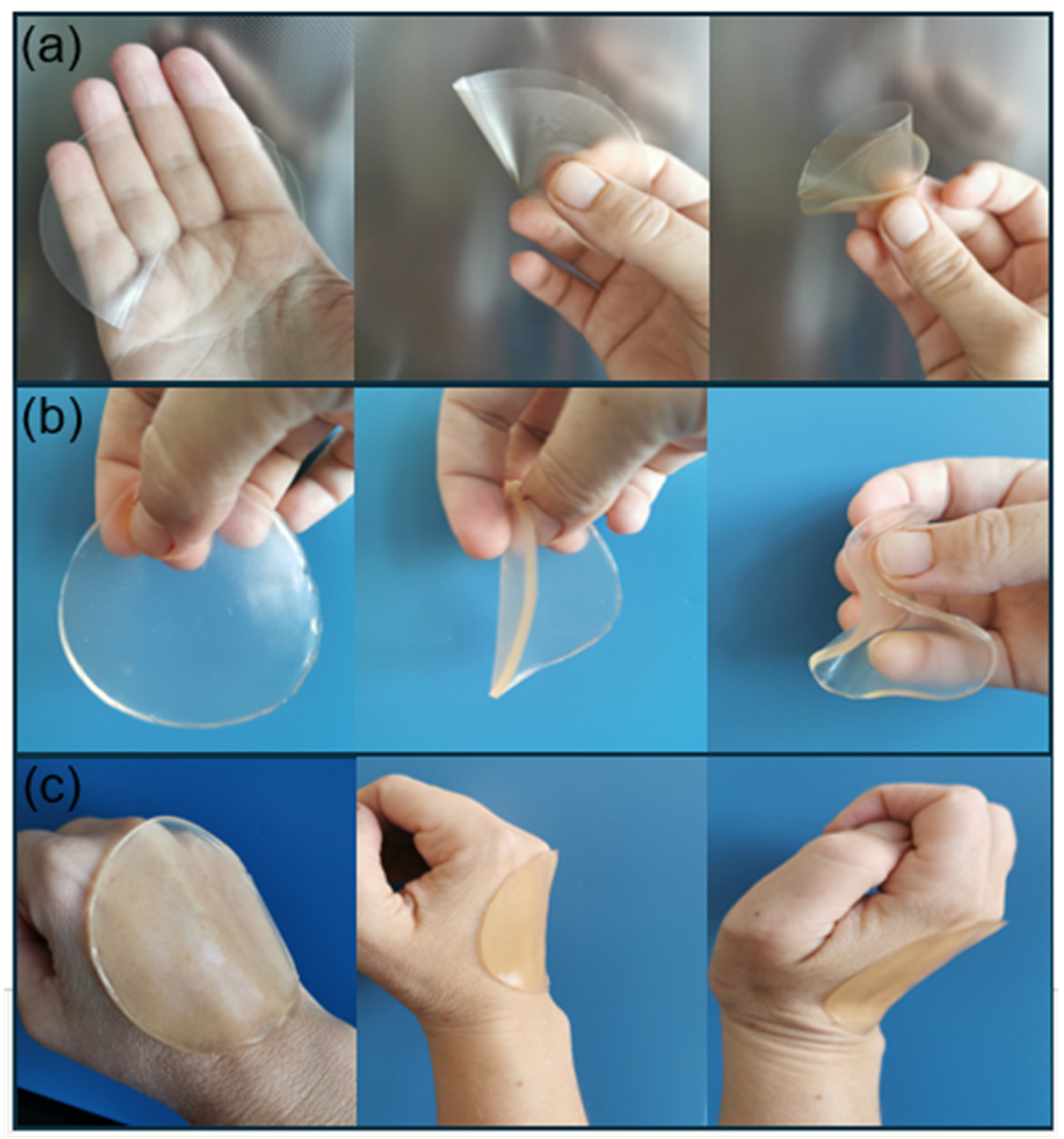
3.2. Thickness, Weight Variation and Folding Endurance
3.3. Gel Fraction
3.4. Swelling of the Films
3.5. Moisture Uptake
3.6. Water Retention
3.7. In Vivo Biocompatibility Assessment
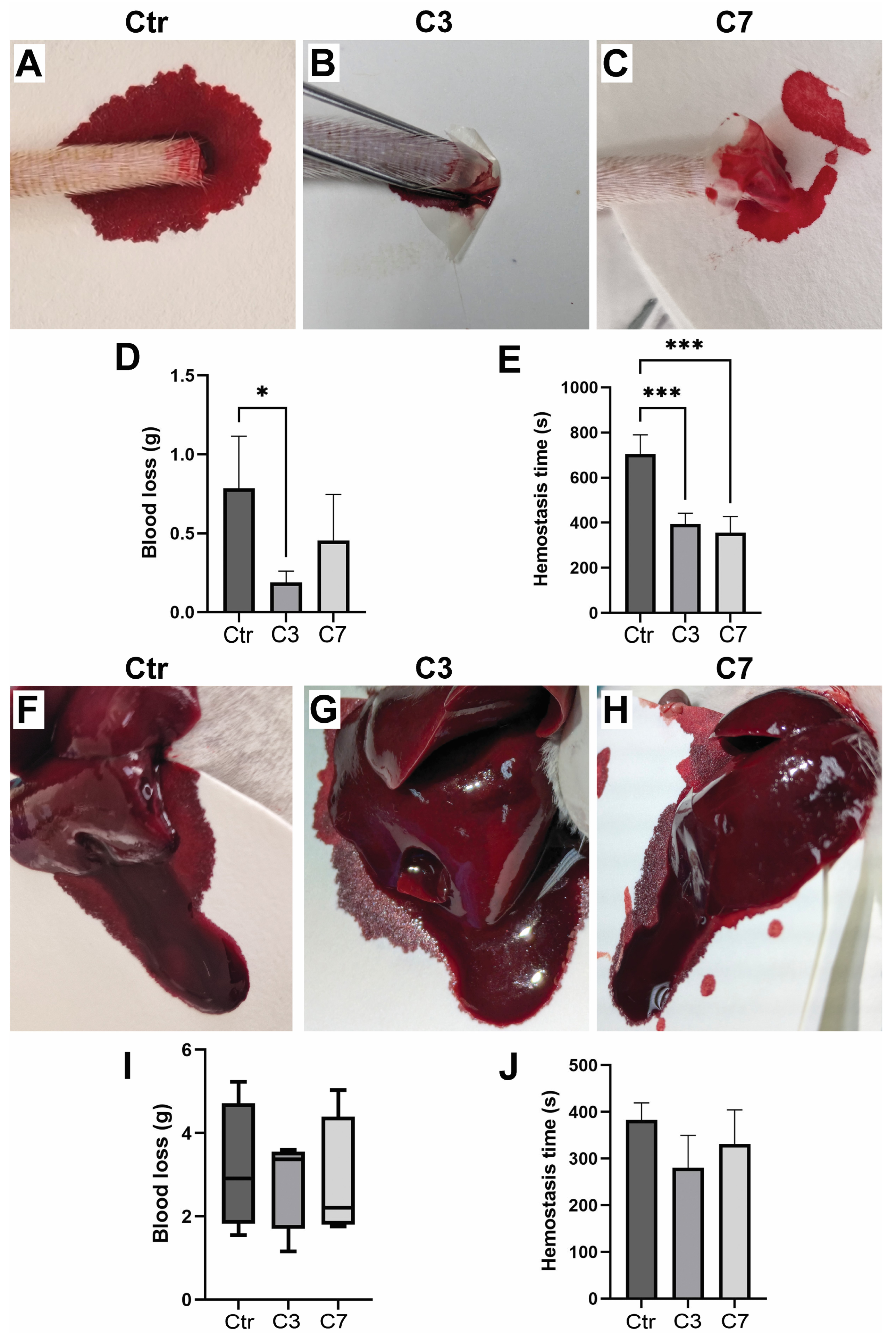
3.8. Assessment of Hemostatic Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sasmal, P.K.; Ganguly, S. Polymer in Hemostasis and Follow-up Wound Healing. J. Appl. Polym. Sci. 2023, 140, e53559. [Google Scholar] [CrossRef]
- Peña, O.A.; Martin, P. Cellular and molecular mechanisms of skin wound healing. Nat. Rev. Mol. Cell. Biol. 2024, 25, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.-Y.; Li, Q.-J.; Hu, J.-J.; Song, Y.-T.; Zhang, Q.-Y.; Nie, R.; Li-Ling, J.; Xie, H.-Q. Design of Biopolymer-Based Hemostatic Material: Starting from Molecular Structures and Forms. Mater. Today Bio 2022, 17, 100468. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, M. Thrombosis and von Willebrand Factor. In Thrombosis and Embolism: From Research to Clinical Practice; Islam, M.S., Ed.; Springer International Publishing: Cham, Switzerland, 2016; Volume 906, pp. 285–306. [Google Scholar]
- Sung, Y.K.; Lee, D.R.; Chung, D.J. Advances in the Development of Hemostatic Biomaterials for Medical Application. Biomater. Res. 2021, 25, 37. [Google Scholar] [CrossRef]
- Sutar, T.; Bangde, P.; Dandekar, P.; Adivarekar, R. Herbal Hemostatic Biopolymeric Dressings of Alginate/Pectin Coated with Croton Oblongifolius Extract. Carbohydr. Polym. Technol. Appl. 2021, 2, 100025. [Google Scholar] [CrossRef]
- Mecwan, M.; Li, J.; Falcone, N.; Ermis, M.; Torres, E.; Morales, R.; Hassani, A.; Haghniaz, R.; Mandal, K.; Sharma, S.; et al. Recent Advances in Biopolymer-Based Hemostatic Materials. Regen. Biomater. 2022, 9, rbac063. [Google Scholar] [CrossRef]
- Suarato, G.; Bertorelli, R.; Athanassiou, A. Borrowing From Nature: Biopolymers and Biocomposites as Smart Wound Care Materials. Front. Bioeng. Biotechnol. 2018, 6, 137. [Google Scholar] [CrossRef]
- Zhang, M.; Han, F.; Duan, X.; Zheng, D.; Cui, Q.; Liao, W. Advances of Biological Macromolecules Hemostatic Materials: A Review. Int. J. Biol. Macromol. 2024, 269, 131772. [Google Scholar] [CrossRef]
- Rothe, R.; Xu, Y.; Thomas, A.K.; Meister, S.; Zhang, Y.; Pietzsch, J.; Hauser, S. A Modular, Injectable, Non-Covalently Assembled Hydrogel System Features Widescale Tunable Degradability for Controlled Release and Tissue Integration. Biomaterials 2021, 269, 120637. [Google Scholar] [CrossRef]
- Fang, Y.; Guo, W.; Ni, P.; Liu, H. Recent Research Advances in Polysaccharide-Based Hemostatic Materials: A Review. Int. J. Biol. Macromol. 2024, 271, 132559. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, L.; Li, P.; Hao, X.; Yang, X.; Xi, G.; Liu, W.; Feng, Y.; He, H.; Shi, C. Polysaccharide Based Hemostatic Strategy for Ultrarapid Hemostasis. Macromol. Biosci. 2020, 20, 1900370. [Google Scholar] [CrossRef] [PubMed]
- Dogaru, B.-I.; Simionescu, B.; Popescu, M.-C. Synthesis and Characterization of κ-Carrageenan Bio-Nanocomposite Films Reinforced with Bentonite Nanoclay. Int. J. Biol. Macromol. 2020, 154, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Mihaila, S.M.; Gaharwar, A.K.; Reis, R.L.; Marques, A.P.; Gomes, M.E.; Khademhosseini, A. Photocrosslinkable Kappa-Carrageenan Hydrogels for Tissue Engineering Applications. Adv. Healthc. Mater. 2013, 2, 895–907. [Google Scholar] [CrossRef]
- Sun, T.; Tao, H.; Xie, J.; Zhang, S.; Xu, X. Degradation and Antioxidant Activity of Κ-carrageenans. J. Appl. Polym. Sci. 2010, 117, 194–199. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Q.; Ning, Z.; Ma, Y.; Huang, Y.; Wu, Y.; Yang, Y.; Xiao, M.; Ye, J. Preparation, Antibacterial Activity, and Structure-Activity Relationship of Low Molecular Weight κ-Carrageenan. Int. J. Biol. Macromol. 2024, 266, 131021. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, A.; Lu, Z.; Qin, C.; Hu, J.; Yin, J. Overview on the Antiviral Activities and Mechanisms of Marine Polysaccharides from Seaweeds. Carbohydr. Res. 2017, 453–454, 1–9. [Google Scholar] [CrossRef]
- Pourshahrestani, S.; Zeimaran, E.; Kadri, N.A.; Mutlu, N.; Boccaccini, A.R. Polymeric Hydrogel Systems as Emerging Biomaterial Platforms to Enable Hemostasis and Wound Healing. Adv Healthc. Mater. 2020, 9, 2000905. [Google Scholar] [CrossRef]
- Li, L.; Ni, R.; Shao, Y.; Mao, S. Carrageenan and Its Applications in Drug Delivery. Carbohydr. Polym. 2014, 103, 1–11. [Google Scholar] [CrossRef]
- Liu, J.; Zhan, X.; Wan, J.; Wang, Y.; Wang, C. Review for Carrageenan-Based Pharmaceutical Biomaterials: Favourable Physical Features versus Adverse Biological Effects. Carbohydr. Polym. 2015, 121, 27–36. [Google Scholar] [CrossRef]
- Pacheco-Quito, E.-M.; Ruiz-Caro, R.; Veiga, M.-D. Carrageenan: Drug Delivery Systems and Other Biomedical Applications. Mar. Drugs 2020, 18, 583. [Google Scholar] [CrossRef]
- Salmasi, S.S.; Ehsani, M.; Zandi, M.; Saeed, M.; Sabeti, M. Polysaccharide-Based (Kappa Carrageenan/Carboxymethyl Chitosan) Nanofibrous Membrane Loaded with Antifibrinolytic Drug for Rapid Hemostasis-In Vitro and In Vivo Evaluation. Int. J. Biol. Macromol. 2023, 247, 125786. [Google Scholar] [CrossRef]
- Biranje, S.S.; Madiwale, P.V.; Patankar, K.C.; Chhabra, R.; Bangde, P.; Dandekar, P.; Adivarekar, R.V. Cytotoxicity and Hemostatic Activity of Chitosan/Carrageenan Composite Wound Healing Dressing for Traumatic Hemorrhage. Carbohydr. Polym. 2020, 239, 116106. [Google Scholar] [CrossRef] [PubMed]
- Arunagiri, V.; Tsai, H.-C.; Darge, H.F.; Chiang, H.W.; Thankachan, D.; Mei, C.-J.; Lai, J.-Y. Preparation of Physically Crosslinked Polyelectrolyte Gelatin-Tannic Acid-κ-Carrageenan (GTC) Microparticles as Hemostatic Agents. Int. J. Biol. Macromol. 2021, 191, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.C.; Aygin, D. Honey dressing in wound treatment: A systematic review. Complement. Ther. Med. 2020, 51, 102388. [Google Scholar] [CrossRef] [PubMed]
- Forrest, R.D. Early History of Wound Treatment. J. R. Soc. Med. 1982, 75, 198–205. [Google Scholar] [CrossRef]
- El-Kased, R.F.; Amer, R.I.; Attia, D.; Elmazar, M.M. Honey-Based Hydrogel: In Vitro and Comparative In Vivo Evaluation for Burn Wound Healing. Sci. Rep. 2017, 7, 9692. [Google Scholar] [CrossRef]
- Nezhad-Mokhtari, P.; Javanbakht, S.; Asadi, N.; Ghorbani, M.; Milani, M.; Hanifehpour, Y.; Gholizadeh, P.; Akbarzadeh, A. Recent Advances in Honey-Based Hydrogels for Wound Healing Applications: Towards Natural Therapeutics. J. Drug Deliv. Sci. Technol. 2021, 66, 102789. [Google Scholar] [CrossRef]
- Kurek-Górecka, A.; Górecki, M.; Rzepecka-Stojko, A.; Balwierz, R.; Stojko, J. Bee Products in Dermatology and Skin Care. Molecules 2020, 25, 556. [Google Scholar] [CrossRef]
- Ahmed, A.-S.A.-A.; Eltregy, S.; Kandil, M.I. Honey Dressing: A Missed Way for Orthopaedic Wound Care. Int. Orthop. SICOT 2022, 46, 2483–2491. [Google Scholar] [CrossRef]
- Rathinamoorthy, R.; Sasikala, L. In Vivo—Wound Healing Studies of Leptospermum Scoparium Honey Loaded Chitosan Bioactive Wound Dressing. Wound Med. 2019, 26, 100162. [Google Scholar] [CrossRef]
- Ahmed, A.; Khan, R.A.; Azim, M.K.; Saeed, S.A.; Mesaik, M.A.; Ahmed, S.; Imran, I. Effect of natural honey on human platelets and blood coagulation proteins, Pak. J. Pharm. Sci. 2011, 24, 389–397. [Google Scholar]
- Ranneh, Y.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A.; Zakaria, Z.A.; Albujja, M.; Bakar, M.F.A. Honey and Its Nutritional and Anti-Inflammatory Value. BMC Complement. Med. Ther. 2021, 21, 30. [Google Scholar] [CrossRef] [PubMed]
- Pleeging, C.C.F.; Wagener, F.A.D.T.G.; De Rooster, H.; Cremers, N.A.J. Revolutionizing Non-Conventional Wound Healing Using Honey by Simultaneously Targeting Multiple Molecular Mechanisms. Drug Resist. Updat. 2022, 62, 100834. [Google Scholar] [CrossRef]
- Muley, B.; Khadabadi, S.; Banarase, N. Phytochemical Constituents and Pharmacological Activities of Calendula officinalis Linn (Asteraceae): A Review. Trop. J. Pharm. Res. 2009, 8, 455–465. [Google Scholar] [CrossRef]
- Arora, D.; Rani, A.; Sharma, A. A Review on Phytochemistry and Ethnopharmacological Aspects of Genus Calendula. Pharmacogn. Rev. 2013, 7, 179. [Google Scholar] [CrossRef]
- Silva, D.; Ferreira, M.S.; Sousa-Lobo, J.M.; Cruz, M.T.; Almeida, I.F. Anti-Inflammatory Activity of Calendula officinalis L. Flower Extract. Cosmetics 2021, 8, 31. [Google Scholar] [CrossRef]
- Venkatesh, D.P.; Gheena, S.; Ramani, P.; Rajeshkumar, S.; Ramalingam, K.; Shanmugam, R. In Vitro Evaluation of Antioxidant and Anti-Inflammatory Potentials of Herbal Formulation Containing Marigold Flower (Calendula officinalis L.) Tea. Cureus 2023, 15, e43308. [Google Scholar] [CrossRef]
- Alnuqaydan, A.M.; Lenehan, C.E.; Hughes, R.R.; Sanderson, B.J. Extracts from Calendula officinalis Offer In Vitro Protection Against H2O2 Induced Oxidative Stress Cell Killing of Human Skin Cells. Phytother. Res. 2015, 29, 120–124. [Google Scholar] [CrossRef]
- Çetin, B.; Kalyoncu, F.; Kurtuluş, B. Antibacterial Activities of Calendula officinalis Callus Extract. Int. J. Second. Metab. 2017, 4, 257–263. [Google Scholar] [CrossRef]
- Chaleshtori, S.H.; Kachoie, M.A.; Pirbalouti, A.G. Phytochemical Analysis and Antibacterial Effects of Calendula officinalis Essential Oil. Biosci. Biotech. Res. Comm. 2016, 9, 517–522. [Google Scholar] [CrossRef]
- Efstratiou, E.; Hussain, A.I.; Nigam, P.S.; Moore, J.E.; Ayub, M.A.; Rao, J.R. Antimicrobial Activity of Calendula officinalis Petal Extracts Against Fungi, as Well as Gram-Negative and Gram-Positive Clinical Pathogens. Complement. Ther. Clin. Pract. 2012, 18, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Shahane, K.; Kshirsagar, M.; Tambe, S.; Jain, D.; Rout, S.; Ferreira, M.K.M.; Mali, S.; Amin, P.; Srivastav, P.P.; Cruz, J.; et al. An Updated Review on the Multifaceted Therapeutic Potential of Calendula officinalis L. Pharmaceuticals 2023, 16, 611. [Google Scholar] [CrossRef] [PubMed]
- Ejiohuo, O.; Folami, S.; Maigoro, A.Y. Calendula in Modern Medicine: Advancements in Wound Healing and Drug Delivery Applications. Eur. J. Med. Chem. Rep. 2024, 12, 100199. [Google Scholar] [CrossRef]
- Buzzi, M.; de Freitas, F.; Winter, M. A Prospective, Descriptive Study to Assess the Clinical Benefits of Using Calendula officinalis Hydroglycolic Extract for the Topical Treatment of Diabetic Foot Ulcers. Ostomy Wound Manag. 2016, 62, 8–24. [Google Scholar]
- Pal, K.; Kundu, S.K.; Mandal, M.K.; Kundu, T.K. Prothrombin time test, Phytochemical screening, Thin layer chromatographic study and Quantitative study of Calendula officinalis leaves. Int. J. Pharm. Pharm. Sci. 2015, 2, 43–49. [Google Scholar]
- Ayyanahalli Matta, B.K.; Kumar, S.; Mehta, C.H.; Nayak, U.Y.; Rodriguez, P.G. Comparative Evaluation of the Effectiveness of a Combination of Absorbable Gelatin Sponge and Calendula officinalis with Absorbable Gelatin Sponge Used Alone as a Hemostatic Agent—An In-Vitro Study. Dent. J. 2022, 10, 76. [Google Scholar] [CrossRef]
- Ishfaq, B.; Khan, I.U.; Khalid, S.H.; Asghar, S. Design and Evaluation of Sodium Alginate-Based Hydrogel Dressings Containing Betula Utilis Extract for Cutaneous Wound Healing. Front. Bioeng. Biotechnol. 2023, 11, 1042077. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Chen, K.-S.; Run-Chu, L. Design and Evaluation of Drug-Loaded Wound Dressing Having Thermoresponsive, Adhesive, Absorptive and Easy Peeling Properties. Biomaterials 2001, 22, 2999–3004. [Google Scholar] [CrossRef]
- Huber, D.; Grzelak, A.; Baumann, M.; Borth, N.; Schleining, G.; Nyanhongo, G.S.; Guebitz, G.M. Anti-Inflammatory and Anti-Oxidant Properties of Laccase-Synthesized Phenolic-O-Carboxymethyl Chitosan Hydrogels. New Biotechnol. 2018, 40, 236–244. [Google Scholar] [CrossRef]
- Lu, G.; Ling, K.; Zhao, P.; Xu, Z.; Deng, C.; Zheng, H.; Huang, J.; Chen, J. A Novel in Situ-Formed Hydrogel Wound Dressing by the Photocross-Linking of a Chitosan Derivative. Wound Repair Regen. 2010, 18, 70–79. [Google Scholar] [CrossRef]
- Morgan, C.E.; Prakash, V.S.; Vercammen, J.M.; Pritts, T.; Kibbe, M.R. Development and Validation of 4 Different Rat Models of Uncontrolled Hemorrhage. JAMA Surg. 2015, 150, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, R.; Ghosh, A.; Barui, A.; Datta, P. Repositing Honey Incorporated Electrospun Nanofiber Membranes to Provide Anti-Oxidant, Anti-Bacterial and Anti-Inflammatory Microenvironment for Wound Regeneration. J. Mater. Sci. Mater. Med. 2018, 29, 31. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Lan, X.; Liang, C.; Zhong, Z.; Xie, R.; Zhou, Y.; Miao, X.; Wang, H.; Wang, W. Honey Loaded Alginate/PVA Nanofibrous Membrane as Potential Bioactive Wound Dressing. Carbohydr. Polym. 2019, 219, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Pelin, I.M.; Silion, M.; Popescu, I.; Rîmbu, C.M.; Fundueanu, G.; Constantin, M. Pullulan/Poly(Vinyl Alcohol) Hydrogels Loaded with Calendula officinalis Extract: Design and In Vitro Evaluation for Wound Healing Applications. Pharmaceutics 2023, 15, 1674. [Google Scholar] [CrossRef]
- Amruth, P.; Joy, J.M.; Visnuvinayagam, S.; Remya, S.; Mathew, S. Development of κ-Carrageenan-Based Transparent and Absorbent Biodegradable Films for Wound Dressing Applications. Int. J. Biol. Macromol. 2024, 282, 137084. [Google Scholar]
- Kosimaningrum, W.E.; Barleany, D.R.; Sako, V.N.; Ristiyanti, R. Preparation of Gelatin-Chitosan-Honey-Based Hydrogel for Potential Active Material of Wound Care Dressing Application. In Materials Science Forum; Trans Tech Publications Ltd.: Zurich, Switzerland, 2020; Volume 988, pp. 162–168. [Google Scholar]
- Oliveira, R.N.; Meleiro, L.A.D.C.; Quilty, B.; McGuinness, G.B. Release of Natural Extracts from PVA and PVA-CMC Hydrogel Wound Dressings: A Power Law Swelling/Delivery. Front. Bioeng. Biotechnol. 2024, 12, 1406336. [Google Scholar] [CrossRef]
- Gounden, V.; Singh, M. Hydrogels and Wound Healing: Current and Future Prospects. Gels 2024, 10, 43. [Google Scholar] [CrossRef]
- Şalva, E.; Akdağ, A.E.; Alan, S.; Arısoy, S.; Akbuğa, F.J. Evaluation of the Effect of Honey-Containing Chitosan/Hyaluronic Acid Hydrogels on Wound Healing. Gels 2023, 9, 856. [Google Scholar] [CrossRef]
- Tomić, S.L.; Vuković, J.S.; Babić Radić, M.M.; Filipović, V.V.; Živanović, D.P.; Nikolić, M.M.; Nikodinovic-Runic, J. Manuka Honey/2-Hydroxyethyl Methacrylate/Gelatin Hybrid Hydrogel Scaffolds for Potential Tissue Regeneration. Polymers 2023, 15, 589. [Google Scholar] [CrossRef]
- Kędzierska, M.; Sala, K.; Bańkosz, M.; Grzela, K.; Potemski, P.; Miernik, K.; Tyliszczak, B. Enhanced Hydrogel Materials: Incorporating Vitamin C and Plant Extracts for Biomedical Applications. Molecules 2024, 29, 2633. [Google Scholar] [CrossRef]
- Morris, A.H.; Stamer, D.K.; Kyriakides, T.R. The host response to naturally-derived extracellular matrix biomaterials. Semin. Immunol. 2017, 29, 72–91. [Google Scholar] [CrossRef] [PubMed]
- Božinovski, T.L.; Marković, D.; Todorović, V.; Bolka, B.P.; Milošević, I.; Drndarević, N.; Nešović, K.; Yop, R.K.; Mišković-Stanković, V. In vivo investigation of soft tissue response of novel silver/poly (vinyl alcohol)/graphene and silver/poly (vinyl alcohol)/chitosan/graphene hydrogels aimed for medical applications–the first experience. Acta Vet. Beogr. 2018, 68, 321–339. [Google Scholar] [CrossRef]
- Popa, E.G.; Carvalho, P.P.; Dias, A.F.; Santos, T.C.; Santo, V.E.; Marques, A.P.; Viegas, C.A.; Dias, I.R.; Gomes, M.E.; Reis, R.L. Evaluation of the in vitro and in vivo biocompatibility of carrageenan-based hydrogels. J. Biomed. Mater. Res. A 2014, 102, 4087–4097. [Google Scholar] [CrossRef] [PubMed]
- Giusto, G.; Beretta, G.; Vercelli, C.; Valle, E.; Iussich, S.; Borghi, R.; Odetti, P.; Monacelli, F.; Tramuta, C.; Grego, E.; et al. Pectin-honey hydrogel: Characterization, antimicrobial activity and biocompatibility. Biomed. Mater. Eng. 2018, 29, 347–356. [Google Scholar] [CrossRef]
- Pedram Rad, Z.; Mokhtari, J.; Abbasi, M. Preparation and characterization of Calendula officinalis-loaded PCL/gum arabic nanocomposite scaffolds for wound healing applications. Iran. Polym. J. 2019, 28, 51–63. [Google Scholar] [CrossRef]
- Givol, O.; Kornhaber, R.; Visentin, D.; Cleary, M.; Haik, J.; Harats, M. A systematic review of Calendula officinalis extract for wound healing. Wound Repair Regen. 2019, 27, 548–561. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, J.; Li, M.; Liu, Z.; Wang, X.; Zhang, L.; Wang, Z. Hydrogel-based biomaterials engineered from natural-derived polysaccharides and proteins for hemostasis and wound healing. Front. Bioeng. Biotech. 2021, 9, 780187. [Google Scholar] [CrossRef]
- Madruga, L.Y.; Popat, K.C.; Balaban, R.C.; Kipper, M.J. Enhanced blood coagulation and antibacterial activities of carboxymethyl-kappa-carrageenan-containing nanofibers. Carbohyd. Polym. 2021, 273, 118541. [Google Scholar] [CrossRef]
- Basch, E.; Bent, S.; Foppa, I.; Haskmi, S.; Kroll, D.; Mele, M.; Szapary, P.; Ulbricht, C.; Vora, M.; Yong, S. Marigold (Calendula officinalis L.) an evidence-based systematic review by the natural standard research collaboration. J. Herb. Pharmacother. 2006, 6, 135–159. [Google Scholar] [CrossRef]
- Suzuki, A.; Tomita, H.; Okada, H. Form follows function: The endothelial glycocalyx. Transl. Res. 2022, 247, 158–167. [Google Scholar] [CrossRef]



| Formulation | Honey (wt% of Polymer Content) | COF (wt% of Polymer Content) | Glycerol (wt% of Polymer Content) |
|---|---|---|---|
| C1 | - | - | 5 |
| C2 | 5 | - | 5 |
| C3 | 5 | 5 | 5 |
| C4 | 5 | 10 | 5 |
| C5 | 10 | - | 5 |
| C6 | 10 | 5 | 5 |
| C7 | 10 | 10 | 5 |
| Formulation | Thickness (μm) ± SD | Weight Variation (g) ± SD | Folding Endurance (Number of Folds) ± SD |
|---|---|---|---|
| C1 | 21.23 ± 3.41 | 0.009 ± 0.001 | 498 ± 5 |
| C2 | 22.05 ± 4.28 | 0.010 ± 0.002 | 501 ± 3 |
| C3 | 22.98 ± 3.77 | 0.011 ± 0.001 | 500 ± 4 |
| C4 | 23.56 ± 3.82 | 0.011 ± 0.003 | 502 ± 5 |
| C5 | 24.15 ± 4.09 | 0.012 ± 0.001 | 503 ± 3 |
| C6 | 24.84 ± 5.01 | 0.014 ± 0.002 | 507 ± 5 |
| C7 | 25.22 ± 4.73 | 0.015 ± 0.001 | 508 ± 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vuković, J.S.; Perišić, S.; Nikolić, A.; Milošević, I.; Mirilović, M.; Bolka Prokić, B.; Lužajić Božinovski, T. Toward Natural Wound Healing Therapy: Honey and Calendula officinalis Loaded κ-Carrageenan Films with Promising Hemostatic Potential. Pharmaceutics 2025, 17, 578. https://doi.org/10.3390/pharmaceutics17050578
Vuković JS, Perišić S, Nikolić A, Milošević I, Mirilović M, Bolka Prokić B, Lužajić Božinovski T. Toward Natural Wound Healing Therapy: Honey and Calendula officinalis Loaded κ-Carrageenan Films with Promising Hemostatic Potential. Pharmaceutics. 2025; 17(5):578. https://doi.org/10.3390/pharmaceutics17050578
Chicago/Turabian StyleVuković, Jovana S., Srđan Perišić, Anja Nikolić, Ivan Milošević, Milorad Mirilović, Bogomir Bolka Prokić, and Tijana Lužajić Božinovski. 2025. "Toward Natural Wound Healing Therapy: Honey and Calendula officinalis Loaded κ-Carrageenan Films with Promising Hemostatic Potential" Pharmaceutics 17, no. 5: 578. https://doi.org/10.3390/pharmaceutics17050578
APA StyleVuković, J. S., Perišić, S., Nikolić, A., Milošević, I., Mirilović, M., Bolka Prokić, B., & Lužajić Božinovski, T. (2025). Toward Natural Wound Healing Therapy: Honey and Calendula officinalis Loaded κ-Carrageenan Films with Promising Hemostatic Potential. Pharmaceutics, 17(5), 578. https://doi.org/10.3390/pharmaceutics17050578







