Clove Oil-Based Nanoemulsion Containing Amphotericin B as a Therapeutic Approach to Combat Fungal Infections
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Chemical Characterization of Syzygium aromaticum Essential Oil
2.2.2. First Experimental Design
2.2.3. Second Experimental Design and Formulation Selection
2.2.4. Physicochemical Characterization of Clove Oil Nanoemulsions
2.2.5. In Vitro Release Analysis
2.2.6. In Vitro Biological Activity
Antifungal Activity by Agar Disc Diffusion of Nanoemulsions
Antifungal Activity by Microdilution of Nanoemulsions
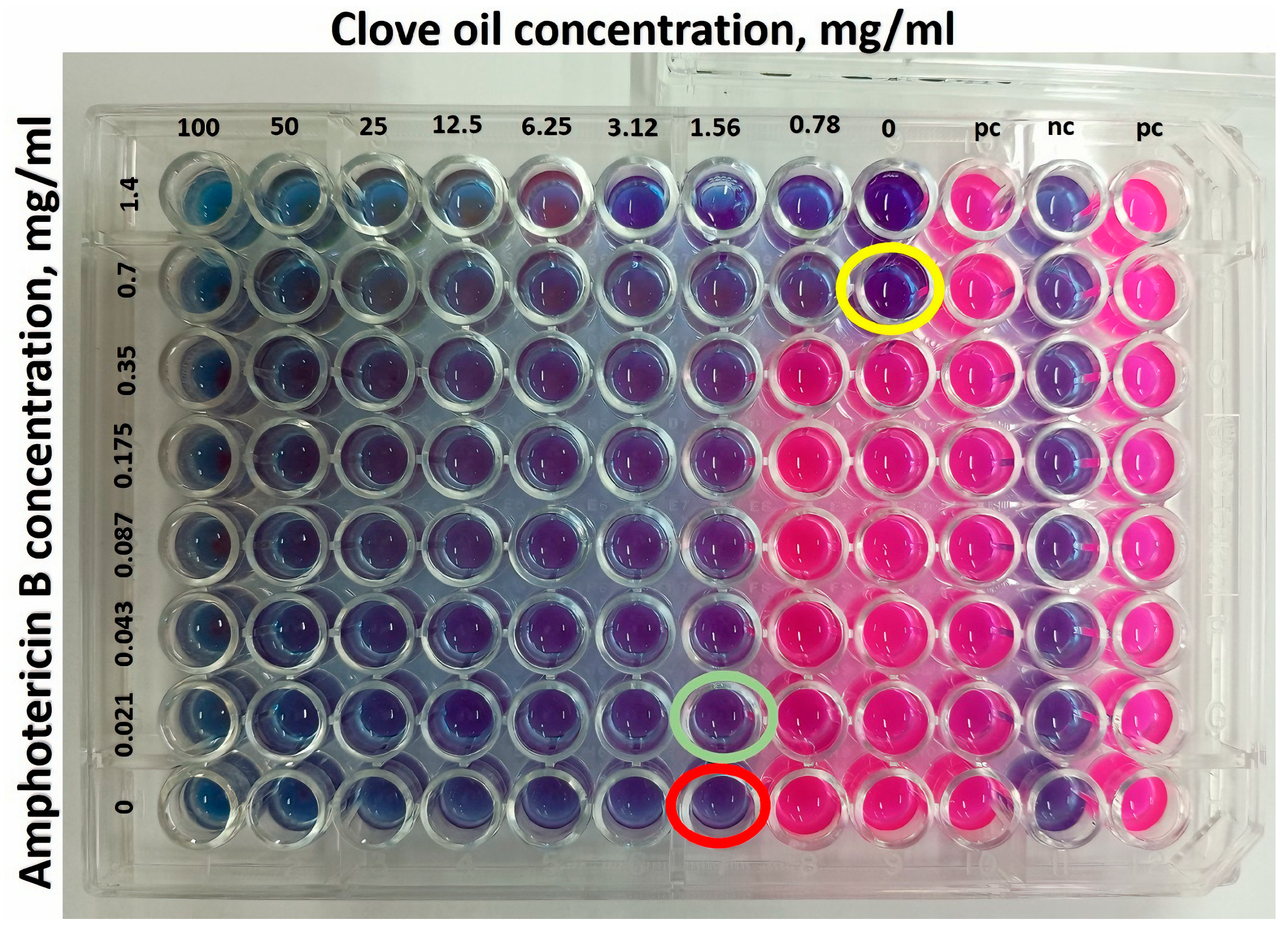
Checkerboard Assay
2.2.7. Statistical Analysis
3. Results and Discussion
3.1. Identification of the Chemical Components of Syzygium aromaticum Essential Oil
3.2. First Experimental Design and Selection of the Amphotericin B-Free Formulation
3.3. Second Experimental Design and Formulation Selection with Drug
3.4. Physicochemical Characterization of the Nanoemulsions
3.5. Stability Studies
3.6. In Vitro Release Studies
3.7. In Vitro Biological Activity of Clove Oil Nanoemulsions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rasool, M.; Mazhar, D.; Afzal, I.; Zeb, A.; Khan, S.; Ali, H. In vitro and in vivo characterization of Miconazole Nitrate loaded transethosomes for the treatment of Cutaneous Candidiasis. Int. J. Pharm. 2023, 647, 123563. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Hong, T.; Jiang, Y.; Whiteway, M.; Zhang, S. Candidiasis: From cutaneous to systemic, new perspectives of potential targets and therapeutic strategies. Adv. Drug Deliv. Rev. 2023, 199, 114960. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.A.; Wilson, C.M.; Pappas, P.G. Invasive Candidiasis. Infect. Dis. Clin. N. Am. 2025, 39, 93–119. [Google Scholar] [CrossRef] [PubMed]
- Holt, A.M.; Nett, J.E. Innate immune response to Candida auris. Curr. Opin. Microbiol. 2024, 80, 102510. [Google Scholar] [CrossRef] [PubMed]
- Dakalbab, S.; Hamdy, R.; Holigová, P.; Abuzaid, E.J.; Abu-Qiyas, A.; Lashine, Y.; Mohammad, M.G.; Soliman, S.S.M. Uniqueness of Candida auris cell wall in morphogenesis, virulence, resistance, and immune evasion. Microbiol. Res. 2024, 286, 127797. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.B.L.; Paes, R.A.; Schubach, A.O. Sporothrix schenckii and Sporotrichosis. Clin. Microbiol. Rev. 2011, 24, 633–654. [Google Scholar] [CrossRef] [PubMed]
- Santiago, M.G.; Silva, C.D.; Souza, B.M.; Assis, B.R.D.; Pinto, P.N.; Keller, K.M.; Vilela, R.V.R.; Oliveira, C.S.F.; Goulart, G.A.C. Topical hydrophilic gel with itraconazole-loaded polymeric nanomicelles improves wound healing in the treatment of feline sporotrichosis. Int. J. Pharm. 2023, 634, 122619. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Aubry, L.; Brandalise, D.; Coste, A.T.; Sanglard, D.; Lamoth, F. Upc2-mediated mechanisms of azole resistance in Candida auris. Microbiol. Spectr. 2024, 12, e03526-23. [Google Scholar] [CrossRef] [PubMed]
- Long, B.; Lacy, A.J.; Koyfman, A.; Liang, S.Y. Candida auris: A focused review for emergency clinicians. Am. J. Emerg. Med. 2024, 84, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Orofino-Costa, R.; Freitas, D.F.S.; Bernardes-Engemann, A.R.; Rodrigues, A.M.; Talhari, C.; Ferraz, C.E.; Veasey, J.V.; Quintella, L.; Sousa, M.S.L.A.; Vettorato, R.; et al. Human sporotrichosis: Recommendations from the Brazilian Society of Dermatology for the clinical, diagnostic and therapeutic management. An. Bras. Dermatol. 2022, 97, 757–777. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Panigrahi, C.; Agarwal, S.; Khuntia, A.; Sahoo, M. Recent trends and advancements in nanoemulsions: Production methods, functional properties, applications in food sector, safety and toxicological effects. Food Phys. 2024, 1, 100024. [Google Scholar] [CrossRef]
- Marena, G.D.; Ramos, M.A.S.; Carvalho, G.C.; Lima, L.C.; Nascimento, A.L.C.S.; Sábio, R.M.; Rodero, C.F.; Spósito, L.; Bauab, T.M.; Chorilli, M. Development and characterization of an amphotericin B—Loaded nanoemulsion applied to Candida auris biofilms control. J. Drug Del. Sci. Tech. 2022, 74, 103566. [Google Scholar] [CrossRef]
- Siqueira, L.B.O.; Matos, A.P.S.; Cardoso, V.S.; Villanova, J.C.O.; Guimarães, B.C.L.R.; Santos, E.P.; Beatriz Vermelho, A.B.; Santos-Oliveira, R.; Junior, E.R. Clove oil nanoemulsion showed potent inhibitory effect against Candida spp. Nanotechnology 2019, 30, 425101. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.K.; Srivastava, S.; Singh, R.; Dar, A.H.; Dash, K.K. Effects of clove essential oil (Caryophyllus aromaticus L.) nanoemulsion incorporated edible coating on shelf-life of fresh cut apple pieces. J. Agric. Food Res. 2023, 14, 100791. [Google Scholar] [CrossRef]
- Pandey, V.K.; Srivastava, S.; Ashish; Dash, K.K.; Singh, R.; Dar, A.H.; Singh, T.; Farooqui, A.; Shaikh, A.M.; Kovacs, B. Bioactive properties of clove (Syzygium aromaticum) essential oil nanoemulsion: A comprehensive review. Heliyon 2024, 10, e22437. [Google Scholar] [CrossRef] [PubMed]
- El amrania, S.; El Ouali Lalami, A.; Ez zoubi, Y.; Moukhafi, K.; Bouslamti, R.; Lairini, S. Evaluation of antibacterial and antioxidant effects of cinnamon and clove essential oils from Madagascar. Mater. Today Proc. 2019, 13, 762–770. [Google Scholar] [CrossRef]
- Braga, P.C.; Dal Sasso, M.; Culici, M.; Alfieri, M. Eugenol and thymol, alone or in combination, induce morphological alterations in the envelope of Candida albicans. Fitoterapia 2007, 78, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Pootong, A.; Chumphon, C.; Jangjaibun, P.; Mungkornkeaw, N.; Norrapong, B. Eugenol Affects the Germ Tube Formation and Cell Adhesion of Candida albicans. J. Pure Appl. Microbiol. 2022, 16, 2802–2809. [Google Scholar] [CrossRef]
- Cuddihy, G.; Wasan, E.K.; Di, Y.; Wasan, K.M. The Development of Oral Amphotericin B to Treat Systemic Fungal and Parasitic Infections: Has the Myth Been Finally Realized? Pharmaceutics 2019, 11, 99. [Google Scholar] [CrossRef] [PubMed]
- Marcelino, H.R.; Solgadi, A.; Chéron, M.; Egito, E.S.T.; Ponchel, G. Exploring the permeability of Amphotericin B trough serum albumin dispersions and lipid nanocarriers for oral delivery. Int. J. Pharm. 2023, 646, 123444. [Google Scholar] [CrossRef] [PubMed]
- Maw, P.D.; Pienpinijtham, P.; Pruksakorn, P.; Jansook, P. Cyclodextrin-based Pickering nanoemulsions containing amphotericin B: Part II. Formulation, antifungal activity, and chemical stability. J. Drug. Deliv. Sci. Technol. 2022, 69, 103174. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An Add-In Program for Modeling and Comparison of Drug Dissolution Profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Lobo, J.M.S. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.J.C. In vitro evaluation of the lyoequivalence of pharmaceutical formulations. Braz. J. Pharm. Sci. 2022, 38, 141–153. [Google Scholar] [CrossRef]
- Peppas, N.A.; Narasimhan, B. Mathematical models in drug delivery: How modeling has shaped the way we design new drug delivery systems. J. Control. Release 2014, 190, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, V.S.; Vermelho, A.B.; Junior, E.R.; Rodrigues, I.A.; Mazotto, A.M.; Supuran, C.T. Antileishmanial activity of sulphonamide nanoemulsions targeting the β-carbonic anhydrase from Leishmania species. J. Enzyme Inhib. Med. Chem. 2018, 33, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Berditsch, M.; Jäger, T.; Strempel, N.; Schwartz, T.; Overhage, J.; Ulrich, A.S. Synergistic Effect of Membrane-Active Peptides Polymyxin B and Gramicidin S on Multidrug-Resistant Strains and Biofilms of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2015, 59, 5288–5296. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Ali, S.M.; Yosipovitch, G. Skin pH: From Basic Science to Basic Skin Care. Acta Derm. Venereol. 2013, 93, 261–267. [Google Scholar] [CrossRef] [PubMed]
- JP, S.B.; Sahu, P.; Vinode, R.; Patel, A.; Alomary, M.N.; Begum, M.Y.; Jamous, Y.F.; Siddiqua, A.; Al Fatease, A.; Ansari, M.A. Antimicrobial Nanoemulsion: A futuristic approach in antibacterial drug delivery system. J. Saudi Chem. Soc. 2024, 28, 101896. [Google Scholar] [CrossRef]
- Sosa, L.; Clares, B.; Alvarado, H.L.; Bozal, N.; Domenech, O.; Calpena, A.C. Amphotericin B releasing topical nanoemulsion for the treatment of candidiasis and aspergillosis. Nanomedicine 2017, 13, 2303–2312. [Google Scholar] [CrossRef] [PubMed]
- Matos, A.P.S.; Lopes, D.C.D.X.P.; Peixoto, M.L.H.; Cardoso, V.S.; Vermelho, A.B.; Santos-Oliveira, R.; Viçosa, A.L.; Holandino, C.; Júnior, E.R. Development, characterization, and anti-leishmanial activity of topical amphotericin B nanoemulsions. Drug Deliv. Transl. Res. 2020, 10, 1552–1570. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Samad, A.; Singh, S.K.; Ahsan, M.N.; Haque, M.W.; Faruk, A.; Ahmed, F.J. Nanoemulsion gel-based topical delivery of an antifungal drug. In vitro activity and in vivo evaluation. Drug Deliv. 2016, 23, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar] [PubMed]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2001, 48, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Whaley, S.G.; Berkow, E.L.; Rybak, J.M.; Nishimoto, A.T.; Barker, K.S.; Rogers, P.D. Azole Antifungal Resistance in Candida albicans and Emerging Non-albicans Candida Species. Front. Microbiol. 2017, 7, 2173. [Google Scholar] [CrossRef] [PubMed]
- Boechat, J.S.; Oliveira, M.M.E.; Gremião, I.D.F.; Almeida-Paes, R.; Machado, A.C.S.; Zancopé-Oliveira, R.M.; Oliveira, R.V.C.; Morgado, D.S.; Corrêa, M.L.; Figueiredo, A.B.F.; et al. Sporothrix brasiliensis and Feline Sporotrichosis in the Metropolitan Region of Rio de Janeiro, Brazil (1998–2018). J. Fungi 2022, 8, 749. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.B.; Gomes, A.R.; Morais, M.H.F.; Bohm, B.C.; Waller, S.B.; Faria, R.O.; Bruhn, N.C.P.; Bruhn, F.R.P. Profile and temporal dynamics of the feline sporotrichosis epidemic in southern Brazil: A forecasting analysis. Vet. Parasitol. Reg. Stud. Reports. 2024, 54, 101091. [Google Scholar] [CrossRef] [PubMed]
- Konuk, H.B.; Bengü, E. Phenolic –OH group is crucial for the antifungal activity of terpenoids via disruption of cell membrane integrity. Folia Microbiol. 2020, 65, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Shahina, Z.; Ndlovu, E.; Persaud, O.; Sultana, T.; Dahms, T.E.S. Candida albicans Reactive Oxygen Species (ROS)-Dependent Lethality and ROS-Independent Hyphal and Biofilm Inhibition by Eugenol and Citral. Microbiol. Spectr. 2022, 10, e0318322. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, M.; Zielińska-Bliźniewska, H.; Kwiatkowski, P.; Łopusiewicz, Ł.; Pruss, A.; Kostek, M.; Kochan, E.; Sienkiewicz, M. Inhibitory Effect of Eugenol and trans-Anethole Alone and in Combination with Antifungal Medicines on Candida albicans Clinical Isolates. Chem. Biodivers. 2021, 18, e2000843. [Google Scholar] [CrossRef] [PubMed]
- Tavvabi-Kashani, N.; Hasanpour, M.; Rahimi, V.B.; Vahdati-Mashhadian, N.; Askari, V.R. Pharmacodynamic, pharmacokinetic, toxicity, and recent advances in Eugenol’s potential benefits against natural and chemical noxious agents: A mechanistic review. Toxicon 2024, 238, 107607. [Google Scholar] [CrossRef] [PubMed]
- Rajkowska, K.; Otlewska, A.; Kunicka-Styczynska, A.; Krajewska, A. Candida albicans Impairments Induced by Peppermint and Clove Oils at Sub-Inhibitory Concentrations. Int. J. Mol. Sci. 2017, 18, 1307. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.A.; Gabriel, K.T.; Graham, K.D.; Butts, B.K.; Cornelison, C.T. Antifungal Activity of Select Essential Oils against Candida auris and Their Interactions with Antifungal Drugs. Pathogens 2022, 11, 821. [Google Scholar] [CrossRef] [PubMed]
- Schuenck-Rodrigues, R.A.; de Oliveira de Siqueira, L.B.; Dos Santos Matos, A.P.; da Costa, S.P.; da Silva Cardoso, V.; Vermelho, A.B.; Colombo, A.P.V.; Oliveira, C.A.; Santos-Oliveira, R.; Ricci-Júnior, E. Development, characterization and photobiological activity of nanoemulsion containing zinc phthalocyanine for oral infections treatment. J. Photochem. Photobiol. B 2020, 211, 112010. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.A.; Malik, A.; Ahmad, I. Anti-candidal activity of essential oils alone and in combination with amphotericin B or fluconazole against multi-drug resistant isolates of Candida albicans. Med. Mycol. 2012, 50, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.N.; Khan, S.; Misba, L.; Sharief, M.; Hashmi, A.; Khan, A.U. Synergistic fungicidal activity with low doses of eugenol and amphotericin B against Candida albicans. Biochem. Biophys. Res. Commun. 2019, 518, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.W.; Su, L.; Yu, D.T.; Chertow, G.M.; Seger, D.L.; Gomes, D.R.; Dasbach, E.J.; Platt, R. Mortality and Costs of Acute Renal Failure Associated with Amphotericin B Therapy. Clin. Infect. Dis. 2001, 32, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Wade, R.L.; Chaudhari, P.; Natoli, J.L.; Taylor, R.J.; Nathanson, B.H.; Horn, D. Comparison of adverse events and hospital length of stay associated with various amphotericin B formulations: Sequential conventional amphotericin b/lipid versus lipid-only therapy for the treatment of invasive fungal infections in hospitalized patients. Pharm. Ther. 2013, 38, 278–287. [Google Scholar]
- Laniado-Laborín, R.; Cabrales-Vargas, M.N. Amphotericin B: Side effects and toxicity. Rev. Iberoam. Micol. 2009, 26, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Shigemi, A.; Matsumoto, K.; Ikawa, K.; Yaji, K.; Shimodozono, Y.; Morikawa, N.; Takeda, Y.; Yamada, K. Safety analysis of liposomal amphotericin B in adult patients: Anaemia, thrombocytopenia, nephrotoxicity, hepatotoxicity and hypokalaemia. Int. J. Antimicrob. Agents. 2011, 38, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Perez, A.P.; Altube, M.J.; Schilrreff, P.; Apezteguia, G.; Celes, F.S.; Zacchino, S.; Oliveira, C.I.; Romero, E.L.; Morilla, M.J. Topical amphotericin B in ultradeformable liposomes: Formulation, skin penetration study, antifungal and antileishmanial activity in vitro. Colloids Surf. B Biointerfaces 2016, 139, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Chime, S.A.; Kenechukwu, F.C.; Attama, A.A. Nanoemulsions—Advances in formulation, characterization and applications in drug delivery. In Application of Nanotechnology in Drug Delivery; IntechOpen: Rijeka, Croatia, 2014; pp. 77–126. [Google Scholar] [CrossRef]

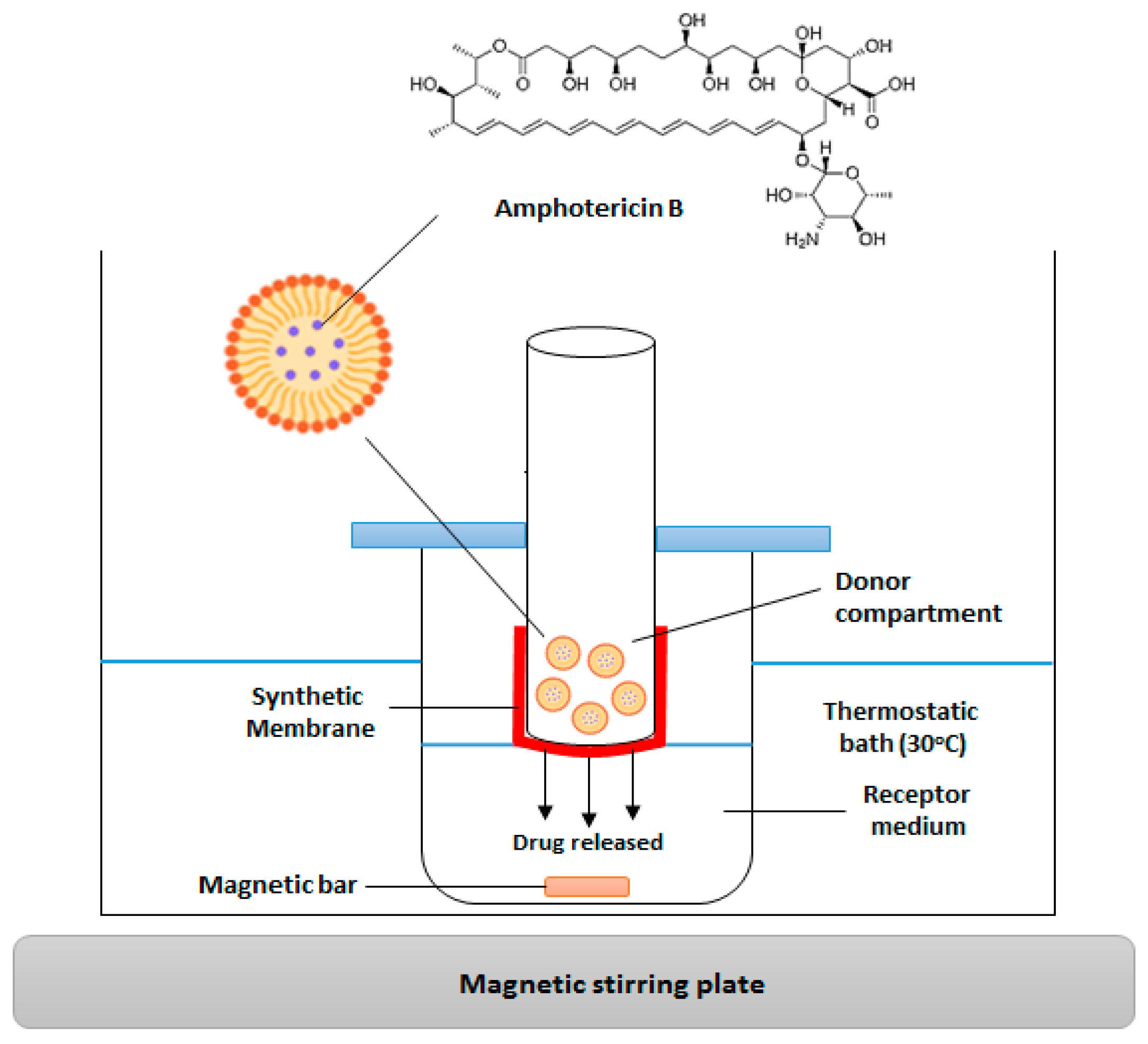


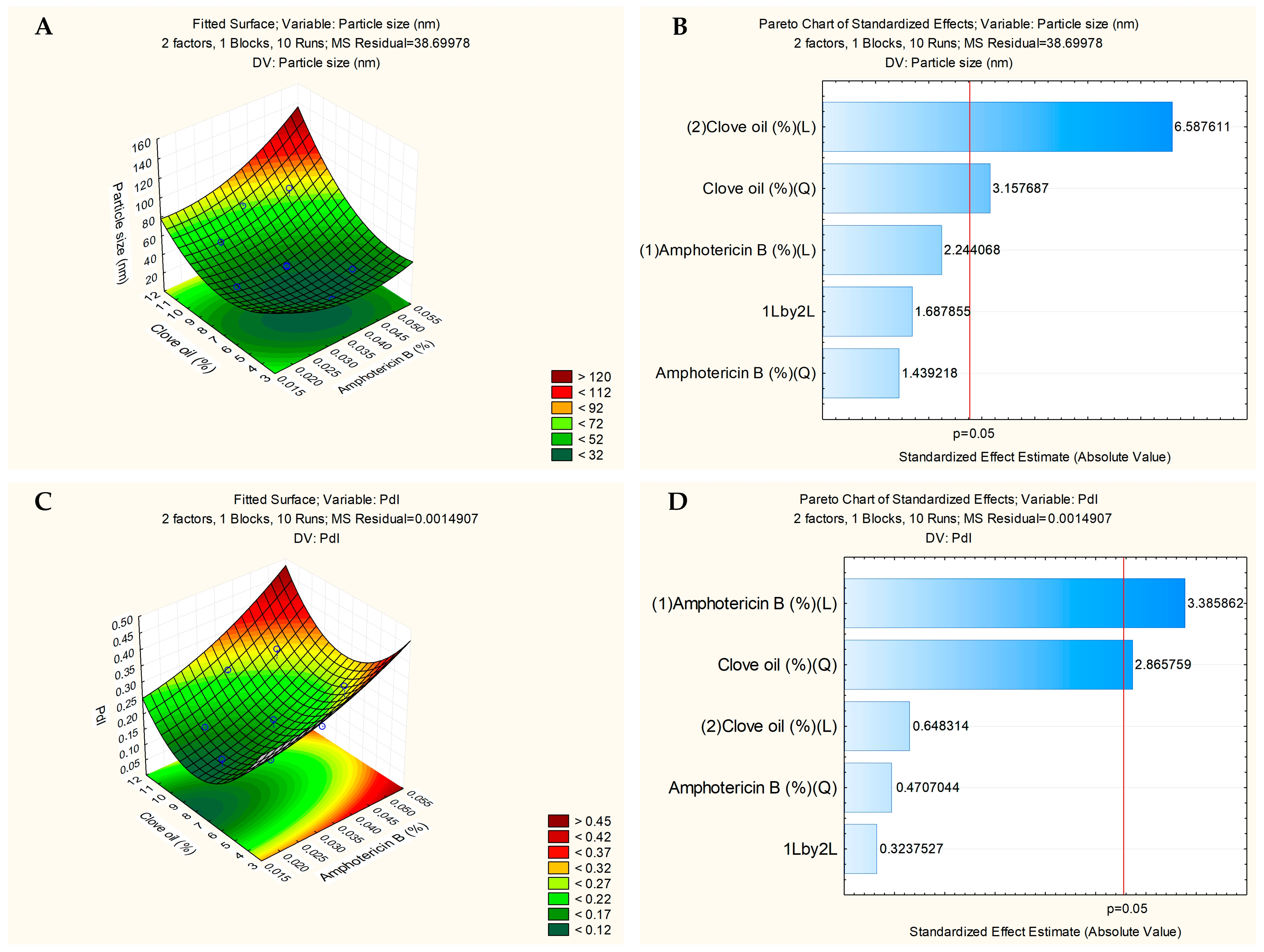
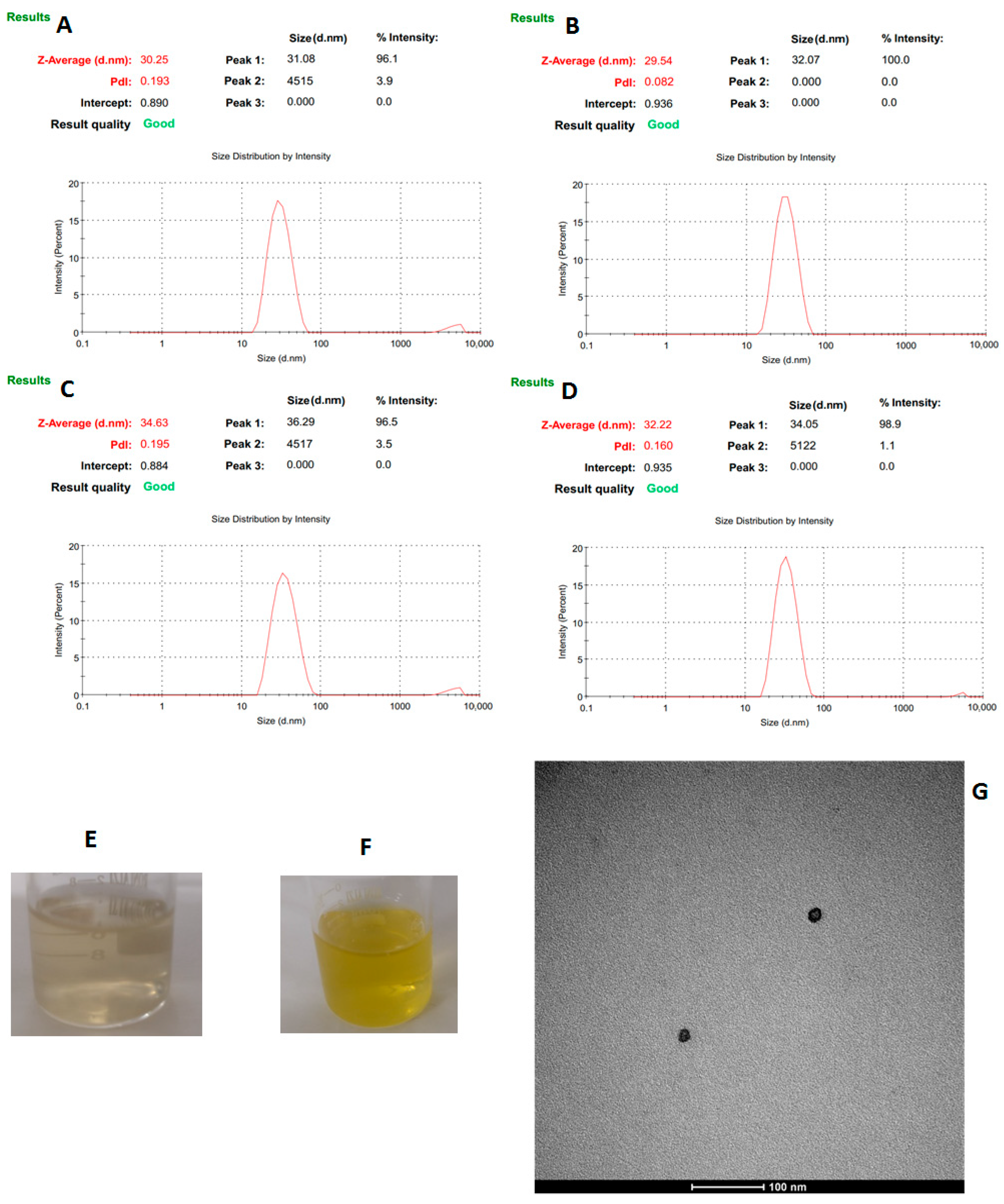
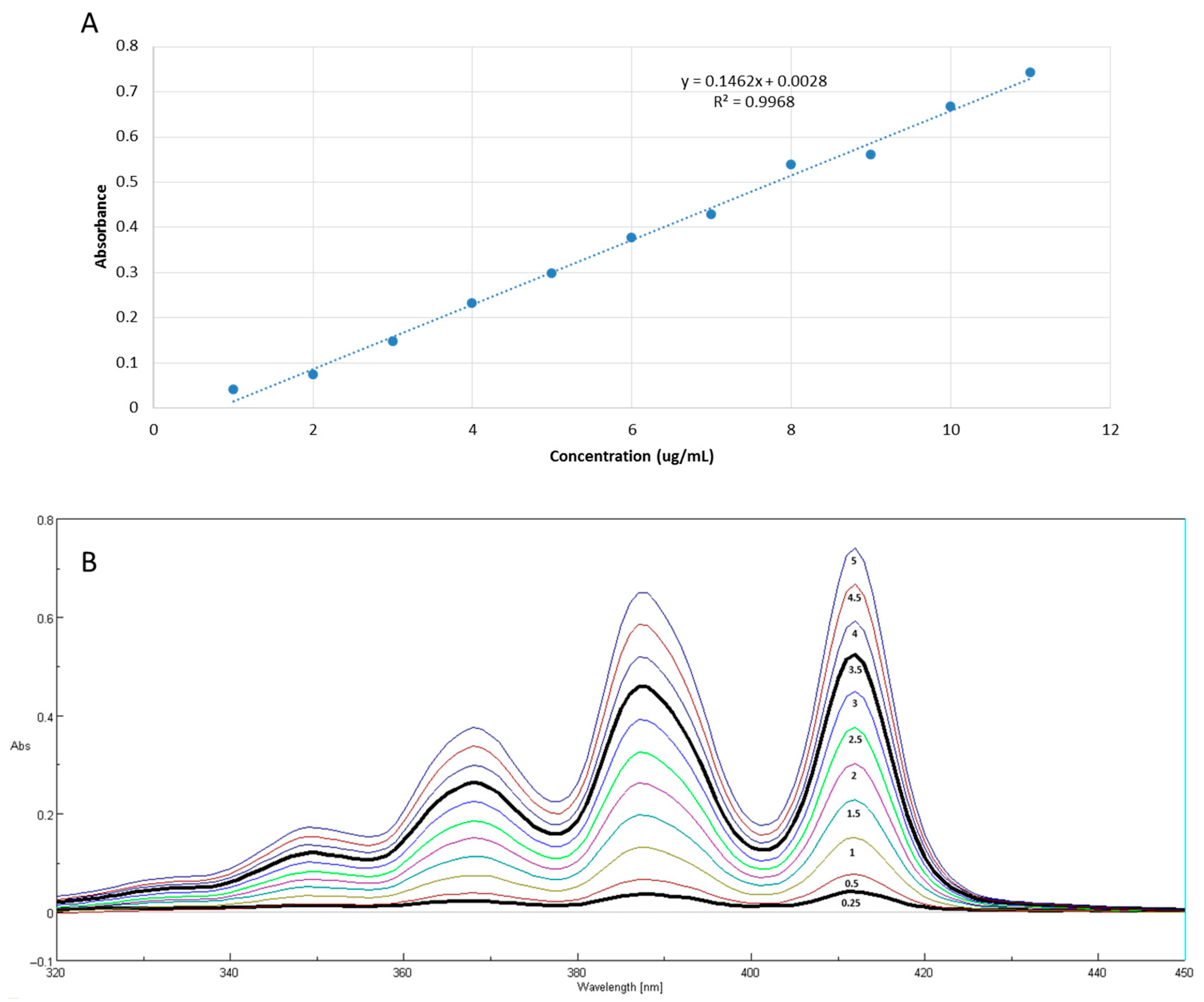


| Independent Factors | (wt/vol) (%) | Level | (wt/vol) (%) | Level | (wt/vol) (%) | Level |
|---|---|---|---|---|---|---|
| Clove oil | 5 | Low (−1) | 7.5 | Center (0) | 10 | High (1) |
| Pluronic® F-127 | 5 | Low (−1) | 7.5 | Center (0) | 10 | High (1) |
| Formulation | Clove Oil (wt/vol) (%) | Pluronic® F-127 (wt/vol) (%) | Particle Size (nm) | PDI |
|---|---|---|---|---|
| NESAF-01 | 5 | 5 | 35.170 ± 3.352 | 0.120 ± 0.045 |
| NESAF-02 | 10 | 5 | 114.800 ± 1.697 | 0.314 ± 0.011 |
| NESAF-03 * | 7.5 | 7.5 | 50.030 ± 3.762 | 0.187 ± 0.041 |
| NESAF-04 | 11 | 7.5 | 88.633 ± 12.258 | 0.271 ± 0.077 |
| NESAF-05 | 4 | 7.5 | 29.825 ± 2.326 | 0.108 ± 0.095 |
| NESAF-06 | 5 | 10 | 28.523 ± 0.675 | 0.072 ± 0.012 |
| NESAF-07 | 10 | 10 | 52.860 ± 0.509 | 0.122 ± 0.028 |
| NESAF-08 | 7.5 | 4.0 | 90.423 ± 5.597 | 0.318 ± 0.030 |
| NESAF-09 | 7.5 | 11 | 35.735 ± 8.436 | 0.117 ± 0.066 |
| Independent Factors | (wt/vol) (%) | Level | (wt/vol) (%) | Level | (wt/vol) (%) | Level |
|---|---|---|---|---|---|---|
| Amphotericin B | 0.025 | Low (−1) | 0.035 | Center (0) | 0.045 | High (1) |
| Clove oil | 5 | Low (−1) | 7.5 | Center (0) | 10 | High (1) |
| Formulation | Clove Oil (wt/vol) (%) | Amphotericin B (wt/vol) (%) | Particle Size (nm) | PDI |
|---|---|---|---|---|
| NEMLB-01 | 10 | 0.045 | 75.870 ± 6.817 | 0.328 ± 0.025 |
| NEMLB-02 | 7.5 | 0.049 | 41.070 ± 3.677 | 0.242 ± 0.032 |
| NEMLB-03 | 10 | 0.025 | 53.265 ± 1.916 | 0.176 ± 0.023 |
| NEMLB-04 | 4 | 0.035 | 32.055 ± 0.587 | 0.247 ± 0.045 |
| NEMLB-05 * | 7.5 | 0.035 | 34.615 ± 0.587 | 0.160 ± 0.006 |
| NEMLB-06 | 5 | 0.025 | 32.155 ± 0.049 | 0.218 ± 0.028 |
| NEMLB-07 | 5 | 0.045 | 38.810 ± 2.036 | 0.397 ± 0.105 |
| NEMLB-08 | 7.5 | 0.021 | 38.080 ± 1.273 | 0.161 ± 0.012 |
| NEMLB-09 | 11 | 0.035 | 67.215 ± 1.421 | 0.258 ± 0.011 |
| N. | RT | AIrep | AIcalc | Substances | Relative Abundance (%) |
|---|---|---|---|---|---|
| 1 | 31.706 | 1362 | 1356 | Phenylpropanoid eugenol | 80.09 |
| 2 | 32.726 | 1384 | - | n.i. | 0.12 |
| 3 | 34.788 | 1431 | 1427 | β-caryophyllene | 7.74 |
| 4 | 36.279 | 1466 | - | n.i. | 0.72 |
| 5 | 38.834 | 1526 | 1524 | Chavibetol acetate | 11.07 |
| 6 | 41.773 | 1599 | - | n.i. | 0.26 |
| Total Identified | 98.90 | ||||
| IC50 (mg/mL) | |||
|---|---|---|---|
| Fungal Strains | NEMLB-05 (mg/mL of Drug) | Free Amphotericin B (mg/mL of Drug) | NESAF-09 (mg/mL of CO) |
| (mg/mL of CO) | |||
| Sporothrix brasiliensis ATCC 5110 (S5) | AmB: 0.0165 ± 0.0160 d | 0.1464 ± 0.0925 d | |
| CO: 2.1900 ± 1.241 c,e | 3.9929 ± 1.0829 a,b,e | ||
| Sporothrix schenkii ATCC 15,383 (S15) | AmB: 0.0090 ± 0.0004 d | 0.1253 ± 0.0504 d | |
| CO: 1.9191 ± 0.0842 c | 2.5102 ± 0.9016 a,b | ||
| Candida albicans ATCC SC5314 | AmB: 0.0207 ± 0.0160 d | 0.0744 ± 0.0630 d | |
| CO: 0.7667 ± 0.3717 c,e | 2.9110 ± 0.2705 a,e | ||
| Candida auris ATCC MYA-5002 | AmB: 0.0117 ± 0.0016 d | >0.25 d | |
| CO: 2.3801 ± 0.1386 c,e | 7.7016 ± 1.0455 a, e | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Almeida, M.L.; Matos, A.P.d.S.; Cardoso, V.d.S.; do Nascimento, T.; Santos-Oliveira, R.; Rocha, L.M.; Machado, F.P.; Kenechukwu, F.C.; Vermelho, A.B.; Ricci-Júnior, E. Clove Oil-Based Nanoemulsion Containing Amphotericin B as a Therapeutic Approach to Combat Fungal Infections. Pharmaceutics 2025, 17, 925. https://doi.org/10.3390/pharmaceutics17070925
de Almeida ML, Matos APdS, Cardoso VdS, do Nascimento T, Santos-Oliveira R, Rocha LM, Machado FP, Kenechukwu FC, Vermelho AB, Ricci-Júnior E. Clove Oil-Based Nanoemulsion Containing Amphotericin B as a Therapeutic Approach to Combat Fungal Infections. Pharmaceutics. 2025; 17(7):925. https://doi.org/10.3390/pharmaceutics17070925
Chicago/Turabian Stylede Almeida, Marcel Lucas, Ana Paula dos Santos Matos, Veronica da Silva Cardoso, Tatielle do Nascimento, Ralph Santos-Oliveira, Leandro Machado Rocha, Francisco Paiva Machado, Franklin Chimaobi Kenechukwu, Alane Beatriz Vermelho, and Eduardo Ricci-Júnior. 2025. "Clove Oil-Based Nanoemulsion Containing Amphotericin B as a Therapeutic Approach to Combat Fungal Infections" Pharmaceutics 17, no. 7: 925. https://doi.org/10.3390/pharmaceutics17070925
APA Stylede Almeida, M. L., Matos, A. P. d. S., Cardoso, V. d. S., do Nascimento, T., Santos-Oliveira, R., Rocha, L. M., Machado, F. P., Kenechukwu, F. C., Vermelho, A. B., & Ricci-Júnior, E. (2025). Clove Oil-Based Nanoemulsion Containing Amphotericin B as a Therapeutic Approach to Combat Fungal Infections. Pharmaceutics, 17(7), 925. https://doi.org/10.3390/pharmaceutics17070925










