From Structure to Function: The Promise of PAMAM Dendrimers in Biomedical Applications
Abstract
1. Introduction
1.1. Nanotechnology and Biomedical Applications
1.2. Properties and Applications of Dendrimers
1.3. Polyamidoamine Dendrimers: Innovations in Drug Delivery and Targeted Therapy
2. Materials and Methods
2.1. PAMAM Dendrimers
2.2. Loading Mechanisms and Functionalization of Dendrimers for Drug Delivery
- Physical Encapsulation
- 2.
- Electrostatic Complexation
- 3.
- Covalent Conjugation
- 4.
- Surface Modification and Targeting Ligands
- 5.
- Alternative Ligands and Stimuli-Responsive Systems
- 6.
- Multifunctionalization: Antibodies, Peptides, Aptamers, Vitamins, and Sugars
- 7.
- Functionalized Dendrimers with Intrinsic Therapeutic Activity
- 8.
- Ligand-Mediated Targeting and Cellular Uptake
2.3. Cellular Internalization Mechanisms of PAMAM Dendrimers
2.4. Intracellular Trafficking of PAMAM Dendrimers
2.4.1. Nucleus-Targeted PAMAM Dendrimers
2.4.2. Mitochondria-Targeted PAMAM Dendrimers
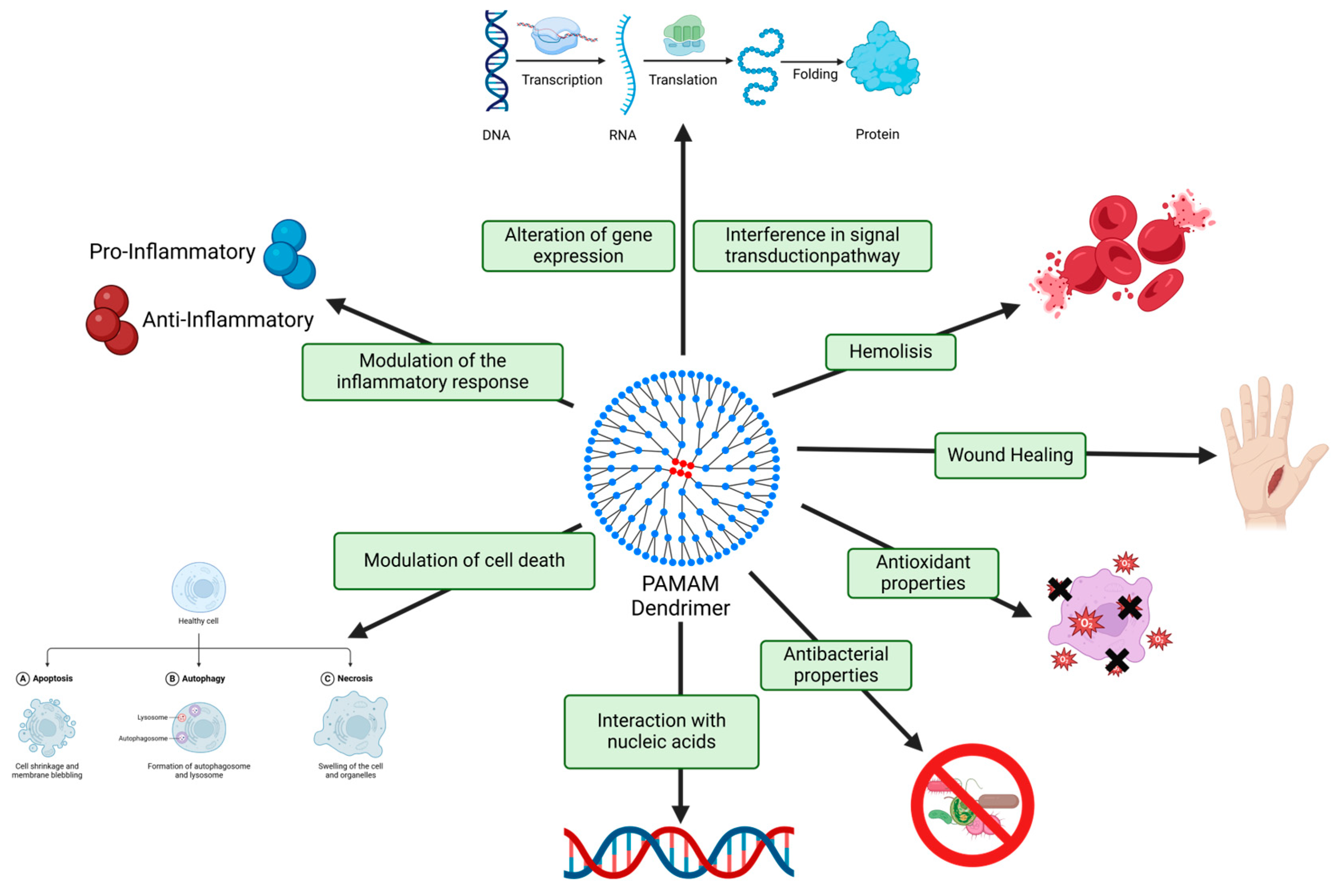
2.5. Harnessing PAMAM Dendrimer Cytotoxicity for Biomedical Applications
2.6. Navigating Biosecurity Challenges of PAMAM Dendrimers for Biomedical Applications
2.7. Applications and Translational Challenges of Surface-Modified PAMAM Dendrimers in Biomedical Therapeutics
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ramos, A.P.; Cruz, M.A.E.; Tovani, C.B.; Ciancaglini, P. Biomedical applications of nanotechnology. Biophys. Rev. 2017, 9, 79–89. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Nanomedicine: Current status and future prospects. FASEB J. 2005, 19, 311–330. [Google Scholar] [CrossRef]
- Sherje, A.P.; Jadhav, M.; Dravyakar, B.R.; Kadam, D. Dendrimers: A versatile nanocarrier for drug delivery and targeting. Int. J. Pharm. 2018, 548, 707–720. [Google Scholar] [CrossRef]
- Hua, Y.; Chen, L.; Hou, C.; Liu, S.; Pei, Z.; Lu, Y. Supramolecular Vesicles Based on Amphiphilic Pillar[n]arenes for Smart Nano-Drug Delivery. Int. J. Nanomed. 2020, 15, 5873–5899. [Google Scholar] [CrossRef]
- Pérez-Ferreiro, M.; MAbelairas, A.; Criado, A.; Gómez, I.J.; Mosquera, J. Dendrimers: Exploring Their Wide Structural Variety and Applications. Polymers 2023, 15, 4369. [Google Scholar] [CrossRef]
- Samad, A.; Alam Md Saxena, K. Dendrimers: A Class of Polymers in the Nanotechnology for the Delivery of Active Pharmaceuticals. Curr. Pharm. Des. 2009, 15, 2958–2969. [Google Scholar] [CrossRef] [PubMed]
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications—Reflections on the field. Adv. Drug Deliv. Rev. 2012, 64, 102–115. [Google Scholar] [CrossRef]
- Kesharwani, P.; Iyer, A.K. Recent advances in dendrimer-based nanovectors for tumor-targeted drug and gene delivery. Drug Discov. Today 2015, 20, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Chis, A.A.; Dobrea, C.; Morgovan, C.; Arseniu, A.M.; Rus, L.L.; Butuca, A.; Juncan, A.M.; Totan, M.; Vonica-Tincu, A.L.; Cormos, G.; et al. Applications and Limitations of Dendrimers in Biomedicine. Molecules 2020, S25, 3982. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abedi-Gaballu, F.; Dehghan, G.; Ghaffari, M.; Yekta, R.; Abbaspour-Ravasjani, S.; Baradaran, B.; Dolatabadi, J.E.N.; Hamblin, M.R. PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Appl. Mater. Today 2018, 12, 177–190. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mignani, S.; Shi, X.; Bryszewska, M.; Shcharbin, D.; Majoral, J.P. Engineered phosphorus dendrimers as powerful non-viral nanoplatforms for gene delivery: A great hope for the future of cancer therapeutics. Explor. Target. Antitumor Ther. 2022, 3, 50–61. [Google Scholar] [CrossRef]
- Wijagkanalan, W.; Kawakami, S.; Hashida, M. Designing Dendrimers for Drug Delivery and Imaging: Pharmacokinetic Considerations. Pharm. Res. 2011, 28, 1500–1519. [Google Scholar] [CrossRef]
- Igartúa, D. Dendrímeros como nanotransportadores de drogas y como drogas per se. Divulg. Perfiles Académicos Posgrado 2020, 4, 48. [Google Scholar] [CrossRef]
- Sanz del Olmo, N.; Carloni, R.; Ortega, P.; García-Gallego, S.; de la Mata, F.J. Metallodendrimers as a promising tool in the biomedical field: An overview. In Advances in Organometallic Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–52. [Google Scholar]
- Nanjwade, B.K.; Bechra, H.M.; Derkar, G.K.; Manvi, F.V.; Nanjwade, V.K. Dendrimers: Emerging polymers for drug-delivery systems. Eur. J. Pharm. Sci. 2009, 38, 185–196. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A New Class of Polymers: Starburst-Dendritic Macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Mintzer, M.A.; Grinstaff, M.W. Biomedical applications of dendrimers: A tutorial. Chem. Soc. Rev. 2011, 40, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A.; Fréchet, J.M.J. Discovery of dendrimers and dendritic polymers: A brief historical perspective*. J. Polym. Sci. A Polym. Chem. 2002, 40, 2719–2728. [Google Scholar] [CrossRef]
- Kukowska-Latallo, J.F.; Bielinska, A.U.; Johnson, J.; Spindler, R.; Tomalia, D.A.; Baker, J.R. Efficient transfer of genetic material into mammalian cells using Starburst polyamidoamine dendrimers. Proc. Natl. Acad. Sci. USA 1996, 93, 4897–4902. [Google Scholar] [CrossRef]
- de Brabander-van den Berg, E.M.M.; Meijer, E.W. Poly(propylene imine) Dendrimers: Large-Scale Synthesis by Hetereogeneously Catalyzed Hydrogenations. Angew. Chem. Int. Ed. Engl. 1993, 32, 1308–1311. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Laurent, R.; Majoral, J.-P. Characterization of dendrimers. Adv. Drug Deliv. Rev. 2005, 57, 2130–2146. [Google Scholar] [CrossRef]
- Zhu, W.; Okollie, B.; Bhujwalla, Z.M.; Artemov, D. PAMAM dendrimer-based contrast agents for MR imaging of Her-2/neu receptors by a three-step pretargeting approach. Magn. Reson. Med. 2008, 59, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.J.; Burgers, T.C.Q.; Vlijm, R.; Roos, W.H.; Åberg, C. Rapid Internalization of Nanoparticles by Human Cells at the Single Particle Level. ACS Nano 2023, 17, 16517–16529. [Google Scholar] [CrossRef] [PubMed]
- Araújo RVde Santos Sda, S.; Igne Ferreira, E.; Giarolla, J. New Advances in General Biomedical Applications of PAMAM Dendrimers. Molecules 2018, 23, 2849. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; D’Emanuele, A.; Attwood, D. Solubility enhancement of paclitaxel using a linear-dendritic block copolymer. Int. J. Pharm. 2013, 452, 173–179. [Google Scholar] [CrossRef]
- Lyu, Z.; Ding, L.; Tintaru, A.; Peng, L. Self-Assembling Supramolecular Dendrimers for Biomedical Applications: Lessons Learned from Poly(amidoamine) Dendrimers. Acc. Chem. Res. 2020, 53, 2936–2949. [Google Scholar] [CrossRef]
- Kharwade, R.; More, S.; Warokar, A.; Agrawal, P.; Mahajan, N. Starburst pamam dendrimers: Synthetic approaches, surface modifications, and biomedical applications. Arab. J. Chem. 2020, 13, 6009–6039. [Google Scholar] [CrossRef]
- Kheraldine, H.; Rachid, O.; Habib, A.M.; Al Moustafa, A.E.; Benter, I.F.; Akhtar, S. Emerging innate biological properties of nano-drug delivery systems: A focus on PAMAM dendrimers and their clinical potential. Adv. Drug Deliv. Rev. 2021, 178, 113908. [Google Scholar] [CrossRef]
- Menjoge, A.R.; Kannan, R.M.; Tomalia, D.A. Dendrimer-based drug and imaging conjugates: Design considerations for nanomedical applications. Drug Discov. Today 2010, 15, 171–185. [Google Scholar] [CrossRef]
- Esfand, R.; Tomalia, D.A. Poly(amidoamine) (PAMAM) dendrimers: From biomimicry to drug delivery and biomedical applications. Drug Discov. Today 2001, 6, 427–436. [Google Scholar] [CrossRef]
- Roberts, J.C.; Bhalgat, M.K.; Zera, R.T. Preliminary biological evaluation of polyamidoamine (PAMAM) StarburstTM dendrimers. J. Biomed. Mater. Res. 1996, 30, 53–65. [Google Scholar] [CrossRef]
- Zenze, M.; Daniels, A.; Singh, M. Dendrimers as Modifiers of Inorganic Nanoparticles for Therapeutic Delivery in Cancer. Pharmaceutics 2023, 15, 398. [Google Scholar] [CrossRef] [PubMed]
- Gou, Y.; Hu, L.; Liao, X.; He, J.; Liu, F. Advances of antimicrobial dressings loaded with antimicrobial agents in infected wounds. Front. Bioeng. Biotechnol. 2024, 12, 1431949. [Google Scholar] [CrossRef] [PubMed]
- Roustazadeh, A.; Askari, M.; Heidari, M.H.; Kowsari, M.; Askari, F.; Mehrzad, J.; Hosseinkhani, S.; Alipour, M.; Bardania, H. Enhancing non-viral gene delivery to human T cells through tuning nanoparticles physicochemical features, modulation cellular physiology, and refining transfection strategies. Biomed. Pharmacother. 2025, 183, 117820. [Google Scholar] [CrossRef] [PubMed]
- Tarach, P.; Janaszewska, A. Recent Advances in Preclinical Research Using PAMAM Dendrimers for Cancer Gene Therapy. Int. J. Mol. Sci. 2021, 22, 2912. [Google Scholar] [CrossRef]
- Lee, I.; Majoros, I.J.; Williams, C.R.; Athey, B.D.; Baker, J.R. Interactive Design Strategy for a Multi-Functional PAMAM Dendrimer-Based Nano-Therapeutic Using Computational Models and Experimental Analysis. J. Comput. Theor. Nanosci. 2009, 6, 54–60. [Google Scholar] [CrossRef]
- Jang, W.D.; Kamruzzaman Selim, K.M.; Lee, C.H.; Kang, I.K. Bioinspired application of dendrimers: From bio-mimicry to biomedical applications. Prog. Polym. Sci. 2009, 34, 1–23. [Google Scholar] [CrossRef]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Surekha, B.; Kommana, N.S.; Dubey, S.K.; Kumar, A.V.P.; Shukla, R.; Kesharwani, P. PAMAM dendrimer as a talented multifunctional biomimetic nanocarrier for cancer diagnosis and therapy. Colloids Surf. B Biointerfaces 2021, 204, 111837. [Google Scholar] [CrossRef]
- Fatima, A.; Naseem, N.; Haider, M.F.; Rahman, M.A.; Mall, J.; Saifi, M.S.; Akhtar, J. A comprehensive review on nanocarriers as a targeted delivery system for the treatment of breast cancer. Intell. Pharm. 2024, 2, 415–426. [Google Scholar] [CrossRef]
- Marquez-Miranda, V.; Araya-Duran, I.; Gonzalez-Nilo, F.D. Multiscale Molecular Simulations Applied to Nucleic Acid-Dendrimer Interactions Studies. Curr. Pharm. Des. 2017, 23, 3062–3075. [Google Scholar] [CrossRef]
- Márquez-Miranda, V.; Camarada, M.B.; Araya-Durán, I.; Varas-Concha, I.; Almonacid, D.E.; González-Nilo, F.D. Biomimetics: From Bioinformatics to Rational Design of Dendrimers as Gene Carriers. PLoS ONE 2015, 10, e0138392. [Google Scholar] [CrossRef] [PubMed]
- Parvin, N.; Kumar, V.; Mandal, T.K.; Joo, S.W. Advancements in Nanoporous Materials for Biomedical Imaging and Diagnostics. J. Funct. Biomater. 2024, 15, 226. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Miranda, V.; Peñaloza, J.P.; Araya-Durán, I.; Reyes, R.; Vidaurre, S.; Romero, V.; Fuentes, J.; Céric, F.; Velásquez, L.; González-Nilo, F.D.; et al. Effect of Terminal Groups of Dendrimers in the Complexation with Antisense Oligonucleotides and Cell Uptake. Nanoscale Res. Lett. 2016, 11, 66. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fant, K.; Esbjörner, E.K.; Jenkins, A.; Grossel, M.C.; Lincoln, P.; Nordén, B. Effects of PEGylation and Acetylation of PAMAM Dendrimers on DNA Binding, Cytotoxicity and in Vitro Transfection Efficiency. Mol. Pharm. 2010, 7, 1734–1746. [Google Scholar] [CrossRef]
- Padash Hooshyar, S.; Mehrabian, R.Z.; Ahmad Panahi, H.; Habibi Jouybari, M.; Jalilian, H. Synthesis and characterization of PEGylated dendrimers based on magnetic nanoparticles for letrozole extraction and determination in body fluids and pharmaceutical samples. Microchem. J. 2018, 143, 190–197. [Google Scholar] [CrossRef]
- Chauhan, A.S. Dendrimers for Drug Delivery. Molecules 2018, 23, 938. [Google Scholar] [CrossRef]
- Singh, J.; Jain, K.; Mehra, N.K.; Jain, N.K. Dendrimers in anticancer drug delivery: Mechanism of interaction of drug and dendrimers. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1626–1634. [Google Scholar] [CrossRef]
- Liu, X.; Hao, W.; Lok, C.N.; Wang, Y.C.; Zhang, R.; Wong, K.K.Y. Dendrimer encapsulation enhances anti-inflammatory efficacy of silver nanoparticles. J. Pediatr. Surg. 2014, 49, 1846–1851. [Google Scholar] [CrossRef]
- Kirkby, M.; Sabri AHBin Holmes, A.; Moss, G.P.J.; Scurr, D. PAMAM dendrimers as mediators of dermal and transdermal drug delivery: A review. J. Pharm. Pharmacol. 2024, 76, 1284–1300. [Google Scholar] [CrossRef]
- Dubey, S.K.; Salunkhe, S.; Agrawal, M.; Kali, M.; Singhvi, G.; Tiwari, S.; Saraf, S.; Saraf, S.; Alexander, A. Understanding the Pharmaceutical Aspects of Dendrimers for the Delivery of Anticancer Drugs. Curr. Drug Targets. 2020, 21, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Xu, Z.; Ma, M.; Xu, T. Dendrimers as Drug Carriers: Applications in Different Routes of Drug Administration. J. Pharm. Sci. 2008, 97, 123–143. [Google Scholar] [CrossRef]
- Cheng, Y.; Wu, Q.; Li, Y.; Xu, T. External Electrostatic Interaction versus Internal Encapsulation between Cationic Dendrimers and Negatively Charged Drugs: Which Contributes More to Solubility Enhancement of the Drugs? J. Phys. Chem. B 2008, 112, 8884–8890. [Google Scholar] [CrossRef]
- Liu, Y.; Tee, J.; Chiu, G. Dendrimers in Oral Drug Delivery Application: Current Explorations, Toxicity Issues and Strategies for Improvement. Curr. Pharm. Des. 2015, 21, 2629–2642. [Google Scholar] [CrossRef] [PubMed]
- Richers, M.T.; Passlick, S.; Agarwal, H.; Ellis-Davies, G.C.R. Dendrimer Conjugation Enables Multiphoton Chemical Neurophysiology Studies with an Extended π-Electron Caging Chromophore. Angew. Chem. Int. Ed. 2019, 58, 12086–12090. [Google Scholar] [CrossRef] [PubMed]
- Taratula, O.; Garbuzenko, O.B.; Kirkpatrick, P.; Pandya, I.; Savla, R.; Pozharov, V.P.; He, H.; Minko, T. Surface-engineered targeted PPI dendrimer for efficient intracellular and intratumoral siRNA delivery. J. Control. Release. 2009, 140, 284–293. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Benchaala, I.; Mishra, M.K.; Wykes, S.M.; Hali, M.; Kannan, R.M.; Whittum-Hudson, J.A. Folate-functionalized dendrimers for targeting Chlamydia-infected tissues in a mouse model of reactive arthritis. Int. J. Pharm. 2014, 466, 258–265. [Google Scholar] [CrossRef]
- Lee, H. Molecular Simulations of PEGylated Biomolecules, Liposomes, and Nanoparticles for Drug Delivery Applications. Pharmaceutics 2020, 12, 533. [Google Scholar] [CrossRef]
- Li, J.; Chen, L.; Liu, N.; Li, S.; Hao, Y.; Zhang, X. EGF-coated nano-dendriplexes for tumor-targeted nucleic acid delivery in vivo. Drug Deliv. 2015, 23, 1718–1725. [Google Scholar]
- Zhang, J.; Li, M.; Wang, M.; Xu, H.; Wang, Z.; Li, Y.; Ding, B.; Gao, J. Effects of the surface charge of polyamidoamine dendrimers on cellular exocytosis and the exocytosis mechanism in multidrug-resistant breast cancer cells. J. Nanobiotechnol. 2021, 19, 135. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, C.; Pan, D.; Li, J.; Hu, J.; Bains, A.; Guys, N.; Zhu, H.; Li, X.; Luo, K.; Gong, Q.; et al. Enzyme-responsive peptide dendrimer-gemcitabine conjugate as a controlled-release drug delivery vehicle with enhanced antitumor efficacy. Acta Biomater. 2017, 55, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Taghdisi, S.M.; Danesh, N.M.; Ramezani, M.; Lavaee, P.; Jalalian, S.H.; Robati, R.Y.; Abnous, K. Double targeting and aptamer-assisted controlled release delivery of epirubicin to cancer cells by aptamers-based dendrimer in vitro and in vivo. Eur. J. Pharm. Biopharm. 2016, 102, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Vagena, I.A.; Malapani, C.; Gatou, M.A.; Lagopati, N.; Pavlatou, E.A. Enhancement of EPR Effect for Passive Tumor Targeting: Current Status and Future Perspectives. Appl. Sci. 2025, 15, 3189. [Google Scholar] [CrossRef]
- Abd-El-Aziz, A.S.; Agatemor, C.; Etkin, N.; Bissessur, R.; Overy, D.; Lanteigne, M.; McQuillan, K.; Kerr, R.G. Quaternized and Thiazole-Functionalized Free Radical-Generating Organometallic Dendrimers as Antimicrobial Platform against Multidrug-Resistant Microorganisms. Macromol Biosci. 2017, 17, 1700020. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Liu, N.; Ma, M.; Wang, L.; Hao, Y.; Zhang, X. A novel EGFR-targeted gene delivery system based on complexes self-assembled by EGF, DNA, and activated PAMAM dendrimers. Int. J. Nanomed. 2012, 7, 4625–4635. [Google Scholar][Green Version]
- Ghaffari, M.; Dehghan, G.; Abedi-Gaballu, F.; Kashanian, S.; Baradaran, B.; Ezzati Nazhad Dolatabadi, J.; Losic, D. Surface functionalized dendrimers as controlled-release delivery nanosystems for tumor targeting. Eur. J. Pharm. Sci. 2018, 122, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Yameen, B.; Choi WIl Vilos, C.; Swami, A.; Shi, J.; Farokhzad, O.C. Insight into nanoparticle cellular uptake and intracellular targeting. J. Control. Release 2014, 190, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Kim Jwon Lee Jjae Choi, J.S.; Kim, H.S. Electrostatically assembled dendrimer complex with a high-affinity protein binder for targeted gene delivery. Int. J. Pharm. 2018, 544, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Albertazzi, L.; Serresi, M.; Albanese, A.; Beltram, F. Dendrimer Internalization and Intracellular Trafficking in Living Cells. Mol. Pharm. 2010, 7, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Prokop, A.; Davidson, J.M. Nanovehicular Intracellular Delivery Systems. J. Pharm. Sci. 2008, 97, 3518–3590. [Google Scholar] [CrossRef]
- Maher, M.A.; Byrne, H.J. Modification of the in vitro uptake mechanism and antioxidant levels in HaCaT cells and resultant changes to toxicity and oxidative stress of G4 and G6 poly(amidoamine) dendrimer nanoparticles. Anal. Bioanal. Chem. 2016, 408, 5295–5307. [Google Scholar] [CrossRef]
- Hwang, M.E.; Keswani, R.K.; Pack, D.W. Dependence of PEI and PAMAM Gene Delivery on Clathrin- and Caveolin-Dependent Trafficking Pathways. Pharm. Res. 2015, 32, 2051–2059. [Google Scholar] [CrossRef]
- Perumal, O.P.; Inapagolla, R.; Kannan, S.; Kannan, R.M. The effect of surface functionality on cellular trafficking of dendrimers. Biomaterials 2008, 29, 3469–3476. [Google Scholar] [CrossRef] [PubMed]
- Seib, F.P.; Jones, A.T.; Duncan, R. Comparison of the endocytic properties of linear and branched PEIs, and cationic PAMAM dendrimers in B16f10 melanoma cells. J. Control. Release 2007, 117, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Han, M.H.; Chen, J.; Wang, J.; Chen, S.L.; Wang, X.T. Blood Compatibility of Polyamidoamine Dendrimers and Erythrocyte Protection. J. Biomed. Nanotechnol. 2010, 6, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Champion, J.A.; Mitragotri, S. Role of target geometry in phagocytosis. Proc. Natl. Acad. Sci. USA 2006, 103, 4930–4934. [Google Scholar] [CrossRef]
- Vidal, F.; Vásquez, P.; Díaz, C.; Nova, D.; Alderete, J.; Guzmán, L. Mechanism of PAMAM Dendrimers Internalization in Hippocampal Neurons. Mol. Pharm. 2016, 13, 3395–3403. [Google Scholar] [CrossRef]
- Kitchens, K.M.; Foraker, A.B.; Kolhatkar, R.B.; Swaan, P.W.; Ghandehari, H. Endocytosis and Interaction of Poly (Amidoamine) Dendrimers with Caco-2 Cells. Pharm. Res. 2007, 24, 2138–2145. [Google Scholar] [CrossRef]
- Patel, P.M.; Malek, M.A.H. Dendrimers for drug solubility enhancement—A review. Int. J. Pharm. Sci. Res. 2020, 11, 507–523. [Google Scholar] [CrossRef]
- Yellepeddi, V.K.; Palakurthi, S.S.; Yellepeddi, V.K. Poly(amido amine) dendrimers in oral delivery. Drug Deliv. Transl. Res. 2016, 6, 661–677. [Google Scholar] [CrossRef]
- Saovapakhiran, A.; D’Emanuele, A.; Attwood, D.; Penny, J. Surface Modification of PAMAM Dendrimers Modulates the Mechanism of Cellular Internalization. Bioconjugate Chem. 2009, 20, 693–701. [Google Scholar] [CrossRef]
- Kannan, R.M.; Nance, E.; Kannan, S.; Tomalia, D.A. Emerging concepts in dendrimer-based nanomedicine: From design principles to clinical applications. J. Intern. Med. 2014, 276, 579–617. [Google Scholar] [CrossRef]
- Yang, Y.; Sunoqrot, S.; Stowell, C.; Ji, J.; Lee, C.W.; Kim, J.W.; Khan, S.A.; Hong, S. Effect of size, surface charge, and hydrophobicity of poly(amidoamine) dendrimers on their skin penetration. Biomacromolecules 2012, 13, 2154–2162. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sahay, G.; Alakhova, D.Y.; Kabanov, A.V. Endocytosis of nanomedicines. J. Control. Release 2010, 145, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Pegoraro, C.; Domingo-Ortí, I.; Conejos-Sánchez, I.; Vicent, M.J. Unlocking the Mitochondria for Nanomedicine-based Treatments: Overcoming Biological Barriers, Improving Designs, and Selecting Verification Techniques. Adv. Drug Deliv. Rev. 2024, 207, 115195. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.F.; Su, C.J.; Wei, M.C.; Chen, C.Y.; Liao, Z.X.; Lee, P.W.; Chen, H.L.; Sung, H.W. Effects of the nanostructure of dendrimer/DNA complexes on their endocytosis and gene expression. Biomaterials 2010, 31, 5660–5670. [Google Scholar] [CrossRef] [PubMed]
- Salloum, G.; Bresnick, A.R.; Backer, J.M. Macropinocytosis: Mechanisms and regulation. Biochem. J. 2023, 480, 335–362. [Google Scholar] [CrossRef]
- Kay, R.R. Macropinocytosis: Biology and mechanisms. Cells Dev. 2021, 168, 203713. [Google Scholar] [CrossRef]
- Palm, W. Signaling Pathways that Regulate Macropinocytosis in Mammalian Cells. In Macropinocytosis; Springer: Cham, Switzerland, 2022; pp. 143–167. [Google Scholar]
- Wen, H.; Li, Y.; Zhao, X. Redox-Sensitive Polymeric Nanoparticles for Intracellular Drug Delivery. In Frontiers in Nanobiomedical Research; World Scientific: Singapore, 2015; pp. 21–48. [Google Scholar]
- Lee, Y.S.; Kim, S.W. Bioreducible polymers for therapeutic gene delivery. J. Control. Release 2014, 190, 424–439. [Google Scholar] [CrossRef]
- Uram, Ł.; Szuster, M.; Filipowicz, A.; Gargasz, K.; Wołowiec, S.; Wałajtys-Rode, E. Different patterns of nuclear and mitochondrial penetration by the G3 PAMAM dendrimer and its biotin–pyridoxal bioconjugate BC-PAMAM in normal and cancer cells in vitro. Int. J. Nanomed. 2015, 10, 5647. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, D.; Zhang, M.; Sun, Y.; Zhang, X.; Guan, G.; Zhao, X.; Qiao, M.; Chen, D.; Hu, H. The cellular uptake mechanism, intracellular transportation, and exocytosis of polyamidoamine dendrimers in multidrug-resistant breast cancer cells. Int. J. Nanomed. 2016, 11, 3677–3690. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shcharbin, D.G.; Klajnert, B.; Bryszewska, M. Dendrimers in gene transfection. Biochemistry 2009, 74, 1070–1079. [Google Scholar] [CrossRef]
- Nandy, B.; Maiti, P.K. DNA Compaction by a Dendrimer. J. Phys. Chem. B 2011, 115, 217–230. [Google Scholar] [CrossRef]
- Shen, X.C.; Zhou, J.; Liu, X.; Wu, J.; Qu, F.; Zhang, Z.L.; Pang, D.W.; Quéléver, G.; Zhang, C.C.; Peng, L. Importance of size-to-charge ratio in construction of stable and uniform nanoscale RNA/dendrimer complexes. Org. Biomol. Chem. 2007, 5, 3674–3681. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.Y.; Liu, C.Y.; Wu, Z.W.; Chien, C.T.; Chiu, W.C.; Lin, S.Y. Designed nucleus penetrating thymine-capped dendrimers: A potential vehicle for intramuscular gene transfection. J. Mater. Chem. B 2015, 3, 9060–9066. [Google Scholar] [CrossRef]
- Wang, G.H.; Chen, H.; Cai, Y.Y.; Li, L.; Yang, H.K.; Li, Q.; He, Z.J.; Lin, J.T. Efficient gene vector with size changeable and nucleus targeting in cancer therapy. Mater Sci. Eng. C Mater Biol. Appl. 2018, 90, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Han, Y.; Lu, Y.; Song, B.; Zhao, M.; Hu, H.; Chen, D. A novel disulfide bond-mediated cleavable RGD-modified PAMAM nanocomplex containing nuclear localization signal HMGB1 for enhancing gene transfection efficiency. Int. J. Nanomed. 2018, 13, 7135–7153. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Ortiz Escarza, J.M.; Medina López, M.E. Estrés oxidativo ¿un asesino silencioso? Educ. Química 2020, 31, 2. [Google Scholar] [CrossRef]
- Sharma, A.; Liaw, K.; Sharma, R.; Zhang, Z.; Kannan, S.; Kannan, R.M. Targeting Mitochondrial Dysfunction and Oxidative Stress in Activated Microglia using Dendrimer-Based Therapeutics. Theranostics 2018, 8, 5529–5547. [Google Scholar] [CrossRef]
- Hernansanz-Agustín, P.; Enríquez, J.A. Generation of Reactive Oxygen Species by Mitochondria. Antioxidants 2021, 10, 415. [Google Scholar] [CrossRef]
- Victoria-Acosta, G.; Martínez-Archundia, M.; Moreno-Vargas, L.; Meléndez-Zajgla, J.; Martínez-Ruiz, G.U. Is there something else besides the proapoptotic AVPI-segment in the Smac/DIABLO protein? Bol. Med. Hosp. Infant. Mex. 2016, 73, 365–371. [Google Scholar] [PubMed]
- Smith, R.A.J.; Porteous, C.M.; Gane, A.M.; Murphy, M.P. Delivery of bioactive molecules to mitochondria in vivo. Proc. Natl. Acad. Sci. USA 2003, 100, 5407–5412. [Google Scholar] [CrossRef] [PubMed]
- Gazzano, E.; Lazzarato, L.; Rolando, B.; Kopecka, J.; Guglielmo, S.; Costamagna, C.; Chegaev, K.; Riganti, C. Mitochondrial Delivery of Phenol Substructure Triggers Mitochondrial Depolarization and Apoptosis of Cancer Cells. Front Pharmacol. 2018, 9, 580. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Biswas, S.; Dodwadkar, N.S.; Piroyan, A.; Torchilin, V.P. Surface conjugation of triphenylphosphonium to target poly(amidoamine) dendrimers to mitochondria. Biomaterials 2012, 33, 4773–4782. [Google Scholar] [CrossRef]
- Liang, S.; Sun, C.; Yang, P.; Ma, P.; Huang, S.; Cheng, Z.; Yu, X.; Lin, J. Core-shell structured upconversion nanocrystal-dendrimer composite as a carrier for mitochondria targeting and catalase enhanced anti-cancer photodynamic therapy. Biomaterials 2020, 240, 119850. [Google Scholar] [CrossRef] [PubMed]
- Maghsoudnia, N.; Baradaran Eftekhari, R.; Naderi Sohi, A.; Norouzi, P.; Akbari, H.; Ghahremani, M.H.; Soleimani, M.; Amini, M.; Samadi, H.; Dorkoosh, F.A. Mitochondrial delivery of microRNA mimic let-7b to NSCLC cells by PAMAM-based nanoparticles. J. Drug. Target. 2020, 28, 818–830. [Google Scholar] [CrossRef] [PubMed]
- Kianamiri, S.; Dinari, A.; Sadeghizadeh, M.; Rezaei, M.; Daraei, B.; Bahsoun, N.E.; Nomani, A. Mitochondria-Targeted Polyamidoamine Dendrimer-Curcumin Construct for Hepatocellular Cancer Treatment. Mol. Pharm. 2020, 17, 4483–4498. [Google Scholar] [CrossRef] [PubMed]
- Janaszewska, A.; Lazniewska, J.; Trzepiński, P.; Marcinkowska, M.; Klajnert-Maculewicz, B. Cytotoxicity of Dendrimers. Biomolecules 2019, 9, 330. [Google Scholar] [CrossRef]
- Spirescu, V.A.; Chircov, C.; Grumezescu, A.M.; Andronescu, E. Polymeric Nanoparticles for Antimicrobial Therapies: An up-to-date Overview. Polymers 2021, 13, 724. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Davoren, M.; Byrne, H.J. In vitro mammalian cytotoxicological study of PAMAM dendrimers—Towards quantitative structure activity relationships. Toxicol. Vitr. 2010, 24, 169–177. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, H.; Wang, S.; Qian, X.; Fan, J.; Wang, Z.; Song, P.; Zhang, X.; Lu, W.; Ju, D. Interplay of Oxidative Stress and Autophagy in PAMAM Dendrimers-Induced Neuronal Cell Death. Theranostics 2015, 5, 1363–1377. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ziemba, B.; Matuszko, G.; Bryszewska, M.; Klajnert, B. Influence of dendrimers on red blood cells. Cell. Mol. Biol. Lett. 2012, 17, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Kurokawa, Y.; Zeng, Q.; Win-Shwe, T.T.; Nansai, H.; Zhang, Z.; Sone, H. Effects of Polyamidoamine Dendrimers on a 3-D Neurosphere System Using Human Neural Progenitor Cells. Toxicol Sci. 2016, 152, 128–144. [Google Scholar] [CrossRef] [PubMed]
- Domański, D.M.; Klajnert, B.; Bryszewska, M. Influence of PAMAM dendrimers on human red blood cells. Bioelectrochemistry 2004, 63, 189–191. [Google Scholar] [CrossRef]
- Akhtar, S.; El-Hashim, A.Z.; Chandrasekhar, B.; Attur, S.; Benter, I.F. Naked Polyamidoamine Polymers Intrinsically Inhibit Angiotensin II-Mediated EGFR and ErbB2 Transactivation in a Dendrimer Generation- and Surface Chemistry-Dependent Manner. Mol. Pharm. 2016, 13, 1575–1586. [Google Scholar] [CrossRef]
- Naha, P.C.; Davoren, M.; Lyng, F.M.; Byrne, H.J. Reactive oxygen species (ROS) induced cytokine production and cytotoxicity of PAMAM dendrimers in J774A.1 cells. Toxicol. Appl. Pharmacol. 2010, 246, 91–99. [Google Scholar] [CrossRef]
- Janaszewska, A.; Mączyńska, K.; Matuszko, G.; Appelhans, D.; Voit, B.; Klajnert, B.; Bryszewska, M. Cytotoxicity of PAMAM, PPI and maltose modified PPIdendrimers in Chinese hamster ovary (CHO) and human ovarian carcinoma (SKOV3) cells. New J. Chem. 2012, 36, 428–437. [Google Scholar] [CrossRef]
- Duncan, R.; Izzo, L. Dendrimer biocompatibility and toxicity. Adv. Drug Deliv. Rev. 2005, 57, 2215–2237. [Google Scholar] [CrossRef]
- Luong, D.; Kesharwani, P.; Deshmukh, R.; Mohd Amin, M.C.I.; Gupta, U.; Greish, K.; Iyer, A.K. PEGylated PAMAM dendrimers: Enhancing efficacy and mitigating toxicity for effective anticancer drug and gene delivery. Acta Biomater. 2016, 43, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Jain, K.; Jain, N.K. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2014, 39, 268–307. [Google Scholar] [CrossRef]
- Opitz, A.W.; Czymmek, K.J.; Wickstrom, E.; Wagner, N.J. Uptake, efflux, and mass transfer coefficient of fluorescent PAMAM dendrimers into pancreatic cancer cells. Biochim. Biophys. Acta (BBA)-Biomembr. 2013, 1828, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Seok, S.H.; Yoon, H.Y.; Ryu, J.H.; Kwon, I.C. Advancing cancer immunotherapy through siRNA-based gene silencing for immune checkpoint blockade. Adv. Drug Deliv. Rev. 2024, 209, 115306. [Google Scholar] [CrossRef]
- Ortiz-Morales, A.A.; García-Vázquez, J.B.; Fragoso-Vázquez, M.J.; Rosales-Hernández, M.C.; Fragoso-Morales, L.G.; Estrada-Pérez, A.R.; Correa-Basurto, J. PAMAM-G4 protect the N-(2-hydroxyphenyl)-2-propylpentanamide (HO-AAVPA) and maintain its antiproliferative effects on MCF-7. Sci. Rep. 2023, 13, 3383. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.; Chandrasekhar, B.; Yousif, M.H.; Renno, W.; Benter, I.F.; El-Hashim, A.Z. Chronic administration of nano-sized PAMAM dendrimers in vivo inhibits EGFR-ERK1/2-ROCK signaling pathway and attenuates diabetes-induced vascular remodeling and dysfunction. Nanomedicine 2019, 18, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.; Kesharwani, P.; Gupta, U.; Jain, N.K. Dendrimer toxicity: Let’s meet the challenge. Int. J. Pharm. 2010, 394, 122–142. [Google Scholar] [CrossRef]
- Labieniec-Watala, M.; Watala, C. PAMAM Dendrimers: Destined for Success or Doomed to Fail? Plain and Modified PAMAM Dendrimers in the Context of Biomedical Applications. J. Pharm. Sci. 2015, 104, 2–14. [Google Scholar] [CrossRef]
- Dehkordi, A.A.; Mollazadeh, S.; Talaie, A.; Yazdimamaghani, M. Engineering PAMAM dendrimers for optimized drug delivery. Nano Trends 2025, 9, 100094. [Google Scholar] [CrossRef]
- Chowdhury, S.; Toth, I.; Stephenson, R.J. Dendrimers in vaccine delivery: Recent progress and advances. Biomaterials 2022, 280, 121303. [Google Scholar] [CrossRef]
- Daftarian, P.; Kaifer, A.E.; Li, W.; Blomberg, B.B.; Frasca, D.; Roth, F.; Chowdhury, R.; Berg, E.A.; Fishman, J.B.; Al Sayegh, H.A.; et al. Peptide-conjugated PAMAM dendrimer as a universal DNA vaccine platform to target antigen-presenting cells. Cancer Res. 2011, 71, 7452–7462. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dai, Y.; Zhao, S.; Tang, J.; Li, H.; Xing, Y.; Qu, G.; Li, X.; Dai, J.; Zhu, Y.; et al. PAMAM-Lys, a novel vaccine delivery vector, enhances the protective effects of the SjC23 DNA vaccine against Schistosoma japonicum infection. PLoS ONE 2014, 9, e86578. [Google Scholar] [CrossRef] [PubMed]
- Sheng, K.C.; Kalkanidis, M.; Pouniotis, D.S.; Esparon, S.; Tang, C.K.; Apostolopoulos, V.; Pietersz, G.A. Delivery of antigen using a novel mannosylated dendrimer potentiates immunogenicity in vitro and in vivo. Eur. J. Immunol. 2008, 38, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.M.; Mano, J.F.; Reis, R.L. Progress in Dendrimer-Based Nanocarriers. In Biomimetic Approaches for Biomaterials Development; Wiley: Hoboken, NJ, USA, 2012; pp. 459–469. [Google Scholar]


| Cell Type | Surface Functionalization | Observed Internalization Pathway(s) | Key Observations | Reference |
|---|---|---|---|---|
| Microglia/Macrophages | Hydroxyl (G4-OH) | Likely macropinocytosis and phagocytosis | Rapid and extensive uptake; >80% in 3 h; >95% in 6 h | [79] |
| Astrocytes | Hydroxyl (G4-OH) | Minimal internalization | Only ~8.5% uptake after 24 h | [79] |
| Hippocampal Neurons | Unmodified | Clathrin-mediated endocytosis | Efficient internalization of unmodified PAMAM | [80] |
| Hippocampal Neurons | Folic acid (PFO) | Clathrin + caveolin-mediated endocytosis | Enhanced uptake via dual mechanisms | [80] |
| Hippocampal Neurons | PEG (50%)/Acrylate (30%) | Minimal uptake | Surface shielding inhibits internalization | [80] |
| HT-29 (Colon Cancer) | Unmodified/Propranolol-G3 | Caveolin + macropinocytosis | Dual pathway uptake; propranolol improves cytotoxicity | [81] |
| HT-29 (Colon Cancer) | Lauryl-modified G3 | Clathrin + caveolin + macropinocytosis | Surface hydrophobicity enhances multi-pathway internalization | [81] |
| Generic (various cells) | Anionic dendrimers | Caveolin-mediated endocytosis | Internalization correlates with charge and membrane interaction | [82] |
| Generic (various cells) | Neutral/Cationic dendrimers | Clathrin-independent endocytosis | Uptake efficiency varies with surface charge and membrane composition | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alamos-Musre, S.; Beltrán-Chacana, D.; Moyano, J.; Márquez-Miranda, V.; Duarte, Y.; Miranda-Rojas, S.; Olguín, Y.; Fuentes, J.A.; González-Nilo, D.; Otero, M.C. From Structure to Function: The Promise of PAMAM Dendrimers in Biomedical Applications. Pharmaceutics 2025, 17, 927. https://doi.org/10.3390/pharmaceutics17070927
Alamos-Musre S, Beltrán-Chacana D, Moyano J, Márquez-Miranda V, Duarte Y, Miranda-Rojas S, Olguín Y, Fuentes JA, González-Nilo D, Otero MC. From Structure to Function: The Promise of PAMAM Dendrimers in Biomedical Applications. Pharmaceutics. 2025; 17(7):927. https://doi.org/10.3390/pharmaceutics17070927
Chicago/Turabian StyleAlamos-Musre, Said, Daniel Beltrán-Chacana, Juan Moyano, Valeria Márquez-Miranda, Yorley Duarte, Sebastián Miranda-Rojas, Yusser Olguín, Juan A. Fuentes, Danilo González-Nilo, and María Carolina Otero. 2025. "From Structure to Function: The Promise of PAMAM Dendrimers in Biomedical Applications" Pharmaceutics 17, no. 7: 927. https://doi.org/10.3390/pharmaceutics17070927
APA StyleAlamos-Musre, S., Beltrán-Chacana, D., Moyano, J., Márquez-Miranda, V., Duarte, Y., Miranda-Rojas, S., Olguín, Y., Fuentes, J. A., González-Nilo, D., & Otero, M. C. (2025). From Structure to Function: The Promise of PAMAM Dendrimers in Biomedical Applications. Pharmaceutics, 17(7), 927. https://doi.org/10.3390/pharmaceutics17070927








