Nasal Residence Depending on the Administered Dosage Form: Impact of Formulation Type on the In Vivo Nasal Retention Time of Drugs in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Regents
2.1.2. Animals
2.2. In Vivo Study for Evaluating the Effect of Drug Application Site on Nasal Residence Time
2.2.1. In Vivo Nasal Residence Study
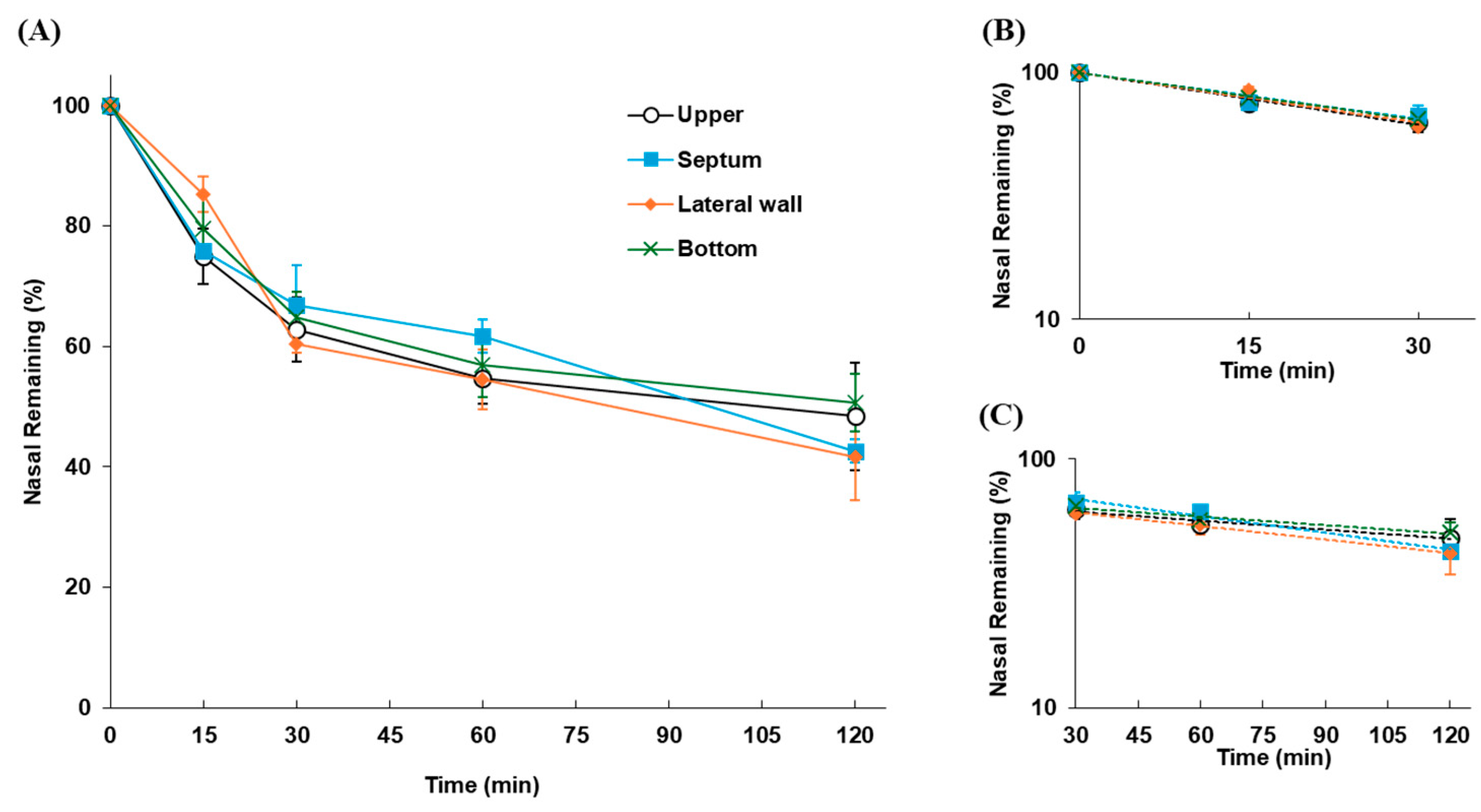
2.2.2. Impact of Drug Deposition Sites in the Nasal Cavity on Nasal Residence
2.2.3. Transfer Dynamics of Drugs After Disappearance from the Nasal Cavity
2.3. Effect of Formulation Type on the Nasal Residence of Drugs
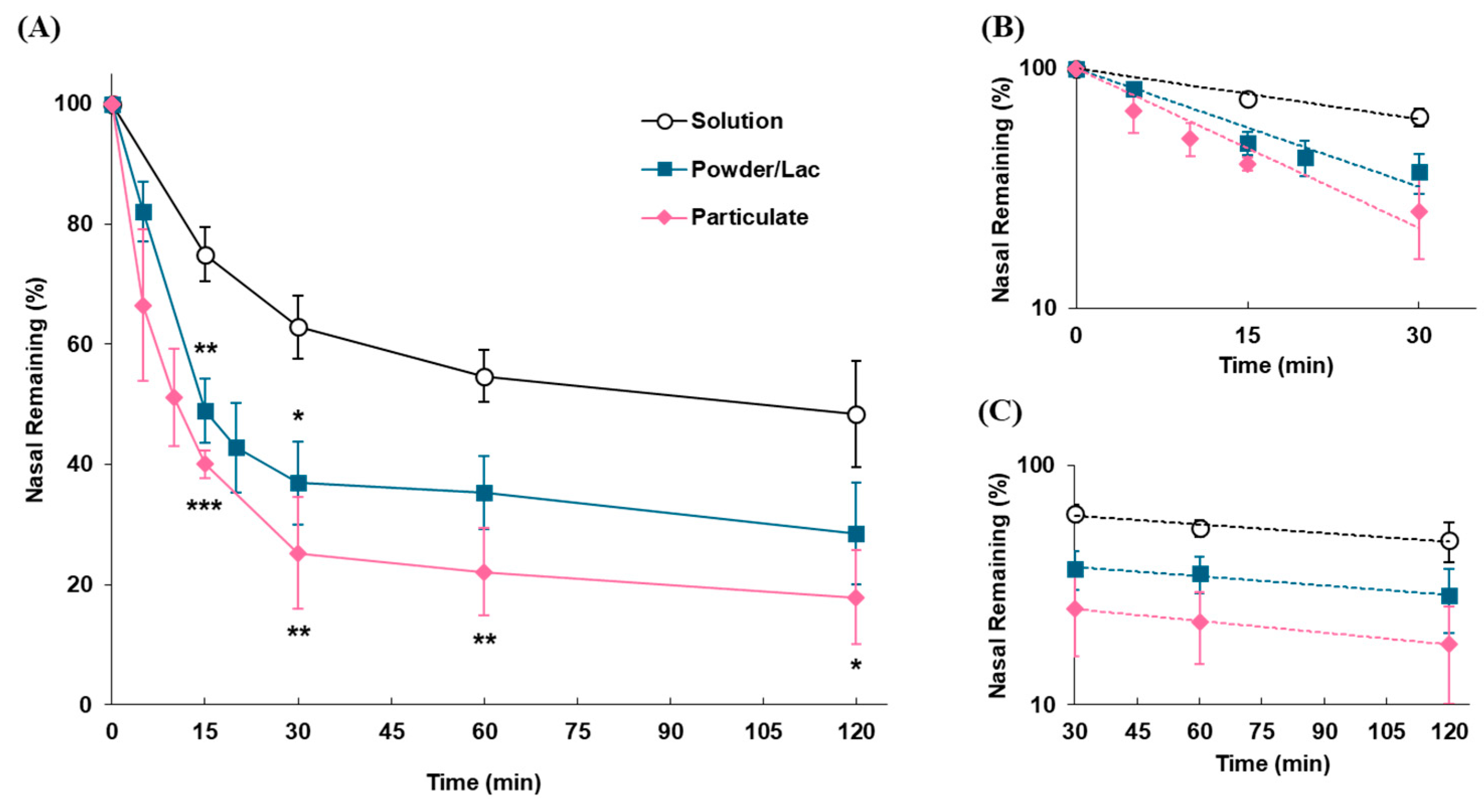
2.3.1. Impact of Dosage Forms on Nasal Retention Time
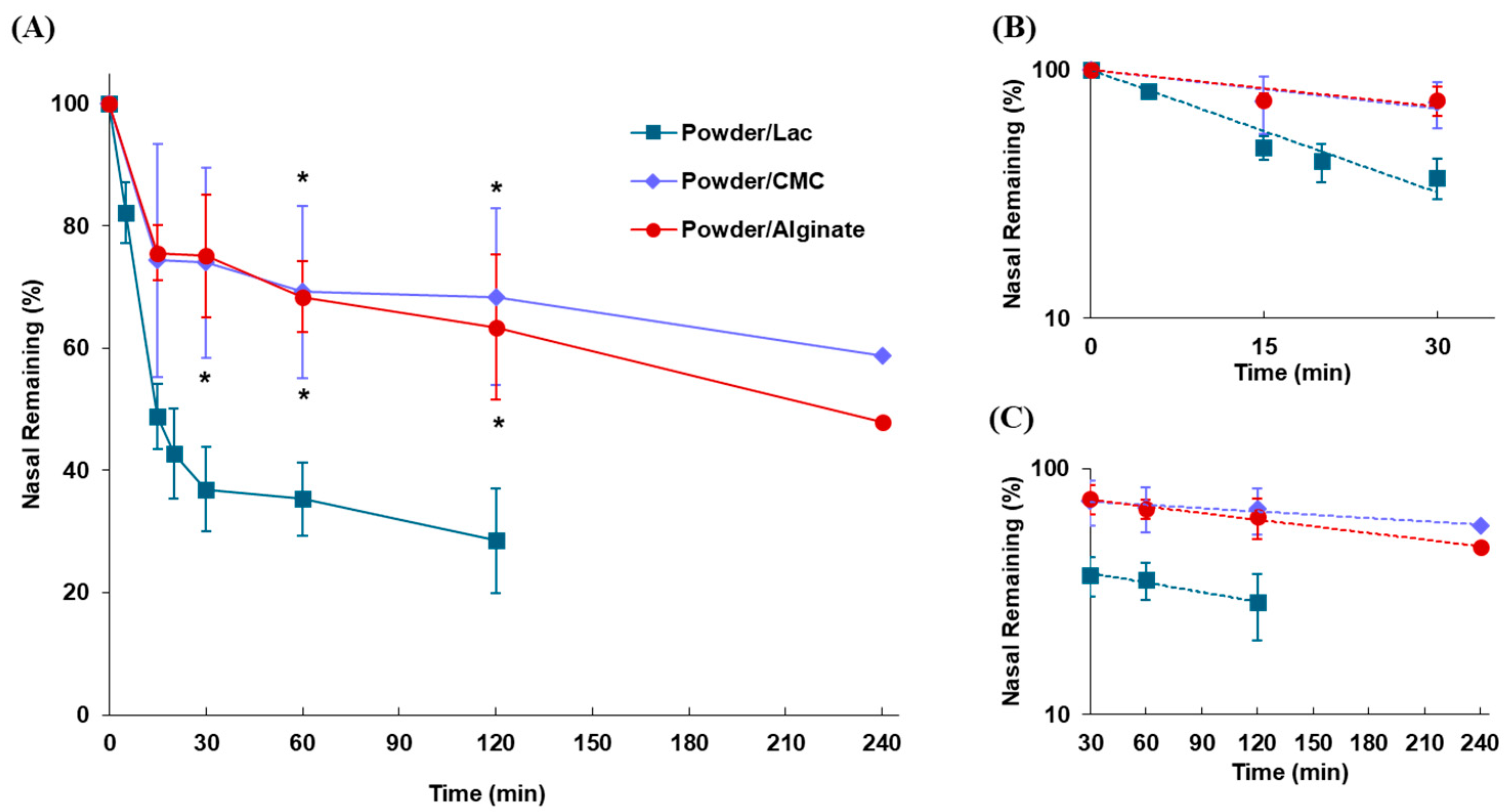
2.3.2. Effect of Mucoadhesive Polymers on Nasal Residence in Powder Formulation
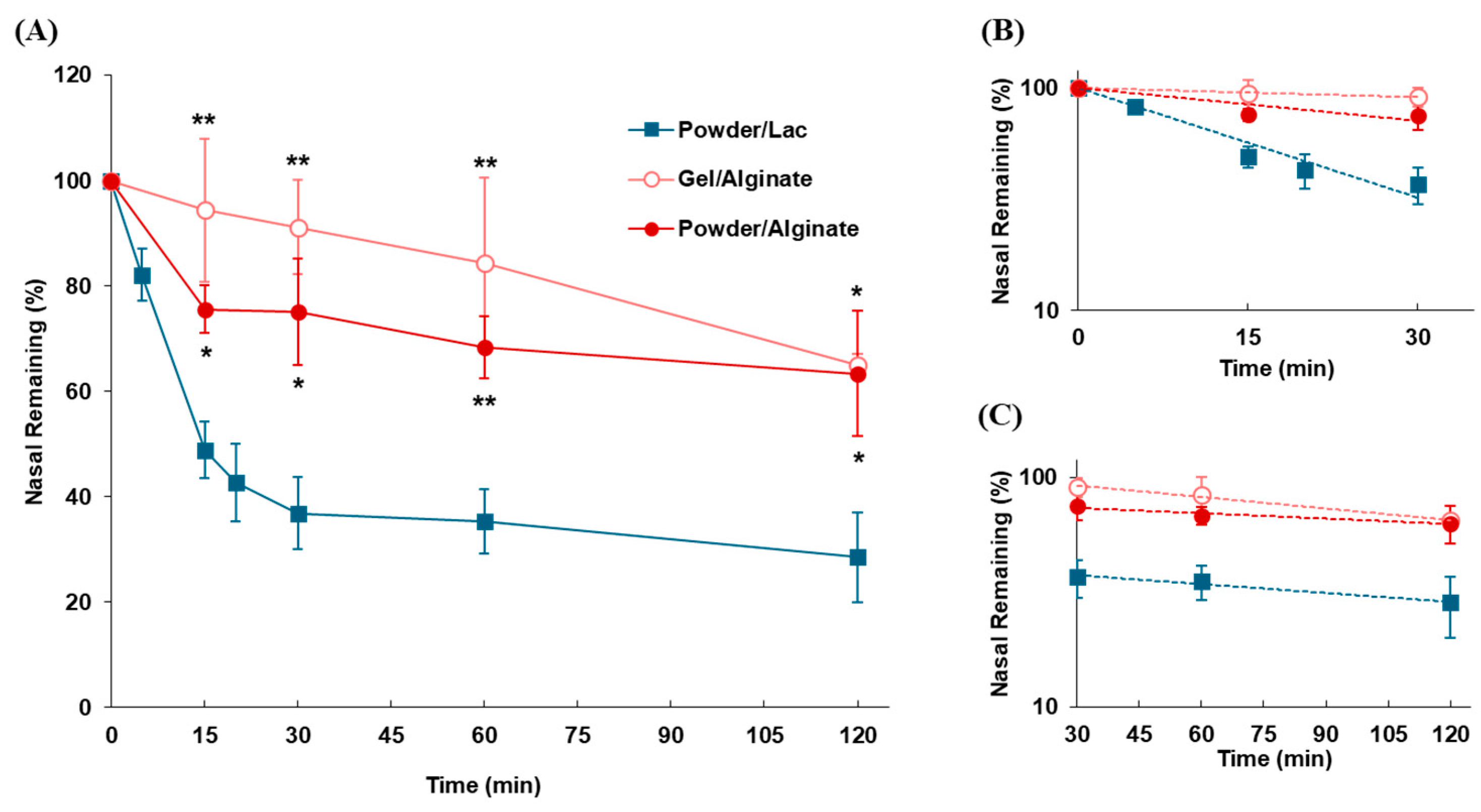
2.3.3. Impact of Dosage Forms on Nasal Residence in Mucoadhesive Formulation
2.4. Analytical Procedure
2.4.1. Calculation of Drug Disappearance Rate Constant by Mucociliary Clearance
2.4.2. Statistical Analysis
3. Results
3.1. In Vivo Nasal Residence of Drugs Following Intranasal Application
3.1.1. Differences in Nasal Residence Depending on the Site of Drug Deposition
3.1.2. Kinetics of FD-70 After Disappearance from the Nasal Cavity
3.2. Impact of Formulation Types on the Nasal Residence of Drugs
3.2.1. Impact of Dosage Forms on the Nasal Residence of Formulations
3.2.2. Effect of Mucoadhesive Polymers on Nasal Residence of Powder Formulations
3.2.3. Impact of Dosage Forms on Nasal Residence for Mucoadhesive Formulations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MC | Mucociliary clearance |
| FMS | Fluorescent microsphere |
| CMC-Na | Carboxymethylcellulose |
| FD-70 | Fluorescein isothiocyanate dextran, 70 kDa |
| kmc | Drug disappearance rate constant by MC (α-phase) |
| klater | Drug disappearance rate constant in later phase (β-phase) |
| t1/2(mc) | Half-life of disappearance by MC (α-phase) |
| t1/2(later) | Half-life of disappearance in later phase (β-phase) |
References
- Illum, L. Nasal Drug Delivery—Recent Developments and Future Prospects. J. Control. Release 2012, 161, 254–263. [Google Scholar] [CrossRef]
- Fortuna, A.; Alves, G.; Serralheiro, A.; Sousa, J.; Falcão, A. Intranasal Delivery of Systemic-Acting Drugs: Small-Molecules and Biomacromolecules. Eur. J. Pharm. Biopharm. 2014, 88, 8–27. [Google Scholar] [CrossRef]
- Bose, M.; Farias Quipildor, G.; Ehrlich, M.E.; Salton, S.R. Intranasal Peptide Therapeutics: A Promising Avenue for Overcoming the Challenges of Traditional CNS Drug Development. Cells 2022, 11, 3629. [Google Scholar] [CrossRef]
- Samaridou, E.; Alonso, M.J. Nose-to-Brain Peptide Delivery—The Potential of Nanotechnology. Bioorg. Med. Chem. 2018, 26, 2888–2905. [Google Scholar] [CrossRef]
- Lin, J.; Yu, Z.; Gao, X. Advanced Noninvasive Strategies for the Brain Delivery of Therapeutic Proteins and Peptides. ACS Nano 2024, 18, 22752–22779. [Google Scholar] [CrossRef]
- Dhaliwal, H.K.; Fan, Y.; Kim, J.; Amiji, M.M. Intranasal Delivery and Transfection of MRNA Therapeutics in the Brain Using Cationic Liposomes. Mol. Pharm. 2020, 17, 1996–2005. [Google Scholar] [CrossRef]
- Shah, P.; Lalan, M.; Barve, K. Intranasal Delivery: An Attractive Route for the Administration of Nucleic Acid Based Therapeutics for CNS Disorders. Front. Pharmacol. 2022, 13, 974666. [Google Scholar] [CrossRef]
- D’Souza, A.; Nozohouri, S.; Bleier, B.S.; Amiji, M.M. CNS Delivery of Nucleic Acid Therapeutics: Beyond the Blood–Brain Barrier and Towards Specific Cellular Targeting. Pharm. Res. 2023, 40, 77–105. [Google Scholar] [CrossRef]
- Yuan, D.; Yi, X.; Zhao, Y.; Poon, C.D.; Bullock, K.M.; Hansen, K.M.; Salameh, T.S.; Farr, S.A.; Banks, W.A.; Kabanov, A.V. Intranasal Delivery of N-Terminal Modified Leptin-Pluronic Conjugate for Treatment of Obesity. J. Control. Release 2017, 263, 172–184. [Google Scholar] [CrossRef]
- Van De Bittner, G.C.; Van De Bittner, K.C.; Wey, H.Y.; Rowe, W.; Dharanipragada, R.; Ying, X.; Hurst, W.; Giovanni, A.; Alving, K.; Gupta, A.; et al. Positron Emission Tomography Assessment of the Intranasal Delivery Route for Orexin A. ACS Chem. Neurosci. 2018, 9, 358–368. [Google Scholar] [CrossRef]
- Röhm, M.; Carle, S.; Maigler, F.; Flamm, J.; Kramer, V.; Mavoungou, C.; Schmid, O.; Schindowski, K. A Comprehensive Screening Platform for Aerosolizable Protein Formulations for Intranasal and Pulmonary Drug Delivery. Int. J. Pharm. 2017, 532, 537–546. [Google Scholar] [CrossRef]
- Abdulhameed, N.; Babin, A.; Hansen, K.; Weaver, R.; Banks, W.A.; Talbot, K.; Rhea, E.M. Comparing Regional Brain Uptake of Incretin Receptor Agonists after Intranasal Delivery in CD-1 Mice and the APP/PS1 Mouse Model of Alzheimer’s Disease. Alzheimer’s Res. Ther. 2024, 16, 173. [Google Scholar] [CrossRef] [PubMed]
- Duong, V.A.; Nguyen, T.T.L.; Maeng, H.J. Recent Advances in Intranasal Liposomes for Drug, Gene, and Vaccine Delivery. Pharmaceutics 2023, 15, 207. [Google Scholar] [CrossRef]
- Rohrer, J.; Lupo, N.; Bernkop-Schnürch, A. Advanced Formulations for Intranasal Delivery of Biologics. Int. J. Pharm. 2018, 553, 8–20. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, Y.; Zhang, W.; Xia, X.; Li, H.; Qin, M.; Gao, H. Nanotechnology for Enhanced Nose-to-Brain Drug Delivery in Treating Neurological Diseases. J. Control. Release 2024, 366, 519–534. [Google Scholar] [CrossRef]
- Keller, L.A.; Merkel, O.; Popp, A. Intranasal Drug Delivery: Opportunities and Toxicologic Challenges during Drug Development. Drug Deliv. Transl. Res. 2022, 12, 735–757. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Thorne, R.G. Intranasal Delivery of Biologics to the Central Nervous System. Adv. Drug Deliv. Rev. 2012, 64, 614–628. [Google Scholar] [CrossRef]
- Singh, S.; Shukla, R. Nanovesicular-Mediated Intranasal Drug Therapy for Neurodegenerative Disease; Springer International Publishing: Berlin/Heidelberg, Germany, 2023; Volume 24, ISBN 0123456789. [Google Scholar]
- Kubek, M.J.; Domb, A.J.; Veronesi, M.C. Attenuation of Kindled Seizures by Intranasal Delivery of Neuropeptide-Loaded Nanoparticles. Neurotherapeutics 2009, 6, 359–371. [Google Scholar] [CrossRef]
- Qu, Y.; Sun, X.; Ma, L.; Li, C.; Xu, Z.; Ma, W.; Zhou, Y.; Zhao, Z.; Ma, D. Therapeutic Effect of Disulfiram Inclusion Complex Embedded in Hydroxypropyl-β-Cyclodextrin on Intracranial Glioma-Bearing Male Rats via Intranasal Route. Eur. J. Pharm. Sci. 2021, 156, 105590. [Google Scholar] [CrossRef]
- Zheng, X.; Kendrick, K.M. Neural and Molecular Contributions to Pathological Jealousy and a Potential Therapeutic Role for Intranasal Oxytocin. Front. Pharmacol. 2021, 12, 652473. [Google Scholar] [CrossRef]
- Patel, D.; Patel, B.; Wairkar, S. Intranasal Delivery of Biotechnology-Based Therapeutics. Drug Discov. Today 2022, 27, 103371. [Google Scholar] [CrossRef] [PubMed]
- Tal, Y.; Ribak, Y.; Rubin, L.; Talmon, A.; Shamriz, O.; Hershko, A.Y.; Blotnick, S.; Bouhajib, M.; Krayz, G.T.; Abrutzky, C.; et al. Fast Acting, Dry Powder, Needle-Free, Intranasal Epinephrine Spray: A Promising Future Treatment for Anaphylaxis. J. Allergy Clin. Immunol. Pract. 2023, 11, 3047–3054. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.; Fortuna, A.; Alves, G.; Falcão, A. Intranasal Drug Delivery: How, Why and What For? J. Pharm. Pharm. Sci. 2009, 12, 288–311. [Google Scholar] [CrossRef] [PubMed]
- Hallschmid, M. Intranasal Insulin for Alzheimer’s Disease. CNS Drugs 2021, 35, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Lipton, R.B.; Croop, R.; Stock, D.A.; Madonia, J.; Forshaw, M.; Lovegren, M.; Mosher, L.; Coric, V.; Goadsby, P.J. Safety, Tolerability, and Efficacy of Zavegepant 10 Mg Nasal Spray for the Acute Treatment of Migraine in the USA: A Phase 3, Double-Blind, Randomised, Placebo-Controlled Multicentre Trial. Lancet Neurol. 2023, 22, 209–217. [Google Scholar] [CrossRef]
- Li, B.V.; Jin, F.; Lee, S.L.; Bai, T.; Chowdhury, B.; Caramenico, H.T.; Conner, D.P. Bioequivalence for Locally Acting Nasal Spray and Nasal Aerosol Products: Standard Development and Generic Approval. AAPS J. 2013, 15, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Achmad, N.A.; Tuna, R.W.; Kurniawan, I.; Khairiyah, N.; Asaf, M.B.; Rahman, L.; Manggau, M.A.; Aliyah, N.; Dominguez-Robles, J.; Aswad, M.; et al. Development of Thermosensitive Mucoadhesive Gel Based Encapsulated Lipid Microspheres as Nose-to-Brain Rivastigmine Delivery System. Langmuir 2025, 41, 314–328. [Google Scholar] [CrossRef]
- Mohananaidu, K.; Chatterjee, B.; Mohamed, F.; Mahmood, S.; Hamed Almurisi, S. Thermoreversible Carbamazepine In Situ Gel for Intranasal Delivery: Development and In Vitro, Ex Vivo Evaluation. AAPS PharmSciTech 2022, 23, 288. [Google Scholar] [CrossRef]
- Cirri, M.; Maestrelli, F.; Nerli, G.; Mennini, N.; D’ambrosio, M.; Luceri, C.; Mura, P.A. Development of a Cyclodextrin-Based Mucoadhesive-Thermo-Sensitive in Situ Gel for Clonazepam Intranasal Delivery. Pharmaceutics 2021, 13, 969. [Google Scholar] [CrossRef]
- Mura, P.; Mennini, N.; Nativi, C.; Richichi, B. In Situ Mucoadhesive-Thermosensitive Liposomal Gel as a Novel Vehicle for Nasal Extended Delivery of Opiorphin. Eur. J. Pharm. Biopharm. 2018, 122, 54–61. [Google Scholar] [CrossRef]
- Matarazzo, A.P.; Elisei, L.M.S.; Carvalho, F.C.; Bonfílio, R.; Ruela, A.L.M.; Galdino, G.; Pereira, G.R. Mucoadhesive Nanostructured Lipid Carriers as a Cannabidiol Nasal Delivery System for the Treatment of Neuropathic Pain. Eur. J. Pharm. Sci. 2021, 159, 105698. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, C.; Huang, Y.; Ma, Y.; Song, Q.; Chen, H.; Jiang, G.; Gao, X. Intranasal Drug Delivery: The Interaction between Nanoparticles and the Nose-to-Brain Pathway. Adv. Drug Deliv. Rev. 2024, 207, 115196. [Google Scholar] [CrossRef] [PubMed]
- Boyuklieva, R.; Pilicheva, B. Micro- and Nanosized Carriers for Nose-to-Brain Drug Delivery in Neurodegenerative Disorders. Biomedicines 2022, 10, 1706. [Google Scholar] [CrossRef]
- Tiozzo Fasiolo, L.; Manniello, M.D.; Tratta, E.; Buttini, F.; Rossi, A.; Sonvico, F.; Bortolotti, F.; Russo, P.; Colombo, G. Opportunity and Challenges of Nasal Powders: Drug Formulation and Delivery. Eur. J. Pharm. Sci. 2018, 113, 2–17. [Google Scholar] [CrossRef]
- Henriques, P.; Fortuna, A.; Doktorovová, S. Spray Dried Powders for Nasal Delivery: Process and Formulation Considerations. Eur. J. Pharm. Biopharm. 2022, 176, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Baldelli, A.; Boraey, M.A.; Oguzlu, H.; Cidem, A.; Rodriguez, A.P.; Ong, H.X.; Jiang, F.; Bacca, M.; Thamboo, A.; Traini, D.; et al. Engineered Nasal Dry Powder for the Encapsulation of Bioactive Compounds. Drug Discov. Today 2022, 27, 2300–2308. [Google Scholar] [CrossRef]
- Wong, C.Y.J.; Baldelli, A.; Tietz, O.; van der Hoven, J.; Suman, J.; Ong, H.X.; Traini, D. An Overview of in Vitro and in Vivo Techniques for Characterization of Intranasal Protein and Peptide Formulations for Brain Targeting. Int. J. Pharm. 2024, 654, 123922. [Google Scholar] [CrossRef]
- Inoue, D.; Furubayashi, T.; Ogawara, K.; Kimura, T.; Higaki, K.; Katsumi, H.; Sakane, T.; Yamamoto, A.; Higashi, Y. In Vitro Evaluation of Nasal Mucociliary Clearance Using Excised Rat Nasal Septum. Biol. Pharm. Bull. 2012, 35, 889–894. [Google Scholar] [CrossRef]
- Shang, Y.; Inthavong, K.; Qiu, D.; Singh, N.; He, F.; Tu, J. Prediction of Nasal Spray Drug Absorption Influenced by Mucociliary Clearance. PLoS ONE 2021, 16, e0246007. [Google Scholar] [CrossRef]
- Schipper, N.G.; Verhoef, J.C.; Merkus, F.W. The Nasal Mucociliary Clearance: Relevance to Nasal Drug Delivery. Pharm. Res. 1991, 8, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Donovan, M.D.; Zhou, M. Drug Effects on in Vivo Nasal Clearance in Rats. Int. J. Pharm. 1995, 116, 77–86. [Google Scholar] [CrossRef]
- Inoue, D.; Tanaka, A.; Kimura, S.; Kiriyama, A.; Katsumi, H.; Yamamoto, A.; Ogawara, K.I.; Kimura, T.; Higaki, K.; Yutani, R.; et al. The Relationship between in Vivo Nasal Drug Clearance and in Vitro Nasal Mucociliary Clearance: Application to the Prediction of Nasal Drug Absorption. Eur. J. Pharm. Sci. 2018, 117, 21–26. [Google Scholar] [CrossRef]
- Wu, J.; Wei, W.; Wang, L.Y.; Su, Z.G.; Ma, G.H. A Thermosensitive Hydrogel Based on Quaternized Chitosan and Poly(Ethylene Glycol) for Nasal Drug Delivery System. Biomaterials 2007, 28, 2220–2232. [Google Scholar] [CrossRef] [PubMed]
- Trenkel, M.; Scherließ, R. Nasal Powder Formulations: In-Vitro Characterisation of the Impact of Powders on Nasal Residence Time and Sensory Effects. Pharmaceutics 2021, 13, 385. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.A.; Hirai, S.; Bawarshi, R. Nasal Absorption of Propranolol in Rats. J. Pharm. Sci. 1979, 68, 1196. [Google Scholar] [CrossRef]
- Ugwoke, M.I.; Agu, R.U.; Verbeke, N.; Kinget, R. Nasal Mucoadhesive Drug Delivery: Background, Applications, Trends and Future Perspectives. Adv. Drug Deliv. Rev. 2005, 57, 1640–1665. [Google Scholar] [CrossRef] [PubMed]
- Seifelnasr, A.; Si, X.; Xi, J. Assessing Nasal Epithelial Dynamics: Impact of the Natural Nasal Cycle on Intranasal Spray Deposition. Pharmaceuticals 2024, 17, 73. [Google Scholar] [CrossRef]
- Chiang, H.; Martin, H.L.; Sicard, R.M.; Frank-Ito, D.O. Olfactory Drug Delivery with Intranasal Sprays after Nasal Midvault Reconstruction. Int. J. Pharm. 2023, 644, 123341. [Google Scholar] [CrossRef]
- Crowe, T.P.; Hsu, W.H. Evaluation of Recent Intranasal Drug Delivery Systems to the Central Nervous System. Pharmaceutics 2022, 14, 629. [Google Scholar] [CrossRef]
- Kublik, H.; Vidgren, M.T. Nasal Delivery Systems and Their Effect on Deposition and Absorption. Adv. Drug Deliv. Rev. 1998, 29, 157–177. [Google Scholar] [CrossRef]
- Hounam, R.F. The Removal of Particles from the Nasopharyngeal (NP) Compartment of the Respiratory Tract by Nose Blowing and Swabbing. Health Phys. 1975, 28, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Inoue, D.; Furubayashi, T.; Ogawara, K.; Kimura, T.; Higaki, K.; Shingaki, T.; Kimura, S.; Tanaka, A.; Katsumi, H.; Sakane, T.; et al. In Vitro Evaluation of the Ciliary Beat Frequency of the Rat Nasal Epithelium Using a High-Speed Digital Imaging System. Biol. Pharm. Bull. 2013, 36, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Pozzoli, M.; Traini, D.; Young, P.M.; Sukkar, M.B.; Sonvico, F. Development of a Soluplus Budesonide Freeze-Dried Powder for Nasal Drug Delivery. Drug Dev. Ind. Pharm. 2017, 43, 1510–1518. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.; Kim, G.; Desai, K.G.H.; Patel, H.; Olsen, K.F.; Curtis-Fisk, J.; Tocce, E.; Jordan, S.; Schwendeman, S.P. Feasibility Investigation of Cellulose Polymers for Mucoadhesive Nasal Drug Delivery Applications. Mol. Pharm. 2015, 12, 2732–2741. [Google Scholar] [CrossRef] [PubMed]
- Deutel, B.; Laffleur, F.; Palmberger, T.; Saxer, A.; Thaler, M.; Bernkop-Schnürch, A. In Vitro Characterization of Insulin Containing Thiomeric Microparticles as Nasal Drug Delivery System. Eur. J. Pharm. Sci. 2016, 81, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Dukovski, B.J.; Plantić, I.; Čunčić, I.; Krtalić, I.; Juretić, M.; Pepić, I.; Lovrić, J.; Hafner, A. Lipid/Alginate Nanoparticle-Loaded in Situ Gelling System Tailored for Dexamethasone Nasal Delivery. Int. J. Pharm. 2017, 533, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Casettari, L.; Illum, L. Chitosan in Nasal Delivery Systems for Therapeutic Drugs. J. Control. Release 2014, 190, 189–200. [Google Scholar] [CrossRef]


| Dosage Form | Solvent/Additive | Marker | Marker Content | Dose per Head |
|---|---|---|---|---|
| Solution | PBS a | FD-70 c | 2.0 (w/v)% | 5.0 µL |
| Particulate | PBS a | FMS d | Diluted 10,000 times | 5.0 µL |
| Powder | Lactose | FD-70 c | 10 (w/w)% | 0.5 mg |
| CMC-Na b | FD-70 c | 10 (w/w)% | 0.5 mg | |
| Sodium alginate | FD-70 c | 10 (w/w)% | 0.5 mg | |
| Gel | Sodium alginate | FD-70 c | 2.0 (w/v)% | 5.0 µL |
| Site Applied | kmc (min−1) | t1/2(mc) (min) | klater (min−1) | t1/2(later) (min) |
|---|---|---|---|---|
| Upper | 0.0162 | 42.8 | 0.00278 | 249.3 |
| Septum | 0.0144 | 48.1 | 0.00518 | 133.8 |
| Lateral wall | 0.0155 | 44.7 | 0.00418 | 165.8 |
| Bottom | 0.0146 | 47.5 | 0.00264 | 262.6 |
| Dosage Form | kmc | t1/2(mc) | klater | t1/2(later) |
|---|---|---|---|---|
| (min−1) | (min) | (min−1) | (min) | |
| Solution | 0.0162 | 42.8 | 0.00278 | 249.3 |
| Particulate | 0.0510 | 13.6 | 0.00297 | 233.4 |
| Powder/Lac | 0.0378 | 18.3 | 0.00378 | 183.4 |
| Powders | kmc | t1/2(mc) | klater | t1/2(later) |
|---|---|---|---|---|
| (min−1) | (min) | (min−1) | (min) | |
| Powder/Lac | 0.0378 | 18.3 | 0.00297 | 233.4 |
| Powder/CMC | 0.0120 | 57.8 | 0.00102 | 679.6 |
| Powder/Alginate | 0.0114 | 60.8 | 0.00207 | 334.9 |
| Dosage Form | kmc | t1/2(mc) | klater | t1/2(later) |
|---|---|---|---|---|
| (min−1) | (min) | (min−1) | (min) | |
| Gel/Alginate | 0.0032 | 215.3 | 0.00386 | 179.6 |
| Powder/Alginate | 0.0114 | 60.8 | 0.00207 | 334.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, D.; Seto, Y.; To, H. Nasal Residence Depending on the Administered Dosage Form: Impact of Formulation Type on the In Vivo Nasal Retention Time of Drugs in Rats. Pharmaceutics 2025, 17, 863. https://doi.org/10.3390/pharmaceutics17070863
Inoue D, Seto Y, To H. Nasal Residence Depending on the Administered Dosage Form: Impact of Formulation Type on the In Vivo Nasal Retention Time of Drugs in Rats. Pharmaceutics. 2025; 17(7):863. https://doi.org/10.3390/pharmaceutics17070863
Chicago/Turabian StyleInoue, Daisuke, Yoshihiro Seto, and Hideto To. 2025. "Nasal Residence Depending on the Administered Dosage Form: Impact of Formulation Type on the In Vivo Nasal Retention Time of Drugs in Rats" Pharmaceutics 17, no. 7: 863. https://doi.org/10.3390/pharmaceutics17070863
APA StyleInoue, D., Seto, Y., & To, H. (2025). Nasal Residence Depending on the Administered Dosage Form: Impact of Formulation Type on the In Vivo Nasal Retention Time of Drugs in Rats. Pharmaceutics, 17(7), 863. https://doi.org/10.3390/pharmaceutics17070863







