Trans-p-Coumaryl Alcohol as a Bioactive Compound and Anti-Inflammatory Agent in Wannachawee Recipe for Psoriasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Materials
2.3. Sample Preparation
2.4. Bioassay-Guided Isolation with Anti-Inflammation Analysis
2.4.1. Separation Technique
2.4.2. Anti-Inflammatory Activity Test [18,19]
- Cell used and Cell Culture
- 2.
- Cytotoxicity Assay with Sulforhodamine B (SRB Assay)
- 3.
- Studying the effect of the extracts on the inhibition of TNF-α and IL-6
- 4.
- Statistical Analysis
2.5. Identification of Chemical Compounds in WCR Extract by HPLC
2.6. Structure Elucidation by Spectroscopy
3. Results
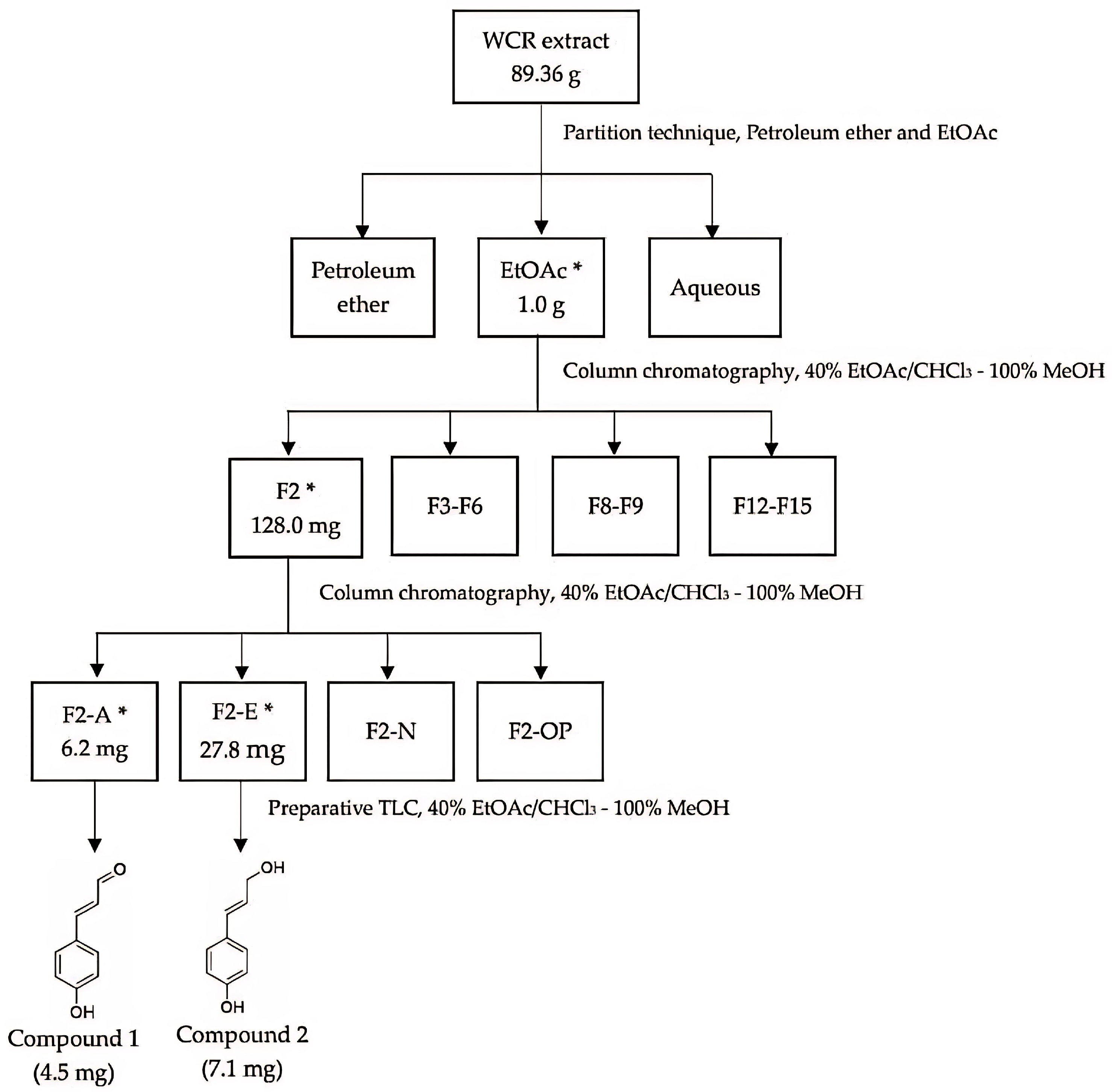
3.1. Preparation of the Extract
3.2. Bioassay-Guided Fractionations
3.2.1. Separation Results
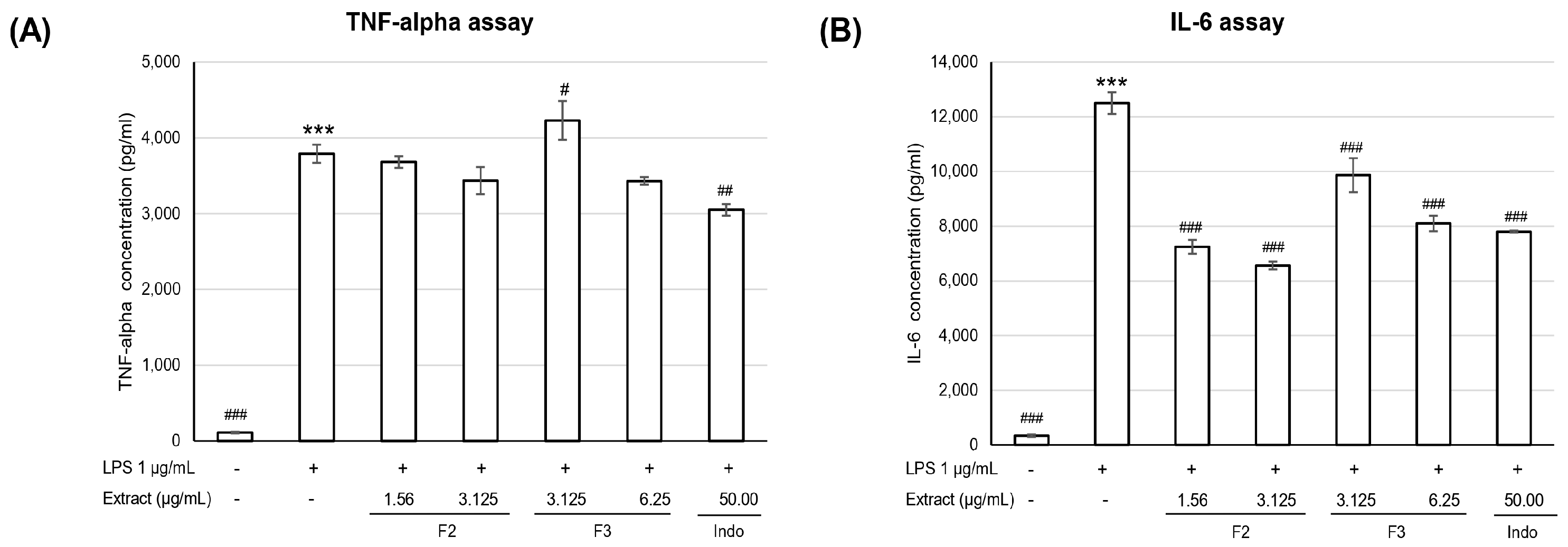
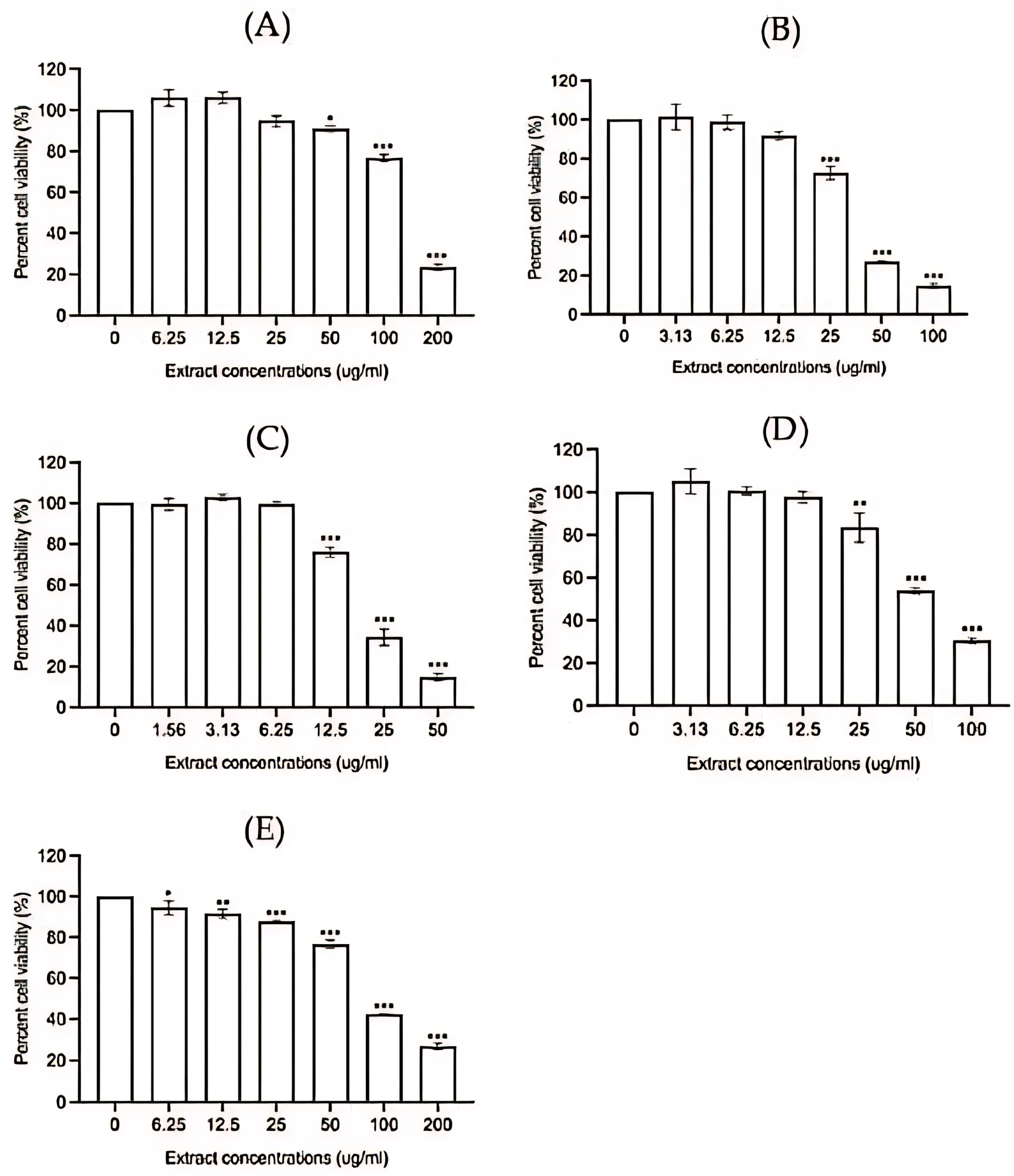
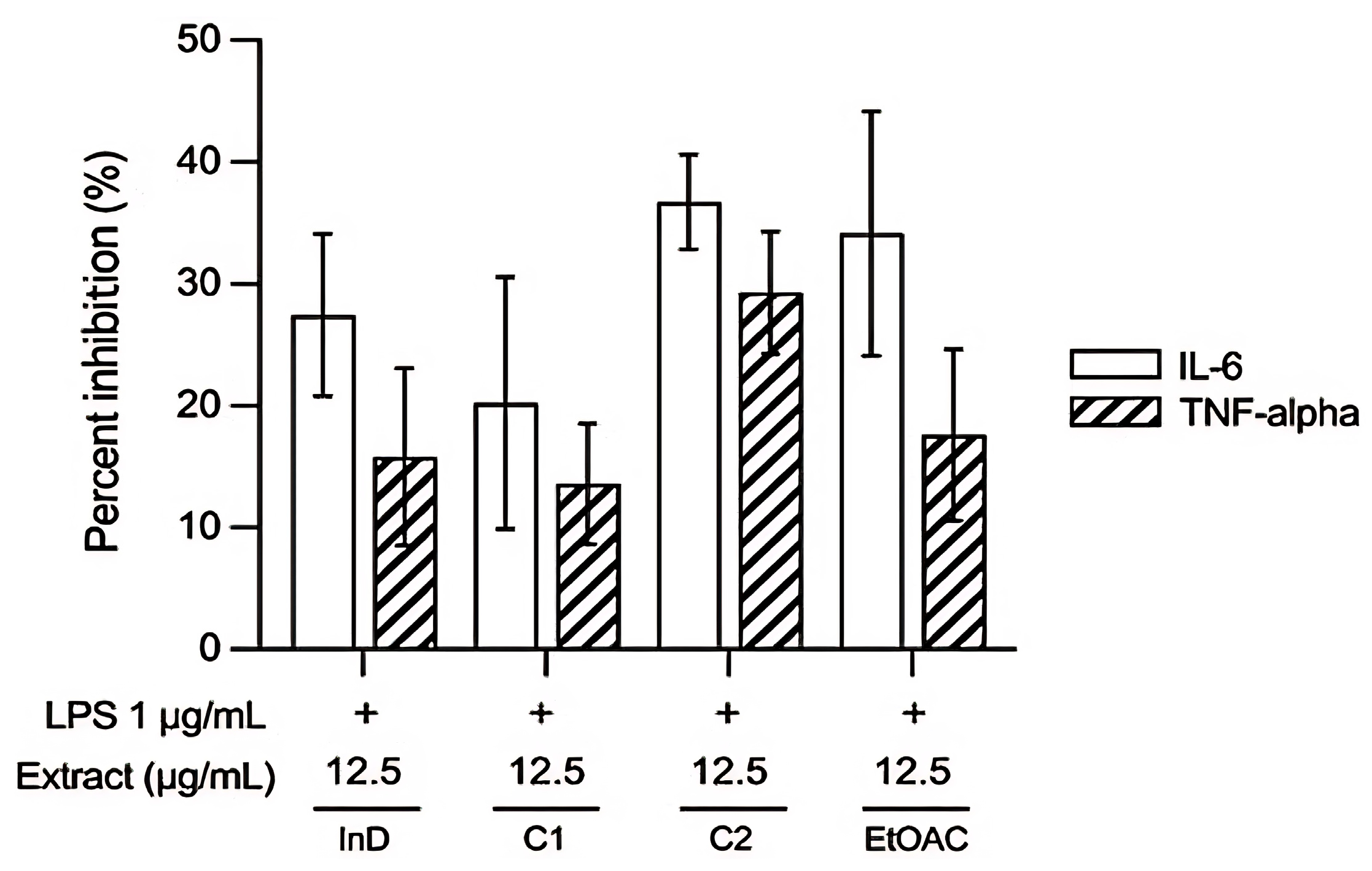
3.2.2. Anti-Inflammatory Activity
- Cytotoxicity assay
- 2.
- Effect of extracts on inhibition of TNF-α and IL-6
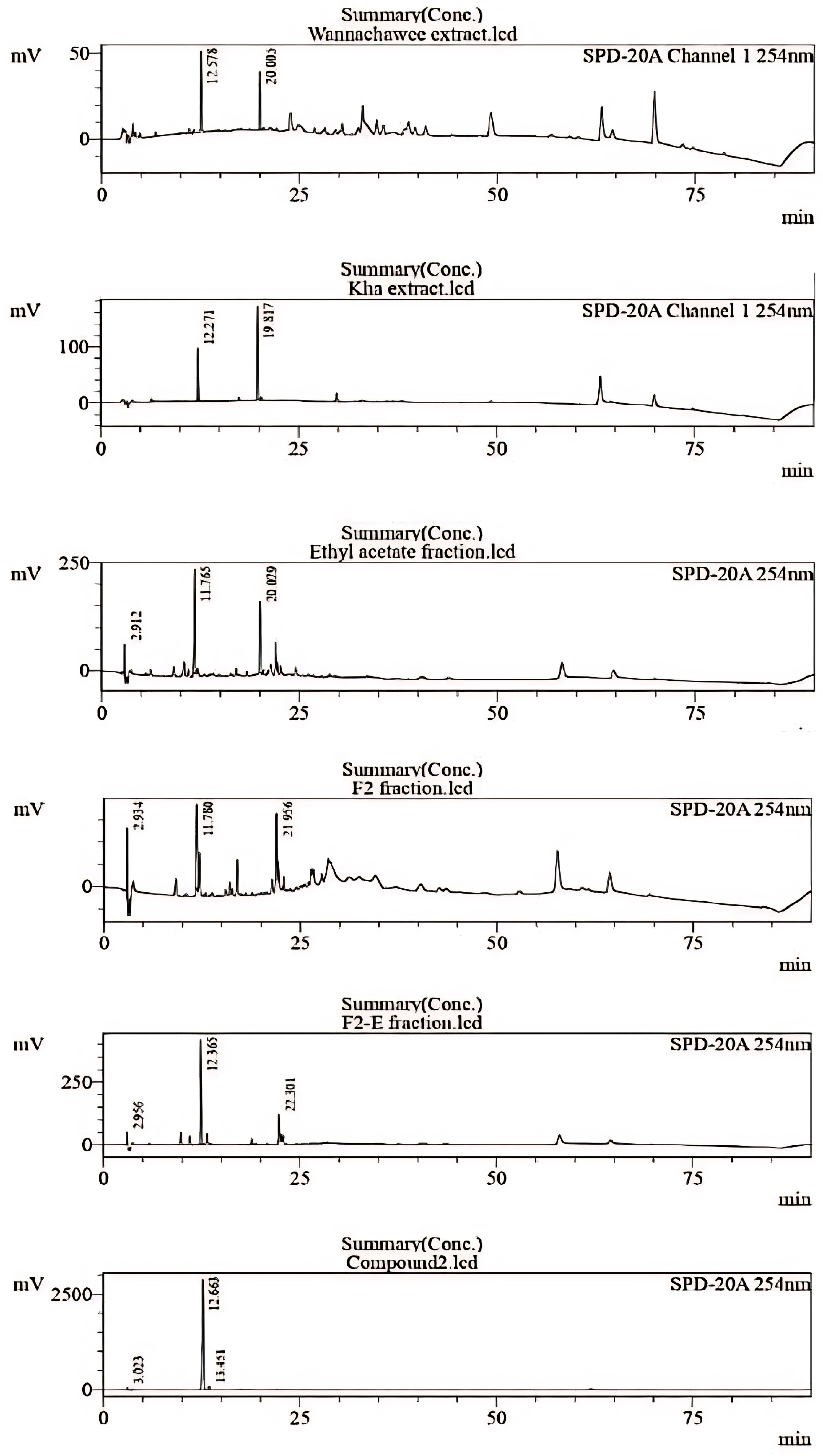
3.3. Chemical Identification of WCR Recipe by HPLC
3.4. Results of Structure Elucidation
| Position | Compound 1 | p-Coumaryl Aldehyde | |
|---|---|---|---|
| δH (mult., J in Hz) | δc, mult | δH (mult., J in Hz) of Ref. [23] | |
| 1 | - | 159.3, C | - |
| 2 | 6.73 (d, 8.5, 2H) | 116.4, CH | 6.72 (d, 8.5, 2H) |
| 3 | 7.27 (d, 8.5, 2H) | 128.8, CH | 7.24 (d, 8.5, 2H) |
| 4 | - | 180.3, C | - |
| 5 | 6.50 (d, 16.0, 1H) | 133.9, CH | 6.50 (d, 15.9, 1H) |
| 6 | 6.11 (dt, 15.5, 6.5, 1H) | 122.4, CH | 6.16 (dt, 15.9, 6.0, 1H) |
| 7 | 8.54 (s, 1H) | 200.08, CHO | 9.56 (d, 7.9, 1H) |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pradhan, M.; Alexander, A.; Singh, M.R.; Singh, D.; Saraf, S.; Saraf, S.; Ajazuddin. Understanding the Prospective of Nano-Formulations towards the Treatment of Psoriasis. Biomed. Pharmacother. 2018, 107, 447–463. [Google Scholar] [PubMed]
- GBD 2021 Forecasting Collaborators. Burden of disease scenarios for 204 countries and territories, 2022–2050: A forecasting analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2204–2256. [Google Scholar]
- Orzan, O.A.; Tutunaru, C.V.; Ianosi, S.L. Understanding the Intricate Pathophysiology of Psoriasis and Related Skin Disorders. Int. J. Mol. Sci. 2025, 26, 749. [Google Scholar] [CrossRef] [PubMed]
- Skayem, C.; Taieb, C.; Halioua, B.; Baissac, C.; Aroman, M.S. Epidemiology of Psoriasis: A Worldwide Global Study. Acta Derm Venereol. 2025, 105, 42945. [Google Scholar] [CrossRef]
- Thailand Develops Herbal Patch Innovation for Psoriasis Treatment. Available online: https://thainews.prd.go.th/nbtworld/news/view/771386/?bid=1 (accessed on 19 June 2025).
- Lee, Y.G.; Jung, Y.; Choi, H.-K.; Lee, J.-I.; Lim, T.-G.; Lee, J. Natural Product-Derived Compounds Targeting Keratinocytes and Molecular Pathways in Psoriasis Therapeutics. Int. J. Mol. Sci. 2024, 25, 6068. [Google Scholar] [PubMed]
- Na Takuathung, M.; Wongnoppavich, A.; Panthong, A.; Khonsung, P.; Chiranthanut, N.; Soonthornchareonnon, N.; Sireeratawong, S. Antipsoriatic Effects of Wannachawee Recipe on Imiquimod-Induced Psoriasis-Like Dermatitis in BALB/c Mice. Evid. Based Complement. Alternat. Med. 2018, 2018, 7931031. [Google Scholar] [CrossRef]
- Na Takuathung, M.; Wongnoppavich, A.; Pitchakarn, P.; Panthong, A.; Khonsung, P.; Chiranthanut, N.; Soonthornchareonnon, N.; Sireeratawong, S. Effects of Wannachawee Recipe with Antipsoriatic Activity on Suppressing Inflammatory Cytokine Production in HaCaT Human Keratinocytes. Evid. Based Complement. Alternat. Med. 2017, 2017, 5906539. [Google Scholar] [CrossRef]
- Lu, C.L.; Zhu, W.; Wang, D.M.; Chen, W.L.; Hu, M.M.; Wang, M.; Xu, X.-J.; Lu, C.-J. Inhibitory Effects of Chemical Compounds Isolated from the Rhizome of Smilax glabra on Nitric Oxide and Tumor Necrosis Factor-alpha Production in Lipopolysaccharide-Induced RAW264.7 Cell. Evid. Based Complement. Alternat. Med. 2015, 2015, 602425. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, R.; Shi, Y.; Zhang, X.; Tian, C.; Xia, D. Antioxidant and Anti-Inflammatory Activities of Six Flavonoids from Smilax glabra Roxb. Molecules 2020, 25, 5295. [Google Scholar] [CrossRef]
- Tewtrakul, S.; Tansakul, P.; Panichayupakaranant, P. Effects of Rhinacanthins from Rhinacanthus nasutus on Nitric Oxide, Prostaglandin E2 and Tumor Necrosis Factor-Alpha Releases Using RAW264.7 Macrophage Cells. Phytomedicine 2009, 16, 581–585. [Google Scholar] [CrossRef]
- Gaspari, A.; Tyring, S. New and emerging biologic therapies for moderate-to-severe plaque psoriasis: Mechanistic rationales and recent clinical data for IL-17 and IL-23 inhibitors. Dermatol. Ther. 2015, 28, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Blauvelt, A.; Langley, R.G.; Branigan, P.J.; Liu, X.; Chen, Y.; DePrimo, S.; Ma, K.; Scott, B.; Campbell, K.; Muñoz-Elías, E.J.; et al. Guselkumab Reduces Disease- and Mechanism Related Biomarkers More Than Adalimumab in Patients with Psoriasis: A VOYAGE 1 Substudy. JID Innov. 2024, 4, 100287. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef] [PubMed]
- Saggini, A.; Chimenti, S.; Chiricozzi, A. IL-6 as a Druggable Target in Psoriasis: Focus on Pustular Variants. J. Immunol. Res. 2014, 2014, 964069. [Google Scholar] [CrossRef]
- Sieminska, I.; Pieniawska, M.; Grzywa, T.M. The Immunology of Psoriasis—Current Concepts in Pathogenesis. Clin. Rev. Allergy Immunol. 2024, 66, 164–191. [Google Scholar]
- Verghese, B.; Bhatnagar, S.; Tanwar, R.; Bhattacharjee, J. Serum Cytokine Profile in Psoriasis-A Case–Control Study in a Tertiary Care Hospital from Northern India. Indian J. Clin. Biochem. 2011, 26, 373–377. [Google Scholar] [CrossRef]
- Soonthornchareonnon, N. Research and Development of Crude Drug and Extractive of Wannachawee Recipes for Psoriasis Treatment; National Research Council of Thailand (NRCT): Bangkok, Thailand, 2014; pp. 1–69. [Google Scholar]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Duarte, D.; Vale, N.; Trindade, T. RAW264.7 cell line as an in vitro model for screening anti-inflammatory and immunomodulatory compounds. Pharmaceuticals 2021, 14, 933. [Google Scholar]
- Ramanunny, A.K.; Wadhwa, S.; Gulati, M.; Gupta, S.; Porwal, O.; Jha, N.K.; Gupta, P.K.; Kumar, D.; Prasher, P.; Dua, K.; et al. Development and validation of RP-HPLC method for 1′-Acetoxychavicol acetate (ACA) and its application in optimizing the yield of ACA during its isolation from Alpinia galanga extract as well as its quantification in nanoemulsion. S. Afr. J. Bot. 2022, 149, 887–898. [Google Scholar] [CrossRef]
- Simon, A.; Nghiem, K.S.; Gampe, N.; Garadi, Z.; Boldizsar, I.; Backlund, A.; Darcsi, A.; Nedves, A.N.; Riethmüller, E. Stability Study of Alpinia galanga Constituents and Investigation of Their Membrane Permeability by ChemGPS-NP and the Parallel Artificial Membrane Permeability Assay. Pharmaceutics 2022, 14, 1967. [Google Scholar] [CrossRef]
- Leem, J.Y.; Jeong, I.J.; Park, K.T.; Park, H.Y. Isolation of p-hydroxycinnamaldehyde as an antibacterial substance from the saw fly, Acantholyda parki S. FEBS Lett. 1999, 442, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Qian, C.; Roman, M.; Glasser, W.G.; Esker, A.R. Surface-initiated dehydrogenative polymerization of monolignols: A quartz crystal microbalance with dissipation monitoring and atomic force microscopy study. Biomacromolecules 2013, 14, 3964–3972. [Google Scholar] [CrossRef] [PubMed]
- Bassanini, I.; Krejzova, J.; Panzeri, W.; Monti, D.; Kren, V.; Riva, S. A sustainable one-Pot, two-enzyme synthesis of naturally occurring arylalkyl glucosides. ChemSusChem 2017, 10, 2040–2045. [Google Scholar] [CrossRef]
- Lesuis, N.; Befrits, R.; Nyberg, F.; van Vollenhoven, R.F. Gender and the treatment of immune-mediated chronic inflammatory diseases: Rheumatoid arthritis, inflammatory bowel disease and psoriasis: An observational study. BMC Med. 2012, 10, 82. [Google Scholar] [CrossRef]
- Reich, K. The concept of psoriasis as a systemic inflammation: Implications for disease management. J. Eur. Acad. Dermatol. Venereol. 2011, 26, 3–11. [Google Scholar] [CrossRef]
- Chronic Inflammation. Available online: https://www.ncbi.nlm.nih.gov/books/NBK493173/ (accessed on 13 March 2025).
- Man, A.-M.; Orăsan, M.S.; Hoteiuc, O.-A.; Olănescu-Vaida-Voevod, M.-C.; Mocan, T. Inflammation and Psoriasis: A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 16095. [Google Scholar] [CrossRef]
- Chudiwal, A.K.; Jain, D.P.; Somani, R.S. Alpinia galanga Willd.—An overview on phyto-pharmacological properties. IJNPR 2010, 1, 143–149. [Google Scholar]
- Pengsook, A.; Puangsomchit, A.; Yooboon, T.; Bullangpoti, V.; Pluempanupat, W. Insecticidal activity of isolated phenylpropanoids from Alpinia galanga rhizomes against Spodoptera litura. Nat. Prod. Res. 2021, 35, 5261–5265. [Google Scholar] [CrossRef]
- Manse, Y.; Ninomiya, K.; Nishi, R.; Kamei, I.; Katsuyama, Y.; Imagawa, T.; Chaipech, S.; Muraoka, O.; Morikawa, T. Melanogenesis inhibitory activity of a 7-O-9’-linked neolignan from Alpinia galanga fruit. Bioorg. Med. Chem. 2016, 24, 6215–6224. [Google Scholar] [CrossRef]
- Lee, D.; Son, S.-R.; Qi, Y.; Kang, K.S.; Jang, D.S. (1S)-1-Acetoxyeugenol Acetate Enhances Glucose-Stimulated Insulin Secretion. Plants 2023, 12, 579. [Google Scholar] [CrossRef] [PubMed]
- Youn, I.; Han, A.-R.; Piao, D.; Lee, H.; Kwak, H.; Lee, Y.; Nam, J.-W.; Seo, E.K. Phytochemical and pharmacological properties of the genus Alpinia from 2016 to 2023. Nat. Prod. Rep. 2024, 41, 1346–1367. [Google Scholar] [CrossRef] [PubMed]
- Ly, T.N.; Shimoyamada, M.; Kato, K.; Yamauchi, R. Isolation and Characterization of Some Antioxidative Compounds from the Rhizome of Smaller Galanga (Alpinia officinarum Hance). J. Agric. Food Chem. 2003, 51, 4924–4929. [Google Scholar] [CrossRef]
- Da Silveira E Sá, R.D.C.; Andrade, L.N.; De Oliveira, R.D.R.B.; De Sousa, D.P. A Review on Anti-Inflammatory Activity of Phenylpropanoids Found in Essential Oils. Molecules 2014, 19, 1459–1480. [Google Scholar] [CrossRef]
- Ambriz-Pérez, D.L.; Leyva-López, N.; Gutierrez-Grijalva, E.P.; Heredia, J.B. Phenolic compounds: Natural alternative in inflammation treatment. A Review. Cogent Food Agric. 2016, 2, 1131412. [Google Scholar]
- Gaffen, S.L.; Jain, R.; Garg, A.V.; Cua, D.J. The IL-23-IL-17 immune axis: From mechanisms to the therapeutic testing. Nat. Rev. Immunol. 2014, 14, 585–600. [Google Scholar] [PubMed]
- Lowes, M.A.; Suarez-Farinas, M.; Krueger, J.G. Immunology of psoriasis. Annu. Rev. Immunol. 2014, 32, 227–255. [Google Scholar] [CrossRef] [PubMed]
- Blauvelt, A.; Chiricozzi, A. The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clin. Rev. Allergy Immunol. 2018, 55, 379–390. [Google Scholar] [PubMed]
- Di Cesare, A.; Di Meglio, P.; Nestle, F.O. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J. Investig. Dermatol. 2009, 129, 1339–1350. [Google Scholar] [CrossRef]
- Tey, H.L.; Bachelez, H. Psoriasis: Target cytokines and therapy. Br. J. Dermatol. 2016, 174, 502–513. [Google Scholar]


| Thai Name | Scientific Name | Family | Part Used | Voucher No. |
|---|---|---|---|---|
| Kha | Alpinia galanga (L.) Willd. | Zingiberaceae | Rhizome | 0023406 |
| Khao YenTai | Smilax glabra Wall.ex Roxb. | Smilacaceae | Rhizome | 0023412 |
| Khao Yen Nuea | Smilax corbularia Kunth. | Smilacaceae | Rhizome | 0023411 |
| Khao Yen Jeen | Smilax sp. | Smilacaceae | Rhizome | 0023413 |
| Hua Ta Pead | Stemona involuta Inthachub. | Stemonaceae | Root | 0023409 |
| Non Tai Yak | Stemona collinsae Craib. | Stemonaceae | Root | 0023408 |
| Thong Pan Chang | Rhinacanthus nasutus (L.) Kurz. | Acanthaceae | Aerial part | 0023410 |
| Ngueak plaamo | Acanthus ilicifolius L. | Acanthaceae | Aerial part | 0023407 |
| Time (min) | A (per cent v/v) | B (per cent v/v) |
|---|---|---|
| 0.01 | 10 | 90 |
| 20.00 | 45 | 55 |
| 40.00 | 50 | 50 |
| 45.00 | 50 | 50 |
| 80.00 | 80 | 20 |
| 85.00 | 10 | 90 |
| 90.00 | 10 | 90 |
| Volume of conc. Water Extract (mL) | % Brix | pH | Dried Water Extract (g) | % Yield (w/w) |
|---|---|---|---|---|
| 2900 * | 3 * | 5.17 * | 91.81 * | 14.34 * |
| Extract Concentration (µg/mL) | TNF-α (pg/mL) | IL-6 (pg/mL) | |
|---|---|---|---|
| Unstimulated cell | 162 ± 25 | 285 ± 27 | |
| LPS-induced cell | 3661 ± 170 ### | 10,912 ± 856 ### | |
| Ethyl acetate | 3.13 | 2796 ± 281 * | 6730 ± 956 *** |
| 6.25 | 2611 ± 446 ** | 5349 ± 1329 *** | |
| F2-A | 1.56 | 2443 ± 167 ** | 5055 ± 157 *** |
| 3.13 | 2215 ± 153 *** | 3929 ± 128 *** | |
| F2-E | 1.56 | 2692 ± 200 ** | 5993 ± 557 *** |
| 3.13 | 2600 ± 363 ** | 5155 ± 524 *** | |
| F2-N | 12.5 | 2607 ± 335 ** | 5292 ± 1483 *** |
| 25 | 2625 ± 48 ** | 6041 ± 318 *** | |
| F2-OP | 12.5 | 2673 ± 146 ** | 6811 ± 123 *** |
| 25 | 2649 ± 281 ** | 6365 ± 454 *** | |
| Indomethacin | 25 | 1851 ± 170 *** | 3910 ± 202 *** |
| 50 | 1705 ± 160 *** | 3094 ± 195 *** | |
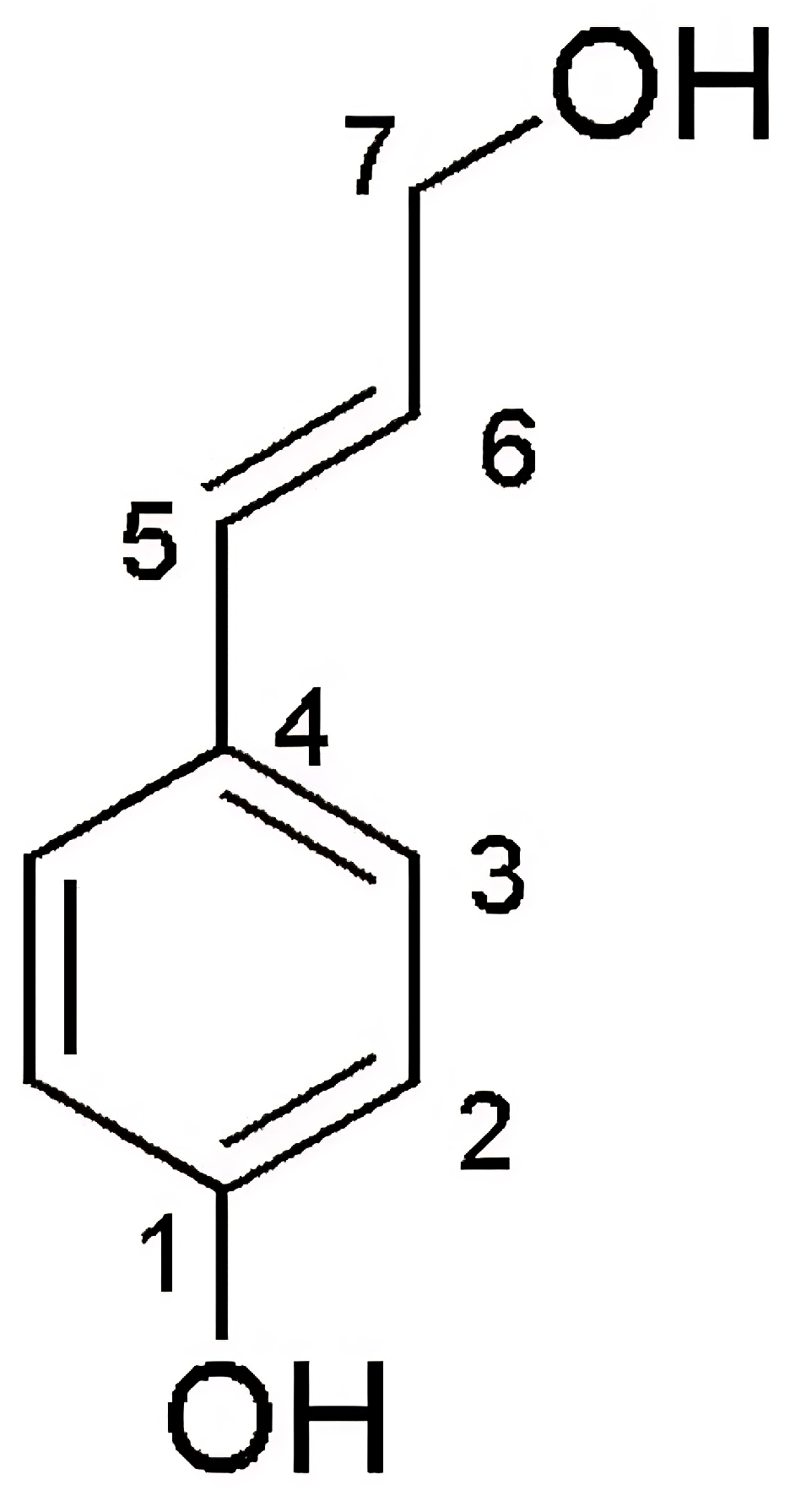
| Position | Compound 2 | trans-p-Coumaryl Alcohol | ||
|---|---|---|---|---|
| δH (mult., J in Hz) | δc, mult | δH (mult., J in Hz) of Ref. [24] | δc, (mult) of Ref. [23] | |
| 1 | - | 158.2, C | - | 157.73, C |
| 2 | 6.72 (d, 8.5, 2H) | 116.3, CH | 6.74 (d, 8.6, 2H) | 116.29, CH |
| 3 | 7.27 (d, 8.5, 2H) | 128.6, CH | 7.25 (d, 8.5, 2H) | 128.31, CH |
| 4 | - | 129.9, C | 128.82, C | |
| 5 | 6.50 (d, 16.0, 1H) | 131.8, CH | 6.52 (d, 15.8, 1H) | 129.59, CH |
| 6 | 6.16 (dt, 16.0, 6.0, 1H) | 126.6, CH | 6.18 (dt, 15.8, 6.0, 1H) | 128.08, CH |
| 7 | 4.18 (dd, 6.0, 1.5, 2H) | 63.9, CH2 | 4.20 (dd, 6.0, 1.4, 2H) | 62.66, CH2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tantipat, S.; Trisuwan, K.; Kunnaja, P.; Sireeratawong, S.; Natakankitkul, S.; Imiam, S.; Chansakaow, S. Trans-p-Coumaryl Alcohol as a Bioactive Compound and Anti-Inflammatory Agent in Wannachawee Recipe for Psoriasis. Pharmaceutics 2025, 17, 864. https://doi.org/10.3390/pharmaceutics17070864
Tantipat S, Trisuwan K, Kunnaja P, Sireeratawong S, Natakankitkul S, Imiam S, Chansakaow S. Trans-p-Coumaryl Alcohol as a Bioactive Compound and Anti-Inflammatory Agent in Wannachawee Recipe for Psoriasis. Pharmaceutics. 2025; 17(7):864. https://doi.org/10.3390/pharmaceutics17070864
Chicago/Turabian StyleTantipat, Supreeya, Kongkiat Trisuwan, Phraepakaporn Kunnaja, Seewaboon Sireeratawong, Surapol Natakankitkul, Surasak Imiam, and Sunee Chansakaow. 2025. "Trans-p-Coumaryl Alcohol as a Bioactive Compound and Anti-Inflammatory Agent in Wannachawee Recipe for Psoriasis" Pharmaceutics 17, no. 7: 864. https://doi.org/10.3390/pharmaceutics17070864
APA StyleTantipat, S., Trisuwan, K., Kunnaja, P., Sireeratawong, S., Natakankitkul, S., Imiam, S., & Chansakaow, S. (2025). Trans-p-Coumaryl Alcohol as a Bioactive Compound and Anti-Inflammatory Agent in Wannachawee Recipe for Psoriasis. Pharmaceutics, 17(7), 864. https://doi.org/10.3390/pharmaceutics17070864








