In Vitro Evaluation of the Efficient Passage of PLGA-Formulated Trastuzumab for Nose-to-Brain Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Trastuzumab Production
2.2. Preparation and Characterization of Trastuzumab-Loaded PLGA NPs
2.2.1. Nanoparticle Preparation
2.2.2. Size Measurement and Zeta Potential Morphology
2.2.3. Trastuzumab Encapsulation Efficacy (EE%) and Drug Loading Capacity (DL%)
2.2.4. Study of TZB Release Kinetics from NPs in PBS Buffer, Nasal Mucus, or Plasma
2.3. ELISA Assay
2.4. Flow Cytometry
2.5. Study of TZB Passage Using In Vitro Models
2.5.1. Epithelium Nasal Barrier Model Air-Liquid Interface
2.5.2. BBB In Vitro Cell-Based Model
2.5.3. In Vitro Permeability Experimental Procedures
2.6. MTT Cell Toxicity Test on RPMI 2650 Cells
2.7. Statistics
3. Results
3.1. Nanoparticle Formulation and Characterization
3.1.1. Stability of TZB During Formulation Process
3.1.2. Physicochemical Characterization of the Formulation
3.1.3. Nanoparticle Release Kinetics and Characterization of TZB Released from PLGA Nanoparticles
3.2. Permeability Studies on In Vitro Models
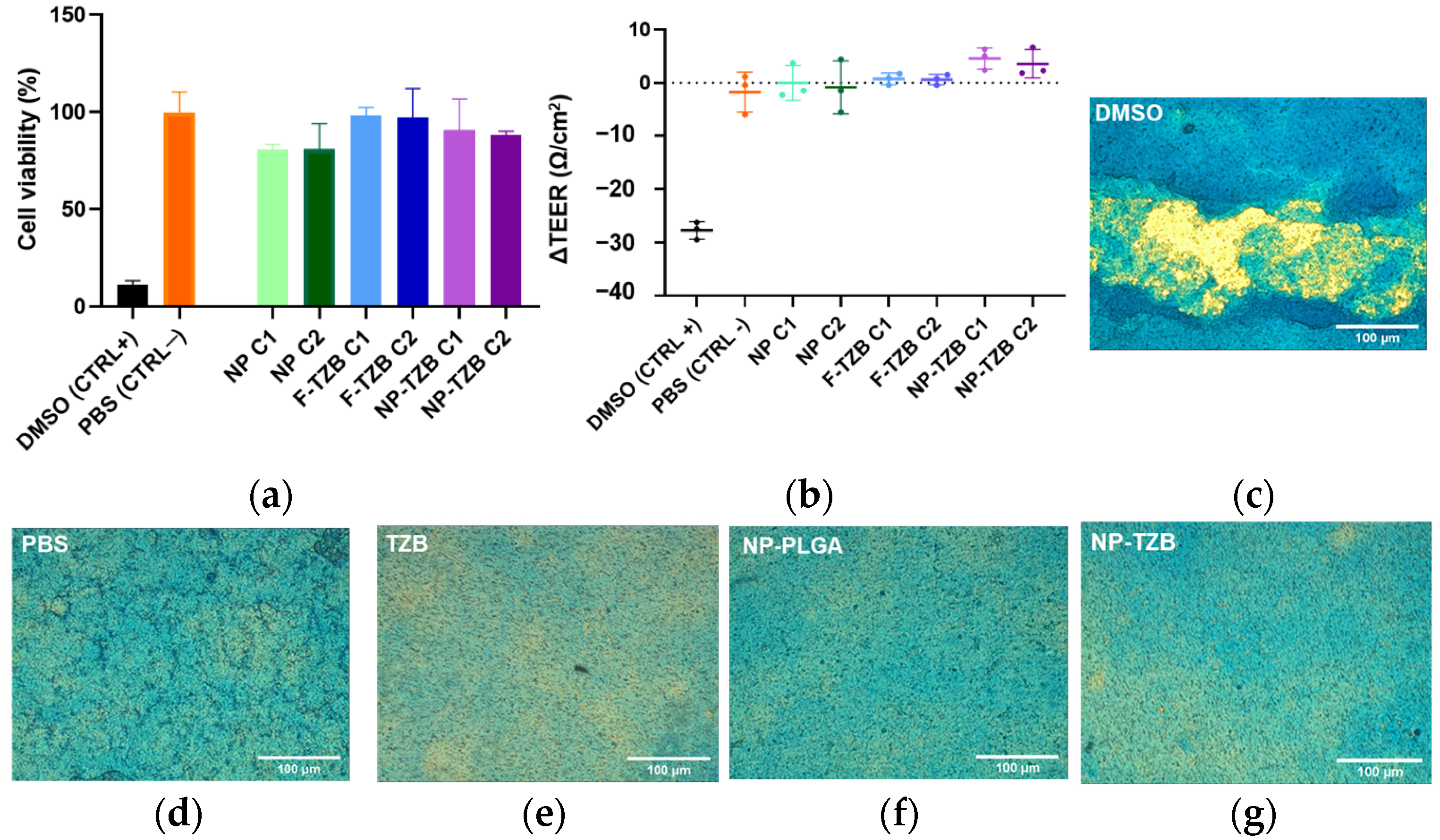
3.2.1. Integrity Studies in ALI Model
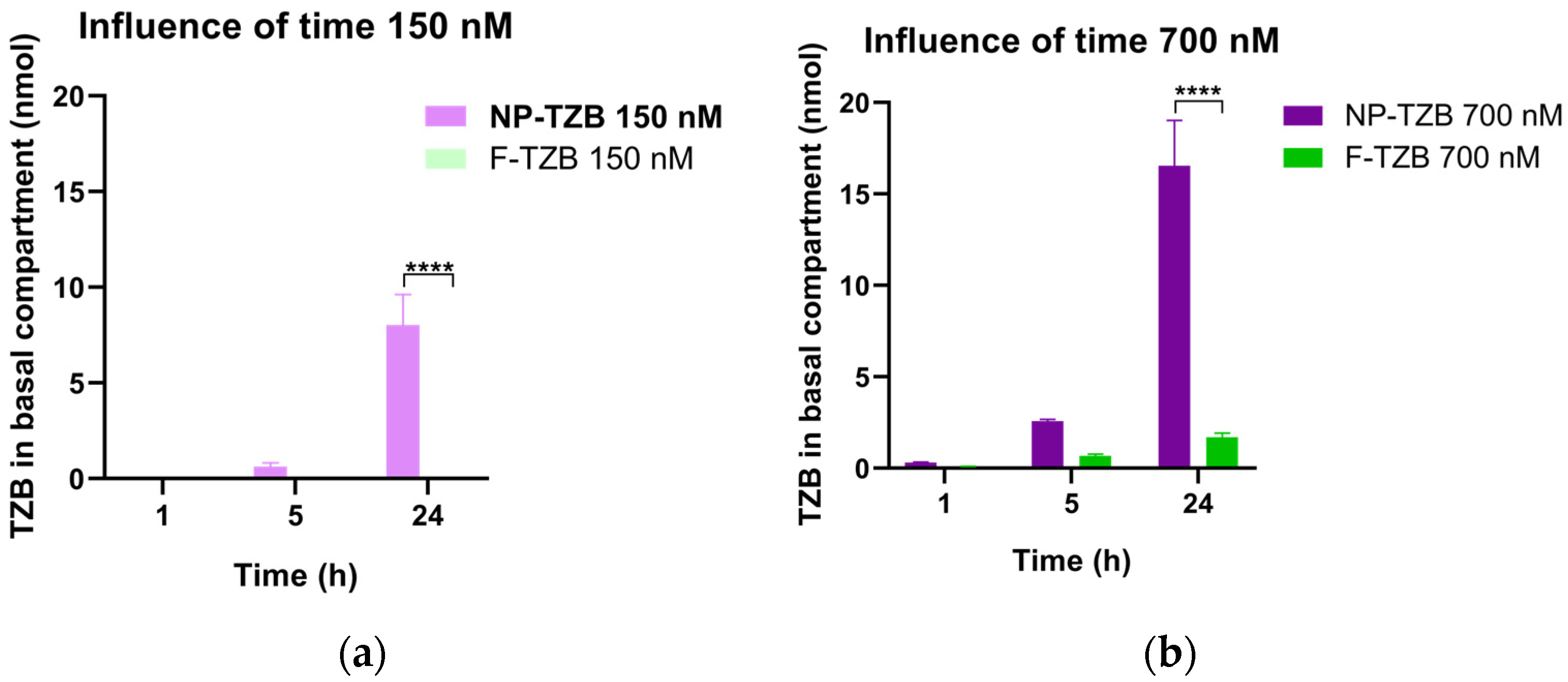
3.2.2. Permeability Across the Nasal Epithelium Study on RPMI 2650 Barrier Model
3.2.3. Binding Specificity of Both Encapsulated and Free TZB After Crossing the In Vitro Nasal Epithelium
3.2.4. Permeability Study on BBB
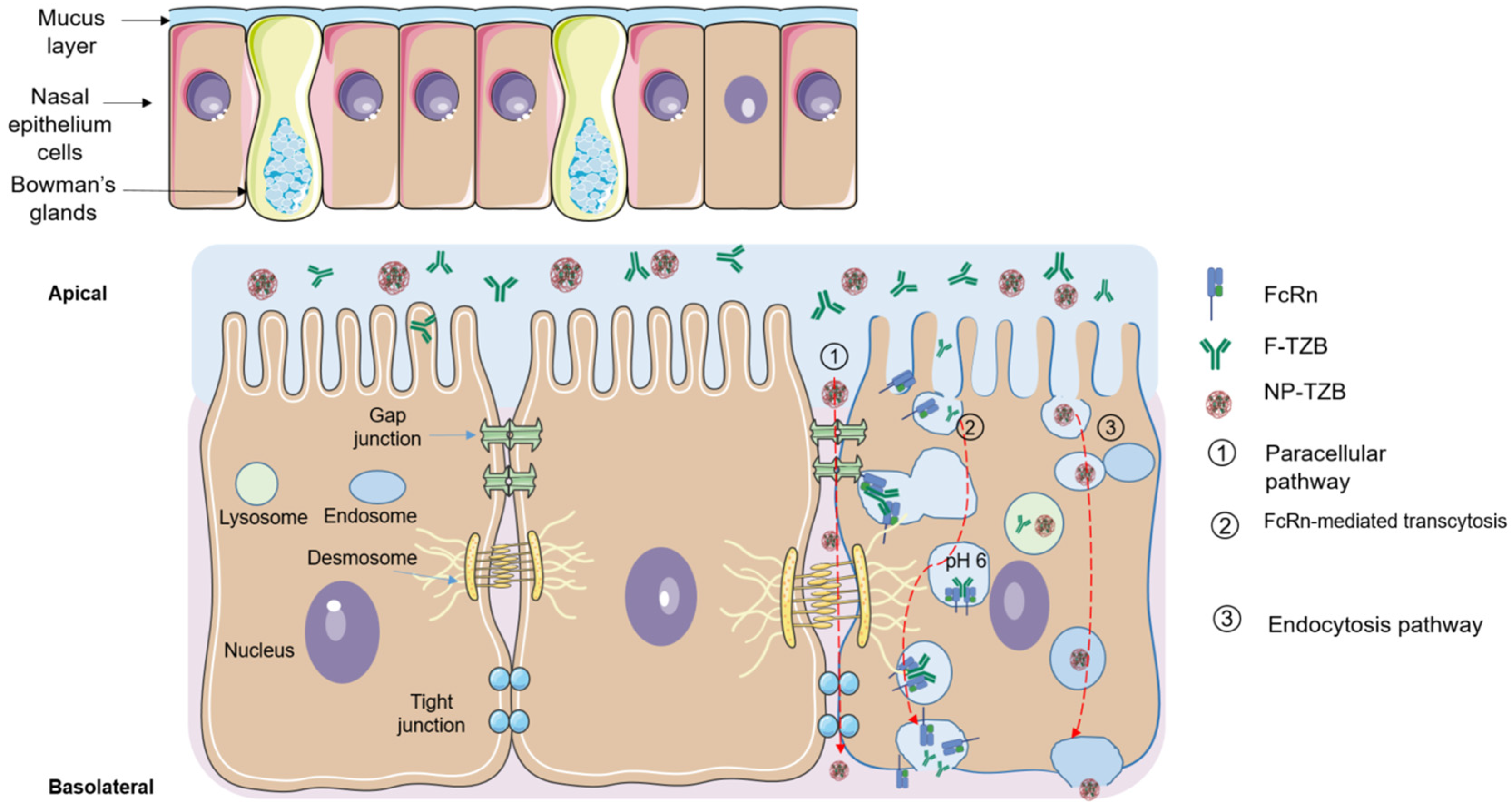
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALI | Air–liquid interface cultivation |
| BBB | Blood–brain barrier |
| BMs | Brain metastases |
| BSA | Bovine serum albumin |
| DLS | Dynamic light scattering |
| EE | Encapsulation efficiency |
| ELISA | Enzyme-linked immunosorbent assay |
| HER2 | Human epidermal growth factor receptor 2 |
| IgG | Immunoglobulin G |
| MFI | Mean fluorescence intensity |
| N2B | Nose-to-brain |
| NPs | Nanoparticles |
| NP-TZBs | Trastuzumab-loaded nanoparticles |
| PBS | Phosphate-buffered saline |
| PLGA | Poly(lactic-co-glycolic) acid |
| PVA | Polyvinyl alcohol |
| SD | Standard deviation |
| SDS-PAGE | Sodium dodecyl–sulfate polyacrylamide gel electrophoresis |
| TEER | Transepithelial electrical resistance |
| TZB | Trastuzumab |
| ZP | Zeta potential |
References
- Amsbaugh, M.J.; Kim, C.S. Brain Metastasis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Caroli, M.; Di Cristofori, A.; Lucarella, F.; Raneri, F.A.; Portaluri, F.; Gaini, S.M. Surgical Brain Metastases: Management and Outcome Related to Prognostic Indexes: A Critical Review of a Ten-Year Series. ISRN Surg. 2011, 2011, 207103. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alvarez, A.; Papakonstantinou, A.; Oliveira, M. Brain Metastases in HER2-Positive Breast Cancer: Current and Novel Treatment Strategies. Cancers 2021, 13, 2927. [Google Scholar] [CrossRef]
- Khan, M.; Lin, J.; Liao, G.; Tian, Y.; Liang, Y.; Li, R.; Liu, M.; Yuan, Y. Whole Brain Radiation Therapy Plus Stereotactic Radiosurgery in the Treatment of Brain Metastases Leading to Improved Survival in Patients with Favorable Prognostic Factors. Front. Oncol. 2019, 9, 205. [Google Scholar] [CrossRef]
- van Grinsven, E.E.; Nagtegaal, S.H.J.; Verhoeff, J.J.C.; van Zandvoort, M.J.E. The Impact of Stereotactic or Whole Brain Radiotherapy on Neurocognitive Functioning in Adult Patients with Brain Metastases: A Systematic Review and Meta-Analysis. Oncol. Res. Treat. 2021, 44, 622–636. [Google Scholar] [CrossRef]
- Pinto, A.C.; Ades, F.; de Azambuja, E.; Piccart-Gebhart, M. Trastuzumab for Patients with HER2 Positive Breast Cancer: Delivery, Duration and Combination Therapies. Breast 2013, 22, S152–S155. [Google Scholar] [CrossRef]
- Swain, S.M.; Shastry, M.; Hamilton, E. Targeting HER2-Positive Breast Cancer: Advances and Future Directions. Nat. Rev. Drug Discov. 2023, 22, 101–126. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The Blood–Brain Barrier: Structure, Regulation, and Drug Delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef]
- Terrell-Hall, T.B.; Nounou, M.I.; El-Amrawy, F.; Griffith, J.I.G.; Lockman, P.R. Trastuzumab Distribution in an In-Vivo and in-Vitro Model of Brain Metastases of Breast Cancer. Oncotarget 2017, 8, 83734–83744. [Google Scholar] [CrossRef]
- Lockman, P.R.; Mittapalli, R.K.; Taskar, K.S.; Rudraraju, V.; Gril, B.; Bohn, K.A.; Adkins, C.E.; Roberts, A.; Thorsheim, H.R.; Gaasch, J.A.; et al. Heterogeneous Blood–Tumor Barrier Permeability Determines Drug Efficacy in Experimental Brain Metastases of Breast Cancer. Clin. Cancer Res. 2010, 16, 5664–5678. [Google Scholar] [CrossRef]
- Dijkers, E.C.; Oude Munnink, T.H.; Kosterink, J.G.; Brouwers, A.H.; Jager, P.L.; de Jong, J.R.; van Dongen, G.A.; Schröder, C.P.; Lub-de Hooge, M.N.; de Vries, E.G. Biodistribution of 89Zr-Trastuzumab and PET Imaging of HER2-Positive Lesions in Patients with Metastatic Breast Cancer. Clin. Pharmacol. Ther. 2010, 87, 586–592. [Google Scholar] [CrossRef]
- Bartsch, R.; Berghoff, A.S.; Furtner, J.; Marhold, M.; Bergen, E.S.; Roider-Schur, S.; Starzer, A.M.; Forstner, H.; Rottenmanner, B.; Dieckmann, K.; et al. Trastuzumab Deruxtecan in HER2-Positive Breast Cancer with Brain Metastases: A Single-Arm, Phase 2 Trial. Nat. Med. 2022, 28, 1840–1847. [Google Scholar] [CrossRef] [PubMed]
- Jerusalem, G.; Park, Y.H.; Yamashita, T.; Hurvitz, S.A.; Modi, S.; Andre, F.; Krop, I.E.; Gonzàlez Farré, X.; You, B.; Saura, C.; et al. Trastuzumab Deruxtecan in HER2-Positive Metastatic Breast Cancer Patients with Brain Metastases: A DESTINY-Breast01 Subgroup Analysis. Cancer Discov. 2022, 12, 2754–2762. [Google Scholar] [CrossRef] [PubMed]
- Patharapankal, E.J.; Ajiboye, A.L.; Mattern, C.; Trivedi, V. Nose-to-Brain (N2B) Delivery: An Alternative Route for the Delivery of Biologics in the Management and Treatment of Central Nervous System Disorders. Pharmaceutics 2024, 16, 66. [Google Scholar] [CrossRef]
- Lee, D.; Minko, T. Nanotherapeutics for Nose-to-Brain Drug Delivery: An Approach to Bypass the Blood Brain Barrier. Pharmaceutics 2021, 13, 2049. [Google Scholar] [CrossRef]
- Jeong, S.-H.; Jang, J.-H.; Lee, Y.-B. Drug Delivery to the Brain via the Nasal Route of Administration: Exploration of Key Targets and Major Consideration Factors. J. Pharm. Investig. 2023, 53, 119–152. [Google Scholar] [CrossRef]
- Sonvico, F.; Clementino, A.; Buttini, F.; Colombo, G.; Pescina, S.; Stanisçuaski Guterres, S.; Raffin Pohlmann, A.; Nicoli, S. Surface-Modified Nanocarriers for Nose-to-Brain Delivery: From Bioadhesion to Targeting. Pharmaceutics 2018, 10, 34. [Google Scholar] [CrossRef]
- Ismail, R.; Sovány, T.; Gácsi, A.; Ambrus, R.; Katona, G.; Imre, N.; Csóka, I. Synthesis and Statistical Optimization of Poly (Lactic-Co-Glycolic Acid) Nanoparticles Encapsulating GLP1 Analog Designed for Oral Delivery. Pharm. Res. 2019, 36, 99. [Google Scholar] [CrossRef]
- Reichl, S.; Becker, K. Cultivation of RPMI 2650 Cells as an In-Vitro Model for Human Transmucosal Nasal Drug Absorption Studies: Optimization of Selected Culture Conditions. J. Pharm. Pharmacol. 2012, 64, 1621–1630. [Google Scholar] [CrossRef]
- Kreft, M.E.; Jerman, U.D.; Lasič, E.; Lanišnik Rižner, T.; Hevir-Kene, N.; Peternel, L.; Kristan, K. The Characterization of the Human Nasal Epithelial Cell Line RPMI 2650 Under Different Culture Conditions and Their Optimization for an Appropriate in Vitro Nasal Model. Pharm. Res. 2015, 32, 665–679. [Google Scholar] [CrossRef]
- Gonçalves, V.S.S.; Matias, A.A.; Poejo, J.; Serra, A.T.; Duarte, C.M.M. Application of RPMI 2650 as a Cell Model to Evaluate Solid Formulations for Intranasal Delivery of Drugs. Int. J. Pharm. 2016, 515, 1–10. [Google Scholar] [CrossRef]
- Sibinovska, N.; Žakelj, S.; Kristan, K. Suitability of RPMI 2650 Cell Models for Nasal Drug Permeability Prediction. Eur. J. Pharm. Biopharm. 2019, 145, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Pozzoli, M.; Sonvico, F.; Ong, H.X.; Traini, D.; Bebawy, M.; Young, P.M. Optimization of RPMI 2650 Cells as a Model for Nasal Mucosa. Respir. Drug Deliv. 2014, 3, 739–742. [Google Scholar]
- Lacombe, O.; Videau, O.; Chevillon, D.; Guyot, A.-C.; Contreras, C.; Blondel, S.; Nicolas, L.; Ghettas, A.; Bénech, H.; Thevenot, E.; et al. In Vitro Primary Human and Animal Cell-Based Blood−Brain Barrier Models as a Screening Tool in Drug Discovery. Mol. Pharm. 2011, 8, 651–663. [Google Scholar] [CrossRef]
- Kouhi, A.; Pachipulusu, V.; Kapenstein, T.; Hu, P.; Epstein, A.L.; Khawli, L.A. Brain Disposition of Antibody-Based Therapeutics: Dogma, Approaches and Perspectives. Int. J. Mol. Sci. 2021, 22, 6442. [Google Scholar] [CrossRef]
- Alshawwa, S.Z.; Kassem, A.A.; Farid, R.M.; Mostafa, S.K.; Labib, G.S. Nanocarrier Drug Delivery Systems: Characterization, Limitations, Future Perspectives and Implementation of Artificial Intelligence. Pharmaceutics 2022, 14, 883. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-Based Nanoparticles: An Overview of Biomedical Applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Bilati, U.; Allémann, E.; Doelker, E. Nanoprecipitation versus Emulsion-Based Techniques for the Encapsulation of Proteins into Biodegradable Nanoparticles and Process-Related Stability Issues. AAPS PharmSciTech 2005, 6, 74. [Google Scholar] [CrossRef]
- Zhang, Z.; Marie Woys, A.; Hong, K.; Grapentin, C.; Khan, T.A.; Zarraga, I.E.; Wagner, N.J.; Liu, Y. Adsorption of Non-Ionic Surfactant and Monoclonal Antibody on Siliconized Surface Studied by Neutron Reflectometry. J. Colloid Interface Sci. 2021, 584, 429–438. [Google Scholar] [CrossRef]
- Le Basle, Y.; Chennell, P.; Tokhadze, N.; Astier, A.; Sautou, V. Physicochemical Stability of Monoclonal Antibodies: A Review. J. Pharm. Sci. 2020, 109, 169–190. [Google Scholar] [CrossRef]
- Formica, M.L.; Real, D.A.; Picchio, M.L.; Catlin, E.; Donnelly, R.F.; Paredes, A.J. On a Highway to the Brain: A Review on Nose-to-Brain Drug Delivery Using Nanoparticles. Appl. Mater. Today 2022, 29, 101631. [Google Scholar] [CrossRef]
- Boyuklieva, R.; Pilicheva, B. Micro- and Nanosized Carriers for Nose-to-Brain Drug Delivery in Neurodegenerative Disorders. Biomedicines 2022, 10, 1706. [Google Scholar] [CrossRef]
- Morrison, E.E.; Costanzo, R.M. Morphology of Olfactory Epithelium in Humans and Other Vertebrates. Microsc. Res. Tech. 1992, 23, 49–61. [Google Scholar] [CrossRef]
- Handa, M.; Singh, A.; Bisht, D.; Kesharwani, P.; Shukla, R. Potential of Particle Size Less than 15 Nm via Olfactory Region for Direct Brain Delivery via Intranasal Route. Health Sci. Rev. 2022, 4, 100038. [Google Scholar] [CrossRef]
- Cone, R.A. Barrier Properties of Mucus. Adv. Drug Deliv. Rev. 2009, 61, 75–85. [Google Scholar] [CrossRef]
- Lieleg, O.; Ribbeck, K. Biological Hydrogels as Selective Diffusion Barriers. Trends Cell Biol. 2011, 21, 543–551. [Google Scholar] [CrossRef]
- Ahmad, E.; Feng, Y.; Qi, J.; Fan, W.; Ma, Y.; He, H.; Xia, F.; Dong, X.; Zhao, W.; Lu, Y.; et al. Evidence of Nose-to-Brain Delivery of Nanoemulsions: Cargoes but Not Vehicles. Nanoscale 2017, 9, 1174–1183. [Google Scholar] [CrossRef]
- Sharma, D.; Sharma, R.K.; Sharma, N.; Gabrani, R.; Sharma, S.K.; Ali, J.; Dang, S. Nose-To-Brain Delivery of PLGA-Diazepam Nanoparticles. AAPS PharmSciTech 2015, 16, 1108–1121. [Google Scholar] [CrossRef]
- Adnet, T.; Groo, A.-C.; Picard, C.; Davis, A.; Corvaisier, S.; Since, M.; Bounoure, F.; Rochais, C.; Le Pluart, L.; Dallemagne, P.; et al. Pharmacotechnical Development of a Nasal Drug Delivery Composite Nanosystem Intended for Alzheimer’s Disease Treatment. Pharmaceutics 2020, 12, 251. [Google Scholar] [CrossRef]
- Groo, A.-C.; Mircheva, K.; Bejaud, J.; Ailhas, C.; Panaiotov, I.; Saulnier, P.; Ivanova, T.; Lagarce, F. Development of 2D and 3D Mucus Models and Their Interactions with Mucus-Penetrating Paclitaxel-Loaded Lipid Nanocapsules. Pharm. Res. 2014, 31, 1753–1765. [Google Scholar] [CrossRef]
- Akpinar Adscheid, S.; Rojas-Rodríguez, M.; Abdel-Hafez, S.M.; Pavone, F.S.; Schneider, M.; Türeli, A.E.; Calamai, M.; Günday-Türeli, N. Scalable Manufacturing Method for Model Protein-Loaded PLGA Nanoparticles: Biocompatibility, Trafficking and Release Properties. Pharmaceutics 2025, 17, 87. [Google Scholar] [CrossRef]
- Ladel, S.; Maigler, F.; Flamm, J.; Schlossbauer, P.; Handl, A.; Hermann, R.; Herzog, H.; Hummel, T.; Mizaikoff, B.; Schindowski, K. Impact of Glycosylation and Species Origin on the Uptake and Permeation of IgGs through the Nasal Airway Mucosa. Pharmaceutics 2020, 12, 1014. [Google Scholar] [CrossRef] [PubMed]
- Foss, S.; Grevys, A.; Sand, K.M.K.; Bern, M.; Blundell, P.; Michaelsen, T.E.; Pleass, R.J.; Sandlie, I.; Andersen, J.T. Enhanced FcRn-Dependent Transepithelial Delivery of IgG by Fc-Engineering and Polymerization. J. Control. Release 2016, 223, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Li, G.; Lin, D.-H.; Wang, J.-T.; Qin, L.-F.; Guo, G.-P. Transport of PLGA nanoparticles across Caco-2/HT29-MTX co-cultured cells. Yao Xue Xue Bao 2013, 48, 1829–1835. [Google Scholar]
- Roux, G.L.; Jarray, R.; Guyot, A.-C.; Pavoni, S.; Costa, N.; Théodoro, F.; Nassor, F.; Pruvost, A.; Tournier, N.; Kiyan, Y.; et al. Proof-of-Concept Study of Drug Brain Permeability Between in Vivo Human Brain and an in Vitro iPSCs-Human Blood-Brain Barrier Model. Sci. Rep. 2019, 9, 16310. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kengne Kamkui, L.; Disdier, C.; Herbet, A.; Costa, N.; Guyot, A.-C.; Boquet, D.; Mabondzo, A. In Vitro Evaluation of the Efficient Passage of PLGA-Formulated Trastuzumab for Nose-to-Brain Delivery. Pharmaceutics 2025, 17, 681. https://doi.org/10.3390/pharmaceutics17060681
Kengne Kamkui L, Disdier C, Herbet A, Costa N, Guyot A-C, Boquet D, Mabondzo A. In Vitro Evaluation of the Efficient Passage of PLGA-Formulated Trastuzumab for Nose-to-Brain Delivery. Pharmaceutics. 2025; 17(6):681. https://doi.org/10.3390/pharmaceutics17060681
Chicago/Turabian StyleKengne Kamkui, Léa, Clémence Disdier, Amaury Herbet, Narciso Costa, Anne-Cécile Guyot, Didier Boquet, and Aloïse Mabondzo. 2025. "In Vitro Evaluation of the Efficient Passage of PLGA-Formulated Trastuzumab for Nose-to-Brain Delivery" Pharmaceutics 17, no. 6: 681. https://doi.org/10.3390/pharmaceutics17060681
APA StyleKengne Kamkui, L., Disdier, C., Herbet, A., Costa, N., Guyot, A.-C., Boquet, D., & Mabondzo, A. (2025). In Vitro Evaluation of the Efficient Passage of PLGA-Formulated Trastuzumab for Nose-to-Brain Delivery. Pharmaceutics, 17(6), 681. https://doi.org/10.3390/pharmaceutics17060681






