Synthesis and In Vitro Evaluation of a Scandium-44 Radiolabeled Nanobody as a PD-L1 PET Imaging Probe
Abstract
1. Introduction
2. Materials and Methods
2.1. Production and Purification of 44Sc
2.2. Quality Control of 44Sc
2.2.1. HPGe Analysis of Radioimpurities
2.2.2. MP-AES Analysis
2.3. Synthesis of (Anti-PD-L1) DTPA-B11-Nanobody and DTPA-B11-IgG
2.4. Radiosynthesis
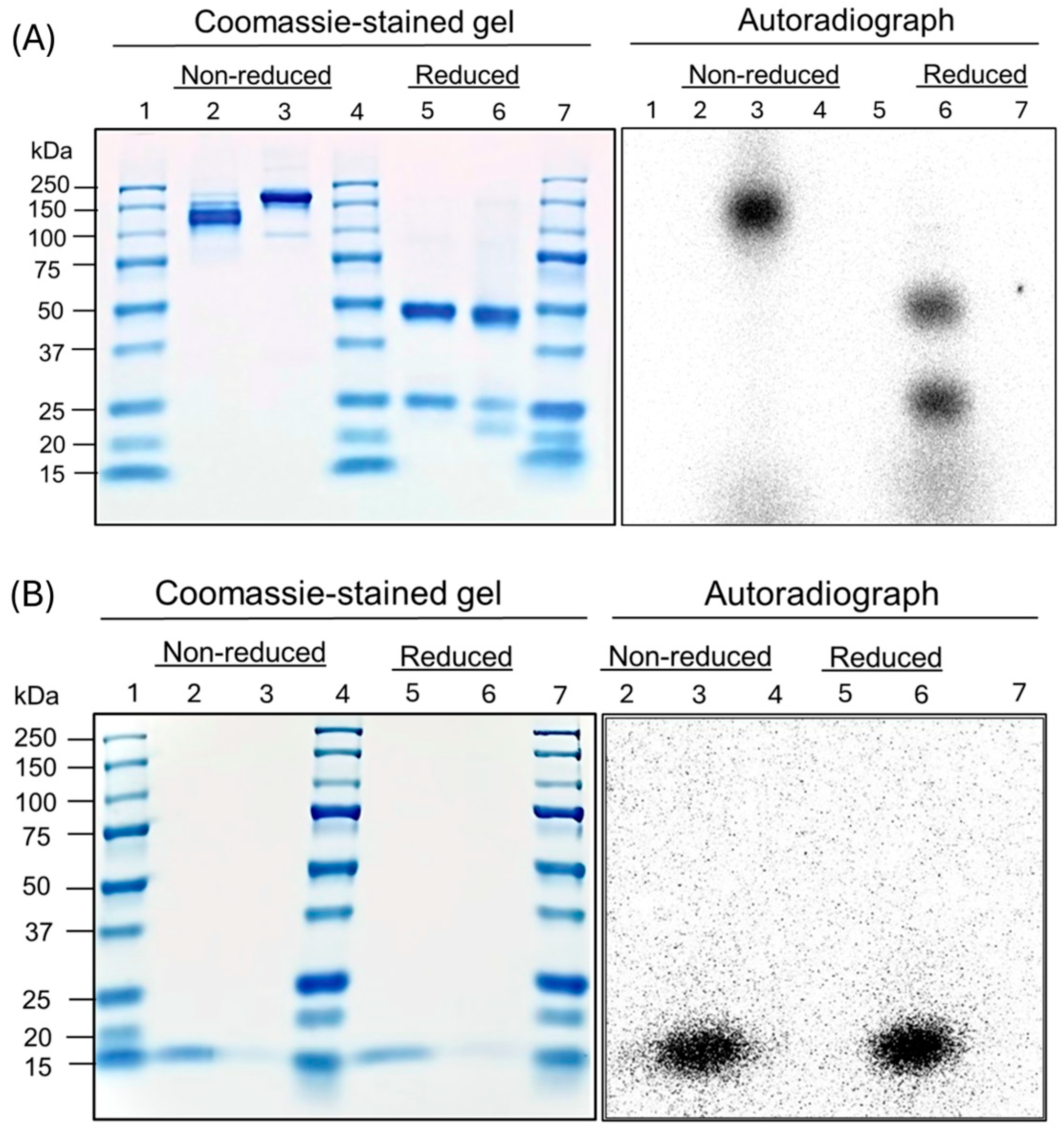
2.5. SDS-PAGE
2.6. Autoradiography
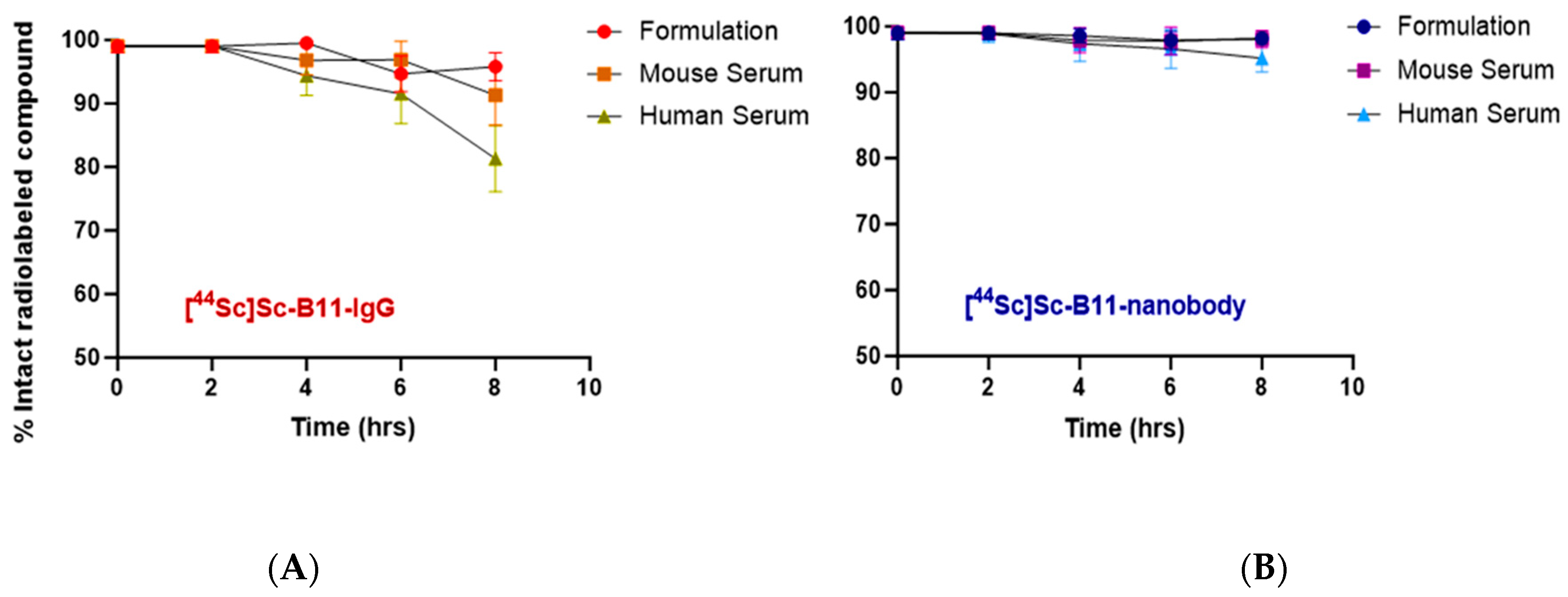
2.7. Serum Stability Analysis
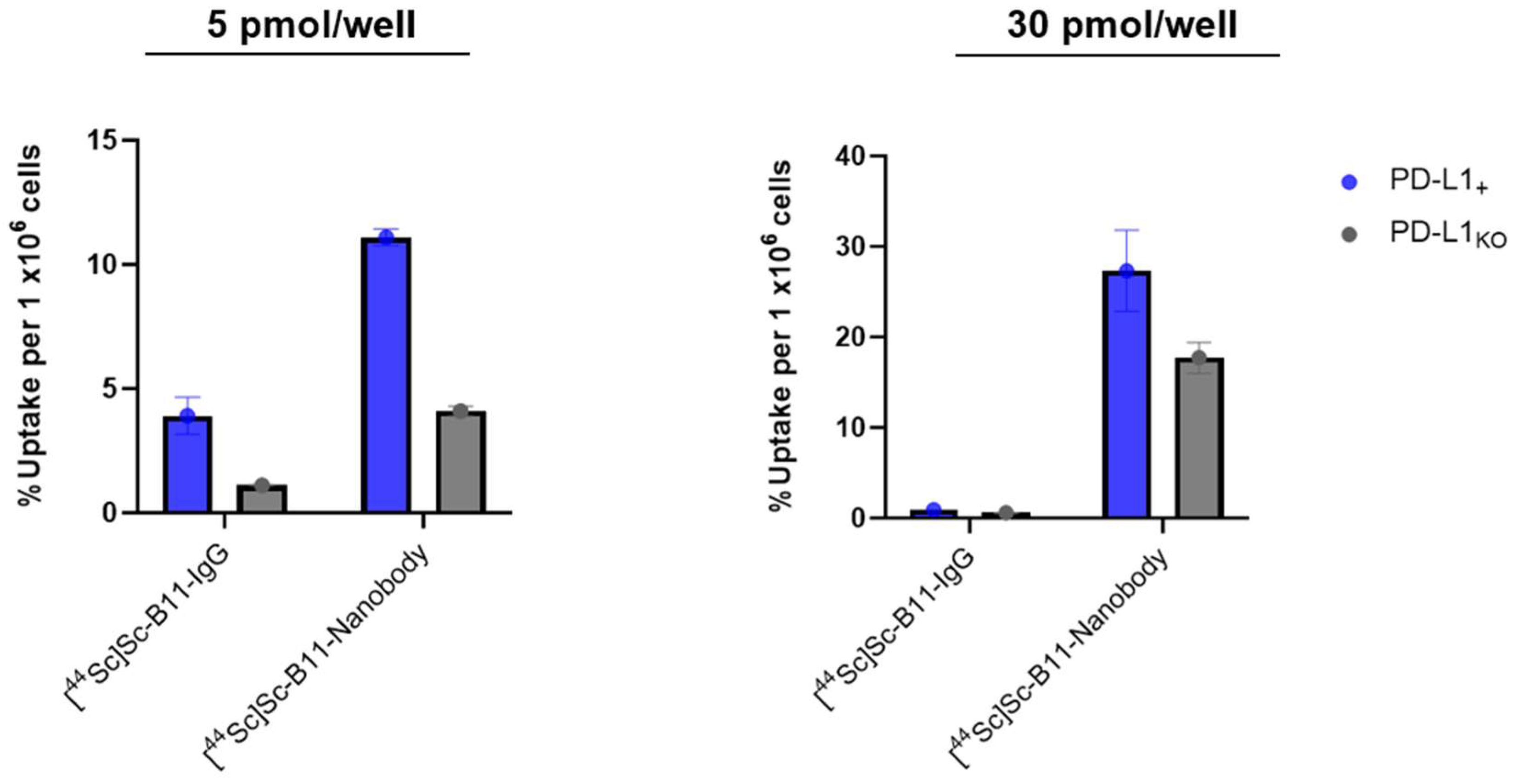
2.8. Cell Studies
3. Results
3.1. Scadium-44 Production
3.2. Antibody and Antibody Fragment Conjugation
3.3. Radiolabeling
3.4. SDS-PAGE and Autoradiography
3.5. Stability of Radiotracers in Serum
3.6. Cell Uptake
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PD-L1 | Programed Death Ligand 1 |
| PD-1 | Programed Death 1 |
| PET | Positron Emission Tomography |
| TRT | Targeted Radionuclide Therapy |
| SBR | Signal to Background Ratio |
| MP-AES | Microwave Plasma Atomic Emission Spectroscopy |
| AUC | Area Under the Curve |
| SDS-PAGE | Sodium Dodecyl Sulfate—PolyacrylAmide Gel Electrophoresis |
| HBSS | Hanks Buffered Salt Solution |
References
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727. [Google Scholar] [PubMed]
- Blank, C.; Gajewski, T.F.; Mackensen, A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: Implications for tumor immunotherapy. Cancer Immunol. Immunother. 2005, 54, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Diskin, B.; Adam, S.; Cassini, M.F.; Sanchez, G.; Liria, M.; Aykut, B.; Buttar, C.; Li, E.; Sundberg, B.; Salas, R.D. PD-L1 engagement on T cells promotes self-tolerance and suppression of neighboring macrophages and effector T cells in cancer. Nat. Immunol. 2020, 21, 442–454. [Google Scholar] [CrossRef]
- Beenen, A.C.; Sauerer, T.; Schaft, N.; Dörrie, J. Beyond cancer: Regulation and function of PD-L1 in health and immune-related diseases. Int. J. Mol. Sci. 2022, 23, 8599. [Google Scholar] [CrossRef]
- Sunshine, J.; Taube, J.M. Pd-1/pd-l1 inhibitors. Curr. Opin. Pharmacol. 2015, 23, 32–38. [Google Scholar] [CrossRef]
- Hamanishi, J.; Mandai, M.; Matsumura, N.; Abiko, K.; Baba, T.; Konishi, I. PD-1/PD-L1 blockade in cancer treatment: Perspectives and issues. Int. J. Clin. Oncol. 2016, 21, 462–473. [Google Scholar] [CrossRef]
- Seliger, B. Basis of PD1/PD-L1 therapies. J. Clin. Med. 2019, 8, 2168. [Google Scholar] [CrossRef]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.H.; Dong, H.; Kwon, E.D. Implications of B7-H1 expression in clear cell carcinoma of the kidney for prognostication and therapy. Clin. Cancer Res. 2007, 13, 709s–715s. [Google Scholar] [CrossRef]
- Gandini, S.; Massi, D.; Mandalà, M. PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies: A systematic review and meta-analysis. Crit. Rev. Oncol./Hematol. 2016, 100, 88–98. [Google Scholar] [CrossRef]
- Vranic, S.; Gatalica, Z. PD-L1 testing by immunohistochemistry in immuno-oncology. Biomol Biomed 2023, 23, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Niemeijer, A.; Leung, D.; Huisman, M.; Bahce, I.; Hoekstra, O.; Van Dongen, G.; Boellaard, R.; Du, S.; Hayes, W.; Smith, R. Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer. Nat. Commun. 2018, 9, 4664. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Lesniak, W.G.; Nimmagadda, S. Noninvasive imaging of immune checkpoint ligand PD-L1 in tumors and metastases for guiding immunotherapy. Mol. Imaging 2017, 16, 1536012117718459. [Google Scholar] [CrossRef]
- Ehlerding, E.B.; England, C.G.; McNeel, D.G.; Cai, W. Molecular Imaging of Immunotherapy Targets in Cancer. J. Nucl. Med. 2016, 57, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Rosenkrans, Z.T.; Liu, J.; Huang, G.; Luo, Q.-Y.; Cai, W. ImmunoPET: Concept, design, and applications. Chem. Rev. 2020, 120, 3787–3851. [Google Scholar] [CrossRef]
- Kikuchi, M.; Clump, D.A.; Srivastava, R.M.; Sun, L.; Zeng, D.; Diaz-Perez, J.A.; Anderson, C.J.; Edwards, W.B.; Ferris, R.L. Preclinical immunoPET/CT imaging using Zr-89-labeled anti-PD-L1 monoclonal antibody for assessing radiation-induced PD-L1 upregulation in head and neck cancer and melanoma. Oncoimmunology 2017, 6, e1329071. [Google Scholar] [CrossRef]
- Jalilian, A.R.; Osso, J.A. Production, applications and status of zirconium-89 immunoPET agents. J. Radioanal. Nucl. Chem. 2017, 314, 7–21. [Google Scholar] [CrossRef]
- Van De Watering, F.C.; Rijpkema, M.; Perk, L.; Brinkmann, U.; Oyen, W.J.; Boerman, O.C. Zirconium-89 labeled antibodies: A new tool for molecular imaging in cancer patients. BioMed Res. Int. 2014, 2014, 203601. [Google Scholar] [CrossRef]
- Rashidian, M.; Ploegh, H. Nanobodies as non-invasive imaging tools. Immuno-Oncol. Technol. 2020, 7, 2–14. [Google Scholar] [CrossRef]
- Fu, R.; Carroll, L.; Yahioglu, G.; Aboagye, E.O.; Miller, P.W. Antibody Fragment and Affibody ImmunoPET Imaging Agents: Radiolabelling Strategies and Applications. ChemMedChem 2018, 13, 2466–2478. [Google Scholar] [CrossRef]
- Bansal, A.; Pandey, M.K.; Barham, W.; Liu, X.; Harrington, S.M.; Lucien, F.; Dong, H.; Park, S.S.; DeGrado, T.R. Non-invasive immunoPET imaging of PD-L1 using anti-PD-L1-B11 in breast cancer and melanoma tumor model. Nucl. Med. Biol. 2021, 100, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Mohr, P.; van Sluis, J.; Lub-de Hooge, M.N.; Lammertsma, A.A.; Brouwers, A.H.; Tsoumpas, C. Advances and challenges in immunoPET methodology. Front. Nucl. Med. 2024, 4, 1360710. [Google Scholar] [CrossRef]
- Ferguson, S.; Jans, H.-S.; Wuest, M.; Riauka, T.; Wuest, F. Comparison of scandium-44 g with other PET radionuclides in pre-clinical PET phantom imaging. EJNMMI Phys. 2019, 6, 1–14. [Google Scholar] [CrossRef]
- Chernysheva, M.; Loveless, S.C.; Brossard, T.; Becker, K.; Cingoranelli, S.; Aluicio-Sarduy, E.; Song, J.; Ellison, P.; Nolen, J.; Rotsch, D.A.; et al. Accelerator Production of Scandium Radioisotopes: Sc-43, Sc-44, and Sc-47. Curr. Radiopharm. 2021, 14, 359–373. [Google Scholar] [CrossRef] [PubMed]
- van der Meulen, N.P.; Bunka, M.; Domnanich, K.A.; Müller, C.; Haller, S.; Vermeulen, C.; Türler, A.; Schibli, R. Cyclotron production of 44Sc: From bench to bedside. Nucl. Med. Biol. 2015, 42, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Umbricht, C.A.; Benešová, M.; Schmid, R.M.; Türler, A.; Schibli, R.; van der Meulen, N.P.; Müller, C. 44Sc-PSMA-617 for radiotheragnostics in tandem with 177Lu-PSMA-617—Preclinical investigations in comparison with 68Ga-PSMA-11 and 68Ga-PSMA-617. EJNMMI Res. 2017, 7, 9. [Google Scholar] [CrossRef]
- Müller, C.; Bunka, M.; Haller, S.; Köster, U.; Groehn, V.; Bernhardt, P.; van der Meulen, N.; Türler, A.; Schibli, R. Promising Prospects for 44Sc-/47Sc-Based Theragnostics: Application of 47Sc for Radionuclide Tumor Therapy in Mice. J. Nucl. Med. 2014, 55, 1658–1664. [Google Scholar] [CrossRef]
- Ziegler, J.F.; Ziegler, M.D.; Biersack, J.P. SRIM—The stopping and range of ions in matter. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2010, 268, 1818–1823. [Google Scholar] [CrossRef]
- Karlsson, S.; Sjöberg, V.; Ogar, A. Comparison of MP AES and ICP-MS for analysis of principal and selected trace elements in nitric acid digests of sunflower (Helianthus annuus). Talanta 2015, 135, 124–132. [Google Scholar] [CrossRef]
- Guerin, A.; Steeg, U.; Abdelnour, Y. Determination of Exchangeable Cations in Soil Extracts Using the Agilent 4100 Microwave Plasma-Atomic Emission Spectrometer. 2012. Available online: https://www.agilent.com/cs/library/applications/5991-0048EN_AppNote_4100MP-AES_Agriculture_soil.pdf (accessed on 11 June 2025).
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Schägger, H.; Von Jagow, G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 1987, 166, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Taubel, J.C.; Nelson, N.R.; Bansal, A.; Curran, G.L.; Wang, L.; Wang, Z.; Berg, H.M.; Vernon, C.J.; Min, H.-K.; Larson, N.B. Design, synthesis, and preliminary evaluation of [68Ga] Ga-NOTA-insulin as a PET probe in an Alzheimer’s disease mouse model. Bioconjugate Chem. 2022, 33, 892–906. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Lavoie, R.R.; Lucien, F.; Kethamreddy, M.; Wootla, B.; Dong, H.; Park, S.S.; Pandey, M.K. Synthesis and evaluation of anti-PD-L1-B11 antibody fragments for PET imaging of PD-L1 in breast cancer and melanoma tumor models. Sci. Rep. 2024, 14, 19561. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.V.; Aluicio-Sarduy, E.; Bradshaw, T.; Hurley, S.A.; Olson, A.P.; Barrett, K.E.; Batterton, J.; Ellison, P.A.; Barnhart, T.E.; Pirasteh, A.; et al. Cyclotron production of 43Sc and 44gSc from enriched 42CaO, 43CaO, and 44CaO targets. Front. Chem. 2023, 11, 1167783. [Google Scholar] [CrossRef]
- Krajewski, S.; Cydzik, I.; Abbas, K.; Bulgheroni, A.; Simonelli, F.; Holzwarth, U.; Bilewicz, A. Cyclotron production of 44Sc for clinical application. Radiochim. Acta 2013, 101, 333–338. [Google Scholar] [CrossRef]
| Element Wavelength (λ) | Nebulizer Flow Rate (L/min) | Viewing Position | Pump Speed | Stabilization Time |
|---|---|---|---|---|
| Ca (317.933 nm) | 0.60 | 0 | 25 rpm | 15 s |
| Al (396.152 nm) | 0.95 | 0 | 25 rpm | 15 s |
| Fe (371.993 nm) | 0.65 | 0 | 25 rpm | 15 s |
| Current (µA) | Irradiation Time (min) | AEOB (MBq) | YEOB (MBq/µAh) |
|---|---|---|---|
| 15 | 15 | 49.95 ± 1.48 (n = 2) | 9.99 ± 0.30 |
| 20 | 30 | 138.75 ± 1.49 (n = 3) | 13.87 ± 0.15 |
| 20 | 60 | 246.42 ± 5.92 (n = 3) | 12.32 ± 0.30 |
| 30 | 60 | 354.09 ± 8.51(n = 5) | 11.80 ± 0.28 |
| 40 | 30 | 257.15 ± 4.44 (n = 3) | 12.86 ± 0.22 |
| 40 | 60 | 483.22 ± 7.41 (n = 31) | 12.08 ± 0.19 |
| 40 | 120 | 943.13 ± 5.98 (n = 3) | 11.79 ± 0.07 |
| Fraction | Ca (ppm) | Fe (ppm) | Al (ppm) | Volume (µL) | Activity (GBq) | Apparent As (GBq/µg) |
|---|---|---|---|---|---|---|
| 1 | 14.7 ± 5.6 | 2.2 ± 1.0 | 3.4 ± 1.6 | 500 | 109.8 ± 15.5 | 10.8 ± 7.7 |
| 2 | 8.7 ± 1.7 | 1.0 ± 0.3 | 1.4 ± 0.8 | 500 | 31.3 ± 4.9 | 5.6 ± 3.7 |
| 3 | 6.0 ± 0.01 | 0.6 ± 0.01 | <0.01 | 500 | 10.1 ± 4.5 | 3.1 ± 2.2 |
| Stability | 0 h | 2 h | 4 h | 6 h | 8 h |
|---|---|---|---|---|---|
| [44Sc]Sc-B11-IgG (% intact) | |||||
| Formulation | >99 | >99 | 99.5 ± 0.9 | 94.7 ± 2.8 | 95.8 ± 2.2 |
| Mouse Serum | >99 | >99 | 96.8 ± 2.3 | 96.8 ± 2.9 | 91.3 ± 4.7 |
| Human Serum | >99 | >99 | 94.4 ± 3.1 | 91.5 ± 4.7 | 81.3 ± 8.3 |
| [44Sc]Sc-B11-Nanobody (% intact) | |||||
| Formulation | >99 | >99 | 98.6 ± 1.1 | 97.9 ± 1.4 | 98.2 ± 0.9 |
| Mouse Serum | >99 | >99 | 97.9 ± 1.9 | 97.8 ± 2.1 | 98.1 ± 1.3 |
| Human Serum | >99 | 98.9 ± 1.3 | 97.4 ± 2.7 | 96.6 ± 2.9 | 95.2 ± 2.1 |
| Compound | Apparent Molar Activity (Am) (GBq/µmol) | % Uptake (PD-L1KO) | % Uptake (PD-L1+) | p-Value | Uptake Ratio (+/KO) |
|---|---|---|---|---|---|
| 5 pmol/ well | |||||
| [44Sc]Sc-B11-IgG | 4.6 | 1.11 ± 0.02 | 3.91 ± 0.75 | 4 × 10−4 | ~3.5 |
| [44Sc]Sc-B11-nanobody | 2.4 | 4.10 ± 0.19 | 11.1 ± 0.33 | 0.03 | 2.7 |
| 30 pmol/ well | |||||
| [44Sc]Sc-B11-IgG | 2.3 | 0.58 ± 0.05 | 0.91 ± 0.01 | 0.003 | ~1.6 |
| [44Sc]Sc-B11-nanobody | 6.9 | 17.70 ± 1.71 | 27.33 ± 4.49 | 6 × 10−6 | ~1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krol, V.E.; Bansal, A.; Kethamreddy, M.; Ellinghuysen, J.R.; Vail, D.J.; Lucien-Matteoni, F.; Dong, H.; Park, S.S.; Pandey, M.K. Synthesis and In Vitro Evaluation of a Scandium-44 Radiolabeled Nanobody as a PD-L1 PET Imaging Probe. Pharmaceutics 2025, 17, 796. https://doi.org/10.3390/pharmaceutics17060796
Krol VE, Bansal A, Kethamreddy M, Ellinghuysen JR, Vail DJ, Lucien-Matteoni F, Dong H, Park SS, Pandey MK. Synthesis and In Vitro Evaluation of a Scandium-44 Radiolabeled Nanobody as a PD-L1 PET Imaging Probe. Pharmaceutics. 2025; 17(6):796. https://doi.org/10.3390/pharmaceutics17060796
Chicago/Turabian StyleKrol, Viktoria E., Aditya Bansal, Manasa Kethamreddy, Jason R. Ellinghuysen, Daniel J. Vail, Fabrice Lucien-Matteoni, Haidong Dong, Sean S. Park, and Mukesh K. Pandey. 2025. "Synthesis and In Vitro Evaluation of a Scandium-44 Radiolabeled Nanobody as a PD-L1 PET Imaging Probe" Pharmaceutics 17, no. 6: 796. https://doi.org/10.3390/pharmaceutics17060796
APA StyleKrol, V. E., Bansal, A., Kethamreddy, M., Ellinghuysen, J. R., Vail, D. J., Lucien-Matteoni, F., Dong, H., Park, S. S., & Pandey, M. K. (2025). Synthesis and In Vitro Evaluation of a Scandium-44 Radiolabeled Nanobody as a PD-L1 PET Imaging Probe. Pharmaceutics, 17(6), 796. https://doi.org/10.3390/pharmaceutics17060796








