Abstract
This review examines the pharmaceutical applications of essential oils (EOs) and terpenes, highlighting their dual role as therapeutic agents and natural penetration enhancers. These volatile, hydrophobic compounds have well-documented antimicrobial, antioxidant, and anti-inflammatory properties. However, their clinical potential is limited by poor water solubility, high volatility, and sensitivity to environmental factors, including light, heat, and oxygen. To address these challenges, various advanced delivery systems have been developed to enhance stability, bioavailability, and controlled release. These systems not only protect chemical integrity but also exploit these compounds’ abilities to interact with lipid membranes, facilitating the transport of active compounds across biological barriers. Additionally, their inherent antimicrobial properties can contribute to the overall stability of formulations. The review critically examines the incorporation of terpenes and major essential oil (EO) components, such as limonene, linalool, eugenol, α-pinene, and menthol, into delivery systems, assessing their performance in enhancing drug permeability and targeting specific tissues. Current challenges and future directions in terpenes and EO-based delivery strategies are discussed, highlighting their promising role in developing multifunctional and efficient pharmaceutical formulations.
1. Introduction
Plant-derived compounds have long been recognized as valuable sources of therapeutic agents, encompassing diverse classes such as terpenoids, glycosides, flavonoids, and polyphenols collectively known as phytoalexins [1]. Among these, essential oils (EOs) represent complex mixtures of volatile aliphatic and cyclic hydrocarbons, predominantly composed of monoterpenoids. These compounds have found wide application in the food, fragrance, and pharmaceutical industries due to their distinctive aromatic profiles and notable antimicrobial activities [2]. Recently, the growing concern over antimicrobial resistance, driven in part by the overuse of conventional antibiotics and antifungal agents, has renewed interest in the antimicrobial potential of terpenes and essential oils (EOs), despite their relatively modest potency compared to synthetic drugs [2]. Beyond their antimicrobial action, EOs also exhibit significant antioxidant properties, effectively scavenging reactive oxygen and nitrogen species (ROS and RNS) that contribute to oxidative stress and the progression of chronic diseases such as cancer, cardiovascular disorders, and arthritis [2]. These broad-spectrum biological activities have positioned essential oils (EOs) as promising candidates for use in both traditional herbal medicine and modern pharmaceutical formulations.
Driven by increasing consumer preference for natural, plant-based therapies over synthetic pharmaceuticals, terpenes, and essential oils (EOs), which are often perceived as safer and more sustainable, are gaining traction as functional and therapeutic ingredients. When used alone or in synergy with other bioactive molecules, terpenes, and essential oils (EOs) demonstrate a range of pharmacological effects, including antimicrobial, anti-inflammatory, and antioxidant actions. Moreover, their ability to modulate the chemical and biological behavior of co-administered compounds underscores the importance of understanding their interactions in complex formulations. However, several physicochemical limitations hinder the clinical translation and broader pharmaceutical application of EOs. These include low water solubility, high volatility, and sensitivity to light, heat, and oxygen [3], as well as poor skin permeation, which is often restricted to superficial layers [4]. To overcome these challenges, recent research has focused on the development of advanced delivery systems (DSs), particularly nano- and lipid-based carriers. Encapsulation of terpenes and essential oils (EOs) in such systems not only enhances their aqueous solubility and chemical stability but also protects them from environmental degradation, reduces toxicity, and improves controlled release and bioavailability [4,5]. Additionally, the use of vegetable-based oils in formulations can further optimize physical properties such as viscosity and heat resistance [4].
The aim of this review is to highlight the potential of essential oils and terpenes in addressing current pharmaceutical challenges by exploring their incorporation into advanced delivery systems. Emphasis is placed on how these systems can enhance the stability, bioavailability, and therapeutic efficacy of essential oils. The review also discusses the emerging role of delivery systems in targeted drug delivery for various disorders, while acknowledging existing barriers to clinical translation, including concerns about toxicity, regulatory limitations, and a lack of comprehensive clinical data.
2. Essential Oils as Pharmaceuticals
Classification of EOs is divided into monoterpenes and phenylpropanoids, and each of these two classes includes esters, aldehydes, ketones, alcohols, phenols, and oxides, while hydrocarbon compounds contain terpenes. Due to their diverse and complex compositions, EOs lack a specific single cellular target, with each complex blend exerting multiple cellular effects through their main components [6]. Moreover, EOs are susceptible to degradation when exposed to high temperatures, light, and oxygen [3]. The degradation of EOs depends on their chemical composition, with more hydrogenated compounds like mono- and sesquiterpenes being particularly susceptible to oxidation.
Another challenge related to EO application is limited penetration due to their physicochemical properties. The chemical structure of the constituents in EOs plays a significant role in the process of compound penetration through the skin. EOs primarily penetrate the outermost layers of the skin, enhancing epidermal water balance through a “filmogenic” process [4]. Mechanisms involve altering the fluidity of the stratum corneum (SC) membrane through the actions of EOs, which break hydrogen bonds and induce modifications in the state, conformation, and structure of both SC lipids and keratin [4]. Recent studies indicate that EOs can act as prooxidants within eukaryotic cells, affecting organelles such as mitochondria and inner cell membranes. These oils induce antigenotoxic effects and change intracellular redox potential and mitochondrial dysfunction [7] (Figure 1). Understanding these biological and physicochemical properties is essential, as they directly influence the design, efficacy, and safety of essential oils when incorporated into drug delivery systems.
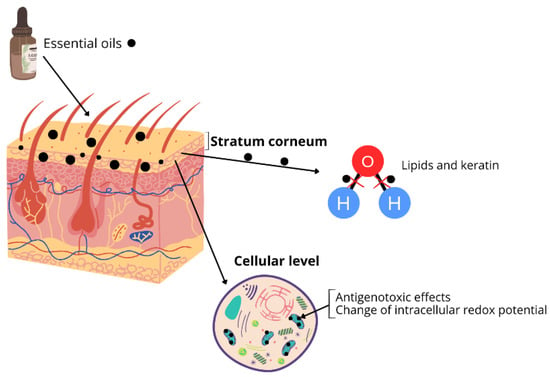
Figure 1.
Essential oils penetration through the skin.
Hydrophilic compounds tend to show improved transdermal absorption with terpenes containing polar functional groups, while hydrocarbon terpenes are more effective in enhancing the absorption of lipophilic compounds, potentially due to differences in thermodynamic activity within gels [8,9]. In subsequent sections, the most common terpenes and their usage cases will be discussed in detail.
2.1. Linalool Usage as a Pharmaceutical
Linalool, an acyclic monoterpene tertiary alcohol, is widely distributed across various plant species, including the Lamiaceae, Lauraceae, and Apiaceae families [10]. Linalool has a small molecular weight and a hydroxyl group, which affect polarity. However, the hydrocarbon-based apolar structure of linalool leads to low solubility in water and high solubility in various organic solvents such as alcohol, chloroform, ether, and propylene glycol [10].
The encapsulation of linalool enhances its stability and augments its antioxidant activity, which is crucial for more effective treatment of dermatological conditions [11]. Additionally, recent studies suggest enhancing linalool’s bioavailability and addressing its volatility and poor water solubility while complexing with β-cyclodextrins. The supramolecular structure of β-cyclodextrins features a hydrophilic outer surface and a hydrophobic inner cavity. This configuration allows cyclodextrins to encapsulate lipophilic compounds like linalool, improving water solubility [10]. Incorporating linalool within β-cyclodextrin enhances its bioavailability and antihypertensive efficacy, suggesting a potential novel pharmaceutical formulation for hypertension treatment in upcoming applications [12].
For example, incorporating linalool into DSs for cancer therapy could elevate its anticancer effects while allowing for further modification to target cancer cells and selectively minimize toxicity to normal cells. Nanotechnology-based approaches in drug delivery research offer innovative design features that can enhance the in vivo efficacy of linalool, presenting promising opportunities for its utilization not only in pharmaceuticals as active pharmaceutical ingredients or adjuvants but also in cosmetics [10].
2.2. α-Pinene Usage as a Pharmaceutical
α-Pinene, a bicyclic monoterpene, is commonly found in coniferous trees and is a major turpentine component. Due to its minty odor and pine scent, it has been extensively used as a fragrance and flavor ingredient in various essential oils, fruits, vegetables, and herbs [13]. α-Pinene use is limited due to its volatility, low aqueous solubility, and chemical instability. To address these challenges, α-pinene has been encapsulated in conventional liposomes and drug-in-cyclodextrin-in-liposomes systems. The complex of α-pinene combined with hydroxypropyl-β-cyclodextrin improves its solubilization, with optimal conditions achieved at a specific molar ratio [14].
Another study aimed to develop and optimize a self-nano-emulsifying DS of α-pinene and evaluate its in vivo anti-Parkinson’s activity. Various excipient components were screened for miscibility with α-pinene to determine the optimized combination, followed by assessments of self-nano emulsification, thermodynamic stability, dilution robustness, optical clarity, viscosity, and conductivity. Two optimized formulations, composed of Anise oil, Tween 80, and Transcutol-HP oil, were selected, showing improved in vitro drug release profiles compared to free α-pinene suspension. Additionally, the system demonstrated enhanced behavioral activities in rodent models of Parkinson’s disease in vivo [15].
2.3. Cineole Usage as Pharmaceutical
1,8-cineole, a natural monoterpene, can serve as a chemical penetrant for transdermal drug delivery, transporting both lipophilic and hydrophilic drugs. For less lipophilic drugs, 1,8-cineole demonstrates notable osmotic-enhancing properties, suggesting its potential to enhance drug permeation. Due to its permeability and low toxicity, 1,8-cineole holds promise for further development in transdermal DSs and could potentially serve as a candidate for brain transport applications [16].
An optimized self-microemulsifying DS containing 1,8-cineole effectively alleviated lipopolysaccharide (LPS)-induced endothelial injury in Kunming mice. The novel 1,8-cineole delivery system demonstrated significant inhibition of inflammatory cytokines and the attenuation of LPS-induced vascular endothelial injury, potentially mediated through modulation of the NF-κB and peroxisome proliferator-activated receptor γ signaling pathways, suggesting its potential as a therapeutic agent for inflammatory cardiovascular diseases [17]. Nanoemulgels loaded with 1,8-cineole showed favorable characteristics, including pH stability, homogeneity, and acceptable droplet size. In both in vitro (a Franz diffusion cell) and in vivo (rabbit) evaluations, nano emulgels demonstrated significant wound contraction compared to standard and control groups, suggesting their potential as an effective wound-healing alternative [18] (Figure 2).
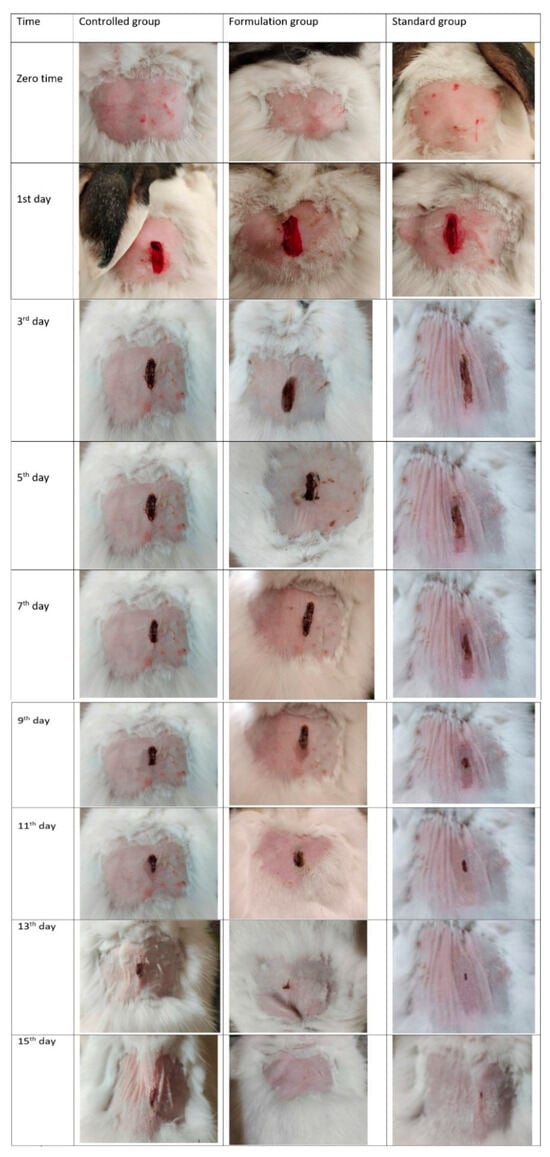
Figure 2.
Contraction of wounds in control group, group treated with F5, and group treated with commercial product. F5 is the nanoemulsion containing eucalyptol:tween80:span-60:propulene glycol:black seed oil mixed as 8:30:7.5:13:15 [18].
2.4. General Trends in Essential Oil Usage as Pharmaceuticals
The low solubility in water [4] and the exhibition of high oil/water partition coefficients leads to limited production of EOs, restricting their incorporation into hydrophilic products. Their lipid-soluble components enable them to traverse the blood–brain barrier and interact with fluids surrounding the brain [4]. The intense odor impacts sensory properties and might diminish acceptance of the final product [3]. To address stability and stemming issues, the incorporation of EOs into nano-formulations such as nano-emulsions or nano-capsules presents a promising approach, enhancing the physicochemical stability, prolonging their persistence, optimizing the release of active components at the target site, and potentially augmenting their in situ bioactivity [19]. These challenges can be solved by integrating EOs into nanometer-scale surfactant structures, offering a potential solution to enhance their stability and applicability in various formulations [3].
3. Design of Novel Essential Oil Delivery Systems
The stability issues of EOs are associated with volatility, oxidation, photosensitivity, heat, humidity, pH, and ion sensitivity. Well-developed DSs should effectively stabilize EOs. Polysaccharides are often chosen as carrier materials due to their safety, versatility, and abundant sources, aligning with the biomimetic concept by mimicking the structure of plant tissue [20]. Nanocarriers can target specific organs and disorders; however, commercialization of them has not been finalized, i.e., toxicity safety has not been granted in clinical studies [21].
Thus, chemical permeation enhancers (CPEs) hold considerable promise for transdermal DSs [16]. The enhanced activity of CPEs is associated with side effects, limiting their progress in transdermal compound delivery. However, advancements in analytical techniques, synthetic chemistry, and combinatorial studies have led to potentially effective CPEs being used in transdermal compound delivery [22]. Biomimetic carrier technology creates emulsions by combining oil, water, and amphiphilic molecules, mimicking natural secretion tissues’ protective mechanisms. This process forms emulsion droplets dispersed within the medium, with EOs encapsulated in the droplets and shielded by an interfacial film, offering protection against volatility and environmental factors [20].
Main Systems for Essential Oil Delivery
Natural compounds often have a low absorption capacity due to their inability to cross lipid membranes, primarily because of their large molecular size, which leads to reduced bioavailability and efficacy. Additionally, their high systemic clearance demands frequent applications or higher doses, diminishing their therapeutic effectiveness [23]. Encapsulation plays a vital role in protecting and delivering unstable or sensitive compounds, with extensive applications in the pharmaceutical industry. With growing interest, innovative encapsulation strategies continue to advance these applications, enriching both functionality and palatability [24]. Various delivery systems, including lipid-based nanoparticles (NPs), polymeric NPs, dendrimers, inorganic NPs, capsules, hydrogels, oil in water emulsions, and liposomes, can be used to deliver EOs (Table 1) [25,26]. Among these, polymeric NPs and liposomes have been most widely tested due to their biocompatibility, biodegradability, and ease of functionalization [25]. Release systems enhance compound solubility, stability, targeted delivery, bioavailability, and action duration, while potentially delaying compound resistance when combined with natural compounds, offering promising avenues for treating diseases with low response to conventional therapies [23].
Despite the abovementioned delivery systems’ advantages, challenges such as rapid elimination by the reticuloendothelial system, potential toxicity, inflammation, and tissue damage persist. Furthermore, these systems must meet stringent regulatory requirements, including characterization, compound release control, stability, scalability, and cost-effectiveness [25]. For example, liposomal DSs have low compound loading, poor stability, high production costs, and potential toxic side effects; hydrogel DSs are highly dependent on the internal microenvironment; and polymer micelle has long-term safety concerns and limitations in clinical application, which pose significant challenges [27].

Table 1.
Types of EO delivery systems: advantages and limitations.
Table 1.
Types of EO delivery systems: advantages and limitations.
| Type of DS | Advantages | Limitations | Examples |
|---|---|---|---|
| Emulsions | Emulsions can be water-in-oil (W/O) or oil-in-water (O/W), based on phase location. Micro and nanoemulsions of EOs show improved stability, controlled release, and enhanced antioxidant and antimicrobial properties [26,28]. The absence of surfactants in these systems minimizes environmental impact, making them ideal for utilizing the bioactivity of EOs [29]. | The main challenge lies in the poor biopolymer properties and potential flavor impacts from high concentrations of EO [30]. Nanoemulsions with low oil content face challenges in preparation and require fillers, binders, or emulsifiers to enhance viscosity [31]. | O/W cinnamon and black pepper emulsions [32], nanoemulsion of eucalyptus oil, Tween 80, and water [33], nanoemulsions of Cymbopogon flexuosus [34]. |
| Capsules | Capsules exhibit improved antioxidant and antimicrobial activities, enhanced stability, and controlled release of entrapped EOs [26]. This DS increases bioavailability by adhering to mucous membranes, protects EOs from hydrolysis and oxidation, reduces toxicity and volatility, and enables the targeted delivery of therapeutic doses, improving patient compliance [5]. | The main limitations of capsules are volatility and degradation, low encapsulation efficiency, poor release control, and environmental sensitivity. | EO delivery systems based on capsules consisting of zeolites to improve chemical and physical EOs properties [35]. |
| Liposomes | Liposomes offer low toxicity, biocompatibility, non-immunogenicity, enhanced antimicrobial and antioxidant activities, and targeted delivery [26,27]. | The use of liposomes is limited by high costs, low compound loading, and poor stability [26,27]. | Liposomes loaded with Barije (Ferula gummosa) EO were evaluated for their physical properties and antibacterial effects against Escherichia coli O157:H7 [36]. |
| Hydrogels | Encapsulating EOs in hydrogels enhances their stability and biological activity [37]. | It heavily depends on the internal microenvironment [27]. In addition, there is limited data on stability, safety, long-term bioactivity, and in vivo studies [37]. | Semi-solid poly(vinyl alcohol) hydrogels with ginger EO encapsulated in chitosan NPs show promise for wound management applications [38]. Alginate–soy protein isolate complex beads encapsulating thyme EO are designed for targeted intestinal delivery [39]. |
| Solid lipid NPs (SNLs) | SNLs offer better protection, controlled release, and cost-effective formation compared to liposomes and emulsions [26,40]. | SNLs exhibit poor stability, aggregation, low encapsulation load, and potential instability under acidic conditions and during storage [26,40,41]. | Zataria multiflora EO-loaded solid lipid NPs demonstrated antifungal activity under in vitro conditions [42]. Chitosan/polyvinyl alcohol hydrogels loaded with EOs of Origanum vulgare and Thymus vulgaris in solid lipid NPs effectively controlled the growth of Botrytis cinerea and Penicillium expansum [43]. |
| Inorganic NPs | EOs and NPs affect gut processes like inflammation, oxidative stress, metabolite synthesis, and microbiota balance [44]. Inorganic NPs have good bioavailability and low toxic side effects tolerance [27] | Potential toxicity depending on dose and exposure, requiring consideration of synergistic and antagonistic interactions [44]. | Silver NPs and lavender oil for a synergistic antibacterial effect [45]. |
| Nanostructured lipid carriers (NLCs) | NLCs were developed to overcome SNLs’ limitations, offering smaller size, higher loading capacity, and preventing crystal formation and expulsion [26,41]. | SLNs and NLCs require high temperatures for lipid melting, risking degradation of heat-sensitive EO components, and have low stability under acidic conditions and high production costs at an industrial scale [26,46]. | NLCs containing 10% w/v lipid and 10% w/v EOs (lavender, rosemary, peppermint) show potential for sustainable insect pest control [47]. Red sacaca EO-loaded nanostructured lipid carriers, optimized by factorial design, were evaluated for cytotoxicity and cellular reactive oxygen species levels [48]. |
4. Essential Oils as Penetration Enhancers
To investigate the penetration-enhancing effect of EOs, the efficacy of the whole oil with individual compounds isolated from it should be compared. In vitro study of permeation facilitation of donepezil hydrochloride using oil extracted from Ledum palustre L. var. angustum N. Busch identified cuminaldehyde (5.72% of the oil composition), which enhances donepezil hydrochloride penetration, demonstrating twice the efficacy of the oil alone. However, other terpenes present in the oil, such as sabinene (33.40%), 4-terpineol (20.33%), and p-cymene (18.31%), exhibited minimal to no efficacy when tested individually [49].
Citrus peels possess availability and nutrient-rich composition, including essential micro and macronutrients such as water, proteins, sugars, minerals, and EOs, leading to the consideration of being valuable matrices [50]. For example, the adsorption capacity of pomelo peel, a natural and versatile bio-absorbent, is able to adsorb epigallocatechin-3-gallate. This offers pomelo peel as a cost-effective and efficient option as a carrier for delivering natural products in functional food and dietary supplement contexts [51]. Eugenol and its derivatives (isoeugenol, methyleugenol, acetyleugenol, and eugenol oxide) from the Cinnamomum tamala leaf interact with lipid bilayers composed of palmitoyl-oleoyl-sn-glycero-3-phosphatidylcholine and palmitoyl-linoleoyl-sn-glycero-3-phosphatidylcholine, showing their antioxidant activity [52]. The antibacterial activity of EOs is often related to their hydrophobic nature, which can disrupt membrane integrity and function. Studies suggest that EOs affect bacterial cells’ external envelopes and cytoplasm through a series of events. While both Gram-positive and Gram-negative bacteria possess peptidoglycans in their cell walls, EOs have demonstrated potent effects against both types of bacteria, offering a promising alternative to traditional antibiotics [53]. Mini-emulsion polymerization offers advantages for producing nano-encapsulated systems, allowing for the development of stable, uniformly-sized droplets that retain their shape throughout the polymerization process [54]. Andriotis et al. used D-limonene in their research, a key component of citrus EOs with enhanced antimicrobial activity, as a model volatile compound to synthesize terpene-loaded NPs via mini-emulsion polymerization to optimize NP properties for potential applications in elevated temperature processes [54] (Figure 3).
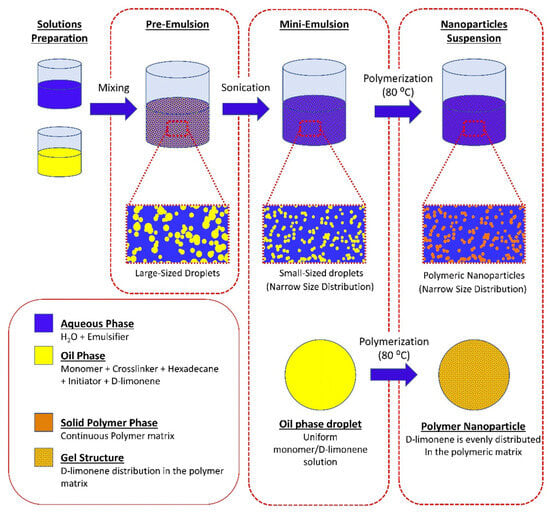
Figure 3.
Schematic representation of the mini-emulsion polymerization process and the possible structure of the synthesized nanoparticles [54].
EOs possess the synergistic effects of their compounds, leading to greater activity when applied as natural EOs compared to the sum of the effects of individual substances, while their qualitative and quantitative composition plays a crucial role in determining their antimicrobial potential [55]. The hydrophobic characteristics of EOs affect their direct integration into aqueous-based matrices and environments, potentially compromising their antimicrobial efficacy and applications. Higher concentrations may be necessary to attain desired functionalities in water-based matrices, potentially leading to changes in organoleptic properties and acceptability [26]. For example, the ideal carrier for oral DSs must be non-toxic and offer both chemical and physical stability for therapeutic proteins. Nano-sized colloidal systems are attractive candidates because they can encapsulate biomolecules, protect them from gastrointestinal degradation, and efficiently navigate through membranes related to hydrophobic/hydrophilic properties [56]. An oral matrix carrier is a self-ordered structure made of hydrophobically modified silica NPs, polysaccharides, biopolymers, and natural oils. Silica NPs attract amphiphilic protein molecules, which bind to them, while polysaccharides in the surrounding oil environment attach to the proteins. Natural oils (olive oil, sea buckthorn oil, or coconut oil) then encapsulate the active compound component, forming a protective complex that shields the protein from digestion and hydrolysis in the gastrointestinal tract [56].
Natural terpenes can be used as penetration enhancers for transdermal compound delivery. Terpenes, abundant in nature, offer promising advantages over synthetic enhancers, demonstrating higher enhancement activity and lower toxicity, making them preferable candidates for enhancing percutaneous compound absorption [57]. In subsequent sections, the most common terpenes and their delivery strategies will be discussed in detail.
4.1. Limonene Usage as a Penetration Enhancer
Orange, grapefruit, and lemon oils contain the main volatile component of citrus peel oil, commonly known as D-limonene [9]. Limonene oil belongs to the terpene family and has various biological properties, including anti-inflammatory, anti-cancer, antioxidant, and gastroprotective effects. It has potential as an adjuvant to modern therapeutic drugs in mitigating the progression of neurological diseases, i.e., limitation of nerve injury spread and reduction of hyperalgesia [58]. Limonene is widely used in DSs, providing drug release into the skin or transdermal release [11].
D-limonene nanoemulsion has been used as a carrier for curcumin insertion and transdermal absorption. Results indicate that the droplet size of the curcumin nanoemulsion influenced the cumulative concentration of its transdermal absorption, with larger droplet sizes showing higher steady-state flux. Additionally, this system showed significant potential as a carrier for transdermal DSs in food and cosmetic products [59]. The synthesis of polymeric NPs loaded with D-limonene demonstrated enhanced antimicrobial potency, suggesting their potential as promising materials for sterilization [54]. A self-microemulsifying DS was used to enhance the oral bioavailability of limonene, which was optimized for stability and release profile. Results demonstrated a significant improvement in oral bioavailability and the tissue distribution of limonene in the system compared to free limonene [60].
Transfersomes containing raloxifene hydrochloride have been optimized by limonene for transdermal delivery, varying phospholipid, sodium cholate, and limonene concentrations. An in vivo study showed that the optimized formulation improved the permeation of raloxifene into deeper layers of Wistar rat skin and significantly enhanced bioavailability compared to an oral raloxifene suspension [61]. A thermo-responsive intranasal limonene-based microemulsion mucoadhesive nano gel aimed to improve the efficacy of propranolol for managing migraine attacks. The nano gel exhibited good mucoadhesive properties, controlled release, and enhanced nasal permeability, leading to significantly improved propranolol brain availability compared to the control group, indicating its potential as a promising strategy for migraine management [58].
4.2. Eugenol Usage as a Penetration Enhancer
Eugenol, a phenol, is a key constituent of clove oil with anti-inflammatory effects, particularly in topical formulations [62]. However, the skin irritation associated with these formulations limits eugenol’s clinical use. To address this challenge, eugenol-loaded calcium citrate NPs (Eu-CaCit NPs) demonstrated biocompatibility and the ability to penetrate the dermis layer of intact human skin without cytotoxic effects. These findings suggest that Eu-CaCit NPs could be an effective carrier for the topical delivery of eugenol, offering a potential alternative for natural pain relief in topical applications [63]. Another example is that an O/W nanoemulsion formulation of eugenol significantly enhanced anti-inflammatory activity compared to a marketed piroxicam gel in a carrageenan-induced paw edema rat model. A higher concentration of eugenol did not correspond to higher anti-inflammatory effects. Additionally, a nanoemulsion containing piroxicam had diminished anti-inflammatory properties compared to formulations without piroxicam [62].
Eugenol microemulsions have been optimized for transdermal delivery of indomethacin, a model drug, by constructing pseudo-ternary phase diagrams using Tween 40 as a surfactant with or without ethanol or propylene glycol as co-surfactants. Results indicated that the presence of ethanol or propylene glycol increased the microemulsion zone. Higher eugenol concentrations and the co-surfactant presence enhanced indomethacin loading, release rate, and transdermal flux, with propylene glycol-containing systems showing superior performance [64]. Another DS was developed for resistant metastatic melanoma. Hyaluronic acid-coated liposomes loaded with a combination of anti-melanoma agents, including dacarbazine and eugenol, were developed using a solvent injection method guided by quality-by-design principles. This system showed higher cytotoxicity, increased late apoptotic cell numbers, and exhibited greater cell migration and proliferation inhibition compared to a dacarbazine solution. It is a promising strategy against aggressive melanoma that has reduced cytotoxicity and lower doses of chemotherapeutic agents [65].
4.3. Borneol Usage as a Penetration Enhancer
Borneol, a natural lipophilic monoterpenoid, has been extensively researched for its potential as a permeation enhancer across the blood–brain barrier, where it alters membrane lipid structures and modulates transport proteins. Additionally, borneol can improve drug delivery across various physiological barriers, including nasal and gastrointestinal layers, transdermal pathways, transcorneal routes, and the blood–optic nerve barrier [66]. Borneol penetrates the blood–brain barrier and modulates signaling pathways within the neurovascular unit. This component is a potential drug delivery agent for neurodegenerative disease therapies by regulating inflammatory and oxidative stress proteins [67].
Borneol has been evaluated as a matrix-forming component for in situ forming matrices (ISMs) targeting crevicular pockets. Drug-free and vancomycin hydrochloride-loaded borneol ISMs were compared. The borneol-based ISM demonstrated promising characteristics, such as low viscosity for improved injectability, sustained drug release over 14 days via a diffusion-controlled mechanism, and potent antimicrobial activity against various pathogens, suggesting its potential as an effective local treatment for periodontitis via crevicular pocket injection [68].
4.4. Menthol Usage as a Penetration Enhancer
Menthol, a cyclic monoterpene alcohol, contains cooling properties and has a lingering minty fragrance reminiscent of its source oil residues. This terpene is effective in enhancing the dermal penetration of pharmaceuticals [69]. At concentrations of 3.5% or lower, menthol altered the SCs’ original structure to varying extents, enhancing their fluidity and facilitating the permeation and retention of menthol. Two mechanisms were found: (1) menthol’s robust hydrogen-bonding capability allows it to compete for lipid-lipid hydrogen bonding sites, thereby weakening the stability of the skin lipid’s hydrogen-bonding network; and (2) menthol’s affinity for cholesterol, likely due to their similar molecular structures, suggests that its incorporation could enhance lipid membrane fluidity akin to cholesterol [70]. Xu et al. aimed to identify the impact of menthol on the permeability of dexamethasone disodium phosphate across the cornea and sclera in vitro. Rabbit eyes were subjected to topical drops and subconjunctival injections of dexamethasone disodium phosphate, both with and without the addition of 0.1% menthol. Results showed that concentrations of 0.05% and 0.1% menthol notably augmented the penetration of dexamethasone into the cornea. However, there was no observed alteration in dexamethasone penetration through the sclera and in the retina–choroid tissue under these conditions. Upon administration of topical drops containing dexamethasone with 0.1% menthol, a notable increase in dexamethasone concentration was observed in the cornea and aqueous humor tissues [71]. Another study by Krishnaiah et al. analyzed the impact of different solvent systems and concentrations of menthol on the permeation of ondansetron hydrochloride across rat epidermis. Solubility tests revealed the highest permeation rate with a 60% v/v ethanol–water system, prompting the preparation of hydroxypropyl cellulose gel formulations containing varying concentrations of menthol. Incorporating 8% w/w menthol into 2% w/w HPC gel resulted in optimal transdermal permeation of ondansetron hydrochloride, with a significant enhancement in drug flux observed [72].
Additionally, supramolecular eutectogels exhibit dynamic properties due to noncovalent interactions, making them responsive to stimuli, thus finding applications across various domains. The supramolecular interactions between the eutectic solvent components thymol–menthol and ethosuximide drug molecules are responsible for the gel structure and dynamics of the small molecules. In vitro release profiles, explored in different pH and temperature conditions, demonstrated the capability of eutectogel formulations to deliver ethosuximide over extended periods [73]. Encapsulating menthol within lipid-core nanocapsules using a nanoprecipitation method assesses its suitability for skin application. Results from the hen’s egg chorioallantoic membrane and cytotoxicity assays indicated the safety of topically applied nanocapsules, suggesting their potential as an aqueous solubility enhancer for menthol with improved thermal stability and as an alternative option for cosmeto-textiles with rapid dermal absorption properties [74].
4.5. Essential Oil Mixtures Usage as Penetration Enhancers
Terpenes, including carvone, 1,8-cineole, menthol, and thymol, combined with ethanol, enhance the percutaneous absorption of tamoxifen through the porcine epidermis in vitro. This mixture improved the partitioning of tamoxifen into the SC, suggesting potential applications for terpenes as enhancers for lipophilic drugs like tamoxifen [75].
In vitro and in vivo studies have identified the transdermal delivery of zidovudine using novel chemical enhancers, including t-anethole, carvacrol, thymol, and linalool, with L-menthol as a reference enhancer. An isopropyl alcohol/water solvent yielded better absorption than propylene glycol/water with most enhancers, indicating the potential of these enhancers for effective transdermal delivery of the drug [76].
The percutaneous absorption-enhancing effects of 11 monoterpenes ((+)-limonene; (-)-menthone; (+)-terpinen-4-ol; alpha-terpineol; 1,8-cineole; (+)-carvone; (-)-verbenone; (-)-fenchone; 9, p-cymene; (+)-neomenthol; and geraniol) were evaluated on the skin of hairless mice using three model drugs with varying lipophilicities: caffeine, hydrocortisone, and triamcinolone acetonide (TA). Terpenes applied at a concentration of 0.4 M in propylene glycol enhanced the permeation of caffeine, menthol, and geraniol, showing the most substantial increase. Additionally, terpenes improved the delivery of hydrocortisone, with both terpineols demonstrating the highest enhancement. However, the examined compounds did not impact the delivery of TA, suggesting their potential selectivity in the transdermal penetration of specific drugs [77]. In vitro and in vivo studies evaluated the permeation-enhancing effects of EOs extracted from Alpinia oxyphylla on indomethacin permeation through Wistar rat skin. Both AO-1 and AO-2 fractions significantly induced indomethacin permeation, with AO-2 exhibiting greater efficacy, which can likely be attributed to increased skin/vehicle partitioning. It also demonstrated limited skin irritation and differing effects on prostaglandin E(2) release from skin fibroblasts and lung epithelial cells [78].
Williams et al. demonstrated that α-pinene, 1,8-cineole, ascaridole, α-terpineol, and carvone have the ability to improve the skin penetration of the polar penetrant 5-fluorouracil (5-FU). Different terpenes showed varying levels of effectiveness; i.e., α-pinene, only modestly increased permeability, 1,8-cineole demonstrated a significant enhancement, suggesting a diverse range of activities among the tested compounds. The terpenes primarily increased drug diffusivity through the membranes via interactions with intercellular SC lipids rather than affecting drug partitioning or keratin interaction [79]. In addition, an in vitro study of poly(acrylic acid) gels incorporating 5-FU and tetrahydrogeraniol (THG) on rat skin showed that THG significantly induced the permeability of 5-FU. Higher THG concentrations increased permeability, indicating a potential application for THG in developing transdermal therapeutic systems for poorly absorbable drugs [80].
Tulsi and turpentine oil have been investigated as potential penetration enhancers for transdermal delivery of flurbiprofen. An optimized binary solvent mixture containing propylene glycol and isopropyl alcohol showed significantly higher permeation rates of flurbiprofen across rat abdominal skin compared to other solvent mixtures, and the addition of tulsi and turpentine oil further enhanced the flux of flurbiprofen [81]. Fang et al. identified the efficacy of sweet basil (Ocimum basilicum, OB) EOs as skin permeation enhancers and their potential for irritancy. Sweet basil EOs, containing various terpenes, notably improved the in vitro (keratinocytes and skin fibroblasts) skin permeation and deposition of indomethacin, with OB-1 exhibiting superior enhancement compared to OB-2, while showing minimal irritation in both in vitro and in vivo analyses [82].
Another study has demonstrated the potential of transferring some formulations’ development using eucalyptus oil to deliver ketoconazole through the skin. Results showed that EO improved the release and permeation of ketoconazole, demonstrating their ability to modify the skin barrier without undergoing any alteration themselves [83].
5. Release Mechanisms of Active Substances from Compound Delivery Systems
Delivery systems are designed to release encapsulated bioactive compounds in response to specific triggers, such as changes in pH, relative humidity, temperature, particle size, carrier system form, and properties of carrier materials and cross-linking agents. Effective encapsulation ensures the protection of bioactive compounds from external conditions until release. The release of encapsulated EOs from carrier systems is regulated by physicochemical mechanisms such as diffusion, swelling, melting, or degradation when exposed to environmental trigger conditions [26]. The topical application of EOs primarily involves skin penetration by interacting with the lipid constituents of the skin’s cell membrane. The depth of penetration depends on the chemical composition of the EO; e.g., oils like jojoba, avocado, soybean, and almond typically penetrate only the upper epidermis, while oxygenated terpenes can reach deeper layers of the skin. Certain oils (basil, tulsi, and tea tree) also can be penetration enhancers, both internally and topically; i.e., various mechanisms involve enhancing compound partitioning, disrupting the highly ordered intercellular lipid structure between corneocytes in the SC, and inducing conformational changes by interacting with intercellular protein domains [84]. Although progress has been made in designing degradable polymers for DSs, the successful translation of such materials is limited due to a lack of knowledge regarding the optimal combination of matrix properties for achieving controlled and sustained release. Poly α-esters are used for fabricating compound carrier matrices because they can degrade into harmless end products, and their degradation rates can be adjusted by modifying parameters like molecular weight, crystallinity, and backbone chemistry, making them versatile for various sustained release applications [85].
Despite significant advancements in diagnostic and therapeutic approaches for pulmonary disorders, effective treatment remains elusive. One of the primary challenges contributing to inadequate therapy is multicompound resistance, coupled with the difficulty of ensuring that active compound molecules reach the lower respiratory tract with minimal side effects. Research suggests that administering compounds directly to the lungs via inhalers may mitigate many side effects of oral or intravenous delivery [86,87]. When inhaled, EOs primarily act through two pathways: olfactory stimulation and respiratory stimulation. Through olfactory stimulation, they activate the olfactory nerve, transmitting signals to the brain. Their structural similarity to neurotransmitters and hormones allows them to stimulate olfactory chemoreceptors, triggering a cascade of physiological responses in the limbic and hypothalamic regions of the brain, ultimately inducing a sense of calmness and alleviating symptoms of anxiety and depression [84]. Due to volatility, EOs can access both the upper and lower regions of the respiratory tract through inhalation. Additionally, their antimicrobial and anti-inflammatory properties make them a potential option for effectively treating respiratory tract infections [88]. In addition to olfactory stimulation, EOs can modulate brain function by being absorbed through the alveoli. This absorption allows EO molecules to enter the bloodstream, traverse the blood–brain barrier, and potentially interact with specific brain areas. Through alveolar diffusion, volatile EO molecules may reach the systemic circulation and subsequently access the brain, particularly if they possess lipophilic properties, thereby eliciting positive psychological and physiological effects that alleviate mood disorders [84]. Over the past decade, inhalation aromatherapy has demonstrated benefits in addressing mood disorders like depression and anxiety. It has been observed that EOs can access brain tissue via the nasal–brain pathway, circumventing the blood–brain barrier. Once within the brain, they affect areas such as the cerebral cortex, thalamus, and limbic system, leading to the amelioration of anxiety and depression and enhancement of sleep quality [89].
Controlled release systems are developed for the specific active agent and designed to regulate compound exposure, facilitate passage through physiological barriers, protect against premature elimination, and direct the compound to its intended site of action, thereby reducing exposure elsewhere in the body and enhancing patient compliance. These systems can enhance the commercial value of existing compounds by prolonging patent protection and minimizing variability in compound product performance [90]. The main processes used for controlled compound release are dissolution, partitioning, diffusion, osmosis, swelling, erosion, and targeting (Table 2) [90].

Table 2.
Processes of controlled compound release systems.
Compound Delivery Routes
The limitations of chemical enhancers due to their efficiency and safety, i.e., exhibiting poor permeation across the SC, cause compound release to the top layers, increasing the risk of skin irritation with higher concentrations [8]. EOs and their constituents are emerging as preferred alternatives due to their safety profile and ability to promote percutaneous absorption of compounds into deeper skin layers, facilitating the permeation of both hydrophilic and lipophilic compounds with low cytotoxicity. Documented by organizations like the Research Institute for Fragrance Materials and the National Toxicology Program, EOs demonstrate relatively low toxicity compared to synthetic penetration enhancers, suggesting their potential as safer alternatives for enhancing compound permeation through the skin [8].
Compound delivery through inhalation offers targeted delivery to the lungs, depending on pulmonary compound concentration, which reduces systemic active compound levels and results in higher pulmonary efficacy and fewer systemic side effects. However, the complex anatomy of the lungs and various pulmonary pharmacokinetic processes (deposition, dissolution, mucociliary clearance, adsorption to lung tissue, pulmonary metabolism, and systemic compound clearance) create challenges for effective compound delivery. The structural characteristics of the lungs, such as airway mucus acting as a barrier, can further impede compound retention and clinical effectiveness by trapping conventional compound delivery particles, necessitating the careful design of newer carriers with specific size and surface properties [86].
Despite the advantages of nose-to-brain compound delivery, the smaller surface area of the nasal cavity limits molecule absorption, compounded by the inherent lipophilicity of the thin mucous lining, which favors only small, lipophilic species transport [58]. Recently, nanotechnology-based carriers have emerged as highly effective DSs capable of targeting multiple organs, shielding compounds from degradation, mitigating rapid clearance, and minimizing side effects. These carriers can be modified or surface-functionalized with various molecules to enhance targeting outcomes [86]. The design of compound nanocarriers loaded within thermo-responsive nanogels has become crucial for achieving the desired therapeutic effects in the brain. For example, microemulsions are promising due to their nanometric globule size and solubilizing properties, as well as their abilities to incorporate both lipophilic and hydrophilic compounds, enhance compound permeation through biological membranes, and prevent enzyme degradation. Additionally, the emerging concept of EO-based microemulsions presents a novel approach, leveraging the intrinsic properties of these oils to enrich the DS [58] (Figure 4).
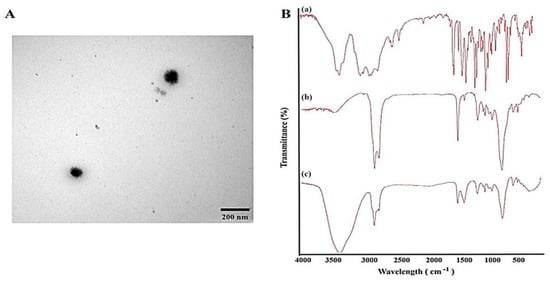
Figure 4.
Example of EO-based microemulsion: propranolol-loaded limonene-based microemulsion system. (A) Transmission electron microscopical image of the optimized propranolol-loaded limonene-based microemulsion system, and (B) Fourier transform infrared spectra of (a) propranolol, (b) physical mixture of ME components, and (c) propranolol-loaded limonene-based ME [58].
Innovation in DSs aims to improve the bioavailability of active pharmaceutical ingredients. Oral delivery systems are preferred due to their diverse dosage forms, ease of administration, safety, and patient compliance. However, oral delivery systems face limitations such as poor compound stability in the gastrointestinal tract, first-pass metabolism, and solubility issues, particularly for peptide or protein-based compounds, leading to low bioavailability. To address these challenges, the transdermal route has emerged as a promising alternative, offering potential advantages such as non-invasive administration, improved patient compliance, and reduced disposal costs [76]. Ingesting EOs is not recommended for absorbing their therapeutic benefits as it is the least efficient method, with the oil typically passing through the digestive tract, including the stomach and small intestine, before reaching the bloodstream [104].
To address such limitations of Eos as hydrophobicity, instability, volatility, and toxicity, encapsulation within delivery systems has emerged as a successful strategy. This approach enhances EO bioavailability, improves chemical stability, reduces volatility and toxicity, and enables targeted delivery to therapeutic sites, thereby enhancing patient compliance. Various strategies, including polymeric NPs and cyclodextrin inclusion complexes, have been explored for EO encapsulation, while lipid-based delivery formulations like micro- and nanoemulsions, liposomes, solid lipid NPs, and nanostructured lipid carriers have shown promise in this regard [5] (Figure 5). Although nanotechnology holds promise for therapeutic applications, it may cause risks to human health and the environment due to its physicochemical properties, dosage, route of administration, and interactions with cellular components. Factors such as particle shape, size, surface area, surface charge, interactions with proteins and lipids, and the ability to traverse biological barriers like the blood–brain barrier or placental barrier contribute to their toxicity. The pharmacokinetic characteristics of NPs play a crucial role in determining their pharmacological and toxicological behavior, with various mechanisms of toxicity including reactive oxygen species generation, lipid peroxidation, genotoxicity, membrane damage, protein denaturation, inflammation, granuloma formation, and disruptions in cellular functions such as lysosomal and mitochondrial dysfunction, apoptosis, and necrosis [105].
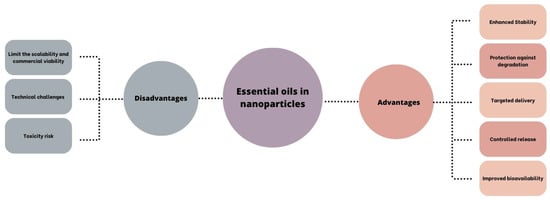
Figure 5.
Advantages and disadvantages of the combination of EOs and nanoparticles [106].
6. Discussion
Over the past five years, essential oils and their constituent terpenes have garnered increasing attention in the fields of medicine and pharmaceutical sciences due to their broad spectrum of biological activities, natural origin, and compatibility with human physiology. These volatile, aromatic compounds—primarily derived from plants—exhibit a wide array of therapeutic properties, including antimicrobial, anti-inflammatory, antioxidant, analgesic, anxiolytic, and anticancer effects. One of the key advantages of essential oils is their multifaceted mechanism of action. Such compounds as eugenol and menthol exert potent antimicrobial activity by disrupting microbial membranes, making them promising candidates for combating antibiotic-resistant strains. Linalool and limonene demonstrate notable anti-inflammatory effects through the modulation of pro-inflammatory cytokines and oxidative stress pathways, leading to their consideration in formulations targeting chronic inflammatory diseases. In oncology, in preclinical studies, monoterpenes such as d-limonene have shown the potential to induce apoptosis and inhibit tumor proliferation. In neuropharmacology, essential oil constituents like linalool, borneol, and α-pinene have been found to modulate the central nervous system, exhibiting sedative, anxiolytic, and neuroprotective effects, which have implications for treating disorders such as anxiety, depression, and neurodegeneration. To enhance the stability and bioavailability of EOs, various innovative delivery systems have been developed. Microencapsulation involves enclosing EOs within protective matrices, preserving their aromatic profiles and therapeutic properties while enabling controlled release. For example, liposome technology improves EO bioavailability by encapsulating both hydrophilic and hydrophobic compounds, ensuring targeted delivery and sustained release in cosmetic formulations. Self-emulsifying systems further enhance EO solubility and absorption, allowing their incorporation into diverse formulations. Overall, essential oils represent a promising, underexploited class of bioactive natural products with substantial potential in modern pharmacotherapy. Their versatility and biocompatibility make them attractive candidates for the development of novel therapeutic agents and delivery systems. As interest in natural and integrative medicine continues to grow, the role of essential oils and terpenes in pharmaceutical science is poised to expand significantly.
7. Conclusions
Since terpenes and essential oils (EOs) exhibit relatively low toxicity compared to synthetic penetration enhancers, they are potentially safer alternatives for improving compound permeation through the skin. This suggests their wider utilization in pharmaceutical formulations due to their safety profile and effectiveness. Integration into delivery systems may offer a promising approach to overcoming key limitations in drug delivery, thereby enhancing stability and bioavailability while facilitating improved penetration. As the pharmaceutical field continues to shift toward more natural, sustainable, and patient-friendly formulations, delivery systems for terpenes and EO are poised to play an increasingly important role. With continued innovation and rigorous scientific validation, terpenes and essential oils may become key components in the next generation of pharmaceutical therapies.
Author Contributions
Conceptualization, G.K., T.I., and U.P.; methodology, U.P.; software, U.P.; validation, G.K. and A.R.; formal analysis, G.K.; investigation, G.K. and S.R.; resources, A.R.; data curation, U.P.; writing—original draft preparation, G.K. and S.R.; writing—review and editing, T.I. and U.P.; visualization, G.K.; supervision, U.P.; project administration, A.R.; funding acquisition, S.R. and U.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data sharing does not apply to this article as no datasets were generated.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| 5-FU | 5-fluorouracil |
| CPE | chemical permeation enhancers |
| DS | delivery system |
| EOs | essential oils |
| Eu-CaCit NPs | eugenol-loaded calcium citrate nanoparticles |
| EβF | (E)-β-farnesene |
| ISMs | in situ forming matrixes |
| LPS | lipopolysaccharide |
| MeSA | methyl salicylate |
| NLC | nanostructured lipid carriers |
| NPs | nanoparticles |
| O/W | oil-in-water phase |
| OB | Ocimum basilicum |
| SC | stratum corneum |
| SNL | solid lipid nanoparticles |
| TA | triamcinolone acetonide |
| THG | tetrahydrogeraniol |
| TZN | trazodone hydrochloride |
| W/O | water-in-oil phase |
References
- Scazzocchio, F.; Garzoli, S.; Conti, C.; Leone, C.; Renaioli, C.; Pepi, F.; Angiolella, L. Properties and Limits of Some Essential Oils: Chemical Characterisation, Antimicrobial Activity, Interaction with Antibiotics and Cytotoxicity. Nat. Prod. Res. 2016, 30, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Barros, A.; de Morais, S.M.; Ferreira, P.A.T.; Vieira, Í.G.P.; Craveiro, A.A.; dos Santos Fontenelle, R.O.; de Menezes, J.E.S.A.; da Silva, F.W.F.; de Sousa, H.A. Chemical Composition and Functional Properties of Essential Oils from Mentha Species. Ind. Crops Prod. 2015, 76, 557–564. [Google Scholar] [CrossRef]
- Cossetin, L.F.; Garlet, Q.I.; Velho, M.C.; Gündel, S.; Ourique, A.F.; Heinzmann, B.M.; Monteiro, S.G. Development of Nanoemulsions Containing Lavandula dentata or Myristica fragrans Essential Oils: Influence of Temperature and Storage Period on Physical-Chemical Properties and Chemical Stability. Ind. Crops Prod. 2021, 160, 113115. [Google Scholar] [CrossRef]
- Kaspute, G.; Arunagiri, B.D.; Alexander, R.; Ramanavicius, A.; Samukaite-Bubniene, U. Development of Essential Oil Delivery Systems by ‘Click Chemistry’ Methods: Possible Ways to Manage Duchenne Muscular Dystrophy. Materials 2023, 16, 6537. [Google Scholar] [CrossRef] [PubMed]
- Cimino, C.; Maurel, O.M.; Musumeci, T.; Bonaccorso, A.; Drago, F.; Souto, E.M.B.; Pignatello, R.; Carbone, C. Essential Oils: Pharmaceutical Applications and Encapsulation Strategies into Lipid-Based Delivery Systems. Pharmaceutics 2021, 13, 327. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Singh, D.; Singh, R.K. Chapter 13—Therapeutic Role of Essential Oils in Malignancies through Drug Delivery Mechanisms. In Drug-Delivery Systems of Phytochemicals and Therapeutic Strategies in Cancer Therapy; Srivastava, A.K., Singh, D., Singh, R.K., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 199–213. ISBN 978-0-443-15960-2. [Google Scholar]
- Mali, S.N.; Pandey, A.; de Oliveira, M.S.; Jawarkar, R.D. A Decade Review of Analysis of Essential Oils from Genus Artocarpus: Its Phytochemistry and Pharmacology. Pharmacol. Res.—Nat. Prod. 2024, 2, 100016. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Essential Oils and Their Constituents as Skin Penetration Enhancer for Transdermal Drug Delivery: A Review. J. Pharm. Pharmacol. 2015, 67, 473–485. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. D-Limonene. In Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins; International Agency for Research on Cancer: Lyon, France, 1993. [Google Scholar]
- Pereira, I.; Severino, P.; Santos, A.C.; Silva, A.M.; Souto, E.B. Linalool Bioactive Properties and Potential Applicability in Drug Delivery Systems. Colloids Surf. B Biointerfaces 2018, 171, 566–578. [Google Scholar] [CrossRef]
- Santos, J.S.; Gonzatto, M.P.; Santos, J.S.; Gonzatto, M.P. Citrus Essential Oils and Nanosystems towards Skin Delivery. In Citrus Research—Horticultural and Human Health Aspects; IntechOpen: London, UK, 2023; ISBN 978-1-80355-805-9. [Google Scholar]
- Camargo, S.F.; Medeiros, C.F.; Santos, V.V.; Quintans-Júnior, L.J.; Azeredo, F.J.; Silva, D.F. Pharmacokinetics and Antihypertensive Effect of Linalool Is Improved after Incorporation in β-Cyclodextrin as a Drug Delivery System. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Waidyanatha, S.; Hackett, M.; Black, S.R.; Stout, M.D.; Fennell, T.R.; Silinski, M.R.; Watson, S.L.; Licause, J.; Robinson, V.G.; Sparrow, B.; et al. Toxicokinetic Evaluation of the Common Indoor Air Pollutant, α-Pinene, and Its Potential Reactive Metabolite, α-Pinene Oxide, Following Inhalation Exposure in Rodents. Toxicol. Appl. Pharmacol. 2021, 418, 115496. [Google Scholar] [CrossRef]
- Hammoud, Z.; Kayouka, M.; Trifan, A.; Sieniawska, E.; Jemâa, J.M.B.; Elaissari, A.; Greige-Gerges, H. Encapsulation of α-Pinene in Delivery Systems Based on Liposomes and Cyclodextrins. Molecules 2021, 26, 6840. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.; Choudhury, P.K.; Dev, S.K.; Rathore, V. Formulation and Evaluation of α-Pinene Loaded Self-Emulsifying Nanoformulation for In-Vivo Anti-Parkinson’s Activity. Recent Pat. Nanotechnol. 2022, 16, 139–159. [Google Scholar] [CrossRef] [PubMed]
- Dao, L.; Dong, Y.; Song, L.; Sa, C. The Fate of 1,8-Cineole as a Chemical Penetrant: A Review. Curr. Drug Deliv. 2024, 21, 697–708. [Google Scholar] [CrossRef]
- Jiang, F.; Wu, G.; Li, W.; Yang, J.; Yan, J.; Wang, Y.; Yao, W.; Zhou, X.; He, Z.; Wu, L.; et al. Preparation and Protective Effects of 1,8-Cineole-Loaded Self-Microemulsifying Drug Delivery System on Lipopolysaccharide-Induced Endothelial Injury in Mice. Eur. J. Pharm. Sci. 2019, 127, 14–23. [Google Scholar] [CrossRef]
- Rehman, A.; Iqbal, M.; Khan, B.A.; Khan, M.K.; Huwaimel, B.; Alshehri, S.; Alamri, A.H.; Alzhrani, R.M.; Bukhary, D.M.; Safhi, A.Y.; et al. Fabrication, In Vitro, and In Vivo Assessment of Eucalyptol-Loaded Nanoemulgel as a Novel Paradigm for Wound Healing. Pharmaceutics 2022, 14, 1971. [Google Scholar] [CrossRef]
- Abbad, I.; Soulaimani, B.; Abbad, A. Chemical Composition, Insecticidal and Allelopathic Properties of Essential Oils Obtained from Wild and Cultivated Moroccan Satureja calamintha (L.). J. Nat. Pestic. Res. 2023, 3, 100021. [Google Scholar] [CrossRef]
- Lim, X.-Y.; Li, J.; Yin, H.-M.; He, M.; Li, L.; Zhang, T. Stabilization of Essential Oil: Polysaccharide-Based Drug Delivery System with Plant-like Structure Based on Biomimetic Concept. Polymers 2023, 15, 3338. [Google Scholar] [CrossRef] [PubMed]
- Din, F.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective Use of Nanocarriers as Drug Delivery Systems for the Treatment of Selected Tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef]
- Hmingthansanga, V.; Singh, N.; Banerjee, S.; Manickam, S.; Velayutham, R.; Natesan, S. Improved Topical Drug Delivery: Role of Permeation Enhancers and Advanced Approaches. Pharmaceutics 2022, 14, 2818. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Gravelle, A.J.; Saffarionpour, S.; Wright, A.J. Editorial: Novel Delivery Systems of Flavors and Essential Oils: Exploring Potential Health Applications in Foods. Front. Nutr. 2023, 10, 1183878. [Google Scholar] [CrossRef] [PubMed]
- Obeid, M.A.; Al Qaraghuli, M.M.; Alsaadi, M.; Alzahrani, A.R.; Niwasabutra, K.; Ferro, V.A. Delivering Natural Products and Biotherapeutics to Improve Drug Efficacy. Ther. Deliv. 2017, 8, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Yammine, J.; Chihib, N.-E.; Gharsallaoui, A.; Ismail, A.; Karam, L. Advances in Essential Oils Encapsulation: Development, Characterization and Release Mechanisms. Polym. Bull. 2024, 81, 3837–3882. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, Z.; Liu, H.; Man, J.; Oladejo, A.O.; Ibrahim, S.; Wang, S.; Hao, B. Novel Drug Delivery Systems: An Important Direction for Drug Innovation Research and Development. Pharmaceutics 2024, 16, 674. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.R.; Malik, M.H.; Biswas, S.; Tam, V.H.; Rumbaugh, K.P.; Li, W.; Liu, X. Nanoemulsion Delivery Systems for Enhanced Efficacy of Antimicrobials and Essential Oils. Biomater. Sci. 2022, 10, 633–653. [Google Scholar] [CrossRef]
- Lucia, A.; Guzmán, E. Emulsions Containing Essential Oils, Their Components or Volatile Semiochemicals as Promising Tools for Insect Pest and Pathogen Management. Adv. Colloid Interface Sci. 2021, 287, 102330. [Google Scholar] [CrossRef] [PubMed]
- Hanan, E.; Dar, A.H.; Shams, R.; Goksen, G. New Insights into Essential Oil Nano Emulsions Loaded Natural Biopolymers Recent Development, Formulation, Characterization and Packaging Applications: A Comprehensive Review. Int. J. Biol. Macromol. 2024, 280, 135751. [Google Scholar] [CrossRef]
- Mushtaq, A.; Mohd Wani, S.; Malik, A.R.; Gull, A.; Ramniwas, S.; Ahmad Nayik, G.; Ercisli, S.; Alina Marc, R.; Ullah, R.; Bari, A. Recent Insights into Nanoemulsions: Their Preparation, Properties and Applications. Food Chem. X 2023, 18, 100684. [Google Scholar] [CrossRef]
- Jiménez, M.; Domínguez, J.A.; Pascual-Pineda, L.A.; Azuara, E.; Beristain, C.I. Elaboration and Characterization of O/W Cinnamon (Cinnamomum zeylanicum) and Black Pepper (Piper nigrum) Emulsions. Food Hydrocoll. 2018, 77, 902–910. [Google Scholar] [CrossRef]
- Sugumar, S.; Nirmala, J.; Ghosh, V.; Anjali, H.; Mukherjee, A.; Chandrasekaran, N. Bio-Based Nanoemulsion Formulation, Characterization and Antibacterial Activity against Food-Borne Pathogens. J. Basic Microbiol. 2013, 53, 677–685. [Google Scholar] [CrossRef]
- Rossi, G.G.; Guterres, K.B.; Bonez, P.C.; da Silva Gundel, S.; Aggertt, V.A.; Siqueira, F.S.; Ourique, A.F.; Wagnerd, R.; Klein, B.; Santos, R.C.V.; et al. Antibiofilm Activity of Nanoemulsions of Cymbopogon flexuosus against Rapidly Growing Mycobacteria. Microb. Pathog. 2017, 113, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.P.; Almeida-Aguiar, C.; Costa, S.P.G.; Neves, I.C. Essential Oils Encapsulated in Zeolite Structures as Delivery Systems (EODS): An Overview. Molecules 2022, 27, 8525. [Google Scholar] [CrossRef]
- Najaf Najafi, M.; Arianmehr, A.; Sani, A.M. Preparation of Barije (Ferula Gummosa) Essential Oil–Loaded Liposomes and Evaluation of Physical and Antibacterial Effect on Escherichia coli O157:H7. J. Food Prot. 2020, 83, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Chelu, M. Hydrogels with Essential Oils: Recent Advances in Designs and Applications. Gels 2024, 10, 636. [Google Scholar] [CrossRef]
- Ngampunwetchakul, L.; Toonkaew, S.; Supaphol, P.; Suwantong, O. Semi-Solid Poly(Vinyl Alcohol) Hydrogels Containing Ginger Essential Oil Encapsulated in Chitosan Nanoparticles for Use in Wound Management. J. Polym. Res. 2019, 26, 224. [Google Scholar] [CrossRef]
- Volić, M.; Pajić-Lijaković, I.; Djordjević, V.; Knežević-Jugović, Z.; Pećinar, I.; Stevanović-Dajić, Z.; Veljović, Đ.; Hadnadjev, M.; Bugarski, B. Alginate/Soy Protein System for Essential Oil Encapsulation with Intestinal Delivery. Carbohydr. Polym. 2018, 200, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Shishir, M.R.I.; Xie, L.; Sun, C.; Zheng, X.; Chen, W. Advances in Micro and Nano-Encapsulation of Bioactive Compounds Using Biopolymer and Lipid-Based Transporters. Trends Food Sci. Technol. 2018, 78, 34–60. [Google Scholar] [CrossRef]
- Katouzian, I.; Faridi Esfanjani, A.; Jafari, S.M.; Akhavan, S. Formulation and Application of a New Generation of Lipid Nano-Carriers for the Food Bioactive Ingredients. Trends Food Sci. Technol. 2017, 68, 14–25. [Google Scholar] [CrossRef]
- Nasseri, M.; Golmohammadzadeh, S.; Arouiee, H.; Jaafari, M.R.; Neamati, H. Antifungal Activity of Zataria Multiflora Essential Oil-Loaded Solid Lipid Nanoparticles in-Vitro Condition. Iran. J. Basic Med. Sci. 2016, 19, 1231–1237. [Google Scholar]
- Fincheira, P.; Espinoza, J.; Levío-Raimán, M.; Vera, J.; Tortella, G.; Brito, A.M.M.; Seabra, A.B.; Diez, M.C.; Quiroz, A.; Rubilar, O. Formulation of Essential Oils-Loaded Solid Lipid Nanoparticles-Based Chitosan/PVA Hydrogels to Control the Growth of Botrytis cinerea and Penicillium expansum. Int. J. Biol. Macromol. 2024, 270, 132218. [Google Scholar] [CrossRef]
- Lazar, V.; Holban, A.-M.; Curutiu, C.; Ditu, L.M. Modulation of Gut Microbiota by Essential Oils and Inorganic Nanoparticles: Impact in Nutrition and Health. Front. Nutr. 2022, 9, 920413. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yu, D.-G.; Williams, G.R.; Bligh, S.W.A. Co-Loading of Inorganic Nanoparticles and Natural Oil in the Electrospun Janus Nanofibers for a Synergetic Antibacterial Effect. Pharmaceutics 2022, 14, 1208. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Vinceković, M.; Jurić, S.; Viskić, M.; Režek Jambrak, A.; Donsì, F. Food-Grade Colloidal Systems for the Delivery of Essential Oils. Food Rev. Int. 2019, 37, 1–45. [Google Scholar] [CrossRef]
- Tortorici, S.; Cimino, C.; Ricupero, M.; Musumeci, T.; Biondi, A.; Siscaro, G.; Carbone, C.; Zappalà, L. Nanostructured Lipid Carriers of Essential Oils as Potential Tools for the Sustainable Control of Insect Pests. Ind. Crops Prod. 2022, 181, 114766. [Google Scholar] [CrossRef]
- Chura, S.S.D.; Memória, K.A.S.; Lopes, A.T.; Pelissari, F.M.; Da Silveira, J.V.W.; Bezerra, J.d.A.; Chaves, F.C.M.; Rodrigues, A.P.; Faria, J.A.Q.A.; Carneiro, G. Red Sacaca Essential Oil-Loaded Nanostructured Lipid Carriers Optimized by Factorial Design: Cytotoxicity and Cellular Reactive Oxygen Species Levels. Front. Pharmacol. 2023, 14, 1176629. [Google Scholar] [CrossRef]
- Schafer, N.; Balwierz, R.; Biernat, P.; Ochędzan-Siodłak, W.; Lipok, J. Natural Ingredients of Transdermal Drug Delivery Systems as Permeation Enhancers of Active Substances through the Stratum Corneum. Mol. Pharm. 2023, 20, 3278–3297. [Google Scholar] [CrossRef] [PubMed]
- Meryem, S.; Mohamed, D.; Nour-eddine, C.; Faouzi, E. Chemical Composition, Antibacterial and Antioxidant Properties of Three Moroccan Citrus Peel Essential Oils. Sci. Afr. 2023, 20, e01592. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, Y.; Guo, Y.; Liu, J.; Wu, L.; Lin, J. The Application of Pomelo Peel as a Carrier for Adsorption of Epigallocatechin-3-Gallate. J. Sci. Food Agric. 2018, 98, 4135–4141. [Google Scholar] [CrossRef]
- Saha, S.; Verma, R.J. Molecular Interactions of Active Constituents of Essential Oils in Zwitterionic Lipid Bilayers. Chem. Phys. Lipids 2018, 213, 76–87. [Google Scholar] [CrossRef]
- Yap, P.S.X.; Yusoff, K.; Lim, S.-H.E.; Chong, C.-M.; Lai, K.-S. Membrane Disruption Properties of Essential Oils—A Double-Edged Sword? Processes 2021, 9, 595. [Google Scholar] [CrossRef]
- Andriotis, E.G.; Papi, R.M.; Paraskevopoulou, A.; Achilias, D.S. Synthesis of D-Limonene Loaded Polymeric Nanoparticles with Enhanced Antimicrobial Properties for Potential Application in Food Packaging. Nanomaterials 2021, 11, 191. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, L.; Chiralt, A.; González-Martínez, C.; Cháfer, M. Effect of Essential Oils on Properties of Film Forming Emulsions and Films Based on Hydroxypropylmethylcellulose and Chitosan. J. Food Eng. 2011, 105, 246–253. [Google Scholar] [CrossRef]
- Vol, A.; Gribova, O.; Shamir, O.; Levy, E.; Ben Ishai, P.; Gutina, A.G.; Feldman, Y. Dielectric Properties of a Novel Colloidal Oral Matrix Drug Carrier. Colloids Surf. B Biointerfaces 2017, 155, 223–228. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, Q.-D.; Chai, Y.-P.; Zhang, H.; Peng, P.; Yang, X.-X. Natural Terpenes as Penetration Enhancers for Transdermal Drug Delivery. Molecules 2016, 21, 1709. [Google Scholar] [CrossRef]
- Abla, K.K.; Domiati, S.; El Majzoub, R.; Mehanna, M.M. Propranolol-Loaded Limonene-Based Microemulsion Thermo-Responsive Mucoadhesive Nasal Nanogel: Design, In Vitro Assessment, Ex Vivo Permeation, and Brain Biodistribution. Gels 2023, 9, 491. [Google Scholar] [CrossRef]
- Chiu, C.-S.; Huang, P.-H.; Chan, Y.-J.; Li, P.-H.; Lu, W.-C. D-Limonene Nanoemulsion as Skin Permeation Enhancer for Curcumin Prepared by Ultrasonic Emulsification. J. Agric. Food Res. 2024, 15, 100932. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, W.; Zhang, J.; Liao, Y.; Firempong, C.K.; Adu-Frimpong, M.; Deng, W.; Zhang, H.; Yu, J.; Xu, X. Self-Microemulsifying Drug Delivery System for Improved Oral Delivery of Limonene: Preparation, Characterization, in Vitro and in Vivo Evaluation. AAPS PharmSciTech 2019, 20, 153. [Google Scholar] [CrossRef]
- Waheed, A.; Aqil, M.; Ahad, A.; Imam, S.S.; Moolakkadath, T.; Iqbal, Z.; Ali, A. Improved Bioavailability of Raloxifene Hydrochloride Using Limonene Containing Transdermal Nano-Sized Vesicles. J. Drug Deliv. Sci. Technol. 2019, 52, 468–476. [Google Scholar] [CrossRef]
- Esmaeili, F.; Rajabnejhad, S.; Partoazar, A.R.; Mehr, S.E.; Faridi-Majidi, R.; Sahebgharani, M.; Syedmoradi, L.; Rajabnejhad, M.R.; Amani, A. Anti-Inflammatory Effects of Eugenol Nanoemulsion as a Topical Delivery System. Pharm. Dev. Technol. 2016, 21, 887–893. [Google Scholar] [CrossRef]
- Cherdchom, S.; Keawsongsaeng, W.; Buasorn, W.; Rimsueb, N.; Pienpinijtham, P.; Sereemaspun, A.; Rojanathanes, R.; Aramwit, P. Development of Eugenol-Embedded Calcium Citrate Nanoparticles as a Local Anesthetic Agent. ACS Omega 2021, 6, 28880–28889. [Google Scholar] [CrossRef]
- El Khayat, N.W.; Donia, A.A.; Mady, O.Y.; El Maghraby, G.M. Optimization of Eugenol Microemulsion for Transdermal Delivery of Indomethacin. J. Drug Deliv. Sci. Technol. 2018, 48, 311–318. [Google Scholar] [CrossRef]
- Mishra, H.; Mishra, P.K.; Iqbal, Z.; Jaggi, M.; Madaan, A.; Bhuyan, K.; Gupta, N.; Gupta, N.; Vats, K.; Verma, R.; et al. Co-Delivery of Eugenol and Dacarbazine by Hyaluronic Acid-Coated Liposomes for Targeted Inhibition of Survivin in Treatment of Resistant Metastatic Melanoma. Pharmaceutics 2019, 11, 163. [Google Scholar] [CrossRef]
- Kulkarni, M.; Sawant, N.; Kolapkar, A.; Huprikar, A.; Desai, N. Borneol: A Promising Monoterpenoid in Enhancing Drug Delivery Across Various Physiological Barriers. AAPS PharmSciTech 2021, 22, 145. [Google Scholar] [CrossRef]
- Chen, N.; Wen, J.; Wang, Z.; Wang, J. Multiple Regulation and Targeting Effects of Borneol in the Neurovascular Unit in Neurodegenerative Diseases. Basic Clin. Pharmacol. Toxicol. 2022, 130, 5–19. [Google Scholar] [CrossRef]
- Lertsuphotvanit, N.; Santimaleeworagun, W.; Narakornwit, W.; Chuenbarn, T.; Mahadlek, J.; Chantadee, T.; Phaechamud, T. Borneol-Based Antisolvent-Induced in Situ Forming Matrix for Crevicular Pocket Delivery of Vancomycin Hydrochloride. Int. J. Pharm. 2022, 617, 121603. [Google Scholar] [CrossRef] [PubMed]
- Kamatou, G.P.P.; Vermaak, I.; Viljoen, A.M.; Lawrence, B.M. Menthol: A Simple Monoterpene with Remarkable Biological Properties. Phytochemistry 2013, 96, 15–25. [Google Scholar] [CrossRef]
- Chen, L.; Ma, L.; Yang, S.; Wu, X.; Dai, X.; Wang, S.; Shi, X. A Multiscale Study of the Penetration-Enhancing Mechanism of Menthol. J. Tradit. Chin. Med. Sci. 2019, 6, 347–354. [Google Scholar] [CrossRef]
- Xu, X.; Yu, N.; Bai, Z.; Xun, Y.; Jin, D.; Li, Z.; Cui, H. Effect of Menthol on Ocular Drug Delivery. Graefes Arch. Clin. Exp. Ophthalmol. 2011, 249, 1503–1510. [Google Scholar] [CrossRef]
- Krishnaiah, Y.S.R.; Kumar, M.S.; Raju, V.; Lakshmi, M.; Rama, B. Penetration-Enhancing Effect of Ethanolic Solution of Menthol on Transdermal Permeation of Ondansetron Hydrochloride Across Rat Epidermis. Drug Deliv. 2008, 15, 227–234. [Google Scholar] [CrossRef]
- Vanoli, V.; Pietrowska, J.; de Araujo Lima e Souza, G.; Di Pietro, M.E.; Briatico Vangosa, F.; Mele, A.; Castiglione, F. Supramolecular Hydrophobic Eutectogels Based on Menthol-Thymol as Thermo- and pH-Responsive Drug Delivery Systems. ACS Appl. Eng. Mater. 2024, 2, 388–396. [Google Scholar] [CrossRef]
- Yingngam, B.; Chiangsom, A.; Pharikarn, P.; Vonganakasame, K.; Kanoknitthiran, V.; Rungseevijitprapa, W.; Prasitpuriprecha, C. Optimization of Menthol-Loaded Nanocapsules for Skin Application Using the Response Surface Methodology. J. Drug Deliv. Sci. Technol. 2019, 53, 101138. [Google Scholar] [CrossRef]
- Gao, S.; Singh, J. In Vitro Percutaneous Absorption Enhancement of a Lipophilic Drug Tamoxifen by Terpenes. J. Control. Release 1998, 51, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Ramadon, D.; McCrudden, M.T.C.; Courtenay, A.J.; Donnelly, R.F. Enhancement Strategies for Transdermal Drug Delivery Systems: Current Trends and Applications. Drug Deliv. Transl. Res. 2022, 12, 758–791. [Google Scholar] [CrossRef]
- Godwin, D.A.; Michniak, B.B. Influence of Drug Lipophilicity on Terpenes as Transdermal Penetration Enhancers. Drug Dev. Ind. Pharm. 1999, 25, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.-Y.; Leu, Y.-L.; Hwang, T.-L.; Cheng, H.-C.; Hung, C.-F. Development of Sesquiterpenes from Alpinia Oxyphylla as Novel Skin Permeation Enhancers. Eur. J. Pharm. Sci. 2003, 19, 253–262. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Terpenes and the Lipid-Protein-Partitioning Theory of Skin Penetration Enhancement. Pharm. Res. 1991, 8, 17–24. [Google Scholar] [CrossRef]
- Hanif, R.M.; Qineng, P.; Zhan, G. Penetration Enhancing Effect of Tetrahydrogeraniol on the Percutaneous Absorption of 5-Fluorouracil from Gels in Excised Rat Skin. J. Control. Release 1998, 55, 297–302. [Google Scholar] [CrossRef]
- Charoo, N.A.; Shamsher, A.A.A.; Kohli, K.; Pillai, K.; Rahman, Z. Improvement in Bioavailability of Transdermally Applied Flurbiprofen Using Tulsi (Ocimum sanctum) and Turpentine Oil. Colloids Surf. B Biointerfaces 2008, 65, 300–307. [Google Scholar] [CrossRef]
- Fang, J.-Y.; Leu, Y.-L.; Hwang, T.-L.; Cheng, H.-C. Essential Oils from Sweet Basil (Ocimum basilicum) as Novel Enhancers to Accelerate Transdermal Drug Delivery. Biol. Pharm. Bull. 2004, 27, 1819–1825. [Google Scholar] [CrossRef]
- Rajan, R.; Vasudevan, D.T. Effect of Permeation Enhancers on the Penetration Mechanism of Transfersomal Gel of Ketoconazole. J. Adv. Pharm. Technol. Res. 2012, 3, 112–116. [Google Scholar] [CrossRef]
- Vora, L.K.; Gholap, A.D.; Hatvate, N.T.; Naren, P.; Khan, S.; Chavda, V.P.; Balar, P.C.; Gandhi, J.; Khatri, D.K. Essential Oils for Clinical Aromatherapy: A Comprehensive Review. J. Ethnopharmacol. 2024, 330, 118180. [Google Scholar] [CrossRef] [PubMed]
- Joy, N.; Samavedi, S. Identifying Specific Combinations of Matrix Properties That Promote Controlled and Sustained Release of a Hydrophobic Drug from Electrospun Meshes. ACS Omega 2020, 5, 15865–15876. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Özcan Bülbül, E.; Miliotou, A.N.; Karantas, I.D.; Okur, M.E.; Üstündağ Okur, N. Nano-Based Carriers for Pulmonary Drug Delivery: A Review on the Available Drug Delivery Applications and Toxicity Issues. J. Drug Deliv. Sci. Technol. 2024, 92, 105381. [Google Scholar] [CrossRef]
- Nainwal, N. Treatment of Respiratory Viral Infections through Inhalation Therapeutics: Challenges and Opportunities. Pulm. Pharmacol. Ther. 2022, 77, 102170. [Google Scholar] [CrossRef]
- Horváth, G.; Ács, K. Essential Oils in the Treatment of Respiratory Tract Diseases Highlighting Their Role in Bacterial Infections and Their Anti-inflammatory Action: A Review. Flavour Fragr. J. 2015, 30, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Li, M.; Wei, Y.; Li, H.; He, X.; Yang, Q.; Li, Z.; Duan, J.; Wu, Z.; Chen, Q.; et al. Inhalation Aromatherapy via Brain-Targeted Nasal Delivery: Natural Volatiles or Essential Oils on Mood Disorders. Front. Pharmacol. 2022, 13, 860043. [Google Scholar] [CrossRef]
- Bruschi, M.L. (Ed.) Main Mechanisms to Control the Drug Release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Cambridge, UK, 2015; pp. 37–62. ISBN 978-0-08-100092-2. [Google Scholar]
- Lu, J.X.; Tupper, C.; Murray, J. Biochemistry, Dissolution and Solubility. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- El-Hawary, S.S.; Ezzat, S.M.; Eid, G.E.; Abd-El Rhman, S.K. Effect of Certain Essential Oils on Dissolution of Three Commercial Gutta-Percha Brands. J. Essent. Oil Bear. Plants 2015, 18, 1126–1137. [Google Scholar] [CrossRef]
- Judy, E.; Pagariya, D.; Kishore, N. Drug Partitioning in Micellar Media and Its Implications in Rational Drug Design: Insights with Streptomycin. Langmuir 2018, 34, 3467–3484. [Google Scholar] [CrossRef]
- Das, M.K.; Bhattacharya, A.; Ghosal, S.K. Effect of Different Terpene-Containing Essential Oils on Percutaneous Absorption of Trazodone Hydrochloride Through Mouse Epidermis. Drug Deliv. 2006, 13, 425–431. [Google Scholar] [CrossRef]
- Sanopoulou, M.; Papadokostaki, K.G. Controlled Drug Release Systems: Mechanisms and Kinetics. In Biomedical Membranes and (Bio)Artificial Organs; World Scientific: Singapore, 2018; pp. 1–33. ISBN 978-981-322-175-8. [Google Scholar]
- Carreño, H.; Stashenko, E.E.; Escobar, P. Essential Oils Distilled from Colombian Aromatic Plants and Their Constituents as Penetration Enhancers for Transdermal Drug Delivery. Molecules 2023, 28, 2872. [Google Scholar] [CrossRef]
- Herrlich, S.; Spieth, S.; Messner, S.; Zengerle, R. Osmotic Micropumps for Drug Delivery. Adv. Drug Deliv. Rev. 2012, 64, 1617–1627. [Google Scholar] [CrossRef] [PubMed]
- Hamamura, K.; Katsuyama, S.; Komatsu, T.; Scuteri, D.; Bagetta, G.; Aritake, K.; Sakurada, T. Behavioral Effects of Continuously Administered Bergamot Essential Oil on Mice With Partial Sciatic Nerve Ligation. Front. Pharmacol. 2020, 11, 1310. [Google Scholar] [CrossRef]
- Adrover, A.; Venditti, C.; Giona, M. Swelling and Drug Release in Polymers through the Theory of Poisson–Kac Stochastic Processes. Gels 2021, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Dima, C.; Pătraşcu, L.; Cantaragiu, A.; Alexe, P.; Dima, Ş. The Kinetics of the Swelling Process and the Release Mechanisms of Coriandrum sativum L. Essential Oil from Chitosan/Alginate/Inulin Microcapsules. Food Chem. 2016, 195, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Choonara, B.F.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Tomar, L.K.; Tyagi, C.; Pillay, V. A Menthol-Based Solid Dispersion Technique for Enhanced Solubility and Dissolution of Sulfamethoxazole from an Oral Tablet Matrix. AAPS PharmSciTech 2014, 16, 771–786. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Q.; Xia, G.; Adilijiang, N.; Li, Y.; Hou, Z.; Fan, Z.; Li, J. Recent Advances in Targeted Drug Delivery Strategy for Enhancing Oncotherapy. Pharmaceutics 2023, 15, 2233. [Google Scholar] [CrossRef]
- Jampilek, J.; Kralova, K. Anticancer Applications of Essential Oils Formulated into Lipid-Based Delivery Nanosystems. Pharmaceutics 2022, 14, 2681. [Google Scholar] [CrossRef]
- Sartori Tamburlin, I.; Roux, E.; Feuillée, M.; Labbé, J.; Aussaguès, Y.; El Fadle, F.E.; Fraboul, F.; Bouvier, G. Toxicological Safety Assessment of Essential Oils Used as Food Supplements to Establish Safe Oral Recommended Doses. Food Chem. Toxicol. 2021, 157, 112603. [Google Scholar] [CrossRef]
- Kang, H.; Mintri, S.; Menon, A.V.; Lee, H.Y.; Choi, H.S.; Kim, J. Pharmacokinetics, Pharmacodynamics and Toxicology of Theranostic Nanoparticles. Nanoscale 2015, 7, 18848–18862. [Google Scholar] [CrossRef]
- El-Gebaly, A.S.; Sofy, A.R.; Hmed, A.A.; Youssef, A.M. Combination of Nanoparticles (NPs) and Essential Oils (EOs) as Promising Alternatives to Non-Effective Antibacterial, Antifungal and Antiviral Agents: A Review. Biocatal. Agric. Biotechnol. 2024, 57, 103067. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).