Revolutionizing Diabetes Management Through Nanotechnology-Driven Smart Systems
Abstract
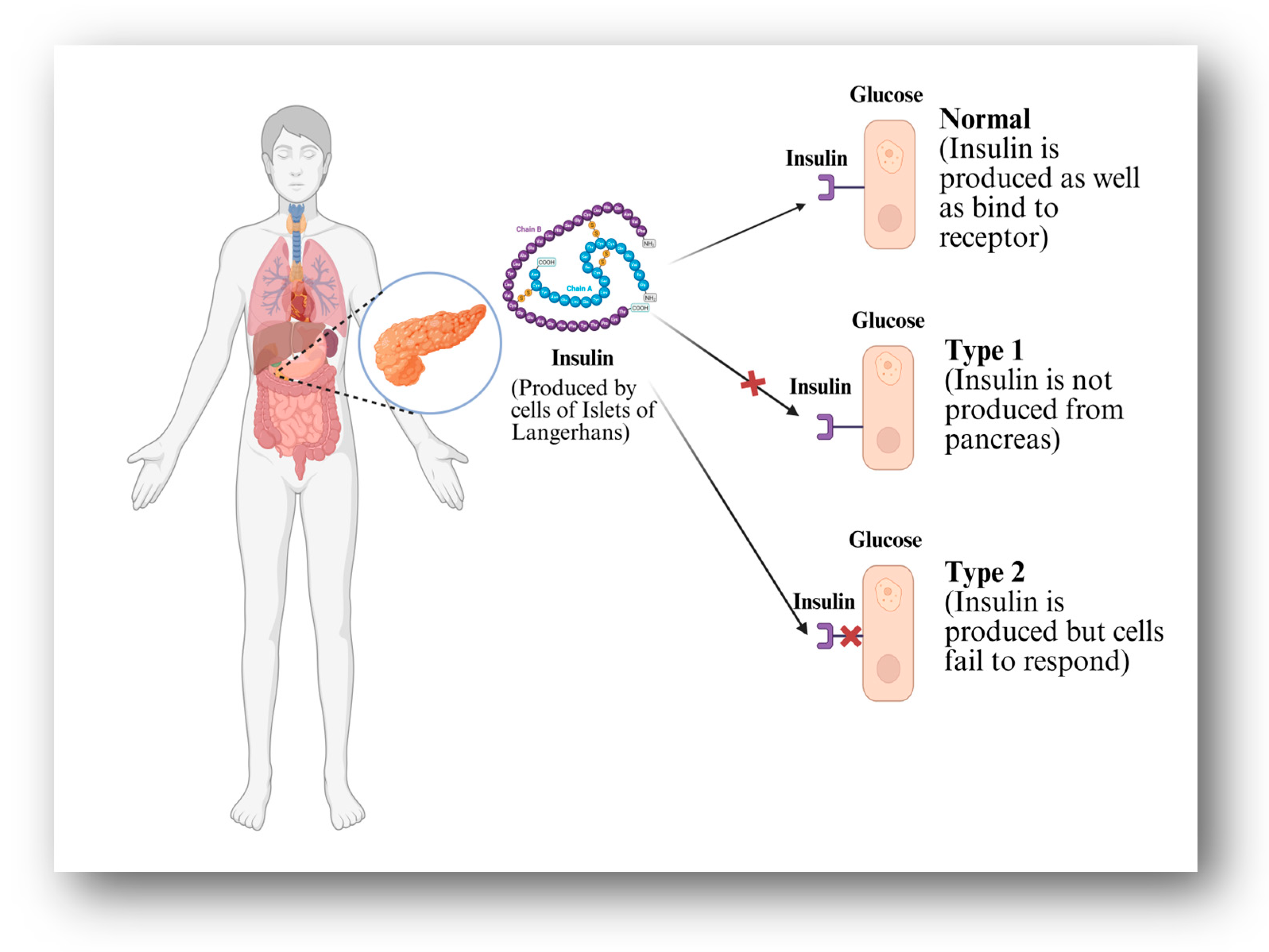
1. Introduction
2. Conventional Antidiabetic Regimens: Mechanisms, Efficacy, and Modern-Day Relevance
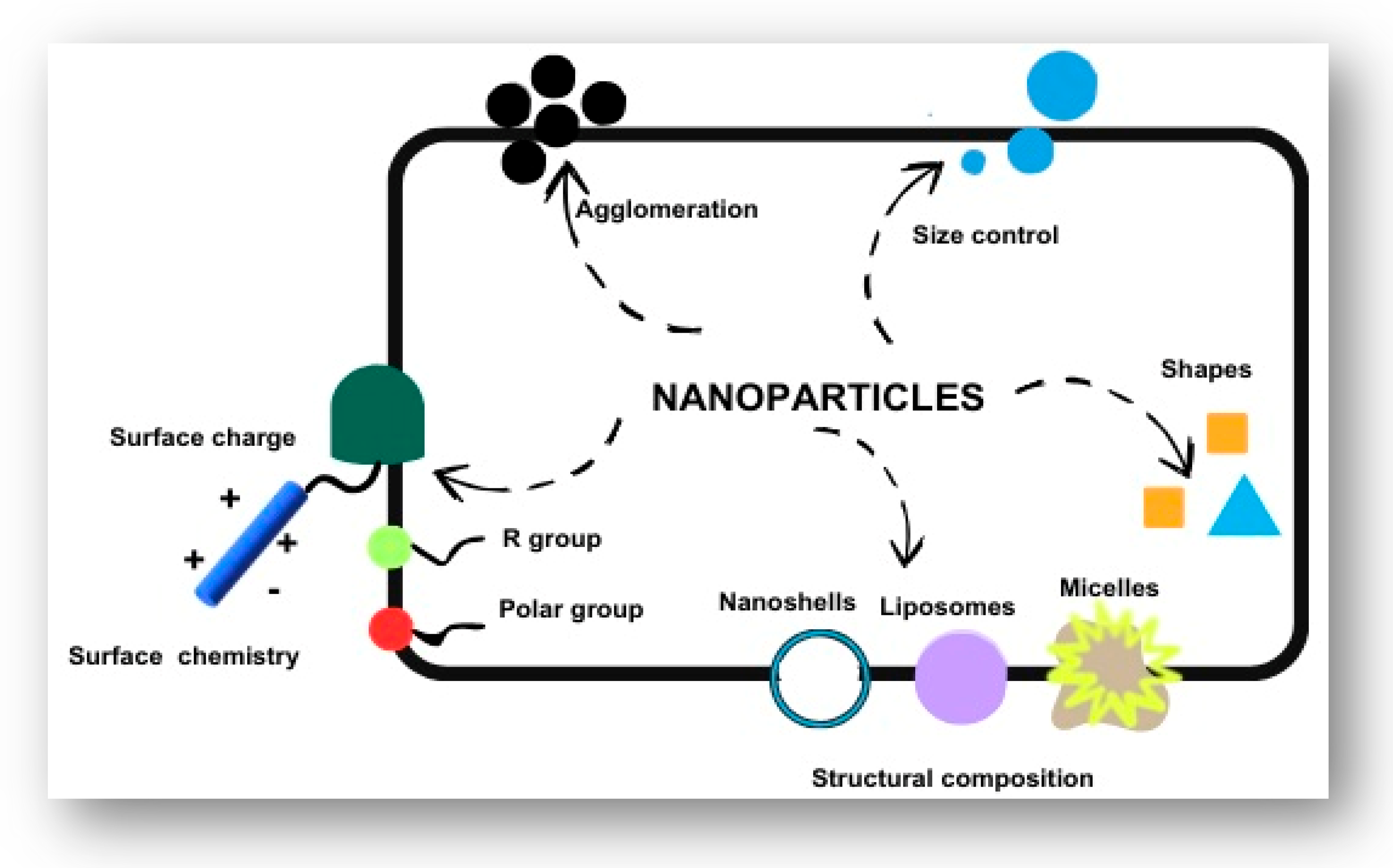
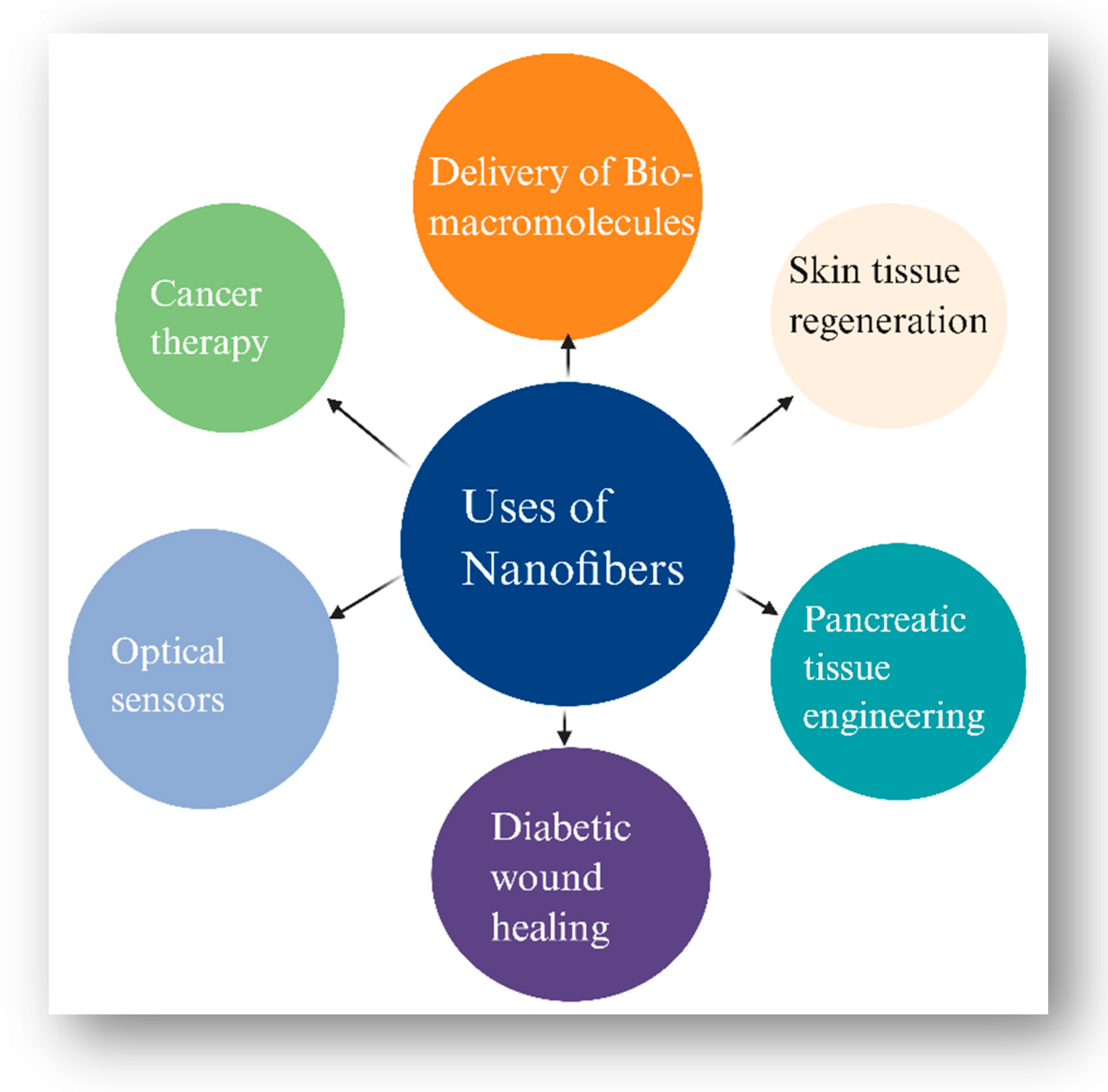
3. Nanoscale Innovations in Diabetes Care
4. Smart Nanocarriers for Precision Insulin Delivery
5. Nanosensors in Glucose Monitoring
6. Peptides in the Treatment of Diabetes
7. AI-Driven Glucose Monitoring
8. Critical Perspective on Clinical Translation, Safety, and Efficacy of Nanotechnologies in Diabetes
9. Future Directions and Emerging Trends
10. Conclusions
Funding
Conflicts of Interest
Abbreviations
| AMPK | AMP-activated protein kinase |
| CGM | Continuous Glucose Monitoring |
| CNF | carbon nanofiber |
| CNMs | carbon nanomaterials |
| CNT | carbon nanotubes |
| DPP-4i | dipeptidyl peptidase-4 inhibitors |
| DM | diabetes mellitus |
| FRET | Förster Resonance Energy Transfer |
| GBP | glucose-binding protein |
| GDH | glucose dehydrogenase |
| GI | gastrointestinal |
| GIP | glucose-dependent insulinotropic polypeptide |
| GLP-1 | glucagon-like peptide-1 |
| GOx | glucose oxidase |
| HA | hyaluronic acid |
| IDDM | insulin-dependent diabetes mellitus |
| ISF | interstitial fluid |
| iPSC | inducible pluripotent stem cells |
| MARD | mean absolute relative difference |
| MWCNT | multi-walled carbon nanotubes |
| NIDDM | non-insulin-dependent diabetes mellitus |
| PBA | phenylboronic acid |
| PL | photoluminescence |
| PLGA | poly (lactic-co-glycolic acid) |
| PVA | polyvinyl alcohol |
| QD | quantum dot |
| SF | silk fibroin |
| SMBG | self-monitoring blood glucose |
| SGLT-2i | sodium–glucose cotransporter-2 inhibitors |
| SU | sulphonyl urea |
| SWCNT | single-walled carbon nanotubes |
| T1DM | Type 1 diabetes mellitus |
| T2DM | Type 2 diabetes mellitus |
| TZD | thiazolidinedione |
References
- Mukhtar, Y.; Galalain, A.; Yunusa, U. A Modern Overview on Diabetes Mellitus: A Chronic Endocrine Disorder. Eur. J. Biol. 2020, 5, 1–14. [Google Scholar] [CrossRef]
- Katsarou, A.; Gudbjörnsdottir, S.; Rawshani, A.; Dabelea, D.; Bonifacio, E.; Anderson, B.J.; Jacobsen, L.M.; Schatz, D.A.; Lernmark, Å. Type 1 diabetes mellitus. Nat. Rev. Dis. Primers 2017, 3, 17016. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers 2015, 1, 15019. [Google Scholar] [CrossRef]
- Alam, S.; Hasan, M.K.; Neaz, S.; Hussain, N.; Hossain, M.F.; Rahman, T. Diabetes Mellitus: Insights from Epidemiology, Biochemistry, Risk Factors, Diagnosis, Complications and Comprehensive Management. Diabetology 2021, 2, 36–50. [Google Scholar] [CrossRef]
- Forouhi, N.G.; Wareham, N.J. Epidemiology of diabetes. Medicine 2022, 50, 638–643. [Google Scholar] [CrossRef]
- Kumar, A.; Gangwar, R.; Zargar, A.A.; Kumar, R.; Sharma, A. Prevalence of Diabetes in India: A Review of IDF Diabetes Atlas 10th Edition. Curr. Diabetes Rev. 2024, 20, 1. [Google Scholar] [CrossRef]
- Arokiasamy, P.; Salvi, S.; Selvamani, Y. Global Burden of Diabetes Mellitus. In Handbook of Global Health; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–44. [Google Scholar] [CrossRef]
- Patil, S.R.; Chavan, A.B.; Patel, A.M.; Chavan, P.D.; Bhopale, J.V. A Review on Diabetes Mellitus its Types, Pathophysiology, Epidermiology and its Global Burden. J. Res. Appl. Sci. Biotechnol. 2023, 2, 73–79. [Google Scholar] [CrossRef]
- Goyal, Y.; Verma, A.K.; Bhatt, D.; Rahmani, A.H.; Yasheshwar; Dev, K. Diabetes: Perspective and challenges in modern era. Gene Rep. 2020, 20, 100759. [Google Scholar] [CrossRef]
- Sinclair, A.J.; Abdelhafiz, A.H.; Rodríguez-Mañas, L. Frailty and sarcopenia—Newly emerging and high impact complications of diabetes. J. Diabetes Complicat. 2017, 31, 1465–1473. [Google Scholar] [CrossRef]
- Pal, K.; Mukadam, N.; Petersen, I.; Cooper, C. Mild cognitive impairment and progression to dementia in people with diabetes, prediabetes and metabolic syndrome: A systematic review and meta-analysis. Soc. Psychiatry Psychiatr. Epidemiol. 2018, 53, 1149–1160. [Google Scholar] [CrossRef]
- Hasan, S.S.; Mamun, A.A.; Clavarino, A.M.; Kairuz, T. Incidence and Risk of Depression Associated with Diabetes in Adults: Evidence from Longitudinal Studies. Community Ment. Health J. 2015, 51, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Vaz, J.; Patnaik, A. Diabetes mellitus: Exploring the challenges in the drug development process. Perspect. Clin. Res. 2012, 3, 109. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.R.; Kashyap, A.S.; Das, S. Diabetes mellitus in India: The modern scourge. Med. J. Armed Forces India 2009, 65, 50–54. [Google Scholar] [CrossRef]
- Chaudhury, A.; Duvoor, C.; Reddy Dendi, V.S.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical Review of Antidiabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front. Endocrinol. 2017, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Consoli, A.; Czupryniak, L.; Duarte, R.; Jermendy, G.; Kautzky-Willer, A.; Mathieu, C.; Melo, M.; Mosenzon, O.; Nobels, F.; Papanas, N.; et al. Positioning sulphonylureas in a modern treatment algorithm for patients with type 2 diabetes: Expert opinion from a European consensus panel. Diabetes Obes. Metab. 2020, 22, 1705–1713. [Google Scholar] [CrossRef]
- Lv, W.; Wang, X.; Xu, Q.; Lu, W. Mechanisms and Characteristics of Sulfonylureas and Glinides. Curr. Top. Med. Chem. 2020, 20, 37–56. [Google Scholar] [CrossRef]
- Hasslacher, C. Safety and Efficacy of Repaglinide in Type 2 Diabetic Patients With and Without Impaired Renal Function. Diabetes Care 2003, 26, 886–891. [Google Scholar] [CrossRef]
- Green, J.B.; Bethel, M.A.; Armstrong, P.W.; Buse, J.B.; Engel, S.S.; Garg, J.; Josse, R.; Kaufman, K.D.; Koglin, J.; Korn, S.; et al. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 232–242. [Google Scholar] [CrossRef]
- Giaccari, A.; Solini, A.; Frontoni, S.; Del Prato, S. Metformin Benefits: Another Example for Alternative Energy Substrate Mechanism? Diabetes Care 2021, 44, 647–654. [Google Scholar] [CrossRef]
- Hundal, R.S.; Krssak, M.; Dufour, S.; Laurent, D.; Lebon, V.; Chandramouli, V.; Inzucchi, S.E.; Schumann, W.C.; Petersen, K.F.; Landau, B.R.; et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 2000, 49, 2063–2069. [Google Scholar] [CrossRef]
- Kernan, W.N.; Viscoli, C.M.; Furie, K.L.; Young, L.H.; Inzucchi, S.E.; Gorman, M.; Guarino, P.D.; Lovejoy, A.M.; Peduzzi, P.N.; Conwit, R.; et al. Pioglitazone after Ischemic Stroke or Transient Ischemic Attack. N. Engl. J. Med. 2016, 374, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- National Kidney Foundation. KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. Am. J. Kidney Dis. 2012, 60, 850–886. [Google Scholar] [CrossRef]
- Shah, M.A.; Shahnaz, T.; Din, Z.U.; Masoodi, J.; Nazir, S.; Qurashi, A.; Ahmed, G.H. Application of nanotechnology in the agricultural and food processing industries: A review. Sustain. Mater. Technol. 2024, 39, e00809. [Google Scholar] [CrossRef]
- Alhadramy, M.S. Diabetes and oral therapies: A review of oral therapies for diabetes mellitus. J. Taibah Univ. Med. Sci. 2016, 11, 317–329. [Google Scholar] [CrossRef]
- Takla, P.G. Glibenclamide. In Analytical Profiles of Drug Substances; Academic Press: Cambridge, MA, USA, 1981; pp. 337–355. [Google Scholar] [CrossRef]
- Rodbard, H.W.; Jellinger, P.S.; Davidson, J.A.; Einhorn, D.; Garber, A.J.; Grunberger, G.; Handelsman, Y.; Horton, E.S.; Lebovitz, H.; Levy, P.; et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology Consensus Panel on Type 2 Diabetes Mellitus: An Algorithm for Glycemic Control. Endocr. Pract. 2009, 15, 540–559. [Google Scholar] [CrossRef]
- Karagiannis, T.; Boura, P.; Tsapas, A. Safety of dipeptidyl peptidase 4 inhibitors: A perspective review. Ther. Adv. Drug Saf. 2014, 5, 138–146. [Google Scholar] [CrossRef]
- Cubeddu, L.; Bönisch, H.; Göthert, M.; Molderings, G.; Racké, K.; Ramadori, G.; Miller, K.; Schwörer, H. Effects of metformin on intestinal 5-hydroxytryptamine (5-HT) release and on 5-HT3 receptors. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2000, 361, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Hirst, J.A.; Farmer, A.J.; Ali, R.; Roberts, N.W.; Stevens, R.J. Quantifying the Effect of Metformin Treatment and Dose on Glycemic Control. Diabetes Care 2012, 35, 446–454. [Google Scholar] [CrossRef]
- Kahn, S.E.; Haffner, S.M.; Heise, M.A.; Herman, W.H.; Holman, R.R.; Jones, N.P.; Kravitz, B.G.; Lachin, J.M.; O’NEill, M.C.; Zinman, B.; et al. Glycemic Durability of Rosiglitazone, Metformin, or Glyburide Monotherapy. N. Engl. J. Med. 2006, 355, 2427–2443. [Google Scholar] [CrossRef]
- Inzucchi, S.E. Oral Antihyperglycemic Therapy for Type 2 Diabetes. JAMA 2002, 287, 360–372. [Google Scholar] [CrossRef]
- Martin, A.E.; Montgomery, P.A. Acarbose: An α-glucosidase inhibitor. Am. J. Health-Syst. Pharm. 1996, 53, 2277–2290. [Google Scholar] [CrossRef] [PubMed]
- Sibony, R.W.; Segev, O.; Dor, S.; Raz, I. Drug Therapies for Diabetes. Int. J. Mol. Sci. 2023, 24, 17147. [Google Scholar] [CrossRef]
- Beringer, J.; Höfer, D. Nanotechnology and its application. Melliand Int. 2004, 10, 295–296. [Google Scholar] [CrossRef]
- Zaib, S.; Iqbal, J. Nanotechnology: Applications, techniques, approaches, & the advancement in toxicology and environmental impact of engineered nanomaterials. Importance Appl. Nanotechnol. 2019, 8, 1–10. [Google Scholar]
- Scognamiglio, V. Nanotechnology in glucose monitoring: Advances and challenges in the last 10 years. Biosens. Bioelectron. 2013, 47, 12–25. [Google Scholar] [CrossRef]
- Borgmann, S.; Schulte, A.; Neugebauer, S.; Schuhmann, W. Amperometric Biosensors. Adv. Electrochem. Sci. Eng. Bioelectrochem. 2011, 13, 1–83. [Google Scholar] [CrossRef]
- Jin, S.; Veetil, J.V.; Garrett, J.R.; Ye, K. Construction of a panel of glucose indicator proteins for continuous glucose monitoring. Biosens. Bioelectron. 2011, 26, 3427–3431. [Google Scholar] [CrossRef]
- Balasubramaniyam, S.; Rathinam, T.; Srinivasan, M.; Jayarani, S.; Elumalai, K. Nanotechnology in Diabetes Management: Advancements in PLGA-Based Drug Delivery. Biomed. Mater. Devices 2025, 1–15. [Google Scholar] [CrossRef]
- Besteman, K.; Lee, J.O.; Wiertz, F.G.M.; Heering, H.A.; Dekker, C. Enzyme-coated carbon nanotubes as single-molecule biosensors. Nano Lett. 2003, 3, 727–730. [Google Scholar] [CrossRef]
- Barone, P.W.; Baik, S.; Heller, D.A.; Strano, M.S. Near-infrared optical sensors based on single-walled carbon nanotubes. Nat. Mater. 2005, 4, 86–92. [Google Scholar] [CrossRef]
- Dang, T.T.; Thai, A.V.; Cohen, J.; Slosberg, J.E.; Siniakowicz, K.; Doloff, J.C.; Ma, M.; Hollister-Lock, J.; Tang, K.M.; Gu, Z.; et al. Enhanced function of immuno-isolated islets in diabetes therapy by co-encapsulation with an anti-inflammatory drug. Biomaterials 2013, 34, 5792–5801. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Xia, C.; Lin, J.; Garalleh, H.A.L.; Alalawi, A.; Pugazhendhi, A. Carbon nanomaterials: A growing tool for the diagnosis and treatment of diabetes mellitus. Environ. Res. 2023, 221, 115250. [Google Scholar] [CrossRef]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as Drug Delivery Systems: A Review of the Implication of Nanoparticles’ Physicochemical Properties on Responses in Biological Systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef]
- Ijaz, I.; Gilani, E.; Nazir, A.; Bukhari, A. Detail review on chemical, physical and green synthesis, classification, characterizations and applications of nanoparticles. Green Chem. Lett. Rev. 2020, 13, 223–245. [Google Scholar] [CrossRef]
- Jia, L.; Jiang, Q.; He, Z.; Wang, Y. Characterization techniques: The stepping stone to liposome lyophilized product development. Int. J. Pharm. 2021, 601, 120519. [Google Scholar] [CrossRef]
- Vasile, C. Polymeric Nanomaterials. In Polymeric Nanomaterials in Nanotherapeutics; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–66. [Google Scholar] [CrossRef]
- Lu, X.-Y.; Wu, D.-C.; Li, Z.-J.; Chen, G.-Q. Polymer Nanoparticles. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2011; pp. 299–323. [Google Scholar] [CrossRef]
- Thakur, M.; Sharma, A.; Chandel, M.; Pathania, D. Modern applications and current status of green nanotechnology in environmental industry. In Green Functionalized Nanomaterials for Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 259–281. [Google Scholar] [CrossRef]
- Saleemi, M.A.; Wong, E.H. Nanoprobes for advanced nanotheranostic applications. In Advanced Nanoformulations; Elsevier: Amsterdam, The Netherlands, 2023; pp. 557–586. [Google Scholar] [CrossRef]
- Kagan, V.; Tyurina, Y.; Tyurin, V.; Konduru, N.; Potapovich, A.; Osipov, A.; Kisin, E.; Schwegler-Berry, D.; Mercer, R.; Castranova, V.; et al. Direct and indirect effects of single walled carbon nanotubes on RAW 264.7 macrophages: Role of iron. Toxicol. Lett. 2006, 165, 88–100. [Google Scholar] [CrossRef]
- Porter, A.E.; Gass, M.; Muller, K.; Skepper, J.N.; Midgley, P.A.; Welland, M. Direct imaging of single-walled carbon nanotubes in cells. Nat. Nanotechnol. 2007, 2, 713–717. [Google Scholar] [CrossRef]
- Melechko, A.V.; Merkulov, V.I.; McKnight, T.E.; Guillorn, M.A.; Klein, K.L.; Lowndes, D.H.; Simpson, M.L. Vertically aligned carbon nanofibers and related structures: Controlled synthesis and directed assembly. J. Appl. Phys. 2005, 97, 041301. [Google Scholar] [CrossRef]
- Grzybowski, B.A.; Huck, W.T.S. The nanotechnology of life-inspired systems. Nat. Nanotechnol. 2016, 11, 585–592. [Google Scholar] [CrossRef]
- Kreupl, F.; Graham, A.; Duesberg, G.; Steinhögl, W.; Liebau, M.; Unger, E.; Hönlein, W. Carbon nanotubes in interconnect applications. Microelectron. Eng. 2002, 64, 399–408. [Google Scholar] [CrossRef]
- Tisch, R.; McDevitt, H. Insulin-Dependent Diabetes Mellitus. Cell 1996, 85, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Khafagy, E.-S.; Morishita, M.; Onuki, Y.; Takayama, K. Current challenges in non-invasive insulin delivery systems: A comparative review. Adv. Drug Deliv. Rev. 2007, 59, 1521–1546. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Ang, B.C.; Andriyana, A.; Afifi, A.M. A review on fabrication of nanofibers via electrospinning and their applications. SN Appl. Sci. 2019, 1, 1248. [Google Scholar] [CrossRef]
- Disanto, R.M.; Subramanian, V.; Gu, Z. Recent advances in nanotechnology for diabetes treatment. WIREs Nanomed. Nanobiotechnology 2015, 7, 548–564. [Google Scholar] [CrossRef]
- Primavera, R.; Kevadiya, B.D.; Swaminathan, G.; Wilson, R.J.; De Pascale, A.; Decuzzi, P.; Thakor, A.S. Emerging Nano- and Micro-Technologies Used in the Treatment of Type-1 Diabetes. Nanomaterials 2020, 10, 789. [Google Scholar] [CrossRef]
- Souto, E.B.; Souto, S.B.; Campos, J.R.; Severino, P.; Pashirova, T.N.; Zakharova, L.Y.; Silva, A.M.; Durazzo, A.; Lucarini, M.; Izzo, A.A.; et al. Nanoparticle Delivery Systems in the Treatment of Diabetes Complications. Molecules 2019, 24, 4209. [Google Scholar] [CrossRef] [PubMed]
- Maleki, H.; Khoshnevisan, K.; Sajjadi-Jazi, S.M.; Baharifar, H.; Doostan, M.; Khoshnevisan, N.; Sharifi, F. Nanofiber-based systems intended for diabetes. J. Nanobiotechnology 2021, 19, 317. [Google Scholar] [CrossRef]
- Shah, S.A.; Sohail, M.; Khan, S.; Minhas, M.U.; de Matas, M.; Sikstone, V.; Hussain, Z.; Abbasi, M.; Kousar, M. Biopolymer-based biomaterials for accelerated diabetic wound healing: A critical review. Int. J. Biol. Macromol. 2019, 139, 975–993. [Google Scholar] [CrossRef]
- Mary, S.A.; Dev, V.R.G. Electrospun herbal nanofibrous wound dressings for skin tissue engineering. J. Text. Inst. 2015, 106, 886–895. [Google Scholar] [CrossRef]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef]
- Ozkan, M. Quantum dots and other nanoparticles: What can they offer to drug discovery? Drug Discov. Today 2004, 9, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Cotta, M.A. Quantum Dots and Their Applications: What Lies Ahead? ACS Appl. Nano Mater. 2020, 3, 4920–4924. [Google Scholar] [CrossRef]
- Petryayeva, E.; Algar, W.R.; Medintz, I.L. Quantum Dots in Bioanalysis: A Review of Applications across Various Platforms for Fluorescence Spectroscopy and Imaging. Appl. Spectrosc. 2013, 67, 215–252. [Google Scholar] [CrossRef]
- Jacak, L.; Wójs, A.; Hawrylak, P. Quantum Dots; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, S.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. Polymer-based nanoparticle strategies for insulin delivery. Polymers 2019, 11, 1380. [Google Scholar] [CrossRef]
- Díaz-Montes, E. Dextran: Sources, Structures, and Properties. Polysaccharides 2021, 2, 554–565. [Google Scholar] [CrossRef]
- Alipal, J.; Pu’AD, N.M.; Lee, T.; Nayan, N.; Sahari, N.; Basri, H.; Idris, M.; Abdullah, H. A review of gelatin: Properties, sources, process, applications, and commercialisation. Mater. Today Proc. 2021, 42, 240–250. [Google Scholar] [CrossRef]
- Lu, Y.; Cheng, D.; Niu, B.; Wang, X.; Wu, X.; Wang, A. Properties of Poly (Lactic-co-Glycolic Acid) and Progress of Poly (Lactic-co-Glycolic Acid)-Based Biodegradable Materials in Biomedical Research. Pharmaceuticals 2023, 16, 454. [Google Scholar] [CrossRef]
- Gaaz, T.; Sulong, A.; Akhtar, M.; Kadhum, A.; Mohamad, A.; Al-Amiery, A. Properties and Applications of Polyvinyl Alcohol, Halloysite Nanotubes and Their Nanocomposites. Molecules 2015, 20, 22833–22847. [Google Scholar] [CrossRef]
- Katchalski, E. Poly-α-Amino Acids. Adv. Protein Chem. 1951, 6, 123–185. [Google Scholar] [CrossRef]
- Zhao, R.; Lu, Z.; Yang, J.; Zhang, L.; Li, Y.; Zhang, X. Drug Delivery System in the Treatment of Diabetes Mellitus. Front. Bioeng. Biotechnol. 2020, 8, 880. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yokoyama, W.; Xu, S.; Zhu, S.; Ma, J.; Zhong, F. Formation and stability of W/O microemulsion formed by food grade ingredients and its oral delivery of insulin in mice. J. Funct. Foods 2017, 30, 134–141. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Jin, X.; Zhu, D.D.; Chen, B.Z.; Ashfaq, M.; Guo, X.D. Insulin delivery systems combined with microneedle technology. Adv. Drug Deliv. Rev. 2018, 127, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Bariya, S.H.; Gohel, M.C.; Mehta, T.A.; Sharma, O.P. Microneedles: An emerging transdermal drug delivery system. J. Pharm. Pharmacol. 2012, 64, 11–29. [Google Scholar] [CrossRef]
- Park, J.-H.; Allen, M.G.; Prausnitz, M.R. Polymer Microneedles for Controlled-Release Drug Delivery. Pharm. Res. 2006, 23, 1008–1019. [Google Scholar] [CrossRef]
- Yan, L.; Alba, M.; Tabassum, N.; Voelcker, N.H. Micro- and Nanosystems for Advanced Transdermal Delivery. Adv. Ther. 2019, 2, 1900141. [Google Scholar] [CrossRef]
- Liu, S.; Jin, M.-N.; Quan, Y.-S.; Kamiyama, F.; Katsumi, H.; Sakane, T.; Yamamoto, A. The development and characteristics of novel microneedle arrays fabricated from hyaluronic acid, and their application in the transdermal delivery of insulin. J. Control. Release 2012, 161, 933–941. [Google Scholar] [CrossRef]
- Lee, I.-C.; Wu, Y.-C.; Tsai, S.-W.; Chen, C.-H.; Wu, M.-H. Fabrication of two-layer dissolving polyvinylpyrrolidone microneedles with different molecular weights for in vivo insulin transdermal delivery. RSC Adv. 2017, 7, 5067–5075. [Google Scholar] [CrossRef]
- Demir, B.; Rosselle, L.; Voronova, A.; Pagneux, Q.; Quenon, A.; Gmyr, V.; Jary, D.; Hennuyer, N.; Staels, B.; Hubert, T.; et al. Innovative transdermal delivery of insulin using gelatin methacrylate-based microneedle patches in mice and mini-pigs. Nanoscale Horiz. 2022, 7, 174–184. [Google Scholar] [CrossRef]
- Ghaywat, S.D.; Mate, P.S.; Parsutkar, Y.M.; Chandimeshram, A.D.; Umekar, M.J. Overview of nanogel and its applications. GSC Biol. Pharm. Sci. 2021, 16, 40–61. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, X.; Guo, H.; Li, C.; Yu, D. An injectable and glucose-sensitive nanogel for controlled insulin release. J. Mater. Chem. 2012, 22, 22788–22796. [Google Scholar] [CrossRef]
- Chou, H.-S.; Larsson, M.; Hsiao, M.-H.; Chen, Y.-C.; Röding, M.; Nydén, M.; Liu, D.-M. Injectable insulin-lysozyme-loaded nanogels with enzymatically-controlled degradation and release for basal insulin treatment: In vitro characterization and in vivo observation. J. Control. Release 2016, 224, 33–42. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2013, 36 (Suppl. S1), S67–S74. [Google Scholar] [CrossRef]
- Omer, A.E.; Shaker, G.; Safavi-Naeini, S.; Kokabi, H.; Alquié, G.; Deshours, F.; Shubair, R.M. Low-cost portable microwave sensor for non-invasive monitoring of blood glucose level: Novel design utilizing a four-cell CSRR hexagonal configuration. Sci. Rep. 2020, 10, 15200. [Google Scholar] [CrossRef]
- Tang, L.; Chang, S.J.; Chen, C.-J.; Liu, J.-T. Non-Invasive Blood Glucose Monitoring Technology: A Review. Sensors 2020, 20, 6925. [Google Scholar] [CrossRef]
- Johns, B.R.; Jones, T.C.; Sink, J.H.; Cooke, C.E. Real-World Assessment of Glycemic Control After V-Go® Initiation in an Endocrine Practice in the Southeastern United States. J. Diabetes Sci. Technol. 2014, 8, 1060–1061. [Google Scholar] [CrossRef]
- Boselli, L.; Pomili, T.; Donati, P.; Pompa, P.P. Nanosensors for Visual Detection of Glucose in Biofluids: Are We Ready for Instrument-Free Home-Testing? Materials 2021, 14, 1978. [Google Scholar] [CrossRef]
- Ardakani, H.K.; Gerami, M.; Chashmpoosh, M.; Omidifar, N.; Gholami, A. Recent Progress in Nanobiosensors for Precise Detection of Blood Glucose Level. Biochem. Res. Int. 2022, 2022, 2964705. [Google Scholar] [CrossRef]
- Eckert, M.A.; Vu, P.Q.; Zhang, K.; Kang, D.; Ali, M.M.; Xu, C.; Zhao, W. Novel Molecular and Nanosensors for In Vivo Sensing. Theranostics 2013, 3, 583–594. [Google Scholar] [CrossRef]
- Vigneshvar, S.; Sudhakumari, C.C.; Senthilkumaran, B.; Prakash, H. Recent Advances in Biosensor Technology for Potential Applications—An Overview. Front. Bioeng. Biotechnol. 2016, 4, 11. [Google Scholar] [CrossRef]
- Safarkhani, M.; Aldhaher, A.; Heidari, G.; Zare, E.N.; Warkiani, M.E.; Akhavan, O.; Huh, Y.; Rabiee, N. Nanomaterial-assisted wearable glucose biosensors for noninvasive real-time monitoring: Pioneering point-of-care and beyond. Nano Mater. Sci. 2024, 6, 263–283. [Google Scholar] [CrossRef]
- Mansour, M.; Darweesh, M.S.; Soltan, A. Wearable devices for glucose monitoring: A review of state-of-the-art technologies and emerging trends. Alex. Eng. J. 2024, 89, 224–243. [Google Scholar] [CrossRef]
- Heller, A.; Feldman, B. Electrochemical Glucose Sensors and Their Applications in Diabetes Management. Chem. Rev. 2008, 108, 2482–2505. [Google Scholar] [CrossRef]
- Lee, H.; Hong, Y.J.; Baik, S.; Hyeon, T.; Kim, D. Enzyme-Based Glucose Sensor: From Invasive to Wearable Device. Adv. Healthc. Mater. 2018, 7, 1701150. [Google Scholar] [CrossRef]
- Freeman, M.H.; Hall, J.R.; Leopold, M.C. Monolayer-Protected Nanoparticle Doped Xerogels as Functional Components of Amperometric Glucose Biosensors. Anal. Chem. 2013, 85, 4057–4065. [Google Scholar] [CrossRef]
- Pham, X.; Bui, M.N.; Li, C.A.; Han, K.N.; Seong, G.H. Electrochemical Patterning of Palladium Nanoparticles on a Single-Walled Carbon Nanotube Platform and Its Application to Glucose Detection. Electroanalysis 2011, 23, 2087–2093. [Google Scholar] [CrossRef]
- Teymourian, H.; Barfidokht, A.; Wang, J. Electrochemical glucose sensors in diabetes management: An updated review (2010–2020). Chem. Soc. Rev. 2020, 49, 7671–7709. [Google Scholar] [CrossRef]
- Zierler, K. Whole body glucose metabolism. Am. J. Physiol.-Endocrinol. Metab. 1999, 276, E409–E426. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; Wang, J. Wearable non-invasive epidermal glucose sensors: A review. Talanta 2018, 177, 163–170. [Google Scholar] [CrossRef]
- Ackerman, N.; Berner, B.; Biegajski, J.; Chen, Q.; Chen, H.; Conn, T.; Dehal, H.; Dunn, T.; Ewing, A.; Fermi, S.; et al. Glucose Monitoring via Reverse Iontophoresis; ACS Publications: Washington, DC, USA, 2000; pp. 273–282. [Google Scholar] [CrossRef]
- Heikenfeld, J. Non-invasive Analyte Access and Sensing through Eccrine Sweat: Challenges and Outlook circa 2016. Electroanalysis 2016, 28, 1242–1249. [Google Scholar] [CrossRef]
- Tierney, M.J.; Tamada, J.A.; Potts, R.O.; Jovanovic, L.; Garg, S. Clinical evaluation of the GlucoWatch® biographer: A continual, non-invasive glucose monitor for patients with diabetes. Biosens. Bioelectron. 2001, 16, 621–629. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Jia, W.; Yardımcı, C.; Wang, X.; Ramirez, J.; Wang, J. Tattoo-Based Noninvasive Glucose Monitoring: A Proof-of-Concept Study. Anal. Chem. 2015, 87, 394–398. [Google Scholar] [CrossRef]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef]
- Emaminejad, S.; Gao, W.; Wu, E.; Davies, Z.A.; Nyein, H.Y.Y.; Challa, S.; Ryan, S.P.; Fahad, H.M.; Chen, K.; Shahpar, Z.; et al. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc. Natl. Acad. Sci. USA 2017, 114, 4625–4630. [Google Scholar] [CrossRef]
- Lee, H.; Choi, T.K.; Lee, Y.B.; Cho, H.R.; Ghaffari, R.; Wang, L.; Choi, H.J.; Chung, T.D.; Lu, N.; Hyeon, T.; et al. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol. 2016, 11, 566–572. [Google Scholar] [CrossRef]
- Lee, H.; Song, C.; Hong, Y.S.; Kim, M.S.; Cho, H.R.; Kang, T.; Shin, K.; Choi, S.H.; Hyeon, T.; Kim, D.-H. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Sci. Adv. 2017, 3, e1601314. [Google Scholar] [CrossRef]
- Emerich, D.F.; Thanos, C.G. The pinpoint promise of nanoparticle-based drug delivery and molecular diagnosis. Biomol. Eng. 2006, 23, 171–184. [Google Scholar] [CrossRef]
- Joseph, J.I. Review of the Long-Term Implantable Senseonics Continuous Glucose Monitoring System and Other Continuous Glucose Monitoring Systems. J. Diabetes Sci. Technol. 2021, 15, 167–173. [Google Scholar] [CrossRef]
- Heo, Y.J.; Kim, S.-H. Toward Long-Term Implantable Glucose Biosensors for Clinical Use. Appl. Sci. 2019, 9, 2158. [Google Scholar] [CrossRef]
- Steiner, M.-S.; Duerkop, A.; Wolfbeis, O.S. Optical methods for sensing glucose. Chem. Soc. Rev. 2011, 40, 4805–4839. [Google Scholar] [CrossRef]
- Garzón, V.; Pinacho, D.; Bustos, R.-H.; Garzón, G.; Bustamante, S. Optical Biosensors for Therapeutic Drug Monitoring. Biosensors 2019, 9, 132. [Google Scholar] [CrossRef]
- Adeel, M.; Rahman, M.M.; Caligiuri, I.; Canzonieri, V.; Rizzolio, F.; Daniele, S. Recent advances of electrochemical and optical enzyme-free glucose sensors operating at physiological conditions. Biosens. Bioelectron. 2020, 165, 112331. [Google Scholar] [CrossRef]
- Li, D.; Liu, Y.; Wu, N. Application progress of nanotechnology in regenerative medicine of diabetes mellitus. Diabetes Res. Clin. Pract. 2022, 190, 109966. [Google Scholar] [CrossRef]
- Ernst, A.U.; Bowers, D.T.; Wang, L.-H.; Shariati, K.; Plesser, M.D.; Brown, N.K.; Mehrabyan, T.; Ma, M. Nanotechnology in cell replacement therapies for type 1 diabetes. Adv. Drug Deliv. Rev. 2019, 139, 116–138. [Google Scholar] [CrossRef]
- Aguayo-Mazzucato, C.; Bonner-Weir, S. Pancreatic β Cell Regeneration as a Possible Therapy for Diabetes. Cell Metab. 2018, 27, 57–67. [Google Scholar] [CrossRef]
- Oran, D.C.; Gokulu, I.S.; Kizilel, S. Nanoengineered biomaterials for pancreas regeneration. In Nanoengineered Biomaterials for Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 443–457. [Google Scholar] [CrossRef]
- Cierpka-Kmiec, K.; Wronska, A.; Kmiec, Z. In vitro generation of pancreatic β-cells for diabetes treatment. I. β-like cells derived from human pluripotent stem cells. Folia Histochem. Cytobiol. 2015, 57, 1–14. [Google Scholar] [CrossRef]
- Xiao, W.; Jiang, W.; Chen, Z.; Huang, Y.; Mao, J.; Zheng, W.; Hu, Y.; Shi, J. Advance in peptide-based drug development: Delivery platforms, therapeutics and vaccines. Signal Transduct. Target. Ther. 2025, 10, 74. [Google Scholar] [CrossRef]
- Vahl, T.P.; D’Alessio, D.A. Gut peptides in the treatment of diabetes mellitus. Expert Opin. Investig. Drugs 2004, 13, 177–188. [Google Scholar] [CrossRef]
- Todd, J.F.; Bloom, S.R. Incretins and other peptides in the treatment of diabetes. Diabet. Med. 2007, 24, 223–232. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.E.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef]
- Frías, J.P.; Davies, M.J.; Rosenstock, J.; Pérez Manghi, F.C.; Fernández Landó, L.; Bergman, B.K.; Liu, B.; Cui, X.; Brown, K. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 503–515. [Google Scholar] [CrossRef]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef]
- Anson, M.; Zhao, S.S.; Austin, P.; Ibarburu, G.H.; Malik, R.A.; Alam, U. SGLT2i and GLP-1 RA therapy in type 1 diabetes and reno-vascular outcomes: A real-world study. Diabetologia 2023, 66, 1869–1881. [Google Scholar] [CrossRef]
- Hope, S.V.; Jones, A.G.; Goodchild, E.; Shepherd, M.; Besser, R.E.J.; Shields, B.; McDonald, T.; Knight, B.A.; Hattersley, A. Urinary C-peptide creatinine ratio detects absolute insulin deficiency in Type 2 diabetes. Diabet. Med. 2013, 30, 1342–1348. [Google Scholar] [CrossRef]
- Maddaloni, E.; Bolli, G.B.; Frier, B.M.; Little, R.R.; Leslie, R.D.; Pozzilli, P.; Buzzetti, R. C-peptide determination in the diagnosis of type of diabetes and its management: A clinical perspective. Diabetes Obes. Metab. 2022, 24, 1912–1926. [Google Scholar] [CrossRef]
- Ekberg, K.; Johansson, B.L. Effect of C-Peptide on Diabetic Neuropathy in Patients with Type 1 Diabetes. Exp. Diabetes Res. 2008, 2008, 457912. [Google Scholar] [CrossRef]
- Patil, S.P.; Goswami, A.; Kalia, K.; Kate, A.S. Plant-Derived Bioactive Peptides: A Treatment to Cure Diabetes. Int. J. Pept. Res. Ther. 2020, 26, 955–968. [Google Scholar] [CrossRef]
- Khalily, M.P.; Soydan, M. Peptide-based diagnostic and therapeutic agents: Where we are and where we are heading? Chem. Biol. Drug Des. 2023, 101, 772–793. [Google Scholar] [CrossRef]
- American Diabetes Association. 11. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes—2021. Diabetes Care, 2021; 44, (Suppl. S1), S151–S167. [CrossRef]
- Ellahham, S. Artificial Intelligence: The Future for Diabetes Care. Am. J. Med. 2020, 133, 895–900. [Google Scholar] [CrossRef]
- Jin, X.; Liu, C.; Xu, T.; Su, L.; Zhang, X. Artificial intelligence biosensors: Challenges and prospects. Biosens. Bioelectron. 2020, 165, 112412. [Google Scholar] [CrossRef]
- Fuchs, J.; Hovorka, R. Closed-loop control in insulin pumps for type-1 diabetes mellitus: Safety and efficacy. Expert Rev. Med. Devices 2020, 17, 707–720. [Google Scholar] [CrossRef]
- Boughton, C.K.; Hovorka, R. Is an artificial pancreas (closed-loop system) for Type 1 diabetes effective? Diabet. Med. 2019, 36, 279–286. [Google Scholar] [CrossRef]
- Jackson, M.; Castle, J.R. Where Do We Stand with Closed-Loop Systems and Their Challenges? Diabetes Technol. Ther. 2020, 22, 485–491. [Google Scholar] [CrossRef]
- Vettoretti, M.; Cappon, G.; Facchinetti, A.; Sparacino, G. Advanced Diabetes Management Using Artificial Intelligence and Continuous Glucose Monitoring Sensors. Sensors 2020, 20, 3870. [Google Scholar] [CrossRef]
- Pérez-Gandía, C.; Facchinetti, A.; Sparacino, G.; Cobelli, C.; Gómez, E.; Rigla, M.; de Leiva, A.; Hernando, M. Artificial Neural Network Algorithm for Online Glucose Prediction from Continuous Glucose Monitoring. Diabetes Technol. Ther. 2010, 12, 81–88. [Google Scholar] [CrossRef]
- Simkó, M.; Mattsson, M.O.; Yokel, R.A. Neurological system. In Adverse Effects of Engineered Nanomaterials: Exposure, Toxicology, and Impact on Human Health, 2nd ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 275–312. [Google Scholar] [CrossRef]
- Riehemann, K.; Schneider, S.W.; Luger, T.A.; Godin, B.; Ferrari, M.; Fuchs, H. Nanomedicine—Challenge and perspectives. Angew. Chem.-Int. Ed. 2009, 48, 872–897. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Sun, B.; Chen, C. Understanding the toxicity of carbon nanotubes. Accounts Chem. Res. 2013, 46, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.K. Past, Present, and Future of Continuous Glucose Monitors. Diabetes Technol. Ther. 2023, 25, S1–S4. [Google Scholar] [CrossRef] [PubMed]
- The Diabetes Research in Children Network (DirecNet) Study Group. Youth and Parent Satisfaction with Clinical Use of the GlucoWatch G2 Biographer in the Management of Pediatric Type 1 Diabetes. Diabetes Care 2005, 28, 1929–1935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chan, H.W.; Shao, Z.; Wang, Q.; Chow, S.; Chow, S.F. Navigating translational research in nanomedicine: A strategic guide to formulation and manufacturing. Int. J. Pharm. 2025, 671, 125202. [Google Scholar] [CrossRef] [PubMed]
- Yasmeen; Mamatha, T.; Zubair, M.; Begum, S.; Muneera, T. Various Emerging Trends in Insulin Drug Delivery Systems. Br. J. Pharm. Res. 2015, 5, 294–308. [Google Scholar] [CrossRef]
- Testa, M.A.; Simonson, D.C.; Turner, R.R. Valuing Quality of Life and Improvements in Glycemic Control in People With Type 2 Diabetes. Diabetes Care 1998, 21 (Suppl. S3), C44–C52. [Google Scholar] [CrossRef]
- Saffran, M.; Kumar, G.S.; Savariar, C.; Burnham, J.C.; Williams, F.; Neckers, D.C. A New Approach to the Oral Administration of Insulin and Other Peptide Drugs. Science 1986, 233, 1081–1084. [Google Scholar] [CrossRef] [PubMed]
- Naftanel, M.A.; Harlan, D.M. Pancreatic Islet Transplantation. PLoS Med. 2004, 1, e58. [Google Scholar] [CrossRef]
- Baron, P.F.; Escalante-Pulido, M. Insulin analogues. Med. Interna Mexico 2007, 23, 310–320. [Google Scholar]


| Category | Drug | Dose | Food Effect | Side Effect | Reference |
|---|---|---|---|---|---|
| Glinides | Repaglinide | 0.6–4 mg | Take 30 min before meals | Drug interactions, upper respiratory infections. | [23] |
| Sulfonylureas | Glibenclamide | 1.25–20 mg/day | Taken with food | Increased body weight, hypoglycaemia | [25,26,27] |
| Glipizide | 2.5–40 mg/day | 30 min before meals | |||
| Gliclazide | Max 15 mg/day | Taken with food | |||
| Dipeptidyl peptidase-4 inhibitors | Sitagliptin | 100 mg daily | Not affected | Angioedema, pancreatitis | [28] |
| Saxagliptin | 2.5–5 mg | Not affected | |||
| Vildagliptin | 50 mg BID | Not affected | |||
| Biguanides | Metformin (IR) | 500 mg BID, Max 3 g | During or after meals | GI disorders, vitamin B12 deficiency, lactic acidosis | [29,30] |
| Metformin (XR) | 750 mg OD, Max 2 g | With evening meals | |||
| Thiazolidinediones | Pioglitazone | 15–45 mg once/day | Not affected | Increased Body weight, anaemia | [31] |
| Acarbose | 25–100 mg | At the start of every meal | Hypoglycaemia, URTI | [32,33] | |
| Sodium–glucose cotransporter-2 inhibitors | Dapagliflozin | 10 mg OD | Irrespective of food | DKA, UTI, hypovolemia | [25,34] |
| Empagliflozin | 10–25 mg OD | Irrespective of food |
| Nanoparticle Type | Properties | References |
|---|---|---|
| Liposomes | Lipid bilayer-encapsulated spherical structures are designed to protect medications from deterioration. They are effortlessly functionalised with targeted segments for a particular delivery and are biocompatible. | [47] |
| Dendrimers | Potentially useful as drug loading systems, these nanoscale polymers are monodispersed and highly branched. They come in a range of sizes, surface qualities, and drug-delivery capacities, making them highly customisable. | [48] |
| Polymeric NPs | Made from biodegradable polymers that can shield medications from deterioration by encasing them. They are frequently employed for the long-term, sustained delivery of medications. | [49] |
| Metal NPs | They are appealing for application in drug delivery because of their distinct optical, electrical, and thermal characteristics, such as those of gold nanoparticles (AuNP) and silver nanoparticles (AgNP). Targeting moieties can functionalise them for a particular delivery method. | [50] |
| Solid Lipid NPs | They are used for packing hydrophobic medications and are composed of solid lipids. Compared to other kinds of nanoparticles, they have several benefits, such as increased bioavailability, stability, and biocompatibility. | [51] |
| Polymer Type | Polymer Name | Route of Administration | Key Properties | Examples of Nanoparticles | Reference |
|---|---|---|---|---|---|
| Natural Polymer | Chitosan | Oral, Nasal | Biocompatibility, antibacterial and antifungal activity, mucoadhesiveness, varying degrees of crosslinking | Reduced gold nanoparticle systems; chitosan polyelectrolyte complex-based nanoparticles | [71] |
| Alginate | Oral | Film-forming ability, gelling capability, hydrophilicity, biodegradability, non-toxicity, ionic crosslinking | Alginate–chitosan–beta-cyclodextrin nanoparticles; alginate–chitosan polyelectrolyte complex nanoparticles; alginate–chitosan-coated nanoparticles | [72] | |
| Dextran | Oral | High branching potential, water solubility, biocompatibility | Dextran–alginate sulphate nanoparticles with chitosan and albumin coating | [73] | |
| Gelatin | Oral, Pulmonary | Biocompatibility, gel-forming ability, adhesiveness, water solubility | Gelatine–glutaraldehyde nanoparticles; gelatine–poloxamer-based nanoparticles | [74] | |
| Synthetic Polymer | Poly (Lactic-co-Glycolic Acid) (PLGA) | Oral, Intraperitoneal, Injectable | Biodegradable copolymer of lactic and glycolic acid; good biocompatibility; non-toxicity; plasticity | zinc–insulin-loaded PLGA nanoparticles; PLGA nanoparticles; PLGA–chitosan conjugated nanoparticles | [75] |
| Polyvinyl Alcohol | Transdermal, Oral | Derived from the hydrolysis of polyvinyl acetate; biodegradable by microorganisms | Polyvinyl alcohol–chitosan hydrogel loaded with insulin; polyvinyl alcohol nanoparticles | [76] | |
| Polyamino Acids | Oral | Obtained by polymerisation of amino acids or derivatives; customisable monomeric structure; high compatibility | Chitosan and poly-gamma-glutamic acid nanoparticles; gelatine-coated chitosan/poly-gamma-glutamic acid nanoparticles; L-valine/poly(butyl acrylate) nanoparticles | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaushal, A.; Musafir, A.; Sharma, G.; Rani, S.; Singh, R.K.; Kumar, A.; Bhadada, S.K.; Barnwal, R.P.; Singh, G. Revolutionizing Diabetes Management Through Nanotechnology-Driven Smart Systems. Pharmaceutics 2025, 17, 777. https://doi.org/10.3390/pharmaceutics17060777
Kaushal A, Musafir A, Sharma G, Rani S, Singh RK, Kumar A, Bhadada SK, Barnwal RP, Singh G. Revolutionizing Diabetes Management Through Nanotechnology-Driven Smart Systems. Pharmaceutics. 2025; 17(6):777. https://doi.org/10.3390/pharmaceutics17060777
Chicago/Turabian StyleKaushal, Aayush, Aanchal Musafir, Gourav Sharma, Shital Rani, Rajat Kumar Singh, Akhilesh Kumar, Sanjay Kumar Bhadada, Ravi Pratap Barnwal, and Gurpal Singh. 2025. "Revolutionizing Diabetes Management Through Nanotechnology-Driven Smart Systems" Pharmaceutics 17, no. 6: 777. https://doi.org/10.3390/pharmaceutics17060777
APA StyleKaushal, A., Musafir, A., Sharma, G., Rani, S., Singh, R. K., Kumar, A., Bhadada, S. K., Barnwal, R. P., & Singh, G. (2025). Revolutionizing Diabetes Management Through Nanotechnology-Driven Smart Systems. Pharmaceutics, 17(6), 777. https://doi.org/10.3390/pharmaceutics17060777







