The present research paper provides evidence related to physicochemical properties, stability, antioxidant capacity, and release kinetics of developed carob extract-loaded liposomes, as well as the influence of UV irradiation and sonication on the mentioned variables.
3.1. Encapsulation Efficiency of Developed Liposomal Vesicles
With the aim of investigating the success of the encapsulation process of active principles from carob extract in developed liposomal formulations, liposome encapsulation efficiency was quantified, and the data are presented in
Table 1. The mass of encapsulated carob polyphenols was determined indirectly by measuring the concentration of non-encapsulated polyphenols in the supernatant of developed liposome suspensions.
Carob polyphenols were successfully encapsulated in liposomes employing the proliposome technique, with an encapsulation efficiency of 80.59 ± 1.29% (
Table 1). The data shown in
Table 1 also indicate that post-preparation modifications significantly changed (decreased) encapsulation efficiency. Namely, the encapsulation efficiency was 74.99 ± 1.02% in the UV-irradiated formulation and 71.05 ± 1.34% in the sonicated formulation (
Table 1). Various studies on the encapsulation of plant extracts in liposomes report encapsulation efficiency in a wide range, from ~40% to ~95%, depending on liposome composition and encapsulated plant-origin compounds [
11,
18,
19,
26,
27]. For example, in our previous study, the entrapment efficiency of ethanol rosehip extract in multilamellar liposomes was 90.8% [
21], while similar values were found for the encapsulation of turmeric extract (over 85%) [
27]. However, the encapsulation efficiency of carob polyphenols in the present study was lower and similar to the values obtained for betel ethanolic extract (71.8–79.9%) [
28], palm seed extract (71.0–86.8%) [
19], pineapple mint extract (73.3–77.5%), and pokeweed extract (81.4–84.0%) encapsulated in liposomes [
29]. The obtained differences are expected due to the proven significant impact of phospholipid and/or sterol contents on encapsulation efficiency [
30]. According to the data in the literature, increasing the phospholipid concentration enhances the incorporation capacity for plant extract within liposomal vesicles [
26]. Hence, several studies have reported that in the case of decreased concentration of phosphatidylcholine, the liposomal particles available for the encapsulation of phenolic compounds were limited. For example, in the studies of Takahashi et al. [
27] and Lu et al. [
17], the encapsulation efficiency of plant extracts increased with the increase in lecithin concentration. Since the concentration of phospholipids in developed liposomes with carob extract was high (5%), it can explain the high values of the entrapment efficiency for carob polyphenols.
Furthermore, the stability of liposome particles can be reduced or degraded because of lipid or other compound breakdown or oxidation caused by exposure to UV light. It can alter the liposomal bilayer structure by affecting the interactions among hydrophobic and hydrophilic regions [
31]. The mentioned alterations can trigger modifications in the liposome’s stability, permeability, and other characteristics as well. Therefore, the changes in the encapsulation efficiency value after UV treatment were also expected. Namely, the decrease in the encapsulation efficiency of carob polyphenols after irradiation by UV light can be explained by the leakage of encapsulated compounds upon irradiation. UV irradiation is known to generate reactive oxygen species (ROS) that can make pores on the phospholipid bilayer, causing UV-induced liposome damage, i.e., a significant leakage rate, which was already shown in the literature [
32]. FT-IR analysis of liposomes with carob extract (shown in
Section 3.4) also confirms the occurrence of oxidation/peroxidation processes after UV light exposure. Additionally, the destabilization of the phospholipid bilayer and, consequently, release of the entrapped bioactives can occur due to the polymerizable domain contraction or polymerized domain distortion because UV lights directly initiate the phospholipid polymerization [
32]. After irradiation, covalent bonds are created in the hydrophobic tails, binding the polymerizable lipids together. Also, the polymerized domain cannot be incorporated into the phospholipid bilayer membrane, forming micelles or disks with unfavorable interactions among the hydrophilic head groups (from the poly (lipid) domains) and the lipophilic tails (from the phospholipid bilayer membrane). Thus, upon UV light treatment, diffusion of the poly (lipid) domain induces the leakage of the encapsulated active principles [
32].
Lower encapsulation efficiency after the sonication of carob extract-loaded liposomes was most likely influenced by the breakdown of liposome membranes under the sonication method via the cavitation mechanism. Further, polyphenols entrapped between the phospholipid bilayers may be partially released, which could result in decreased encapsulation efficiency [
33]. Chotphruethipong et al. study [
33] showed that higher levels of amplitude contributed to the higher reduction in liposome encapsulation efficiency at all employed periods of the sonication process. In the present study, a 70% amplitude of the ultrasound probe was employed. Machado et al. [
34] have also reported that the liposomal vesicles subjected to homogenization provided 10% higher encapsulation efficiency in comparison to those subjected to ultrasound waves, as in the case of liposomes with carob extract. The mentioned results can be due to the excessive amount of energy delivered to the liposomal system, disrupting the liposomal particles [
33,
35]. Additionally, the temperature in the system increased during the sonication process (despite the ice coating of the flask with the liposome sample), consequently increasing the liposome membrane fluidity and inducing a higher polyphenol release from the liposomal vesicles, thus lowering the encapsulation efficiency [
35]. The two key limiting factors for the utilization of ultrasound waves in the preparation of liposomes are low and restricted encapsulation efficiency, as well as the lack of reproducibility throughout various systems.
After 60 days of storage, encapsulation efficiency did not change in non-treated liposomes (78.84 ± 1.95%,
Table 1), while a significant drop was noticed in UV-treated and particularly in sonicated liposomes (69.51 ± 0.91% and 48.62 ± 1.78%, respectively). The obtained results were expected due to the above-mentioned changes that occur during post-processing treatments, resulting in a more fluid membrane, as well as facilitated leakage of carob polyphenols from liposomes.
3.2. Size, Size Distribution, Mobility, and Zeta Potential of Developed Liposomal Vesicles
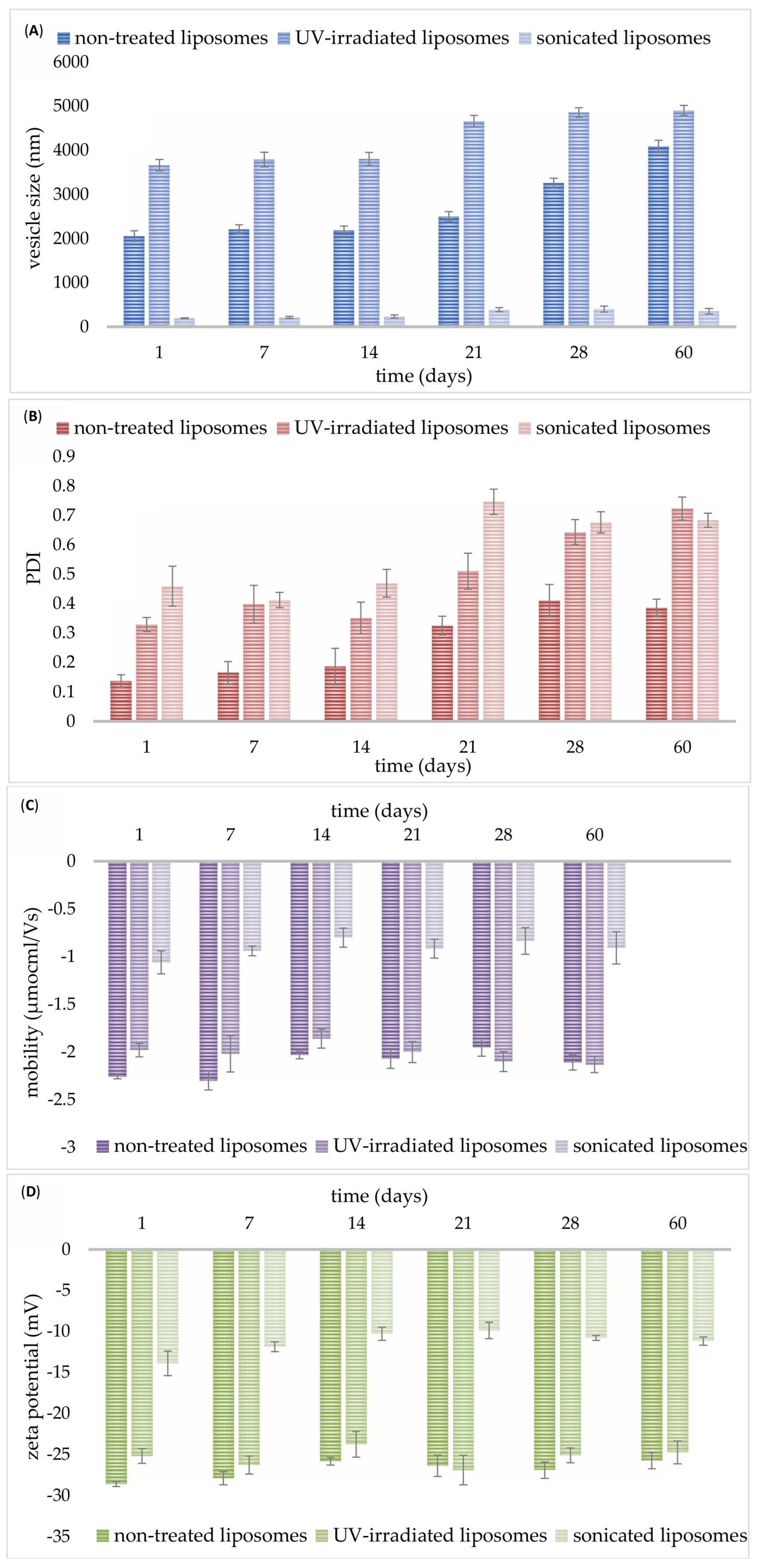
As can be seen from
Figure 2A, on the 1st day, non-treated liposomes with carob extract had a diameter of 2055 ± 120 nm, while UV irradiation treatment caused a significant increase in the mentioned parameter (3662 ± 131 nm). As expected, the sonication treatment significantly reduced the size of developed liposomes with carob extract up to 194.0 ± 11.0 nm (
Figure 2A). A graphical presentation of row data obtained using the dynamic light scattering method is shown in
Figures S1–S3. On the 1st day, the PDI value for the non-treated sample was low (0.137 ± 0.021), whereas both modification treatments resulted in an increase in the PDI values of the liposomal system, 0.329 ± 0.024 (for UV-irradiated) and 0.459 ± 0.068 (for sonicated) (
Figure 2B). The mobility was −2.26 ± 0.02 µmcm/Vs for non-treated, −1.98 ± 0.07 µmcm/Vs for UV-irradiated, and −1.06 ± 0.12 µmcm/Vs for sonicated liposomes on the 1st day (
Figure 2C). The zeta potential possessed negative values for all developed liposomal systems: −28.6 ± 0.3 mV for non-treated, −25.2 ± 0.9 mV for UV-irradiated, and −13.9 ± 1.5 mV for sonicated liposomes (measured immediately after the preparation,
Figure 2D). Both UV light and ultrasound waves cause a significant drop in the mobility and zeta potential of liposomes with carob extract.
The development of large liposomes with carob extract can be explained by the presence of carob polyphenols. Namely, polyphenols have a negative charge; therefore, higher extract content results in increased electrical repulsive forces and liposome size, consequently causing structural instability of developed lipid particles [
11]. In the case of higher encapsulation efficiency (which was also in carob extract-loaded liposomes), with a decreasing volume of liposome core, the number of polyphenol compounds located interior liposomal bilayer was high, and accordingly, the diameter of the liposomal particles increased [
11,
17]. The study of Jahanfar et al. [
11] also showed that the placement of non-polar compounds in the liposomal bilayer can affect the increase in vesicle diameter, as well as a rupture in their membrane, which can also result in enhanced permeability. The obtained value of liposome diameter was in agreement with our previous study, where rosehip oil-loaded liposome size was also high, 2145.7 nm [
36]. However, the size of natural extracts-loaded liposomes after sonication at 70% amplitude for 30 min (as in the case of sonicated liposomes with carob extract) was significantly lower (61.4 nm) compared to our result (194 nm). The reason can lie in differences in lipid compounds and their concentration, the nature of the extracts, and the initial vesicle size. Nevertheless, sonicated grape pomace extract-loaded liposomes had a size of 245 ± 26 nm [
37], while the ginger ethanolic extract loaded-nanoliposome size was 164.5 nm without sonication [
16]. The increased liposome size under UV irradiation was also proven in the literature, suggesting the liposome photochemical destruction across the photon energy absorption, which results in drastic alterations of the bilayer conformation [
38]. UV light exposure triggers the changes in the physical characteristics of phospholipid bilayers via disturbing phospholipid order and packing, thus inducing a size increase, as well as modifications of membrane permeability [
38]. Additionally, as a result of the dominant effect of UV irradiation on the liposome membrane damage, i.e., photodegradation, highly disordered chains of polyunsaturated fatty acids and consequently exposed hydrophobic patches can promote vesicle aggregation as well [
38]. According to Freitas and Müller [
39], the introduction of energy, including temperature or light, to the liposome system led to vesicle growth accompanied by a lowering in zeta potential values (which is shown for carob extract-loaded liposomes as well) and gelation.
The measured value of size distribution agrees with the study of Ganji et al. [
16], where the PDI of liposomes with ginger extract amounted to 0.186. PDI values equal to or less than 0.2 are acceptable for polymer-based carriers, while in applications of liposomes for drug delivery, PDI values of 0.3 and below can be acceptable. Additionally, post-formation processing of liposomes with carob extract (UV irradiation and sonication) significantly affected the mentioned variable, causing its increase. The measured values agree with the literature data, where the PDI values of sonicated liposomes with encapsulated doum fruit extract were between 0.425 and 0.469 [
40], and for sonicated rosehip oil-loaded liposomes, PDI was 0.439 [
36], as in the case of sonicated liposomes with carob extract. The highest PDI was recorded for the sonicated sample, which indicates the presence of multilamellar vesicles along with small unilamellar vesicles. It was also shown in a graphical presentation of row data obtained using the DLS method for sonicated carob extract-loaded liposomes (
Figure S3A), where apart from small unilamellar vesicles (size of around 100 nm), peaks after 1000 nm appear as well, causing an increase in the PDI values. Moreover, larger liposomes (with vesicle sizes exceeding 1000 nm) tended to exhibit lower PDI values compared to smaller liposome populations (ranging from 100 to 400 nm in size) [
41]. Zeta potential serves as a crucial parameter for assessing the colloidal stability of liposomal dispersions, as it reflects the extent of electrostatic repulsion between nanoparticles within the system. Particles with high absolute zeta potential values, whether positive or negative, are well stabilized due to strong repulsive forces, whereas those with low zeta potentials are more prone to aggregation or flocculation [
42]. Since the absolute values of the mentioned variables were the lowest for sonicated samples (shown later in
Figure 2D), this can explain the formation of aggregates, apart from smaller vesicles, which resulted in a higher size distribution, i.e., PDI values. Lower mobility values for sonicated vesicles (shown later in
Figure 2C) also confirm this claim of the aggregate occurrence, since aggregates show reduced mobility. UV light exposure also significantly increased the size heterogeneity of the liposomal system with carob extract, which can be explained by the above-mentioned modifications of the membrane conformation and liposome size, as well as the potential aggregation of lipid particles [
38].
The liposome mobility measurement is important to evaluate their stability in an aqueous medium. Namely, high mobility values lead to a repulsion among liposomal vesicles, preventing vesicle aggregation and proving liposome system stability [
43]. Additionally, mobility value is important to formulate adequate drug delivery systems (including liposomes) under optimized encapsulation conditions, figure out mechanisms of drug release, and predict their behavior in vivo [
44]. According to the literature data, the values of liposome mobility varied in a wide range depending on the used phospholipids, encapsulated compounds, and liposome size [
12,
43]. The data of carob extract-loaded liposome mobility shows a negative value of mobility because phospholipids are negatively charged. Jacquot et al. study [
43] demonstrated that higher mobility possessed larger liposomes, which was also the case with non-treated and UV-treated liposomes with carob extract (larger liposomes with higher mobility in comparison to smaller sonicated parallels). Furthermore, UV-irradiated and sonicated liposomes possessed a significantly lower value of encapsulation efficiency; therefore, the non-encapsulated fraction of carob polyphenols can be located on the outer surface of liposomes due to proven interactions between lipids and polyphenols [
45], which consequently can reduce the liposome mobility.
Zeta potential, as one of the main parameters in the investigation of the stability of liposomal formulations, should be higher than +30 mV or lower than −30 mV with the aim of preventing or minimizing liposome aggregation and fusion of liposomal particles [
11,
17]. Hence, higher values of zeta potential of the liposomal system demonstrate its higher stability due to more repulsive forces that prevent vesicle aggregation and fusion. However, the measured zeta potential of all developed liposomes with carob extract was at a middle level. A wide range of different zeta potential values obtained in numerous studies depends on various characteristics of phospholipid molecules used in liposome structures [
11]. In addition, circumferential temperature and ionic power affect the positive and negative values of zeta potential. In the case of lower circumference ionic power, phosphatidyl groups are located in the outer part of the polar head of phospholipids, resulting in a negative zeta potential of vesicles [
11,
17], as in our study. As outlined by Ben-Fadhel et al. [
46], sonication treatment significantly decreased the zeta potential of natural extracts-loaded liposomes by 6–10 mV, as in the case of carob extract-loaded liposomes. Freitas and Müller’s study [
39] reported that the consequence of changing the liposomal system after UV irradiation is the reduction in the values of zeta potential, which can also be the reason for a higher possibility of aggregation occurrence. Namely, supported by a decreased zeta potential, i.e., sufficient reduction in repulsive forces, lipid vesicles may interact and form a gel network [
38].
Figure 2A also shows that in the first 14 days of storage, the diameter of all developed liposomes did not change (2187 ± 96 nm for non-treated, 3805 ± 147 nm for UV-treated, and 236 ± 35 nm for sonicated liposomes). However, up to the 60th day, the size of liposomal particles increased and amounted to 4085 ± 139 nm for non-treated, 4902 ± 114 nm for UV-irradiated, and 354 ± 62.5 nm for sonicated liposomes. PDI values did not alter during a two-week period as well: 0.186 ± 0.062 for non-treated, 0.351 ± 0.054 for UV-irradiated, and 0.469 ± 0.045 for sonicated sample, while changes were noticed on the 21st day of the measurement: 0.325 ± 0.032, 0.510 ± 0.061 for UV-irradiated, and 0.746 ± 0.043, respectively (
Figure 2B). In higher phospholipid concentrations (as in carob extract-loaded liposomes), more phospholipid molecules entered into each liposomal particle; thus, the diameter and instability of the liposome structure increased [
11,
30]. Namely, the employment of high phospholipid content reduced their solubility and enhanced the possibility of phospholipid aggregation because of the more consumed process energy necessary for the distribution of phospholipids compared to smaller vesicles [
30]. Additionally, the drop in the viscosity values of developed carob extract-loaded liposomes after storage (shown in
Table 1) can be responsible for liposome system instability: alteration/increase in the particle size, i.e., potential aggregation of lipid vesicles, and consequently creating a more heterogeneous system, i.e., enhancement in the values of polydispersity [
47,
48].
Mobility and zeta potential values did not modify in the first 7 days of storage (
Figure 2C and
Figure 2D, respectively) but lower values were measured after 14 days of storage at 4 °C (−2.03 ± 0.04 µmcm/Vs and −25.8 ± 0.5 mV for non-treated, −1.80 ± 0.10 µmcm/Vs and −23.8 ± 1.56 mV for UV-irradiated, as well as −0.80 ± 0.10 µmcm/Vs and −10.3 ± 0.8 mV for sonicated particles). After that period, no additional changes in the zeta potential values of all developed liposomes were noticed on the 60th day (−25.7 ± 1.0 mV for non-treated, −24.7 ± 1.4 mV for UV-irradiated, and −11.2 ± 0.5 mV for sonicated particles). However, during the 60-day storage study, zeta potential values of post-processing modified liposomes varied in a narrow range in comparison to the non-treated sample. The same trend was observed for the mobility after 60 days: −2.11 ± 0.08 µmcm/Vs for non-treated, −2.00 ± 0.09 µmcm/Vs for UV-treated, and −0.91 ± 0.17 mV for sonicated systems. Wolfram et al.’s study [
12] also showed a slight alteration in the mobility and zeta potential of phospholipid liposomes after two weeks. The fact that the noticed alterations in mobility and zeta potential of liposomes with carob extract (after 7 days of storage) had no effect on the liposome size (without significant changes during 14 days) can be explained by the more significant contribution of zeta potential to the liposomal mobility than to the liposome size.
3.4. Rheological Properties of Developed Liposomal Formulations
The viscosity of the liposomal system has an essential role in its long-term storage and represents a relevant factor of stability and controlled release of encapsulated components [
49]. Therefore, the viscosity of developed liposomal formulations with carob extract was measured on the 1st and 60th days of storage at 4 °C. Initial data on viscometry measurements are presented in
Table S1. The results are presented as mean ± standard deviation in
Table 1. The values of viscosity of non-treated and UV-irradiated liposomes with carob extract amount to 18.40 ± 1.22 mPa·s and 16.90 ± 0.85 mPa·s, respectively, while the sonicated sample showed significantly lower viscosity (5.17 ± 0.09 mPa·s). The study of Nareni et al. [
48] showed that liposomes of smaller size possessed a lower viscosity, as in the case of sonicated carob extract-loaded liposomes. Karaz and Senses [
49] also reported that the liposomes with a high fraction of multilamellar structures had increased viscosity, while the viscosity was lower for the unilamellar vesicles due to the reduction in volume fraction. According to the literature data, the increased particle size of liposome formulations can result in increased viscosity and, hence, higher PDI values. Namely, an increase in liposome size can induce the formation of complex linkages and crystallinity of particles in the central core because of their solid property, contributing to higher viscosity values of the liposome system. Higher concentrations of phospholipids in liposomal formulation, such as lipid-based vesicles (which is also the case with developed carob extract-loaded liposomes), can lead to higher aggregation because of decreased steric stabilization and increased molecular interactions, consequently resulting in a higher viscosity value [
47]. Changes in the liposome viscosity can also induce changes in liposome stability, potential separation or sedimentation, Brownian motion of particles in the system, delivery of bioactives from liposomes, etc. [
48]. As can be seen from the results of the stability study (
Figure 2), in all liposome samples (with lower and higher viscosity values), alterations in physical properties, such as size and size distribution, have occurred during storage. Hence, during the 60-day storage at 4 °C, the viscosity of developed liposomes significantly decreased: 13.93 ± 0.82 mPa·s for non-treated, 14.20 ± 1.03 mPa·s for UV-irradiated, and 4.46 ± 0.14 mPa·s for sonicated samples (
Table 1). The decrease in viscosity of all developed liposomal systems can be attributed to the occurrence of the reactions of hydrolysis that are specific to the water environment, also presented in carob extract-loaded liposomes [
36].
Surface tension has an important role in interfacial dynamics, water penetration within the phospholipid membrane bilayers, as well as lateral phospholipid packing of liposomes [
50]. Therefore, the surface tension of developed liposomal preparations with carob extract was determined on the 1st and 60th days of storage at 4 °C (
Table 1). Initial data on tensiometry measurements are presented in
Table S1. The surface tension of non-treated and UV-irradiated liposomes was 41.75 ± 1.98 mN/m and 39.57 ± 1.06 mN/m, respectively, while the sonicated liposomes possessed a significantly lower surface tension value, 24.83 ± 1.27 mN/m. The surface tension of all prepared liposomes altered during 60 days, showing a decreasing trend. Namely, the measured values were significantly lower in comparison to initial measurements and amounted to 30.20 ± 0.93 mN/m (non-treated), 29.27 ± 1.00 mN/m (UV-treated), and 22.03 ± 0.92 mN/m (sonicated) (
Table 1). The presence of various ingredients in water surroundings can trigger structural changes in aqueous mediums. Depending on their influence on the water hydrogen-bonded networks, the mentioned compounds are classified as “structure breakers” and “structure makers” [
50]. “Structure breakers” accumulate in the interface and decrease surface tension, while “structure makers” increase the surface free energy, decrease the interfacial area, and consequently increase surface tension compared to the bulk phase. Since the measured surface tension of pure water (the medium used for the liposome preparation) was 69.9 ± 1.16 mN/m, phospholipids belong to the class of “structure breakers” because the surface tension of developed liposomes with carob extract was significantly lower in comparison to water. The obtained phenomenon is in agreement with the literature data, where small unilamellar liposomes reduce the value of water surface tension due to amphiphiles from the liposomal vesicles and spreading as a monomolecular layer at the interface between the high-dielectric-constant polar water and the low-dielectric-constant nonpolar oil [
51]. At higher surface tension levels, there is a reduced number of interfacial water molecules because of the increased lipid packing [
50]. Thus, it can be concluded that non-sonicated liposomes with carob extract (multilamellar liposomal vesicles) possessed augmented lipid packing compared to reduced-size, sonicated liposomes. However, the mentioned decrease in the mean molecular area per lipid (augmented packing) can be reduced with the increase in the repulsion at the headgroups and acyl chains [
50]. Rivera et al. demonstrated that lipid packing and surface tension affect the zeta potential of liposomes as well [
52]. In the case of liposomes with encapsulated carob polyphenols, the sample with a lower zeta potential also possessed a lower surface tension (sonicated system). The decrease in the surface tension value of sonicated liposomes with carob extract should be attributed to impurities (debris) produced by the employed ultrasound probe [
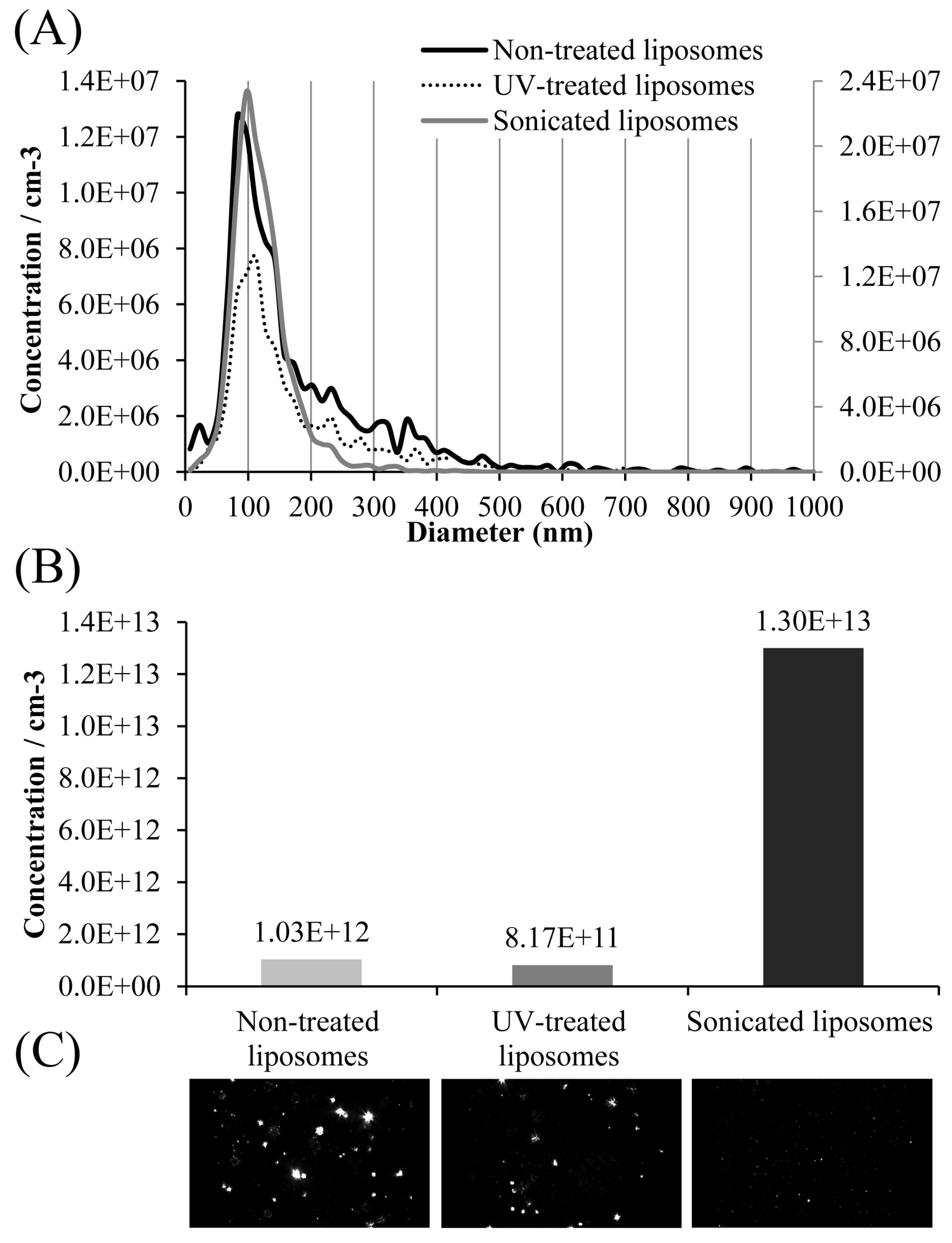
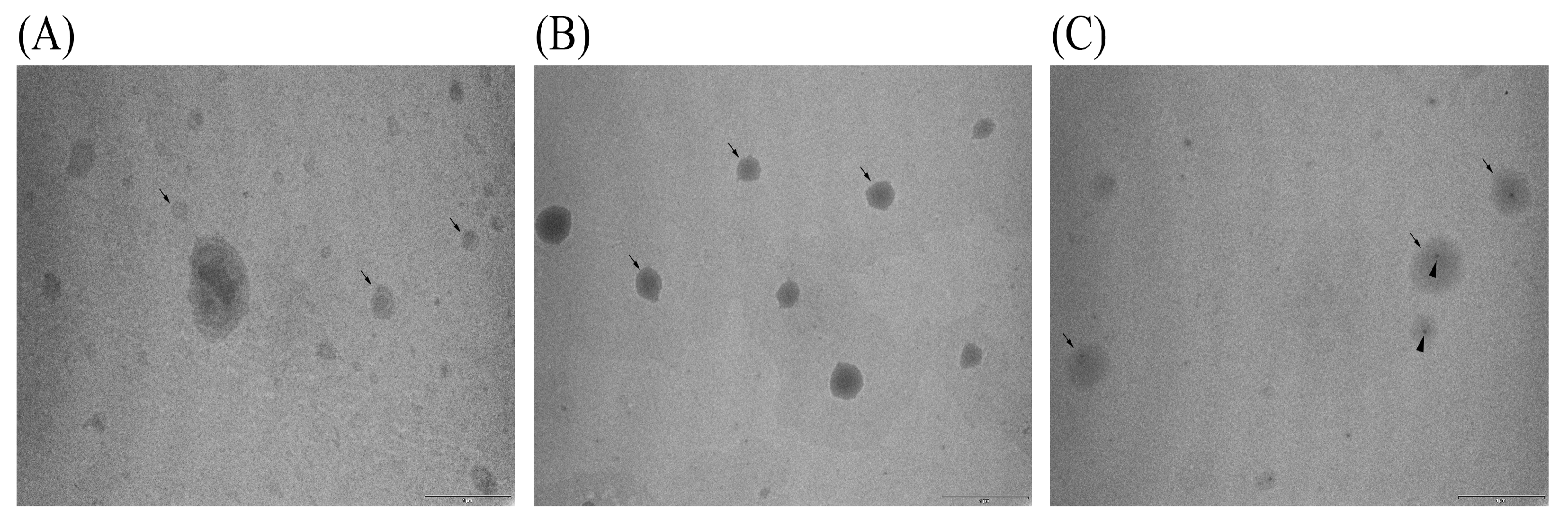
53]. TEM analysis of the obtained sonicated liposomal sample with carob extract also showed the presence of the ultrasound probe debris (presented later in Figure 5). Lombardo and Kiselev’s study also reported that a major drawback of the sonication technique is the potential for titanium particle shedding from the probe tip, which can lead to contamination of the lipid formulations [
42]. Wei et al.’s study [
54] showed the absence of correlation between surface tension and viscosity, as the two fluid characteristics, as well as a significant impact of the intermolecular attraction of water molecules on the surface tension; therefore, the decrease in viscosity did not trigger changes in the surface tension of liposomes. Thus, the alterations in the surface tension of developed liposomes during storage can be explained by the potential occurrence of nanobubbles at the liquid’s surface. Namely, according to the literature, with time, the nanobubble quantity in the bulk liquid significantly reduces, while the number of nanobubbles gradually adsorbed at the liquid surface increases. Therefore, the drop in surface tension values can be attributed to the Janus-like structure of nanobubbles, which can break the water molecules’ hydrogen bonding network at the surface of the liquid [
53].
The density of the carob extract-liposome system was also monitored on the 1st and 60th days of storage. As can be seen in
Table 1, the density of non-treated and UV-irradiated samples was the same, 1.04 g/cm
3, whereas the density of the small, ultrasound-treated liposomal population amounted to 1.02 g/cm
3. However, there was no statistically significant difference between the measured values. The mentioned variable of all developed liposomal populations did not alter from the 1st to the 60th day (
Table 1). The absence of a significant difference in the density values of the three liposomal systems was expected since the density of liposomes and other liquid systems is affected by the type and concentration of employed lipids and solvents for their preparation. In the case of carob extract-loaded liposomes, the same type and content of phospholipids, as well as solvent, were used in all samples.
3.5. FT-IR Spectroscopic Analysis of Developed Liposomal Formulations
FT-IR spectroscopic analysis was employed to examine potential chemical changes in phospholipids and carob extract compounds that can occur during liposome preparation, as well as changes in lipid bilayers during modifications, such as treatments by UV irradiation or sonication. The data are presented graphically in
Figure 4.
The most common route of administration of different compounds loaded in liposomes is per os and parenteral applications; therefore, developed encapsulates must be sterilized employing different processes, such as autoclaving, ultraviolet, or gamma ionizing irradiation, filtration, etc. Some of the mentioned techniques can lead to liposomes’ bilayer destabilization via oxidation of the liposomal membrane, i.e., peroxidation of unsaturated lipids, hydrolysis, lipid fragmentation, and pH changes in the liposome system [
31]. Thus, UV-irradiated liposomes were subjected to FT-IR spectroscopy analysis, and their spectra were compared to the non-treated parallels.
The spectrum of the extract displays broad bands at 3295 cm
−1 assigned to O-H stretching vibrations of hydroxyl, phenolic, and carboxyl groups. The bands in the region 2978–2880 cm
−1 and bands at 1446, 1418–1375, and 1328 cm
−1 correspond to stretching vibration of methylene and methyl, bending vibrations of methylene, rocking vibrations of C-H bonds, and bending vibrations of methyl groups, respectively [
55]. In addition, a C=C stretching band of aromatic and vinyl parts in the structures of the components of extract appears at 1602 cm
−1, while asymmetric and symmetric C–O/C-O-C/C-OH stretching vibrations of ether, phenol, alcohol, and alkyl ester groups at 1266–924 cm
−1, and =CH deformational vibrations of aromatic and vinyl structures at 761–720 cm
−1 are observed [
3]. All these bands confirm the presence of the components of carob extract: flavonoids, phenolic acids, and carbohydrates [
1,
2].
The FT-IR spectra of L-α-phosphatidylcholine (Ph) show peaks observed at 3012, 2925–2850, and 1464–1333 cm
−1, related to =CH stretching vibrations, -CH
2 and -CH
3 stretching and bending vibration of fatty acid residue [
56]. Additionally, the bands observed at 1735 and 1652 cm
−1 are due to the stretching vibrations of C=O in the ester group and O-H deformations overlapped with C=C stretching vibration in fatty acid residue. The ether C-O-C/C-O and phosphate P=O/P-O-C groups were observed in the 1261–965 cm
−1 region [
56]. The peaks at 874 and 720–500 cm
−1 are related to P-O asymmetric stretching vibration and =C-H out-of-plane in
cis-unsaturated double bond.
The spectra of liposomes, i.e., non-treated, UV-irradiated, and sonicated samples, indicated the presence of characteristic peaks from phospholipids and extract. In addition, intra/intermolecular interaction in the structure of phospholipids and intermolecular/hydrogen bonding interaction between phospholipids and extract lead to a change in the structure of the peak (intensity and shape) or peak shifting. The spectra of non-treated and sonicated liposomes are similar, while the spectrum of UV-irradiated liposomes shows spectral change, as observed in
Figure 4. The addition of extract to the phospholipids leads to a decrease in bands assigned to hydroxyl, carboxyl, ethylene, methylene, methyl, and ether groups for non-treated and sonicated liposomes. In addition, new bands, observed at 1609, 1133, and 924 cm
−1, appear as a result of the extract addition. The peak shifting from 1261 cm
−1 in phospholipids to 1236 cm
−1 in liposomes and the disappearance of the bands at 1652, 1071, and 1091 cm
−1 indicate the incorporation of the extract into the phospholipid liposome structure and the creation of different intermolecular interactions. The opposite trend of the peak structure change in the region 1200–965 cm
−1 is observed for liposomes treated by UV irradiation. In this region, the intensity of the peak at 1045 cm
−1 increases, and a new band at 990 cm
−1 appears. Also, the increase in broadband for aromatic and vinyl structures, observed at 1609 cm
−1, is noticed. This increase in intensity peaks is probably related to oxidation/peroxidation processes, causing the formation of oxygen-containing functionalities. Further, small peaks at 874 and 816 cm
−1 suggest the presence of hydroperoxide species. Accordingly, UV irradiation and ultrasound treatment of liposomes caused a decrease in the double bond at 3012 m
−1, a change in the structure of peaks between 1735 and 1600 cm
−1, and the existence of small bands at 874–816 cm
−1 as a result of forming low-stability oxygen species. The results obtained from FT-IR analysis are consistent with radical scavenging activity analysis (
Section 3.5), where non-treated liposomes with extract showed higher antioxidant capacity in the ABTS assay compared to post-processing modified liposomes.
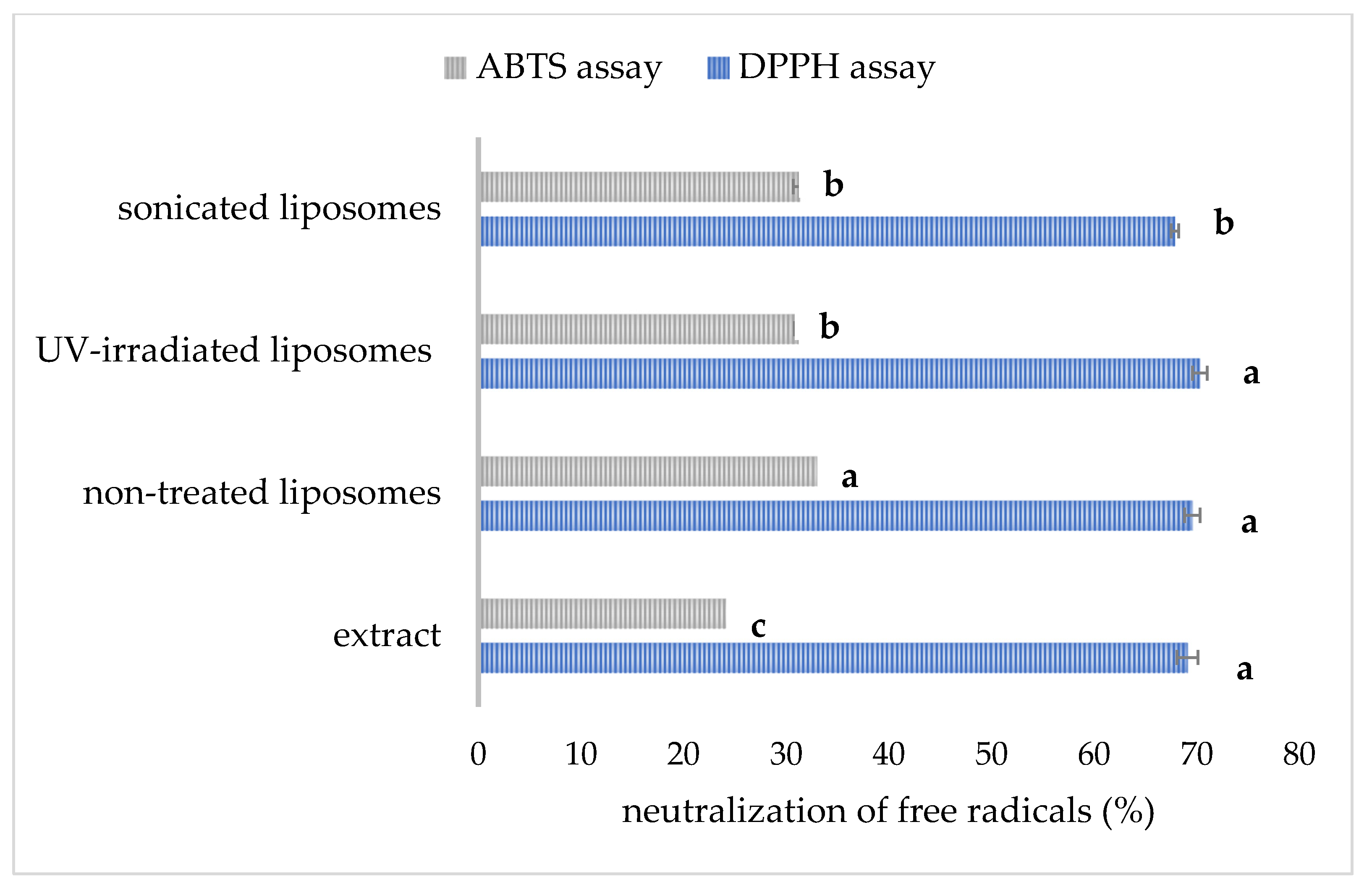
3.7. The Radical Scavenging Activity of Developed Liposomal Formulations
The data related to the radical scavenging capacity of pure carob extract and developed liposomes are presented in
Figure 6. The antioxidant capacity of the extract and liposomal formulations determined in the DPPH and ABTS assays is presented as the percentage of free radical neutralization.
The anti-DPPH activity of pure extract (diluted to achieve the same concentration as in the liposome samples) amounted to 69.1 ± 1.0% (
Figure 6). The mentioned activity was not significantly different in non-treated and UV-irradiated liposomal formulations (69.6 ± 0.8% and 70.3 ± 0.7%, respectively), indicating the ability of the prepared liposomal vesicles to retain anti-DPPH radical activity via encapsulation. The developed liposomes can retain a considerable level of the antioxidant effect of carob extract, which was also the case with the antioxidant activity of unencapsulated betel extract and its encapsulated counterpart [
28]. Additionally, anti-DPPH capacity was significantly lower in sonicated liposomes (67.9 ± 0.4%). In our preliminary study [
59], shortened sonication time (15 min) and lower amplitude (40%) provided the liposomes with higher anti-DPPH potential (69.4 ± 0.6%) in comparison to liposomes after prolonged exposure time and higher amplitude obtained in the present study. The antioxidant activity of carob extract determined in the ABTS test was 25.6 ± 0.6%, while the antioxidant potential of extract-loaded multilamellar vesicles was significantly higher, 33.8 ± 0.2%. The mentioned activity was significantly lower upon UV light treatment (31.2 ± 0.4%) and sonication (31.4 ± 0.7%). Noudoost et al. study [
60] also demonstrated that green tea encapsulated in a liposomal carrier provided a higher antioxidant effect in comparison to the free extract. Results of the Jahanfar et al. study [
11] demonstrated that the inhibition percentage of rosemary extract-loaded glycerosomes was higher compared to pure extract. The modified antioxidant potential of plant extracts during their entrapment in liposomal vesicles was expected due to new physicochemical properties and consequently changed biological activity of the complex (liposome bilayer–extract compounds), depending on the structure, diameter, as well as zeta potential of the formed liposomes. The data of Cortie and Else’s research [
61] suggest that non-peroxidizable phospholipid components can exert an
antioxidant-like action in membranes, and the mentioned potential can augment the action of traditional antioxidant compounds. The antioxidant potential of empty liposomes originating from antioxidants added to the initial phospholipid mixture has already been published [
21], which can be the reason for the higher antioxidant capacity of carob extract-loaded liposomes compared to free extract. A significant drop in the antioxidant potential of sonicated liposomes was expected due to the possibility of ultrasound waves generating free radicals due to the extreme conditions, such as the vigorous collapse of cavitation bubbles and the sonolysis of water and other medium components [
62], and consequently decreasing the antioxidant effect of developed liposomes. Sonicated liposomes with carob extract showed a lower antioxidant effect, also due to a lower encapsulation efficiency, and thus, their non-encapsulated carob extract compounds can undergo destruction caused by environmental conditions, which can later result in a decrease in antioxidant capacity [
28]. Also, as mentioned earlier, UV irradiation can trigger ROS damage in the liposomal bilayer [
32], decreasing the antioxidant potential of the developed liposomal preparation.
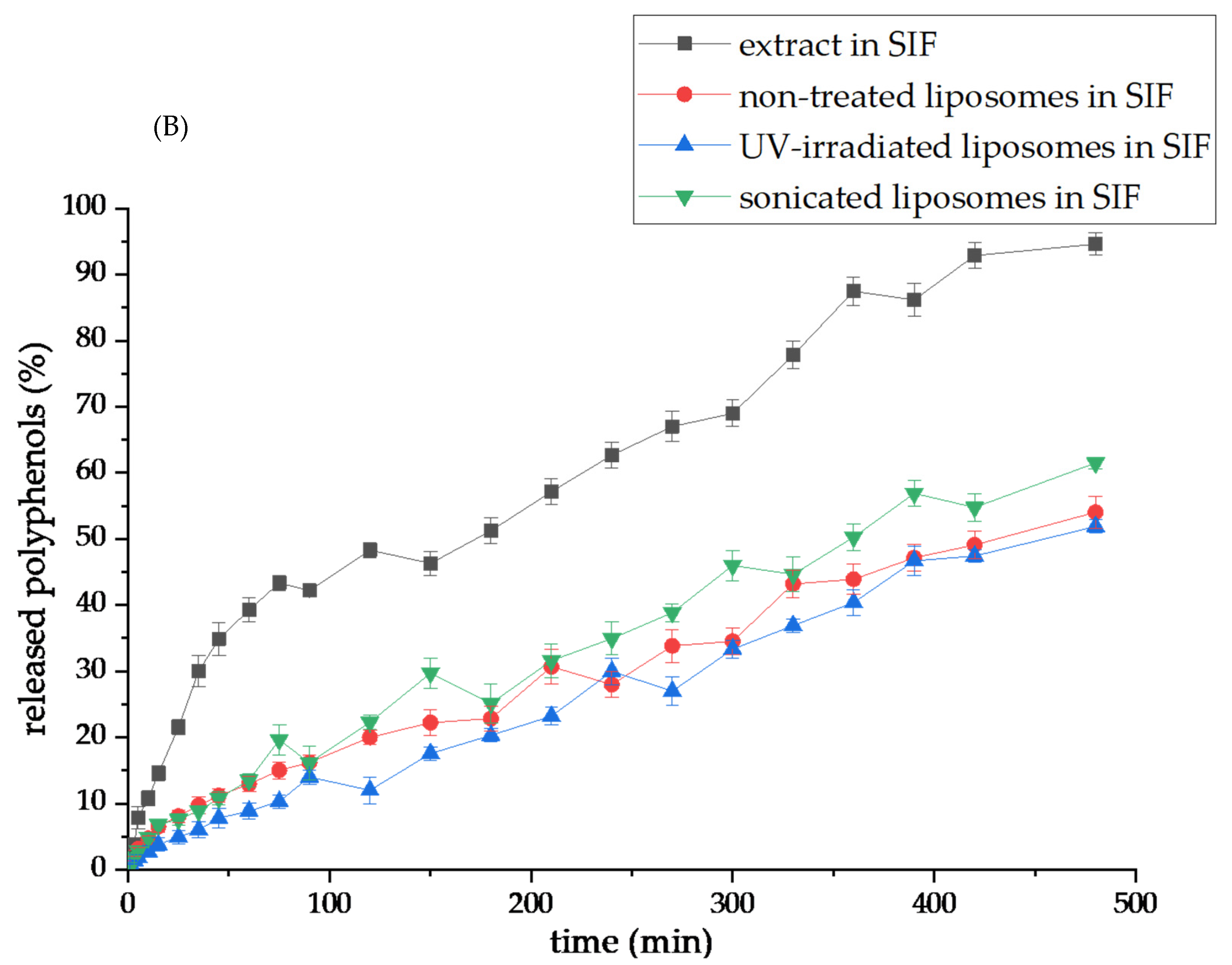
3.8. Carob Extract Polyphenol Release Under Simulated Gastrointestinal Conditions
The carob extract polyphenols’ release from the pure extract and the encapsulation systems (non-treated, UV-irradiated, and sonicated liposomes) toward SGF (pH~1.5) and SIF (pH~7.4) were performed, and the release profiles are displayed in
Figure 7A and
Figure 7B, respectively. Results obtained from the polyphenol release studies of carob extract and carob extract-loaded liposomes were analyzed to examine the diffusion coefficients and diffusion resistances derived from liposomes in simulated gastric and intestinal fluids and the calculation flow is presented in the
Supplementary Material. The data on the diffusion coefficients and diffusion resistances are presented in
Table 2. UV spectra of carob polyphenols in SGF and SIF medium are presented in
Figure S4.
As can be seen from
Figure 7A, the release of polyphenols within SGF from the pure extract and sonicated liposomes was faster and higher compared to larger-sized non-treated, and UV-irradiated liposomal vesicles. Namely, the amount of released polyphenol compounds after 3 h is 33.14% from the pure extract and 29.91% from sonicated liposomes, while the level of distributed polyphenols from non-treated and UV-irradiated liposomes in the SGF medium was 11.22% and 12.37%, respectively. Diffusion coefficients of non-treated and UV-irradiated liposomes in the SGF medium were similar and amounted to 9.44 × 10
−9 and 9.77 × 10
−9 m
2/s, respectively, while the mentioned variable was higher for other tested samples: 1.61 × 10
−8 m
2/s for pure extract and 1.65 × 10
−8 m
2/s for sonicated liposomes (
Table 2). Consequently, diffusion resistance in the SGF surrounding follows the same trend: 2.54 × 10
5 s/m (extract), 4.32 × 10
5 s/m (non-treated), 4.17 × 10
5 s/m (UV-irradiated), and 2.47 × 10
5 s/m (sonicated) (
Table 2). A slightly higher diffusion coefficient, as well as a lower diffusion resistance value of UV-treated liposomes in comparison to non-treated parallels, can be explained by the potential creation of pores within liposomal membranes induced by UV irradiation [
63]. Several studies have shown the higher release of encapsulated active compounds from liposome particles upon photoinitiated destabilization using UV lights [
32,
64,
65]. The reason for the lower capacity of sonicated liposomes to hold carob polyphenols lies in a greater contact surface between smaller liposomal particles and the simulated fluids. In the case of small liposomes, such as those after sonication, the contact surface with the surrounding medium increases. Hence, small particles (a high number of particles), as well as high-porosity samples, are more exposed to the surrounding medium influences due to an increase in the available areas for surface contact [
66]. Additionally, the significantly lower viscosity of the sonicated liposomal system compared to other liposomes (shown in
Table 1) can also be responsible for a higher percentage of released polyphenols in the environmental medium. As the degree of retention of carob polyphenols in liposomes was high in SGF (88.78% for non-treated and 87.62% for UV-irradiated), the data demonstrated the potential of liposomal vesicles to retain and protect entrapped bioactives in the presence of pepsin and low pH value in the gastric surrounding, which agrees with the literature [
67]. On the other hand, enzymes present in the SIF medium, including lipase and phospholipase A2, as well as bile salts, may destabilize the liposomal bilayer membrane due to the catalysis of lipid hydrolysis [
24], resulting in a more rapid and more abundant release of carob phenolics, which is presented in the next paragraph. Namely, the presence and absence of enzymes or bile salts in the investigated medium for the release kinetics, as well as medium pH, significantly affect the release properties of the liposomal carriers [
24,
67].
It can be observed that the polyphenol release from the pure extract was faster and complete in the SIF medium (94.7% after 8 h,
Figure 7B), while all liposomal particles allowed a more retarded release of encapsulated extract bioactives: 54.0% for non-treated, 51.9% for UV-irradiated, and 61.5% for sonicated liposomes. The reason for the lower level of distributed carob polyphenols from all liposomal systems obtained in the SIF medium, i.e., their incomplete recovery, is in the rigid liposomal membrane. Namely, in this study, only phospholipid compounds (in the absence of sterols) were used for the preparation of liposomes. According to the references, the addition of sterols during liposome formation can change the fluidity and permeability of membranes [
41,
68]. Nevertheless, cholesterol can affect the mechanical properties of membranes due to modifications of the acyl chain order, as well as the interfacial membrane region, but the mentioned effects are not universal and depend on the type of present lipids and cholesterol concentration [
68,
69]. At low and intermediate percentages, cholesterol enhances the membrane fluidity in the upper region [
68]. Therefore, more fluid membranes are able to release a higher amount of encapsulated components, but at the same time, their undesirable leakage during the storage period. Furthermore, cholesterol, as a commonly used sterol in liposome systems, can exert negative effects on health as well. Since the effects of developed liposomes with carob extracts on the metabolism of rat models will be included in future experiments, plant-based sterols, such as β-sitosterol, and fungus-based sterols, such as ergosterol, can be taken into account to improve the recovery of polyphenols from liposomal vesicles. Also, the liposome bilayer membrane containing β-sitosterol shows a higher level of fluidity in comparison to cholesterol parallels [
41]. Furthermore, diffusion coefficients determined in the SIF were higher compared to the values obtained for SGF: 3.14 × 10
−8 m
2/s (extract), 1.90 × 10
−8 m
2/s (non-treated), 1.35 × 10
−8 m
2/s (UV-irradiated), and 2.07 × 10
−8 m
2/s (sonicated), while diffusion resistance values were 1.29 × 10
5 s/m (extract), 2.14 × 10
5 s/m (non-treated), 3.01 × 10
5 s/m (UV-irradiated), and 1.97 × 10
5 s/m (sonicated) (
Table 2). Although the diffusion coefficient for UV-irradiated liposomes was lower in comparison to the non-treated parallel, the percentage of distributed carob polyphenols was the same after the 8 h period in the SIF medium. At the beginning of the diffusion process, the access of enzymes and salts from the SIF medium to liposomal membranes was probably slower and more difficult due to larger particles and/or formed agglomerates in the UV-irradiated sample, causing slow diffusion, i.e., higher diffusion resistance. On the other hand, the diffusion coefficient and the level of distributed polyphenols from sonicated parallel in SIF were higher due to a higher surface area between the liposome bilayer and medium enzymes, including lipases, and bile salts, as well as reduced viscosity, as mentioned above. Shashidhar and Manohar’s study [
70] reported that a higher viscosity liposome system provided a slower release of encapsulated active principles. Namely, in the case of reduced viscosity, diffusion and mass transfer of molecules are improved, which consequently provides their faster and more intensive release into the surrounding medium.
Due to the shown results of carob polyphenol release in simulated gastrointestinal conditions and similar conclusions obtained in several studies [
24,
37,
67], liposomal encapsulation of herbal extract formulations and plant-based ingredients can be proposed for increased gastric stability and delayed/targeted recovery of plant bioactives in the intestine.
Although carob pulp extracts encapsulated in liposomes demonstrate significant potential, it is crucial to advance toward pharmacological validation of their health-promoting activities. The encapsulation of carob pulp extracts in liposomes holds significant promise for enhancing their bioactive properties, particularly antioxidant and anti-inflammatory effects, which could be utilized effectively in therapeutic applications, such as reducing oxidative stress and inflammation or preventing cellular damage in various disease models [
71]. Advanced in vivo studies could investigate their biodistribution, pharmacokinetics, and targeted release mechanisms, paving the way for novel treatments, dietary supplements, and functional foods designed to optimize health benefits. Furthermore, the potential of liposome-encapsulated carob extracts to address specific conditions, like gut inflammation, vascular oxidative damage, or mitigating toxic treatment side effects, could add to their innovative applications in both pharmacology and nutraceuticals.