Intranasal Drug Delivery Technology in the Treatment of Central Nervous System Diseases: Challenges, Advances, and Future Research Directions
Abstract
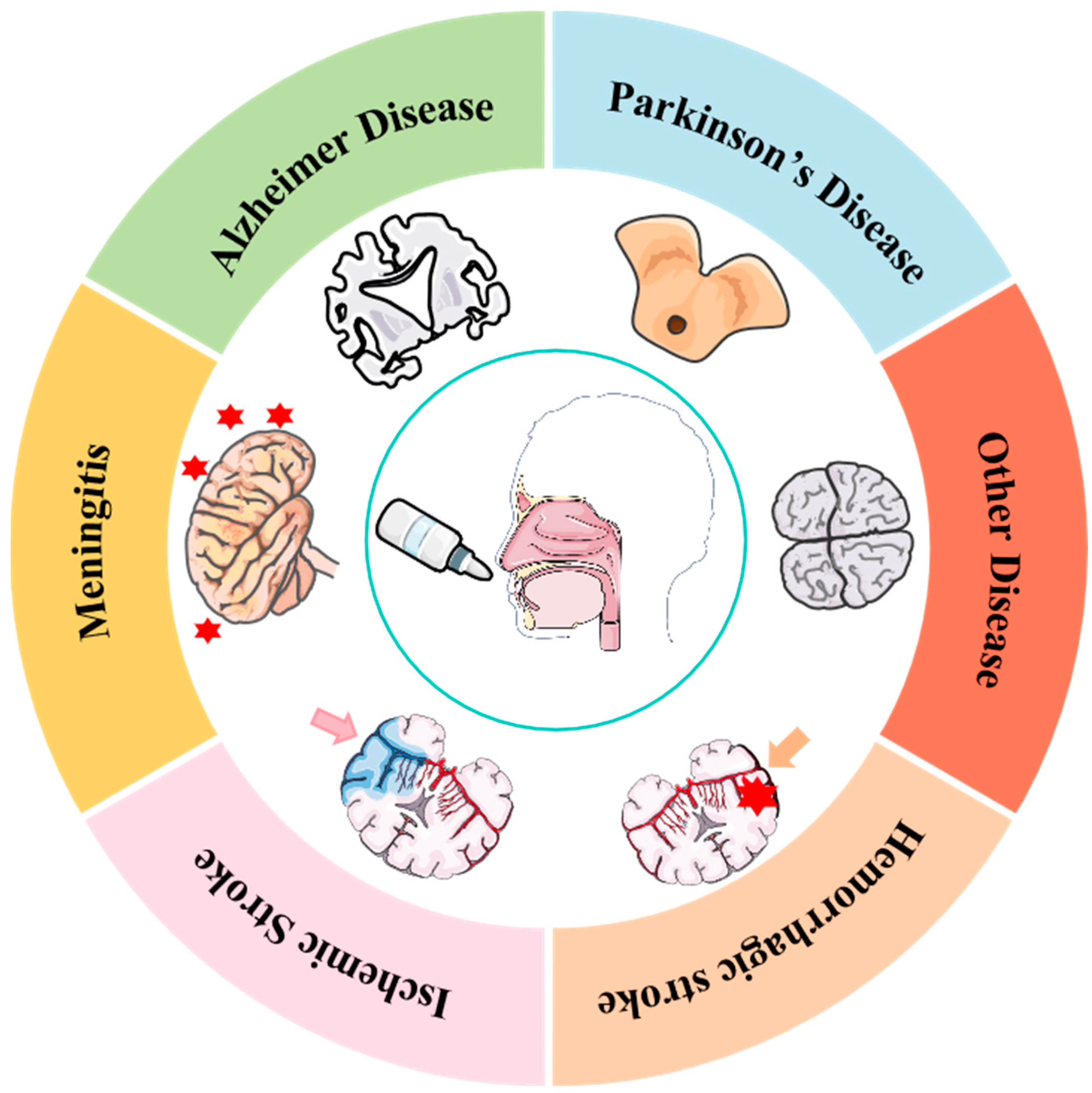
1. Introduction
2. Molecular Mechanisms of Intranasal Drug Delivery
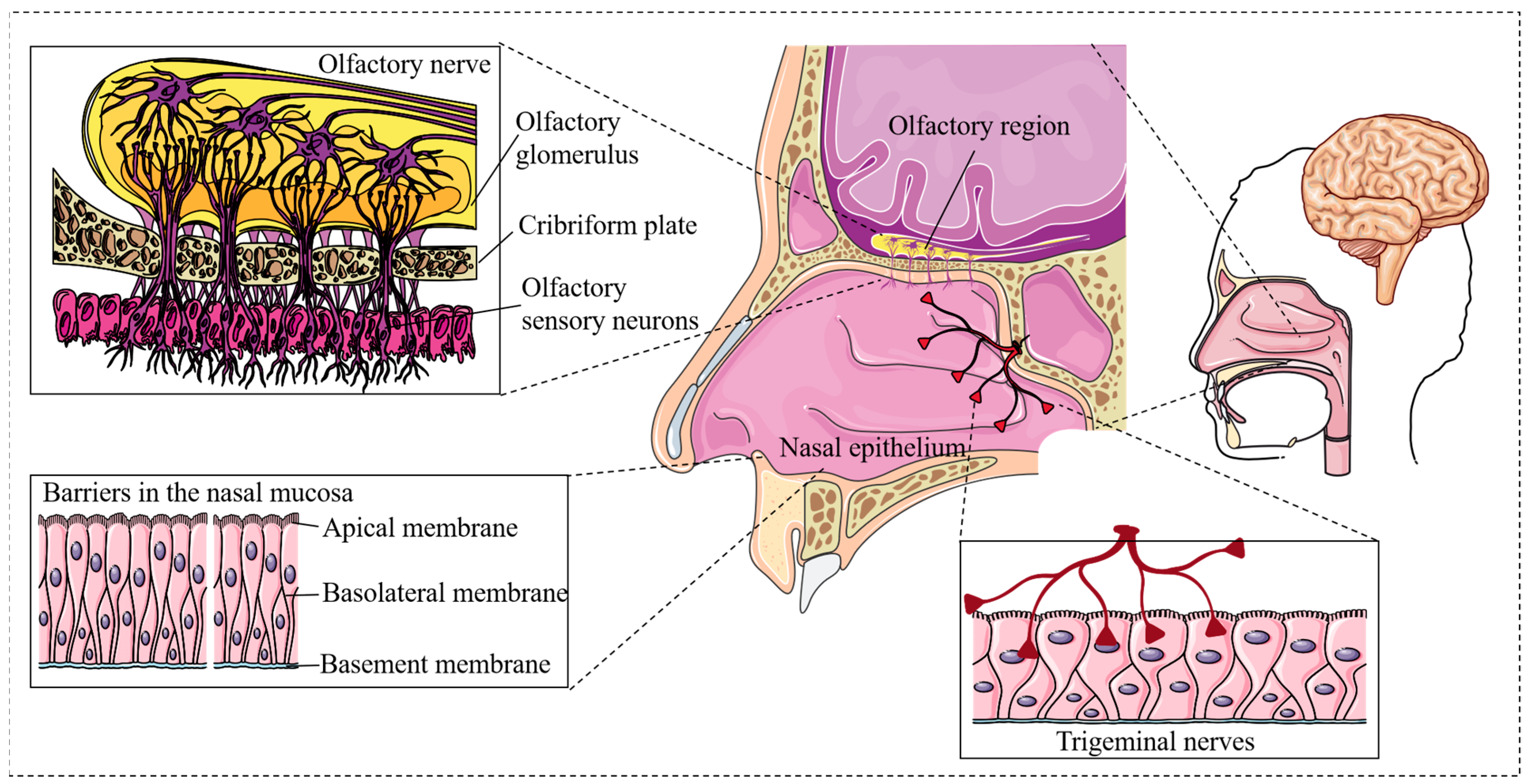
2.1. Olfactory Nerve Pathway
2.2. Trigeminal Nerve Pathway
2.3. Transduction Mechanisms of Paracellular and Transcellular Pathways in Intranasal Drug Delivery
3. Advances in Intranasal Drug Delivery Applications
3.1. Progress in Intranasal Delivery of Small Molecule Drugs
3.2. Research Progress of Intranasal Delivery of Biomacromolecule Drugs
3.2.1. Progress in Intranasal Delivery of Growth Factors and Peptide Drugs
3.2.2. Research Progress in Intranasal Vaccine Delivery
3.2.3. Research Progress in Intranasal Delivery of Nucleic Acid Drugs
3.3. Research Progress in Intranasal Delivery of Cell-Derived Therapeutic Drugs
3.3.1. Research Progress in Intranasal Delivery of Cell Therapy Drugs
3.3.2. Research Progress in Exosome Intranasal Delivery
3.4. Promising Formulations and Translational Potential
4. Advancements in Strategies to Enhance Intranasal Drug Delivery
4.1. Advances in Drug Delivery Devices for Intranasal Administration
4.2. Progress in Intranasal Drug Delivery with Nanotechnology
4.3. Research Progress in Hydrogels for Intranasal Drug Delivery
4.4. Research Progress on Permeation Enhancers in Intranasal Drug Delivery
5. Challenges and Future Research Directions in Intranasal Drug Delivery
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAVs | Adeno-associated viruses |
| AD | Alzheimer’s disease |
| ASO | Antisense oligonucleotide |
| BBB | Blood–brain barrier |
| BMSCs | Bone marrow-derived mesenchymal stem cells |
| BPD | Bronchopulmonary dysplasia |
| CSF | Cerebrospinal fluid |
| CNS | Central nervous system |
| DPSCs | Deciduous dental pulp stem cells |
| EVs | Extracellular vesicles |
| FUSIN | Focused ultrasound-mediated nasal drug delivery |
| GLP-1 | Glucagon-like peptide-1 |
| IGF-1 | Insulin-like growth factor 1 |
| hNSCs | Human neural stem cells |
| LIF | Leukemia inhibitory factor |
| MSC | Mesenchymal stem cell |
| NGF | Nerve growth factor |
| OMPCs | Olfactory mucosal progenitor cells |
| PD | Parkinson’s disease |
| PEG | Polyethylene glycol |
| PTD | Protein transduction domain |
| SAD | Social anxiety disorder |
| SCI | Spinal cord injury |
| TBI | Traumatic brain injury |
| ZO-1 | Zonula occludens-1 |
References
- Agrawal, M.; Saraf, S.; Saraf, S.; Antimisiaris, S.; Chougule, M.; Shoyele, S.; Alexander, A. Nose-to-brain drug delivery: An update on clinical challenges and progress towards approval of anti-Alzheimer drugs. J. Control. Release 2018, 281, 139–177. [Google Scholar] [CrossRef] [PubMed]
- Kou, D.; Gao, Y.; Li, C.; Zhou, D.; Lu, K.; Wang, N.; Zhang, R.; Yang, Z.; Zhou, Y.; Chen, L.; et al. Intranasal Pathway for Nanoparticles to Enter the Central Nervous System. Nano Lett. 2023, 23, 5381–5390. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, C.C.; Acosta, C.; Wu, S.Y.; Sun, T.; Konofagou, E.E. A new brain drug delivery strategy: Focused ultrasound-enhanced intranasal drug delivery. PLoS ONE 2014, 9, e108880. [Google Scholar] [CrossRef] [PubMed]
- Lochhead, J.J.; Davis, T.P. Perivascular and Perineural Pathways Involved in Brain Delivery and Distribution of Drugs after Intranasal Administration. Pharmaceutics 2019, 11, 598. [Google Scholar] [CrossRef]
- Wu, J.; Li, A.; Shi, Y.; Wang, Y.; Luo, J.; Zhuang, W.; Ma, X.; Qiao, Z.; Xiu, X.; Lang, X.; et al. Intranasal delivery of mesenchymal stem cell-derived exosomes ameliorates experimental autoimmune encephalomyelitis. Int. Immunopharmacol. 2025, 146, 113853. [Google Scholar] [CrossRef]
- Jia, Y.; Xu, L.; Leng, S.; Sun, Y.; Huang, X.; Wang, Y.; Ren, H.; Li, G.; Bai, Y.; Zhang, Z.; et al. Nose-to-Brain Delivery of Circular RNA SCMH1-Loaded Lipid Nanoparticles for Ischemic Stroke Therapy. Adv. Mater. 2025, 37, 2500598. [Google Scholar] [CrossRef]
- Snyers, D.; Tribolet, S.; Rigo, V. Intranasal Analgosedation for Infants in the Neonatal Intensive Care Unit: A Systematic Review. Neonatology 2022, 119, 273–284. [Google Scholar] [CrossRef]
- Keller, L.-A.; Merkel, O.; Popp, A. Intranasal drug delivery: Opportunities and toxicologic challenges during drug development. Drug Deliv. Transl. Res. 2021, 12, 735–757. [Google Scholar] [CrossRef]
- Gotoh, S.; Kawabori, M.; Fujimura, M. Intranasal administration of stem cell-derived exosomes for central nervous system diseases. Neural Regen. Res. 2024, 19, 1249–1255. [Google Scholar] [CrossRef]
- Gandhi, S.; Shastri, D.H.; Shah, J.; Nair, A.B.; Jacob, S. Nasal Delivery to the Brain: Harnessing Nanoparticles for Effective Drug Transport. Pharmaceutics 2024, 16, 481. [Google Scholar] [CrossRef]
- Zha, S.; Wong, K.L.; All, A.H. Intranasal Delivery of Functionalized Polymeric Nanomaterials to the Brain. Adv. Healthc. Mater. 2022, 11, 2102610. [Google Scholar] [CrossRef] [PubMed]
- Awad, R.; Avital, A.; Sosnik, A. Polymeric nanocarriers for nose-to-brain drug delivery in neurodegenerative diseases and neurodevelopmental disorders. Acta Pharm. Sin. B 2023, 13, 1866–1886. [Google Scholar] [CrossRef] [PubMed]
- Kosyakovsky, J.; Witthuhn, B.A.; Svitak, A.L.; Frey, W.H.; Hanson, L.R.; Fine, J.M. Quantifying Intranasally Administered Deferoxamine in Rat Brain Tissue with Mass Spectrometry. ACS Chem. Neurosci. 2019, 10, 4571–4578. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.A.; Patel, R.J.; Patel, S.R. Nanomedicine for Intranasal Delivery to Improve Brain Uptake. Curr. Drug Deliv. 2018, 15, 461–469. [Google Scholar] [CrossRef]
- Duong, V.-A.; Nguyen, T.-T.-L.; Maeng, H.-J. Recent Advances in Intranasal Liposomes for Drug, Gene, and Vaccine Delivery. Pharmaceutics 2023, 15, 207. [Google Scholar] [CrossRef]
- Pandya, J.D.; Musyaju, S.; Modi, H.R.; Okada-Rising, S.L.; Bailey, Z.S.; Scultetus, A.H.; Shear, D.A. Intranasal delivery of mitochondria targeted neuroprotective compounds for traumatic brain injury: Screening based on pharmacological and physiological properties. J. Transl. Med. 2024, 22, 167. [Google Scholar] [CrossRef]
- Ojo, A.S.; Odipe, O.G.; Owoseni, O. Improving the Emergency Department Management of Sickle Cell Vaso-Occlusive Pain Crisis: The Role and Options of Sublingual and Intranasally Administered Analgesia. J. Clin. Med. Res. 2023, 15, 10–22. [Google Scholar] [CrossRef]
- Martirosov, A.; Giuliano, C.; Shupp, M.; Channey, S.; Kale-Pradhan, P.B. Zavegepant Intranasal Spray for Migraines. Ann. Pharmacother. 2024, 58, 827–833. [Google Scholar] [CrossRef]
- Mehta, M.U.; Uppoor, R.S.; Roca, R.A.; Sabarinath, S.; Florian, J.; Xu, Y.; Nallani, S.C.; Li, Z.; Brescia-Oddo, T. FDA Approval Summary: Nalmefene Nasal Spray for the Emergency Treatment of Known or Suspected Opioid Overdose. Clin. Pharmacol. Ther. 2024, 117, 620–626. [Google Scholar] [CrossRef]
- Sposito, B.; Broggi, A.; Pandolfi, L.; Crotta, S.; Clementi, N.; Ferrarese, R.; Sisti, S.; Criscuolo, E.; Spreafico, R.; Long, J.; et al. The interferon landscape along the respiratory tract impacts the severity of COVID-19. Cell 2021, 184, 4953–4968.e16. [Google Scholar] [CrossRef]
- Buyze, J.; Llorca, P.-M.; Messer, T.; Rive, B.; Young, A.H.; Frey, R.; Bitter, I.; Fu, D.-J.; Kambarov, Y.; Reif, A.; et al. Esketamine Nasal Spray versus Quetiapine for Treatment-Resistant Depression. N. Engl. J. Med. 2023, 389, 1298–1309. [Google Scholar] [CrossRef]
- Illum, L. Transport of drugs from the nasal cavity to the central nervous system. Eur. J. Pharm. Sci. 2000, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Thorne, R.G.; Lochhead, J.J. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 2011, 64, 614–628. [Google Scholar] [CrossRef]
- Crowe, T.P.; Greenlee, M.H.W.; Kanthasamy, A.G.; Hsu, W.H. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018, 195, 44–52. [Google Scholar] [CrossRef]
- Mestre, H.; Rasmussen, M.K.; Nedergaard, M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018, 17, 1016–1024. [Google Scholar] [CrossRef]
- Illum, L. pharmacology. Is nose-to-brain transport of drugs in man a reality? J. Pharm. Pharmacol. 2004, 56, 3–17. [Google Scholar] [CrossRef]
- Bagley, J.H.; Geltzeiler, M.; Liu, J.J.; Sanusi, O.R.; Piantino, J.; Dogan, A.; Yamamoto, E.A. The perivascular space is a conduit for cerebrospinal fluid flow in humans: A proof-of-principle report. Proc. Natl. Acad. Sci. USA 2024, 121, e2407246121. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, Y.; Wang, Y.; Tong, L.; Wang, F.; Song, S.; Xu, L.; Liu, B.; Yan, H.; Sun, Z. Current State and Future Directions of Intranasal Delivery Route for Central Nervous System Disorders: A Scientometric and Visualization Analysis. Front. Pharmacol. 2021, 12, 717192. [Google Scholar] [CrossRef]
- Saka, R.; Chella, N.; Khan, W. Development of Imatinib Mesylate-Loaded Liposomes for Nose to Brain Delivery: In Vitro and In Vivo Evaluation. AAPS PharmSciTech 2021, 22, 192. [Google Scholar] [CrossRef]
- Katona, G.; Csóka, I.; Sipos, B. Risk-Assessment-Based Optimization Favours the Development of Albumin Nanoparticles with Proper Characteristics Prior to Drug Loading. Pharmaceutics 2022, 14, 2036. [Google Scholar] [CrossRef]
- Baboota, S.; Kumar, S.; Arora, A.; Ali, J. Intranasal delivery of tetrabenazine nanoemulsion via olfactory region for better treatment of hyperkinetic movement associated with Huntington’s disease: Pharmacokinetic and brain delivery study. Chem. Phys. Lipids 2020, 230, 104917. [Google Scholar] [CrossRef]
- Agrawal, M.; Ajazuddin; Murty, U.S.; Ravichandiran, V.; Dubey, S.K.; Saraf, S.; Saraf, S.; Alexander, A.; Patel, R.J.; Puri, A. Recent strategies and advances in the fabrication of nano lipid carriers and their application towards brain targeting. J. Control. Release 2020, 321, 372–415. [Google Scholar] [CrossRef]
- Jing, T.; Xinchen, Y.; Jiaoqiong, G. Lipid-based nanoparticles via nose-to-brain delivery: A mini review. Front. Cell Dev. Biol. 2023, 11, 1214450. [Google Scholar] [CrossRef]
- Préat, V.; Beloqui, A.; Rieux, A.D. Mechanisms of transport of polymeric and lipidic nanoparticles across the intestinal barrier. Adv. Drug Deliv. Rev. 2016, 106, 242–255. [Google Scholar] [CrossRef]
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H. Intranasal delivery to the central nervous system: Mechanisms and experimental considerations. J. Pharm. Sci. 2010, 99, 1654–1673. [Google Scholar] [CrossRef]
- Hanson, L.R.; Frey, W.H. Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008, 9, S5. [Google Scholar] [CrossRef]
- Andresen, M.J.F. Understanding diverse TRPV1 signaling—An update. F1000Research 2019, 8, 1978. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.H.; Crowe, T.P. Evaluation of Recent Intranasal Drug Delivery Systems to the Central Nervous System. Pharmaceutics 2022, 14, 629. [Google Scholar] [CrossRef]
- Dighe, S.; Jog, S.; Momin, M.; Sawarkar, S.; Omri, A. Intranasal Drug Delivery by Nanotechnology: Advances in and Challenges for Alzheimer’s Disease Management. Pharmaceutics 2024, 16, 58. [Google Scholar] [CrossRef]
- de Lange, E.C.M.; Ruigrok, M.J.R. Emerging Insights for Translational Pharmacokinetic and Pharmacokinetic-Pharmacodynamic Studies: Towards Prediction of Nose-to-Brain Transport in Humans. AAPS J. 2015, 17, 493–505. [Google Scholar] [CrossRef]
- Padmanabhan, V.; Frey, W.; Thorne, R.; Pronk, G. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience 2004, 127, 481–496. [Google Scholar] [CrossRef]
- Gänger, S.; Schindowski, K. Tailoring Formulations for Intranasal Nose-to-Brain Delivery: A Review on Architecture, Physico-Chemical Characteristics and Mucociliary Clearance of the Nasal Olfactory Mucosa. Pharmaceutics 2018, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Yu, S.; Gong, G.; Chen, X.; Shu, H. Research progress in brain-targeted nasal drug delivery. Front. Aging Neurosci. 2023, 15, 1341295. [Google Scholar] [CrossRef]
- Chiono, V.; Marcello, E. Biomaterials-Enhanced Intranasal Delivery of Drugs as a Direct Route for Brain Targeting. Int. J. Mol. Sci. 2023, 24, 3390. [Google Scholar] [CrossRef]
- Shen, H.; Aggarwal, N.; Cui, B.; Foo, G.; He, Y.; Srivastava, S.; Li, S.; Seah, M.; Wun, K.; Ling, H.; et al. Engineered commensals for targeted nose-to-brain drug delivery. Cell 2025, 188, 1545–1562.e16. [Google Scholar] [CrossRef]
- Ladel, S.; Flamm, J.; Schlossbauer, P.; Mizaikoff, B.; Luksch, H.; Schindowski, K. Improved In Vitro Model for Intranasal Mucosal Drug Delivery: Primary Olfactory and Respiratory Epithelial Cells Compared with the Permanent Nasal Cell Line RPMI 2650. Pharmaceutics 2019, 11, 367. [Google Scholar] [CrossRef] [PubMed]
- Lundgaard, I.; Munk, A.S.F.; Nedergaard, M.; Jessen, N.A. The Glymphatic System: A Beginner’s Guide. Neurochem. Res. 2015, 40, 2583–2599. [Google Scholar] [CrossRef]
- Illum, L.; Exposito-Harris, R.; Heras, A.; Vllasaliu, D.; Garnett, M.; Stolnik, S.; Casettari, L. Tight junction modulation by chitosan nanoparticles: Comparison with chitosan solution. Int. J. Pharm. 2010, 400, 183–193. [Google Scholar] [CrossRef]
- Craft, S.; Raman, R.; Chow, T.W.; Rafii, M.S.; Sun, C.K.; Rissman, R.A.; Donohue, M.C.; Brewer, J.B.; Jenkins, C.; Harless, K.; et al. Safety, Efficacy, and Feasibility of Intranasal Insulin for the Treatment of Mild Cognitive Impairment and Alzheimer Disease Dementia: A Randomized Clinical Trial. JAMA Neurol. 2020, 77, 1099–1109. [Google Scholar] [CrossRef]
- Sotomayor, P.; Morales, P.; Acuña, E.; González, L.F.; Arellano, G.; Oyarzun-Ampuero, F.; Naves, R. Intranasal delivery of interferon-β-loaded nanoparticles induces control of neuroinflammation in a preclinical model of multiple sclerosis: A promising simple, effective, non-invasive, and low-cost therapy. J. Control. Release 2021, 331, 443–459. [Google Scholar] [CrossRef]
- Nanaki, S.G.; Spyrou, K.; Papadopoulos, G.C.; Bekiari, C.; Veneti, P.; Karouta, N.; Bikiaris, D.N.; Baroud, T.N.; Gournis, D.; Grivas, I. Hierarchical Porous Carbon-PLLA and PLGA Hybrid Nanoparticles for Intranasal Delivery of Galantamine for Alzheimer’s Disease Therapy. Pharmaceutics 2020, 12, 227. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.; Alves, G.; Fonseca, C.; Carona, A.; Bicker, J.; Falcão, A.; Fortuna, A. Is intranasal administration an opportunity for direct brain delivery of lacosamide? Eur. J. Pharm. Sci. 2021, 157, 105632. [Google Scholar] [CrossRef] [PubMed]
- Zolkowska, D.; Wu, C.-Y.; Rogawski, M.A. Intranasal Allopregnanolone Confers Rapid Seizure Protection: Evidence for Direct Nose-to-Brain Delivery. Neurotherapeutics 2021, 18, 544–555. [Google Scholar] [CrossRef]
- Hong, Z.; Shuo, L.; Zhuo, L.; Shuo, Y.; Dandan, L.; Jiaolin, Z.; Hongmei, G.; Ling, Y.; YingLi, G.; Longxuan, L.; et al. Intranasal delivery of 9-cis retinoic acid reduces beta-amyloid deposition via inhibiting astrocyte-mediated inflammation. Aging 2020, 12, 5469–5478. [Google Scholar] [CrossRef]
- Bhargavi, M.; Sai Sarath, G.; Surana, P.; Dhull, K.S.; Shaikh, M.; Rajan, M. Efficacy of Intranasal Atomized Dexmedetomidine for Sedation in Surgical Removal of Impacted Mandibular Third Molars: A Prospective Study. Cureus 2023, 15, e36721. [Google Scholar] [CrossRef]
- Korczeniewska, O.A.; Tatineni, K.; Faheem, S.; Fresin, W.; Bonitto, J.; Khan, J.; Eliav, E.; Benoliel, R. Effects of intra-nasal melanocortin-4 receptor antagonist on trigeminal neuropathic pain in male and female rats. Neurosci. Lett. 2023, 796, 137054. [Google Scholar] [CrossRef] [PubMed]
- Abolfazl, F.; Nafiseh, F.-M.; Zeinab, N.; Mouna Faghani, A. Effect of Intranasal Ketamine on Pain Intensity after Cesarean Section: A Single-Center, Double Blind, Randomized Controlled Trial. Ethiop. J. Health Sci. 2023, 33, 55–64. [Google Scholar] [CrossRef]
- Siegler, B.H.; Gruß, M.; Oehler, B.; Keßler, J.; Fluhr, H.; Weis, C.; Schulz, F.; Weigand, M.A. Intranasale Lidocainvernebelung als neue und nichtinvasive Therapieoption des Postpunktionskopfschmerzes. Der Anaesthesist 2020, 70, 392–397. [Google Scholar] [CrossRef]
- Micheli, L.; Di Cesare Mannelli, L.; Lucarini, E.; Parisio, C.; Toti, A.; Fiorentino, B.; Rigamonti, M.A.; Calosi, L.; Ghelardini, C. Intranasal Low-Dose Naltrexone Against Opioid Side Effects: A Preclinical Study. Front. Pharmacol. 2020, 11, 576624. [Google Scholar] [CrossRef]
- Ingielewicz, A.; Szymczak, R.K. Intranasal Therapy in Palliative Care. Pharmaceutics 2024, 16, 519. [Google Scholar] [CrossRef]
- Nicholas, T.G.; Husbands, E.L. Benefits of Intranasal Administration of Diamorphine and Midazolam in the Management of Patients Receiving Palliative Care in the Community: A Case Series. J. Pain Palliat. Care Pharmacother. 2022, 36, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Osorio, I.N.; Espinosa, A.; Giraldo Velázquez, M.; Padilla, P.; Bárcena, B.; Fragoso, G.; Jung-Cook, H.; Besedovsky, H.; Meneses, G.; Sciutto Conde, E.L. Nose-to-Brain Delivery of Dexamethasone: Biodistribution Studies in Mice. J. Pharmacol. Exp. Ther. 2021, 378, 244–250. [Google Scholar] [CrossRef]
- Xu, D.; Lu, Y.-R.; Kou, N.; Hu, M.-J.; Wang, Q.-S.; Cui, Y.-L. Intranasal delivery of icariin via a nanogel-thermoresponsive hydrogel compound system to improve its antidepressant-like activity. Int. J. Pharm. 2020, 586, 119550. [Google Scholar] [CrossRef] [PubMed]
- Tiozzo Fasiolo, L.; Manniello, M.D.; Banella, S.; Napoli, L.; Bortolotti, F.; Quarta, E.; Colombo, P.; Balafas, E.; Kostomitsopoulos, N.; Rekkas, D.M.; et al. Flurbiprofen sodium microparticles and soft pellets for nose-to-brain delivery: Serum and brain levels in rats after nasal insufflation. Int. J. Pharm. 2021, 605, 120827. [Google Scholar] [CrossRef]
- Harris, H.M.; Boyet, K.L.; Liu, H.; Dwivedi, R.; Ashpole, N.M.; Tandon, R.; Bidwell, G.L.; Cheng, Z.; Fassero, L.A.; Yu, C.S.; et al. Safety and Pharmacokinetics of Intranasally Administered Heparin. Pharm. Res. 2022, 39, 541–551. [Google Scholar] [CrossRef]
- Gallegos, C.E.; Bartos, M.; Gumilar, F.; Minetti, A.; Baier, C.J. Behavioral and neurochemical impairments after intranasal administration of chlorpyrifos formulation in mice. Pestic. Biochem. Physiol. 2023, 189, 105315. [Google Scholar] [CrossRef] [PubMed]
- Chiaretti, A.; Eftimiadi, G.; Soligo, M.; Manni, L.; Di Giuda, D.; Calcagni, M. Topical delivery of nerve growth factor for treatment of ocular and brain disorders. Neural Regen. Res. 2021, 16, 1740–1750. [Google Scholar] [CrossRef]
- Manni, L.; Conti, G.; Chiaretti, A.; Soligo, M. Intranasal Delivery of Nerve Growth Factor in Neurodegenerative Diseases and Neurotrauma. Front. Pharmacol. 2021, 12, 754502. [Google Scholar] [CrossRef]
- Soligo, M.; Manni, L.; Conti, G.; Chiaretti, A. Intranasal nerve growth factor for prevention and recovery of the outcomes of traumatic brain injury. Neural Regen. Res. 2023, 18, 773–778. [Google Scholar] [CrossRef]
- Antonio, G.; Lavinia, C.; Giorgio, C.; Gemma, E.; Serena, F.; Luigi, M.; Antonietta, C.; Benedetta, G.; Lorenzo, D.S.; Maria Lucia, C.; et al. Intranasal human-recombinant NGF administration improves outcome in children with post-traumatic unresponsive wakefulness syndrome. Biol. Direct 2023, 18, 61. [Google Scholar] [CrossRef]
- D’Mello, V.; Subramaniam, M.; Bhalla, A.P.; Saavedra, S.; Leiba, O.; Levison, S.W. Intranasal Leukemia Inhibitory Factor Attenuates Gliosis and Axonal Injury and Improves Sensorimotor Function After a Mild Pediatric Traumatic Brain Injury. Neurotrauma Rep. 2023, 4, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Zorina, I.I.; Avrova, N.F.; Zakharova, I.O.; Shpakov, A.O. Prospects for the Use of Intranasally Administered Insulin and Insulin-Like Growth Factor-1 in Cerebral Ischemia. Biochemistry 2023, 88, 374–391. [Google Scholar] [CrossRef] [PubMed]
- Tashima, T. Shortcut Approaches to Substance Delivery into the Brain Based on Intranasal Administration Using Nanodelivery Strategies for Insulin. Molecules 2020, 25, 5188. [Google Scholar] [CrossRef]
- Attama, A.A.; Ofokansi, K.C.; Mumuni, M.A.; Díaz, D.D.; Kenechukwu, F.C. Insulin-loaded mucoadhesive nanoparticles based on mucin-chitosan complexes for oral delivery and diabetes treatment. Carbohydr. Polym. 2019, 229, 115506. [Google Scholar] [CrossRef]
- Shen, X.; Mao, S.; Gao, M.; Kou, Y.; Huo, Y.; Sun, Z.; Liu, C.; Sun, Y.; Zhang, X. Effect of Glyceryl Monocaprylate-Modified Chitosan on the Intranasal Absorption of Insulin in Rats. J. Pharm. Sci. 2019, 108, 3623–3629. [Google Scholar] [CrossRef]
- Wong, C.Y.J.; Baldelli, A.; Hoyos, C.M.; Tietz, O.; Ong, H.X.; Traini, D. Insulin Delivery to the Brain via the Nasal Route: Unraveling the Potential for Alzheimer’s Disease Therapy. Drug Deliv. Transl. Res. 2024, 14, 1776–1793. [Google Scholar] [CrossRef] [PubMed]
- Hallschmid, M. Intranasal Insulin for Alzheimer’s Disease. CNS Drugs 2021, 35, 21–37. [Google Scholar] [CrossRef]
- Fine, J.M.; Stroebel, B.M.; Faltesek, K.A.; Terai, K.; Haase, L.; Knutzen, K.E.; Kosyakovsky, J.; Bowe, T.J.; Fuller, A.K.; Frey, W.H.; et al. Intranasal delivery of low-dose insulin ameliorates motor dysfunction and dopaminergic cell death in a 6-OHDA rat model of Parkinson’s Disease. Neurosci. Lett. 2020, 714, 134567. [Google Scholar] [CrossRef]
- Bae, H.-D.; Lee, J.-S.; Pyun, H.; Kim, M.; Lee, K. Optimization of formulation for enhanced intranasal delivery of insulin with translationally controlled tumor protein-derived protein transduction domain. Drug Deliv. 2019, 26, 622–628. [Google Scholar] [CrossRef]
- Salah, M.; Mahmoud, S.; Hammam, O.A.; Kandeel, W.; Attia, Y.M.; Maher, M.A. Histopathological evaluation of insulin-DMSO formula designed for direct nose-to-brain delivery. Histol. Histopathol. 2022, 37, 431–439. [Google Scholar] [CrossRef]
- Zhang, T.-T.; Cai, Q.-L.; Gu, Z.-Y.; Song, T.-Y. Novel ACE-inhibiting peptides from soybean protein hydrolysates by peptidomics combined with in silico analysis and their inhibitory effects on proliferation and migration of Ang II-induced VSMCs. Food Med. Homol. 2024, 1, 9420023. [Google Scholar] [CrossRef]
- Novak, V.; Mantzoros, C.; Novak, P.; McGlinchey, R.; Dai, W.; Lioutas, V.; Buss, S.; Fortier, C.; Khan, F.; Aponte Becerra, L.; et al. MemAID: Memory advancement with intranasal insulin vs. placebo in type 2 diabetes and control participants: A randomized clinical trial. J. Neurol. 2022, 269, 4817–4835. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.T.S.; Sheikh, Z.; Maleknia, S.; Oveissi, F.; Fathi, A.; Abrams, T.; Ong, H.X.; Traini, D. Intranasal delivery of glucagon-like peptide-1 to the brain for obesity treatment: Opportunities and challenges. Expert Opin. Drug Deliv. 2024, 21, 1081–1101. [Google Scholar] [CrossRef]
- De Luca, V.; Ishii, D.; Kageyama, M.; Umeda, S. Cerebral and extracerebral distribution of radioactivity associated with oxytocin in rabbits after intranasal administration: Comparison of TTA-121, a newly developed oxytocin formulation, with Syntocinon. PLoS ONE 2021, 16, e0261451. [Google Scholar] [CrossRef]
- DuBois, M.; Tseng, A.; Francis, S.M.; Haynos, A.F.; Peterson, C.B.; Jacob, S. Utility of Downstream Biomarkers to Assess and Optimize Intranasal Delivery of Oxytocin. Pharmaceutics 2022, 14, 1178. [Google Scholar] [CrossRef] [PubMed]
- Guastella, A.J.; Howard, A.L.; Mitchell, P.; Carson, D.S.; Dadds, M. A randomized controlled trial of intranasal oxytocin as an adjunct to exposure therapy for social anxiety disorder. Psychoneuroendocrinology 2009, 34, 917–923. [Google Scholar] [CrossRef]
- Rayala, R.; Tiller, A.; Majumder, S.A.; Stacy, H.M.; Eans, S.O.; Nedovic, A.; McLaughlin, J.P.; Cudic, P. Solid-Phase Synthesis of the Bicyclic Peptide OL-CTOP Containing Two Disulfide Bridges, and an Assessment of Its In Vivo μ-Opioid Receptor Antagonism after Nasal Administration. Molecules 2023, 28, 1822. [Google Scholar] [CrossRef]
- Hiroki, T.; Keiko, I.; Bin, J.; Kayoko, T.; Takako, E.; Keizo, T.; Tsuyoshi, M.; Masato, H.; Naruhiko, S.; Nobuhisa, I.; et al. Nasal vaccine delivery attenuates brain pathology and cognitive impairment in tauopathy model mice. npj Vaccines 2020, 5, 28. [Google Scholar] [CrossRef]
- Tonelli, L.H.; Postolache, T.T. Airborne inflammatory factors: “From the nose to the brain”. Front. Biosci. 2010, 2, 135–152. [Google Scholar] [CrossRef]
- Barnett, E.M.; Perlman, S. The olfactory nerve and not the trigeminal nerve is the major site of CNS entry for mouse hepatitis virus, strain JHM. Virology 1993, 194, 185–191. [Google Scholar] [CrossRef]
- Kristensson, K. Microbes’ roadmap to neurons. Nat. Rev. Neurosci. 2011, 12, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Belur, L.; Temme, A.; Podetz-Pedersen, K.; Riedl, M.; Vulchanova, L.; Robinson, N.; Hanson, L.; Kozarsky, K.; Orchard, P.; Frey, W.; et al. Intranasal Adeno-Associated Virus Mediated Gene Delivery and Expression of Human Iduronidase in the Central Nervous System: A Noninvasive and Effective Approach for Prevention of Neurologic Disease in Mucopolysaccharidosis Type I. Hum. Gene Ther. 2017, 28, 576–587. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Chukwu, C.; Yang, Y.; Hu, Z.; Chen, H. Adeno-associated virus vector delivery to the brain: Technology advancements and clinical applications. Adv. Drug Deliv. Rev. 2024, 211, 115363. [Google Scholar] [CrossRef]
- Maguire, C.A.; Peters, C.W.; Hanlon, K.S. Delivering AAV to the Central Nervous and Sensory Systems. Trends Pharmacol. Sci. 2021, 42, 461–474. [Google Scholar] [CrossRef]
- Cossette, B.; Kelly, S.H.; Collier, J.H. Intranasal Subunit Vaccination Strategies Employing Nanomaterials and Biomaterials. ACS Biomater. Sci. Eng. 2020, 7, 1765–1779. [Google Scholar] [CrossRef]
- Calzas, C.; Chevalier, C. Innovative Mucosal Vaccine Formulations Against Influenza A Virus Infections. Front. Immunol. 2019, 10, 1605. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-S.; Xu, H.; AboulFotouh, K.; Williams, G.; Suman, J.; Sahakijpijarn, S.; Cano, C.; Warnken, Z.N.; Wu, K.C.W.; Williams, R.O.; et al. Intranasal delivery of thin-film freeze-dried monoclonal antibodies using a powder nasal spray system. Int. J. Pharm. 2024, 653, 123892. [Google Scholar] [CrossRef]
- Smith, C.A.; Kulkarni, U.; Chen, J.; Goldstein, D.R. Influenza virus inoculum volume is critical to elucidate age—Dependent mortality in mice. Aging Cell 2019, 18, e12893. [Google Scholar] [CrossRef]
- Xu, Y.; Yan, X.; Wei, T.; Chen, M.; Zhu, J.; Gao, J.; Liu, B.; Zhu, W.; Liu, Z. Transmucosal Delivery of Nasal Nanovaccines Enhancing Mucosal and Systemic Immunity. Nano Lett. 2023, 23, 10522–10531. [Google Scholar] [CrossRef]
- Xu, H.; Cai, L.; Hufnagel, S.; Cui, Z. Intranasal vaccine: Factors to consider in research and development. Int. J. Pharm. 2021, 609, 121180. [Google Scholar] [CrossRef]
- Seefeld, M.L.; Templeton, E.L.; Lehtinen, J.M.; Sinclair, N.; Yadav, D.; Hartwell, B.L. Harnessing the potential of the NALT and BALT as targets for immunomodulation using engineering strategies to enhance mucosal uptake. Front. Immunol. 2024, 15, 1419527. [Google Scholar] [CrossRef]
- Meinhardt, J.; Radke, J.; Dittmayer, C.; Franz, J.; Thomas, C.; Mothes, R.; Laue, M.; Schneider, J.; Brünink, S.; Greuel, S.; et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021, 24, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Ezeasor, C.; Shoyinka, S.; Emikpe, B.; Bodjo, C. Intranasal Peste des petits ruminants virus vaccination of goats using Irvingia gabonensis gum as delivery system: Hematological and humoral immune responses. J. Immunoass. Immunochem. 2020, 42, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Kim, J.; Jeong, Y.; Jang, Y.-S. Intranasal immunization with a Middle East respiratory syndrome-coronavirus antigen conjugated to the M-cell targeting ligand Co4B enhances antigen-specific mucosal and systemic immunity and protects against infection. Vaccine 2022, 40, 714–725. [Google Scholar] [CrossRef]
- Kraus, A.; Huertas, M.; Ellis, L.; Boudinot, P.; Levraud, J.-P.; Salinas, I. Intranasal delivery of SARS-CoV-2 spike protein is sufficient to cause olfactory damage, inflammation and olfactory dysfunction in zebrafish. Brain Behav. Immun. 2022, 102, 341–359. [Google Scholar] [CrossRef]
- Neary, M.; Sharp, J.; Gallardo-Toledo, E.; Herriott, J.; Kijak, E.; Bramwell, C.; Cox, H.; Tatham, L.; Box, H.; Curley, P.; et al. Evaluation of Nafamostat as Chemoprophylaxis for SARS-CoV-2 Infection in Hamsters. Viruses 2023, 15, 1744. [Google Scholar] [CrossRef] [PubMed]
- Jearanaiwitayakul, T.; Seesen, M.; Chawengkirttikul, R.; Limthongkul, J.; Apichirapokey, S.; Sapsutthipas, S.; Phumiamorn, S.; Sunintaboon, P.; Ubol, S. Intranasal Administration of RBD Nanoparticles Confers Induction of Mucosal and Systemic Immunity against SARS-CoV-2. Vaccines 2021, 9, 768. [Google Scholar] [CrossRef]
- Teng, Z.; Meng, L.-Y.; Yang, J.-K.; He, Z.; Chen, X.-G.; Liu, Y. Bridging nanoplatform and vaccine delivery, a landscape of strategy to enhance nasal immunity. J. Control. Release 2022, 351, 456–475. [Google Scholar] [CrossRef]
- Alzhrani, R.F.; Xu, H.; Valdes, S.A.; Cui, Z. Intranasal delivery of a nicotine vaccine candidate induces antibodies in mouse blood and lung mucosal secretions that specifically neutralize nicotine. Drug Dev. Ind. Pharm. 2020, 46, 1656–1664. [Google Scholar] [CrossRef]
- Yi, E.-J.; Kim, Y.-I.; Song, J.-H.; Ko, H.-J.; Chang, S.-Y. Intranasal immunization with curdlan induce Th17 responses and enhance protection against enterovirus 71. Vaccine 2023, 41, 2243–2252. [Google Scholar] [CrossRef]
- Tolman, L.E.; Yates, J.L.; Rong, Y.; Reynolds-Peterson, C.; Ehrbar, D.; Torres-Velez, F.J.; Mantis, N.J. Durable Immunity to Ricin Toxin Elicited by Intranasally Administered Monoclonal Antibody–Based Immune Complexes. ImmunoHorizons 2022, 6, 324–333. [Google Scholar] [CrossRef]
- Ryu, J.-Y.; Cerecedo-Lopez, C.; Yang, H.; Ryu, I.; Du, R. Brain-targeted intranasal delivery of protein-based gene therapy for treatment of ischemic stroke. Theranostics 2024, 14, 4773–4786. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.; Kim, M.; Lee, Y.; Lee, M. Intranasal delivery of self-assembled nanoparticles of therapeutic peptides and antagomirs elicits anti-tumor effects in an intracranial glioblastoma model. Nanoscale 2021, 13, 14745–14759. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.E.E.; Caron, N.S.; Black, H.F.; Schmidt, M.E.; Anderson, C.; Ko, S.; Baddeley, H.J.E.; Anderson, L.; Casal, L.L.; Rahavi, R.S.M.; et al. Delivery of mutant huntingtin-lowering antisense oligonucleotides to the brain by intranasally administered apolipoprotein A-I nanodisks. J. Control. Release 2023, 360, 913–927. [Google Scholar] [CrossRef]
- Petkova, A.I.; Kubajewska, I.; Vaideanu, A.; Schätzlein, A.G.; Uchegbu, I.F. Gene Targeting to the Cerebral Cortex Following Intranasal Administration of Polyplexes. Pharmaceutics 2022, 14, 1136. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Perets, N.; Betzer, O.; Ben-Shaul, S.; Sheinin, A.; Michaelevski, I.; Popovtzer, R.; Offen, D.; Levenberg, S. Intranasal Delivery of Mesenchymal Stem Cell Derived Exosomes Loaded with Phosphatase and Tensin Homolog siRNA Repairs Complete Spinal Cord Injury. ACS Nano 2019, 13, 10015–10028. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Wu, S.; Zhang, R.; Zhou, B.; Zhang, X.; Tang, L.; Tian, Y.; Men, K.; Yang, L. Enhanced nose-to-brain delivery of siRNA using hyaluronan-enveloped nanomicelles for glioma therapy. J Control. Release 2022, 342, 66–80. [Google Scholar] [CrossRef]
- Dhaliwal, H.K.; Fan, Y.; Kim, J.; Amiji, M.M. Intranasal Delivery and Transfection of mRNA Therapeutics in the Brain Using Cationic Liposomes. Mol. Pharm. 2020, 17, 1996–2005. [Google Scholar] [CrossRef]
- Lee, D.; Minko, T. Nanotherapeutics for Nose-to-Brain Drug Delivery: An Approach to Bypass the Blood Brain Barrier. Pharmaceutics 2021, 13, 2049. [Google Scholar] [CrossRef]
- Kronner, J.M.; Folbe, A.; Meythaler, J.; Nelson, J.O.; Borisov, A.; Peduzzi, J.D. Intranasally Applied Human Olfactory Mucosa Neural Progenitor Cells Migrate to Damaged Brain Regions. Future Sci. OA 2022, 8, FSO806. [Google Scholar] [CrossRef]
- Lu, S.; Li, K.; Yang, Y.; Wang, Q.; Yu, Y.; Wang, Z.; Luan, Z. Optimization of an Intranasal Route for the Delivery of Human Neural Stem Cells to Treat a Neonatal Hypoxic-Ischemic Brain Injury Rat Model. Neuropsychiatr. Dis. Treat. 2022, 18, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.-H.; Ji, W.-L.; Chen, H.; Sun, Y.-Y.; Zhao, X.-Y.; Wang, F.; Shi, Y.; Hu, Y.-N.; Liu, B.-X.; Wu, J.-W.; et al. Intranasal Transplantation of Human Neural Stem Cells Ameliorates Alzheimer’s Disease-Like Pathology in a Mouse Model. Front. Aging Neurosci. 2021, 13, 650103. [Google Scholar] [CrossRef]
- Tang, Y.; Han, L.; Bai, X.; Liang, X.; Zhao, J.; Huang, F.; Wang, J. Intranasal Delivery of Bone Marrow Stromal Cells Preconditioned with Fasudil to Treat a Mouse Model of Parkinson’s Disease. Neuropsychiatr. Dis. Treat. 2020, 16, 249–262. [Google Scholar] [CrossRef]
- Simon, C.; Gan, Q.F.; Kathivaloo, P.; Mohamad, N.A.; Dhamodharan, J.; Krishnan, A.; Sengodan, B.; Palanimuthu, V.R.; Marimuthu, K.; Rajandas, H.; et al. Deciduous DPSCs Ameliorate MPTP-Mediated Neurotoxicity, Sensorimotor Coordination and Olfactory Function in Parkinsonian Mice. Int. J. Mol. Sci. 2019, 20, 568. [Google Scholar] [CrossRef]
- Moreira, A.; Winter, C.; Joy, J.; Winter, L.; Jones, M.; Noronha, M.; Porter, M.; Quim, K.; Corral, A.; Alayli, Y.; et al. Intranasal delivery of human umbilical cord Wharton’s jelly mesenchymal stromal cells restores lung alveolarization and vascularization in experimental bronchopulmonary dysplasia. Stem Cells Transl. Med. 2020, 9, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Hay, A.J.D.; Latham, A.S.; Mumford, G.; Hines, A.D.; Risen, S.; Gordon, E.; Siebenaler, C.; Gilberto, V.S.; Zabel, M.D.; Moreno, J.A. Intranasally delivered mesenchymal stromal cells decrease glial inflammation early in prion disease. Front. Neurosci. 2023, 17, 1158408. [Google Scholar] [CrossRef]
- Yu, S.P.; Gu, X.; Chau, M.J.; Deveau, T.C.; Kim, Y.S.; Wei, L.; Xu, Y. Delayed and repeated intranasal delivery of bone marrow stromal cells increases regeneration and functional recovery after ischemic stroke in mice. BMC Neurosci. 2018, 19, 20. [Google Scholar] [CrossRef]
- Parvez, S.; A Puchowicz, M.; Parveen, K.; Stayton, A.S.; Bajwa, A.; Salman, M.; Parveen, A.; Ishrat, T. Intranasal Delivery of Mitochondria Attenuates Brain Injury by AMPK and SIRT1/PGC-1α Pathways in a Murine Model of Photothrombotic Stroke. Mol. Neurobiol. 2023, 61, 2822–2838. [Google Scholar] [CrossRef]
- Price, T.J.; Alexander, J.F.; Heijnen, C.J.; Wangzhou, A.; Ray, P.R.; Arroyo, L.D.; Seua, A.V.; Heiβ-Lückemann, L.; Schedlowski, M.; Kavelaars, A. Nasal administration of mitochondria reverses chemotherapy-induced cognitive deficits. Theranostics 2021, 11, 3109–3130. [Google Scholar] [CrossRef]
- Sánchez, S.V.; Otavalo, G.N.; Gazeau, F.; Silva, A.K.A.; Morales, J.O. Intranasal delivery of extracellular vesicles: A promising new approach for treating neurological and respiratory disorders. J. Control. Release 2025, 379, 489–523. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, N.; Liu, J.; Ren, H.; Jiang, W.; Lei, Y.; Fu, X.; Hao, M.; Lang, X.; Liu, Y.; et al. Intranasal delivery of hMSC-derived supernatant for treatment of ischemic stroke by inhibiting the pro-inflammatory polarization of neutrophils. Stem Cell Res. Ther. 2025, 16, 43. [Google Scholar] [CrossRef]
- Ikeda, T.; Kawabori, M.; Zheng, Y.; Yamaguchi, S.; Gotoh, S.; Nakahara, Y.; Yoshie, E.; Fujimura, M. Intranasal Administration of Mesenchymal Stem Cell-Derived Exosome Alleviates Hypoxic-Ischemic Brain Injury. Pharmaceutics 2024, 16, 446. [Google Scholar] [CrossRef] [PubMed]
- El-Nabarawy, N.A.; Teaima, M.H.; Helal, D.A. Assessment of Spanlastic Vesicles of Zolmitriptan for Treating Migraine In Rats. Drug Des. Dev. Ther. 2019, 13, 3929–3937. [Google Scholar] [CrossRef] [PubMed]
- Kouhjani, M.; Jaafari, M.R.; Saberi, A.; Gholami, L.; Tafaghodi, M. Nose to brain delivery of insulin loaded in PLGA and chitosan-coated PLGA nanoparticles: A promising approach for Alzheimer’s disease therapy. J. Drug Deliv. Sci. Technol. 2025, 108, 106857. [Google Scholar] [CrossRef]
- Alves, G.; Falcão, A.; Meirinho, S.; Rodrigues, M.; Santos, A.O. Self-Emulsifying Drug Delivery Systems: An Alternative Approach to Improve Brain Bioavailability of Poorly Water-Soluble Drugs through Intranasal Administration. Pharmaceutics 2022, 14, 1487. [Google Scholar] [CrossRef]
- Lim, C.; Koo, J.; Oh, K.T. Recent Advances in Intranasal Administration for Brain-Targeting Delivery: A Comprehensive Review of Lipid-Based Nanoparticles and Stimuli-Responsive Gel Formulations. Int. J. Nanomed. 2024, 19, 1767–1807. [Google Scholar] [CrossRef]
- Shah, P.; Lalan, M.; Barve, K. Intranasal delivery: An attractive route for the administration of nucleic acid based therapeutics for CNS disorders. Front Pharmacol. 2022, 13, 974666. [Google Scholar] [CrossRef]
- Xu, J.; Hsu, S.-H. Self-healing hydrogel as an injectable implant: Translation in brain diseases. J. Biomed. Sci. 2023, 30, 43. [Google Scholar] [CrossRef]
- Zhao, W.; Yu, F.; Sun, K.; Zheng, X.; Jin, H. Exosomes as CNS Drug Delivery Tools and Their Applications. Pharmaceutics 2022, 14, 2252. [Google Scholar] [CrossRef]
- Shi, B.; Liu, Y.; Zheng, M.; Rehman, F.U. Exosomes based strategies for brain drug delivery. Biomaterials 2022, 293, 121949. [Google Scholar] [CrossRef]
- Huang, Y.; Qin, S.; Liu, J.; Wen, Y.; Li, C.; Zhao, W. Overcoming the blood-brain barrier: Exosomes as theranostic nanocarriers for precision neuroimaging. Release 2022, 349, 902–916. [Google Scholar] [CrossRef]
- Weinberg, M.S.; Samulski, R.J.; McCown, T.J. Adeno-associated virus (AAV) gene therapy for neurological disease. Neuropharmacology 2013, 69, 82–88. [Google Scholar] [CrossRef]
- Grimm, D.; Gadenstaetter, A.J.; Schmutzler, L.; Landegger, L.D. Intranasal application of adeno-associated viruses: A systematic review. Transl. Res. 2022, 248, 87–110. [Google Scholar] [CrossRef]
- Price, R.J.; Mead, B.P.; Timbie, K.F. Drug and gene delivery across the blood-brain barrier with focused ultrasound. J. Control. Release 2015, 219, 61–75. [Google Scholar] [CrossRef]
- Hafner, A. Advances in Development, Characterisation and Application of Nasal Drug Delivery Systems. Pharmaceutics 2022, 14, 1562. [Google Scholar] [CrossRef]
- Kashyap, K.; Shukla, R. Drug Delivery and Targeting to the Brain Through Nasal Route: Mechanisms, Applications and Challenges. Curr. Drug Deliv. 2019, 16, 887–901. [Google Scholar] [CrossRef] [PubMed]
- Herzog, H.; Glöckler, S.; Flamm, J.; Ladel, S.; Maigler, F.; Pitzer, C.; Schindowski, K. Intranasal Nose-to-Brain Drug Delivery via the Olfactory Region in Mice: Two In-Depth Protocols for Region-Specific Intranasal Application of Antibodies and for Expression Analysis of Fc Receptors via In Situ Hybridization in the Nasal Mucosa. In Tau Protein; Methods in Molecular Biology; Springer: New York, NY, USA, 2024; pp. 387–410. [Google Scholar]
- Chen, J.; Finlay, W.H.; Vehring, R.; Martin, A.R. Characterizing regional drug delivery within the nasal airways. Expert Opin. Drug Deliv. 2024, 21, 537–551. [Google Scholar] [CrossRef]
- Flamm, J.; Hartung, S.; Gänger, S.; Maigler, F.; Pitzer, C.; Schindowski, K. Establishment of an Olfactory Region-specific Intranasal Delivery Technique in Mice to Target the Central Nervous System. Front. Pharmacol. 2022, 12, 789780. [Google Scholar] [CrossRef]
- Simón, J.A.; Utomo, E.; Pareja, F.; Collantes, M.; Quincoces, G.; Otero, A.; Ecay, M.; Domínguez-Robles, J.; Larrañeta, E.; Peñuelas, I. Radiolabeled Risperidone microSPECT/CT Imaging for Intranasal Implant Studies Development. Pharmaceutics 2023, 15, 843. [Google Scholar] [CrossRef]
- Ye, D.; Chen, H. Focused ultrasound-mediated intranasal brain drug delivery technique (FUSIN). MethodsX 2021, 8, 101266. [Google Scholar] [CrossRef]
- Ye, D.; Luan, J.; Pang, H.; Yang, Y.; Nazeri, A.; Rubin, J.B.; Chen, H. Characterization of focused ultrasound-mediated brainstem delivery of intranasally administered agents. J. Control. Release 2020, 328, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Yuan, J.; Yang, Y.; Yue, Y.; Hu, Z.; Fadera, S.; Chen, H. Incisionless targeted adeno-associated viral vector delivery to the brain by focused ultrasound-mediated intranasal administration. eBioMedicine 2022, 84, 104277. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Park, S.H.; Kim, E.; Kim, J.y.; Kim, S.W.; Choi, H. A Magnetically Powered Stem Cell—Based Microrobot for Minimally Invasive Stem Cell Delivery via the Intranasal Pathway in a Mouse Brain. Adv. Healthc. Mater. 2021, 10, 2100801. [Google Scholar] [CrossRef]
- Wang, J.; Liao, Z.-X. Research progress of microrobots in tumor drug delivery. Food Med. Homol. 2024, 1, 9420025. [Google Scholar] [CrossRef]
- Stenslik, M.; Evans, A.; Pomerleau, F.; Weeks, R.; Huettl, P.; Foreman, E.; Turchan-Cholewo, J.; Andersen, A.; Cass, W.; Zhang, Z.; et al. Methodology and effects of repeated intranasal delivery of DNSP-11 in awake Rhesus macaques. J. Neurosci. Methods 2018, 303, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Wingrove, J.; Swedrowska, M.; Scherließ, R.; Parry, M.; Ramjeeawon, M.; Taylor, D.; Gauthier, G.; Brown, L.; Amiel, S.; Zelaya, F.; et al. Characterisation of nasal devices for delivery of insulin to the brain and evaluation in humans using functional magnetic resonance imaging. J. Control. Release 2019, 302, 140–147. [Google Scholar] [CrossRef]
- Ye, E.; Park, E.; Kim, E.; Lee, J.E.; Yang, S.H.; Park, S.-M. Transcranial application of magnetic pulses for improving brain drug delivery efficiency via intranasal injection of magnetic nanoparticles. Biomed. Eng. Lett. 2023, 13, 417–427. [Google Scholar] [CrossRef]
- Hong, S.-S.; Oh, K.T.; Choi, H.-G.; Lim, S.-J. Liposomal Formulations for Nose-to-Brain Delivery: Recent Advances and Future Perspectives. Pharmaceutics 2019, 11, 540. [Google Scholar] [CrossRef]
- Sawarkar, S.P.; Tanna, V.; Ravikumar, P. Exploring Nose to Brain Nano Delivery for Effective Management of Migraine. Curr. Drug Deliv. 2023, 20, 144–157. [Google Scholar] [CrossRef]
- Bai, J.; Wei, Y.-S.; He, Y.; Zhang, H.; Ma, X. Preparation and biological activity of polysaccharide metal ion complex and feasibility analysis of Tremella fuciformis polysaccharide with metal ions. Food Med. Homol. 2025, 2, 9420031. [Google Scholar] [CrossRef]
- Nasr, M.; Wahdan, S.A. Neuroprotective effects of novel nanosystems simultaneously loaded with vinpocetine and piracetam after intranasal administration. Life Sci. 2019, 226, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.F.; Aboud, H.M.; Abdellatif, M.M.; Abou-Taleb, H.A. Nose-to-Brain Targeted Delivery of Donepezil Hydrochloride via Novel Hyaluronic Acid-Doped Nanotransfersomes for Alzheimer’s Disease Mitigation. J. Pharm. Sci. 2024, 113, 1934–1945. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Gabrani, R.; Nigam, K.; Kaur, A.; Nematullah; Khan, F.; Dang, S. Nose-to-brain delivery of lamotrigine-loaded PLGA nanoparticles. Drug Deliv. Transl. Res. 2019, 9, 879–890. [Google Scholar] [CrossRef]
- Wasan, E.K.; Cuddihy, G.; Rai, R.; Aibani, N.; Patel, P. Chitosan Nanoparticles at the Biological Interface: Implications for Drug Delivery. Pharmaceutics 2021, 13, 1686. [Google Scholar] [CrossRef]
- Walgrave, H.; Zhou, L.; De Strooper, B.; Salta, E. The promise of microRNA-based therapies in Alzheimer’s disease: Challenges and perspectives. Mol. Neurodegener. 2021, 16, 76. [Google Scholar] [CrossRef]
- Oppong-Damoah, A.; Zaman, R.U.; D’Souza, M.J.; Murnane, K.S. Nanoparticle encapsulation increases the brain penetrance and duration of action of intranasal oxytocin. Horm. Behav. 2019, 108, 20–29. [Google Scholar] [CrossRef]
- Pomatto, M.A.C.; Gai, C.; Negro, F.; Massari, L.; Deregibus, M.C.; Grange, C.; De Rosa, F.G.; Camussi, G. Plant-Derived Extracellular Vesicles as a Delivery Platform for RNA-Based Vaccine: Feasibility Study of an Oral and Intranasal SARS-CoV-2 Vaccine. Pharmaceutics 2023, 15, 974. [Google Scholar] [CrossRef] [PubMed]
- Gupta, I.; Adin, S.N.; Aqil, M.; Mujeeb, M. Nose to brain delivery of naringin loaded transniosomes for epilepsy: Formulation, characterisation, blood-brain distribution and invivo pharmacodynamic evaluation. J. Liposome Res. 2023, 34, 60–76. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, R.; Wang, Y.; Zhang, X.; Yang, Y.; Zhao, B.; Yang, L. A simple self-assembly nanomicelle based on brain tumor-targeting peptide-mediated siRNA delivery for glioma immunotherapy via intranasal administration. Acta Biomater. 2023, 155, 521–537. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, X.; Zhou, Z.; Ferguson, T.; Obliosca, J.; Luo, C.C.; Chan, K.W.; Kong, X.P.; Tison, C.K. Mucosal Delivery of HIV-1 Glycoprotein Vaccine Candidate Enabled by Short Carbon Nanotubes. Part. Part. Syst. Charact. 2022, 39, 2200011. [Google Scholar] [CrossRef]
- Hua, T.; Li, S.; Han, B. Nanomedicines for intranasal delivery: Understanding the nano-bio interactions at the nasal mucus-mucosal barrier. Expert Opin. Drug Deliv. 2024, 21, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Ramot, Y.; Rottenberg, Y.; Domb, A.J.; Kubek, M.J.; Williams, K.D.; Nyska, A. Preclinical In-Vivo Safety of a Novel Thyrotropin-Releasing Hormone-Loaded Biodegradable Nanoparticles After Intranasal Administration in Rats and Primates. Int. J. Toxicol. 2023, 42, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Allegritti, E.; Battista, S.; Maggi, M.A.; Marconi, C.; Galantini, L.; Giansanti, L. Novel liposomal formulations for protection and delivery of levodopa: Structure-properties correlation. Int. J. Pharm. 2023, 643, 123230. [Google Scholar] [CrossRef]
- Elsheikh, M.A.; El-Feky, Y.A.; Al-Sawahli, M.M.; Ali, M.E.; Fayez, A.M.; Abbas, H. A Brain-Targeted Approach to Ameliorate Memory Disorders in a Sporadic Alzheimer’s Disease Mouse Model via Intranasal Luteolin-Loaded Nanobilosomes. Pharmaceutics 2022, 14, 576. [Google Scholar] [CrossRef]
- Soligo, M.; Felsani, F.M.; Da Ros, T.; Bosi, S.; Pellizzoni, E.; Bruni, S.; Isopi, J.; Marcaccio, M.; Manni, L.; Fiorito, S. Distribution in the brain and possible neuroprotective effects of intranasally delivered multi-walled carbon nanotubes. Nanoscale Adv. 2021, 3, 418–431. [Google Scholar] [CrossRef]
- Hayes, S.H.; Liu, Q.; Selvakumaran, S.; Haney, M.J.; Batrakova, E.V.; Allman, B.L.; Walton, P.A.; Kiser, P.; Whitehead, S.N. Brain Targeting and Toxicological Assessment of the Extracellular Vesicle-Packaged Antioxidant Catalase-SKL Following Intranasal Administration in Mice. Neurotox. Res. 2021, 39, 1418–1429. [Google Scholar] [CrossRef]
- Hao, R.; Sun, B.; Yang, L.; Ma, C.; Li, S. RVG29-modified microRNA-loaded nanoparticles improve ischemic brain injury by nasal delivery. Drug Deliv. 2020, 27, 772–781. [Google Scholar] [CrossRef]
- Liao, K.; Niu, F.; Dagur, R.S.; He, M.; Tian, C.; Hu, G. Intranasal Delivery of lincRNA-Cox2 siRNA Loaded Extracellular Vesicles Decreases Lipopolysaccharide-Induced Microglial Proliferation in Mice. J. Neuroimmune Pharmacol. 2019, 15, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Sun, B.; Gao, X.; Dong, X.; Fu, L.; Zhang, Y.; Li, Z.; Wang, Y.; Jiang, H.; Han, B. Intranasal Delivery of Targeted Nanoparticles Loaded With miR-132 to Brain for the Treatment of Neurodegenerative Diseases. Front. Pharmacol. 2020, 11, 1165. [Google Scholar] [CrossRef]
- Diao, Y.; Wu, X.; Zang, R.; Liu, M.; Wei, S.; Yang, N.; Qiu, Y.; Xu, X. Self-Assembly of Rhein and Matrine Nanoparticles for Enhanced Wound Healing. Molecules 2024, 29, 3326. [Google Scholar] [CrossRef]
- Miao, Y.-H.; Wang, X.; Zhao, X.-M.; Hu, Y.-W.; Liu, X.; Deng, D.-W. Co-assembly strategies of natural plant compounds for improving their bioavailability. Food Med. Homol. 2025, 2, 9420022. [Google Scholar] [CrossRef]
- Ali, M.M.; Shoukri, R.A.; Yousry, C. Thin film hydration versus modified spraying technique to fabricate intranasal spanlastic nanovesicles for rasagiline mesylate brain delivery: Characterization, statistical optimization, and in vivo pharmacokinetic evaluation. Drug Deliv. Transl. Res. 2022, 13, 1153–1168. [Google Scholar] [CrossRef] [PubMed]
- Kadakia, E.; Bottino, D.; Amiji, M. Mathematical Modeling and Simulation to Investigate the CNS Transport Characteristics of Nanoemulsion-Based Drug Delivery Following Intranasal Administration. Pharm. Res. 2019, 36, 75. [Google Scholar] [CrossRef]
- Sipos, B.; Csóka, I.; Szivacski, N.; Budai-Szűcs, M.; Schelcz, Z.; Zupkó, I.; Szabó-Révész, P.; Volk, B.; Katona, G. Mucoadhesive meloxicam-loaded nanoemulsions: Development, characterization and nasal applicability studies. Eur. J. Pharm. Sci. 2022, 175, 106229. [Google Scholar] [CrossRef]
- Pailla, S.; Sampathi, S.; Junnuthula, V.; Maddukuri, S.; Dodoala, S.; Dyawanapelly, S. Brain-Targeted Intranasal Delivery of Zotepine Microemulsion: Pharmacokinetics and Pharmacodynamics. Pharmaceutics 2022, 14, 978. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Nigam, K.; Srivastava, S.; Tyagi, A.; Dang, S. Memantine nanoemulsion: A new approach to treat Alzheimer’s disease. J. Microencapsul. 2020, 37, 355–365. [Google Scholar] [CrossRef]
- Khunt, D.; Shrivas, M.; Polaka, S.; Gondaliya, P.; Misra, M. Role of Omega-3 Fatty Acids and Butter Oil in Targeting Delivery of Donepezil Hydrochloride Microemulsion to Brain via the Intranasal Route: A Comparative Study. AAPS PharmSciTech 2020, 21, 45. [Google Scholar] [CrossRef]
- Gaba, B.; Khan, T.; Haider, M.F.; Alam, T.; Baboota, S.; Parvez, S.; Ali, J. Vitamin E Loaded Naringenin Nanoemulsion via Intranasal Delivery for the Management of Oxidative Stress in a 6-OHDA Parkinson’s Disease Model. BioMed Res. Int. 2019, 2019, 2382563. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, C.; Zhai, W.; Zhuang, N.; Han, T.; Ding, Z. The Optimization Design Of Lactoferrin Loaded HupA Nanoemulsion For Targeted Drug Transport Via Intranasal Route. Int. J. Nanomed. 2019, 14, 9217–9234. [Google Scholar] [CrossRef]
- Pragya; Bisht, S.; Parashar, P. Nanotechnology-driven Microemulsion Based Intranasal Delivery to Neurotechnology-driven Neuralink: Strategies to Improve Management of Neurodegenerative Disorders. AAPS PharmSciTech 2024, 25, 215. [Google Scholar] [CrossRef]
- Liu, N.; Yang, C.; Liang, X.; Cao, K.; Xie, J.; Luo, Q.; Luo, H. Mesoporous silica nanoparticle-encapsulated Bifidobacterium attenuates brain Aβ burden and improves olfactory dysfunction of APP/PS1 mice by nasal delivery. J. Nanobiotechnology 2022, 20, 439. [Google Scholar] [CrossRef] [PubMed]
- Latif, R.; Makar, R.R.; Hosni, E.A.; El Gazayerly, O.N. The potential of intranasal delivery of nanocrystals in powder form on the improvement of zaleplon performance: In-vitro, in-vivo assessment. Drug Dev. Ind. Pharm. 2021, 47, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-S.; Li, K.; Gao, L.-N.; Zhang, Y.; Lin, K.-M.; Cui, Y.-L. Intranasal delivery of berberine via in situ thermoresponsive hydrogels with non-invasive therapy exhibits better antidepressant-like effects. Biomater. Sci. 2020, 8, 2853–2865. [Google Scholar] [CrossRef]
- Salem, H.F.; Ali, A.A.; Hegazy, A.M.; Sadek, A.-R.A.; Aboud, H.M. Harnessing of Doxylamine Succinate/Pyridoxine Hydrochloride-Dual Laden Bilosomes as a Novel Combinatorial Nanoparadigm for Intranasal Delivery: In Vitro Optimization and In Vivo Pharmacokinetic Appraisal. J. Pharm. Sci. 2022, 111, 794–809. [Google Scholar] [CrossRef]
- Xu, D.; Qiao, T.; Wang, Y.; Wang, Q.-S.; Cui, Y.-L. Alginate nanogels-based thermosensitive hydrogel to improve antidepressant-like effects of albiflorin via intranasal delivery. Drug Deliv. 2021, 28, 2137–2149. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tan, Y.; Dai, Y.; Hu, K.; Tan, X.; Jiang, S.; Li, G.; Zhang, X.; Kang, L.; Wang, X.; et al. Intranasal Delivery of Endothelial Cell-Derived Extracellular Vesicles with Supramolecular Gel Attenuates Myocardial Ischemia-Reperfusion Injury. Int. J. Nanomed. 2023, 18, 5495–5510. [Google Scholar] [CrossRef]
- Liu, L.; Hao, H.; Wu, X.; Li, P.; Ye, H.; Zheng, Q.; Yang, H. Protocatechuic aldehyde protects cardiomycoytes against ischemic injury via regulation of nuclear pyruvate kinase M2. Acta Pharm. Sin. B 2021, 11, 3553–3566. [Google Scholar] [CrossRef]
- Liu, L.; Hao, H.; Ye, H.; Wu, X.; Yang, H.; Li, P.; Zheng, Q. Dihydrotanshinone I preconditions myocardium against ischemic injury via PKM2 glutathionylation sensitive to ROS. Acta Pharm. Sin. B 2023, 13, 113–127. [Google Scholar] [CrossRef]
- Mohanty, D.; Alsaidan, O.A.; Zafar, A.; Dodle, T.; Gupta, J.K.; Yasir, M.; Mohanty, A.; Khalid, M. Development of Atomoxetine-Loaded NLC In Situ Gel for Nose-to-Brain Delivery: Optimization, In Vitro, and Preclinical Evaluation. Pharmaceutics 2023, 15, 1985. [Google Scholar] [CrossRef]
- Popescu, R.; Dinu-Pîrvu, C.-E.; Ghica, M.V.; Anuța, V.; Popa, L. Physico-Chemical Characterization and Initial Evaluation of Carboxymethyl Chitosan–Hyaluronan Hydrocolloid Systems with Insulin Intended for Intranasal Administration. Int. J. Mol. Sci. 2024, 25, 10452. [Google Scholar] [CrossRef]
- Wang, J.T.W.; Rodrigo, A.C.; Patterson, A.K.; Hawkins, K.; Aly, M.M.S.; Sun, J.; Al Jamal, K.T.; Smith, D.K. Enhanced Delivery of Neuroactive Drugs via Nasal Delivery with a Self-Healing Supramolecular Gel. Adv. Sci. 2021, 8, 2101058. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Li, A.; Ma, L.; Iqbal, S.; Sun, X.; Ma, W.; Li, C.; Zheng, D.; Xu, Z.; Zhao, Z.; et al. Nose-to-brain delivery of disulfiram nanoemulsion in situ gel formulation for glioblastoma targeting therapy. Int. J. Pharm. 2021, 597, 120250. [Google Scholar] [CrossRef] [PubMed]
- Radwan, M.A.; El Khatib, M.M.; Alavi, S.E.; Al Harthi, S.; AlSarra, I.A. Nasal delivery of donepezil HCl-loaded hydrogels for the treatment of Alzheimer’s disease. Sci. Rep. 2019, 9, 9563. [Google Scholar] [CrossRef]
- Tan, Y.; Liu, Y.; Liu, Y.; Ma, R.; Luo, J.; Hong, H.; Chen, X.; Wang, S.; Liu, C.; Zhang, Y.; et al. Rational Design of Thermosensitive Hydrogel to Deliver Nanocrystals with Intranasal Administration for Brain Targeting in Parkinson’s Disease. Research 2021, 2021, 9812523. [Google Scholar] [CrossRef]
- Mulè, F.; Picone, P.; Biagio, P.L.S.; Dispenza, C.; Amato, A.; Ditta, L.A.; Sabatino, M.A.; Di Carlo, M.; Giacomazza, D. Nose-to-brain delivery of insulin enhanced by a nanogel carrier. J. Control. Release 2018, 270, 23–36. [Google Scholar] [CrossRef]
- Ulusoy, S.; Konstantinidis, I.; Passali, D.; Cingi, C.; Negm, H.; Milkov, M.; Muluk, N.B.; Karpischenko, S.; Passali, G.C.; Dilber, M.; et al. Mechanisms and solutions for nasal drug delivery—A narrative review. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 72–81. [Google Scholar] [CrossRef]
- Carrazana, E.; Rabinowicz, A.L.; Madden, S. Optimizing Absorption for Intranasal Delivery of Drugs Targeting the Central Nervous System Using Alkylsaccharide Permeation Enhancers. Pharmaceutics 2023, 15, 2119. [Google Scholar] [CrossRef]
- Almahmoud, A.; Parekh, H.S.; Paterson, B.M.; Tupally, K.R.; Vegh, V. Intranasal delivery of imaging agents to the brain. Theranostics 2024, 14, 5022–5101. [Google Scholar] [CrossRef]
- Kamei, N.; Suwabe, S.; Arime, K.; Bando, H.; Murata, K.; Yamaguchi, M.; Yokoyama, N.; Tanaka, E.; Hashimoto, A.; Kanazawa, T.; et al. Investigation of the Transport Pathways Associated with Enhanced Brain Delivery of Peptide Drugs by Intranasal Coadministration with Penetratin. Pharmaceutics 2021, 13, 1745. [Google Scholar] [CrossRef]
- Clow, F.; Peterken, K.; Pearson, V.; Proft, T.; Radcliff, F.J. PilVax, a novel Lactococcus lactis—Based mucosal vaccine platform, stimulates systemic and mucosal immune responses to Staphylococcus aureus. Immunol. Cell Biol. 2020, 98, 369–381. [Google Scholar] [CrossRef]
- Idoudi, S.; Saleh, A.; Akkbik, M.; Amine, L.; Alansari, K.; Rachid, O.; Alkilany, A.M. Investigating Strategies to Enhance the Aqueous Solubility of Ketamine HCl for Intranasal Delivery. Pharmaceutics 2024, 16, 1502. [Google Scholar] [CrossRef]
- Prakapenka, A.V.; Peña, V.L.; Strouse, I.; Northup-Smith, S.; Schrier, A.; Ahmed, K.; Bimonte-Nelson, H.A.; Sirianni, R.W. Intranasal 17β-Estradiol Modulates Spatial Learning and Memory in a Rat Model of Surgical Menopause. Pharmaceutics 2020, 12, 1225. [Google Scholar] [CrossRef]
- Gerard Lee, S.; Florencio Jr, A.; Sabrina, D.; Akie, O.; Muhammad Fikri Bin Mohd, F.; Ichiro, H.; Shoko, I.; Masanori, K.; Hiroaki, T.; Kenji, S. Enhanced nose-to-brain delivery of tranilast using liquid crystal formulations. J. Control. Release 2020, 325, 1–9. [Google Scholar] [CrossRef]
- Kim, N.A.; Thapa, R.; Jeong, S.H.; Bae, H.-d.; Maeng, J.; Lee, K.; Park, K. Enhanced intranasal insulin delivery by formulations and tumor protein-derived protein transduction domain as an absorption enhancer. J. Control. Release 2019, 294, 226–236. [Google Scholar] [CrossRef]
- Fukakusa, S.; Suzuki, C.; Sasaki, K.; Sonoda, Y.; Hatano, Y.; Haruta, S.; Magata, Y. Brain drug delivery from the nasal olfactory region is enhanced using lauroylcholine chloride: An estimation using in vivo PET imaging. Nucl. Med. Biol. 2024, 138–139, 108968. [Google Scholar] [CrossRef] [PubMed]
- Maeng, J.; Lee, K. Systemic and brain delivery of antidiabetic peptides through nasal administration using cell-penetrating peptides. Front Pharmacol. 2022, 13, 1068495. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.M.; Mercenier, A. Mucosal delivery of therapeutic and prophylactic molecules using lactic acid bacteria. Nat. Rev. Microbiol. 2008, 6, 349–362. [Google Scholar] [CrossRef]
- Zhang, H.; Sheng, S.; Li, C.; Bao, X.; Zhao, L.; Chen, J.; Guan, P.; Li, X.; Pan, N.; Liang, Y.; et al. Mucosal immunization with the lung Lactobacillus-derived amphiphilic exopolysaccharide adjuvanted recombinant vaccine improved protection against P. aeruginosa infection. PLoS Pathog. 2024, 20, e1012696. [Google Scholar] [CrossRef]
- Marchetti, J.M.; Barcellos, J.P.A.; Lee, R.J.; de Souza, M.C.; Petrilli, R.; Eloy, J.O. Liposomes as carriers of hydrophilic small molecule drugs: Strategies to enhance encapsulation and delivery. Colloids Surf. B Biointerfaces 2014, 123, 345–363. [Google Scholar] [CrossRef]
- Shalaby, K.; Abdelgawad, M.A.; Alsaidan, O.A.; Elkomy, M.H.; Gomaa, H.A.M.; Elmowafy, M.; Mostafa, E.M. Polymeric Nanoparticles for Delivery of Natural Bioactive Agents: Recent Advances and Challenges. Polymers 2023, 15, 1123. [Google Scholar] [CrossRef]
- Hatton, T.A.; Gupta, A.; Eral, H.B.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef]
- Kurczewska, J.; Dobosz, B. Recent Progress and Challenges Regarding Magnetite-Based Nanoparticles for Targeted Drug Delivery. Appl. Sci. 2024, 14, 1132. [Google Scholar] [CrossRef]
- Veronese, F.M.; Pasut, G. PEGylation, successful approach to drug delivery. Drug Discov. Today 2005, 10, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.; Jain, A.; Charbe, N.; Thalla, M.; Immanuel, S.; Kapre, S.; Palakurthi, S.S.; Pasham, S. A comprehensive review of challenges and advances in exosome-based drug delivery systems. Nanoscale Adv. 2024, 6, 5803–5826. [Google Scholar] [CrossRef]
- Zelkó, R.; Kazsoki, A.; Kürtösi, B. A Systematic Review on Plant-Derived Extracellular Vesicles as Drug Delivery Systems. Int. J. Mol. Sci. 2024, 25, 7559. [Google Scholar] [CrossRef]
- Choi, W.; Kohane, D.S. Hybrid Nanoparticle-Hydrogel Systems for Drug Delivery Depots and Other Biomedical Applications. ACS Nano 2024, 18, 22780–22792. [Google Scholar] [CrossRef]
- Frank-Ito, D.O. Olfaction and drug delivery to the human olfactory airspace: Current challenges and recent advances. Expert Opin. Drug Deliv. 2025, 22, 511–524. [Google Scholar] [CrossRef]
- Chen, J.; Martin, A.R.; Finlay, W.H. Recent In Vitro and In Silico Advances in the Understanding of Intranasal Drug Delivery. Curr. Pharm. Des. 2021, 27, 1482–1497. [Google Scholar] [CrossRef]
- League-Pascual, J.C.; Lester-McCully, C.M.; Shandilya, S.; Ronner, L.; Rodgers, L.; Cruz, R.; Peer, C.J.; Figg, W.D.; Warren, K.E. Plasma and cerebrospinal fluid pharmacokinetics of select chemotherapeutic agents following intranasal delivery in a non-human primate model. J. Neuro-Oncol. 2017, 132, 401–407. [Google Scholar] [CrossRef]
- Aderibigbe, B.A.; Naki, T. Chitosan-Based Nanocarriers for Nose to Brain Delivery. Appl. Sci. 2019, 9, 2219. [Google Scholar] [CrossRef]

| Drug Category | Specific Type | Relevant Examples |
|---|---|---|
| Chemical drugs | Solution dispersion | Solutions and suspensions |
| Nanocarriers/polymers | Liposomes, nanoparticles, micelles, and hydrogels | |
| Biomacromolecule drugs | Protein drugs | Recombinant proteins, antibodies, hormones, cytokines, and vaccines |
| Nucleic acid drugs | DNA, RNA, and viruses | |
| Cell-derived drugs | Cell-derived drugs | Stem cells, immune cells, and mitochondria |
| Exosome-derived drugs | Exosomes from different sources |
| Drug/ System Name | Application/Disease | Research Model | Key Findings | Ref. |
|---|---|---|---|---|
| Mixed Nanoparticle System | AD | Mouse Model | Rapid absorption, effective drug delivery to the brain, and high organ-specific drug concentration. | [51] |
| 9-cis Retinoic Acid | AD | AD Transgenic Mouse | Reduces Aβ deposition and improves neuroinflammation and synaptic function. | [54] |
| Fluorobiprofene Microspheres/Soft Particles | AD | Rat Model | Intranasal powder delivery superior to solution, significant olfactory bulb concentration, and early intervention potential. | [64] |
| Lacosamide | Epilepsy | Mouse Model | Better pharmacokinetics compared to intravenous injection. | [52] |
| Opioids/Sedatives | Palliative Care | Clinical Study | Rapid onset, good patient tolerance, suitable for late-stage patients unable to take oral drugs. | [60] |
| Dihydrocodeine/Midazolam | Community Palliative Care (Pain/Agitation) | Clinical Practice | Easy to administer, improves patient comfort, and reduces medical delay. | [61] |
| Heparin | COVID-19 Prevention | Mouse and Human Trials | No significant toxicity and maintains effective concentration for 12 h. | [65] |
| Dexmedetomidine | Sedation for Extractions | Anxiety Patient Study | Onset in 30–45 min, lasts 60–75 min, no respiratory suppression, suitable for day surgeries. | [55] |
| MC4R Antagonist HS014 | Trigeminal Neuralgia | Rat Model | Significant relief of hyperalgesia, upregulation of MC4R protein levels. | [56] |
| Ketamine | Post-Cesarean Pain Relief | Maternal RCT Study | Significantly reduces postoperative pain and morphine demand, and good tolerance. | [57] |
| Isocyanomethane | Epilepsy | Mouse Model | Rapidly increases seizure threshold and no motor/sedation side effects. | [53] |
| Lidocaine Spray | Post-Epidural Headache | Clinical Case | Non-invasive treatment, rapid symptom relief, replaces epidural blood patch. | [58] |
| Dexamethasone | Neuroinflammation (e.g., Stroke) | Mouse Model | Higher brain concentration, faster onset, and suitable for acute treatment. | [62] |
| Icariin-NGSTH System | Depression | Chronic Stress Rat Model | Faster antidepressant effect and organ-specific drug concentration significantly better than oral administration. | [63] |
| Naltrexone | Opioid Side Effects | Rodent Model | Alleviates gastrointestinal and central side effects without affecting analgesic effect. | [59] |
| Chlorpyrifos | Neurotoxicity | Adult Male Mouse | High doses cause memory impairment, anxiety, and brain oxidative stress. | [65] |
| Drug/ System Name | Application/ Disease | Research Model | Key Findings | Ref. |
|---|---|---|---|---|
| NGF | TBI | Clinical Trial | Bypasses the BBB to directly affect brain tissue, reducing systemic side effects. | [67,70] |
| Leukemia Inhibitory Factor (LIF) | Mild TBI (mTBI) in Children | CD1 Mice | Alleviates glial proliferation and axonal damage, improves sensory-motor function, and has no side effects. | [71] |
| Insulin | AD | Rat Model | Intranasal insulin rapidly distributes to the brain, improves cognitive function, and optimized formulation reduces systemic side effects. | [80] |
| Insulin | AD | Multicenter Clinical Trial | Improves memory performance in patients with mild cognitive impairment or AD. | [77] |
| Insulin (Nanocarrier Technology) | CNS Diseases | Rat Model | Enhances delivery efficiency with nanotechnology, bypasses the BBB through olfactory or trigeminal nerves, and significantly improves cognitive function. | [74,75] |
| Insulin | PD | Rat Model | Directly targets the brain and minimizes systemic side effects. | [78] |
| IGF-1 | Brain Ischemia | Rat Model | Reduces neural damage and inflammation and bypasses the BBB to directly affect the brain. | [72] |
| Oxytocin Intranasal Spray TTA-121 | ASD | Rabbit Model | Significantly higher brain-specific drug concentration compared to Syntocinon, higher concentrations in the prefrontal cortex and cuneus. | [84] |
| Insulin (PTD-Modified Formulation) | Diabetes | Rat Experiment | Enhances absorption with Protein Transduction Domain (PTD) and optimizes intranasal delivery formulation to improve efficacy. | [79] |
| Bicyclic Peptide OL-CTOP | Morphine Side Effect Antagonism | Mouse | Intranasal delivery effectively antagonizes morphine’s analgesic and respiratory suppression side effects, demonstrating potential for brain-targeted delivery. | [87] |
| Drug/System Name | Application/Disease | Research Model | Key Findings | References |
|---|---|---|---|---|
| Self-Assembled Antagomir-21/RAP Nanoparticles | Glioblastoma | Mouse Model | Enhanced efficacy with a non-toxic carrier, effectively inhibiting tumor growth. | [113] |
| ApoA-I Nanodisk-Loaded ASO (Antisense Oligonucleotide) | Huntington’s Disease | HD Mouse Model | Single intranasal delivery significantly reduces mutated Huntington protein (mHTT) levels in the striatum and cortex. | [114] |
| Glycerol Chitosan-DNA Complex (GCP/GCPH) | Neurological Diseases (e.g., AD) | Mouse Model | GCP targets gene delivery to the cerebral cortex; GCPH (hyaluronidase-coated) enhances brain distribution. | [115] |
| MSC-Exo-loaded PTEN siRNA (Mesenchymal Stem Cell Exosomes) | Complete SCI | Animal Model | Non-invasive intranasal delivery promotes functional recovery after SCI. | [116] |
| Drug/System Name | Application/ Disease | Research Model | Key Findings | Ref. |
|---|---|---|---|---|
| Mitochondria | Chemotherapy-induced Cognitive Deficits | Mouse Model | Intranasal delivery of mitochondrial-targeted compounds provides neuroprotective effects. | [128,129] |
| Human Olfactory Mucosal Progenitor Cells (OMPCs) | Brain Injury | Rat Diffuse Axonal Injury Model | OMPCs migrate to the vicinity of damaged neurons and axons via intranasal delivery, supporting non-invasive stem cell therapy. | [120] |
| Human Neural Stem Cells (hNSCs) | AD | AD Mouse Model | hNSCs survive and differentiate into neurons, reducing β-amyloid plaque accumulation and synapse loss, improving cognitive function. | [122] |
| Bone Marrow-Derived Mesenchymal Stem Cells (BMSCs) | PD | PD Mouse Model | Pre-treated BMSCs enhance efficacy, improving motor function and reducing dopaminergic neuron loss. | [123] |
| Human Umbilical Cord-Derived Mesenchymal Stromal Cells (MSCs) | Bronchopulmonary Dysplasia (BPD) | Experimental BPD Model | Intranasal delivery of MSCs repairs lung damage caused by BPD with simple methods and clinical potential. | [125] |
| Deciduous Dental Pulp Stem Cells (DPSCs) | PD | MPTP-induced PD Mouse | DPSCs improve motor coordination and olfactory function, reducing dopaminergic neuron degeneration. | [124] |
| Delayed Repeated Intranasal Delivery of Bone Marrow Stromal Cells | Ischemic Stroke | Mouse Stroke Model | Delayed repeated intranasal delivery promotes regeneration and functional recovery after stroke. | [127] |
| Nanocarrier Type | Advantages | Limitations | Ref. |
|---|---|---|---|
| Liposomes | High biocompatibility; good drug loading for hydrophilic drugs. | Rapid clearance; low stability. | [220] |
| Polymeric Nanoparticles (PLGA, Chitosan) | High drug loading; sustained release; improved stability; versatile surface modification. | Potential toxicity; difficulty in large-scale production. | [221] |
| Nanoemulsions | Enhanced solubility and permeability of lipophilic drugs. | Thermodynamic instability, need stabilizers. | [222] |
| Magnetic Nanoparticles | Magnetic targeting, imaging compatibility. | Potential safety concerns, complex formulation. | [223] |
| PEGylated Nanoparticles | Extended circulation time, improved CNS penetration. | Expensive, potential immune response. | [224] |
| Exosomes | Endogenous origin; high biocompatibility; excellent penetration across biological barriers. | Low production yield; difficulty in drug loading; high cost. | [225] |
| Plant-derived Extracellular Vesicles (EVs) | Natural origin; low toxicity; immune modulation potential. | Scalability; batch variability. | [226] |
| Hydrogel-nanoparticle hybrids | Mucoadhesion; biocompatibility; prolonged residence time. | Formulation optimization challenges; drug release control. | [227] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Zang, R.; Qiu, Y.; Zhang, Y.; Peng, J.; Cheng, Z.; Wei, S.; Liu, M.; Diao, Y. Intranasal Drug Delivery Technology in the Treatment of Central Nervous System Diseases: Challenges, Advances, and Future Research Directions. Pharmaceutics 2025, 17, 775. https://doi.org/10.3390/pharmaceutics17060775
Wu X, Zang R, Qiu Y, Zhang Y, Peng J, Cheng Z, Wei S, Liu M, Diao Y. Intranasal Drug Delivery Technology in the Treatment of Central Nervous System Diseases: Challenges, Advances, and Future Research Directions. Pharmaceutics. 2025; 17(6):775. https://doi.org/10.3390/pharmaceutics17060775
Chicago/Turabian StyleWu, Xunxun, Ranqing Zang, Yiting Qiu, Yufang Zhang, Junbin Peng, Zhiyun Cheng, Site Wei, Meiyan Liu, and Yong Diao. 2025. "Intranasal Drug Delivery Technology in the Treatment of Central Nervous System Diseases: Challenges, Advances, and Future Research Directions" Pharmaceutics 17, no. 6: 775. https://doi.org/10.3390/pharmaceutics17060775
APA StyleWu, X., Zang, R., Qiu, Y., Zhang, Y., Peng, J., Cheng, Z., Wei, S., Liu, M., & Diao, Y. (2025). Intranasal Drug Delivery Technology in the Treatment of Central Nervous System Diseases: Challenges, Advances, and Future Research Directions. Pharmaceutics, 17(6), 775. https://doi.org/10.3390/pharmaceutics17060775






