Nanoparticles for Glioblastoma Treatment
Abstract
1. Introduction
- qualifying articles relating to the use of nanoparticles in the treatment of GBM
- qualifying articles relating to the use of nanoparticles in the radiosensitization of GBM
- qualifying both in vivo and in vitro studies
- qualifying both abstracts and full text articles
- articles in a language other than English or Polish
- articles from before 2020
- articles with content that does not correspond to the subject of the article
- articles no clearly defining effect of nanoparticles on GBM
2. Nanoparticles: General Characteristics
3. Organic Nanoparticles
3.1. Polymers
3.2. Liposomes
3.3. Dendrimers
3.4. Extracellular Vesicles EVs
3.5. Lipid Nanoparticles
4. Inorganic Nanoparticles
4.1. Carbon Nanotubes
4.2. Carbon and Quantum Dots
4.3. Magnetic NPs
4.4. Metal Oxide NPs
4.5. Silica NPs
4.6. Gold NPs
4.7. Silver NPs
4.8. Nanodiamonds
5. Other Forms of Nanoparticle Supply
5.1. Hydrogels
5.2. Nanosponges
5.3. Nanomotors
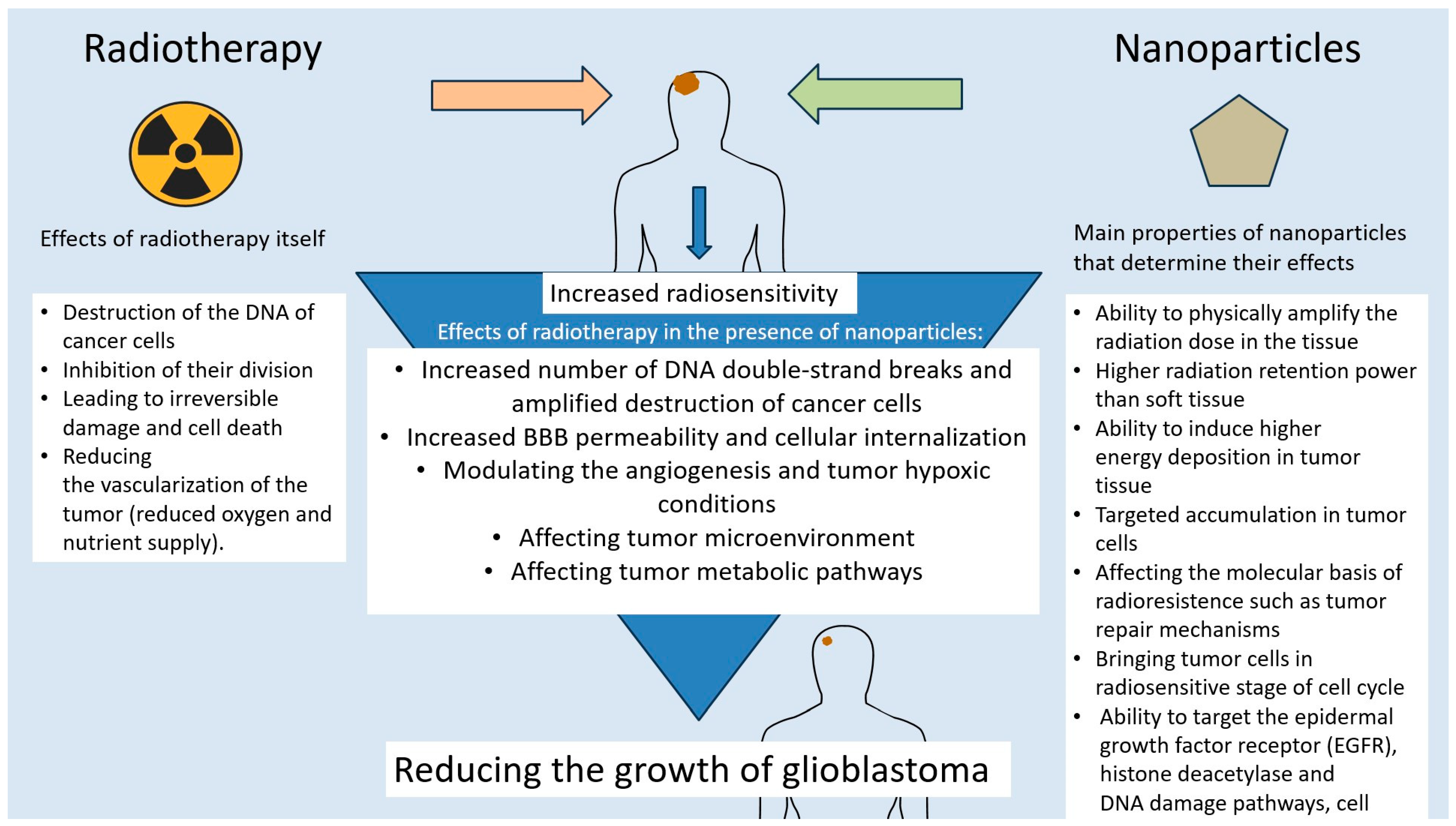
6. The Role of Nanotechnology in GBM Radiosensitization
7. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Pouyan, A.; Ghorbanlo, M.; Eslami, M.; Jahanshahi, M.; Ziaei, E.; Salami, A.; Mokhtari, K.; Shahpasand, K.; Farahani, N.; Meybodi, T.E.; et al. Glioblastoma multiforme: Insights into pathogenesis, key signaling pathways, and therapeutic strategies. Mol. Cancer 2025, 24, 58. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Weller, M.; Lee, E.Q.; Alexander, B.M.; Barnholtz-Sloan, J.S.; Barthel, F.P.; Batchelor, T.T.; Bindra, R.S.; Chang, S.M.; Chiocca, E.A.; et al. Glioblastoma in adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro-Oncology 2020, 22, 1073–1113. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.; Palmer, K.; Campion, T.; O Millner, T.; Scott, E.; Lorimer, C.; Paraskevopoulos, D.; McKenna, G.; Marino, S.; Lewis, R.; et al. Gliomas in adults: Guidance on investigations, diagnosis, treatment and surveillance. Clin. Med. 2024, 24, 100240. [Google Scholar] [CrossRef]
- Roda, D.; Veiga, P.; Melo, J.B.; Carreira, I.M.; Ribeiro, I.P. Principles in the Management of Glioblastoma. Genes 2024, 15, 501. [Google Scholar] [CrossRef]
- Schaff, L.R.; Mellinghoff, I.K. Glioblastoma and Other Primary Brain Malignancies in Adults: A Review. JAMA 2023, 329, 574–587. [Google Scholar] [CrossRef]
- Hamad, A.; Yusubalieva, G.M.; Baklaushev, V.P.; Chumakov, P.M.; Lipatova, A.V. Recent Developments in Glioblastoma Therapy: Oncolytic Viruses and Emerging Future Strategies. Viruses 2023, 15, 547. [Google Scholar] [CrossRef]
- Bartusik-Aebisher, D.; Serafin, I.; Dynarowicz, K.; Aebisher, D. Photodynamic therapy and associated targeting methods for treatment of brain cancer. Front. Pharmacol. 2023, 14, 1250699. [Google Scholar] [CrossRef]
- Pinto-Fraga, J.; García-Chico, C.; Lista, S.; Lacal, P.M.; Carpenzano, G.; Salvati, M.; Santos-Lozano, A.; Graziani, G.; Ceci, C. Protein kinase inhibitors as targeted therapy for glioblastoma: A meta-analysis of randomized controlled clinical trials. Pharmacol. Res. 2025, 212, 107528. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Kong, Z.; Ma, W. PD-1/PD-L1 immune checkpoint inhibitors in glioblastoma: Clinical studies, challenges and potential. Hum. Vaccines Immunother. 2021, 17, 546–553. [Google Scholar] [CrossRef]
- Chou, C.-J.; Lin, C.-F.; Chen, Y.-W.; Huang, P.-I.; Yang, Y.-P.; Wang, M.-L.; Hung, K.-F.; Lee, Y.-Y. The update of chimeric antigen receptor-T cell therapy in glioblastoma. J. Chin. Med. Assoc. JCMA 2020, 83, 442–445. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, D.Y. Nanomedicine in Clinical Photodynamic Therapy for the Treatment of Brain Tumors. Biomedicines 2022, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, L. Natural-product-based, carrier-free, noncovalent nanoparticles for tumor chemo-photodynamic combination therapy. Pharmacol. Res. 2024, 203, 107150. [Google Scholar] [CrossRef]
- Quinlan, J.A.; Inglut, C.T.; Srivastava, P.; Rahman, I.; Stabile, J.; Gaitan, B.; Del Valle, C.A.; Baumiller, K.; Gaur, A.; Chiou, W.; et al. Carrier-Free, Amorphous Verteporfin Nanodrug for Enhanced Photodynamic Cancer Therapy and Brain Drug Delivery. Adv. Sci. 2024, 11, e2302872. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Zhao, X.; Xiao, S. Application prospect of peptide-modified nano targeting drug delivery system combined with PD-1/PD-L1-based immune checkpoint blockade in glioblastoma. Int. J. Pharm. 2020, 589, 119865. [Google Scholar] [CrossRef]
- Zhu, N.; Chen, S.; Jin, Y.; Wang, M.; Fang, L.; Xue, L.; Hua, D.; Zhang, Z.; Jia, M.; Hao, M.; et al. Enhancing Glioblastoma Immunotherapy with Integrated Chimeric Antigen Receptor T Cells through the Re-Education of Tumor-Associated Microglia and Macrophages. ACS Nano 2024, 18, 11165–11182. [Google Scholar] [CrossRef]
- Shabani, L.; Abbasi, M.; Amini, M.; Amani, A.M.; Vaez, A. The brilliance of nanoscience over cancer therapy: Novel promising nanotechnology-based methods for eradicating glioblastoma. J. Neurol. Sci. 2022, 440, 120316. [Google Scholar] [CrossRef]
- Geng, T.; Leung, E.; Chamley, L.W.; Wu, Z. Functionalisation of extracellular vesicles with cyclic-RGDyC potentially for glioblastoma targeted intracellular drug delivery. Biomater. Adv. 2023, 149, 213388. [Google Scholar] [CrossRef]
- Luo, W.; Yan, D.; Song, Z.; Zhu, X.; Liu, X.; Li, X.; Zhao, S. miR-126-3p sensitizes glioblastoma cells to temozolomide by inactivating Wnt/β-catenin signaling via targeting SOX2. Life Sci. 2019, 226, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Garcia, H.; Ramirez-Loera, C.; Malouff, T.D.; Seneviratne, D.S.; Palmer, J.D.; Trifiletti, D.M. Novel Strategies for Nanoparticle-Based Radiosensitization in Glioblastoma. Int. J. Mol. Sci. 2021, 22, 9673. [Google Scholar] [CrossRef]
- Grzegorzewski, J.; Michalak, M.; Wołoszczuk, M.; Bulicz, M.; Majchrzak-Celińska, A. Nanotherapy of Glioblastoma-Where Hope Grows. Int. J. Mol. Sci. 2025, 26, 1814. [Google Scholar] [CrossRef]
- Jhaveri, J.; Raichura, Z.; Khan, T.; Momin, M.; Omri, A. Chitosan Nanoparticles-Insight into Properties, Functionalization and Applications in Drug Delivery and Theranostics. Molecules 2021, 26, 272. [Google Scholar] [CrossRef] [PubMed]
- Neganova, M.E.; Aleksandrova, Y.R.; Sukocheva, O.A.; Klochkov, S.G. Benefits and limitations of nanomedicine treatment of brain cancers and age-dependent neurodegenerative disorders. Semin. Cancer Biol. 2022, 86 Pt 2, 805–833. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Garcia, H.; Alvarado-Estrada, K.; Schiapparelli, P.; Quinones-Hinojosa, A.; Trifiletti, D.M. Engineering Three-Dimensional Tumor Models to Study Glioma Cancer Stem Cells and Tumor Microenvironment. Front. Cell. Neurosci. 2020, 14, 558381. [Google Scholar] [CrossRef]
- Soleymani, S.; Doroudian, M.; Soezi, M.; Beladi, A.; Asgari, K.; Mobarakshahi, A.; Aghaeipour, A.; Macloughlin, R. Engendered nanoparticles for treatment of brain tumors. Oncol. Res. 2024, 33, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Alam, K.T. Biomimetic Nanoparticle Based Targeted mRNA Vaccine Delivery as a Novel Therapy for Glioblastoma Multiforme. AAPS PharmSciTech 2025, 26, 68. [Google Scholar] [CrossRef]
- Ghorbanizamani, F.; Moulahoum, H.; Celik, E.G.; Zihnioglu, F.; Beduk, T.; Goksel, T.; Turhan, K.; Timur, S. Design of Polymeric Surfaces as Platforms for Streamlined Cancer Diagnostics in Liquid Biopsies. Biosensors 2023, 13, 400. [Google Scholar] [CrossRef]
- Ramalho, M.J.; Serra, É.; Lima, J.; Loureiro, J.A.; Pereira, M.C. Chitosan-PLGA mucoadhesive nanoparticles for gemcitabine repurposing for glioblastoma therapy. Eur. J. Pharm. Biopharm. 2024, 200, 114326. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Liu, Y.; Zhong, Y.; Shangguan, P.; Liu, J.; Luo, Z.; Qi, C.; Guo, J.; Li, X.; Lin, D.; et al. A Brain-Targeting NIR-II Polymeric Phototheranostic Nanoplatform toward Orthotopic Drug-Resistant Glioblastoma. Nano Lett. 2025, 25, 3445–3454. [Google Scholar] [CrossRef]
- He, Y.; Pan, Y.; Zhao, X.; Fan, W.; Cai, Y.; Mou, X. NIR-II absorptive dithienopyrrole-thiadiazolobenzotriazole conjugated polymer for photoacoustic imaging-guided glioblastoma multiforme photothermal therapy. Acta Biomater. 2022, 152, 546–561. [Google Scholar] [CrossRef]
- Hsu, F.-T.; Chen, Y.-T.; Chin, Y.-C.; Chang, L.-C.; Chiang, S.-C.; Yang, L.-X.; Liu, H.-S.; Yueh, P.-F.; Tu, H.-L.; He, R.-Y.; et al. Harnessing the Power of Sugar-Based Nanoparticles: A Drug-Free Approach to Enhance Immune Checkpoint Inhibition against Glioblastoma and Pancreatic Cancer. ACS Nano 2024, 18, 28764–28781. [Google Scholar] [CrossRef]
- Zhao, M.; van Straten, D.; Broekman, M.L.; Préat, V.; Schiffelers, R.M. Nanocarrier-based drug combination therapy for glioblastoma. Theranostics 2020, 10, 1355–1372. [Google Scholar] [CrossRef] [PubMed]
- Kabir, T.; Rahman, H.; Akter, R.; Behl, T.; Kaushik, D.; Mittal, V.; Pandey, P.; Akhtar, M.F.; Saleem, A.; Albadrani, G.M.; et al. Potential Role of Curcumin and Its Nanoformulations to Treat Various Types of Cancers. Biomolecules 2021, 11, 392. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, K.; Chauhan, P.; Kumar, S.; Pahwa, R.; Verma, R.; Goyal, R.; Singh, G.; Sharma, A.; Rao, N.; Kaushik, D. Targeting brain tumors with innovative nanocarriers: Bridging the gap through the blood-brain barrier. Oncol. Res. 2024, 32, 877–897. [Google Scholar] [CrossRef] [PubMed]
- Reva, M.; Mendes, M.; Sousa, J.J.; Pais, A.; Vitorino, C. Boron neutron capture therapy for glioblastoma: The delivery dilemma. Life Sci. 2025, 364, 123435. [Google Scholar] [CrossRef]
- Singh, V.; Kumar, K.; Purohit, D.; Verma, R.; Pandey, P.; Bhatia, S.; Malik, V.; Mittal, V.; Rahman, H.; Albadrani, G.M.; et al. Exploration of therapeutic applicability and different signaling mechanism of various phytopharmacological agents for treatment of breast cancer. Biomed. Pharmacother. = Biomed. Pharmacother. 2021, 139, 111584. [Google Scholar] [CrossRef]
- Chen, E.; Chen, B.M.; Su, Y.C.; Chang, Y.C.; Cheng, T.L.; Barenholz, Y.; Roffler, S.R. Premature Drug Release from Polyethylene Glycol (PEG)-Coated Liposomal Doxorubicin via Formation of the Membrane Attack Complex. ACS Nano 2020, 14, 7808–7822. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Zhao, Y.; Kang, X.; Zhao, P.; Fu, X.; Mo, X.; Wan, Y.; Huang, Y. BBB-penetrating codelivery liposomes treat brain metastasis of non-small cell lung cancer with EGFRT790M mutation. Theranostics 2020, 10, 6122–6135. [Google Scholar] [CrossRef]
- Sharma, R.; Liaw, K.; Sharma, A.; Jimenez, A.; Chang, M.; Salazar, S.; Amlani, I.; Kannan, S.; Kannan, R.M. Glycosylation of PAMAM dendrimers significantly improves tumor macrophage targeting and specificity in glioblastoma. J. Control. Release 2021, 337, 179–192. [Google Scholar] [CrossRef]
- Knauer, N.; Arkhipova, V.; Li, G.; Hewera, M.; Pashkina, E.; Nguyen, P.-H.; Meschaninova, M.; Kozlov, V.; Zhang, W.; Croner, R.S.; et al. In Vitro Validation of the Therapeutic Potential of Dendrimer-Based Nanoformulations against Tumor Stem Cells. Int. J. Mol. Sci. 2022, 23, 5691. [Google Scholar] [CrossRef]
- Mignani, S.; Shi, X.; Karpus, A.; Majoral, J.-P. Non-invasive intranasal administration route directly to the brain using dendrimer nanoplatforms: An opportunity to develop new CNS drugs. Eur. J. Med. Chem. 2021, 209, 112905. [Google Scholar] [CrossRef]
- Wu, Y.; Lloveras, V.; Lope-Piedrafita, S.; Mulero-Acevedo, M.; Candiota, A.P.; Vidal-Gancedo, J. Synthesis and Relaxivity study of amino acid-branched radical dendrimers as MRI contrast agents for potential brain tumor imaging. Acta Biomater. 2025, 192, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Lokumcu, T.; Iskar, M.; Schneider, M.; Helm, D.; Klinke, G.; Schlicker, L.; Bethke, F.; Müller, G.; Richter, K.; Poschet, G.; et al. Proteomic, Metabolomic, and Fatty Acid Profiling of Small Extracellular Vesicles from Glioblastoma Stem-Like Cells and Their Role in Tumor Heterogeneity. ACS Nano 2024, 18, 2500–2519. [Google Scholar] [CrossRef] [PubMed]
- Butreddy, A.; Kommineni, N.; Dudhipala, N. Exosomes as Naturally Occurring Vehicles for Delivery of Biopharmaceuticals: Insights from Drug Delivery to Clinical Perspectives. Nanomaterials 2021, 11, 1481. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; You, J.Y.; Paek, K.; Park, J.; Kang, S.J.; Han, E.H.; Choi, N.; Chung, S.; Rhee, W.J.; Kim, J.A. Inhibition of tumor progression and M2 microglial polarization by extracellular vesicle-mediated microRNA-124 in a 3D microfluidic glioblastoma microenvironment. Theranostics 2021, 11, 9687–9704. [Google Scholar] [CrossRef]
- Dong, S.; Liu, X.; Bi, Y.; Wang, Y.; Antony, A.; Lee, D.; Huntoon, K.; Jeong, S.; Ma, Y.; Li, X.; et al. Adaptive design of mRNA-loaded extracellular vesicles for targeted immunotherapy of cancer. Nat. Commun. 2023, 14, 6610. [Google Scholar] [CrossRef]
- Chattrairat, K.; Yasui, T.; Suzuki, S.; Natsume, A.; Nagashima, K.; Iida, M.; Zhang, M.; Shimada, T.; Kato, A.; Aoki, K.; et al. All-in-One Nanowire Assay System for Capture and Analysis of Extracellular Vesicles from an ex Vivo Brain Tumor Model. ACS Nano 2023, 17, 2235–2244. [Google Scholar] [CrossRef]
- Salviano-Silva, A.; Wollmann, K.; Brenna, S.; Reimer, R.; Neumann, J.E.; Dottermusch, M.; Woythe, L.; Maire, C.L.; Puig, B.; Schüller, U.; et al. Extracellular Vesicles Carrying Tenascin-C Are Clinical Biomarkers and Improve Tumor-Derived DNA Analysis in Glioblastoma Patients. ACS Nano 2025, 19, 9844–9859. [Google Scholar] [CrossRef]
- Ruan, S.; Zhou, Y.; Jiang, X.; Gao, H. Rethinking CRITID Procedure of Brain Targeting Drug Delivery: Circulation, Blood Brain Barrier Recognition, Intracellular Transport, Diseased Cell Targeting, Internalization, and Drug Release. Adv. Sci. 2021, 8, 2004025. [Google Scholar] [CrossRef]
- Brüßeler, M.M.T.; Zam, A.; Moreno-Zafra, V.M.; Rouatbi, N.; Hassuneh, O.W.M.; Marrocu, A.; Liam-Or, R.; Abdel-Bar, H.M.; Walters, A.A.; Al-Jamal, K.T. Polyinosinic/Polycytidylic Lipid Nanoparticles Enhance Immune Cell Infiltration and Improve Survival in the Glioblastoma Mouse Model. Mol. Pharm. 2024, 21, 6339–6352. [Google Scholar] [CrossRef]
- De Gaetano, F.; Cristiano, M.C.; Venuti, V.; Crupi, V.; Majolino, D.; Paladini, G.; Acri, G.; Testagrossa, B.; Irrera, A.; Paolino, D.; et al. Rutin-Loaded Solid Lipid Nanoparticles: Characterization and In Vitro Evaluation. Molecules 2021, 26, 1039. [Google Scholar] [CrossRef]
- Rouatbi, N.; Walters, A.A.; Costa, P.M.; Qin, Y.; Liam-Or, R.; Grant, V.; Pollard, S.M.; Wang, J.T.-W.; Al-Jamal, K.T. RNA lipid nanoparticles as efficient in vivo CRISPR-Cas9 gene editing tool for therapeutic target validation in glioblastoma cancer stem cells. J. Control. Release 2024, 375, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ma, F.; Liu, F.; Chen, J.; Zhao, X.; Xu, Q. Efficient delivery of antisense oligonucleotides using bioreducible lipid nanoparticles in vitro and in vivo. Mol. Ther. Nucleic Acids 2020, 19, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- Schulze, J.; Schöne, L.; Ayoub, A.M.; Librizzi, D.; Amin, M.U.; Engelhardt, K.; Yousefi, B.H.; Bender, L.; Schaefer, J.; Preis, E.; et al. Modern Photodynamic Glioblastoma Therapy Using Curcumin- or Parietin-Loaded Lipid Nanoparticles in a CAM Model Study. ACS Appl. Bio Mater. 2023, 6, 5502–5514. [Google Scholar] [CrossRef] [PubMed]
- Pucci, C.; De Pasquale, D.; Marino, A.; Martinelli, C.; Lauciello, S.; Ciofani, G. Hybrid Magnetic Nanovectors Promote Selective Glioblastoma Cell Death through a Combined Effect of Lysosomal Membrane Permeabilization and Chemotherapy. ACS Appl. Mater. Interfaces 2020, 12, 29037–29055. [Google Scholar] [CrossRef]
- Shukla, R.; Singh, A.; Singh, K.K. Vincristine-based nanoformulations: A preclinical and clinical studies overview. Drug Deliv. Transl. Res. 2024, 14, 1–16. [Google Scholar] [CrossRef]
- Minchenko, D.O.; Rudnytska, O.V.; Khita, O.O.; Kulish, Y.V.; Viletska, Y.M.; Halkin, O.V.; Danilovskyi, S.V.; Ratushna, O.O.; Minchenko, O.H. Expression of DNAJB9 and some other genes is more sensitive to SWCNTs in normal human astrocytes than glioblastoma cells. Endocr. Regul. 2023, 57, 162–172. [Google Scholar] [CrossRef]
- Jiang, T.; Amadei, C.A.; Gou, N.; Lin, Y.; Lan, J.; Vecitis, C.D.; Gu, A.Z. Toxicity of Single-Walled Carbon Nanotubes (SWCNTs): Effect of Lengths, Functional Groups and Electronic Structures Revealed by a Quantitative Toxicogenomics Assay. Environ. Sci. Nano 2020, 7, 1348–1364. [Google Scholar] [CrossRef]
- Rudnytska, O.V.; Khita, O.O.; Minchenko, D.O.; Tsymbal, D.O.; Yefimova, Y.V.; Sliusar, M.Y.; Minchenko, O. The low doses of SWCNTs affect the expression of proliferation and apoptosis related genes in normal human astrocytes. Curr. Res. Toxicol. 2021, 2, 64–71. [Google Scholar] [CrossRef]
- Marhuenda, E.; Fabre, C.; Zhang, C.; Martin-Fernandez, M.; Iskratsch, T.; Saleh, A.; Bauchet, L.; Cambedouzou, J.; Hugnot, J.-P.; Duffau, H.; et al. Glioma stem cell invasive phenotype at optimal stiffness is driven by MGAT5 dependent mechanosensing. J. Exp. Clin. Cancer Res. CR 2021, 40, 139. [Google Scholar] [CrossRef]
- Fuster, E.; Candela, H.; Estévez, J.; Vilanova, E.; Sogorb, M.A. A Transcriptomic Analysis of T98G Human Glioblastoma Cells after Exposure to Cadmium-Selenium Quantum Dots Mainly Reveals Alterations in Neuroinflammation Processes and Hypothalamus Regulation. Int. J. Mol. Sci. 2022, 23, 2267. [Google Scholar] [CrossRef]
- Perini, G.; Palmieri, V.; Ciasca, G.; D’ascenzo, M.; Gervasoni, J.; Primiano, A.; Rinaldi, M.; Fioretti, D.; Prampolini, C.; Tiberio, F.; et al. Graphene Quantum Dots’ Surface Chemistry Modulates the Sensitivity of Glioblastoma Cells to Chemotherapeutics. Int. J. Mol. Sci. 2020, 21, 6301. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.L.; Xu, H.L.; Xiong, C.; Lan, Q.H.; Fang, M.L.; Cai, J.H.; Li, H.; Zhu, S.T.; Xu, J.H.; Tao, F.Y.; et al. c(RGDyk)-modified nanoparticles encapsulating quantum dots as a stable fluorescence probe for imaging-guided surgical resection of glioma under the auxiliary UTMD. Artif. Cells Nanomed. Biotechnol. 2020, 48, 143–158. [Google Scholar] [CrossRef]
- Semyachkina-Glushkovskaya, O.; Sokolovski, S.; Fedosov, I.; Shirokov, A.; Navolokin, N.; Bucharskaya, A.; Blokhina, I.; Terskov, A.; Dubrovski, A.; Telnova, V.; et al. Transcranial Photosensitizer-Free Laser Treatment of Glioblastoma in Rat Brain. Int. J. Mol. Sci. 2023, 24, 13696. [Google Scholar] [CrossRef]
- Madani, F.; Morovvati, H.; Webster, T.J.; Asaadi, S.N.; Rezayat, S.M.; Hadjighassem, M.; Khosravani, M.; Adabi, M. Combination chemotherapy via poloxamer 188 surface-modified PLGA nanoparticles that traverse the blood-brain-barrier in a glioblastoma model. Sci. Rep. 2024, 14, 19516. [Google Scholar] [CrossRef]
- Garcia-Millan, T.; Ramos-Soriano, J.; Ghirardello, M.; Liu, X.; Santi, C.M.; Eloi, J.-C.; Pridmore, N.; Harniman, R.L.; Morgan, D.J.; Hughes, S.; et al. Multicolor Photoluminescent Carbon Dots à La Carte for Biomedical Applications. ACS Appl. Mater. Interfaces 2023, 15, 44711–44721. [Google Scholar] [CrossRef] [PubMed]
- Dhenadhayalan, N.; Lin, K.; Saleh, T.A. Recent Advances in Functionalized Carbon Dots toward the Design of Efficient Materials for Sensing and Catalysis Applications. Small 2020, 16, e1905767. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Liu, Y.; You, Q.; Lei, Z.; Ji, J.; Zhang, F.; Dong, W.; Li, L. Metal-Doping Strategy for Carbon-Based Sonosensitizer in Sonodynamic Therapy of Glioblastoma. Adv. Sci. 2024, 11, e2404230. [Google Scholar] [CrossRef] [PubMed]
- Luta, G.; Butura, M.; Tiron, A.; Tiron, C.E. Enhancing Anti-Tumoral Potential of CD-NHF by Modulating PI3K/Akt Axis in U87 Ex Vivo Glioma Model. Int. J. Mol. Sci. 2021, 22, 3873. [Google Scholar] [CrossRef]
- Qiao, R.; Fu, C.; Forgham, H.; Javed, I.; Huang, X.; Zhu, J.; Whittaker, A.K.; Davis, T.P. Magnetic iron oxide nanoparticles for brain imaging and drug delivery. Adv. Drug Deliv. Rev. 2023, 197, 114822. [Google Scholar] [CrossRef]
- Li, B.; Chen, X.; Qiu, W.; Zhao, R.; Duan, J.; Zhang, S.; Pan, Z.; Zhao, S.; Guo, Q.; Qi, Y.; et al. Synchronous Disintegration of Ferroptosis Defense Axis via Engineered Exosome-Conjugated Magnetic Nanoparticles for Glioblastoma Therapy. Adv. Sci. 2022, 9, e2105451. [Google Scholar] [CrossRef]
- Beola, L.; Iturrioz-Rodríguez, N.; Pucci, C.; Bertorelli, R.; Ciofani, G. Drug-Loaded Lipid Magnetic Nanoparticles for Combined Local Hyperthermia and Chemotherapy against Glioblastoma Multiforme. ACS Nano 2023, 17, 18441–18455. [Google Scholar] [CrossRef] [PubMed]
- Manescu, V.; Antoniac, I.; Paltanea, G.; Nemoianu, I.V.; Mohan, A.G.; Antoniac, A.; Rau, J.V.; Laptoiu, S.A.; Mihai, P.; Gavrila, H.; et al. Magnetic Hyperthermia in Glioblastoma Multiforme Treatment. Int. J. Mol. Sci. 2024, 25, 10065. [Google Scholar] [CrossRef]
- Souiade, L.; Domingo-Diez, J.; Alcaide, C.; Gámez, B.; Gámez, L.; Ramos, M.; Olmedo, J.J.S. Improving the Efficacy of Magnetic Nanoparticle-Mediated Hyperthermia Using Trapezoidal Pulsed Electromagnetic Fields as an In Vitro Anticancer Treatment in Melanoma and Glioblastoma Multiforme Cell Lines. Int. J. Mol. Sci. 2023, 24, 15933. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, B.A.; Kim, Y.-J. Modernistic and Emerging Developments of Nanotechnology in Glioblastoma-Targeted Theranostic Applications. Int. J. Mol. Sci. 2022, 23, 1641. [Google Scholar] [CrossRef] [PubMed]
- Alphandéry, E. Natural Metallic Nanoparticles for Application in Nano-Oncology. Int. J. Mol. Sci. 2020, 21, 4412. [Google Scholar] [CrossRef]
- Lu, V.M.; Jue, T.R.; McDonald, K.L. Cytotoxic lanthanum oxide nanoparticles sensitize glioblastoma cells to radiation therapy and temozolomide: An in vitro rationale for translational studies. Sci. Rep. 2020, 10, 18156. [Google Scholar] [CrossRef]
- Fuster, E.; Candela, H.; Estévez, J.; Vilanova, E.; Sogorb, M.A. Titanium Dioxide, but Not Zinc Oxide, Nanoparticles Cause Severe Transcriptomic Alterations in T98G Human Glioblastoma Cells. Int. J. Mol. Sci. 2021, 22, 2084. [Google Scholar] [CrossRef]
- Petronek, M.S.; Allen, B.G.; Luthe, G.; Stolwijk, J.M. Polyoxometalate Nanoparticles as a Potential Glioblastoma Therapeutic via Lipid-Mediated Cell Death. Int. J. Mol. Sci. 2022, 23, 8263. [Google Scholar] [CrossRef]
- Chen, L.; Liu, M.; Wang, Y.; Wei, W.; Li, Y.; Bai, Y.; Yu, X.; Jiao, L.; Wang, M. TME-Activated MnO2/Pt Nanoplatform of Hydroxyl Radical and Oxygen Generation to Synergistically Promote Radiotherapy and MR Imaging of Glioblastoma. Int. J. Nanomed. 2024, 19, 11055–11070. [Google Scholar] [CrossRef]
- Newham, G.; Mathew, R.K.; Wurdak, H.; Evans, S.D.; Ong, Z.Y. Polyelectrolyte complex templated synthesis of monodisperse, sub-100 nm porous silica nanoparticles for cancer targeted and stimuli-responsive drug delivery. J. Colloid Interface Sci. 2021, 584, 669–683. [Google Scholar] [CrossRef]
- McCabe, S.M.; Wallace, G.Q.; Sloan-Dennison, S.; Tipping, W.J.; Shand, N.C.; Graham, D.; Boyd, M.; Faulds, K. Evaluating nanoparticle localization in glioblastoma multicellular tumor spheroids by surface enhanced Raman scattering. Analyst 2023, 148, 3247–3256. [Google Scholar] [CrossRef] [PubMed]
- Krętowski, R.; Kusaczuk, M.; Naumowicz, M.; Cechowska-Pasko, M. The Pro-Apoptotic Effect of Silica Nanoparticles Depends on Their Size and Dose, as Well as the Type of Glioblastoma Cells. Int. J. Mol. Sci. 2021, 22, 3564. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.-I.; Chen, Y.-P.; Zhang, R.-L.; Chen, Z.-A.; Wu, C.-H.; Chang, W.-C.; Mou, C.-Y.; Chan, H.W.-H.; Wu, S.-H. Overcoming the Blood-Brain Tumor Barrier with Docetaxel-Loaded Mesoporous Silica Nanoparticles for Treatment of Temozolomide-Resistant Glioblastoma. ACS Appl. Mater. Interfaces 2024, 16, 21722–21735. [Google Scholar] [CrossRef]
- Martínez-Carmona, M.; Ho, Q.P.; Morand, J.; García, A.; Ortega, E.; Erthal, L.C.S.; Ruiz-Hernandez, E.; Santana, M.D.; Ruiz, J.; Vallet-Regí, M.; et al. Amino-Functionalized Mesoporous Silica Nanoparticle-Encapsulated Octahedral Organoruthenium Complex as an Efficient Platform for Combatting Cancer. Inorg. Chem. 2020, 59, 10275–10284. [Google Scholar] [CrossRef] [PubMed]
- Hosseinalizadeh, H.; Mahmoodpour, M.; Bahabadi, Z.R.; Hamblin, M.R.; Mirzaei, H. Neutrophil mediated drug delivery for targeted glioblastoma therapy: A comprehensive review. Biomed. Pharmacother. = Biomed. Pharmacother. 2022, 156, 113841. [Google Scholar] [CrossRef]
- Kerr, B.N.; Duffy, D.; McInerney, C.E.; Hutchinson, A.; Dabaja, I.; Bazzi, R.; Roux, S.; Prise, K.M.; Butterworth, K.T. Evaluation of Radiosensitization and Cytokine Modulation by Differentially PEGylated Gold Nanoparticles in Glioblastoma Cells. Int. J. Mol. Sci. 2023, 24, 10032. [Google Scholar] [CrossRef]
- Kostka, K.; Sokolova, V.; El-Taibany, A.; Kruse, B.; Porada, D.; Wolff, N.; Prymak, O.; Seeds, M.C.; Epple, M.; Atala, A.J. The Application of Ultrasmall Gold Nanoparticles (2 nm) Functionalized with Doxorubicin in Three-Dimensional Normal and Glioblastoma Organoid Models of the Blood-Brain Barrier. Molecules 2024, 29, 2469. [Google Scholar] [CrossRef]
- Durand, M.; Chateau, A.; Jubréaux, J.; Devy, J.; Paquot, H.; Laurent, G.; Bazzi, R.; Roux, S.; Richet, N.; Reinhard-Ruch, A.; et al. Radiosensitization with Gadolinium Chelate-Coated Gold Nanoparticles Prevents Aggressiveness and Invasiveness in Glioblastoma. Int. J. Nanomed. 2023, 18, 243–261. [Google Scholar] [CrossRef]
- Kumthekar, P.; Ko, C.H.; Paunesku, T.; Dixit, K.; Sonabend, A.M.; Bloch, O.; Tate, M.; Schwartz, M.; Zuckerman, L.; Lezon, R.; et al. A first-in-human phase 0 clinical study of RNA interference-based spherical nucleic acids in patients with recurrent glioblastoma. Sci. Transl. Med. 2021, 13, eabb3945. [Google Scholar] [CrossRef]
- Jing, Z.; Li, M.; Wang, H.; Yang, Z.; Zhou, S.; Ma, J.; Meng, E.; Zhang, H.; Liang, W.; Hu, W.; et al. Gallic acid-gold nanoparticles enhance radiation-induced cell death of human glioma U251 cells. IUBMB Life 2021, 73, 398–407. [Google Scholar] [CrossRef]
- Bastiancich, C.; Danhier, P.; Préat, V.; Danhier, F. Anticancer drug-loaded hydrogels as drug delivery systems for the local treatment of glioblastoma. J. Control. Release 2016, 243, 29–42. [Google Scholar] [CrossRef] [PubMed]
- López Ruiz, A.; Bartomeu Garcia, C.; Navarro Gallón, S.; Webster, T.J. Novel Silver-Platinum Nanoparticles for Anticancer and Antimicrobial Applications. Int. J. Nanomed. 2020, 15, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Skóra, B.; Masicz, M.; Nowak, P.; Lachowska, J.; Sołtysek, P.; Biskup, J.; Matuszewska, P.; Szychowski, K.A. Suppression of sonic hedgehog pathway-based proliferation in glioblastoma cells by small-size silver nanoparticles in vitro. Arch. Toxicol. 2023, 97, 2385–2398. [Google Scholar] [CrossRef]
- Kabir, S.R.; Dai, Z.; Nurujjaman, M.; Cui, X.; Asaduzzaman, A.K.M.; Sun, B.; Zhang, X.; Dai, H.; Zhao, X. Biogenic silver/silver chloride nanoparticles inhibit human glioblastoma stem cell growth in vitro and Ehrlich ascites carcinoma cell growth in vivo. J. Cell. Mol. Med. 2020, 24, 13223–13234. [Google Scholar] [CrossRef]
- Müller, N.; Eugenio, M.; Romão, L.F.; de Souza, J.M.; Alves-Leon, S.V.; Campanati, L.; Sant’anna, C. Assessing the antiproliferative effect of biogenic silver chloride nanoparticles on glioblastoma cell lines by quantitative image-based analysis. IET Nanobiotechnology 2021, 15, 558–564. [Google Scholar] [CrossRef]
- Manaloto, E.; Gowen, A.A.; Lesniak, A.; He, Z.; Casey, A.; Cullen, P.J.; Curtin, J.F. Cold atmospheric plasma induces silver nanoparticle uptake, oxidative dissolution and enhanced cytotoxicity in glioblastoma multiforme cells. Arch. Biochem. Biophys. 2020, 689, 108462. [Google Scholar] [CrossRef]
- Naveed, M.; Mahmood, S.; Aziz, T.; Azeem, A.; Rajpoot, Z.; Rehman, S.U.; Al-Asmari, F.; Alahmari, A.S.; Saleh, O.; Sameeh, M.Y.; et al. Green-synthesis of silver nanoparticles AgNPs from Podocarpus macrophyllus for targeting GBM and LGG brain cancers via NOTCH2 gene interactions. Sci. Rep. 2024, 14, 25489. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.M.; Lau, C.H.; Ho, J.W.-T.; Chan, M.S.; Chang, T.J.H.; Law, L.H.; Wang, F.; Tam, D.Y.; Liu, L.S.; Chan, K.W.Y.; et al. Targeted brain tumor imaging by using discrete biopolymer-coated nanodiamonds across the blood-brain barrier. Nanoscale 2021, 13, 3184–3193. [Google Scholar] [CrossRef]
- Chen, Z.; Yuan, S.-J.; Li, K.; Zhang, Q.; Li, T.-F.; An, H.-C.; Xu, H.-Z.; Yue, Y.; Han, M.; Xu, Y.-H.; et al. Doxorubicin-polyglycerol-nanodiamond conjugates disrupt STAT3/IL-6-mediated reciprocal activation loop between glioblastoma cells and astrocytes. J. Control. Release 2020, 320, 469–483. [Google Scholar] [CrossRef]
- Gazaille, C.; Sicot, M.; Saulnier, P.; Eyer, J.; Bastiat, G. Local Delivery and Glioblastoma: Why Not Combining Sustained Release and Targeting? Front. Med. Technol. 2021, 3, 791596. [Google Scholar] [CrossRef]
- Liu, R.; Liang, Q.; Luo, J.; Li, Y.; Zhang, X.; Fan, K.; Du, J. Ferritin-Based Nanocomposite Hydrogel Promotes Tumor Penetration and Enhances Cancer Chemoimmunotherapy. Adv. Sci. 2024, 11, e2305217. [Google Scholar] [CrossRef] [PubMed]
- Erthal, L.C.S.; Shi, Y.; Sweeney, K.J.; Gobbo, O.L.; Ruiz-Hernandez, E. Nanocomposite formulation for a sustained release of free drug and drug-loaded responsive nanoparticles: An approach for a local therapy of glioblastoma multiforme. Sci. Rep. 2023, 13, 5094. [Google Scholar] [CrossRef] [PubMed]
- Gazaille, C.; Bozzato, E.; Madadian-Bozorg, N.; Mellinger, A.; Sicot, M.; Farooq, U.; Saulnier, P.; Eyer, J.; Préat, V.; Bertrand, N.; et al. Glioblastoma-targeted, local and sustained drug delivery system based on an unconventional lipid nanocapsule hydrogel. Biomater. Adv. 2023, 153, 213549. [Google Scholar] [CrossRef]
- Shadab, A.; Farokhi, S.; Fakouri, A.; Mohagheghzadeh, N.; Noroozi, A.; Razavi, Z.S.; Rouzbahani, A.K.; Zalpoor, H.; Mahjoor, M. Hydrogel-based nanoparticles: Revolutionizing brain tumor treatment and paving the way for future innovations. Eur. J. Med. Res. 2025, 30, 71. [Google Scholar] [CrossRef] [PubMed]
- Bouché, M.; Dong, Y.C.; Sheikh, S.; Taing, K.; Saxena, D.; Hsu, J.C.; Chen, M.H.; Salinas, R.D.; Song, H.; Burdick, J.A.; et al. Novel Treatment for Glioblastoma Delivered by a Radiation Responsive and Radiopaque Hydrogel. ACS Biomater. Sci. Eng. 2021, 7, 3209–3220. [Google Scholar] [CrossRef]
- Yin, Y.; Tian, N.; Deng, Z.; Wang, J.; Kuang, L.; Tang, Y.; Zhu, S.; Dong, Z.; Wang, Z.; Wu, X.; et al. Targeted Microglial Membrane-Coated MicroRNA Nanosponge Mediates Inhibition of Glioblastoma. ACS Nano 2024, 18, 29089–29105. [Google Scholar] [CrossRef]
- Fan, N.; Bian, X.; Li, M.; Chen, J.; Wu, H.; Peng, Q.; Bai, H.; Cheng, W.; Kong, L.; Ding, S.; et al. Hierarchical self-uncloaking CRISPR-Cas13a-customized RNA nanococoons for spatial-controlled genome editing and precise cancer therapy. Sci. Adv. 2022, 8, eabn7382. [Google Scholar] [CrossRef]
- Chen, H.; Li, T.; Liu, Z.; Tang, S.; Tong, J.; Tao, Y.; Zhao, Z.; Li, N.; Mao, C.; Shen, J.; et al. A nitric-oxide driven chemotactic nanomotor for enhanced immunotherapy of glioblastoma. Nat. Commun. 2023, 14, 941. [Google Scholar] [CrossRef]
- Süngü Akdoğan, Ç.Z.; Akbay Çetin, E.; Onur, M.A.; Onel, S.; Tuncel, A. Copper(II) Oxide Spindle-like Nanomotors Decorated with Calcium Peroxide Nanoshell as a New Nanozyme with Photothermal and Chemodynamic Functions Providing ROS Self-Amplification, Glutathione Depletion, and Cu(I)/Cu(II) Recycling. ACS Appl. Mater. Interfaces 2025, 17, 632–649. [Google Scholar] [CrossRef]
- Choi, J.; Kim, G.; Bin Cho, S.; Im, H.-J. Radiosensitizing high-Z metal nanoparticles for enhanced radiotherapy of glioblastoma multiforme. J. Nanobiotechnol. 2020, 18, 122. [Google Scholar] [CrossRef]
- Ali, Y.; Oliva, C.R.; Noman, A.S.M.; Allen, B.G.; Goswami, P.C.; Zakharia, Y.; Monga, V.; Spitz, D.R.; Buatti, J.M.; Griguer, C.E. Radioresistance in Glioblastoma and the Development of Radiosensitizers. Cancers 2020, 12, 2511. [Google Scholar] [CrossRef] [PubMed]
- Alhaddad, L.; Osipov, A.N.; Leonov, S. The Molecular and Cellular Strategies of Glioblastoma and Non-Small-Cell Lung Cancer Cells Conferring Radioresistance. Int. J. Mol. Sci. 2022, 23, 13577. [Google Scholar] [CrossRef] [PubMed]
- Alphandéry, E. Nano-Therapies for Glioblastoma Treatment. Cancers 2020, 12, 242. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, F.; Vallis, K.A.; Vellayappan, B.A.; Bandla, A.; Yukun, D.; Carlisle, R. Megavoltage Radiosensitization of Gold Nanoparticles on a Glioblastoma Cancer Cell Line Using a Clinical Platform. Int. J. Mol. Sci. 2020, 21, 429. [Google Scholar] [CrossRef]
- Cen, B.; Zhang, J.; Pan, X.; Xu, Z.; Li, R.; Chen, C.; Wang, B.; Li, Z.; Zhang, G.; Ji, A.; et al. Stimuli-Responsive Peptide/siRNA Nanoparticles as a Radiation Sensitizer for Glioblastoma Treatment by Co-Inhibiting RELA/P65 and EGFR. Int. J. Nanomed. 2024, 19, 11517–11537. [Google Scholar] [CrossRef]

| Type of NP Under the Clinical Trial | Description of the NP | Target Patients’ Group | ClinicalTrials.gov ID | Aim of the Study | Estimated Primary Completion |
|---|---|---|---|---|---|
| RNA–lipid particle (RNA-LP) vaccine. | Autologous total tumor mRNA and pp65 full-length lysosomal-associated membrane protein (LAMP) mRNA loaded DOTAP liposome vaccine administered intravenously | Adult patients with newly diagnosed GBM and pediatric patients with newly diagnosed HGG (high-grade glioma) | NCT04573140 | To manufacture feasibility and safety and to determine the maximum tolerated dose (MTD) of RNA-LP vaccines | September 2026 |
| Rhenium nanoliposomes | 186 rhenium nanoliposomes (186RNL) administered through a convection-enhanced delivery catheter | Patients with recurrent or progressive malignant glioma after standard surgical, radiation, and/or chemotherapy treatment | NCT01906385 | To assess the safety, tolerability, and distribution of 186RNL given by convection-enhanced delivery | December 2025 |
| Liposomal curcumin | Liposomal curcumin (LC) in combination with radiotherapy (XRT), and TMZ and adjuvant TMZ delivered intravenously | Patients with newly diagnosed high-grade malignant gliomas | NCT05768919 | To assess the tolerability, safety, and efficacy of liposomal curcumin (LC) in combination with radiotherapy (RT) and temozolomide (TMZ) | February 2026 |
| Liposomal transcrocetin (L-TC) | Liposomal transcrocetin (L-TC) added intravenously to hypofractionated radiotherapy and concomitant temozolomide followed by adjuvant temozolomide | Patients with histologically confirmed diagnosis of glioblastoma (GBM) | NCT06477939 | To evaluate the efficacy and safety of adding or not adding liposomal transcrocetin (L-TC) to hypofractionated radiation therapy and concomitant temozolomide | 31 December 2032 |
| Type of NP Under the Clinical Trial | Description of the NP | Target Patients’ Group | ClinicalTrials.gov ID | Aim of the Study | Estimated Primary Completion |
|---|---|---|---|---|---|
| RNA–lipid particle (RNA-LP) vaccines | pp65 RNA-loaded lipid particles, (Drug Product 1), RNA-loaded lipid particles, RNA-LPs (Drug Product 2) | Adult patients with recurrent glioblastoma | NCT06389591 | To demonstrate the manufacturing feasibility and safety and to determine the maximum tolerated dose (MTD) of RNA-LP vaccines | December 2026 |
| NanoTherm therapy system—a sterile suspension of iron oxide nanoparticles | A sterile suspension of iron oxide nanoparticles | Patients with recurrent GBM | NCT06271421 | To evaluate the efficacy and tolerance of using the NanoTherm therapy system in recurrent GBM | February 2027 |
| Aguix gadolinium-based nanoparticles | AGuIX (Activation and Guidance of Irradiation by X-ray) gadolinium-based nanoparticles | Patients with brain metastases at higher risk of local recurrence with radiation alone | NCT04899908 | To determine whether AGuIX (Activation and Guidance of Irradiation by X-ray) gadolinium-based nanoparticles make radiation work more effectively in the treatment of patients with brain metastases that are more difficult to control with stereotactic radiation alone | February 2025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartusik-Aebisher, D.; Rogóż, K.; Aebisher, D. Nanoparticles for Glioblastoma Treatment. Pharmaceutics 2025, 17, 688. https://doi.org/10.3390/pharmaceutics17060688
Bartusik-Aebisher D, Rogóż K, Aebisher D. Nanoparticles for Glioblastoma Treatment. Pharmaceutics. 2025; 17(6):688. https://doi.org/10.3390/pharmaceutics17060688
Chicago/Turabian StyleBartusik-Aebisher, Dorota, Kacper Rogóż, and David Aebisher. 2025. "Nanoparticles for Glioblastoma Treatment" Pharmaceutics 17, no. 6: 688. https://doi.org/10.3390/pharmaceutics17060688
APA StyleBartusik-Aebisher, D., Rogóż, K., & Aebisher, D. (2025). Nanoparticles for Glioblastoma Treatment. Pharmaceutics, 17(6), 688. https://doi.org/10.3390/pharmaceutics17060688









