Lung Delivery of Lactose-Free Microparticles Loaded with Azithromycin for the Treatment of Bacterial Infections
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Design of Experiments (DoE)
2.2.2. Solid State Characterization
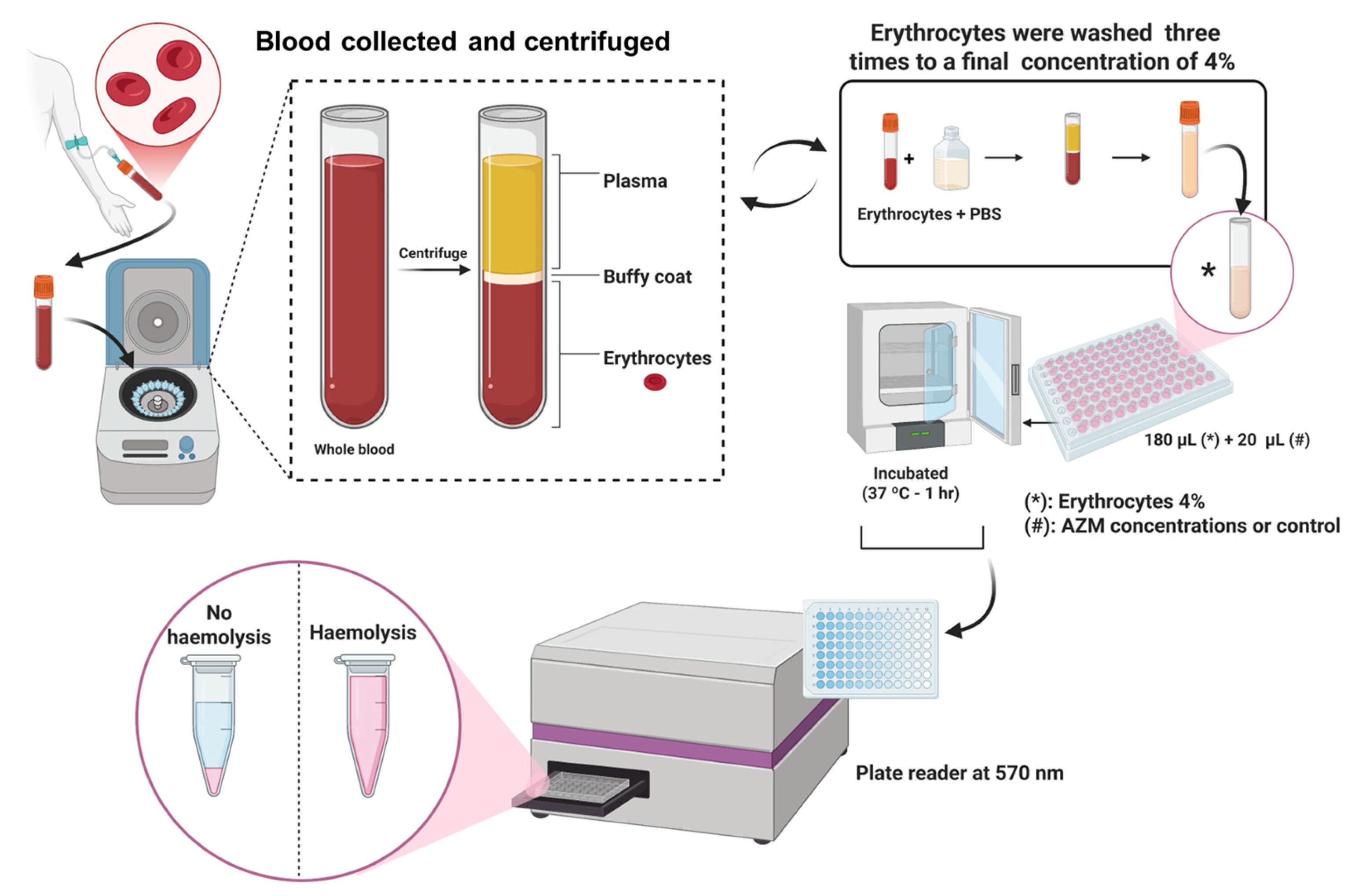
2.2.3. In Vitro Haemolysis Assay
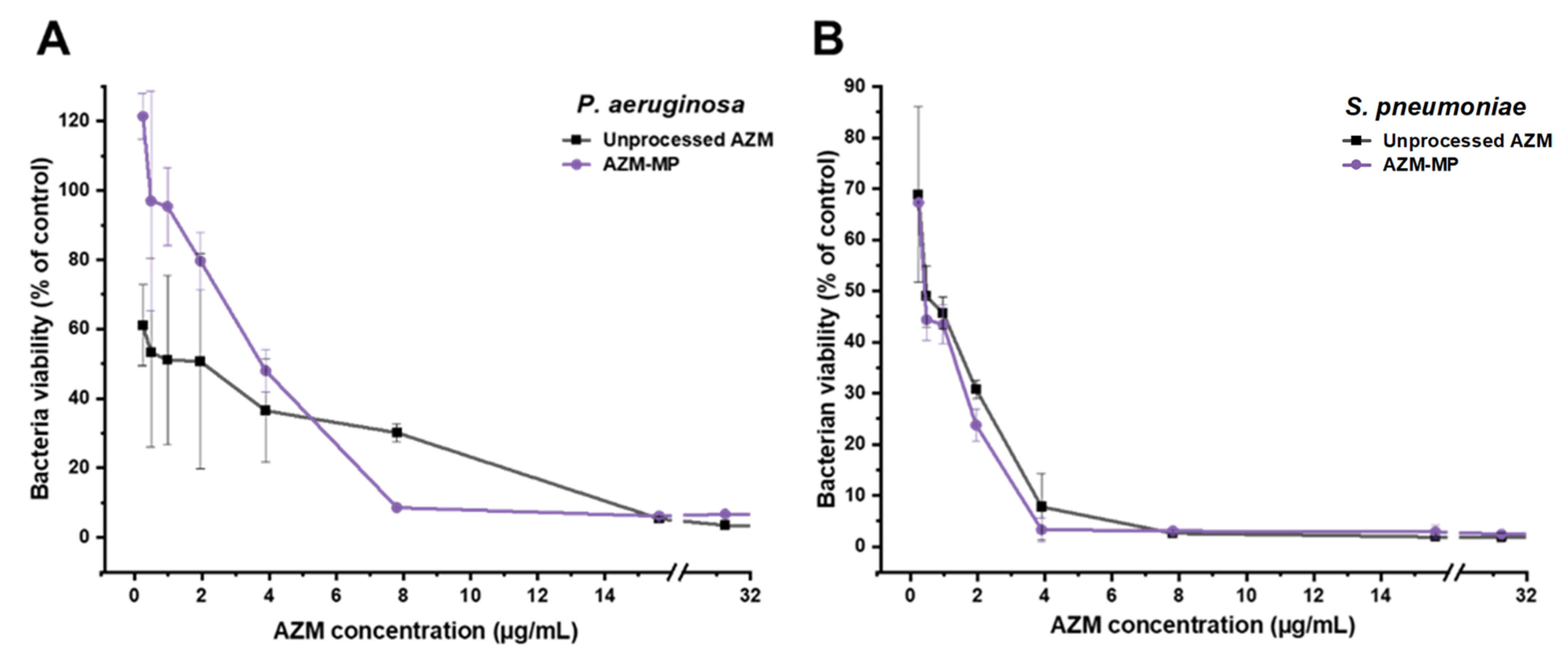
2.2.4. Antibacterial In Vitro Assay
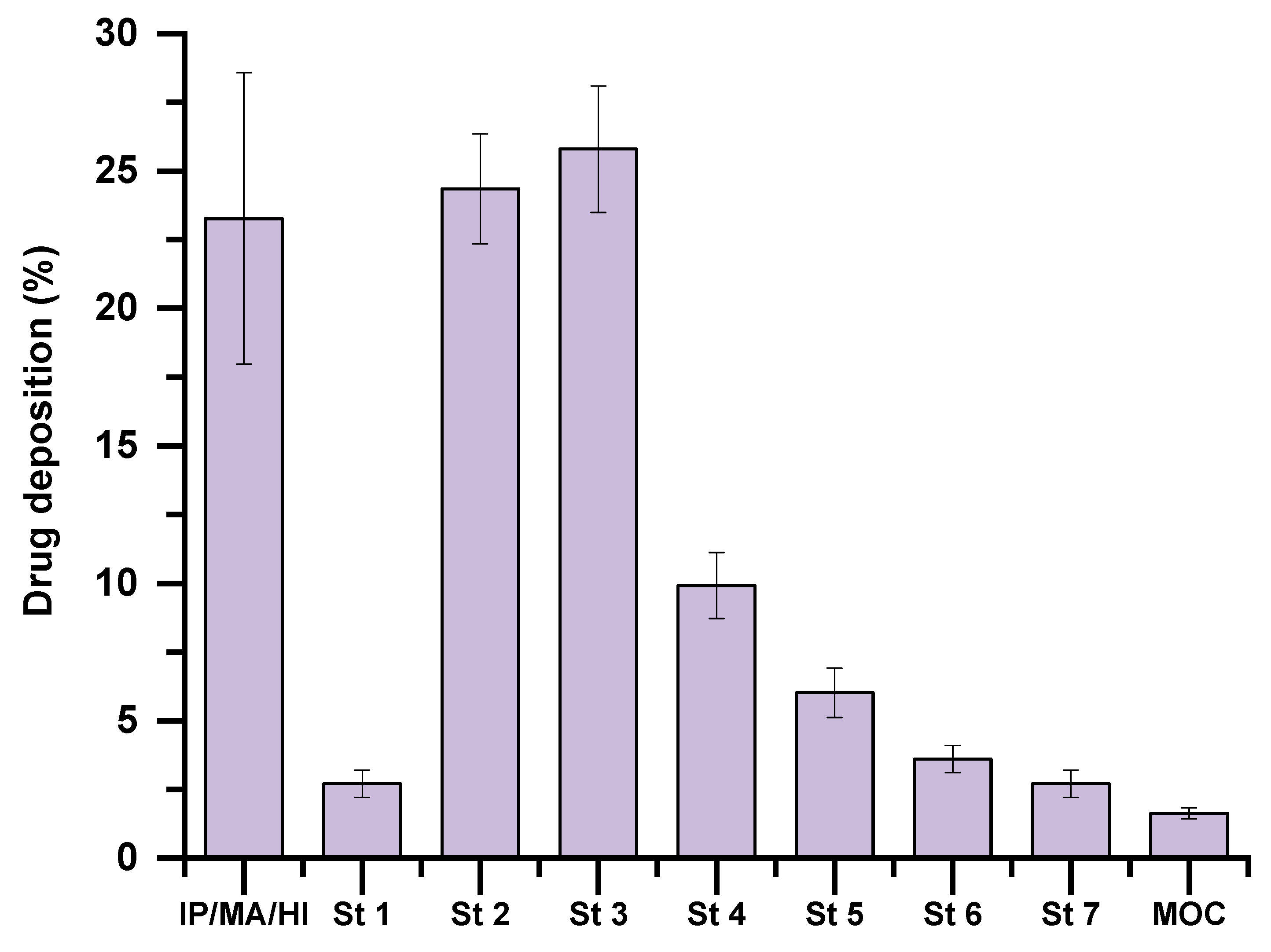
2.2.5. In Vitro Lung Deposition
2.2.6. In Vitro Cell Culture Assays
2.2.7. Statistical Analysis
3. Results
3.1. DoE and Optimization of AZM Formulation
3.2. Microparticle Characterization
3.3. Solid State Characterization
3.4. In Vitro Assessment of Aerodynamic Performance
3.5. Antibacterial In Vitro Assay
3.6. In Vitro Haemolysis and In Vitro Cytotoxicity MTT Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kradin, R.L.; Digumarthy, S. The pathology of pulmonary bacterial infection. Semin. Diagn. Pathol. 2017, 34, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Troeger, C.; Blacker, B.; Khalil, I.A.; Rao, P.C.; Cao, J.; Zimsen, S.R.; Albertson, S.B.; Deshpande, A.; Farag, T.; Abebe, Z. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1191–1210. [Google Scholar] [CrossRef] [PubMed]
- Firth, A.; Prathapan, P. Broad-spectrum therapeutics: A new antimicrobial class. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100011. [Google Scholar] [CrossRef] [PubMed]
- Parnham, M.J.; Haber, V.E.; Giamarellos-Bourboulis, E.J.; Perletti, G.; Verleden, G.M.; Vos, R. Azithromycin: Mechanisms of action and their relevance for clinical applications. Pharmacol. Ther. 2014, 143, 225–245. [Google Scholar] [CrossRef]
- Burke, D.; Harrison, M.; Fleming, C.; McCarthy, M.; Shortt, C.; Sulaiman, I.; Murphy, D.; Eustace, J.; Shanahan, F.; Hill, C. Clostridium difficile carriage in adult cystic fibrosis (CF); implications for patients with CF and the potential for transmission of nosocomial infection. J. Cyst. Fibros. 2017, 16, 291–298. [Google Scholar] [CrossRef]
- Wenzler, E.; Fraidenburg, D.R.; Scardina, T.; Danziger, L.H. Inhaled antibiotics for gram-negative respiratory infections. Clin. Microbiol. Rev. 2016, 29, 581–632. [Google Scholar] [CrossRef]
- Chan, S.H.Y.; Sheikh, K.; Zariwala, M.G.; Somavarapu, S. Dry powder formulation of azithromycin for COVID-19 therapeutics. J. Microencapsul. 2023, 40, 217–232. [Google Scholar] [CrossRef]
- Anaya, B.J.; Kara, A.; Raposo, R.; Tirado, D.F.; Lalatsa, A.; González-Burgos, E.; Serrano, D.R. Integration of 3D-printed micromixers and spray drying for pulmonary delivery of antimicrobial microparticles. Int. J. Pharm. 2025, 674, 125493. [Google Scholar] [CrossRef]
- Cheng, Y.S. Mechanisms of pharmaceutical aerosol deposition in the respiratory tract. AAPS PharmSciTech 2014, 15, 630–640. [Google Scholar] [CrossRef]
- Khan, I.; Elhissi, A.; Shah, M.; Alhnan, M.A.; Ahmed, W. Liposome-based carrier systems and devices used for pulmonary drug delivery. In Biomaterials and Medical Tribology; Elsevier: Amsterdam, The Netherlands, 2013; pp. 395–443. [Google Scholar]
- Hebbink, G.A.; Jaspers, M.; Peters, H.J.; Dickhoff, B.H. Recent developments in lactose blend formulations for carrier-based dry powder inhalation. Adv. Drug Deliv. Rev. 2022, 189, 114527. [Google Scholar] [CrossRef]
- Gholizadeh-Hashjin, A.; Hamishehkar, H.; Monajjemzadeh, F. Detection of Excipient–Excipient Interaction in Dry Powder Inhaler Formulation Prepared by Spray Drying. Pharm. Sci. 2024, 30, 197–203. [Google Scholar] [CrossRef]
- Agnihotri, V.V.; Gorle, A.P. Quality by design based synthesis and characterization of novel maleyl functionalized albumin solid dry powder for pulmonary targeting. Dry. Technol. 2024, 42, 712–727. [Google Scholar] [CrossRef]
- Bar-On, O.; Levine, H.; Stafler, P.; Shmueli, E.; Jacobi, E.; Goldberg, O.; Steuer, G.; Prais, D.; Mei-Zahav, M. Lactose-Containing Dry-Powder Inhalers for Patients with Cow’s Milk Protein Allergy—The Conundrum; A National Survey of Pediatric Pulmonologists and Allergologists. J. Clin. Med. 2022, 11, 7346. [Google Scholar] [CrossRef]
- Molina, C.; Kaialy, W.; Nokhodchi, A. The crucial role of leucine concentration on spray dried mannitol-leucine as a single carrier to enhance the aerosolization performance of albuterol sulfate. J. Drug Deliv. Sci. Technol. 2019, 49, 97–106. [Google Scholar] [CrossRef]
- Muralidharan, P.; Mallory, E.K.; Malapit, M.; Phan, H.; Ledford, J.G.; Hayes, D.; Mansour, H.M. Advanced design and development of nanoparticle/microparticle dual-drug combination lactose carrier-free dry powder inhalation aerosols. RSC Adv. 2020, 10, 41846–41856. [Google Scholar] [CrossRef]
- Banat, H.; Csóka, I.; Paróczai, D.; Burian, K.; Farkas, Á.; Ambrus, R. A Novel Combined Dry Powder Inhaler Comprising Nanosized Ketoprofen-Embedded Mannitol-Coated Microparticles for Pulmonary Inflammations: Development, In Vitro–In Silico Characterization, and Cell Line Evaluation. Pharmaceuticals 2024, 17, 75. [Google Scholar] [CrossRef]
- Ordoubadi, M.; Shepard, K.B.; Wang, H.; Wang, Z.; Pluntze, A.M.; Churchman, J.P.; Vehring, R. On the physical stability of leucine-containing spray-dried powders for respiratory drug delivery. Pharmaceutics 2023, 15, 435. [Google Scholar] [CrossRef]
- Ferdynand, M.S.; Nokhodchi, A. Co-spraying of carriers (mannitol-lactose) as a method to improve aerosolization performance of salbutamol sulfate dry powder inhaler. Drug Deliv. Transl. Res. 2020, 10, 1418–1427. [Google Scholar] [CrossRef]
- Lamy, B.; Serrano, D.R.; O’connell, P.; Couet, W.; Marchand, S.; Healy, A.M.; Tewes, F. Use of leucine to improve aerodynamic properties of ciprofloxacin-loaded maltose microparticles for inhalation. Eur. J. Pharm. Res. 2019, 1, 2–11. [Google Scholar] [CrossRef]
- Shukla, A.; Mishra, V.; Bhoop, B.S.; Katare, O.P. Alginate coated chitosan microparticles mediated oral delivery of diphtheria toxoid (Part A). Systematic optimization, development and characterization. Int. J. Pharm. 2015, 495, 220–233. [Google Scholar] [CrossRef]
- Al-Hakkani, M.F. A rapid, developed and validated RP-HPLC method for determination of azithromycin. SN Appl. Sci. 2019, 1, 222. [Google Scholar] [CrossRef]
- Serrano, D.R.; Lalatsa, A.; Dea-Ayuela, M.A.; Bilbao-Ramos, P.E.; Garrett, N.L.; Moger, J.; Guarro, J.; Capilla, J.; Ballesteros, M.P.; Schatzlein, A.G. Oral particle uptake and organ targeting drives the activity of amphotericin B nanoparticles. Mol. Pharm. 2015, 12, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.; Serrano, D.R.; Mauger, M.; Bolás-Fernández, F.; Dea-Ayuela, M.A.; Lalatsa, A. Orally bioavailable and effective buparvaquone lipid-based nanomedicines for visceral leishmaniasis. Mol. Pharm. 2018, 15, 2570–2583. [Google Scholar] [CrossRef] [PubMed]
- Rapti, C.; Luciano, F.C.; Anaya, B.J.; Ramirez, B.I.; Ongoren, B.; Dea-Ayuela, M.A.; Lalatsa, A.; Serrano, D.R. Amphotericin B Ocular Films for Fungal Keratitis and a Novel 3D-Printed Microfluidic Ocular Lens Infection Model. J. Fungi 2024, 10, 762. [Google Scholar] [CrossRef]
- Anaya, B.J.; Cerda, J.R.; D’Atri, R.M.; Yuste, I.; Luciano, F.C.; Kara, A.; Ruiz, H.K.; Ballesteros, M.P.; Serrano, D.R. Engineering of 3D printed personalized polypills for the treatment of the metabolic syndrome. Int. J. Pharm. 2023, 642, 123194. [Google Scholar] [CrossRef]
- Anaya, B.J.; Raudone, L.; Ureña-Vacas, I.; Sanz-Perez, A.; Marksa, M.; Vilkickyte, G.; García-Rodríguez, J.J.; Serrano, D.R.; González-Burgos, E. Origanum vulgare ssp. hirtum: From Plant to 3D-Printed Gummies with Antioxidant and Anti-Inflammatory Properties. Gels 2025, 11, 246. [Google Scholar] [CrossRef]
- Santamaría, K.J.; Anaya, B.J.; Lalatsa, A.; González-Barranco, P.; Cantú-Cárdenas, L.; Serrano, D.R. Engineering 3D printed gummies loaded with metformin for paediatric use. Gels 2024, 10, 620. [Google Scholar] [CrossRef]
- Pineros, I.; Slowing, K.; Serrano, D.R.; de Pablo, E.; Ballesteros, M.P. Analgesic and anti-inflammatory controlled-released injectable microemulsion: Pseudo-ternary phase diagrams, in vitro, ex vivo and in vivo evaluation. Eur. J. Pharm. Sci. 2017, 101, 220–227. [Google Scholar] [CrossRef]
- Altman, P.; Wehbe, L.; Dederichs, J.; Guerin, T.; Ament, B.; Moronta, M.C.; Pino, A.V.; Goyal, P. Comparison of peak inspiratory flow rate via the Breezhaler®, Ellipta® and HandiHaler® dry powder inhalers in patients with moderate to very severe COPD: A randomized cross-over trial. BMC Pulm. Med. 2018, 18, 100. [Google Scholar] [CrossRef]
- Van Noord, J.; Cornelissen, P.; Aumann, J.-L.; Platz, J.; Mueller, A.; Fogarty, C. The efficacy of tiotropium administered via Respimat® Soft MistTM Inhaler or HandiHaler® in COPD patients. Respir. Med. 2009, 103, 22–29. [Google Scholar] [CrossRef][Green Version]
- Marple, V.A.; Roberts, D.L.; Romay, F.J.; Miller, N.C.; Truman, K.G.; Van Oort, M.; Olsson, B.; Holroyd, M.J.; Mitchell, J.P.; Hochrainer, D. Next generation pharmaceutical impactor (a new impactor for pharmaceutical inhaler testing). Part I: Design. J. Aerosol Med. 2003, 16, 283–299. [Google Scholar] [CrossRef] [PubMed]
- de Pablo, E.; O’Connell, P.; Fernández-García, R.; Marchand, S.; Chauzy, A.; Tewes, F.; Dea-Ayuela, M.A.; Kumar, D.; Bolás, F.; Ballesteros, M. Targeting lung macrophages for fungal and parasitic pulmonary infections with innovative amphotericin B dry powder inhalers. Int. J. Pharm. 2023, 635, 122788. [Google Scholar] [CrossRef] [PubMed]
- Celi, S.S.; Fernández-García, R.; Afonso-Urich, A.I.; Ballesteros, M.P.; Healy, A.M.; Serrano, D.R. Co-delivery of a high dose of amphotericin B and Itraconazole by means of a dry powder inhaler formulation for the treatment of severe fungal pulmonary infections. Pharmaceutics 2023, 15, 2601. [Google Scholar] [CrossRef] [PubMed]
- Anaya, B.J.; D’Angelo, D.; Bettini, R.; Molina, G.; Sanz-Perez, A.; Dea-Ayuela, M.A.; Galiana, C.; Rodríguez, C.; Tirado, D.F.; Lalatsa, A. Heparin-azithromycin microparticles show anti-inflammatory effects and inhibit SARS-CoV-2 and bacterial pathogens associated to lung infections. Carbohydr. Polym. 2025, 348, 122930. [Google Scholar] [CrossRef]
- Arauzo, B.; Lopez-Mendez, T.B.; Lobera, M.P.; Calzada-Funes, J.; Pedraz, J.L.; Santamaria, J. Excipient-free inhalable microparticles of azithromycin produced by electrospray: A novel approach to direct pulmonary delivery of antibiotics. Pharmaceutics 2021, 13, 1988. [Google Scholar] [CrossRef]
- Young, P.M.; Salama, R.O.; Zhu, B.; Phillips, G.; Crapper, J.; Chan, H.-K.; Traini, D. Multi-breath dry powder inhaler for delivery of cohesive powders in the treatment of bronchiectasis. Drug Dev. Ind. Pharm. 2015, 41, 859–865. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Z.; Ren, Y.; Mei, X. Effects of formulation and operating variables on zanamivir dry powder inhalation characteristics and aerosolization performance. Drug Deliv. 2014, 21, 480–486. [Google Scholar] [CrossRef]
- Wu, H.-T.; Li, T.-H.; Tsai, H.-M.; Chien, L.-J.; Chuang, Y.-H. Formulation of inhalable beclomethasone dipropionate-mannitol composite particles through low-temperature supercritical assisted atomization. J. Supercrit. Fluids 2021, 168, 105095. [Google Scholar] [CrossRef]
- Mangal, S.; Nie, H.; Xu, R.; Guo, R.; Cavallaro, A.; Zemlyanov, D.; Zhou, Q. Physico-chemical properties, aerosolization and dissolution of co-spray dried azithromycin particles with l-leucine for inhalation. Pharm. Res. 2018, 35, 28. [Google Scholar] [CrossRef]
- Peng, T.; Zhang, X.; Huang, Y.; Zhao, Z.; Liao, Q.; Xu, J.; Huang, Z.; Zhang, J.; Wu, C.-Y.; Pan, X. Nanoporous mannitol carrier prepared by non-organic solvent spray drying technique to enhance the aerosolization performance for dry powder inhalation. Sci. Rep. 2017, 7, 46517. [Google Scholar] [CrossRef]
- Wu, H.-T.; Su, Y.-C.; Wang, Y.-M.; Tsai, H.-M. Characterization and aerosolization performance of mannitol particles produced using supercritical assisted atomization. Chem. Eng. Res. Des. 2018, 137, 308–318. [Google Scholar] [CrossRef]
- Darquenne, C. Aerosol deposition in health and disease. J. Aerosol Med. Pulm. Drug Deliv. 2012, 25, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Welp, A.L.; Bomberger, J.M. Bacterial community interactions during chronic respiratory disease. Front. Cell. Infect. Microbiol. 2020, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- D’Anna, S.E.; Maniscalco, M.; Cappello, F.; Carone, M.; Motta, A.; Balbi, B.; Ricciardolo, F.L.; Caramori, G.; Di Stefano, A. Bacterial and viral infections and related inflammatory responses in chronic obstructive pulmonary disease. Ann. Med. 2021, 53, 135–150. [Google Scholar] [CrossRef]
- Motos, A.; Kuti, J.L.; Li Bassi, G.; Torres, A.; Nicolau, D.P. Is one sample enough? β-lactam target attainment and penetration into epithelial lining fluid based on multiple bronchoalveolar lavage sampling time points in a swine pneumonia model. Antimicrob. Agents Chemother. 2019, 63, e01922-18. [Google Scholar] [CrossRef]
- Asempa, T.E.; Motos, A.; Abdelraouf, K.; Bissantz, C.; Zampaloni, C.; Nicolau, D.P. Efficacy of human-simulated epithelial lining fluid exposure of meropenem-nacubactam combination against class A serine β-lactamase-producing Enterobacteriaceae in the neutropenic murine lung infection model. Antimicrob. Agents Chemother. 2019, 63, e02382-18. [Google Scholar] [CrossRef]
- Adivitiya; Kaushik, M.S.; Chakraborty, S.; Veleri, S.; Kateriya, S. Mucociliary respiratory epithelium integrity in molecular defense and susceptibility to pulmonary viral infections. Biology 2021, 10, 95. [Google Scholar] [CrossRef]
- Plaunt, A.J.; Nguyen, T.L.; Corboz, M.R.; Malinin, V.S.; Cipolla, D.C. Strategies to overcome biological barriers associated with pulmonary drug delivery. Pharmaceutics 2022, 14, 302. [Google Scholar] [CrossRef]
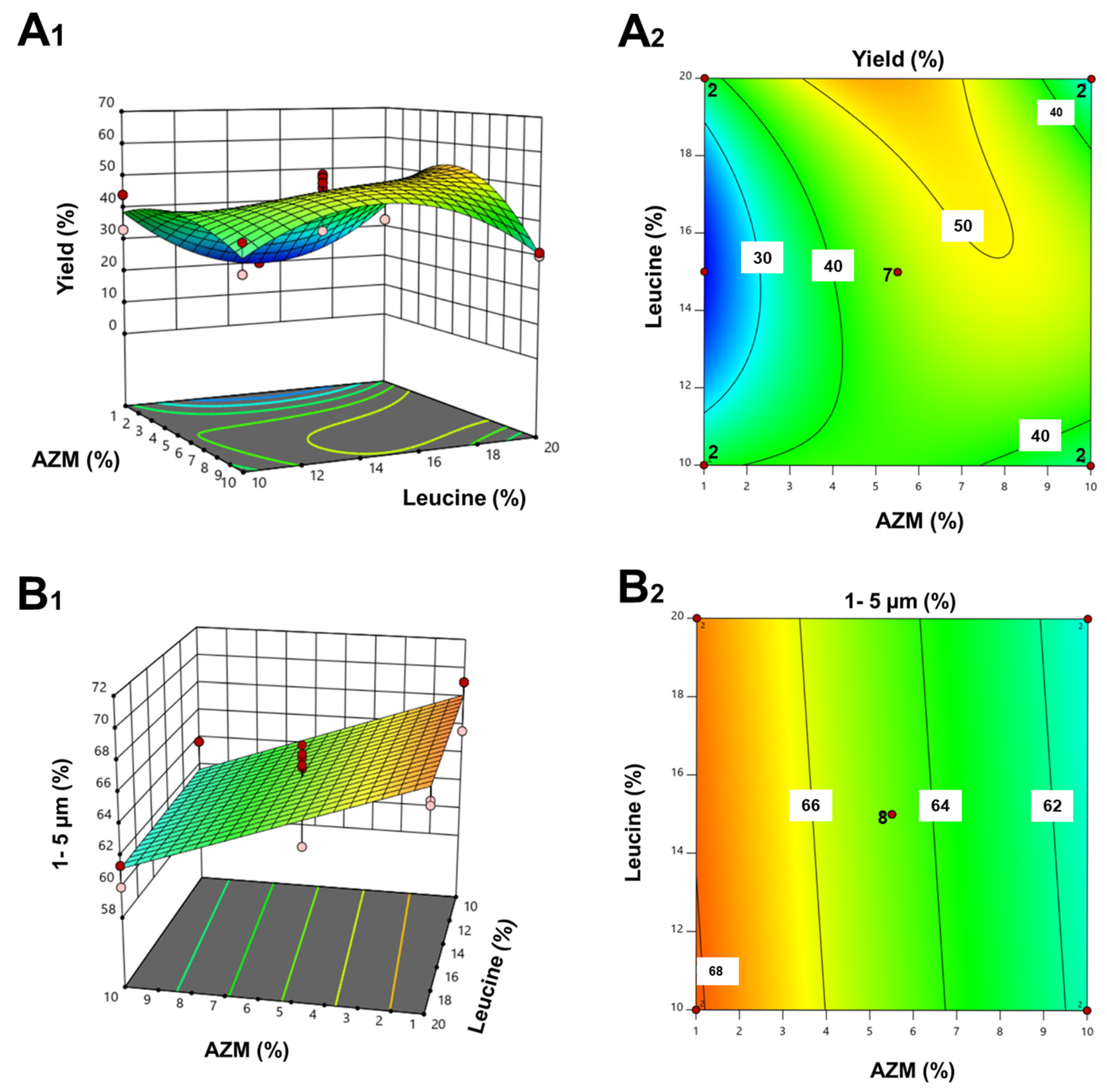




| Experiment | AZM (%) | Leucine (%) | Yield (%) | Percentage of Particle Size in the Range Between 1 and 5 µm (%) |
|---|---|---|---|---|
| 1 | 1.0 | 10.0 | 33.0 | 65.6 |
| 2 | 10.0 | 10.0 | 30.0 | 58.1 |
| 3 | 1.0 | 20.0 | 32.3 | 66.5 |
| 4 | 10.0 | 20.0 | 31.0 | 59.9 |
| 5 | 5.5 | 15.0 | - | 59.9 |
| 6 | 1.0 | 15.0 | 20.0 | - |
| 7 | 13.1 | 15.0 | 33.2 | 58.5 |
| 8 | 5.5 | 6.6 | 40.7 | 67.4 |
| 9 | 5.5 | 23.4 | 59.8 | 66.6 |
| 10 | 5.5 | 15.0 | 44.8 | 66.1 |
| 11 | 5.5 | 15.0 | 48.0 | 65.3 |
| 12 | 5.5 | 15.0 | 52.0 | 65.9 |
| 13 | 5.5 | 15.0 | 51.0 | 66.8 |
| 14 | 5.5 | 15.0 | 49.5 | 66.2 |
| 15 | 5.5 | 15.0 | 42.0 | 65.5 |
| 16 | 1.0 | 10.0 | 44.0 | 69.1 |
| 17 | 10.0 | 10.0 | 38.5 | 63.7 |
| 18 | 1.0 | 20.0 | 41.7 | 66.8 |
| 19 | 10.0 | 20.0 | 30.0 | 61.4 |
| 20 | 5.5 | 15.0 | 35.0 | 63.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina, G.; Serrano, D.R.; Dea-Ayuela, M.A.; Rodriguez, C.; González-Burgos, E.; Anaya, B.J. Lung Delivery of Lactose-Free Microparticles Loaded with Azithromycin for the Treatment of Bacterial Infections. Pharmaceutics 2025, 17, 770. https://doi.org/10.3390/pharmaceutics17060770
Molina G, Serrano DR, Dea-Ayuela MA, Rodriguez C, González-Burgos E, Anaya BJ. Lung Delivery of Lactose-Free Microparticles Loaded with Azithromycin for the Treatment of Bacterial Infections. Pharmaceutics. 2025; 17(6):770. https://doi.org/10.3390/pharmaceutics17060770
Chicago/Turabian StyleMolina, Gracia, Dolores R. Serrano, María Auxiliadora Dea-Ayuela, Carmina Rodriguez, Elena González-Burgos, and Brayan J. Anaya. 2025. "Lung Delivery of Lactose-Free Microparticles Loaded with Azithromycin for the Treatment of Bacterial Infections" Pharmaceutics 17, no. 6: 770. https://doi.org/10.3390/pharmaceutics17060770
APA StyleMolina, G., Serrano, D. R., Dea-Ayuela, M. A., Rodriguez, C., González-Burgos, E., & Anaya, B. J. (2025). Lung Delivery of Lactose-Free Microparticles Loaded with Azithromycin for the Treatment of Bacterial Infections. Pharmaceutics, 17(6), 770. https://doi.org/10.3390/pharmaceutics17060770








