Natural Products for Improving Soft Tissue Healing: Mechanisms, Innovations, and Clinical Potential
Abstract
1. Introduction
2. The Biology of Scar Formation
2.1. Extracellular Matrix
2.2. Cellular and Molecular Players
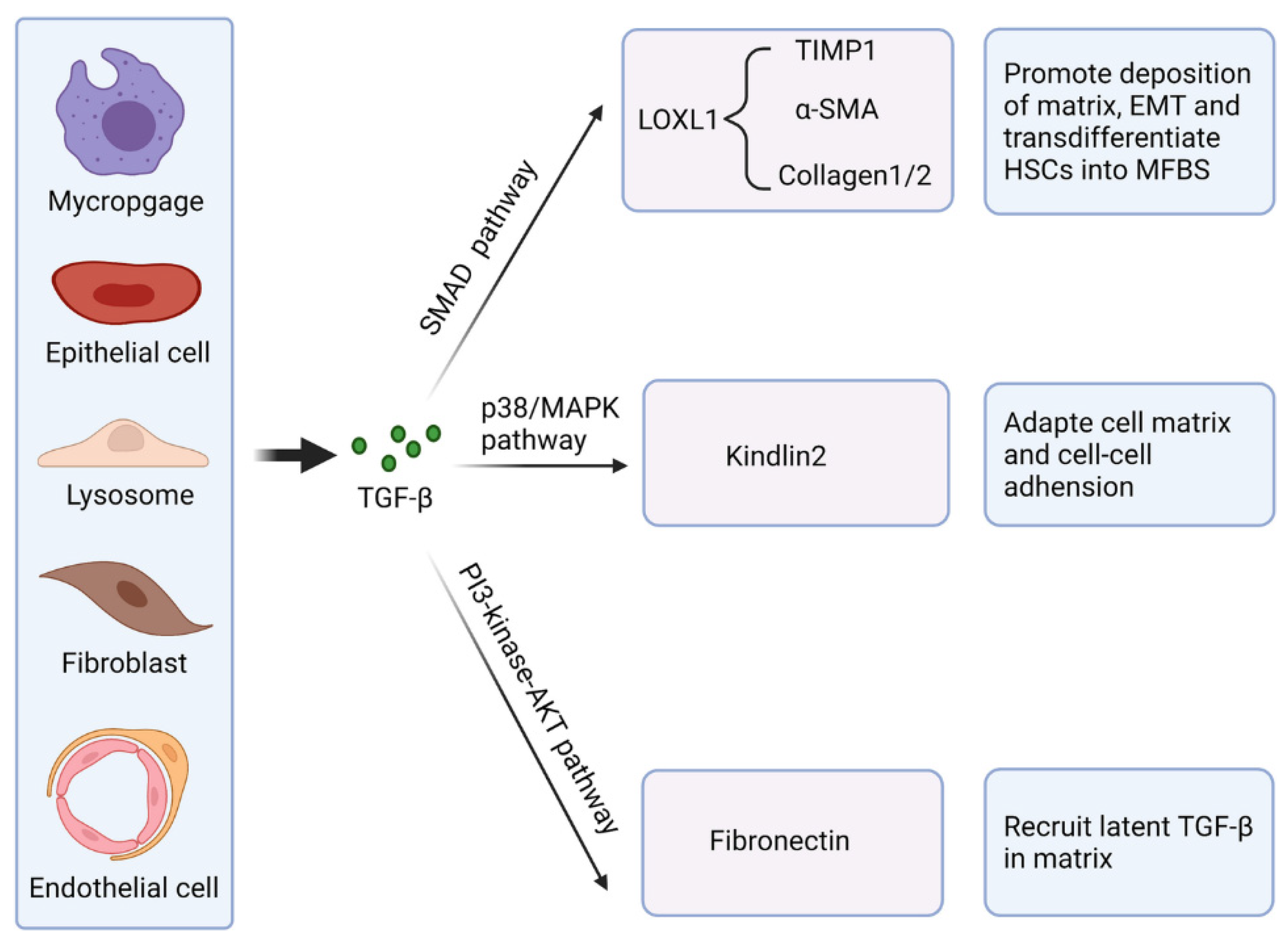
2.3. The Influence of Cytokines in Fibrosis
2.4. Role of Oxidative Stress
3. Natural Products in Soft Tissue Healing
3.1. Historical and Traditional Uses
3.2. Key Natural Products
3.3. Categories of Compounds of Interest for Tissue Healing
3.3.1. Antioxidants
3.3.2. Anti-Inflammatory
3.3.3. Antimicrobials
4. Natural Materials for ECM Restoration
4.1. Polysaccharides
4.2. Proteins
4.3. Bioactive Peptides
5. Cellular Mechanisms of Natural Products in Scar-Free Healing
5.1. Regulation of Inflammation
5.2. Balancing Oxidative Stress
5.3. Antibacterial
5.4. Antifungal
5.5. Modulating Fibroblast-to-Myofibroblast Transition
6. Emerging Innovations in Scar-Free Healing
6.1. Micro- and Nanomaterials
6.2. Composite Materials
6.3. Smart Dressings
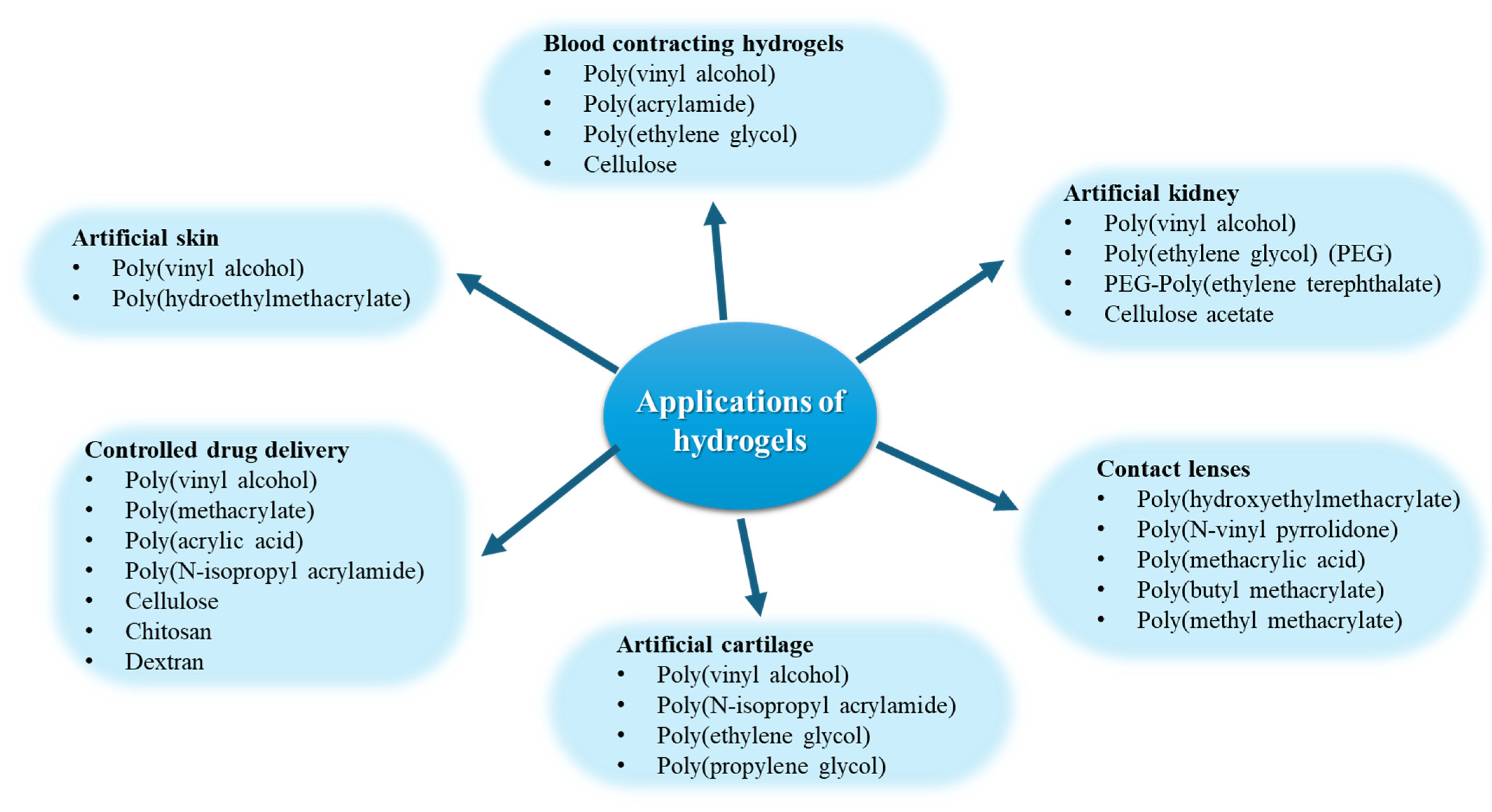
6.4. Injectable Hydrogels
7. Clinical Applications and Case Studies
7.1. Patches Based on Natural Products
7.2. Natural Treatments for Diabetic Foot Ulcers
7.3. Natural Products in Venous Leg Ulcers
7.4. Applications of Natural Products in Burn Wounds
7.5. Natural and Essential Oils in Surgical Wound Care
8. Challenges and Limitations
9. Future Directions
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gonzalez, A.C.d.O.; Costa, T.F.; Andrade, Z.d.A.; Medrado, A.R.A.P. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Li, F.; Han, Z.; Qu, X.; Li, J.; Zhou, Z.; Chen, S.; Wang, H.; Lv, X. Bacterial cellulose-based hydrogel with regulated rehydration and enhanced antibacterial activity for wound healing. Int. J. Biol. Macromol. 2024, 267, 131291. [Google Scholar] [CrossRef] [PubMed]
- Gushiken, L.F.S.; Beserra, F.P.; Bastos, J.K.; Jackson, C.J.; Pellizzon, C.H. Cutaneous Wound Healing: An Update from Physiopathology to Current Therapies. Life 2021, 11, 665. [Google Scholar] [CrossRef] [PubMed]
- Corr, D.T.; Hart, D.A. Biomechanics of scar tissue and uninjured skin. Adv. Wound Care 2013, 2, 37–43. [Google Scholar] [CrossRef]
- Frank, C.; Shrive, N.; Hiraoka, H.; Nakamura, N.; Kaneda, Y.; Hart, D. Optimisation of the biology of soft tissue repair. J. Sci. Med. Sport 1999, 2, 190–210. [Google Scholar] [CrossRef]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Hildebrand, K.A.; Gallant-Behm, C.L.; Kydd, A.S.; Hart, D.A. The basics of soft tissue healing and general factors that influence such healing. Sports Med. Arthrosc. Rev. 2005, 13, 136–144. [Google Scholar] [CrossRef]
- Jones, N. Scar tissue. Curr. Opin. Otolaryngol. Head Neck surgery 2010, 18, 261–265. [Google Scholar] [CrossRef]
- Alberts, A.; Bratu, A.G.; Niculescu, A.-G.; Grumezescu, A.M. Collagen-Based Wound Dressings: Innovations, Mechanisms, and Clinical Applications. Gels 2025, 11, 271. [Google Scholar] [CrossRef]
- Marshall, C.D.; Hu, M.S.; Leavitt, T.; Barnes, L.A.; Lorenz, H.P.; Longaker, M.T. Cutaneous Scarring: Basic Science, Current Treatments, and Future Directions. Adv. Wound Care 2018, 7, 29–45. [Google Scholar] [CrossRef]
- Khanna, A.; Nelmes, R.T.; Gougoulias, N.; Maffulli, N.; Gray, J. The effects of LIPUS on soft-tissue healing: A review of literature. Br. Med. Bull. 2009, 89, 169–182. [Google Scholar] [CrossRef] [PubMed]
- El Ayadi, A.; Jay, J.W.; Prasai, A. Current approaches targeting the wound healing phases to attenuate fibrosis and scarring. Int. J. Mol. Sci. 2020, 21, 1105. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Kwan, J.Y.Y.; Yip, K.; Liu, P.P.; Liu, F.-F. Targeting metabolic dysregulation for fibrosis therapy. Nat. Rev. Drug Discov. 2020, 19, 57–75. [Google Scholar] [CrossRef]
- Katoulis, A.C.; Christodoulou, C.; Liakou, A.I.; Kouris, A.; Korkoliakou, P.; Kaloudi, E.; Kanelleas, A.; Papageorgiou, C.; Rigopoulos, D. Quality of life and psychosocial impact of scarring and non-scarring alopecia in women. JDDG J. Der Dtsch. Dermatol. Ges. 2015, 13, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.W.; Mason, S.T.; Schomer, K.; Klein, M.B. Epidemiology and impact of scarring after burn injury: A systematic review of the literature. J. Burn. Care Res. 2012, 33, 136–146. [Google Scholar] [CrossRef]
- Mobley, S.R.; Sjogren, P.P. Soft tissue trauma and scar revision. Facial Plast. Surg. Clin. 2014, 22, 639–651. [Google Scholar] [CrossRef]
- Trinh, X.T.; Long, N.V.; Van Anh, L.T.; Nga, P.T.; Giang, N.N.; Chien, P.N.; Nam, S.Y.; Heo, C.Y. A Comprehensive Review of Natural Compounds for Wound Healing: Targeting Bioactivity Perspective. Int. J. Mol. Sci. 2022, 23, 9573. [Google Scholar] [CrossRef]
- Monavarian, M.; Kader, S.; Moeinzadeh, S.; Jabbari, E. Regenerative scar-free skin wound healing. Tissue Eng. Part B Rev. 2019, 25, 294–311. [Google Scholar] [CrossRef]
- Harn, H.I.C.; Ogawa, R.; Hsu, C.K.; Hughes, M.W.; Tang, M.J.; Chuong, C.M. The tension biology of wound healing. Exp. Dermatol. 2019, 28, 464–471. [Google Scholar] [CrossRef]
- Qing, C.; Wang, Z.Y.; Song, F.; Wang, X.Q. Dynamic biological changes in fibroblasts during hypertrophic scar formation and regression. Int. Wound J. 2016, 13, 257–262. [Google Scholar]
- Alam, W.; Hasson, J.; Reed, M. Clinical approach to chronic wound management in older adults. J. Am. Geriatr. Soc. 2021, 69, 2327–2334. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef]
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Rýglová, Š.; Braun, M.; Suchý, T. Collagen and Its Modifications—Crucial Aspects with Concern to Its Processing and Analysis. Macromol. Mater. Eng. 2017, 302, 1600460. [Google Scholar] [CrossRef]
- Plikus, M.V.; Wang, X.; Sinha, S.; Forte, E.; Thompson, S.M.; Herzog, E.L.; Driskell, R.R.; Rosenthal, N.; Biernaskie, J.; Horsley, V. Fibroblasts: Origins, definitions, and functions in health and disease. Cell 2021, 184, 3852–3872. [Google Scholar] [CrossRef]
- Rockey, D.C.; Bell, P.D.; Hill, J.A. Fibrosis—A common pathway to organ injury and failure. N. Engl. J. Med. 2015, 372, 1138–1149. [Google Scholar] [CrossRef] [PubMed]
- Shook, B.A.; Wasko, R.R.; Rivera-Gonzalez, G.C.; Salazar-Gatzimas, E.; López-Giráldez, F.; Dash, B.C.; Muñoz-Rojas, A.R.; Aultman, K.D.; Zwick, R.K.; Lei, V.; et al. Myofibroblast proliferation and heterogeneity are supported by macrophages during skin repair. Science 2018, 362, eaar2971. [Google Scholar] [CrossRef]
- Darby, I.A.; Laverdet, B.; Bonté, F.; Desmoulière, A. Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig. Dermatol. 2014, 7, 301–311. [Google Scholar] [CrossRef]
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte—Fibroblast Interactions in Wound Healing. J. Investig. Dermatol. 2007, 127, 998–1008. [Google Scholar] [CrossRef]
- Distler, J.H.; Györfi, A.-H.; Ramanujam, M.; Whitfield, M.L.; Königshoff, M.; Lafyatis, R. Shared and distinct mechanisms of fibrosis. Nat. Rev. Rheumatol. 2019, 15, 705–730. [Google Scholar] [CrossRef]
- Hinz, B. The role of myofibroblasts in wound healing. Curr. Res. Transl. Med. 2016, 64, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Talbott, H.E.; Mascharak, S.; Griffin, M.; Wan, D.C.; Longaker, M.T. Wound healing, fibroblast heterogeneity, and fibrosis. Cell Stem Cell 2022, 29, 1161–1180. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef]
- Peng, D.; Fu, M.; Wang, M.; Wei, Y.; Wei, X. Targeting TGF-β signal transduction for fibrosis and cancer therapy. Mol. Cancer 2022, 21, 104. [Google Scholar] [CrossRef] [PubMed]
- Bonniaud, P.; Margetts, P.J.; Ask, K.; Flanders, K.; Gauldie, J.; Kolb, M. TGF-β and Smad3 Signaling Link Inflammation to Chronic Fibrogenesis1. J. Immunol. 2005, 175, 5390–5395. [Google Scholar] [CrossRef]
- Vander Ark, A.; Cao, J.; Li, X. TGF-β receptors: In and beyond TGF-β signaling. Cell. Signal. 2018, 52, 112–120. [Google Scholar] [CrossRef]
- Houldsworth, A. Role of oxidative stress in neurodegenerative disorders: A review of reactive oxygen species and prevention by antioxidants. Brain Commun. 2024, 6, fcad356. [Google Scholar] [CrossRef]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef]
- Sharma, A.; Khanna, S.; Kaur, G.; Singh, I. Medicinal plants and their components for wound healing applications. Future J. Pharm. Sci. 2021, 7, 53. [Google Scholar] [CrossRef]
- Lusby, P.; Coombes, A.; Wilkinson, J. Honey: A potent agent for wound healing? J. WOCN 2002, 29, 295–300. [Google Scholar] [CrossRef]
- Bezerra, A.; Fonseca, H.; Rodrigues, F.; Delerue-Matos, C.; Gouvinhas, I.; Garcia, J. Honey Therapy in Diabetic Foot Ulcers: A Promising Strategy for Effective Wound Healing. Appl. Sci. 2023, 13, 12820. [Google Scholar] [CrossRef]
- Minden-Birkenmaier, B.A.; Bowlin, G.L. Honey-based templates in wound healing and tissue engineering. Bioengineering 2018, 5, 46. [Google Scholar] [CrossRef]
- Yupanqui Mieles, J.; Vyas, C.; Aslan, E.; Humphreys, G.; Diver, C.; Bartolo, P. Honey: An advanced antimicrobial and wound healing biomaterial for tissue engineering applications. Pharmaceutics 2022, 14, 1663. [Google Scholar] [CrossRef]
- Lee, D.S.; Sinno, S.; Khachemoune, A. Honey and wound healing: An overview. Am. J. Clin. Dermatol. 2011, 12, 181–190. [Google Scholar] [CrossRef]
- Saikaly, S.K.; Khachemoune, A. Honey and wound healing: An update. Am. J. Clin. Dermatol. 2017, 18, 237–251. [Google Scholar] [CrossRef]
- Mancuso, C. Panax notoginseng: Pharmacological Aspects and Toxicological Issues. Nutrients 2024, 16, 2120. [Google Scholar] [CrossRef] [PubMed]
- Potenza, M.A.; Montagnani, M.; Santacroce, L.; Charitos, I.A.; Bottalico, L. Ancient herbal therapy: A brief history of Panax ginseng. J. Ginseng Res. 2023, 47, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, I.M. Ambroise Paré’s accounts of new methods for treating gunshot wounds and burns. J. R. Soc. Med. 2015, 108, 457–461. [Google Scholar] [CrossRef]
- Dai, Y.L.; Li, Y.; Wang, Q.; Niu, F.J.; Li, K.W.; Wang, Y.Y.; Wang, J.; Zhou, C.Z.; Gao, L.N. Chamomile: A Review of Its Traditional Uses, Chemical Constituents, Pharmacological Activities and Quality Control Studies. Molecules 2023, 28, 133. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, K.M.; Torres, B.B.; Sanfelice, R.C.; da Costa, M.M.; Assis, L.; Marques, R.B.; Maia Filho, A.L.M.; Tim, C.R.; Pavinatto, A. Chitosan and chitosan/turmeric-based membranes for wound healing: Production, characterization and application. Int. J. Biol. Macromol. 2023, 253, 127425. [Google Scholar] [CrossRef]
- Mahmudi, G.; Nikpour, M.; Azadbackt, M.; Zanjani, R.; Jahani, M.; Aghamohammadi, A.; Jannati, Y. The impact of turmeric cream on healing of caesarean scar. West Indian Med. J. 2016, 64, 400. [Google Scholar]
- Tejada, S.; Manayi, A.; Daglia, M.; F Nabavi, S.; Sureda, A.; Hajheydari, Z.; Gortzi, O.; Pazoki-Toroudi, H.; M Nabavi, S. Wound healing effects of curcumin: A short review. Curr. Pharm. Biotechnol. 2016, 17, 1002–1007. [Google Scholar] [CrossRef]
- Eteraf-Oskouei, T.; Najafi, M. Traditional and modern uses of natural honey in human diseases: A review. Iran. J. Basic Med. Sci. 2013, 16, 731–742. [Google Scholar] [PubMed]
- Srivastava, J.K.; Shankar, E.; Gupta, S. Chamomile: A herbal medicine of the past with a bright future. Mol. Med. Rep. 2010, 3, 895–901. [Google Scholar] [CrossRef]
- Dat, A.D.; Poon, F.; Pham, K.B.; Doust, J. Aloe vera for treating acute and chronic wounds. Cochrane Database Syst. Rev. 2012, 2012, CD008762. [Google Scholar] [CrossRef]
- Melnyk, N.; Nyczka, A.; Piwowarski, J.P.; Granica, S. Traditional Use of Chamomile Flowers (Matricariae flos) in Inflammatory-Associated Skin Disorders. Prospects in Pharmaceutical Sciences 2024, 22, 59–73. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, D.H.; Park, S.J.; Kim, J.M.; Ryu, J.H. Ginseng in traditional herbal prescriptions. J. Ginseng Res. 2012, 36, 225–241. [Google Scholar] [CrossRef]
- Orhan, I.E. Centella asiatica L. Urban: From Traditional Medicine to Modern Medicine with Neuroprotective Potential. Evid.-Based Complement. Altern. Med. Ecam 2012, 2012, 946259. [Google Scholar] [CrossRef]
- Simbirtsev, A.S.; Konusova, V.G.; McHelidze, G.; Fidarov, E.Z.; Paramonov, B.A.; Chebotarev, V.Y. Pine resin and Biopin ointment: Effects on repair processes in tissues. Bull. Exp. Biol. Med. 2002, 133, 457–460. [Google Scholar] [CrossRef]
- Shah, J.B. The history of wound care. J. Am. Col. Certif. Wound Spec. 2011, 3, 65–66. [Google Scholar] [CrossRef]
- Doersch, K.M.; Newell-Rogers, M.K. The impact of quercetin on wound healing relates to changes in αV and β1 integrin expression. Exp. Biol. Med. 2017, 242, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Martinez, E.J.; Flores-Hernández, F.Y.; Salazar-Montes, A.M.; Nario-Chaidez, H.F.; Hernández-Ortega, L.D. Quercetin, a Flavonoid with Great Pharmacological Capacity. Molecules 2024, 29, 1000. [Google Scholar] [CrossRef] [PubMed]
- Nalini, T.; Khaleel Basha, S.; Mohamed Sadiq, A.; Sugantha Kumari, V. Fabrication and evaluation of nanoencapsulated quercetin for wound healing application. Polym. Bull. 2023, 80, 515–540. [Google Scholar] [CrossRef]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D. Turmeric and its major compound curcumin on health: Bioactive effects and safety profiles for food, pharmaceutical, biotechnological and medicinal applications. Front. Pharmacol. 2020, 11, 550909. [Google Scholar] [CrossRef] [PubMed]
- Fereydouni, N.; Darroudi, M.; Movaffagh, J.; Shahroodi, A.; Butler, A.E.; Ganjali, S.; Sahebkar, A. Curcumin nanofibers for the purpose of wound healing. J. Cell. Physiol. 2019, 234, 5537–5554. [Google Scholar] [CrossRef]
- Farhat, F.; Sohail, S.S.; Siddiqui, F.; Irshad, R.R.; Madsen, D.Ø. Curcumin in wound healing—A bibliometric analysis. Life 2023, 13, 143. [Google Scholar] [CrossRef]
- Gong, C.; Wu, Q.; Wang, Y.; Zhang, D.; Luo, F.; Zhao, X.; Wei, Y.; Qian, Z. A biodegradable hydrogel system containing curcumin encapsulated in micelles for cutaneous wound healing. Biomaterials 2013, 34, 6377–6387. [Google Scholar] [CrossRef]
- Barchitta, M.; Maugeri, A.; Favara, G.; Magnano San Lio, R.; Evola, G.; Agodi, A.; Basile, G. Nutrition and wound healing: An overview focusing on the beneficial effects of curcumin. Int. J. Mol. Sci. 2019, 20, 1119. [Google Scholar] [CrossRef]
- Kasprzak-Drozd, K.; Niziński, P.; Hawrył, A.; Gancarz, M.; Hawrył, D.; Oliwa, W.; Pałka, M.; Markowska, J.; Oniszczuk, A. Potential of Curcumin in the Management of Skin Diseases. Int. J. Mol. Sci. 2024, 25, 3617. [Google Scholar] [CrossRef]
- Mo, Z.; Yuan, J.; Guan, X.; Peng, J. Advancements in dermatological applications of curcumin: Clinical efficacy and mechanistic insights in the management of skin disorders. Clin. Cosmet. Investig. Dermatol. 2024, 17, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Raina, N.; Wahi, A.; Goh, K.W.; Sharma, P.; Nagpal, R.; Jain, A.; Ming, L.C.; Gupta, M. Wound-Healing Effects of Curcumin and Its Nanoformulations: A Comprehensive Review. Pharmaceutics 2022, 14, 2288. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, D.; Mahmood, S.; Singh, V.; Chopra, S.; Hilles, A.R.; Bhatia, A. Nanotechnology-driven wound healing potential of asiaticoside: A comprehensive review. RSC Pharm. 2024, 1, 9–36. [Google Scholar] [CrossRef]
- Witkowska, K.; Paczkowska-Walendowska, M.; Garbiec, E.; Cielecka-Piontek, J. Topical Application of Centella asiatica in Wound Healing: Recent Insights into Mechanisms and Clinical Efficacy. Pharmaceutics 2024, 16, 1252. [Google Scholar] [CrossRef] [PubMed]
- Diniz, L.R.L.; Calado, L.L.; Duarte, A.B.S.; de Sousa, D.P. Centella asiatica and its metabolite asiatic acid: Wound healing effects and therapeutic potential. Metabolites 2023, 13, 276. [Google Scholar] [CrossRef]
- Albahri, G.; Badran, A.; Hijazi, A.; Daou, A.; Baydoun, E.; Nasser, M.; Merah, O. The therapeutic wound healing bioactivities of various medicinal plants. Life 2023, 13, 317. [Google Scholar] [CrossRef]
- Mazzoni, E.; Iaquinta, M.R.; Lanzillotti, C.; Mazziotta, C.; Maritati, M.; Montesi, M.; Sprio, S.; Tampieri, A.; Tognon, M.; Martini, F. Bioactive materials for soft tissue repair. Front. Bioeng. Biotechnol. 2021, 9, 613787. [Google Scholar] [CrossRef]
- Kreimendahl, F.; Marquardt, Y.; Apel, C.; Bartneck, M.; Zwadlo-Klarwasser, G.; Hepp, J.; Jockenhoevel, S.; Baron, J.M. Macrophages significantly enhance wound healing in a vascularized skin model. J. Biomed. Mater. Res. Part A 2019, 107, 1340–1350. [Google Scholar] [CrossRef]
- Van Loey, N.E. Psychological impact of living with scars following burn injury. In Textbook on Scar Management: State of the Art Management and Emerging Technologies; Springer Nature Switzerland AG: Cham, Switzerland, 2020; pp. 429–434. [Google Scholar]
- Mohammed, B.M.; Fisher, B.J.; Kraskauskas, D.; Ward, S.; Wayne, J.S.; Brophy, D.F.; Fowler, A.A., 3rd; Yager, D.R.; Natarajan, R. Vitamin C promotes wound healing through novel pleiotropic mechanisms. Int. Wound J. 2016, 13, 572–584. [Google Scholar] [CrossRef]
- Alberts, A.; Moldoveanu, E.-T.; Niculescu, A.-G.; Grumezescu, A.M. Vitamin C: A Comprehensive Review of Its Role in Health, Disease Prevention, and Therapeutic Potential. Molecules 2025, 30, 748. [Google Scholar] [CrossRef]
- Hobson, R. Vitamin E and wound healing: An evidence-based review. Int. Wound J. 2016, 13, 331–335. [Google Scholar] [CrossRef] [PubMed]
- El-Sakhawy, M.; Salama, A.; Tohamy, H.S. Applications of propolis-based materials in wound healing. Arch. Dermatol. Res. 2023, 316, 61. [Google Scholar] [CrossRef]
- Roney, M.; Aluwi, M.F.F.M.; Zamri, N.B. An In-Silico Approach to Evaluate Bioactive Molecules of Aloe Vera Leaf Extracts in Inhibiting the Glycogen Synthase Kinase-3β (GSK3-β) Protein for Faster Diabetic Wound Healing Potential. Biointerface Res. Appl. Chem. 2024, 14, 115. [Google Scholar]
- Bahadoram, M.; Hassanzadeh, S.; Bahadoram, S.; Mowla, K. Effects of Pomegranate on Wound Repair and Regeneration. World J. Plast. Surg. 2022, 11, 157–159. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Biological properties and therapeutic activities of honey in wound healing: A narrative review and meta-analysis. J. Tissue Viability 2016, 25, 98–118. [Google Scholar] [CrossRef] [PubMed]
- Deli, J.; González-Beiras, C.; Guldan, G.S.; Moses, R.L.; Dally, J.; Moseley, R.; Lundy, F.T.; Corbacho-Monne, M.; Walker, S.L.; Cazorla, M.U.; et al. Ficus septica exudate, a traditional medicine used in Papua New Guinea for treating infected cutaneous ulcers: In vitro evaluation and clinical efficacy assessment by cluster randomised trial. Phytomedicine 2022, 99, 154026. [Google Scholar] [CrossRef]
- Singh, P.; Verma, C.; Mukhopadhyay, S.; Gupta, A.; Gupta, B. Preparation of thyme oil loaded κ-carrageenan-polyethylene glycol hydrogel membranes as wound care system. Int. J. Pharm. 2022, 618, 121661. [Google Scholar] [CrossRef]
- Gourishetti, K.; Raghuvir, K.; Ganesh, N.P.; Reddy, J.S.; Ajitkumar, B.N.; Lalit, K.; Nitesh, K.; Nandakumar, K.; Shenoy, R.R. Sesamol-Loaded PLGA Nanosuspension for Accelerating Wound Healing in Diabetic Foot Ulcer in Rats. Int. J. Nanomed. 2020, 15, 9265–9282. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi-Nesheli, M.; Alizadeh, S.; Solhi, H.; Mohseni, J.; Mahmoodi-Nesheli, M. Adjuvant effect of oral Silymarin on patients’ wound healing process caused by thermal injuries. Casp. J. Intern. Med. 2018, 9, 341–346. [Google Scholar] [CrossRef]
- Kharat, Z.; Amiri Goushki, M.; Sarvian, N.; Asad, S.; Dehghan, M.M.; Kabiri, M. Chitosan/PEO nanofibers containing Calendula officinalis extract: Preparation, characterization, in vitro and in vivo evaluation for wound healing applications. Int. J. Pharm. 2021, 609, 121132. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, M.; Lin, T.; Wang, L.; Wang, G.; Chen, T.; Su, S. Royal jelly from different floral sources possesses distinct wound-healing mechanisms and ingredient profiles. Food Funct. 2021, 12, 12059–12076. [Google Scholar] [CrossRef] [PubMed]
- Olczyk, P.; Koprowski, R.; Kaźmierczak, J.; Mencner, L.; Wojtyczka, R.; Stojko, J.; Olczyk, K.; Komosinska-Vassev, K. Bee Pollen as a Promising Agent in the Burn Wounds Treatment. Evid.-Based Complement. Altern. Med. Ecam 2016, 2016, 8473937. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Liu, C.; Zheng, L. Preparation of photo-crosslinked microalgae-carboxymethyl chitosan composite hydrogels for enhanced wound healing. Carbohydr. Polym. 2025, 348, 122803. [Google Scholar] [CrossRef]
- Jia, Y.; Shao, J.-H.; Zhang, K.-W.; Zou, M.-L.; Teng, Y.-Y.; Tian, F.; Chen, M.-N.; Chen, W.-W.; Yuan, Z.-D.; Wu, J.-J.; et al. Emerging Effects of Resveratrol on Wound Healing: A Comprehensive Review. Molecules 2022, 27, 6736. [Google Scholar] [CrossRef]
- Lu, L.; Ying, K.; Wei, S.; Fang, Y.; Liu, Y.; Lin, H.; Ma, L.; Mao, Y. Asiaticoside induction for cell-cycle progression, proliferation and collagen synthesis in human dermal fibroblasts. Int. J. Dermatol. 2004, 43, 801–807. [Google Scholar] [CrossRef]
- Yang, D.J.; Moh, S.H.; Son, D.H.; You, S.; Kinyua, A.W.; Ko, C.M.; Song, M.; Yeo, J.; Choi, Y.H.; Kim, K.W. Gallic Acid Promotes Wound Healing in Normal and Hyperglucidic Conditions. Molecules 2016, 21, 899. [Google Scholar] [CrossRef]
- Karatas, O.; Gevrek, F. Gallic acid liposome and powder gels improved wound healing in wistar rats. Ann. Med. Res. 2019, 26, 2720–2727. [Google Scholar] [CrossRef]
- Dryden, M. Reactive oxygen species: A novel antimicrobial. Int. J. Antimicrob. Agents 2018, 51, 299–303. [Google Scholar] [CrossRef]
- Motawea, M.H.; Abd Elmaksoud, H.A.; Elharrif, M.G.; Desoky, A.A.E.; Ibrahimi, A. Evaluation of Anti-inflammatory and Antioxidant Profile of Oleuropein in Experimentally Induced Ulcerative Colitis. Int. J. Mol. Cell Med. 2020, 9, 224–233. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in Skin Health, Repair, and Disease: A Comprehensive Review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef] [PubMed]
- Manciula, L.-G.; Berce, C.; Tabaran, F.; Trombitaș, V.; Albu, S. The Effects of Postoperative Astaxanthin Administration on Nasal Mucosa Wound Healing. J. Clin. Med. 2019, 8, 1941. [Google Scholar] [CrossRef] [PubMed]
- Capasso, L.; De Masi, L.; Sirignano, C.; Maresca, V.; Basile, A.; Nebbioso, A.; Rigano, D.; Bontempo, P. Epigallocatechin Gallate (EGCG): Pharmacological Properties, Biological Activities and Therapeutic Potential. Molecules 2025, 30, 654. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J. Burn injuries: The social and emotional impact of scarring. Encycl. Body Image Hum. Appear. 2012, 1, 300–306. [Google Scholar]
- Tredget, E.E.; Shupp, J.W.; Schneider, J.C. Scar management following burn injury. J. Burn. Care Res. 2017, 38, 146–147. [Google Scholar] [CrossRef]
- Liu, E.; Gao, H.; Zhao, Y.; Pang, Y.; Yao, Y.; Yang, Z.; Zhang, X.; Wang, Y.; Yang, S.; Ma, X.; et al. The potential application of natural products in cutaneous wound healing: A review of preclinical evidence. Front. Pharmacol. 2022, 13, 900439. [Google Scholar] [CrossRef]
- Amatto, P.; Chaves, L.; França, S.; Carvalho, J.; Carmona, F.; Pereira, A. Efficacy of different pharmaceutical forms of Curcuma longa or curcumin in reducing oral mucositis severity and incidence in cancer patients: A systematic review and meta-analysis. Front. Pharmacol. 2025, 16, 1560729. [Google Scholar] [CrossRef]
- Arribas-López, E.; Zand, N.; Ojo, O.; Snowden, M.J.; Kochhar, T. A Systematic Review of the Effect of Centella asiatica on Wound Healing. Int. J. Environ. Res. Public Health 2022, 19, 3266. [Google Scholar] [CrossRef]
- Gao, M.; Guo, H.; Dong, X.; Wang, Z.; Yang, Z.; Shang, Q.; Wang, Q. Regulation of inflammation during wound healing: The function of mesenchymal stem cells and strategies for therapeutic enhancement. Front. Pharmacol. 2024, 15, 1345779. [Google Scholar] [CrossRef]
- Wieczorek, P.P.; Hudz, N.; Yezerska, O.; Horčinová-Sedláčková, V.; Shanaida, M.; Korytniuk, O.; Jasicka-Misiak, I. Chemical Variability and Pharmacological Potential of Propolis as a Source for the Development of New Pharmaceutical Products. Molecules 2022, 27, 1600. [Google Scholar] [CrossRef]
- Stojko, M.; Wolny, D.; Włodarczyk, J. Nonwoven Releasing Propolis as a Potential New Wound Healing Method—A Review. Molecules 2021, 26, 5701. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, M.L.; McDermott, A.M.; Zasloff, M. Antimicrobial peptides and wound healing: Biological and therapeutic considerations. Exp. Dermatol. 2016, 25, 167–173. [Google Scholar] [CrossRef]
- Sarheed, O.; Ahmed, A.; Shouqair, D.; Boateng, J. Antimicrobial dressings for improving wound healing. In Wound Healing—New Insights Into Ancient Challenges; IntechOpen Limited: London, UK, 2016; pp. 374–398. [Google Scholar]
- Punjataewakupt, A.; Napavichayanun, S.; Aramwit, P. The downside of antimicrobial agents for wound healing. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 39–54. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Wang, S.; Xie, J.; Fu, T.; Li, S. In situ gelling hydrogel loaded with berberine liposome for the treatment of biofilm-infected wounds. Front. Bioeng. Biotechnol. 2023, 11, 1189010. [Google Scholar] [CrossRef]
- Akhter, M.H.; Al-Keridis, L.A.; Saeed, M.; Khalilullah, H.; Rab, S.O.; Aljadaan, A.M.; Rahman, M.A.; Jaremko, M.; Emwas, A.-H.; Ahmad, S. Enhanced drug delivery and wound healing potential of berberine-loaded chitosan-alginate nanocomposite gel: Characterization and in vivo assessment. Front. Public Health 2023, 11, 1238961. [Google Scholar] [CrossRef] [PubMed]
- Alhashim, M.; Lombardo, J. Mechanism of Action of Topical Garlic on Wound Healing. Dermatol. Surg. 2018, 44, 630–634. [Google Scholar] [CrossRef]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (Tea Tree) oil: A review of antimicrobial and other medicinal properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef]
- Iacovelli, F.; Romeo, A.; Lattanzio, P.; Ammendola, S.; Battistoni, A.; La Frazia, S.; Vindigni, G.; Unida, V.; Biocca, S.; Gaziano, R.; et al. Deciphering the Broad Antimicrobial Activity of Melaleuca alternifolia Tea Tree Oil by Combining Experimental and Computational Investigations. Int. J. Mol. Sci. 2023, 24, 12432. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vannucci, L. Biological properties of andrographolide, an active ingredient of Andrographis Paniculata: A narrative review. Ann. Transl. Med. 2021, 9, 1186. [Google Scholar] [CrossRef]
- Wen, Y.; Wang, Y.; Zhao, C.; Zhao, B.; Wang, J. The Pharmacological Efficacy of Baicalin in Inflammatory Diseases. Int. J. Mol. Sci. 2023, 24, 9317. [Google Scholar] [CrossRef]
- Ma, C.; Mei, C.; Liu, J.; Li, H.; Jiao, M.; Hu, H.; Zhang, Y.; Xiong, J.; He, Y.; Wei, W.; et al. Effect of baicalin on eradicating biofilms of bovine milk derived Acinetobacter lwoffii. BMC Vet. Res. 2024, 20, 212. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Ye, J.; Gao, L.; Liu, Y. The main bioactive compounds of Scutellaria baicalensis Georgi. for alleviation of inflammatory cytokines: A comprehensive review. Biomed. Pharmacother. 2021, 133, 110917. [Google Scholar] [CrossRef]
- Sarig, U.; Sarig, H.; de-Berardinis, E.; Chaw, S.-Y.; Nguyen, E.B.; Ramanujam, V.S.; Thang, V.D.; Al-Haddawi, M.; Liao, S.; Seliktar, D. Natural myocardial ECM patch drives cardiac progenitor based restoration even after scarring. Acta Biomater. 2016, 44, 209–220. [Google Scholar] [CrossRef]
- Neuman, M.G.; Nanau, R.M.; Oruña-Sanchez, L.; Coto, G. Hyaluronic acid and wound healing. J. Pharm. Pharm. Sci. 2015, 18, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Hussey, G.S.; Dziki, J.L.; Badylak, S.F. Extracellular matrix-based materials for regenerative medicine. Nat. Rev. Mater. 2018, 3, 159–173. [Google Scholar] [CrossRef]
- Al-Mamari, A.; Shahitha, F.; Al-Sibani, M.; Al Saadi, A.; Al Harrasi, A.; Ahmad, A. Novel antibacterial wound healing hydrogels based on HEC/SA/HA using gree n chemistry approach. Lett. Appl. NanoBioSci. 2023, 12, 69. [Google Scholar]
- Ahmed, S.; Ikram, S. Chitosan based scaffolds and their applications in wound healing. Achiev. Life Sci. 2016, 10, 27–37. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Martinotti, S.; Laforenza, U.; Patrone, M.; Moccia, F.; Ranzato, E. Honey-Mediated Wound Healing: H₂O₂ Entry through AQP3 Determines Extracellular Ca(2+) Influx. Int. J. Mol. Sci. 2019, 20, 764. [Google Scholar] [CrossRef]
- Winter, R. Chronic Wounds and Their Therapy with Alginate-Based Dressings. J. Pers. Med. 2022, 12, 1356. [Google Scholar] [CrossRef]
- Frank, L.; Lebreton-Decoster, C.; Godeau, G.; Coulomb, B.; Jozefonvicz, J. Dextran derivatives modulate collagen matrix organization in dermal equivalent. J. Biomater. Sci. Polym. Ed. 2006, 17, 499–517. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.; Silcock, D.; Gunnigle, S.; Cullen, B.; Light, N.D.; Watt, P.W. The role of oxidised regenerated cellulose/collagen in wound repair: Effects in vitro on fibroblast biology and in vivo in a model of compromised healing. Int. J. Biochem. Cell Biol. 2002, 34, 1557–1570. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.L.; Marshall, C.D.; Longaker, M.T. Minimizing Skin Scarring through Biomaterial Design. J. Funct. Biomater. 2017, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Bock, A.; Peters, F.; Heitzer, M.; Winnand, P.; Kniha, K.; Katz, M.S.; Hölzle, F.; Modabber, A. Assessing the Influence of Hyaluronan Dressing on Wound Healing on Split-Thickness Skin Graft Donor Sites Using a Three-Dimensional Scanner. J. Clin. Med. 2024, 13, 6433. [Google Scholar] [CrossRef]
- Dai, T.; Tanaka, M.; Huang, Y.Y.; Hamblin, M.R. Chitosan preparations for wounds and burns: Antimicrobial and wound-healing effects. Expert. Rev. Anti Infect. Ther. 2011, 9, 857–879. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Bîrcă, A.C.; Mogoşanu, G.D.; Rădulescu, M.; Holban, A.M.; Manuc, D.; Alberts, A.; Grumezescu, A.M.; Mogoantă, L. Zinc Alginate Hydrogel-Coated Wound Dressings: Fabrication, Characterization, and Evaluation of Anti-Infective and In Vivo Performance. Gels 2025, 11, 427. [Google Scholar] [CrossRef]
- Kallis, P.J.; Friedman, A.J. Collagen powder in wound healing. J. Drugs Dermatol. JDD 2018, 17, 403–408. [Google Scholar]
- Davison-Kotler, E.; Marshall, W.S.; García-Gareta, E. Sources of collagen for biomaterials in skin wound healing. Bioengineering 2019, 6, 56. [Google Scholar] [CrossRef]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in wound healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef]
- Ndlovu, S.P.; Ngece, K.; Alven, S.; Aderibigbe, B.A. Gelatin-Based Hybrid Scaffolds: Promising Wound Dressings. Polymers 2021, 13, 2959. [Google Scholar] [CrossRef]
- Sultan, M.T.; Lee, O.J.; Kim, S.H.; Ju, H.W.; Park, C.H. Silk fibroin in wound healing process. In Novel Biomaterials for Regenerative Medicine; Springer Nature Singapore Pte Ltd.: Singapore, 2018; pp. 115–126. [Google Scholar]
- Zhang, W.; Chen, L.; Chen, J.; Wang, L.; Gui, X.; Ran, J.; Xu, G.; Zhao, H.; Zeng, M.; Ji, J. Silk fibroin biomaterial shows safe and effective wound healing in animal models and a randomized controlled clinical trial. Adv. Healthc. Mater. 2017, 6, 1700121. [Google Scholar] [CrossRef]
- Shen, Y.; Ning, J.; Zhao, L.; Liu, W.; Wang, T.; Yu, J.; Wang, Y. Matrix remodeling associated 7 proteins promote cutaneous wound healing through vimentin in coordinating fibroblast functions. Inflamm. Regen. 2023, 43, 5. [Google Scholar] [CrossRef]
- Ansari, M.; Darvishi, A. A review of the current state of natural biomaterials in wound healing applications. Front. Bioeng. Biotechnol. 2024, 12, 1309541. [Google Scholar] [CrossRef] [PubMed]
- Konop, M.; Rybka, M.; Drapała, A. Keratin Biomaterials in Skin Wound Healing, an Old Player in Modern Medicine: A Mini Review. Pharmaceutics 2021, 13, 2029. [Google Scholar] [CrossRef] [PubMed]
- Bayer, I.S. Advances in Fibrin-Based Materials in Wound Repair: A Review. Molecules 2022, 27, 4504. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.; Vázquez, A. Bioactive peptides: A review. Food Qual. Saf. 2017, 1, 29–46. [Google Scholar] [CrossRef]
- Li, R.; Liu, K.; Huang, X.; Li, D.; Ding, J.; Liu, B.; Chen, X. Bioactive materials promote wound healing through modulation of cell behaviors. Adv. Sci. 2022, 9, 2105152. [Google Scholar] [CrossRef]
- Hao, Z.W.; Zhang, Z.Y.; Wang, Z.P.; Wang, Y.; Chen, J.Y.; Chen, T.H.; Shi, G.; Li, H.K.; Wang, J.W.; Dong, M.C.; et al. Bioactive peptides and proteins for tissue repair: Microenvironment modulation, rational delivery, and clinical potential. Military Medical Research 2024, 11, 75. [Google Scholar] [CrossRef]
- Tsala, D.E.; Amadou, D.; Habtemariam, S. Natural wound healing and bioactive natural products. Phytopharmacology 2013, 4, 532–560. [Google Scholar]
- Criollo-Mendoza, M.S.; Contreras-Angulo, L.A.; Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Jiménez-Ortega, L.A.; Heredia, J.B. Wound Healing Properties of Natural Products: Mechanisms of Action. Molecules 2023, 28, 598. [Google Scholar] [CrossRef]
- Bernal Vaca, L.; Mendoza, S.D.; Vergel, J.C.; Rueda, X.; Bruges, R. Hyperprogression in Pediatric Melanoma Metastatic to the Breast Treated with a Checkpoint Inhibitor. Cureus 2019, 11, e3859. [Google Scholar] [CrossRef]
- Dhall, S.; Do, D.C.; Garcia, M.; Kim, J.; Mirebrahim, S.H.; Lyubovitsky, J.; Lonardi, S.; Nothnagel, E.A.; Schiller, N.; Martins-Green, M. Generating and reversing chronic wounds in diabetic mice by manipulating wound redox parameters. J. Diabetes Res. 2014, 2014, 562625. [Google Scholar] [CrossRef] [PubMed]
- Onifade, M.; Zvarivadza, T.; Adebisi, J.A.; Said, K.O.; Dayo-Olupona, O.; Lawal, A.I.; Khandelwal, M. Advancing toward sustainability: The emergence of green mining technologies and practices. Green Smart Min. Eng. 2024, 1, 157–174. [Google Scholar] [CrossRef]
- Ghelani, H. Sustainable Manufacturing Engineering: Enhancing Product Quality through Green Process Innovations. Int. J. Eng. Comput. Sci. 2024, 11, 25632–25649. [Google Scholar] [CrossRef]
- Bao, M.; Liang, M.; Sun, X.; Mohyuddin, S.G.; Chen, S.; Wen, J.; Yong, Y.; Ma, X.; Yu, Z.; Ju, X.; et al. Baicalin Alleviates LPS-Induced Oxidative Stress via NF-κB and Nrf2-HO1 Signaling Pathways in IPEC-J2 Cells. Front. Vet. Sci. 2021, 8, 808233. [Google Scholar] [CrossRef]
- Kim, E.; Ham, S.; Jung, B.K.; Park, J.W.; Kim, J.; Lee, J.H. Effect of Baicalin on Wound Healing in a Mouse Model of Pressure Ulcers. Int. J. Mol. Sci. 2022, 24, 329. [Google Scholar] [CrossRef]
- Bal-Öztürk, A.; Özkahraman, B.; Özbaş, Z.; Yaşayan, G.; Tamahkar, E.; Alarçin, E. Advancements and future directions in the antibacterial wound dressings—A review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Mirhaj, M.; Labbaf, S.; Tavakoli, M.; Seifalian, A. An overview on the recent advances in the treatment of infected wounds: Antibacterial wound dressings. Macromol. Biosci. 2022, 22, 2200014. [Google Scholar] [CrossRef]
- Shukla, S.K.; Sharma, A.K.; Gupta, V.; Yashavarddhan, M. Pharmacological control of inflammation in wound healing. J. Tissue Viability 2019, 28, 218–222. [Google Scholar] [CrossRef]
- Cushnie, T.T.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef]
- Shehabeldine, A.M.; Salem, S.S.; Ali, O.M.; Abd-Elsalam, K.A.; Elkady, F.M.; Hashem, A.H. Multifunctional silver nanoparticles based on chitosan: Antibacterial, antibiofilm, antifungal, antioxidant, and wound-healing activities. J. Fungi 2022, 8, 612. [Google Scholar] [CrossRef] [PubMed]
- Sultan, A.S.; Vila, T.; Hefni, E.; Karlsson, A.J.; Jabra-Rizk, M.A. Evaluation of the antifungal and wound-healing properties of a novel peptide-based bioadhesive hydrogel formulation. Antimicrob. Agents Chemother. 2019, 63, e00888-19. [Google Scholar] [CrossRef]
- Seydi, N.; Mahdavi, B.; Paydarfard, S.; Zangeneh, A.; Zangeneh, M.M.; Najafi, F.; Jalalvand, A.R.; Pirabbasi, E. Preparation, characterization, and assessment of cytotoxicity, antioxidant, antibacterial, antifungal, and cutaneous wound healing properties of titanium nanoparticles using aqueous extract of Ziziphora clinopodioides Lam leaves. Appl. Organomet. Chem. 2019, 33, e5009. [Google Scholar] [CrossRef]
- Navarro-Martínez, M.D.; García-Cánovas, F.; Rodríguez-López, J.N. Tea polyphenol epigallocatechin-3-gallate inhibits ergosterol synthesis by disturbing folic acid metabolism in Candida albicans. J. Antimicrob. Chemother. 2006, 57, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- D’Urso, M.; Kurniawan, N.A. Mechanical and physical regulation of fibroblast–myofibroblast transition: From cellular mechanoresponse to tissue pathology. Front. Bioeng. Biotechnol. 2020, 8, 609653. [Google Scholar] [CrossRef]
- Xing, X.; Rodeo, S.A. Emerging roles of non-coding RNAs in fibroblast to myofibroblast transition and fibrotic diseases. Front. Pharmacol. 2024, 15, 1423045. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.; Woods, E.L.; Dally, J.; Kong, D.; Steadman, R.; Moseley, R.; Midgley, A.C. Myofibroblasts: Function, formation, and scope of molecular therapies for skin fibrosis. Biomolecules 2021, 11, 1095. [Google Scholar] [CrossRef]
- Souto, E.B.; Cano, A.; Martins-Gomes, C.; Coutinho, T.E.; Zielińska, A.; Silva, A.M. Microemulsions and nanoemulsions in skin drug delivery. Bioengineering 2022, 9, 158. [Google Scholar] [CrossRef]
- de Assis, K.M.A.; da Silva Leite, J.M.; de Melo, D.F.; Borges, J.C.; Santana, L.M.B.; Dos Reis, M.M.L.; Moreira, V.M.; da Rocha, W.R.V.; Catao, R.M.R.; Dos Santos, S.G. Bicontinuous microemulsions containing Melaleuca alternifolia essential oil as a therapeutic agent for cutaneous wound healing. Drug Deliv. Transl. Res. 2020, 10, 1748–1763. [Google Scholar] [CrossRef]
- Scano, A.; Ebau, F.; Cabras, V.; Sini, F.; Ennas, G. Alternative Silica Sources in the Synthesis of Ordered Mesoporous Silica. J. Nanosci. Nanotechnol. 2021, 21, 2847–2854. [Google Scholar] [CrossRef]
- Paocharoen, V. The efficacy and side effects of oral Centella asiatica extract for wound healing promotion in diabetic wound patients. J. Med. Assoc. Thail. 2010, 93, S166-70. [Google Scholar]
- Wichayapreechar, P.; Anuchapreeda, S.; Phongpradist, R.; Rungseevijitprapa, W.; Ampasavate, C. Dermal targeting of Centella asiatica extract using hyaluronic acid surface modified niosomes. J. Liposome Res. 2019, 30, 197–207. [Google Scholar] [CrossRef]
- Mahapatra, A.; Prasad, N.; Panda, N.; Paul, B.; Rout, A. Increasing Efficiency of Transdermal Drug Delivery Systems Using Some Novel Penetration Enhancement Techniques—A Critical Review: Pharmaceutical sciences- Pharmaceutical technology. Int. J. Life Sci. Pharma Res. 2023, 13, P51–P77. [Google Scholar] [CrossRef]
- Cao, X.; Wu, X.; Zhang, Y.; Qian, X.; Sun, W.; Zhao, Y. Emerging biomedical technologies for scarless wound healing. Bioact. Mater. 2024, 42, 449–477. [Google Scholar] [CrossRef] [PubMed]
- Taheri, P.; Jahanmardi, R.; Koosha, M.; Abdi, S. Physical, mechanical and wound healing properties of chitosan/gelatin blend films containing tannic acid and/or bacterial nanocellulose. Int. J. Biol. Macromol. 2020, 154, 421–432. [Google Scholar] [CrossRef]
- Sakthiguru, N.; Sithique, M.A. Fabrication of bioinspired chitosan/gelatin/allantoin biocomposite film for wound dressing application. Int. J. Biol. Macromol. 2020, 152, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Akhavan-Kharazian, N.; Izadi-Vasafi, H. Preparation and characterization of chitosan/gelatin/nanocrystalline cellulose/calcium peroxide films for potential wound dressing applications. Int. J. Biol. Macromol. 2019, 133, 881–891. [Google Scholar] [CrossRef]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Preparation and optimization of chitosan-gelatin films for sustained delivery of lupeol for wound healing. Int. J. Biol. Macromol. 2018, 107, 1888–1897. [Google Scholar] [CrossRef]
- Oliveira, C.; Sousa, D.; Teixeira, J.A.; Ferreira-Santos, P.; Botelho, C.M. Polymeric biomaterials for wound healing. Front. Bioeng. Biotechnol. 2023, 11, 1136077. [Google Scholar] [CrossRef]
- Xin, H.; Al Maruf, D.S.A.; Akin-Ige, F.; Amin, S. Stimuli-responsive hydrogels for skin wound healing and regeneration. Emergent Mater. 2024. [Google Scholar] [CrossRef]
- Fan, X.; Yang, L.; Wang, T.; Sun, T.; Lu, S. pH-responsive cellulose-based dual drug-loaded hydrogel for wound dressing. Eur. Polym. J. 2019, 121, 109290. [Google Scholar] [CrossRef]
- Zhang, Y.; Si, T.; Cai, S.; Gao, X.; Tang, X.; Peng, L.; Chen, Z.; Hu, Q.; Li, J.; Zhang, H. Novel ROS-scavenging hydrogel produced in situ crosslinking between cyclodextrin and cellulose for promoting diabetic wound healing. Chem. Eng. J. 2024, 486, 150373. [Google Scholar] [CrossRef]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.D.; Selvanathan, V.; Sonsudin, F.; Naceur Abouloula, C. pH Sensitive Hydrogels in Drug Delivery: Brief History, Properties, Swelling, and Release Mechanism, Material Selection and Applications. Polymers 2017, 9, 137. [Google Scholar] [CrossRef]
- Ribeiro, M.; Simões, M.; Vitorino, C.; Mascarenhas-Melo, F. Hydrogels in Cutaneous Wound Healing: Insights into Characterization, Properties, Formulation and Therapeutic Potential. Gels 2024, 10, 188. [Google Scholar] [CrossRef]
- Alberts, A.; Moldoveanu, E.-T.; Niculescu, A.-G.; Grumezescu, A.M. Hydrogels for Wound Dressings: Applications in Burn Treatment and Chronic Wound Care. J. Compos. Sci. 2025, 9, 133. [Google Scholar] [CrossRef]
- Dimatteo, R.; Darling, N.J.; Segura, T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Garbern, J.C.; Hoffman, A.S.; Stayton, P.S. Injectable pH- and temperature-responsive poly(N-isopropylacrylamide-co-propylacrylic acid) copolymers for delivery of angiogenic growth factors. Biomacromolecules 2010, 11, 1833–1839. [Google Scholar] [CrossRef]
- Nguyen, V.-L.; Truong, C.-T.; Nguyen, B.C.Q.; Vo, T.-N.V.; Dao, T.-T.; Nguyen, V.-D.; Trinh, D.-T.T.; Huynh, H.K.; Bui, C.-B. Anti-inflammatory and wound healing activities of calophyllolide isolated from Calophyllum inophyllum Linn. PLoS ONE 2017, 12, e0185674. [Google Scholar] [CrossRef]
- Zakerikhoob, M.; Abbasi, S.; Yousefi, G.; Mokhtari, M.; Noorbakhsh, M.S. Curcumin-incorporated crosslinked sodium alginate-g-poly (N-isopropyl acrylamide) thermo-responsive hydrogel as an in-situ forming injectable dressing for wound healing: In vitro characterization and in vivo evaluation. Carbohydr. Polym. 2021, 271, 118434. [Google Scholar] [CrossRef]
- Zhao, C.C.; Zhu, L.; Wu, Z.; Yang, R.; Xu, N.; Liang, L. Resveratrol-loaded peptide-hydrogels inhibit scar formation in wound healing through suppressing inflammation. Regen. Biomater. 2020, 7, 99–107. [Google Scholar] [CrossRef]
- Comotto, M.; Saghazadeh, S.; Bagherifard, S.; Aliakbarian, B.; Kazemzadeh-Narbat, M.; Sharifi, F.; Mousavi Shaegh, S.A.; Arab-Tehrany, E.; Annabi, N.; Perego, P.; et al. Breathable hydrogel dressings containing natural antioxidants for management of skin disorders. J. Biomater. Appl. 2019, 33, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Zhou, H.; Shu, J.; Fu, S.; Yang, Z. Skin wound healing promoted by novel curcumin-loaded micelle hydrogel. Ann. Transl. Med. 2021, 9, 1152. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yuan, W.; Liu, H.; Huang, S.; Bian, L.; Guo, R. Injectable supramolecular gelatin hydrogel loading of resveratrol and histatin-1 for burn wound therapy. Biomater. Sci. 2020, 8, 4810–4820. [Google Scholar] [CrossRef]
- Zhu, W.; Dong, Y.; Xu, P.; Pan, Q.; Jia, K.; Jin, P.; Zhou, M.; Xu, Y.; Guo, R.; Cheng, B. A composite hydrogel containing resveratrol-laden nanoparticles and platelet-derived extracellular vesicles promotes wound healing in diabetic mice. Acta Biomater. 2022, 154, 212–230. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Jiang, C.; Yu, T.; Chen, K. A multi-purpose dressing based on resveratrol-loaded ionic liquids/gelatin methacryloyl hydrogel for enhancing diabetic wound healing. Int. J. Biol. Macromol. 2024, 283, 136773. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, X.; Li, F.; Li, B.; Zhang, M.; Li, W.; Zhuge, P.; Yao, J.; Zhang, Y.; Chen, S.; et al. Thermosensitive Hydrogel Integrated with Bimetallic Nano-Enzymes for Modulating the Microenvironment in Diabetic Wound Beds. Adv. Sci. 2025, 12, e2411575. [Google Scholar] [CrossRef]
- Chaushu, L.; Weinreb, M.; Beitlitum, I.; Moses, O.; Nemcovsky, C.E. Evaluation of a topical herbal patch for soft tissue wound healing: An animal study. J. Clin. Periodontol. 2015, 42, 288–293. [Google Scholar] [CrossRef]
- Bîrcă, A.C.; Chircov, C.; Niculescu, A.G.; Hildegard, H.; Baltă, C.; Roșu, M.; Mladin, B.; Gherasim, O.; Mihaiescu, D.E.; Vasile, B.Ș.; et al. H2O2-PLA-(Alg)2Ca Hydrogel Enriched in Matrigel® Promotes Diabetic Wound Healing. Pharmaceutics 2023, 15, 857. [Google Scholar] [CrossRef]
- O’Meara, S.; Cullum, N.; Majid, M.; Sheldon, T. Systematic reviews of wound care management: (3) antimicrobial agents for chronic wounds; (4) diabetic foot ulceration. Health Technol. Assess. 2000, 4, 1–237. [Google Scholar] [CrossRef]
- O’Meara, S.; Al-Kurdi, D.; Ologun, Y.; Ovington, L.G.; Martyn-St James, M.; Richardson, R. Antibiotics and antiseptics for venous leg ulcers. Cochrane Database Syst. Rev. 2013, 1, Cd003557. [Google Scholar] [CrossRef]
- Dardari, D.; Piaggesi, A.; Potier, L.; Sultan, A.; Diener, H.; Francois, M.; Dorweiler, B.; Bouillet, B.; M’Bemba, J.; Chaillous, L.; et al. Intact Fish Skin Graft to Treat Deep Diabetic Foot Ulcers. NEJM Evid. 2024, 3, EVIDoa2400171. [Google Scholar] [CrossRef] [PubMed]
- Damayanti, E.; Mustofa; Nirwati, H.; Suputa; Widada, J. The evaluation of antimicrobial, antioxidant, and antiproliferative activity of stingless bee honey (Heterotrigona itama and Tetragonula laeviceps) as functional food. AIP Conf. Proc. 2024, 2957, 060025. [Google Scholar] [CrossRef]
- Saeed, Z.F.; Kadhim, B.A.; Alsaadawi, M.A.; Kheder, R.K.; Manshad, D.R.; Hassan, A.A.; Hassan, Y.S.; Hobkirk, J. Locally immunological effects of honey applications in patients with rheumatoid arthritis. AIP Conf. Proc. 2024, 3051, 020002. [Google Scholar] [CrossRef]
- Mohd, M.-A.; Edros, R.; Hamzah, N.A. Antibacterial properties of Kelulut, Tualang and Acacia honey against fourteen clinically-isolated strains of bacteria-infecting wound. AIP Conf. Proc. 2020, 2252, 020001. [Google Scholar] [CrossRef]
- Medhi, B.; Puri, A.; Upadhyay, S.; Kaman, L. Topical application of honey in the treatment of wound healing: A metaanalysis. JK Sci. 2008, 10, 166–169. [Google Scholar]
- Putri, N.M.; Kreshanti, P.; Tunjung, N.; Indania, A.; Basuki, A.; Sukasah, C.L. Efficacy of honey dressing versus hydrogel dressing for wound healing. AIP Conf. Proc. 2021, 2344, 020022. [Google Scholar] [CrossRef]
- Al Saeed, M. Prospective randomized comparison of controlled release ionic silver hydrophilic dressings and medicated honey-impregnated dressings in treating neuropathic diabetic foot ulcer. Saudi J. Health Sci. 2019, 8, 25–30. [Google Scholar] [CrossRef]
- Mahboub, M.; Aghazadeh Attari, A.M.; Sheikhalipour, Z.; Mirza Aghazadeh Attari, M.; Davami, B.; Amidfar, A.; Lotfi, M. A Comparative Study of the Impacts of Aloe vera Gel and Silver Sulfadiazine Cream 1% on Healing, Itching and Pain of Burn Wounds: A Randomized Clinical Trial. J. Caring Sci. 2022, 11, 132–138. [Google Scholar] [CrossRef]
- Lima Júnior, E.M.; de Moraes Filho, M.O.; Costa, B.A.; Fechine, F.V.; Vale, M.L.; Diógenes, A.K.L.; Neves, K.R.T.; Uchôa, A.; Soares, M.; de Moraes, M.E.A. Nile Tilapia Fish Skin-Based Wound Dressing Improves Pain and Treatment-Related Costs of Superficial Partial-Thickness Burns: A Phase III Randomized Controlled Trial. Plast. Reconstr. Surg. 2021, 147, 1189–1198. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Potential role of propolis in wound healing: Biological properties and therapeutic activities. Biomed. Pharmacother. 2018, 98, 469–483. [Google Scholar] [CrossRef]
- Givol, O.; Kornhaber, R.; Visentin, D.; Cleary, M.; Haik, J.; Harats, M. A systematic review of Calendula officinalis extract for wound healing. Wound Repair. Regen. 2019, 27, 548–561. [Google Scholar] [CrossRef] [PubMed]
- Samuelson, R.; Lobl, M.; Higgins, S.; Clarey, D.; Wysong, A. The Effects of Lavender Essential Oil on Wound Healing: A Review of the Current Evidence. J. Altern. Complement. Med. 2020, 26, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Abedian, S.; Abedi, P.; Jahanfar, S.; Iravani, M.; Zahedian, M. The effect of Lavender on pain and healing of episiotomy: A systematic review. Complement. Ther. Med. 2020, 53, 102510. [Google Scholar] [CrossRef] [PubMed]
- Tonaco, L.A.B.; Gomes, F.L.; Velasquez-Melendez, G.; Lopes, M.T.P.; Salas, C.E. The Proteolytic Fraction from Latex of Vasconcellea cundinamarcensis (P1G10) Enhances Wound Healing of Diabetic Foot Ulcers: A Double-Blind Randomized Pilot Study. Adv. Ther. 2018, 35, 494–502. [Google Scholar] [CrossRef]
- Buzzi, M.; de Freitas, F.; Winter, M. A Prospective, Descriptive Study to Assess the Clinical Benefits of Using Calendula officinalis Hydroglycolic Extract for the Topical Treatment of Diabetic Foot Ulcers. Ostomy Wound Manag. 2016, 62, 8–24. [Google Scholar]
- Lammoglia-Ordiales, L.; Vega-Memije, M.E.; Herrera-Arellano, A.; Rivera-Arce, E.; Agüero, J.; Vargas-Martinez, F.; Contreras-Ruiz, J. A randomised comparative trial on the use of a hydrogel with tepescohuite extract (Mimosa tenuiflora cortex extract-2G) in the treatment of venous leg ulcers. Int. Wound J. 2012, 9, 412–418. [Google Scholar] [CrossRef]
- Ullah, F.; Liang, A.; Rangel, A.; Gyengesi, E.; Niedermayer, G.; Münch, G. High bioavailability curcumin: An anti-inflammatory and neurosupportive bioactive nutrient for neurodegenerative diseases characterized by chronic neuroinflammation. Arch. Toxicol. 2017, 91, 1623–1634. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef]
- Wang, Y.J.; Pan, M.H.; Cheng, A.L.; Lin, L.I.; Ho, Y.S.; Hsieh, C.Y.; Lin, J.K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Stohs, S.J.; Chen, C.Y.O.; Preuss, H.G.; Ray, S.D.; Bucci, L.R.; Ji, J.; Ruff, K.J. The fallacy of enzymatic hydrolysis for the determination of bioactive curcumin in plasma samples as an indication of bioavailability: A comparative study. BMC Complement. Altern. Med. 2019, 19, 293. [Google Scholar] [CrossRef]
- Garcea, G.; Berry, D.P.; Jones, D.J.; Singh, R.; Dennison, A.R.; Farmer, P.B.; Sharma, R.A.; Steward, W.P.; Gescher, A.J. Consumption of the putative chemopreventive agent curcumin by cancer patients: Assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol. Biomark. Prev. 2005, 14, 120–125. [Google Scholar] [CrossRef]
- Olotu, F.; Agoni, C.; Soremekun, O.; Soliman, M.E.S. An Update on the Pharmacological Usage of Curcumin: Has it Failed in the Drug Discovery Pipeline? Cell Biochem. Biophys. 2020, 78, 267–289. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Ichikawa, H. Curcumin: The Indian solid gold. Adv. Exp. Med. Biol. 2007, 595, 1–75. [Google Scholar] [CrossRef]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Yoga Latha, L. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef]
- Zeng, R.; Lin, C.; Lin, Z.; Chen, H.; Lu, W.; Lin, C.; Li, H. Approaches to cutaneous wound healing: Basics and future directions. Cell Tissue Res. 2018, 374, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin wound healing: An update on the current knowledge and concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Chouhan, D.B.; Mandal, B. Tissue engineered skin and wound healing: Current strategies and future directions. Curr. Pharm. Des. 2017, 23, 3455–3482. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Burn wound healing: Present concepts, treatment strategies and future directions. J. Wound Care 2017, 26, 5–19. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef]
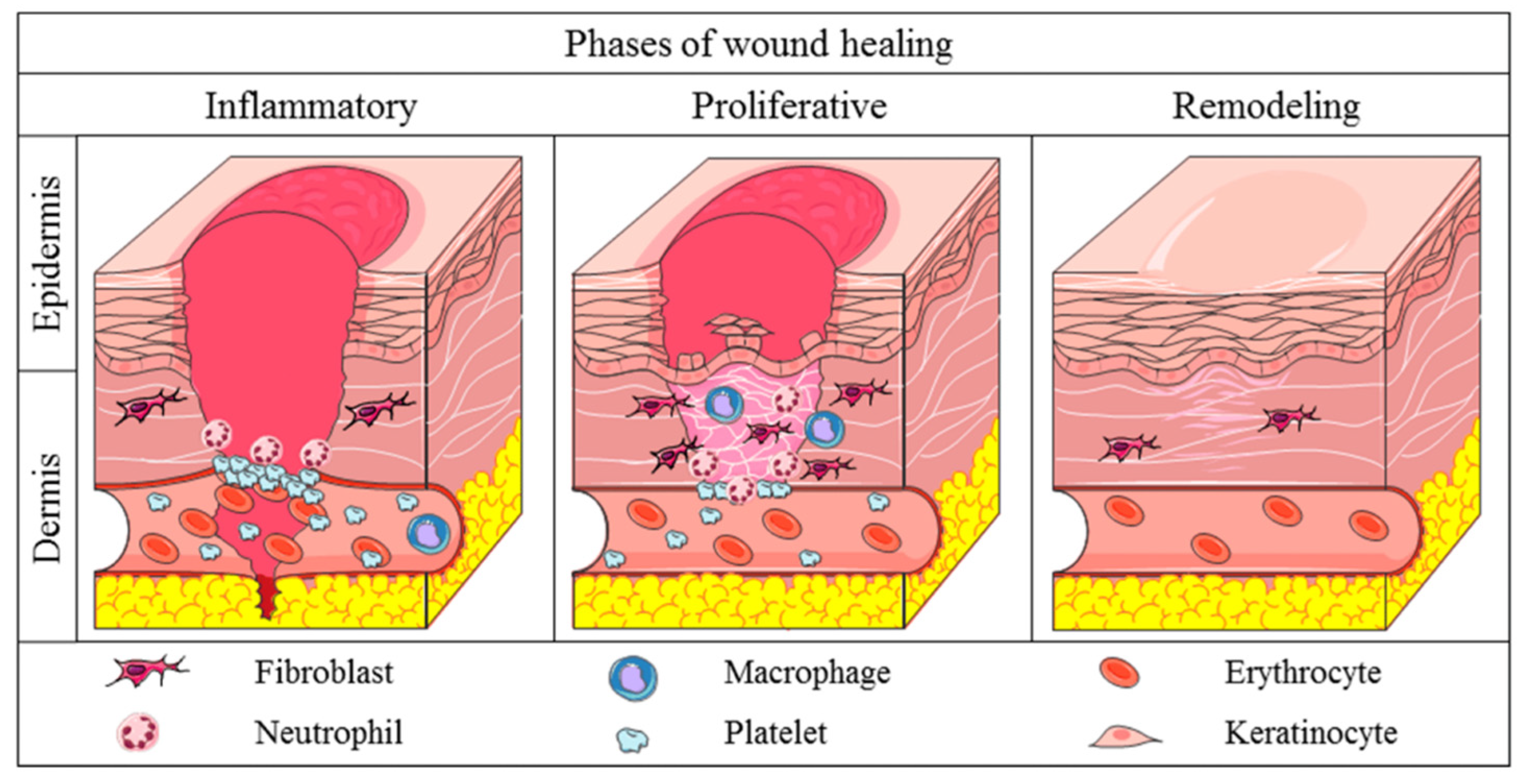




| Phase | Cellular and Biophysiological Events | Phase Description | Refs. |
|---|---|---|---|
| Hemostasis |
|
| [6] |
| Inflammation |
|
| [6,7] |
| Proliferation |
|
| [8] |
| Remodeling |
|
| [9,10,11] |
| Mechanism | Problem | Natural Compounds/Interventions | Refs. |
|---|---|---|---|
| Regulation of inflammatory cytokines | Dysregulated inflammation causes sustained expression of TNF-α, IL-6, IL-1β, promoting fibroblast proliferation and collagen synthesis. | Curcumin and green tea polyphenols reduce inflammatory cytokines, promoting regulated healing. | [10,11] |
| Oxidative stress inhibition | Accumulation of ROS during wound healing leads to fibroblast hyperactivation and excessive collagen production. | Antioxidants such as EGCG (green tea) and resveratrol (grapes) neutralize ROS, reducing oxidative damage and fibrosis. | [11] |
| Collagen accumulation control | Excessive collagen accumulation results in hypertrophic scarring through modulation of collagen synthesis and degradation. | Asiaticoside (Centella asiatica) and curcumin modulate MMPs and TGF-β1 expression to regulate collagen deposition and remodeling. | [6] |
| Angiogenesis regulation | Excessive angiogenesis can result in fibrotic tissue formation, compromising wound healing. | Polyphenols (green tea) and flavonoids (chamomile) regulate VEGF expression, facilitating optimal angiogenesis and reducing fibrosis. | [6,7] |
| Natural Substance | Traditional Use | Claimed Healing Effects | Historical Context Notes | References |
|---|---|---|---|---|
| Honey | Ancient Egypt, Greece, China, WWI-era Europe (and globally) | Antimicrobial, anti-inflammatory, debriding; promotes granulation and healing | Used since antiquity (Stone Age/Egyptian tombs, ~3000 BC); Egyptians/Greeks applied honey to wounds and burns. Tested in WWI to prevent infection; recognized in Ayurvedic and Chinese tradition. | [53] |
| Chamomile (Matricaria chamomilla) | Old World (Europe, Middle East, India) | Mild anti-inflammatory, antimicrobial; soothes ulcers, cuts, and skin lesions | One of the oldest recorded medicinal herbs. Ancient Egyptians, Greeks, and Romans used chamomile poultices for inflammatory skin conditions and ulcers. | [54,55,56] |
| Aloe vera | Africa/India (tropical Asia, cultivated globally) | Anti-inflammatory, cooling, moisturizing; used on burns, minor cuts, skin irritations | Documented in ancient Egyptian and Ayurvedic medicine (e.g., Ebers Papyrus); “prescribed for thousands of years” for wounds and burns. Widely used in 18th–19th C. Western herbalism. | [55] |
| Panax ginseng | East Asia (China, Korea, Japan) | Adaptogenic tonic; claimed to improve circulation and tissue repair | Revered in ancient TCM and Korean medicine (e.g., Shennong’s Herbal Classic, 1st C. AD). Believed to restore vitality (“Panax” = “all-healing”). Modern studies note superior wound healing effects of P. ginseng vs. American ginseng. | [57] |
| Centella asiatica (Gotu kola) | South/Southeast Asia (India, Sri Lanka, China) | Stimulates collagen synthesis and angiogenesis; anti-inflammatory | Featured in Ayurvedic and Chinese pharmacopeias for centuries. Official monographs (WHO, EU) cite its wound healing and scar-reduction use. Traditional poultices of centella leaves were applied to burns and ulcers. | [58] |
| Turpentine oil (pine resin distillate) | Western folk medicine (Europe/N. America) | Antiseptic, counterirritant; warms tissue, relieves pain | Historical remedy (age of sail/naval medicine; 18th–19th C.) for infected wounds and skin diseases. Traditional brands mixed turpentine with lard or sugar as poultices. Modern evidence (pine resin ointment “Biopin”) shows antibacterial effects and immune modulation in wounds. | [59] |
| Compound | Source | Biological Activity | Mechanism | Wound Healing Effects | Refs. |
|---|---|---|---|---|---|
| Curcumin | Turmeric (Curcuma longa) | Anti-inflammatory, antioxidant, anticoagulant | Inhibits proinflammatory NF-κB/COX-2 signaling; activates Nrf2-dependent antioxidant defence | Protects keratinocytes/fibroblasts from oxidative damage; enhances granulation and re-epithelialization in animal wounds. | [17] |
| Quercetin | Many fruits/vegetables (e.g., onions, apples, Ginkgo biloba) | Anti-inflammatory, antioxidant | Scavenges ROS; downregulates TNF-α, IL-1β, IL-6, and other cytokines | Promotes fibroblast proliferation and collagen deposition; accelerates vascularization and collagen matrix formation in wound models. | [17] |
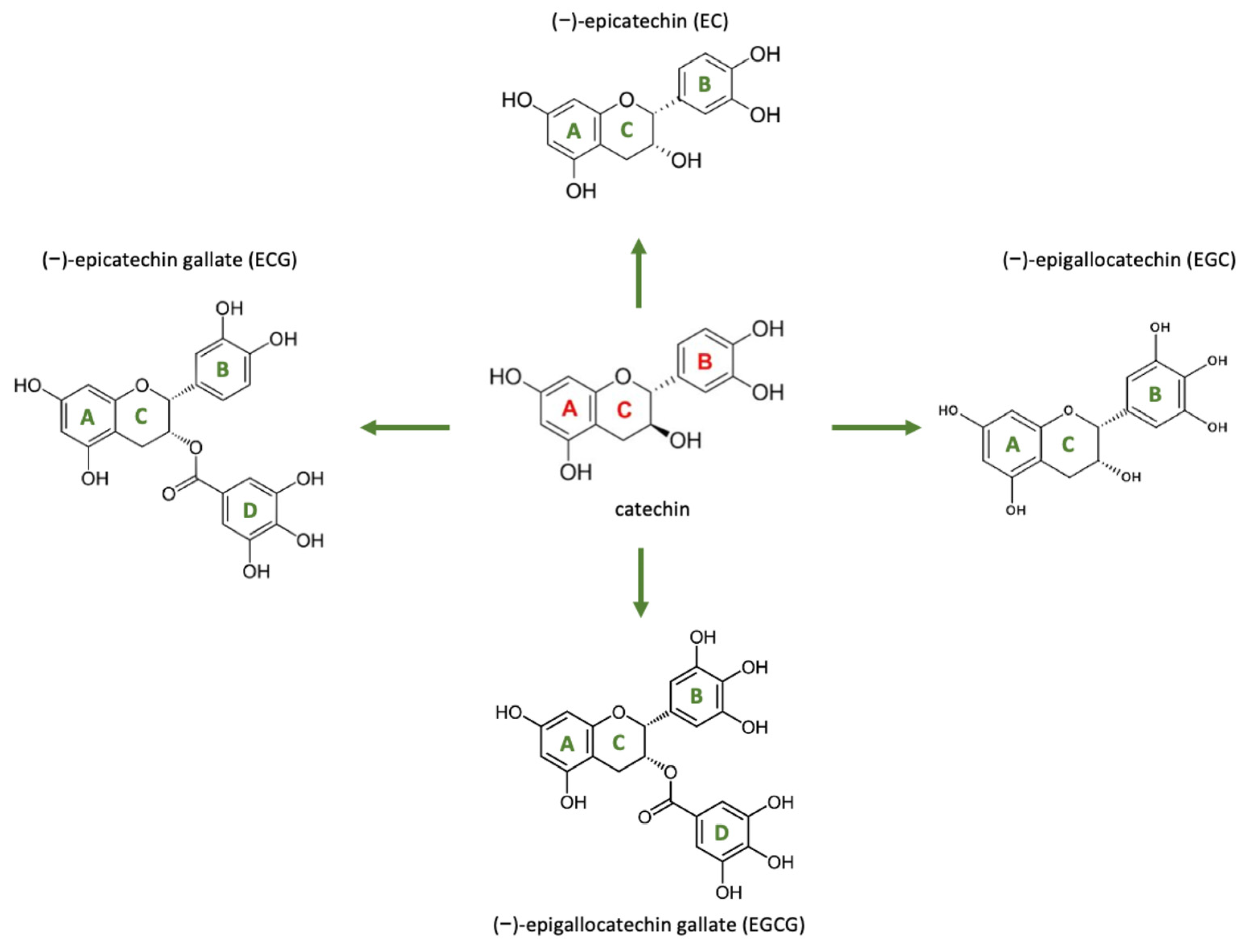
| Catechins (e.g., EGCG) | Green tea (Camellia sinensis), cocoa | Antioxidant, antimicrobial | Potent free-radical scavengers; modulates metalloproteinases and cytokines | Reduces oxidative stress in wound bed; supports fibroblast activity and epithelial growth. Accelerates closure in treated wounds (often via catechin-enriched dressings). | [17] |
| Resveratrol | Grapes (skins), berries, peanuts | Anti-inflammatory, antioxidant, proangiogenic | Inhibits NF-κB and proinflammatory cytokine pathways; activates SIRT1, enhances nitric oxide/VEGF signaling | Enhances re-epithelialization and neovascularization; improves wound contraction and tensile strength (model studies). Improves diabetic wound healing via antioxidation. | [95] |
| Asiaticoside | Centella asiatica (gotu kola) | Promotes fibroblast growth and ECM synthesis | Upregulates collagen I/III and cell-cycle genes in dermal fibroblasts | Stimulates granulation tissue formation and collagen deposition. Speeds epidermal regeneration and increases tensile strength of healing skin. | [73,96] |
| Verbascoside (Acteoside) | Plantago spp. (plantain), Lamiaceae herbs | Anti-inflammatory, antioxidant | Inhibits proinflammatory mediators (↓TNF-α, IL-6, NO, PGE2) | Reduces inflammation in wound tissue; accelerates healing and re-epithelialization. (Studies show verbascoside dressings improved wound closure via reduced cytokine levels.) | [17] |
| Lupeol | Mango, olive, and many fruits (Bowdichia virgilioides, Mangifera indica, Olea europaea) | Anti-inflammatory, antioxidant | Suppresses NF-κB and IL-6; increases IL-10; upregulates angiogenic/growth factors (VEGF, EGF, TGF-β1) | Promotes angiogenesis and collagen synthesis; speeds inflammatory resolution. In animal wounds, topical lupeol creams enhance vessel growth and collagen deposition. | [17] |
| Gallic acid | Tea, gallnuts, berries, oak | Antioxidant, anti-inflammatory | Induces antioxidant enzymes (↑SOD2, CAT, GPx1); modulates cytokine production | Improves wound healing rate and quality. In diabetic wound models, gallic acid gels accelerated closure by boosting antioxidant defences. | [97,98] |
| Biomaterial | Key Properties | Role in Tissue Repair | Evidence (In Vivo/Clinical) | Refs. |
|---|---|---|---|---|
| Collagen |
| Provides structural support; guides neovascularization and re-epithelialization; temporary skin substitute (especially in burns). | In animal/burn models, collagen scaffolds allow organized cell ingrowth and early wound coverage. Clinical use (dermal matrices, cultured grafts) shows improved healing in chronic ulcers. | [135] |
| Hyaluronic Acid (HA) |
| Maintains moist milieu; promotes cell migration via CD44; high-MW HA is anti-inflammatory (low-MW proinflammatory). | Topical HA enhances cell proliferation/angiogenesis in chronic wounds. In a split-thickness graft study, HA dressing accelerated closure and improved 6-month scar quality vs. control. | [135,136] |
| Chitosan |
| Acts as porous 3D scaffold (e.g., electrospun or gel); promotes hemostasis, fibroblast migration, and drug delivery. | Widely studied in vitro/in vivo: chitosan dressings show reduced infection and inflammation, improved healing and collagen content. Clinical gels/gauzes are used for burns and ulcers (hemostatic and antimicrobial). | [137] |
| Alginate |
| Creates moist wound environment; acts as scaffold for cell infiltration; stimulates granulation tissue and fibroblast proliferation. | Alginate dressings promote new granulation and collagen synthesis in chronic wounds. Numerous clinical trials confirm alginate sheets/foams accelerate healing of ulcers by enhancing closure rate. | [132,138] |
| Natural Compound | Source | Anti-Inflammatory Mechanism | Effect on Wound Healing | Refs. |
|---|---|---|---|---|
| Epigallocatechin Gallate (EGCG) | Green tea | Inhibits NF-κB and MAPK pathways; reduces TNF-α, IL-1β, IL-6. | Promotes fibroblast and keratinocyte migration; reduces oxidative damage. | [110] |
| Berberine | Berberis species | Suppresses COX-2, nitric oxide, and cytokines via AMPK/NF-κB inhibition. | Improves re-epithelialization; supports healing of infected wounds. | [153] |
| Andrographolide | Andrographis paniculata | Blocks NF-κB; downregulates IL-6, TNF-α. | Accelerates inflammation resolution and granulation tissue formation. | [153] |
| Allicin | Garlic (Allium sativum) | Scavenges ROS; downregulates TNF-α, IL-1β. | Enhances macrophage activity; supports wound contraction. | [118] |
| Tea Tree Oil | Melaleuca alternifolia | Reduces IL-8 and PGE2; inhibits histamine-induced inflammation. | Reduces inflammation and infection risk in topical applications. | [119] |
| Flavonoids (e.g., Apigenin, Quercetin, Catechins) | Various fruits, vegetables, tea, herbs | Inhibit phosphodiesterases to delay cAMP signaling; reduce NO production; suppress IL-6 and TNF-α; modulate NF-κB activity; impair dendritic cell maturation via CD80/CD86 inhibition; target macrophage activation and differentiation. | Reduce inflammatory cell infiltration; decrease CRP levels; promote dense connective tissue formation; improve wound healing quality; show synergistic effects (e.g., quercetin + catechin) on cytokine suppression and scarring reduction. | [154] |
| Type of Wound Dressing | Polymer(s) | Loaded Bioactive Agent(s) | Application/Outcome | Refs. |
|---|---|---|---|---|
| Films | Chitosan + Gelatin | Tannic acid + Bacterial nanocellulose | Good mechanical performance and faster full-thickness wound closure | [142,182] |
| Films | Chitosan | Allantoin | Excellent biocompatibility and non-toxicity with superior antibacterial efficacy | [182] |
| Films | Chitosan | Nanocrystalline cellulose + Calcium peroxide | Moderate WVTR and excellent cytocompatibility | [182] |
| Films | Chitosan | Lupeol | Initial rapid drug release followed by sustained release and good antioxidant efficacy | [182] |
| Films | Alginate | Hydroxyapatite | High antibacterial activity | [182] |
| Films | Chitosan | ZnO nanoparticles | Good antibacterial effects and increased full-thickness wound contraction rate | [142] |
| Films | Chitosan | Bone ash + Ciprofloxacin | Superior antimicrobial effects | [142,182] |
| Films | PCL | Catechin | High cell proliferation of skin cells | [182] |
| Films | Chitosan | - | High swelling capacity and accelerated wound healing | [142] |
| Films | Cellulose | - | Excellent fluid absorbing effect and fast wound closure | [182] |
| Membranes | CM Chitosan + HA | - | High cell viability and proliferation | [142,182] |
| Membranes | Chitosan | - | High growth inhibition against several bacterial strains | [182] |
| Membranes | Polymyxin B sulfate | Ciprofloxacin + HNTs | High swelling capacity, non-toxic, and good antibacterial activity | [182] |
| Films | Poly(e-caprolactone) | Usnic acid | Full-thickness wounds | [182] |
| Films | Chitosan/Gelatin | Silver nanoparticles/Phosphotungstic-acid–polydopamine nanoflowers | Wound healing | [142] |
| Films | Collagen/HPMC | Povidone–iodide | Regenerative tissue engineering | [182] |
| Films | Chitosan/PVA/PVP/Maltodextrin | Satureja mutica/Oliveria decumbens essential oil | Wound dressing | [182] |
| Films | Zein/PCL/Collagen | Zinc oxide nanoparticles + Aloe vera | Wound healing | [182] |
| Films | Collagen | Polydatin | Chronic non-healing wounds | [182] |
| Films | Collagen/PLA-glycolide | Glucophage | Diabetic wounds | [142,182] |
| Films | Sodium alginate/Gelatin | Paeoniflorin | Diabetic wounds | [142,182] |
| Films | Calcium alginate/PCL/Gelatin | Coconut oil | Wound healing | [182] |
| Films | Chitosan/Gelatin | Platelet-rich plasma | Chronic wounds | [142] |
| Films | Fibrin/Chitosan/Keratin | Ferulic-acid-loaded silica microspheres | Chronic and infected wounds | [142] |
| Films | PEG diacrylate/Catechol-HA | Ag-doped mesoporous silica nanoparticles | Wound dressing | [182] |
| Films | Thiolate hyaluronic acid/Silk fibroin | Bioactive glass nanoparticles | Wound healing | [142,182] |
| Films | Polyurethane/Chitosan | Linezolid | Diabetic wounds | [182] |
| Technology Type | Formulation and Key Components | Mechanism | Stage | Reported Benefits | Ref. |
|---|---|---|---|---|---|
| Injectable Hydrogel | Crosslinked sodium alginate–poly(N-isopropylacrylamide) copolymer loaded with curcumin |
| Preclinical | Accelerated wound contraction; significantly reduced inflammation, enhanced collagen deposition, increased fibroblast proliferation. | [192] |
| Smart Dressing | Self-assembling Fmoc-FFGGRGD peptide hydrogel containing resveratrol |
| Preclinical | Faster wound closure, well-organized collagen deposition; marked reduction of inflammatory cytokines and prevention of scar formation. | [193] |
| Smart Dressing | Breathable sodium alginate hydrogel incorporating curcumin and t-resveratrol |
| Preclinical | Superior antioxidant and antibacterial activity; improved cell viability under oxidative stress. | [194] |
| Injectable Hydrogel | Hyaluronic acid gel with PCL–PEG–PCL nanomicelles loaded with curcumin |
| Preclinical | ~96% wound closure by day 14; earlier re-epithelialization, increased collagen fiber formation and enhanced angiogenesis in skin wounds. | [195] |
| Injectable Hydrogel | Supramolecular gelatin (host–guest) hydrogel containing resveratrol and histatin-1 |
| Preclinical | Promoted burn-wound healing comparable to commercial Tegaderm™; suppressed IL-6, IL-1β, TNF-α and upregulated TGF-β1/CD31 (angiogenesis). | [196] |
| Smart Dressing | GelMA/silk-fibroin glycidyl-methacrylate hydrogel with mesoporous silica NPs loaded with resveratrol and platelet-derived vesicles |
| Preclinical | In diabetic wounds: reduced TNF-α, iNOS; increased anti-inflammatory TGF-β1, Arg-1; enhanced angiogenesis and accelerated healing. | [197] |
| Smart Dressing | Gelatin-methacryloyl (GelMA)/ionic-liquid hydrogel loaded with resveratrol |
| Preclinical | Accelerated diabetic wound closure with reduced inflammation and robust neovascularization; activated proangiogenic PI3K/AKT pathways. | [198] |
| Injectable Hydrogel | Thermosensitive chitosan/ε-polylysine hydrogel integrated with Cu/Mg bimetallic nanoenzymes |
| Preclinical | ~90.6% wound closure by day 14 (vs. 55.4% untreated); enhanced collagen deposition, re-epithelialization, angiogenesis, and immunomodulation in diabetic wounds. | [199] |
| Natural Product or Formulation | Study Design and Patient Population | Outcome Measures | Main Findings | Ref. |
|---|---|---|---|---|
| Centella/Echinacea/Sambucus patch (PerioPatch®) | RCT in rats (gingival incisions, n = 48) | Epithelial gap; collagen content; cell proliferation | Herbal-patch group had smaller epithelial gaps and higher collagen content and cell proliferation versus placebo. | [200] |
| Papaya (Vasconcellea) latex protease (P1G10) ointment | Double-blind RCT, n = 50 (neuropathic diabetic foot ulcer patients) | % of wounds with 100% and ≥80% closure; healing time | More patients healed with P1G10 (11/50 full closures vs. 5/50 controls) and higher ≥80% closures; healing occurred in a shorter time with no adverse effects. | [217] |
| Calendula officinalis hydroglycolic extract | Prospective open-label pilot, n = 41 (diabetic foot ulcer patients) | % wound closure; time to heal; bacterial load; pain | 78% of ulcers achieved complete closure by 30 weeks (mean ~15.5 weeks); bacterial colonization and patient pain were reduced after treatment. | [218] |
| North Atlantic cod fish skin graft | Multinational open-label RCT, n = 255 (deep diabetic foot ulcers) | % healed at 16/24 weeks; time to healing | 44% healed at 16 weeks with fish skin vs. 26% with standard care (p < 0.001); at 24 weeks 55% vs. 38%. Fish skin treatment accelerated healing (hazard ratio ~1.59). | [204] |
| Mimosa tenuiflora (tepezcohuite) bark hydrogel | RCT, n = 41 (venous leg ulcer patients; extract gel vs. hydrogel) | Ulcer area reduction; re-epithelialization | Both groups showed similar ulcer area reduction; the mimosa extract gel was not superior to placebo hydrogel (no significant differences). | [219] |
| Combined herbal ointments (Plantoderm® + Fitoven®) | Pilot RCT, n = 34 (17 combined herb vs. 17 standard care, venous ulcers) | Ulcer area reduction; bacterial load | Herbal-treatment group saw ~42.7% ulcer area reduction vs. ~35.6% in controls at 7 weeks; also greater bacterial clearance (four ulcers became culture-negative). | [211] |
| Aloe vera gel | RCT, n = 68 (first/second-degree burns; 34 aloe vs. 34 silver sulfadiazine) | Time to epithelialization; pain; itch | Both groups fully epithelialized by ~2 weeks, but the aloe group healed faster and had significantly less pain and itch, especially noticeable by day 7. | [211] |
| Glycerol-preserved tilapia fish skin dressing | Phase III RCT, n = 115 (partial-thickness burns; tilapia vs. SSD) | Re-epithelialization time; dressing changes; pain; cost | Tilapia grafts accelerated wound closure (mean ~9.7 days vs. 10.2 days with SSD, p = 0.001). Patients needed fewer dressing changes, reported lower pain, and treatment cost was ~42% lower. | [217] |
| Lavender (Lavandula) essential oil (sitz bath/spray) | Systematic review of 5 RCTs (postpartum episiotomy wounds) | Wound healing (REEDA score); pain score | Lavender baths significantly sped wound closure and improved REEDA scores versus controls and reduced perineal pain in all trials (no reported adverse effects). | [215] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alberts, A.; Lungescu, I.A.; Niculescu, A.-G.; Grumezescu, A.M. Natural Products for Improving Soft Tissue Healing: Mechanisms, Innovations, and Clinical Potential. Pharmaceutics 2025, 17, 758. https://doi.org/10.3390/pharmaceutics17060758
Alberts A, Lungescu IA, Niculescu A-G, Grumezescu AM. Natural Products for Improving Soft Tissue Healing: Mechanisms, Innovations, and Clinical Potential. Pharmaceutics. 2025; 17(6):758. https://doi.org/10.3390/pharmaceutics17060758
Chicago/Turabian StyleAlberts, Adina, Ioana Alexandra Lungescu, Adelina-Gabriela Niculescu, and Alexandru Mihai Grumezescu. 2025. "Natural Products for Improving Soft Tissue Healing: Mechanisms, Innovations, and Clinical Potential" Pharmaceutics 17, no. 6: 758. https://doi.org/10.3390/pharmaceutics17060758
APA StyleAlberts, A., Lungescu, I. A., Niculescu, A.-G., & Grumezescu, A. M. (2025). Natural Products for Improving Soft Tissue Healing: Mechanisms, Innovations, and Clinical Potential. Pharmaceutics, 17(6), 758. https://doi.org/10.3390/pharmaceutics17060758