Considerations Regarding the Cytotoxicity of Certain Classes of Fungal Polyketides—Potential Raw Materials for Skincare Products for Healthy and Diseased Skin
Abstract
1. Introduction
- In silico prediction of the properties of six of the most well-known polyketides biosynthesized by Monascus, and an evaluation of their dermal and oral absorption capacity;
- In vitro evaluation of the effect of bioproducts containing yellow and orange polyketides on the viability of standardised HaCaT cell lines;
- Evaluation of the IC50 value (the concentration at which a substance exerts half of its maximum effect) based on in vitro data, using linear and nonlinear models supplied by the SYSTAT version 13.2, (Inpixon, Palo Alto, CA, USA) or ATT Bioquest programme version 1.0.0, (Pleasanton, CA, USA);
- Evaluation of the cytotoxicity of three types of bioproducts enriched with coloured polyketides, derived from Monascus purpureus, Monascus ruber, and a high-productive Monascus sp.
2. Materials and Methods
2.1. Bioproducts Obtaining
2.2. In Silico Studies
2.3. In Vitro Studies
2.4. IC50 Evaluation
3. Results
3.1. In Silico Studies
- The six analysed molecules exhibit a pronounced lipophilic character (Figure 5); the partition coefficients between octanol and water (WlogPo/w) for the six derived Monascus polyketides are in the range of (3 ÷ 4) [13]. As a result, these polyketides have a greater affinity for the lipid phase than the aqueous phase, which presents them as potential excipients or valuable active ingredients for dermato-cosmetic formulations, a fact also confirmed by the other scientists [19,20,21,22,23];
- The polyketides rubropunctatin, rubropunctamine, monascorubramine, and monascorubrin are molecules with a high degree of unsaturation (Figure 5), which provides them with a high level of chemical instability. This characteristic may limit their use in topical pharmaceutical formulations due to the possibility of rapid degradation or undesirable interactions with other compounds in the formulation.
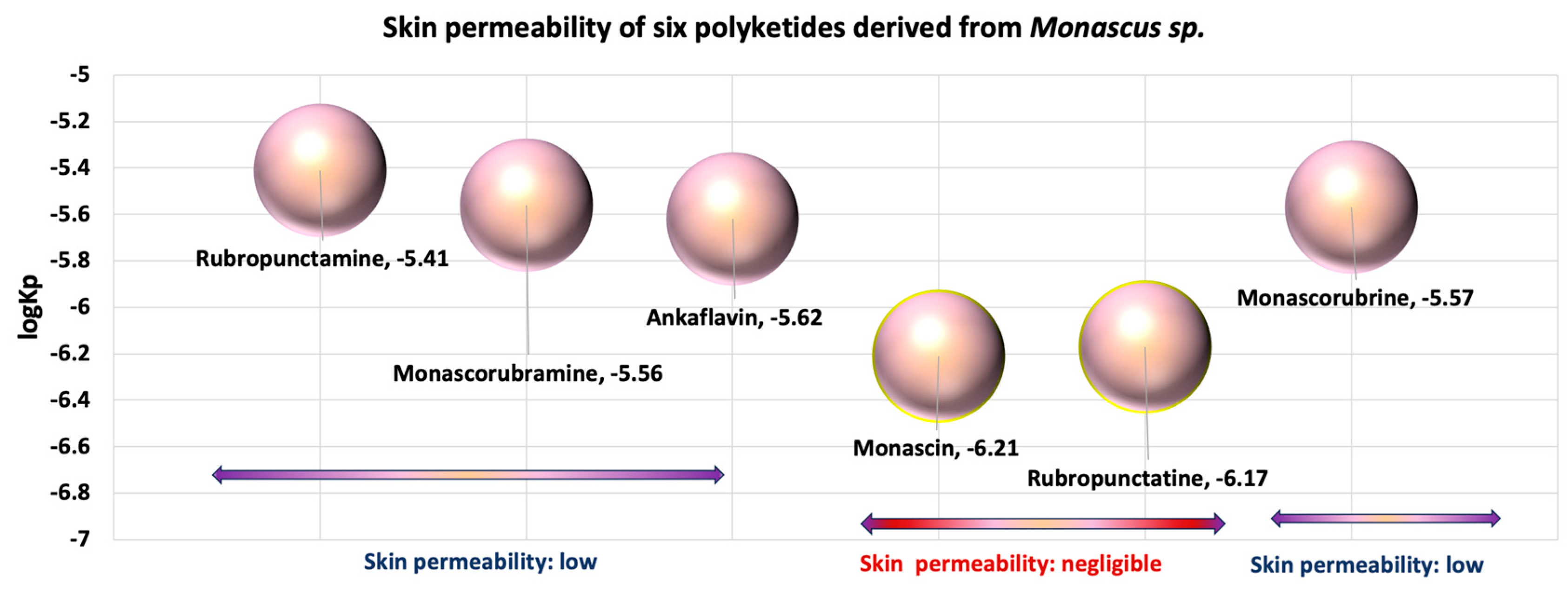
- Given that in silico tests indicated a potential for increased cytotoxicity, the analysis of the permeability coefficients (logKp), determined in silico by Albișoru and collaborators [13], shows that they fall within the range of [−5 ÷ −7] (Figure 6). These values suggest the low permeation of these molecules through skin layers [33,34]. In general, the six polyketides have a low capacity to pass through skin layers, with monascin and rubropunctatin exhibiting negligible permeation (logKp < −6); The “boiled egg” diagrams [35], generated in silico (Figure 7), indicate that the six analysed polyketides are not substrates for P-glycoprotein (PGP), suggesting that they are not easily eliminated from cells. The analysed molecules are easily absorbed from the gastrointestinal tract, with most of them having the ability to cross the blood–brain barrier, except for rubropunctamine.
3.2. In Vitro Studies
- (a)
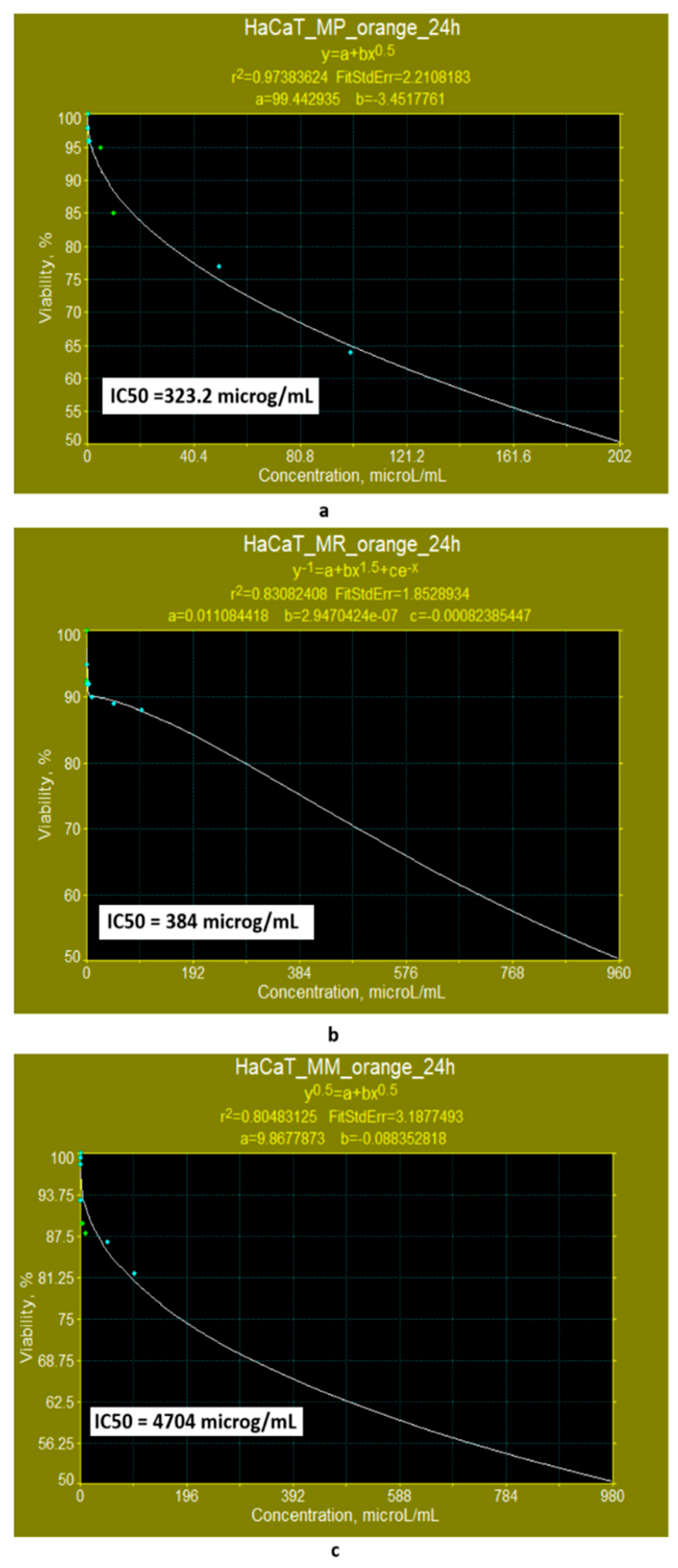
- When the cell line is exposed for 24 h to yellow polyketides biosynthesised by the three Monascus species, the viability measured is greater than 71% in all three cases (Figure 8a–c). The use of linear models for evaluating the IC50 parameter shows that the yellow polyketides isolated from biopreparations derived from Monascus purpureus and Monascus ruber are cytotoxic (IC50 < 20 μg/mL). The yellow polyketides derived from high-productive Monascus sp. exhibit weak cytotoxicity (IC50 = 144.45 μg/mL). The evaluation of the IC50 parameter with nonlinear models provided by Microsoft SYSTAT version 13.2 (Figure 9a–c) shows that the yellow polyketides derived from Monascus purpureus and Monascus ruber (Figure 9a,b) exhibit moderate cytotoxicity (IC50 = 46.45 μg/mL and 31.29 μg/mL, respectively). In contrast, the IC50 value obtained for the yellow polyketides derived from highly productive Monascus sp. indicates weak cytotoxicity (IC50 = 660.49 μg/mL) (Figure 9c).
- (b)
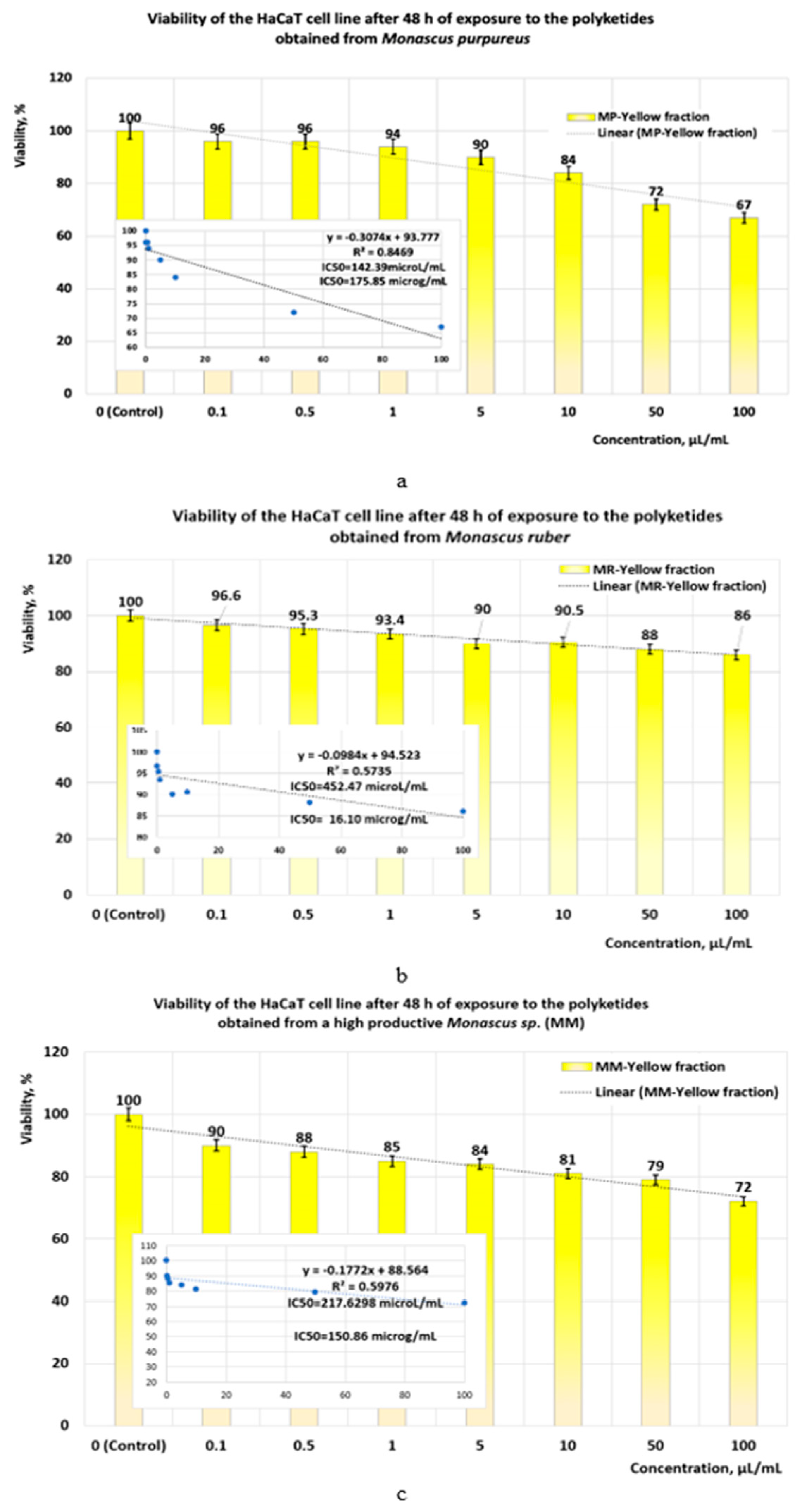
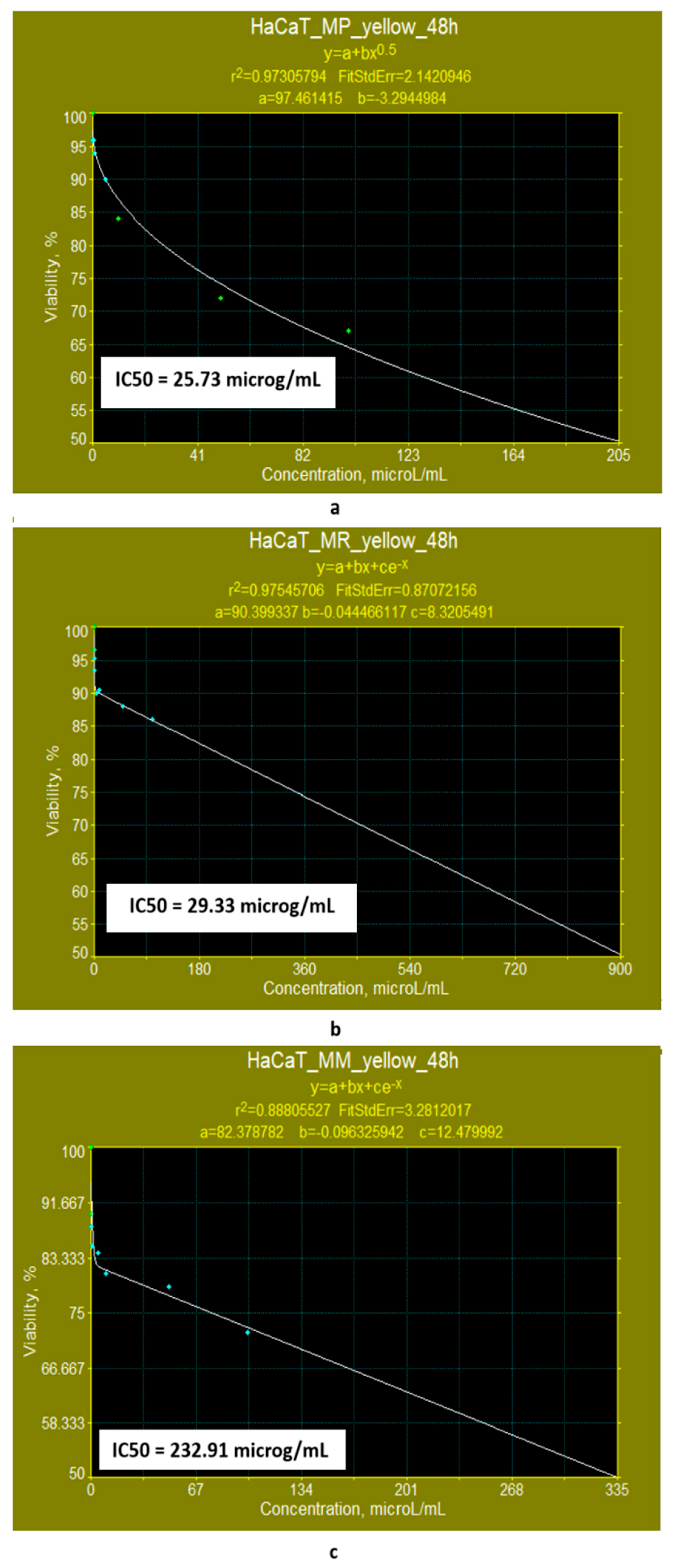
- If the exposure time is increased to 48 h, the linear models for evaluating the IC50 parameter (Figure 10a–c) show that the yellow polyketides derived from Monascus ruber retain their cytotoxic character (IC50 = 16.10 μg/mL) (Figure 10b). However, the yellow polyketides derived from Monascus purpureus and highly productive Monascus sp. exhibit weak cytotoxicity (IC50 = 175.85 μg/mL and IC50 = 150.86 μg/mL, respectively) (Figure 10a,c). Math modelling with the Systat programme confirms the in vitro results (Figure 11a–c), indicating that the yellow polyketides derived from Monascus purpureus and Monascus ruber exhibit moderate cytotoxicity (IC50 = 25.73 μg/mL and 29.33 μg/mL, respectively) (Figure 10a,b). The data obtained for yellow polyketides derived from highly productive Monascus sp. show that they have low cytotoxicity (IC50 = 232.91 μg/mL) (Figure 11c).
- (1)
- The yellow polyketides derived from Monascus ruber and Monascus purpureus (Figure 16a) exhibit high cytotoxicity. In contrast, the red polyketides derived from Monascus purpureus and Monascus ruber show moderate cytotoxicity (20 μg/mL < IC50 < 100 μg/mL). The yellow and red polyketides derived from high-productivity Monascus sp., as well as the orange polyketides derived from Monascus purpureus and Monascus ruber, exhibit low cytotoxicity. Orange polyketides derived from high-productivity Monascus sp. show no cytotoxicity (Figure 16a and Table 1).
- (2)
- The data obtained after 48 h of exposure show that the yellow polyketides derived from Monascus ruber (Figure 16b, Table 1) exhibit high cytotoxicity. The red polyketides derived from Monascus purpureus and Monascus ruber exhibit moderate cytotoxicity. The orange polyketides derived from Monascus ruber and Monascus purpureus, the yellow polyketides derived from Monascus purpureus and from high-productivity Monascus sp., as well as the red polyketides derived from high-productivity Monascus sp. (Figure 16b, Table 1) exhibit low cytotoxicity (100 μg/mL < IC50 < 1000 μg/mL). The orange polyketides derived from high-productivity Monascus sp. are non-cytotoxic (IC50 > 1000 μg/mL).
- The red polyketides derived from Monascus ruber and the yellow polyketides derived from Monascus purpureus and Monascus ruber (Figure 17a) show moderate cytotoxicity. The other polyketides show low cytotoxicity, except for the orange and red polyketides derived from high-productive Monascus sp., which are non-cytotoxic (IC50 > 1000 μg/mL);
- The data obtained after 48 h of exposure show that the yellow polyketides derived from Monascus purpureus and Monascus ruber, as well as the red polyketides derived from Monascus purpureus (Figure 17b, Table 1), exhibit moderate cytotoxicity (20 μg/mL < IC50 < 100 μg/mL). The orange polyketides derived from Monascus purpureus and Monascus ruber, as well as the yellow polyketides derived from high-productive Monascus sp. (Figure 17b), exhibit low cytotoxicity (100 μg/mL < IC50 < 1000 μg/mL). The orange polyketides and the red polyketides derived from high-productive Monascus sp., and the red polyketides derived from Monascus ruber, are non-cytotoxic (IC50 > 1000 μg/mL).
4. Discussions
- (1)
- Polyketides biosynthesised by Monascus species are attracting increasing interest in dermato-cosmetic formulations, due to their wide spectrum of biological activities and natural origin. In silico and in vitro analyses reveal that these compounds, particularly red, orange, and yellow pigments, possess promising features suitable for topical formulations. However, their application must be carefully balanced with their chemical and biological limitations.
- (2)
- From a functional point of view, Monascus-derived polyketides demonstrate multiple dermatological benefits. Compounds such as ankaflavin, monascorubramine, and monascorubrin exhibit high molecular flexibility, which supports potent antioxidant activity, contributing to skin protection against oxidative stress and premature ageing. These polyketides modulate genic expression in keratinocytes and fibroblasts, suggesting their potential to stimulate skin regeneration, improve hydration, and support tissue repair. Moreover, these polyketides exhibit antimicrobial properties, making them suitable candidates for formulations targeting acne-prone skin. The photoprotective and antiproliferative effects of these polyketides, especially for melanoma cell lines, highlight their capacity to protect the skin from ultraviolet radiation and reduce melanin synthesis. These features make Monascus-derived polyketides promising ingredients for formulations designed to address skin hyperpigmentation and UV-induced damage.
- (3)
- A significant safety advantage of these compounds lies in their poor transdermal penetration. In silico predictions indicate very low skin permeability, with logKp values ranging from –5 to –7, and negligible permeation reported for compounds such as monascin and rubropunctatin. This limited ability to cross the skin barrier significantly reduces systemic exposure and toxicity, even for compounds that display moderate-to-high cytotoxicity in vitro.
- (4)
- Additionally, predictions based on the “boiled egg” model suggest that these compounds are not substrates for P-glycoprotein, indicating a low likelihood of efflux-related bioaccumulation in skin cells.
- (1)
- To evaluate the IC50 of bioproducts containing different classes of coloured Monascus polyketides, the linear or nonlinear model provided by the SYSTAT programme is more suitable than the ATT Bioquest model, as the latter is not designed for bioproducts containing multiple bioactive molecules.
- (2)
- The polyketides derived from a new, high-productive Monascus sp. exhibit low or no cytotoxicity.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aruldass, C.A.; Dufossé, L.; Ahmad, W.A. Current perspective of yellowish-orange pigments from microorganisms: A review. J. Clean. Prod. 2018, 180, 168–182. [Google Scholar] [CrossRef]
- Egea, M.B.; Dantas, L.A.; Sousa, T.L.; Lima, A.G.; Lemes, A.C. The potential, strategies, and challenges of Monascus pigment for food application. Front. Sustain. Food Syst. 2023, 7, 1141644. [Google Scholar] [CrossRef]
- Abdel-Raheam, H.E.F.; Alrumman, S.A.; Gadow, S.I.; El-Sayed, M.H.; Hikal, D.M.; Hesham, A.E.-L.; Ali, M.M.A. Optimization of Monascus purpureus for Natural Food Pigments Production on Potato Wastes and Their Application in Ice Lolly. Front. Microbiol. 2022, 13, 862080. [Google Scholar] [CrossRef]
- Vendruscolo, F.; Bühler, R.M.M.; de Carvalho, J.C.; de Oliveira, D.; Estevez Mortitz, D.; Schmidell, W.; Ninow, J.L. Monascus: A Reality on the Production and Application of Microbial Pigments. Appl. Biochem. Biotechnol. 2016, 178, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Radu, N.; Corobea, M.C.; Coman, O.A.; Tanasescu, C. Nanomaterials with antioxidant properties, obtained via biotechnology, using the solid-state biosynthesis. Mol. Cryst. Liq. Cryst. 2016, 627, 210–219. [Google Scholar] [CrossRef]
- Radu, N.; Salageanu, A.; Ferdes, M.; Rau, I. Cytotoxicity Study Regarding Some Products Derived from Monascus sp. Mol. Cryst. Liq. Cryst. 2012, 555, 189–194. [Google Scholar] [CrossRef]
- Radu, N.; Salageanu, A.; Tănăsescu, C. Studies regarding the mechanisms involved in tissue regeneration, due to application of biomaterials obtained via biotechnology. Mol. Cryst. Liq. Cryst. 2016, 628, 204–212. [Google Scholar] [CrossRef]
- Radu, N.; Badea, M.N.; Ferdes, M.; Rau, I. Antioxidant Properties of Fungal Biomaterial. Mol. Cryst. Liq. Cryst. 2012, 555, 202–207. [Google Scholar] [CrossRef]
- Radu, N.; Doncea, S.; Ferdes, M.; Salageanu, A.; Rau, I. Biostimulatory Properties of Monascus sp. Bioproducts. Mol. Cryst. Liq. Cryst. 2012, 555, 195–201. [Google Scholar] [CrossRef]
- Radu, N.; Ghita, I.; Caloian, F.; Rau, I. Biopigments, Obtaining and Properties. Mol. Cryst. Liq. Cryst. 2010, 523, 1–10. [Google Scholar] [CrossRef]
- Chen, G.; Wu, Z. Production and biological activities of yellow pigments from Monascus fungi. World J. Microbiol. Biotechnol. 2016, 32, 136. [Google Scholar] [CrossRef]
- Lin, C.S.; Hur, H.F.; Lin, C.C. Antioxidant properties and antibacterial activity of fermented Monascus purpureus extracts. MOJ Food Process Technol. 2019, 7, 49–54. [Google Scholar] [CrossRef]
- Albisoru, D.; Radu, N.; Pirvu, L.C.; Stefaniu, A.; Băbeanu, N.; Stoica, R.; Mihai, D.P. Studies Regarding Antimicrobial Properties of Some Microbial Polyketides Derived from Monascus Strains. Antibiotics 2024, 13, 1092. [Google Scholar] [CrossRef]
- Stefanutti, C.; Mazza, F.; Mesce, D.; Morozzi, C.; Di Giacomo, S.; Vitale, M.; Pergolini, M. Monascus purpureus for statin and ezetimibe intolerant heterozygous familial hypercholesterolaemia patients: A clinical study. Atherosclerosis. Suppl. 2017, 30, 86–91. [Google Scholar] [CrossRef]
- Vitiello, A.; Izzo, L.; Castaldo, L.; d’Angelo, I.; Ungaro, F.; Miro, A.; Ritieni, A.; Quaglia, F. The Questionable Quality Profile of Food Supplements: The Case of Red Yeast Rice Marketed Products. Foods 2023, 12, 2142. [Google Scholar] [CrossRef]
- Vrolijk, M.F.; van de Koppel, S.; van Hunsel, F. Red yeast rice (Monascus purpureus) supplements: Case series assessment of spontaneously reported cases to The Netherlands Pharmacovigilance Centre Lareb. Br. J. Clin. Pharmacol. 2021, 87, 2146–2151. [Google Scholar] [CrossRef]
- Steffen, C. Rotschimmelreis: Ein bedenkliches Nahrungsergänzungsmittel? [Red yeast rice: An unsafe food supplement?]. Bundesgesundheitsblatt-Gesundheitsforschung-Gesundheitsschutz 2017, 60, 292–296. [Google Scholar] [CrossRef]
- Twarużek, M.; Ałtyn, I.; Kosicki, R. Dietary Supplements Based on Red Yeast Rice—A Source of Citrinin? Toxins 2021, 13, 497. [Google Scholar] [CrossRef]
- Lee, S.-H. Anti-Aging Cosmetic Composition Containing Monascus Fermented Oil. Patent KR101578464B1, 18 December 2015. [Google Scholar]
- Forestier, S.; Candau, D.; Seyler, N.; Elguidj, I. Compositions for Giving the Skin a Coloration Similar to that of a Natural Tan, Based on a Pigment of the Monascus Type, and Uses Thereof. Patent US6740313B2, 2004. [Google Scholar]
- Koli, S.H.; Suryawanshi, R.K.; Mohite, B.V.; Patil, S.V. Prospective of Monascus Pigments as an Additive to Commercial Sunscreens. Nat. Prod. Commun. 2019, 14, 1934578X19894095. [Google Scholar] [CrossRef]
- Jin, Y.J.; Pyo, Y.H. Effect of Monascus-Fermented Soybean Extracts on Antioxidant and Skin Aging-Related Enzymes Inhibitory Activities. Prev. Nutr. Food Sci. 2017, 22, 376–380. [Google Scholar] [CrossRef][Green Version]
- Wu, H.C.; Chen, Y.F.; Cheng, M.J.; Wu, M.D.; Chen, Y.L.; Chang, H.S. Investigations into Chemical Components from Monascus purpureus with Photoprotective and Anti-Melanogenic Activities. J. Fungi 2021, 7, 619. [Google Scholar] [CrossRef]
- de Oliveira, F.; Pedrolli, D.B.; Teixeira, M.F.S.; de Carvalho Santos-Ebinuma, V. Water-soluble fluorescent red colorant production by Talaromyces amestolkiae. Appl. Microbiol. Biotechnol. 2019, 103, 6529–6541. [Google Scholar] [CrossRef]
- Bostan, M.; Mihaila, M.; Petrica-Matei, G.G.; Radu, N.; Hainarosie, R.; Stefanescu, C.D.; Roman, V.; Diaconu, C.C. Resveratrol Modulation of Apoptosis and Cell Cycle Response to Cisplatin in Head and Neck Cancer Cell Lines. Int. J. Mol. Sci. 2021, 22, 6322. [Google Scholar] [CrossRef]
- Ciric, A.; Radu, N.; Zaharie, M.G.O.; Neagu, G.; Pirvu, L.C.; Begea, M.; Stefaniu, A. Potential Antitumor Effect of Functional Yogurts Formulated with Prebiotics from Cereals and a Consortium of Probiotic Bacteria. Foods 2023, 12, 1250. [Google Scholar] [CrossRef]
- Alrabiah, M.A.; Hassan, A.A.; Mubaraki, M.A.; Albarrag, A.M.; Dkhil, M.A.; Somily, A.M.; Delic, D.; Hafiz, T.A. Unexpected Efficacy of Albumin-Bound Glycerol Monolaurate Against Multidrug-Resistant Bacterial Isolates: A Time-Kill Assay Study. Infect. Drug Resist. 2025, 18, 1581–1593. [Google Scholar] [CrossRef]
- Forouz, F.; Mohammed, Y.; Shobeiri Nejad, H.S.A.; Roberts, M.S.; Grice, J.E. In vitro screening of topical formulation excipients for epithelial toxicity in cancerous and non-cancerous cell lines. EXCLI J. 2023, 22, 1173–1199. [Google Scholar] [CrossRef]
- Vijayarathna, S.; Sasidharan, S. Cytotoxicity of methanol extracts of Elaeis guineensis on MCF-7 and Vero cell lines. Asian Pac. J. Trop. Biomed. 2012, 2, 826–829. [Google Scholar] [CrossRef]
- Onyancha, J.M.; Gikonyo, N.K.; Wachira, S.W.; Mwitari, P.G.; Gicheru, M.M. Anticancer activities and safety evaluation of selected Kenyan plant extracts against breast cancer cell lines. J. Pharmacogn. Phytother. 2018, 10, 21–26. [Google Scholar] [CrossRef]
- Available online: https://ntp.niehs.nih.gov/sites/default/files/iccvam/docs/acutetox_docs/brd_tmer/brdvol1_nov2006.pdf (accessed on 26 March 2025).
- Albisoru, D.; Radu, N.; Muleasa, O.; Begea, M.; Roman, V. Preliminary studies regarding the cytotoxicity of red polyketides used as a dye in the food industry. Sci. Papers. Ser. D Anim. Sci. 2025; in press. [Google Scholar]
- Roberts, M.S. (Ed.) Dermal Absorption and Toxicity Assessment, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]
- Tregear, R.T. The permeability of mammalian skin to ions. J. Investig. Dermatol. 1966, 46, 16–23. [Google Scholar] [CrossRef]
- Antoine, D.; Vincent, Z. A Boiled-Egg To Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. Chem. Med. Chem. 2016, 11, 1117. [Google Scholar] [CrossRef]
- Babich, H.; Zuckerbraun, H.L.; Wurzburger, B.J.; Rubin, Y.L.; Borenfreund, E.; Blau, L. Benzoyl peroxide cytotoxicity evaluated in vitro with the human keratinocyte cell line, RHEK-1. Toxicology 1996, 106, 187–196. [Google Scholar] [CrossRef]
- Long, P.; Zhu, L.; Lai, H.; Xu, S.; Dong, X.; Shao, Y.; Wang, L.; Cheng, S.; Liu, G.; He, J.; et al. Monascus Red Pigment Liposomes: Microstructural Characteristics, Stability, and Anticancer Activity. Foods 2023, 12, 447. [Google Scholar] [CrossRef]
- Xue, Y.; Zhang, X.; Wang, Z. Improving the color stability of red Monascus pigments by direct protein microgelation. Food Res. Int. 2025, 199, 115389. [Google Scholar] [CrossRef]
- Dima, G.; Radu, N.; Chirvase, A.; Pena, E.L. Studies regarding using biomass in bioremediation of wastewater with heavy metal. Rev. Chim. 2007, 58, 561–565. [Google Scholar]
- Radu, N.; Meghea, A. Studies concerning the use of microbial biomass by fertilizers manufacture. J. Environ. Prot. Ecol. 2007, 8, 875–890. [Google Scholar]
- Radu, N.; Chirvase, A.A.; Babeanu, N.; Popa, O.; Cornea, P.; Pirvu, L.; Bostan, M.; Ciric, A.; Mathe, E.; Radu, E.; et al. Study Regarding the Potential Use of a Spent Microbial Biomass in Fertilizer Manufacturing. Agronomy 2020, 10, 299. [Google Scholar] [CrossRef]
- Sebaugh, J.L. Guidelines for accurate EC50/IC50 estimation. Pharmaceut. Statist. 2011, 10, 128–134. [Google Scholar] [CrossRef]
- Tallarida, R.J. Drug synergism: Its detection and applications. J. Pharmacol. Exp. Ther. 2001, 298, 865–872. [Google Scholar] [CrossRef]
- Alturki, M.S.; Tawfeeq, N.; Alissa, A.; Ahbail, Z.; Gomaa, M.S.; Al Khzem, A.H.; Rants’o, T.A.; Akbar, M.J.; Alharbi, W.S.; Alshehri, B.Y.; et al. Targeting KRAS G12C and G12S mutations in lung cancer: In silico drug repurposing and antiproliferative assessment on A549 cells. Inform. Med. Unlocked 2025, 52, 101612. [Google Scholar] [CrossRef]
- Davani, L.; Terenzi, C.; De Simone, A.; Tumiatti, V.; Andrisano, V.; Montanari, S. Design of Experiments and Optimization of Monacolin K Green Extraction from Red Yeast Rice by Ultra-High-Performance Liquid Chromatography. Foods 2024, 13, 2509. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, X.; Duan, W.; Wang, X.; Du, J. Accelerated solvent extraction of monacolin K from red yeast rice and purification by high-speed counter-current chromatography. J. Chromatogr. B 2010, 878, 2881–2885. [Google Scholar] [CrossRef]
- Liu, X.; Sun, A.; Li, Q.; Du, Y.; Zhao, T. A systematic study of the production of Monacolin K. by solid state fermentation of Monascus ruber. AMB Express 2022, 12, 29. [Google Scholar] [CrossRef]
- Sweeny, J.G.; Estrada-Valdes, M.C.; Iacobucci, G.A.; Sato, H.; Sadao Sakamura, S. Photoprotection of the red pigments of Monascus anka in aqueous media by 1,4,6-trihydroxynaphthalene. J. Agric. Food Chem. 1981, 29, 1189–1193. [Google Scholar] [CrossRef]
- Musso, L.; Dallavalle, S.; Merlini, L.; Bava, A.; Nasini, G.; Penco, S.; Giannini, G.; Giommarelli, C.; De Cesare, A.; Zuco, V.; et al. Natural and semisynthetic azaphilones as a new scaffold for Hsp90 inhibitors. Bioorg. Med. Chem. 2010, 18, 6031–6043. [Google Scholar] [CrossRef]
- Tan, H.; Xing, Z.; Chen, G.; Tian, X.; Wu, Z. Evaluating Antitumor and Antioxidant Activities of Yellow Monascus Pigments from Monascus ruber Fermentation. Molecules 2018, 23, 3242. [Google Scholar] [CrossRef]
- Singgih, M.; Permana, B.; Maulidya, S.A.; Yuliana, A. Studi In Silico Metabolit Sekunder Kapang Monascus sp. sebagai Kandidat Obat Antikolesterol dan Antikanker. Alchemy J. Penelit. Kim. 2019, 15, 104–123. [Google Scholar] [CrossRef]
- Rizkuloh, L.R.; Pratita, A.T.; Mediana, M.; Yuliana, A. In silico study in toxicity parameters of Pigment-Derived Compounds of Monascus sp. mold as a cervical anti-cancer drugs candidate. J. Teknol. Lab. 2021, 10, 93–100. [Google Scholar] [CrossRef]
- Zain, D.N.; Yuliana, A. In Silico Study of Monascus sp. Pigment. Derivatives as Anticardiovascular Candidates. J. Ilm. Farm. 2023, 19, 1–14. Available online: http://journal.uii.ac.id/index.php/JIF (accessed on 25 September 2024). [CrossRef]

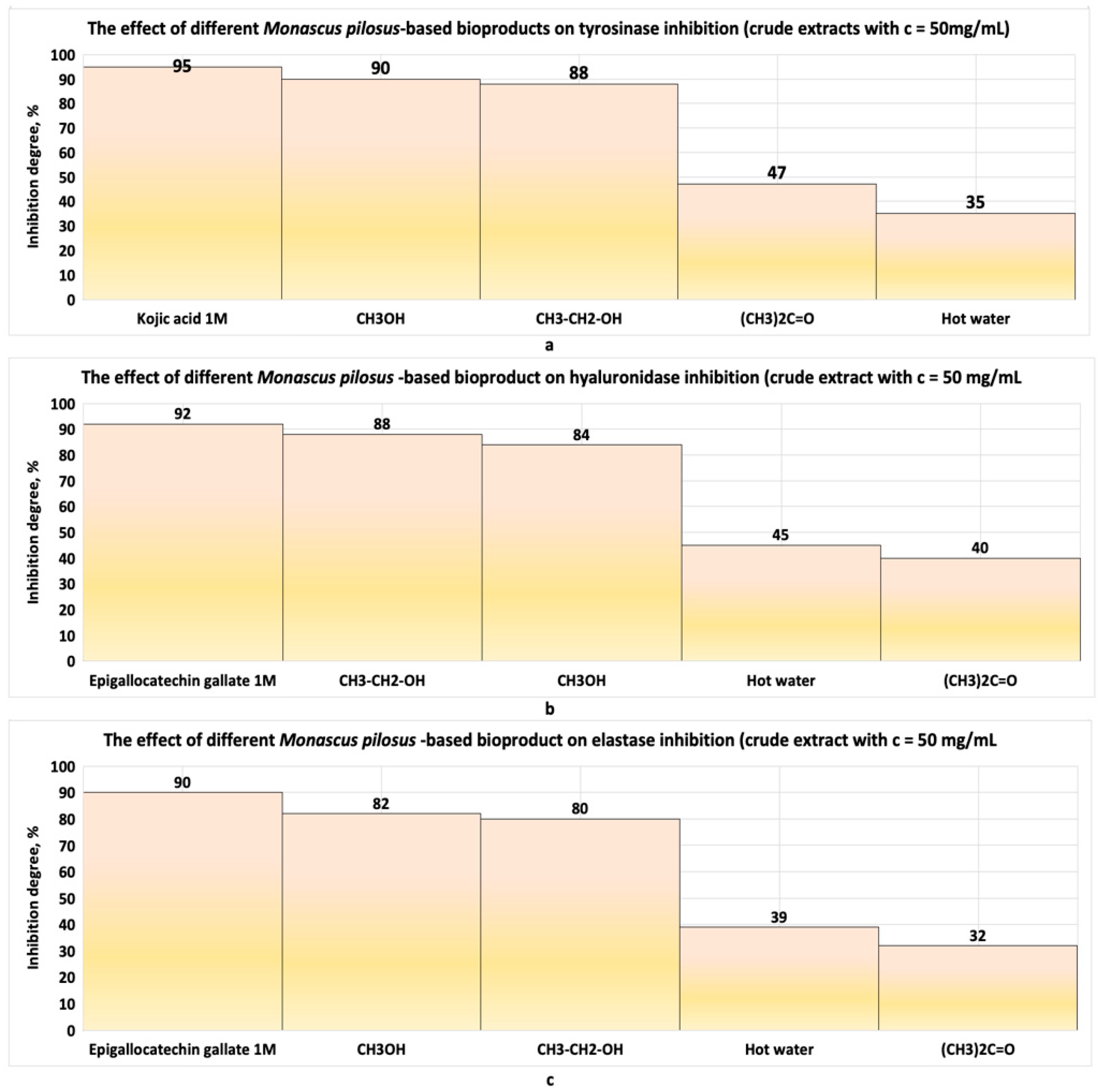
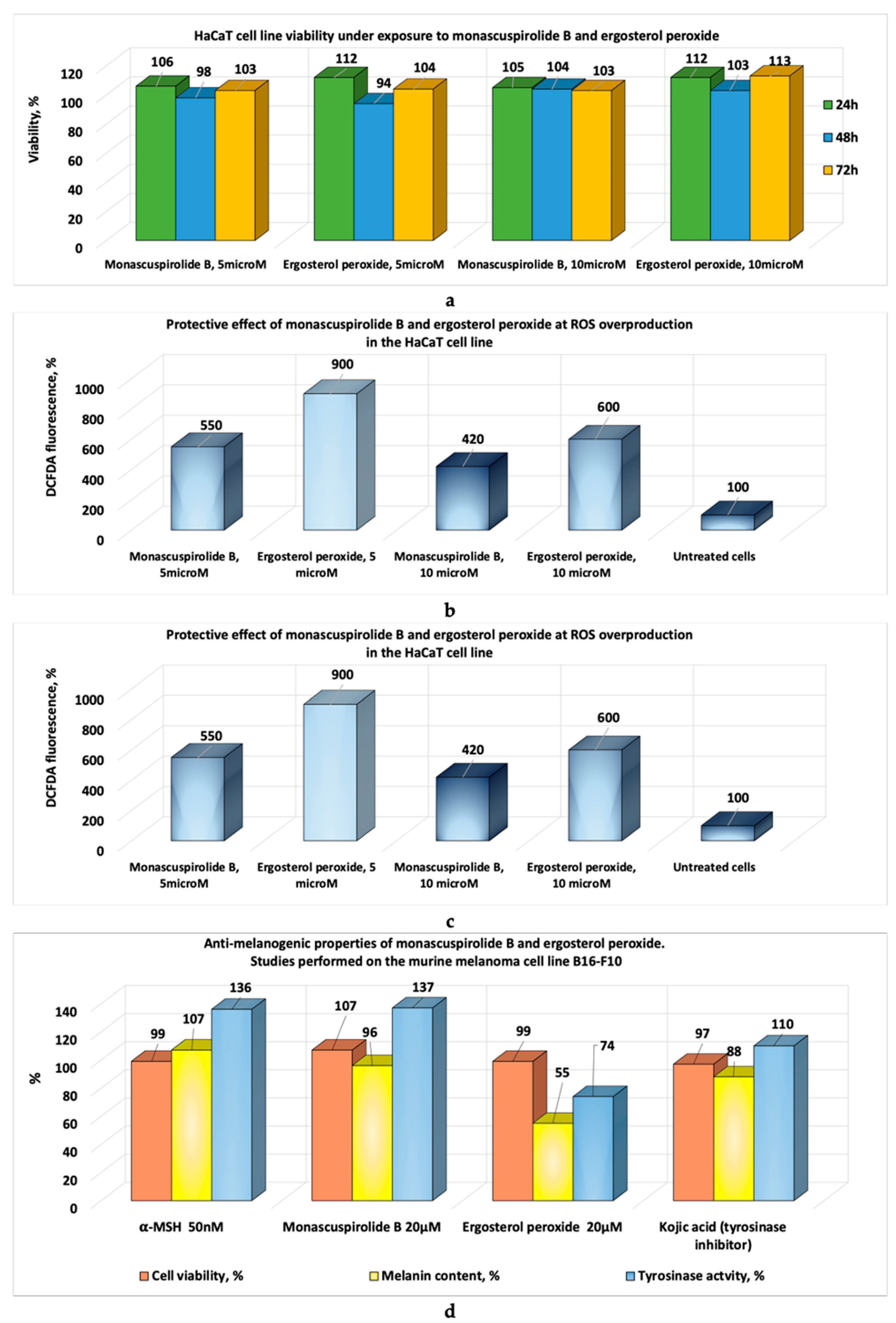
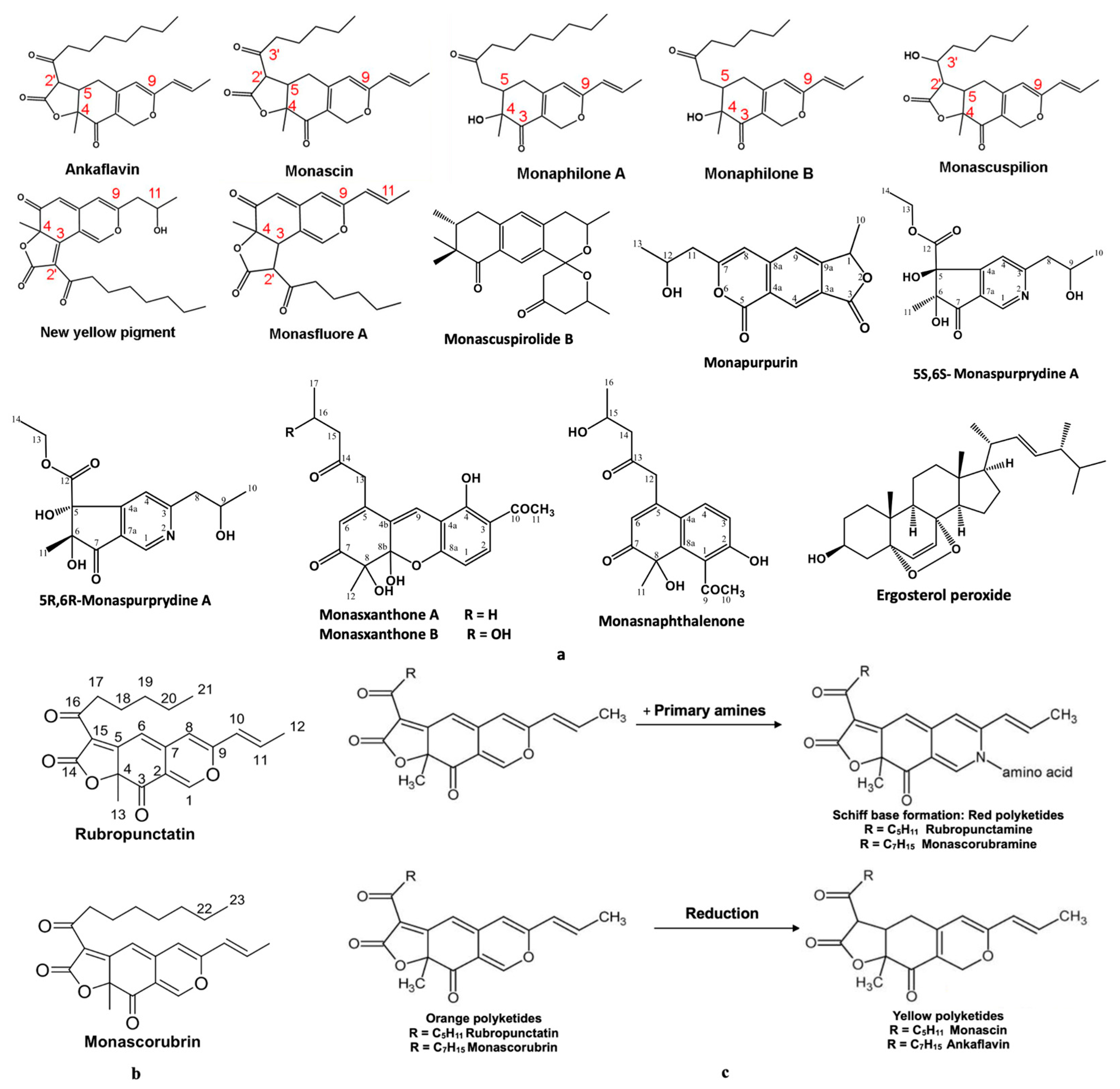
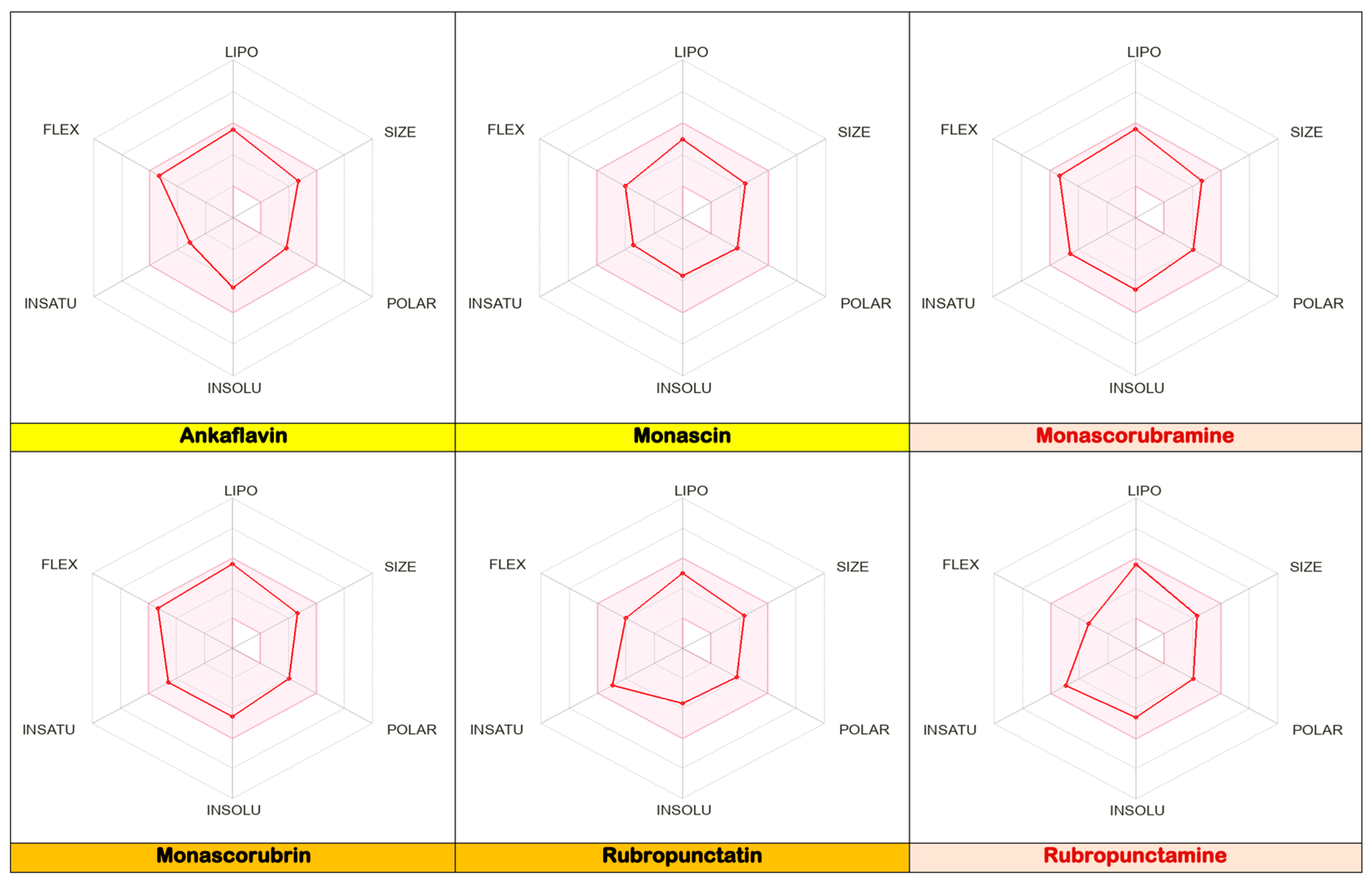




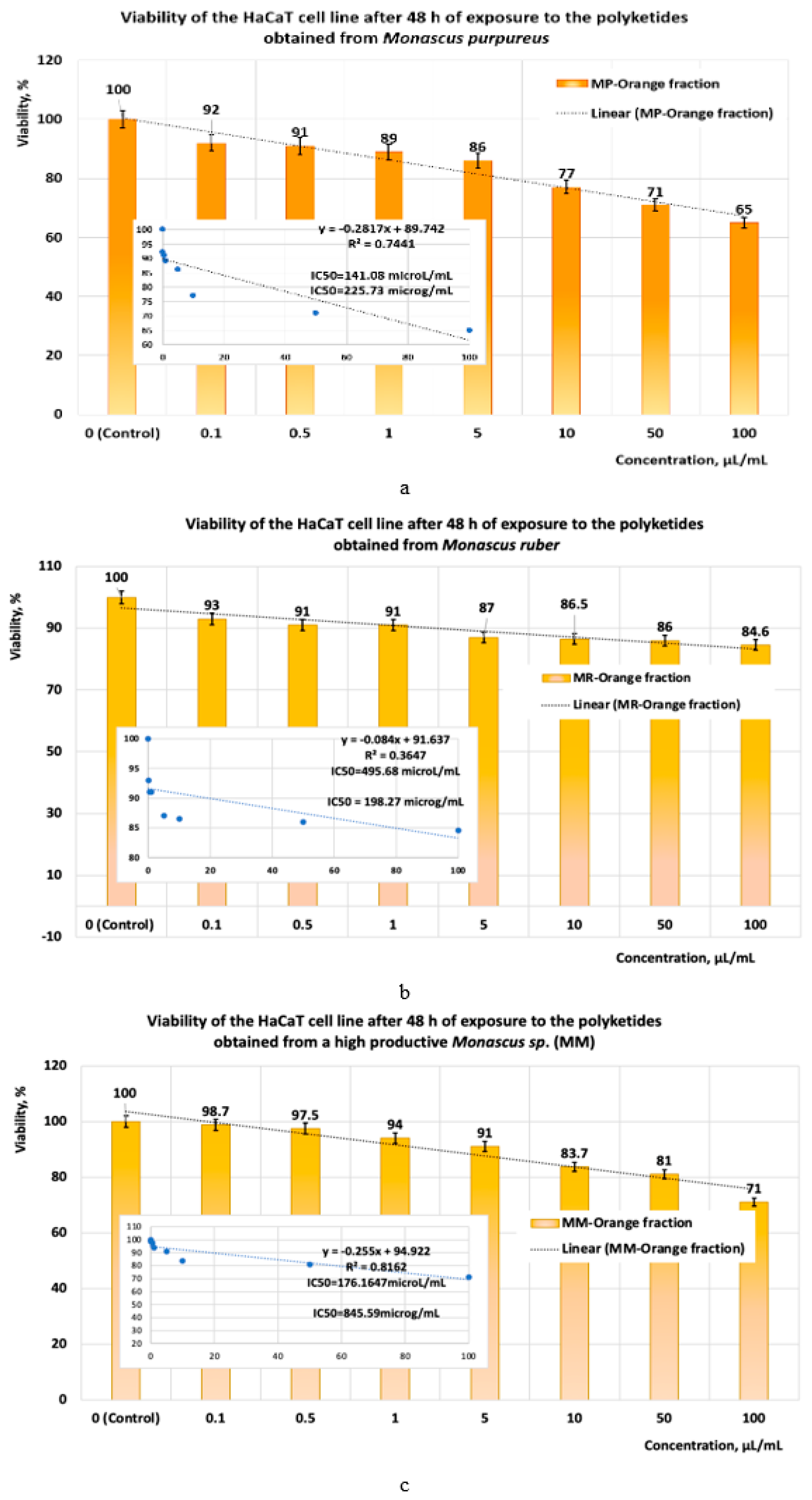

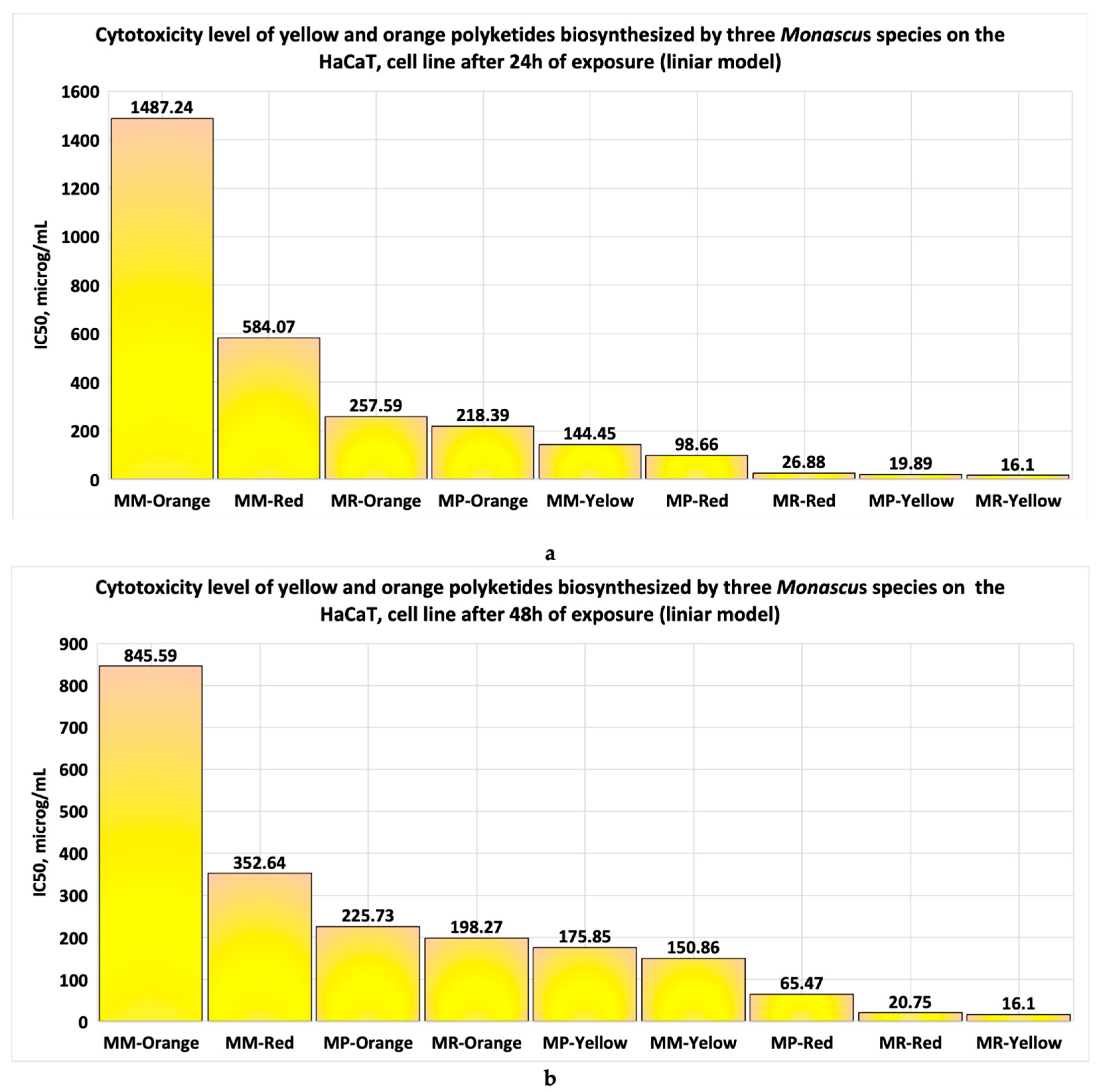
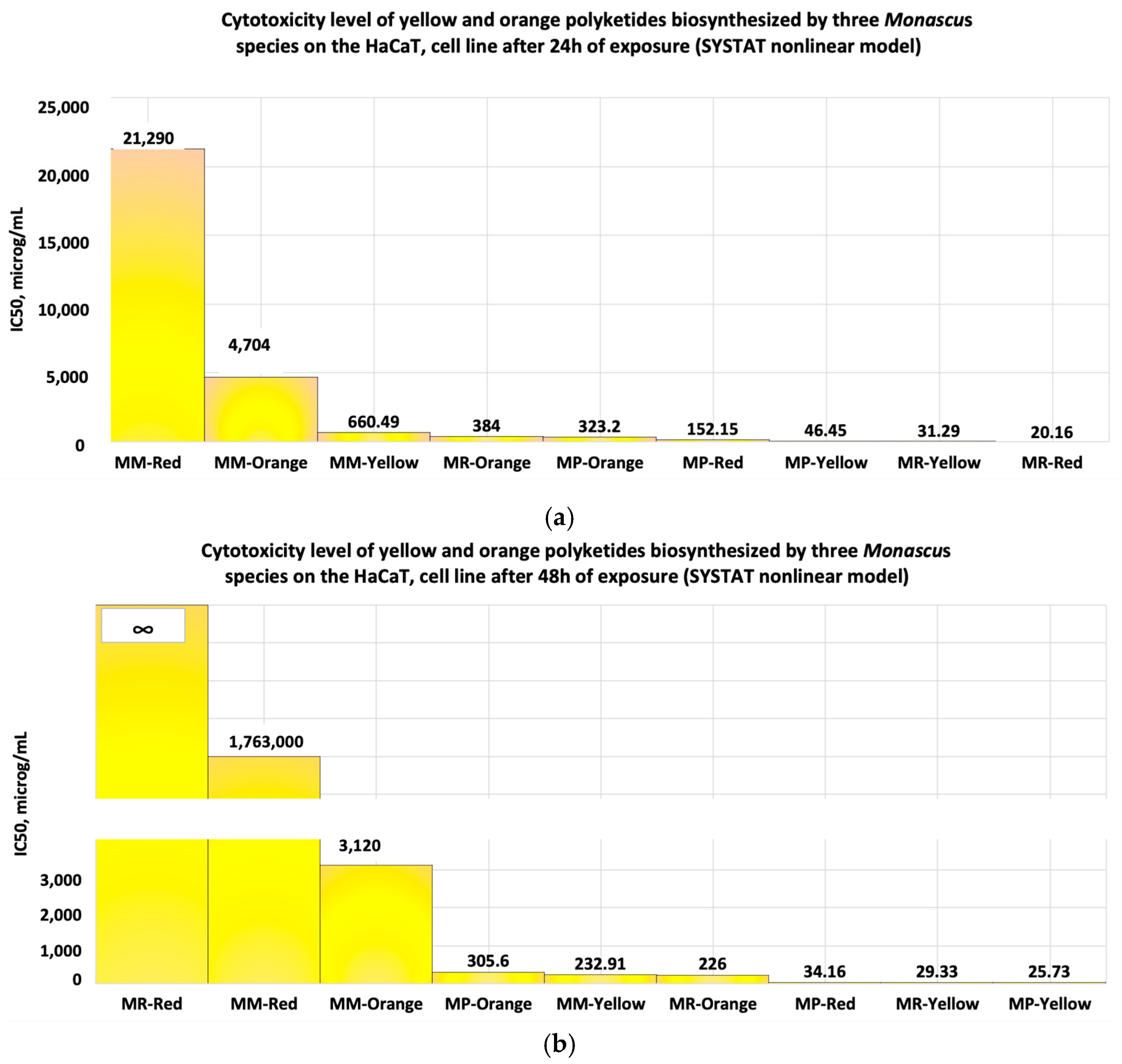
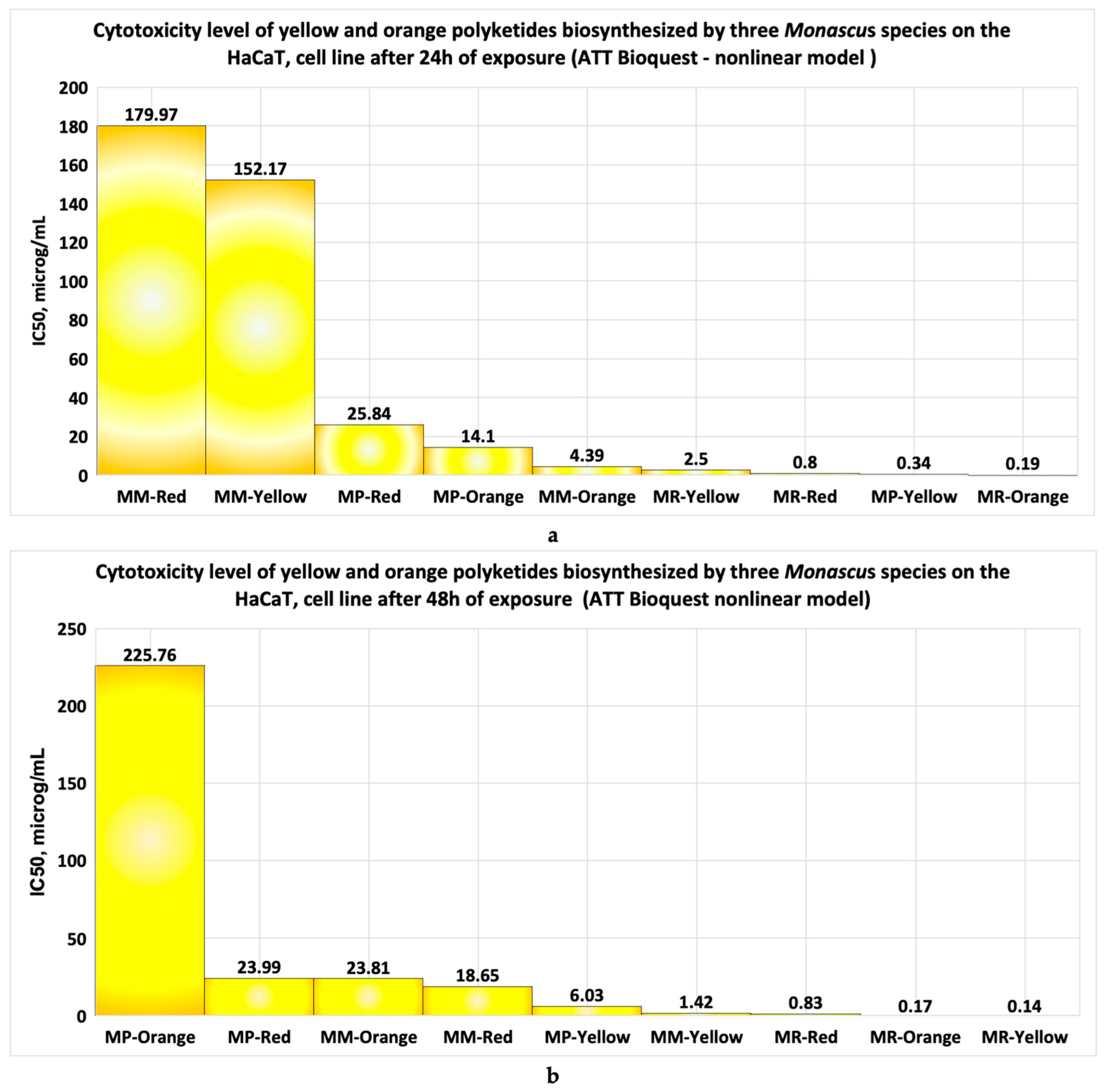
| Strain | Product | IC50, μg/mL Linear Model | IC50, µg/mL Nonlinear Model | Observations | References |
|---|---|---|---|---|---|
| Exposure time: 24 h | |||||
| Monascus purpureus | MP-Red | 98.66 | 152.15 | Moderate cytotoxicity–low cytotoxicity | [26,28,29,30,36] |
| Monascus ruber | MR-Red | 26.88 | 20.60 | Moderate cytotoxicity for both models | |
| Monascus high-productive strain | MM-Red | 584.07 | 21,290.00 | Low cytotoxicity–no cytotoxcity | |
| Monascus purpureus | MP-Yellow | 19.89 | 46.50 | High cytotoxicity–moderate cytotoxicity | |
| Monascus ruber | MR-Yellow | 16.10 | 31.29 | High cytotoxicity–moderate cytotoxicity | |
| Monascus high-productive strain | MM-Yellow | 144.45 | 660.49 | Low cytotoxicity for both models | |
| Monascus purpureus | MP-Orange | 218.39 | 323.20 | Low cytotoxicity for both models | |
| Monascus ruber | MR-Orange | 257.60 | 384.00 | Low cytotoxicity for both models | |
| Monascus high-productive strain | MM-Orange | 1487.24 | 4704.00 | No cytotoxicity for both models | |
| Exposure time: 48 h | |||||
| Monascus purpureus | MP-Red | 65.47 | 34.16 | Moderate cytotoxicity for both models | [26,28,29,30,36] |
| Monascus ruber | MR-Red | 20.75 | ∞ | Moderate cytotoxicity–no cytotoxicity | |
| Monascus high-productive strain | MM-Red | 352.64 | 1763 × 103 | Low cytotoxicity–no cytotoxicity | |
| Monascus purpureus | MP-Yellow | 175.85 | 25.73 | Low cytotoxicity–moderate cytotoxicity | |
| Monascus ruber | MR-Yellow | 16.10 | 29.33 | High cytotoxicity–moderate cytotoxicity | |
| Monascus high-productive strain | MM-Yellow | 150.86 | 232.91 | Low cytotoxicity for both models | |
| Monascus purpureus | MP-Orange | 225.73 | 305.60 | Low cytotoxicity for both models | |
| Monascus ruber | MR-Orange | 198.27 | 226.00 | Low cytotoxicity for both models | |
| Monascus high-productive strain | MM-Orange | 845.59 | 3120.00 | Low cytotoxicity–no cytotoxicity | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albisoru, D.; Radu, N.; Senin, R.; Caramihai, M.D.; Begea, M.; Mulesa, O.; Roman, V.; Bostan, M. Considerations Regarding the Cytotoxicity of Certain Classes of Fungal Polyketides—Potential Raw Materials for Skincare Products for Healthy and Diseased Skin. Pharmaceutics 2025, 17, 759. https://doi.org/10.3390/pharmaceutics17060759
Albisoru D, Radu N, Senin R, Caramihai MD, Begea M, Mulesa O, Roman V, Bostan M. Considerations Regarding the Cytotoxicity of Certain Classes of Fungal Polyketides—Potential Raw Materials for Skincare Products for Healthy and Diseased Skin. Pharmaceutics. 2025; 17(6):759. https://doi.org/10.3390/pharmaceutics17060759
Chicago/Turabian StyleAlbisoru, Daniela, Nicoleta Radu, Raluca Senin, Mihai Dan Caramihai, Mihaela Begea, Oksana Mulesa, Viviana Roman, and Marinela Bostan. 2025. "Considerations Regarding the Cytotoxicity of Certain Classes of Fungal Polyketides—Potential Raw Materials for Skincare Products for Healthy and Diseased Skin" Pharmaceutics 17, no. 6: 759. https://doi.org/10.3390/pharmaceutics17060759
APA StyleAlbisoru, D., Radu, N., Senin, R., Caramihai, M. D., Begea, M., Mulesa, O., Roman, V., & Bostan, M. (2025). Considerations Regarding the Cytotoxicity of Certain Classes of Fungal Polyketides—Potential Raw Materials for Skincare Products for Healthy and Diseased Skin. Pharmaceutics, 17(6), 759. https://doi.org/10.3390/pharmaceutics17060759









