Medulloblastoma: Molecular Targets and Innovative Theranostic Approaches
Abstract
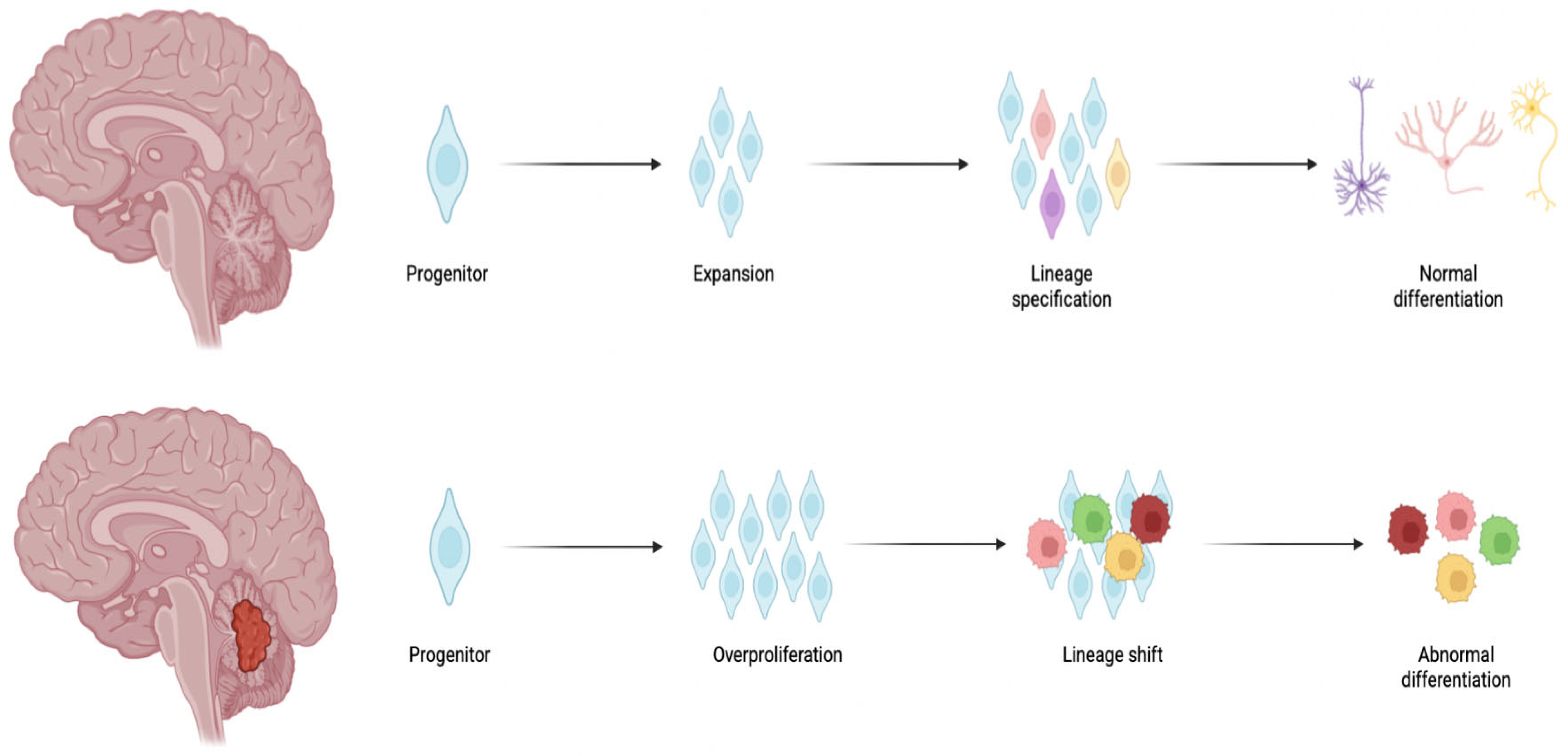
1. Introduction
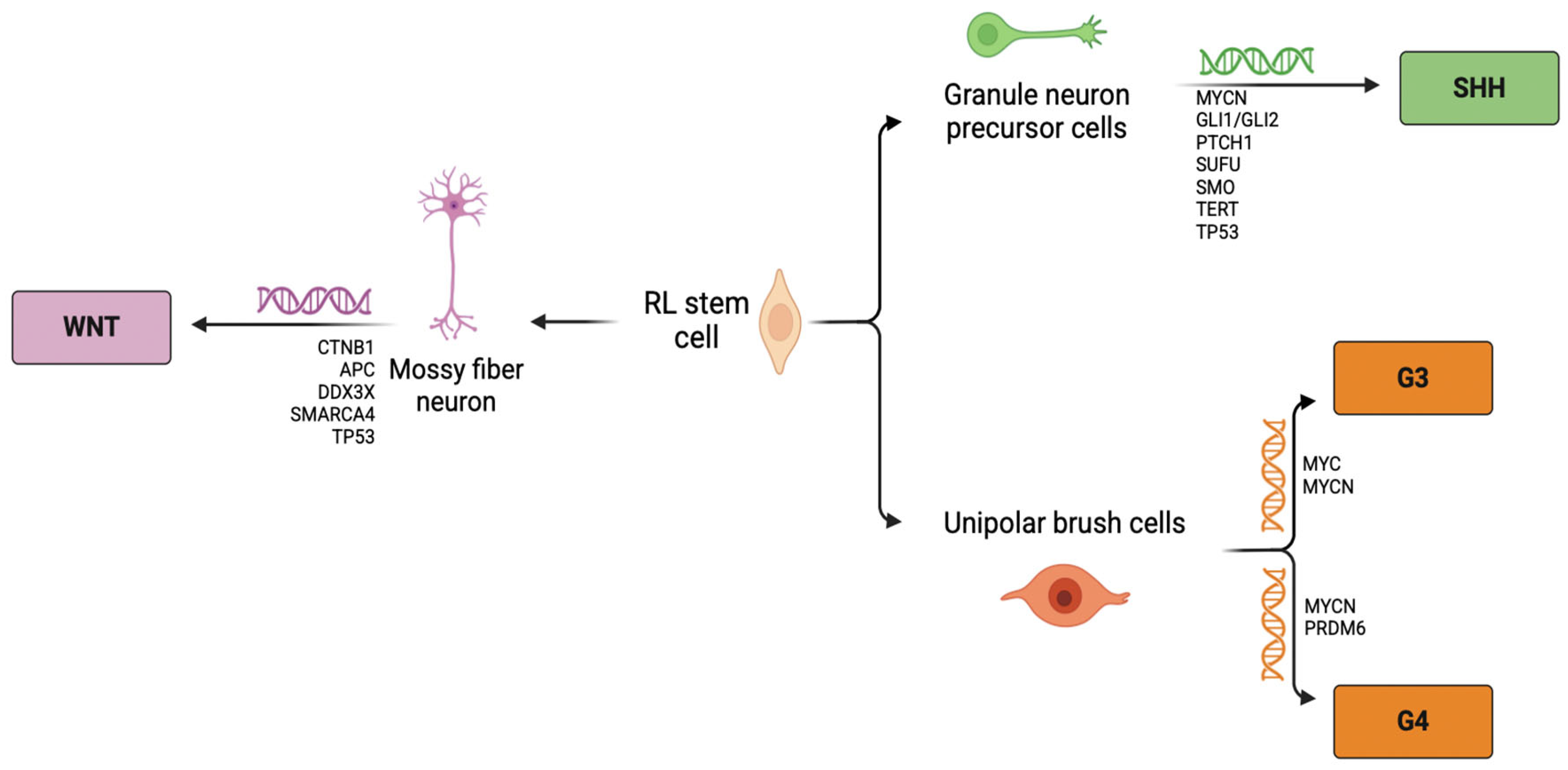
2. Medulloblastoma: The Molecular Subgroups
2.1. Wingless
2.2. Sonic Hedgehog
2.3. Group 3
2.4. Group 4
3. Nanomedicine for Brain Tumors
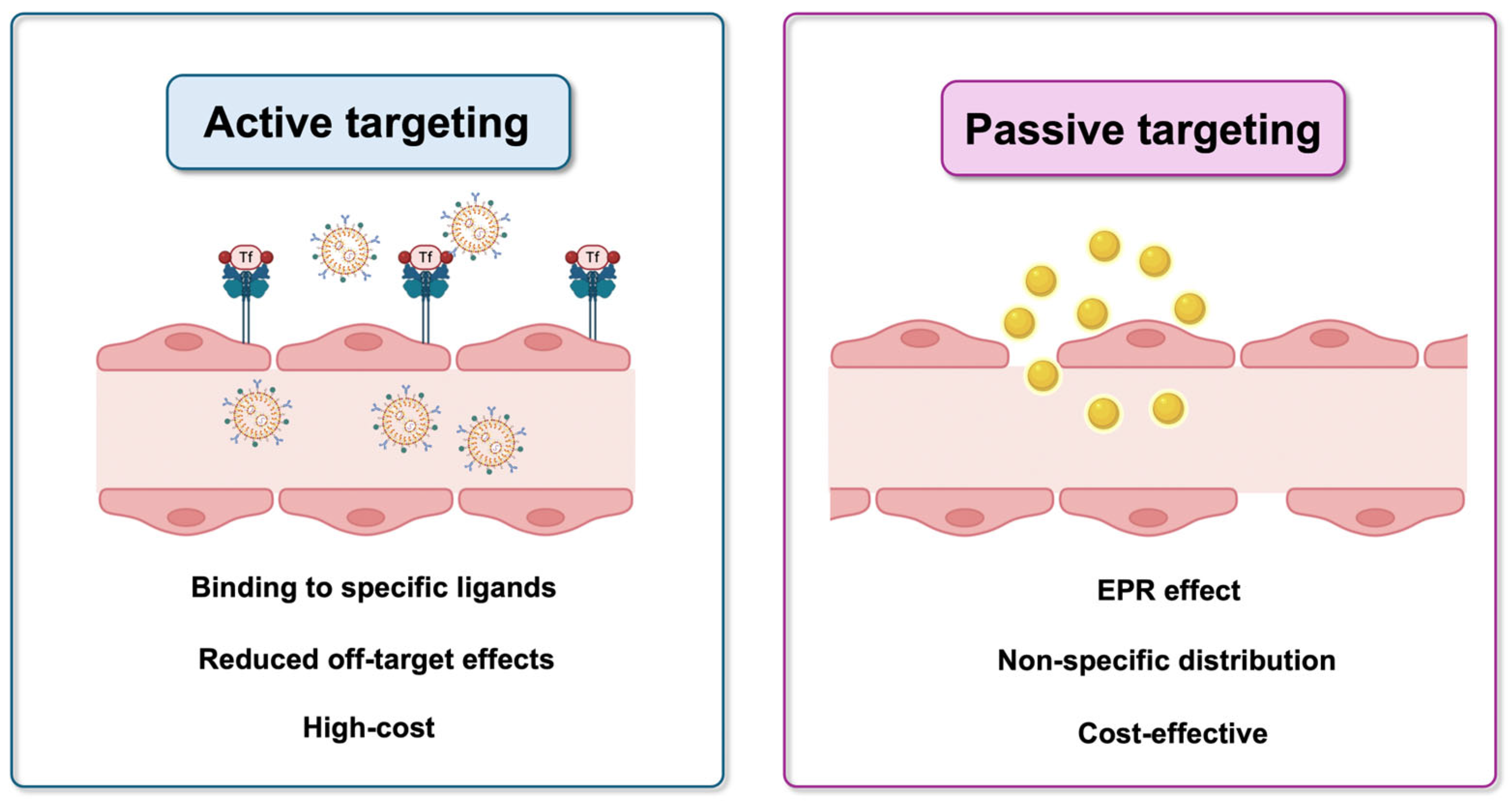
3.1. Nanomedicine for Brain Tumors Targeting Strategies
3.1.1. Passive Targeting
3.1.2. Active Targeting
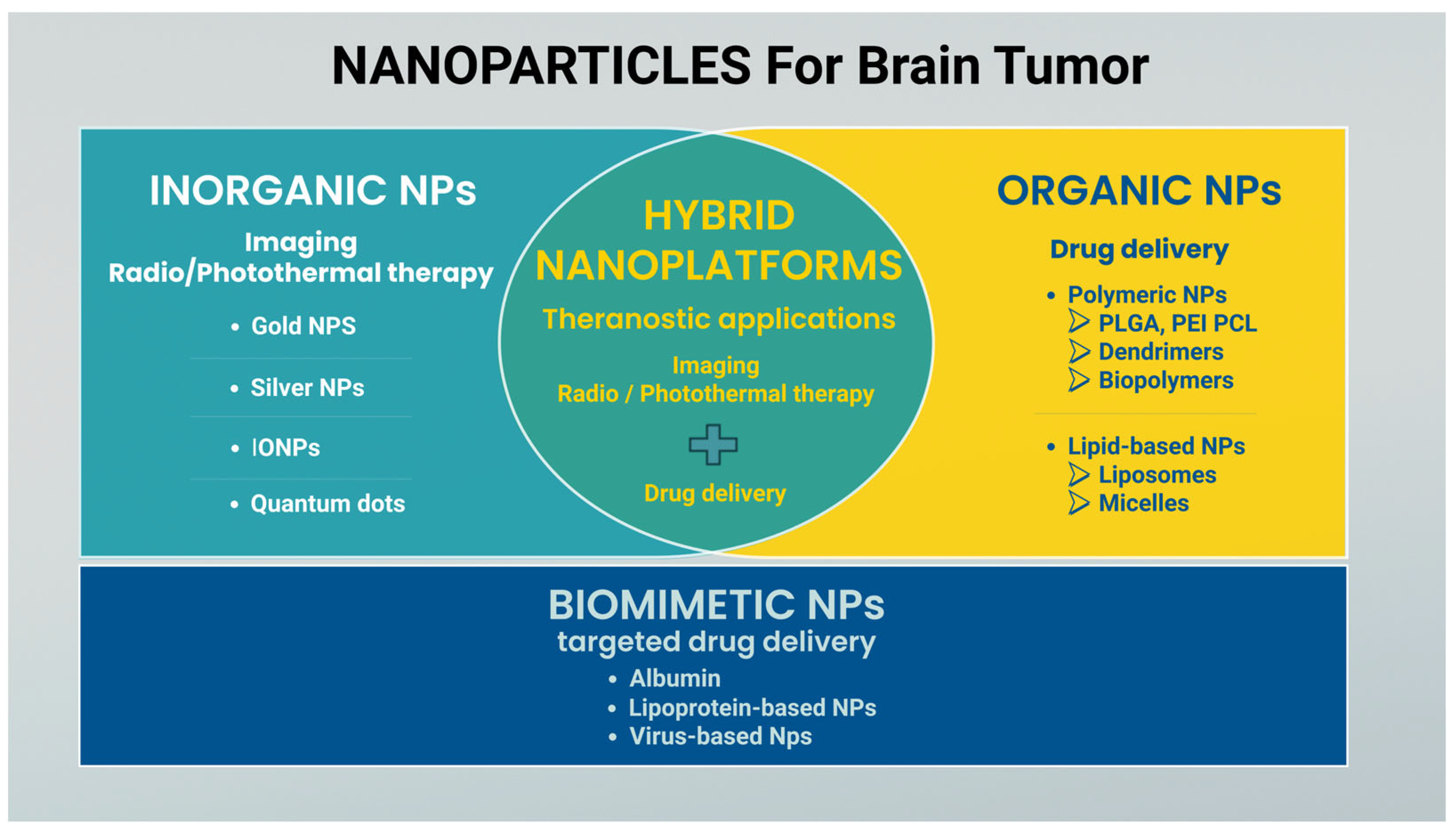
3.2. Nanoparticle Classification
3.2.1. Polymeric Nanoparticles
3.2.2. Lipid-Based Nanoparticles
3.2.3. Inorganic Nanoparticles
3.2.4. Biomimetic NPs
4. Nanomedicine and Medulloblastoma: A Chronological Overview
4.1. Polymeric Nanoparticles for MB
4.2. Lipid Nanoparticles
4.3. Inorganic NPs for MB
| Targeting Mechanism | Year | Inorganic NPs or Hybrid | Tested Models | Application/Results | Ref. |
|---|---|---|---|---|---|
| Passive | 2015 | Iron oxide core coated with chitosan, PEG, PEI, loaded with Ape1 siRNA (NP:siApe1) | In vitro UW228-1 cells (SHH) | Radiosensitization combined with gene therapy/ Efficient silencing of DNA repair systems and increased DNA damage after irradiation | [116] |
| 2017 | Gold NPs coated with PEG chitosan, PEI loaded with Ape1 siRNA | In vitro UW228-1 cells (SHH) | Radiosensitization combined with gene therapy/ Efficient silencing of DNA repair systems and increased DNA damage after irradiation | [117] | |
| 2020 | AuFe core with streptavidin binding sites conjugated with biotinylated etoposide | In vitro D283 (G3/G4) | Drug delivery/ Remote control of drug distribution by rotating magnet | [119] | |
| 2023 | Nanodiamonds | In vitro DAOY cells (SHH) | Radiosensitization/ Induction of apoptosis | [118] | |
| 2024 | Spherical IONP compared to IONR | In vitro D556 (G3) | Imaging probes and drug delivery/ Better internalization of IONR via clathrin/caveolae and phagocytosis | [129] | |
| 2024 | Perfluoropolyether (PFPE) polymer-engineered IONPs complexed electrostatically with Plk1 siRNA | In vitro D425 (G3) | Theranostic/ Improved delivering and protection of siRNA providing an agent contrast for MRI | [133] | |
| 2024 | AuNPs associated with alternating current | In vitro DAOY cells (SHH) D283 (G3) | Bioelectronic medicines/ Endosomal escape | [120] | |
| Active | 2008 | Gold-silica nanoshells targeting HER2 | In vitro DAOY cells (SHH) | Photothermal therapy/ Selective killing of cancer cells after exposure to NIR light | [121] |
| 2010 | Dextran-coated iron oxide nanoparticles targeting JC virus T-antigen | In vitro BSB8 mouse MB | Putative MRI probe/ Cellular internalization | [122] | |
| 2011 | Qdots binding activated EGFR | In vitro DAOY cells (SHH) | Diagnosis/ Identify level of activated, intracellular EGFR populations | [123] | |
| 2012 | Sendai virus-based liposome: Qdots binding intracellular EGFR | In vitro DAOY cells (SHH) | Delivery and bioimaging/ intracellular labeling of EGFR bypassing endosomal entrapment | [124] | |
| 2015 | Tf-conjugated PEG-b-AGE-coated IONPs targeting TfR | In vitro DAOY cells (SHH) D556 (G3) | Delivery and bioimaging/ coating and stabilization of the IONPs with anti-biofouling effect | [125] | |
| 2017 | Tf-conjugated PEG-b-AGE-coated IONPs targeting TfR | In vitro D556 (G3) | Biomarker-targeted imaging for cell separation/ Detection of circulating tumor cells with IONPs and anti-biofouling effect | [128] | |
| 2017 | PEG-coated spherical IONP compared to PEG-coated IONR targeting TfR | In vitro D556 (G3) | Diagnosis, biomarker targeted imaging for cell separation/ Better detection and immunomagnetic cell separation by Tf-IONR | [130] | |
| 2019 | Tf-conjugated IONPs targeting TfR | In vitro D556 (G3) | Diagnosis/ Improved efficiency of TfR-positive cells compared with commonly used commercial magnetic separation agents | [132] |
4.4. Biomimetic Nanoparticles
| Targeting Mechanism | Year | Biomimetic NPs | Tested Models | Application/Results | Ref. |
|---|---|---|---|---|---|
| Passive, (functionally- addressed toward cancers) | 2017 | Redox-responsive BSA-NPs loaded with cisplatin | In vitro DAOY (SHH) | Drug delivery and targeted release/ Cancer toxicity according to glutathione content | [134] |
| 2023 | Polynitroxylated albumin (PNA) | In vivo transgenic SHH MB mice (MAP mice) | Prevention of metastasis | [135] | |
| Active | 2018 | HDL-mimetic NPs targeting SCARB1 | In vitro DAOY cells (SHH) D283 (G3) | Therapy/ Modulated cellular cholesterol, reduction in cell viability and depletion of cancer stem cell populations | [59] |
| 2020 | HDL-mimetic NPs loaded with sonidegib, targeting endothelial SR-B1 and MB-stem cell CD15 | In vitro DAOY (human) and PZp53 (mouse) MB (SHH) Ex vivo and in vivo transgenic SHH MB mice | Drug delivery and dual targeting/ Facilitated and targeted cellular uptake of drugs | [138] | |
| 2021 | Chimeric tomato bushy stunt virus NPs (surface genetically engineered with peptides to target brain cells) loaded with Doxorubicin | In vitro primary murine cell culture (SHH) In vivo transgenic SHH MB mice | Targeted drug delivery/ Reduced drug dose for cell death; reached the tumor in a specific manner. | [139] | |
| 2023 | Chimeric tomato bushy stunt virus NPs (surface genetically engineered with Coop peptides) | In vivo transgenic SHH MB mice | Targeted drug delivery/ Reduced drug dose for therapeutic effects. | [140] |
4.5. Molecular Target of Nanomedicine for MB
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MB | medulloblastoma |
| WNT | Wingless |
| SHH | Sonic Hedgehog |
| G3 | Group 3 |
| G4 | Group 4 |
| CNS | central nervous system |
| BBB | blood–brain barrier |
| CTNNB1 | catenin beta 1 |
| APC | adenomatous polyposis coli |
| GNP | granule neuron progenitors |
| PTCH1 | Patched1 |
| SUFU | suppressor of fused homologue |
| Smo | Smoothened |
| DN | desmoplastic/nodular |
| LCA | large cell/anaplastic |
| CDK | cyclin-dependent kinase |
| PRDM6 | PR/SET domain 6 protein |
| NPs | nanoparticles |
| GLUT | glucose transporter |
| EAAT1–3 | excitatory amino acid transporters |
| TfR | transferrin receptor |
| FDA | Food and Drug Administration |
| EPR | enhanced permeability and retention |
| EGFR | epidermal growth factor receptor |
| GBM | glioblastoma |
| IONP | iron oxide nanoparticle |
| LDL | low-density lipoprotein |
| PLGA | polylactic-co-glycolic acid |
| PEG | polyethylene glycol |
| AuNP | gold nanoparticle |
| NIR | near-infrared |
| Bcl2L12 | oncoprotein Bcl2Like12 |
| AgNP | silver nanoparticle |
| ROS | reactive oxygen species |
| MRI | magnetic resonance imaging |
| PGA | poly(glycerol-adipate) |
| RBITC | rhodamine B isothiocyanate |
| PEI | polyethylenimine |
| CTX | chlorotoxin |
| IGF1 | insulin-like growth factor 1 |
| STAT3 | signal transducer and activator of transcription 3 |
| AKT | serine/threonine kinase |
| Hh | Hedgehog |
| Qdots | quantum dots |
| TEM | transmission electron microscopy |
| PEG-b-AGE | poly(ethylene glycol and allyl glycidyl ether) |
| RT | radiotherapy |
| BSA | bovine serum albumin |
| BSA | glutathione |
| IONR | iron oxide nanorods |
| DSF | disulfiram |
| SCARB1 | scavenger receptor type B-1 |
| PBAE | poly(beta-amino ester) |
| HSVtk | herpes simplex virus type I thymidine kinase |
| BRD4 | bromodomain-containing protein 4 |
| ApoE | apolipoprotein |
| VLDL | very-low-density lipoprotein |
| eHNPs | high-density lipoprotein-mimetic nanoparticles |
| SSEA-1+ | stage-specific embryonic antigen-1 |
| BCC | basal cell carcinoma |
| IB | imipramine blue |
| NOX4 | NADPH oxidase 4 |
| DOX | Doxorubicin |
| PNA | polynitroxylated albumin |
| PLA-HPG | poly(lactic acid) with a grafted hyperbranched polyglycerol |
| PARP | poly(ADP-ribose) polymerase |
| PET | positron emission tomography |
| PDA | polydopamine |
| TMZ | temozolomide |
| siRNA | small interfering RNA |
| HIF-1α | hypoxia-inducible factor 1-alpha |
| NVA622 | N-isopropylacrylamide (NIPAAM), vinylpyrrolidone (VP) and acrylic acid (AA) 60:20:20 |
References
- Thomas, A.; Noël, G. Medulloblastoma: Optimizing Care with a Multidisciplinary Approach. J. Multidiscip. Heal. 2019, 12, 335–347. [Google Scholar] [CrossRef] [PubMed]
- King, A.A.; Seidel, K.; Di, C.; Leisenring, W.M.; Perkins, S.M.; Krull, K.R.; Sklar, C.A.; Green, D.M.; Armstrong, G.T.; Zeltzer, L.K.; et al. Long-Term Neurologic Health and Psychosocial Function of Adult Survivors of Childhood Medulloblastoma/PNET: A Report from the Childhood Cancer Survivor Study. Neuro Oncol. 2017, 19, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Burger, P.; Ellison, D.W.; Reifenberger, G.; von Deimling, A.; Aldape, K.; Brat, D.; Collins, V.P.; Eberhart, C.; et al. International Society of Neuropathology-Haarlem Consensus Guidelines for Nervous System Tumor Classification and Grading. Brain Pathol. 2014, 24, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.D.; Northcott, P.A.; Korshunov, A.; Remke, M.; Cho, Y.-J.; Clifford, S.C.; Eberhart, C.G.; Parsons, D.W.; Rutkowski, S.; Gajjar, A.; et al. Molecular Subgroups of Medulloblastoma: The Current Consensus. Acta Neuropathol. 2012, 123, 465–472. [Google Scholar] [CrossRef]
- Cavalli, F.M.G.; Remke, M.; Rampasek, L.; Peacock, J.; Shih, D.J.H.; Luu, B.; Garzia, L.; Torchia, J.; Nor, C.; Morrissy, A.S.; et al. Intertumoral Heterogeneity within Medulloblastoma Subgroups. Cancer Cell 2017, 31, 737–754.e6. [Google Scholar] [CrossRef]
- Ramaswamy, V.; Remke, M.; Bouffet, E.; Bailey, S.; Clifford, S.C.; Doz, F.; Kool, M.; Dufour, C.; Vassal, G.; Milde, T.; et al. Risk Stratification of Childhood Medulloblastoma in the Molecular Era: The Current Consensus. Acta Neuropathol. 2016, 131, 821–831. [Google Scholar] [CrossRef]
- Hersh, A.M.; Alomari, S.; Tyler, B.M. Crossing the Blood-Brain Barrier: Advances in Nanoparticle Technology for Drug Delivery in Neuro-Oncology. Int. J. Mol. Sci. 2022, 23, 4153. [Google Scholar] [CrossRef]
- Northcott, P.A.; Robinson, G.W.; Kratz, C.P.; Mabbott, D.J.; Pomeroy, S.L.; Clifford, S.C.; Rutkowski, S.; Ellison, D.W.; Malkin, D.; Taylor, M.D.; et al. Medulloblastoma. Nat. Rev. Dis. Primers 2019, 5, 11. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Tischfield, M.; Williams, J.; Smallwood, P.M.; Rattner, A.; Taketo, M.M.; Nathans, J. Canonical WNT Signaling Components in Vascular Development and Barrier Formation. J. Clin. Investig. 2014, 124, 3825–3846. [Google Scholar] [CrossRef]
- Phoenix, T.N.; Patmore, D.M.; Boop, S.; Boulos, N.; Jacus, M.O.; Patel, Y.T.; Roussel, M.F.; Finkelstein, D.; Goumnerova, L.; Perreault, S.; et al. Medulloblastoma Genotype Dictates Blood Brain Barrier Phenotype. Cancer Cell 2016, 29, 508–522. [Google Scholar] [CrossRef]
- Waszak, S.M.; Northcott, P.A.; Buchhalter, I.; Robinson, G.W.; Sutter, C.; Groebner, S.; Grund, K.B.; Brugières, L.; Jones, D.T.W.; Pajtler, K.W.; et al. Spectrum and Prevalence of Genetic Predisposition in Medulloblastoma: A Retrospective Genetic Study and Prospective Validation in a Clinical Trial Cohort. Lancet Oncol. 2018, 19, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Clifford, S.C.; Lusher, M.E.; Lindsey, J.C.; Langdon, J.A.; Gilbertson, R.J.; Straughton, D.; Ellison, D.W. Wnt/Wingless Pathway Activation and Chromosome 6 Loss Characterize a Distinct Molecular Sub-Group of Medulloblastomas Associated with a Favorable Prognosis. Cell Cycle 2006, 5, 2666–2670. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Wang, Y.; Broaddus, R.; Sun, L.; Xue, F.; Zhang, W. Exon 3 Mutations of CTNNB1 Drive Tumorigenesis: A Review. Oncotarget 2017, 9, 5492–5508. [Google Scholar] [CrossRef]
- Juraschka, K.; Taylor, M.D. Medulloblastoma in the Age of Molecular Subgroups: A Review: JNSPG 75th Anniversary Invited Review Article. J. Neurosurg. Pediatr. 2019, 24, 353–363. [Google Scholar] [CrossRef]
- Wu, S.M.; Choo, A.B.H.; Yap, M.G.S.; Chan, K.K.-K. Role of Sonic Hedgehog Signaling and the Expression of Its Components in Human Embryonic Stem Cells. Stem Cell Res. 2010, 4, 38–49. [Google Scholar] [CrossRef]
- Gibson, P.; Tong, Y.; Robinson, G.; Thompson, M.C.; Currle, D.S.; Eden, C.; Kranenburg, T.A.; Hogg, T.; Poppleton, H.; Martin, J.; et al. Subtypes of Medulloblastoma Have Distinct Developmental Origins. Nature 2010, 468, 1095–1099. [Google Scholar] [CrossRef] [PubMed]
- Dahmane, N.; Ruiz i Altaba, A. Sonic Hedgehog Regulates the Growth and Patterning of the Cerebellum. Development 1999, 126, 3089–3100. [Google Scholar] [CrossRef]
- Schüller, U.; Heine, V.M.; Mao, J.; Kho, A.T.; Dillon, A.K.; Han, Y.-G.; Huillard, E.; Sun, T.; Ligon, A.H.; Qian, Y.; et al. Acquisition of Granule Neuron Precursor Identity Is a Critical Determinant of Progenitor Cell Competence to Form Shh-Induced Medulloblastoma. Cancer Cell 2008, 14, 123–134. [Google Scholar] [CrossRef]
- Park, A.K.; Lee, J.Y.; Cheong, H.; Ramaswamy, V.; Park, S.-H.; Kool, M.; Phi, J.H.; Choi, S.A.; Cavalli, F.; Taylor, M.D.; et al. Subgroup-Specific Prognostic Signaling and Metabolic Pathways in Pediatric Medulloblastoma. BMC Cancer 2019, 19, 571. [Google Scholar] [CrossRef]
- Zhukova, N.; Ramaswamy, V.; Remke, M.; Pfaff, E.; Shih, D.J.H.; Martin, D.C.; Castelo-Branco, P.; Baskin, B.; Ray, P.N.; Bouffet, E.; et al. Subgroup-Specific Prognostic Implications of TP53 Mutation in Medulloblastoma. J. Clin. Oncol. 2013, 31, 2927–2935. [Google Scholar] [CrossRef]
- Smith, K.S.; Bihannic, L.; Gudenas, B.L.; Haldipur, P.; Tao, R.; Gao, Q.; Li, Y.; Aldinger, K.A.; Iskusnykh, I.Y.; Chizhikov, V.V.; et al. Unified Rhombic Lip Origins of Group 3 and Group 4 Medulloblastoma. Nature 2022, 609, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Amati, B.; Alevizopoulos, K.; Vlach, J. Myc and the Cell Cycle. Front. Biosci. 1998, 3, d250–d268. [Google Scholar] [CrossRef]
- Dhanasekaran, R.; Deutzmann, A.; Mahauad-Fernandez, W.D.; Hansen, A.S.; Gouw, A.M.; Felsher, D.W. The MYC Oncogene—the Grand Orchestrator of Cancer Growth and Immune Evasion. Nat. Rev. Clin. Oncol. 2022, 19, 23–36. [Google Scholar] [CrossRef]
- Ray, S.; Chaturvedi, N.K.; Bhakat, K.K.; Rizzino, A.; Mahapatra, S. Subgroup-Specific Diagnostic, Prognostic, and Predictive Markers Influencing Pediatric Medulloblastoma Treatment. Diagnostics 2021, 12, 61. [Google Scholar] [CrossRef]
- Colafati, G.S.; Voicu, I.P.; Carducci, C.; Miele, E.; Carai, A.; Di Loreto, S.; Marrazzo, A.; Cacchione, A.; Cecinati, V.; Tornesello, A.; et al. MRI Features as a Helpful Tool to Predict the Molecular Subgroups of Medulloblastoma: State of the Art. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418775375. [Google Scholar] [CrossRef] [PubMed]
- Hendrikse, L.D.; Haldipur, P.; Saulnier, O.; Millman, J.; Sjoboen, A.H.; Erickson, A.W.; Ong, W.; Gordon, V.; Coudière-Morrison, L.; Mercier, A.L.; et al. Failure of Human Rhombic Lip Differentiation Underlies Medulloblastoma Formation. Nature 2022, 609, 1021–1028. [Google Scholar] [CrossRef]
- Northcott, P.A.; Buchhalter, I.; Morrissy, A.S.; Hovestadt, V.; Weischenfeldt, J.; Ehrenberger, T.; Gröbner, S.; Segura-Wang, M.; Zichner, T.; Rudneva, V.A.; et al. The Whole-Genome Landscape of Medulloblastoma Subtypes. Nature 2017, 547, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Cao, Y.; Cao, M.; Wang, Y.; Cao, Y.; Gong, T. Nanomedicine in Cancer Therapy. Signal Transduct. Target. Ther. 2023, 8, 293. [Google Scholar] [CrossRef]
- Guo, Z.-H.; Khattak, S.; Rauf, M.A.; Ansari, M.A.; Alomary, M.N.; Razak, S.; Yang, C.-Y.; Wu, D.-D.; Ji, X.-Y. Role of Nanomedicine-Based Therapeutics in the Treatment of CNS Disorders. Molecules 2023, 28, 1283. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Su, Y.-L.; Hu, S.-H. Functional Nanoparticles for Tumor Penetration of Therapeutics. Pharmaceutics 2018, 10, 193. [Google Scholar] [CrossRef]
- Zhu, D.; Tao, W.; Zhang, H.; Liu, G.; Wang, T.; Zhang, L.; Zeng, X.; Mei, L. Docetaxel (DTX)-Loaded Polydopamine-Modified TPGS-PLA Nanoparticles as a Targeted Drug Delivery System for the Treatment of Liver Cancer. Acta Biomater. 2016, 30, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.A.; Feng, S.-S. Effects of Particle Size and Surface Modification on Cellular Uptake and Biodistribution of Polymeric Nanoparticles for Drug Delivery. Pharm. Res. 2013, 30, 2512–2522. [Google Scholar] [CrossRef]
- Wang, Y.; Li, P.; Kong, L. Chitosan-Modified PLGA Nanoparticles with Versatile Surface for Improved Drug Delivery. AAPS PharmSciTech 2013, 14, 585–592. [Google Scholar] [CrossRef]
- Son, J.; Lee, D.; Yoo, J.; Park, C.; Koo, H. A Comparative Study of the Effect of Drug Hydrophobicity on Nanoparticle Drug Delivery In Vivo Using Two Photosensitizers. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102151. [Google Scholar] [CrossRef] [PubMed]
- Sigot, V.; Arndt-Jovin, D.J.; Jovin, T.M. Targeted Cellular Delivery of Quantum Dots Loaded on and in Biotinylated Liposomes. Bioconjugate Chem. 2010, 21, 1465–1472. [Google Scholar] [CrossRef]
- Maruyama, K.; Takizawa, T.; Yuda, T.; Kennel, S.J.; Huang, L.; Iwatsuru, M. Targetability of Novel Immunoliposomes Modified with Amphipathic Poly(Ethylene Glycol)s Conjugated at Their Distal Terminals to Monoclonal Antibodies. Biochim. Biophys. Acta 1995, 1234, 74–80. [Google Scholar] [CrossRef]
- Tiwari, G.; Tiwari, R.; Sriwastawa, B.; Bhati, L.; Pandey, S.; Pandey, P.; Bannerjee, S.K. Drug Delivery Systems: An Updated Review. Int. J. Pharm. Investig. 2012, 2, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Eker, F.; Akdaşçi, E.; Duman, H.; Bechelany, M.; Karav, S. Gold Nanoparticles in Nanomedicine: Unique Properties and Therapeutic Potential. Nanomaterials 2024, 14, 1854. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The Blood–Brain Barrier: Structure, Regulation and Drug Delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef]
- Kaya, M.; Ahishali, B. Basic Physiology of the Blood-Brain Barrier in Health and Disease: A Brief Overview. Tissue Barriers 2020, 9, 1840913. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Gendelman, H.E. Nanomedicine in the Diagnosis and Therapy of Neurodegenerative Disorders. Prog. Polym. Sci. 2007, 32, 1054–1082. [Google Scholar] [CrossRef]
- Pardridge, W.M. The Blood-Brain Barrier: Bottleneck in Brain Drug Development. NeuroRx 2005, 2, 3–14. [Google Scholar] [CrossRef]
- Hong, C.S.; Ho, W.; Piazza, M.G.; Ray-Chaudhury, A.; Zhuang, Z.; Heiss, J.D. Characterization of the Blood Brain Barrier in Pediatric Central Nervous System Neoplasms. J. Interdiscip. Histopathol. 2016, 4, 29–33. [Google Scholar] [CrossRef]
- Menyhárt, O.; Győrffy, B. Molecular Stratifications, Biomarker Candidates and New Therapeutic Options in Current Medulloblastoma Treatment Approaches. Cancer Metastasis Rev. 2020, 39, 211–233. [Google Scholar] [CrossRef]
- Wohlfart, S.; Gelperina, S.; Kreuter, J. Transport of Drugs across the Blood–Brain Barrier by Nanoparticles. J. Control. Release 2012, 161, 264–273. [Google Scholar] [CrossRef]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR Effect: Unique Features of Tumor Blood Vessels for Drug Delivery, Factors Involved, and Limitations and Augmentation of the Effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Ejigah, V.; Owoseni, O.; Bataille-Backer, P.; Ogundipe, O.D.; Fisusi, F.A.; Adesina, S.K. Approaches to Improve Macromolecule and Nanoparticle Accumulation in the Tumor Microenvironment by the Enhanced Permeability and Retention Effect. Polymers 2022, 14, 2601. [Google Scholar] [CrossRef]
- Perrault, S.D.; Walkey, C.; Jennings, T.; Fischer, H.C.; Chan, W.C.W. Mediating Tumor Targeting Efficiency of Nanoparticles through Design. Nano Lett. 2009, 9, 1909–1915. [Google Scholar] [CrossRef]
- Ikeda-Imafuku, M.; Wang, L.L.-W.; Rodrigues, D.; Shaha, S.; Zhao, Z.; Mitragotri, S. Strategies to Improve the EPR Effect: A Mechanistic Perspective and Clinical Translation. J. Control. Release 2022, 345, 512–536. [Google Scholar] [CrossRef] [PubMed]
- Deivayanai, V.C.; Thamarai, P.; Karishma, S.; Saravanan, A.; Yaashikaa, P.R.; Vickram, A.S.; Hemavathy, R.V.; Kumar, R.R.; Rishikesavan, S.; Shruthi, S. A Comprehensive Review on Advances in Nanoparticle-Mediated Cancer Therapeutics: Current Research and Future Perspectives. Cancer Pathog. Ther. 2024, 2, E01–E16. [Google Scholar] [CrossRef]
- Alexis, F.; Basto, P.; Levy-Nissenbaum, E.; Radovic-Moreno, A.F.; Zhang, L.; Pridgen, E.; Wang, A.Z.; Marein, S.L.; Westerhof, K.; Molnar, L.K.; et al. HER-2-Targeted Nanoparticle–Affibody Bioconjugates for Cancer Therapy. ChemMedChem 2008, 3, 1839–1843. [Google Scholar] [CrossRef]
- Kaluzova, M.; Bouras, A.; Machaidze, R.; Hadjipanayis, C.G. Targeted Therapy of Glioblastoma Stem-like Cells and Tumor Non-Stem Cells Using Cetuximab-Conjugated Iron-Oxide Nanoparticles. Oncotarget 2015, 6, 8788–8806. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhao, Z.; Gao, H.; Rostami, I.; You, Q.; Jia, X.; Wang, C.; Zhu, L.; Yang, Y. Enhanced Blood-Brain-Barrier Penetrability and Tumor-Targeting Efficiency by Peptide-Functionalized Poly(Amidoamine) Dendrimer for the Therapy of Gliomas. Nanotheranostics 2019, 3, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Voth, B.; Nagasawa, D.T.; Pelargos, P.E.; Chung, L.K.; Ung, N.; Gopen, Q.; Tenn, S.; Kamei, D.T.; Yang, I. Transferrin Receptors and Glioblastoma Multiforme: Current Findings and Potential for Treatment. J. Clin. Neurosci. 2015, 22, 1071–1076. [Google Scholar] [CrossRef]
- Li, S.; Amat, D.; Peng, Z.; Vanni, S.; Raskin, S.; Angulo, G.D.; Othman, A.M.; Graham, R.M.; Leblanc, R.M. Transferrin Conjugated Nontoxic Carbon Dots for Doxorubicin Delivery to Target Pediatric Brain Tumor Cells. Nanoscale 2016, 8, 16662–16669. [Google Scholar] [CrossRef]
- Nikanjam, M.; Blakely, E.A.; Bjornstad, K.A.; Shu, X.; Budinger, T.F.; Forte, T.M. Synthetic Nano-Low Density Lipoprotein as Targeted Drug Delivery Vehicle for Glioblastoma Multiforme. Int. J. Pharm. 2007, 328, 86–94. [Google Scholar] [CrossRef]
- Bell, J.B.; Rink, J.S.; Eckerdt, F.; Clymer, J.; Goldman, S.; Thaxton, C.S.; Platanias, L.C. HDL Nanoparticles Targeting Sonic Hedgehog Subtype Medulloblastoma. Sci. Rep. 2018, 8, 1211. [Google Scholar] [CrossRef]
- Yanar, F.; Carugo, D.; Zhang, X. Hybrid Nanoplatforms Comprising Organic Nanocompartments Encapsulating Inorganic Nanoparticles for Enhanced Drug Delivery and Bioimaging Applications. Molecules 2023, 28, 5694. [Google Scholar] [CrossRef]
- Gupta, A.; Wang, S.; Pera, P.; Rao, K.V.R.; Patel, N.; Ohulchanskyy, T.Y.; Missert, J.; Morgan, J.; Koo-Lee, Y.-E.; Kopelman, R.; et al. Multifunctional Nanoplatforms for Fluorescence Imaging and Photodynamic Therapy Developed by Post-Loading Photosensitizer and Fluorophore to Polyacrylamide Nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Saneja, A.; Kumar, R.; Mintoo, M.J.; Dubey, R.D.; Sangwan, P.L.; Mondhe, D.M.; Panda, A.K.; Gupta, P.N. Gemcitabine and Betulinic Acid Co-Encapsulated PLGA−PEG Polymer Nanoparticles for Improved Efficacy of Cancer Chemotherapy. Mater. Sci. Eng. C 2019, 98, 764–771. [Google Scholar] [CrossRef]
- Fukui, N.; Yawata, T.; Nakajo, T.; Kawanishi, Y.; Higashi, Y.; Yamashita, T.; Aratake, T.; Honke, K.; Ueba, T. Targeting CD146 Using Folic Acid-Conjugated Nanoparticles and Suppression of Tumor Growth in a Mouse Glioma Model. J. Neurosurg. 2021, 134, 1772–1782. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Cao, N.; Chen, J.; Yu, X.; Shuai, X. Multifunctional Nanocarrier Mediated Co-Delivery of Doxorubicin and siRNA for Synergistic Enhancement of Glioma Apoptosis in Rat. Biomaterials 2012, 33, 1170–1179. [Google Scholar] [CrossRef]
- Ekladious, I.; Colson, Y.L.; Grinstaff, M.W. Polymer-Drug Conjugate Therapeutics: Advances, Insights and Prospects. Nat. Rev. Drug Discov. 2019, 18, 273–294. [Google Scholar] [CrossRef]
- Kheraldine, H.; Rachid, O.; Habib, A.M.; Al Moustafa, A.-E.; Benter, I.F.; Akhtar, S. Emerging Innate Biological Properties of Nano-Drug Delivery Systems: A Focus on PAMAM Dendrimers and Their Clinical Potential. Adv. Drug Deliv. Rev. 2021, 178, 113908. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Li, J.; Jiang, C.; Hong, B.; Hao, B. Plasmid pORF-hTRAIL Targeting to Glioma Using Transferrin-Modified Polyamidoamine Dendrimer. Drug Des. Dev. Ther. 2016, 10, 1–11. [Google Scholar] [CrossRef]
- Xu, X.; Li, J.; Han, S.; Tao, C.; Fang, L.; Sun, Y.; Zhu, J.; Liang, Z.; Li, F. A Novel Doxorubicin Loaded Folic Acid Conjugated PAMAM Modified with Borneol, a Nature Dual-Functional Product of Reducing PAMAM Toxicity and Boosting BBB Penetration. Eur. J. Pharm. Sci. 2016, 88, 178–190. [Google Scholar] [CrossRef]
- Shah, N.; Steptoe, R.J.; Parekh, H.S. Low-Generation Asymmetric Dendrimers Exhibit Minimal Toxicity and Effectively Complex DNA. J. Pept. Sci. 2011, 17, 470–478. [Google Scholar] [CrossRef]
- Choi, D.Y.; Ha, C.H.; Lee, S.J.; Cheon, S.H.; Seong, G.H. Fucoidan-Chitosan Nanocarriers for Anticancer Therapy through Chemodynamic, Photothermal, and Glucose Starvation Strategies. Colloids Surf. B Biointerfaces 2025, 253, 114726. [Google Scholar] [CrossRef]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric Micelles in Drug Delivery: An Insight of the Techniques for Their Characterization and Assessment in Biorelevant Conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of Liposomes as Drug Delivery System for Therapeutic Applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef]
- Deshpande, P.P.; Biswas, S.; Torchilin, V.P. Current Trends in the Use of Liposomes for Tumor Targeting. Nanomedicine 2013, 8, 1509–1528. [Google Scholar] [CrossRef] [PubMed]
- Juliano, R.L.; Stamp, D. The Effect of Particle Size and Charge on the Clearance Rates of Liposomes and Liposome Encapsulated Drugs. Biochem. Biophys. Res. Commun. 1975, 63, 651–658. [Google Scholar] [CrossRef]
- Werner, J.; Umstätter, F.; Hertlein, T.; Beijer, B.; Kleist, C.; Mühlberg, E.; Zimmermann, S.; Haberkorn, U.; Ohlsen, K.; Fricker, G.; et al. Improved Pharmacokinetics and Enhanced Efficacy of the Vancomycin Derivative FU002 Using a Liposomal Nanocarrier. Nanomed. Nanotechnol. Biol. Med. 2024, 56, 102731. [Google Scholar] [CrossRef]
- Arabi, L.; Badiee, A.; Mosaffa, F.; Jaafari, M.R. Targeting CD44 Expressing Cancer Cells with Anti-CD44 Monoclonal Antibody Improves Cellular Uptake and Antitumor Efficacy of Liposomal Doxorubicin. J. Control. Release 2015, 220, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Vakhshiteh, F.; Khabazian, E.; Atyabi, F.; Ostad, S.N.; Madjd, Z.; Dinarvand, R. Peptide-Conjugated Liposomes for Targeted miR-34a Delivery to Suppress Breast Cancer and Cancer Stem-like Population. J. Drug Deliv. Sci. Technol. 2020, 57, 101687. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.S.; Kim, W.; Lee, J.H.; Jun, B.-H.; Kim, K.-S.; Kim, D.-E. Aptamer-Conjugated Nano-Liposome for Immunogenic Chemotherapy with Reversal of Immunosuppression. J. Control. Release 2022, 348, 893–910. [Google Scholar] [CrossRef]
- Liong, M.; Lu, J.; Kovochich, M.; Xia, T.; Ruehm, S.G.; Nel, A.E.; Tamanoi, F.; Zink, J.I. Multifunctional Inorganic Nanoparticles for Imaging, Targeting, and Drug Delivery. ACS Nano 2008, 2, 889–896. [Google Scholar] [CrossRef]
- Sharifi, S.; Behzadi, S.; Laurent, S.; Forrest, M.L.; Stroeve, P.; Mahmoudi, M. Toxicity of Nanomaterials. Chem. Soc. Rev. 2012, 41, 2323–2343. [Google Scholar] [CrossRef]
- Sela, H.; Cohen, H.; Elia, P.; Zach, R.; Karpas, Z.; Zeiri, Y. Spontaneous Penetration of Gold Nanoparticles through the Blood Brain Barrier (BBB). J. Nanobiotechnol. 2015, 13, 71. [Google Scholar] [CrossRef]
- Foti, A.; Clépoint, B.; Fraix, A.; D’Urso, L.; De Bonis, A.; Satriano, C. A Simple Approach for CTAB-Free and Biofunctionalized Gold Nanorods to Construct Photothermal Active Nanomedicine for Potential in Vivo Applications in Cancer Cells and Scar Treatment. Front. Mater. 2024, 11, 1381176. [Google Scholar] [CrossRef]
- Jensen, S.A.; Day, E.S.; Ko, C.H.; Hurley, L.A.; Luciano, J.P.; Kouri, F.M.; Merkel, T.J.; Luthi, A.J.; Patel, P.C.; Cutler, J.I.; et al. Spherical Nucleic Acid Nanoparticle Conjugates as an RNAi-Based Therapy for Glioblastoma. Sci. Transl. Med. 2013, 5, 209ra152. [Google Scholar] [CrossRef]
- Meola, A.; Rao, J.; Chaudhary, N.; Sharma, M.; Chang, S.D. Gold Nanoparticles for Brain Tumor Imaging: A Systematic Review. Front. Neurol. 2018, 9, 328. [Google Scholar] [CrossRef]
- Foti, A.; Calì, L.; Petralia, S.; Satriano, C. Green Nanoformulations of Polyvinylpyrrolidone-Capped Metal Nanoparticles: A Study at the Hybrid Interface with Biomimetic Cell Membranes and In Vitro Cell Models. Nanomaterials 2023, 13, 1624. [Google Scholar] [CrossRef] [PubMed]
- Salazar-García, S.; García-Rodrigo, J.F.; Martínez-Castañón, G.A.; Ruiz-Rodríguez, V.M.; Portales-Pérez, D.P.; Gonzalez, C. Silver Nanoparticles (AgNPs) and Zinc Chloride (ZnCl2) Exposure Order Determines the Toxicity in C6 Rat Glioma Cells. J. Nanoparticle Res. 2020, 22, 253. [Google Scholar] [CrossRef]
- Israel, L.L.; Galstyan, A.; Holler, E.; Ljubimova, J.Y. Magnetic Iron Oxide Nanoparticles for Imaging, Targeting and Treatment of Primary and Metastatic Tumors of the Brain. J. Control. Release 2020, 320, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, G.; Lorkowski, M.E.; Sims, H.M.; Loutrianakis, G.; Rahmy, A.; Cha, A.; Abenojar, E.; Wickramasinghe, S.; Moon, T.J.; Samia, A.C.S.; et al. Hyperthermia-Mediated Changes in the Tumor Immune Microenvironment Using Iron Oxide Nanoparticles. Nanoscale Adv. 2021, 3, 5890–5899. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayis, C.G.; Machaidze, R.; Kaluzova, M.; Wang, L.; Schuette, A.J.; Chen, H.; Wu, X.; Mao, H. EGFRvIII Antibody-Conjugated Iron Oxide Nanoparticles for Magnetic Resonance Imaging-Guided Convection-Enhanced Delivery and Targeted Therapy of Glioblastoma. Cancer Res. 2010, 70, 6303–6312. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, X.; Niu, Y.; He, K.; Tang, M. Application of Quantum Dots in Brain Diseases and Their Neurotoxic Mechanism. Nanoscale Adv. 2024, 6, 3733–3746. [Google Scholar] [CrossRef]
- Manshian, B.B.; Jiménez, J.; Himmelreich, U.; Soenen, S.J. Personalized Medicine and Follow-up of Therapeutic Delivery through Exploitation of Quantum Dot Toxicity. Biomaterials 2017, 127, 1–12. [Google Scholar] [CrossRef]
- Wang, X.; Song, B.; Wu, M.; Qin, L.; Liang, W. Immune Cell Targeting-Mediated Cytomimetic Drug Delivery System for BBB-Penetrating and Precise Therapy of in Situ Glioma. Mater. Today Bio 2025, 32, 101694. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.; Brayden, D.J.; Cheung, D.L.; Liew, A.; Fitzgerald, M.; Pandit, A. Albumin-Based Delivery Systems: Recent Advances, Challenges, and Opportunities. J. Control. Release 2025, 380, 375–395. [Google Scholar] [CrossRef] [PubMed]
- Ruan, C.; Liu, L.; Lu, Y.; Zhang, Y.; He, X.; Chen, X.; Zhang, Y.; Chen, Q.; Guo, Q.; Sun, T.; et al. Substance P-Modified Human Serum Albumin Nanoparticles Loaded with Paclitaxel for Targeted Therapy of Glioma. Acta Pharm. Sin. B 2018, 8, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, X.; Feng, K.; Zeng, W.; Wu, J.; Sun, D.; Lu, Z.; Feng, H.; Di, L. Nanotechnologies Meeting Natural Sources: Engineered Lipoproteins for Precise Brain Disease Theranostics. Asian J. Pharm. Sci. 2023, 18, 100857. [Google Scholar] [CrossRef]
- Wang, R.; Wang, X.; Li, J.; Di, L.; Zhou, J.; Ding, Y. Lipoprotein-Biomimetic Nanostructure Enables Tumor-Targeted Penetration Delivery for Enhanced Photo-Gene Therapy towards Glioma. Bioact. Mater. 2022, 13, 286–299. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Parker, T.L.; Kallinteri, P.; Walker, D.A.; Higgins, S.; Hutcheon, G.A.; Garnett, M.C. Uptake and Metabolism of Novel Biodegradable Poly (Glycerol-Adipate) Nanoparticles in DAOY Monolayer. J. Control. Release 2006, 116, 314–321. [Google Scholar] [CrossRef]
- Lim, K.J.; Bisht, S.; Bar, E.E.; Maitra, A.; Eberhart, C.G. A Polymeric Nanoparticle Formulation of Curcumin Inhibits Growth, Clonogenicity and Stem-like Fraction in Malignant Brain Tumors. Cancer Biol. Ther. 2011, 11, 464–473. [Google Scholar] [CrossRef]
- Svalina, M.N.; Kikuchi, K.; Abraham, J.; Lal, S.; Davare, M.A.; Settelmeyer, T.P.; Young, M.C.; Peckham, J.L.; Cho, Y.-J.; Michalek, J.E.; et al. IGF1R as a Key Target in High Risk, Metastatic Medulloblastoma. Sci. Rep. 2016, 6, 27012. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, H.; Liu, J.; Malhotra, A.; Dey, A.; Yu, B.; Jella, K.K.; McSwain, L.F.; Schniederjan, M.J.; MacDonald, T.J. STAT3 Is Required for Smo-Dependent Signaling and Mediates Smo-Targeted Treatment Resistance and Tumorigenesis in Shh Medulloblastoma. Mol. Oncol. 2022, 16, 1009–1025. [Google Scholar] [CrossRef]
- Lukoseviciute, M.; Need, E.; Birgersson, M.; Dalianis, T.; Kostopoulou, O.N. Enhancing Targeted Therapy by Combining PI3K and AKT Inhibitors with or without Cisplatin or Vincristine in Medulloblastoma Cell Lines in Vitro. Biomed. Pharmacother. 2024, 180, 117500. [Google Scholar] [CrossRef] [PubMed]
- Chenna, V.; Hu, C.; Pramanik, D.; Aftab, B.T.; Karikari, C.; Campbell, N.R.; Hong, S.-M.; Zhao, M.; Rudek, M.A.; Khan, S.R.; et al. A Polymeric Nanoparticle Encapsulated Small Molecule Inhibitor of Hedgehog Signaling (NanoHHI) Bypasses Secondary Mutational Resistance to Smoothened Antagonists. Mol. Cancer Ther. 2012, 11, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Madala, H.R.; Punganuru, S.R.; Ali-Osman, F.; Zhang, R.; Srivenugopal, K.S. Brain- and Brain Tumor-Penetrating Disulfiram Nanoparticles: Sequence of Cytotoxic Events and Efficacy in Human Glioma Cell Lines and Intracranial Xenografts. Oncotarget 2017, 9, 3459. [Google Scholar] [CrossRef]
- Choi, J.; Rui, Y.; Kim, J.; Gorelick, N.; Wilson, D.R.; Kozielski, K.; Mangraviti, A.; Sankey, E.; Brem, H.; Tyler, B.; et al. Nonviral Polymeric Nanoparticles for Gene Therapy in Pediatric CNS Malignancies. Nanomedicine 2020, 23, 102115. [Google Scholar] [CrossRef]
- Hwang, D.; Dismuke, T.; Tikunov, A.; Rosen, E.P.; Kagel, J.R.; Ramsey, J.D.; Lim, C.; Zamboni, W.; Kabanov, A.V.; Gershon, T.R.; et al. Poly(2-Oxazoline) Nanoparticle Delivery Enhances the Therapeutic Potential of Vismodegib for Medulloblastoma by Improving CNS Pharmacokinetics and Reducing Systemic Toxicity. Nanomedicine 2021, 32, 102345. [Google Scholar] [CrossRef]
- Lim, C.; Dismuke, T.; Malawsky, D.; Ramsey, J.D.; Hwang, D.; Godfrey, V.L.; Kabanov, A.V.; Gershon, T.R.; Sokolsky-Papkov, M. Enhancing CDK4/6 Inhibitor Therapy for Medulloblastoma Using Nanoparticle Delivery and scRNA-Seq–Guided Combination with Sapanisertib. Sci. Adv. 2022, 8, eabl5838. [Google Scholar] [CrossRef]
- Khang, M.; Lee, J.H.; Lee, T.; Suh, H.-W.; Lee, S.; Cavaliere, A.; Rushing, A.; Geraldo, L.H.; Belitzky, E.; Rossano, S.; et al. Intrathecal Delivery of Nanoparticle PARP Inhibitor to the Cerebrospinal Fluid for the Treatment of Metastatic Medulloblastoma. Sci. Transl. Med. 2023, 15, eadi1617. [Google Scholar] [CrossRef]
- Veiseh, O.; Kievit, F.; Gunn, J.; Ratner, B.; Zhang, M. A Ligand-Mediated Nanovector for Targeted Gene Delivery and Transfection in Cancer Cells. Biomaterials 2009, 30, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Kokil, G.R.; Veedu, R.N.; Le, B.T.; Ramm, G.A.; Parekh, H.S. Self-Assembling Asymmetric Peptide-Dendrimer Micelles—a Platform for Effective and Versatile in Vitro Nucleic Acid Delivery. Sci. Rep. 2018, 8, 4832. [Google Scholar] [CrossRef]
- Tjandra, K.C.; Forest, C.R.; Wong, C.K.; Alcantara, S.; Kelly, H.G.; Ju, Y.; Stenzel, M.H.; McCarroll, J.A.; Kavallaris, M.; Caruso, F.; et al. Modulating the Selectivity and Stealth Properties of Ellipsoidal Polymersomes through a Multivalent Peptide Ligand Display. Adv. Heal. Mater. 2020, 9, e2000261. [Google Scholar] [CrossRef]
- Wang, Q.; Kumar, V.; Lin, F.; Sethi, B.; Coulter, D.W.; McGuire, T.R.; Mahato, R.I. ApoE Mimetic Peptide Targeted Nanoparticles Carrying a BRD4 Inhibitor for Treating Medulloblastoma in Mice. J. Control. Release 2020, 323, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Wang, Q.; Sethi, B.; Lin, F.; Kumar, V.; Coulter, D.W.; Dong, Y.; Mahato, R.I. Polymeric Nanomedicine for Overcoming Resistance Mechanisms in Hedgehog and Myc-Amplified Medulloblastoma. Biomaterials 2021, 278, 121138. [Google Scholar] [CrossRef] [PubMed]
- Tylawsky, D.E.; Kiguchi, H.; Vaynshteyn, J.; Gerwin, J.; Shah, J.; Islam, T.; Boyer, J.A.; Boué, D.R.; Snuderl, M.; Greenblatt, M.B.; et al. P-Selectin-Targeted Nanocarriers Induce Active Crossing of the Blood-Brain Barrier via Caveolin-1-Dependent Transcytosis. Nat. Mater. 2023, 22, 391–399. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, T.J.; Liu, J.; Yu, B.; Malhotra, A.; Munson, J.; Park, J.C.; Wang, K.; Fei, B.; Bellamkonda, R.; Arbiser, J. Liposome-Imipramine Blue Inhibits Sonic Hedgehog Medulloblastoma In Vivo. Cancers 2021, 13, 1220. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Lee, H.; Fang, Z.; Velalopoulou, A.; Kim, J.; Thomas, M.B.; Liu, J.; Abramowitz, R.G.; Kim, Y.; Coskun, A.F.; et al. Single-Cell Analysis Reveals Effective siRNA Delivery in Brain Tumors with Microbubble-Enhanced Ultrasound and Cationic Nanoparticles. Sci. Adv. 2021, 7, eabf7390. [Google Scholar] [CrossRef]
- Kievit, F.M.; Stephen, Z.R.; Wang, K.; Dayringer, C.J.; Sham, J.G.; Ellenbogen, R.G.; Silber, J.R.; Zhang, M. Nanoparticle Mediated Silencing of DNA Repair Sensitizes Pediatric Brain Tumor Cells to γ-irradiation. Mol. Oncol. 2015, 9, 1071–1080. [Google Scholar] [CrossRef]
- Liu, Z.; Yan, H.; Li, H. Silencing of DNA Repair Sensitizes Pediatric Brain Tumor Cells to γ-Irradiation Using Gold Nanoparticles. Environ. Toxicol. Pharmacol. 2017, 53, 40–45. [Google Scholar] [CrossRef]
- Varzi, V.; Fratini, E.; Falconieri, M.; Giovannini, D.; Cemmi, A.; Scifo, J.; Di Sarcina, I.; Aprà, P.; Sturari, S.; Mino, L.; et al. Nanodiamond Effects on Cancer Cell Radiosensitivity: The Interplay between Their Chemical/Physical Characteristics and the Irradiation Energy. Int. J. Mol. Sci. 2023, 24, 16622. [Google Scholar] [CrossRef]
- Engelhard, H.H.; Willis, A.J.; Hussain, S.I.; Papavasiliou, G.; Banner, D.J.; Kwasnicki, A.; Lakka, S.S.; Hwang, S.; Shokuhfar, T.; Morris, S.C.; et al. Etoposide-Bound Magnetic Nanoparticles Designed for Remote Targeting of Cancer Cells Disseminated Within Cerebrospinal Fluid Pathways. Front. Neurol. 2020, 11, 596632. [Google Scholar] [CrossRef]
- Jain, A.; Wade, P.; Stolnik, S.; Hume, A.N.; Kerr, I.D.; Coyle, B.; Rawson, F. Tackling Anticancer Drug Resistance and Endosomal Escape in Aggressive Brain Tumors Using Bioelectronics. ACS Omega 2024, 9, 42923–42931. [Google Scholar] [CrossRef]
- Bernardi, R.J.; Lowery, A.R.; Thompson, P.A.; Blaney, S.M.; West, J.L. Immunonanoshells for Targeted Photothermal Ablation in Medulloblastoma and Glioma: An In Vitro Evaluation Using Human Cell Lines. J. Neurooncol. 2008, 86, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Knight, L.C.; Romano, J.E.; Krynska, B.; Faro, S.; Mohamed, F.B.; Gordon, J. Binding and Internalization of Iron Oxide Nanoparticles Targeted to Nuclear Oncoprotein. J. Mol. Biomark. Diagn. 2010, 1, 10000102. [Google Scholar] [CrossRef]
- Dudu, V.; Rotari, V.; Vazquez, M. Targeted Extracellular Nanoparticles Enable Intracellular Detection of Activated Epidermal Growth Factor Receptor in Living Brain Cancer Cells. Nanomedicine 2011, 7, 896–903. [Google Scholar] [CrossRef]
- Dudu, V.; Rotari, V.; Vazquez, M. Sendai Virus-Based Liposomes Enable Targeted Cytosolic Delivery of Nanoparticles in Brain Tumor-Derived Cells. J. Nanobiotechnol. 2012, 10, 9. [Google Scholar] [CrossRef]
- Li, Y.; Lin, R.; Wang, L.; Huang, J.; Wu, H.; Cheng, G.; Zhou, Z.; MacDonald, T.; Yang, L.; Mao, H. PEG-b-AGE Polymer Coated Magnetic Nanoparticle Probes with Facile Functionalization and Anti-Fouling Properties for Reducing Non-Specific Uptake and Improving Biomarker Targeting. J. Mater. Chem. B 2015, 3, 3591–3603. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.B.; Klungland, A.; Rognes, T.; Leiros, I. DNA Repair in Mammalian Cells: Base Excision Repair: The Long and Short of It. Cell Mol. Life Sci. 2009, 66, 981–993. [Google Scholar] [CrossRef]
- Uribe, M.L.; Marrocco, I.; Yarden, Y. EGFR in Cancer: Signaling Mechanisms, Drugs, and Acquired Resistance. Cancers 2021, 13, 2748. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Li, Y.; MacDonald, T.; Wu, H.; Provenzale, J.; Peng, X.; Huang, J.; Wang, L.; Wang, A.Y.; Yang, J.; et al. Improving Sensitivity and Specificity of Capturing and Detecting Targeted Cancer Cells with Anti-Biofouling Polymer Coated Magnetic Iron Oxide Nanoparticles. Colloids Surf. B Biointerfaces 2017, 150, 261–270. [Google Scholar] [CrossRef]
- Thamizhchelvan, A.M.; Ma, H.; Wu, T.; Nguyen, D.; Padelford, J.; Whitworth, T.J.; Li, Y.; Yang, L.; Mao, H. Shape-Dependent Cellular Uptake of Iron Oxide Nanorods: Mechanisms of Endocytosis and Implications on Cell Labeling and Cellular Delivery. Nanoscale 2024, 16, 21398–21415. [Google Scholar] [CrossRef]
- Orza, A.; Wu, H.; Xu, Y.; Lu, Q.; Mao, H. One-Step Facile Synthesis of Highly Magnetic and Surface Functionalized Iron Oxide Nanorods for Biomarker-Targeted Applications. ACS Appl. Mater. Interfaces 2017, 9, 20719–20727. [Google Scholar] [CrossRef]
- Xie, X.; Liao, J.; Shao, X.; Li, Q.; Lin, Y. The Effect of Shape on Cellular Uptake of Gold Nanoparticles in the Forms of Stars, Rods, and Triangles. Sci. Rep. 2017, 7, 3827. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wu, H.; Xiong, Q.; Ji, B.; Yi, H.; Duan, H.; Mao, H. Size-Controllable Magnetic Iron Oxide Nanorods for Biomarker Targeting and Improving Microfluidic Mixing. ACS Appl. Bio Mater. 2019, 2, 3362–3371. [Google Scholar] [CrossRef] [PubMed]
- Forgham, H.; Zhu, J.; Huang, X.; Zhang, C.; Biggs, H.; Liu, L.; Wang, Y.C.; Fletcher, N.; Humphries, J.; Cowin, G.; et al. Multifunctional Fluoropolymer-Engineered Magnetic Nanoparticles to Facilitate Blood-Brain Barrier Penetration and Effective Gene Silencing in Medulloblastoma. Adv. Sci. 2024, 11, 2401340. [Google Scholar] [CrossRef]
- Catanzaro, G.; Curcio, M.; Cirillo, G.; Spizzirri, U.G.; Besharat, Z.M.; Abballe, L.; Vacca, A.; Iemma, F.; Picci, N.; Ferretti, E. Albumin Nanoparticles for Glutathione-Responsive Release of Cisplatin: New Opportunities for Medulloblastoma. Int. J. Pharm. 2017, 517, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Soltys, B.J.; Grausam, K.B.; Messerli, S.M.; Hsia, C.J.C.; Zhao, H. Inhibition of Metastatic Brain Cancer in Sonic Hedgehog Medulloblastoma Using Caged Nitric Oxide Albumin Nanoparticles. Front. Oncol. 2023, 13, 1129533. [Google Scholar] [CrossRef]
- Hentschel, A.; Zahedi, R.P.; Ahrends, R. Protein Lipid Modifications—More than Just a Greasy Ballast. Proteomics 2016, 16, 759–782. [Google Scholar] [CrossRef]
- Xiao, X.; Tang, J.-J.; Peng, C.; Wang, Y.; Fu, L.; Qiu, Z.-P.; Xiong, Y.; Yang, L.-F.; Cui, H.-W.; He, X.-L.; et al. Cholesterol Modification of Smoothened Is Required for Hedgehog Signaling. Mol. Cell 2017, 66, 154–162.e10. [Google Scholar] [CrossRef]
- Kim, J.; Dey, A.; Malhotra, A.; Liu, J.; Ahn, S.I.; Sei, Y.J.; Kenney, A.M.; MacDonald, T.J.; Kim, Y. Engineered Biomimetic Nanoparticle for Dual Targeting of the Cancer Stem-like Cell Population in Sonic Hedgehog Medulloblastoma. Proc. Natl. Acad. Sci. USA 2020, 117, 24205–24212. [Google Scholar] [CrossRef]
- Lico, C.; Tanno, B.; Marchetti, L.; Novelli, F.; Giardullo, P.; Arcangeli, C.; Pazzaglia, S.; Podda, M.S.; Santi, L.; Bernini, R.; et al. Tomato Bushy Stunt Virus Nanoparticles as a Platform for Drug Delivery to Shh-Dependent Medulloblastoma. Int. J. Mol. Sci. 2021, 22, 10523. [Google Scholar] [CrossRef]
- Marchetti, L.; Novelli, F.; Tanno, B.; Leonardi, S.; Hizam, V.M.; Arcangeli, C.; Santi, L.; Baschieri, S.; Lico, C.; Mancuso, M. Peptide-Functionalized and Drug-Loaded Tomato Bushy Stunt Virus Nanoparticles Counteract Tumor Growth in a Mouse Model of Shh-Dependent Medulloblastoma. Int. J. Mol. Sci. 2023, 24, 8911. [Google Scholar] [CrossRef]

| Targeting Mechanism | Year | Polymeric NPs | Tested Models | Application/Results | Ref. |
|---|---|---|---|---|---|
| Passive | 2006 | Spherical PGA labeled with Rhodamine B Isothiocyanate | In vitro DAOY cells (SHH) | Drug delivery/ NPs retention and metabolism | [97] |
| 2011 | Synthetic polymer “NVA622” loaded with curcumin | In vitro DAOY (SHH), D283-MED (G3/G4) | Drug delivery/ Cell cycle arrest and apoptosis | [98] | |
| 2012 | PLGA conjugated with PEG encapsulating HPI-1 | In vivo MB mouse model | Drug delivery/ Inhibition of allograft growth | [102] | |
| 2017 | Monomethoxy PEG and PLGA loaded with disulfiram | In vitro DAOY cells (SHH) In vivo MB mouse xenografts | Drug delivery/ BBB crossing and sustained drug supply by EPR | [103] | |
| 2020 | PBAE loaded with a suicide gene (herpes simplex virus type I thymidine kinase) | In vitro D425 cells (G3) In vivo MB mouse xenografts | Gene therapy/ Cell apoptosis and greater median overall survival in mice | [104] | |
| 2021 | poly(2-oxazoline) micelles to deliver vismodegib | In vivo transgenic mice that develop SHH-driven MB (G-Smo MB mice) | Drug delivery/ Extended overall survival and reduced systemic drug toxicity | [105] | |
| 2022 | poly(2-oxazoline) micelles to deliver palbociclib and sapanisertib | In vivo transgenic mice that develop SHH-driven MB (G-Smo MB mice) | Drug delivery and combinational therapy/ Extended mouse survival | [106] | |
| 2023 | PLA-HPG NPs dye-conjugated encapsulating talazoparib | In vivo xenograft MB mouse models | Drug delivery and bioimaging/ Metastasis treatment PET | [107] | |
| Active | 2009 | PEI functionalized with PEG, Alexa Fluor 647 and chlorotoxin (CTX) to target MMP-2 (P-PEG-AF-CTX) | In vitro DAOY cells (SHH) | Gene delivery system for a broad array of cancer types | [108] |
| 2018 | Lipid-conjugated peptide dendrimers (biopolymer, amino acids) | In vitro DAOY cells (SHH) | Delivery systems/ Effective improvement of the nucleic acid cargo and internalization | [109] | |
| 2020 | PEG-PBC loaded with JQ1 decorated with COG-133 peptide to target LDL receptor | In vitro DAOY (SHH), HD-MB03 (G3) In vivo orthotopic MB tumor in mice | Drug delivery/ Improved anticancer efficiency, inhibited MB progression | [111] | |
| 2021 | PEG-b-PCC-g-DC loaded with MDB5 and SF2523 (alone or in combination) decorated with COG-133 peptide to target LDL receptor | In vitro DAOY (SHH), HD-MB03 (G3) In vivo orthotopic MB tumor in mice | Drug delivery/ Hh pathway impairment and significant antitumor efficacy in chemoresistant MB | [112] | |
| 2023 | Fucoidan-based NPs (biopolymer polysaccharides) targeting endothelial P-selectin and encapsulating vismodegib | In vivo genetically engineered mouse SHH-MB model | Drug delivery and BBB targeting/ BBB crossing/ Hh pathway impairment/ Extended overall survival and reduced systemic drug toxicity | [113] |
| Targeting Mechanism | Year | Lipid NPs | Tested Models | Application/Results | Ref. |
|---|---|---|---|---|---|
| Passive | 2021 | Liposome encapsulating Imipramine blue (Lipo-IB) | In vitro DAOY cells (SHH subgroup) In vivo SmoA1 transgenic mice (SHH) | Drug delivery/ Induction of cell necrosis and prolonged survival in mice. | [114] |
| 2021 | Fluorescently labeled LPH delivering SMO-siRNA combined with MB-FUS | In vivo SmoA1 transgenic mice (SHH) | Gene therapy and combined strategy/ Enhanced siRNA delivery and efficiency (LPH) and increased BBB permeability (MB-FUS) | [115] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foti, A.; Allia, F.; Briglia, M.; Malaguarnera, R.; Tamburrini, G.; Cecconi, F.; Pagliarini, V.; Nazio, F.; Graziano, A.C.E. Medulloblastoma: Molecular Targets and Innovative Theranostic Approaches. Pharmaceutics 2025, 17, 736. https://doi.org/10.3390/pharmaceutics17060736
Foti A, Allia F, Briglia M, Malaguarnera R, Tamburrini G, Cecconi F, Pagliarini V, Nazio F, Graziano ACE. Medulloblastoma: Molecular Targets and Innovative Theranostic Approaches. Pharmaceutics. 2025; 17(6):736. https://doi.org/10.3390/pharmaceutics17060736
Chicago/Turabian StyleFoti, Alice, Fabio Allia, Marilena Briglia, Roberta Malaguarnera, Gianpiero Tamburrini, Francesco Cecconi, Vittoria Pagliarini, Francesca Nazio, and Adriana Carol Eleonora Graziano. 2025. "Medulloblastoma: Molecular Targets and Innovative Theranostic Approaches" Pharmaceutics 17, no. 6: 736. https://doi.org/10.3390/pharmaceutics17060736
APA StyleFoti, A., Allia, F., Briglia, M., Malaguarnera, R., Tamburrini, G., Cecconi, F., Pagliarini, V., Nazio, F., & Graziano, A. C. E. (2025). Medulloblastoma: Molecular Targets and Innovative Theranostic Approaches. Pharmaceutics, 17(6), 736. https://doi.org/10.3390/pharmaceutics17060736










