Combined Effects of Atorvastatin and Glucose Deprivation on Metabolic Stress and Lipid-Raft Disruption in Glioblastoma and Breast Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Cell Culture and Morphological Observations
2.3. Cell Viability
2.4. Apoptosis
2.5. Cell Cycle
2.6. RT-qPCR
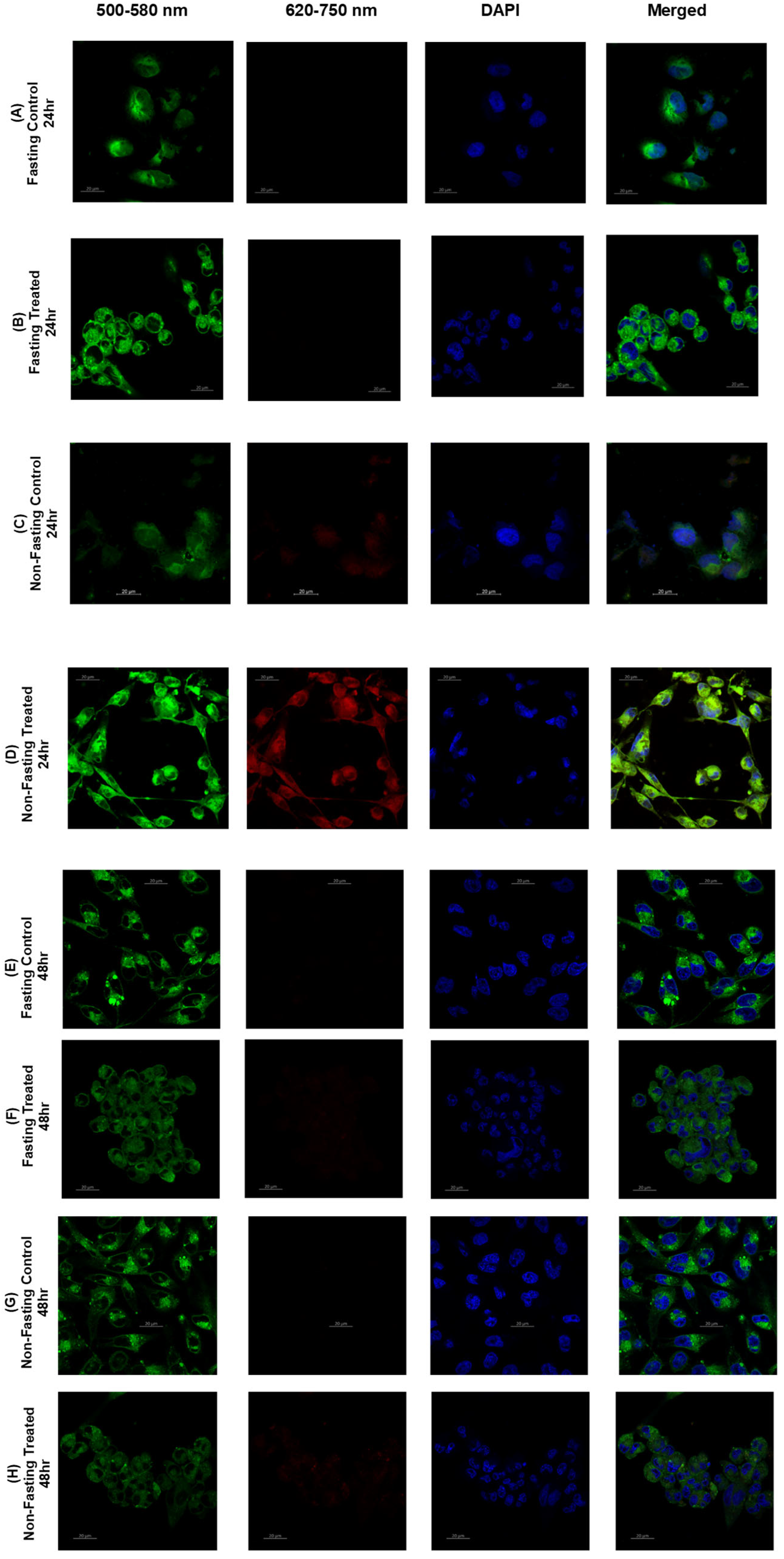
2.7. Lipid Raft: Confocal Imaging and Analysis
2.8. Statistical Analysis
3. Results and Discussion
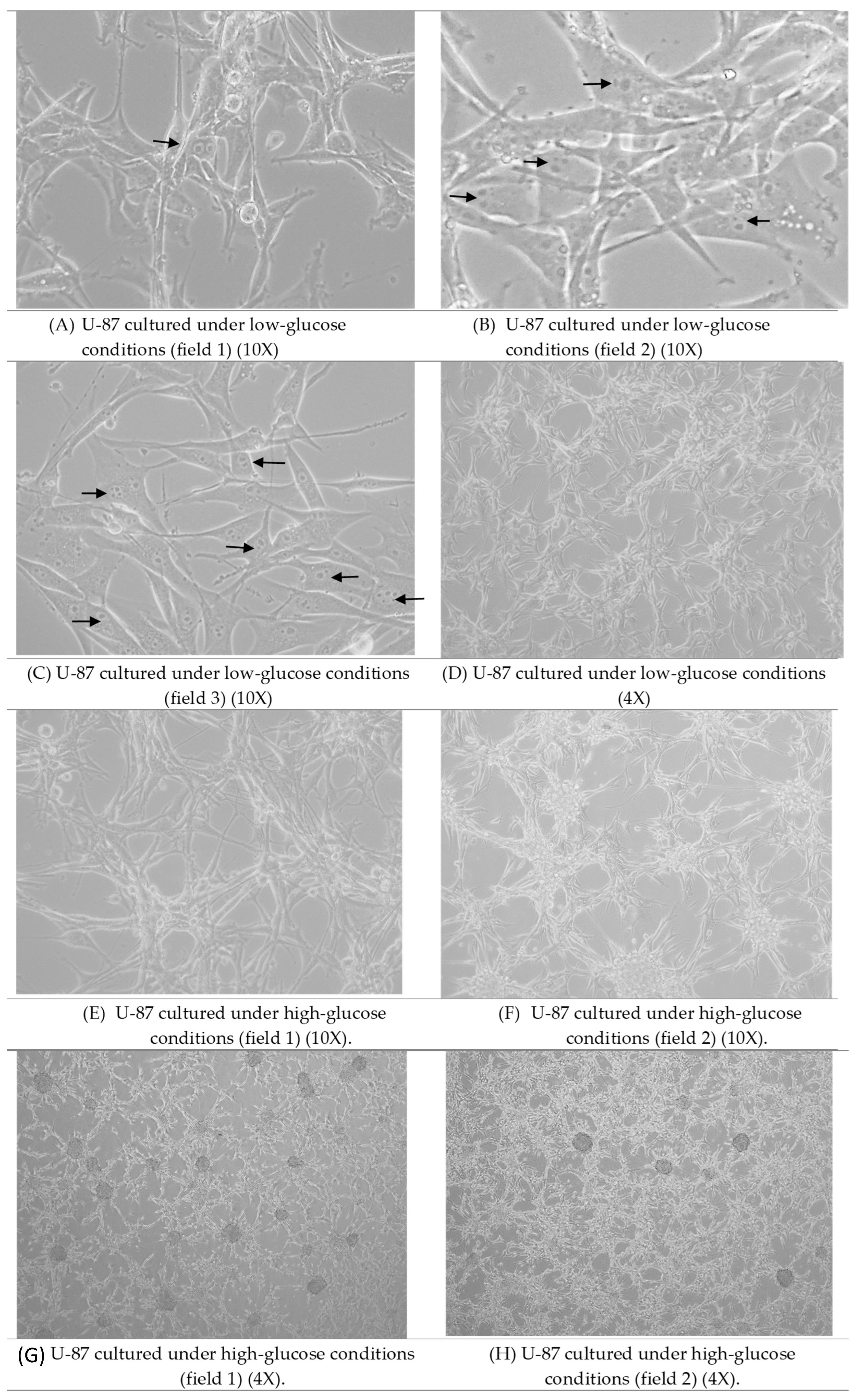
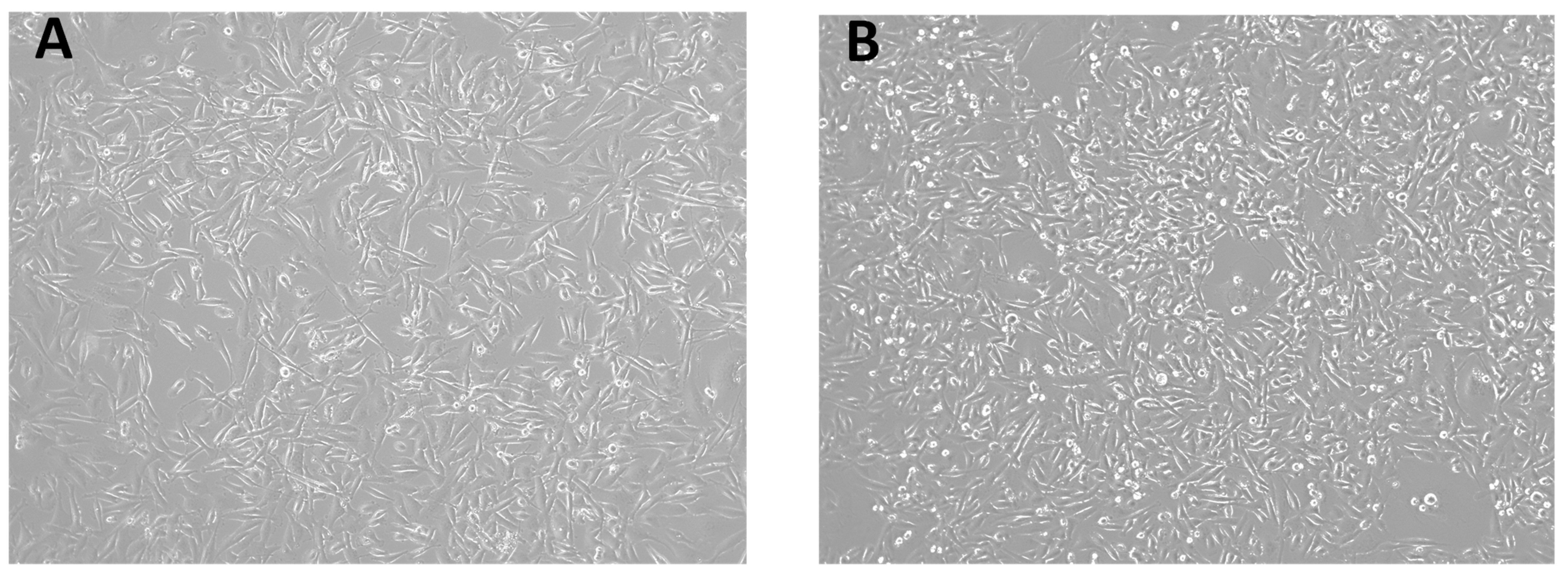
3.1. Morphological Observations
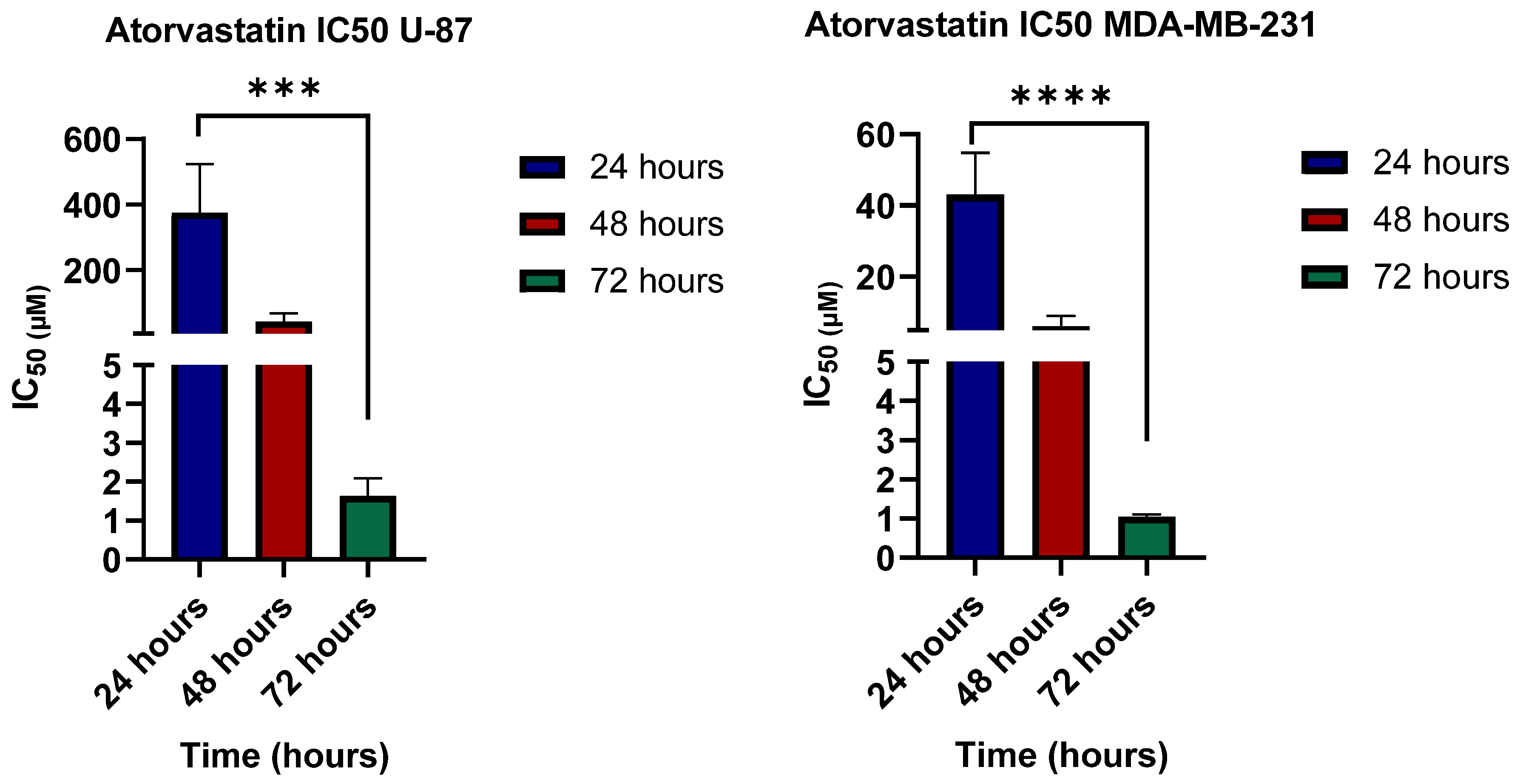
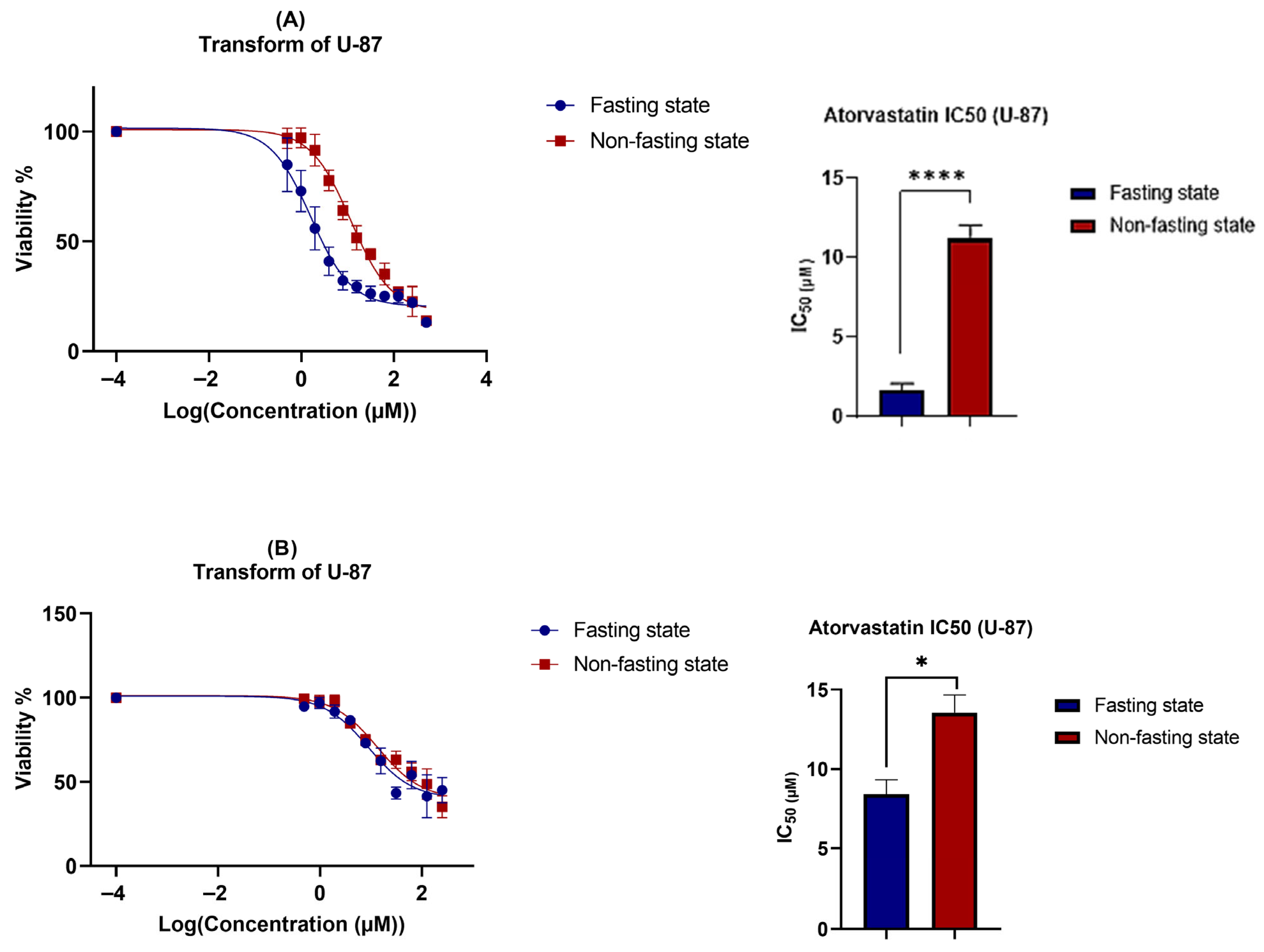
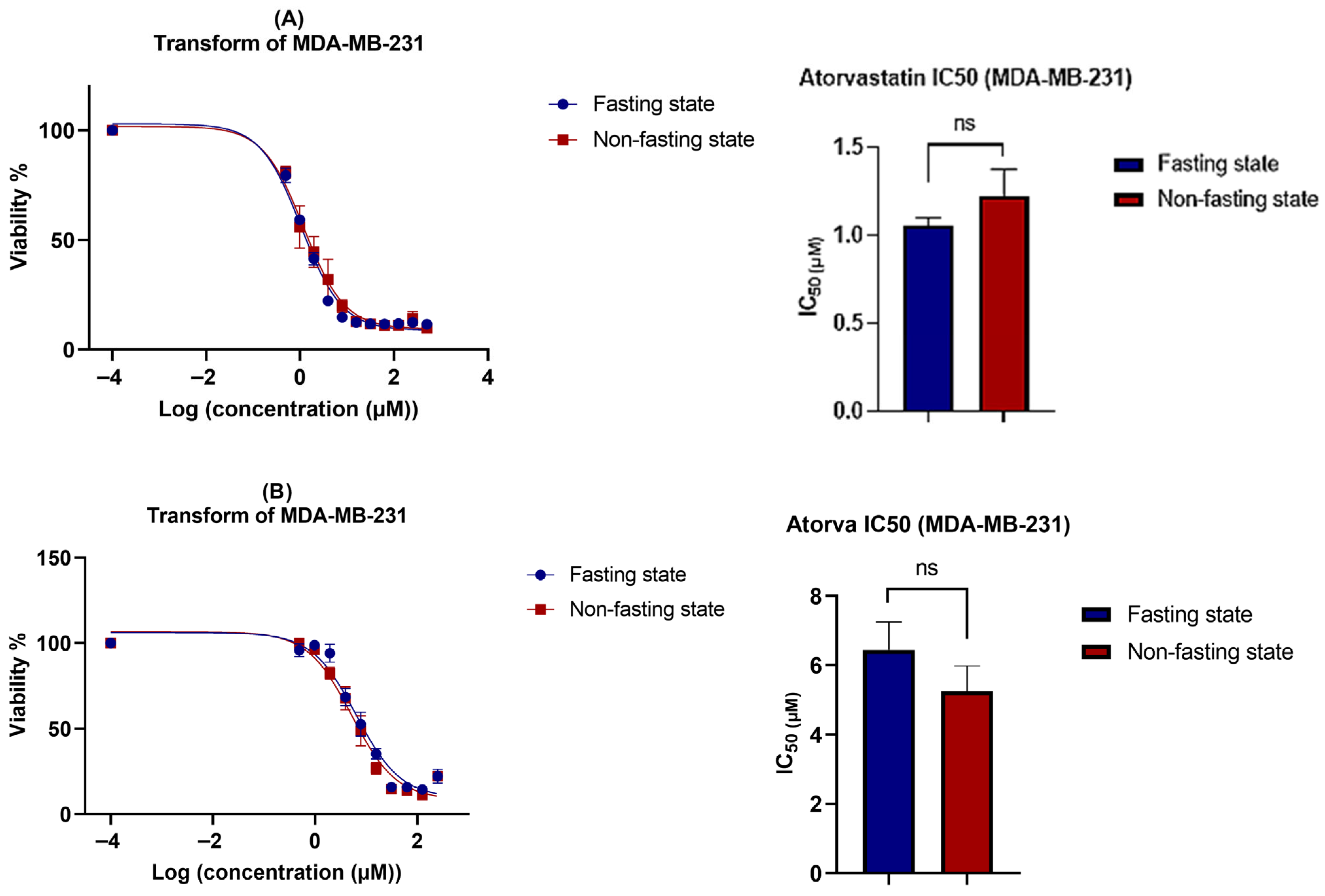
3.2. Cell Viability
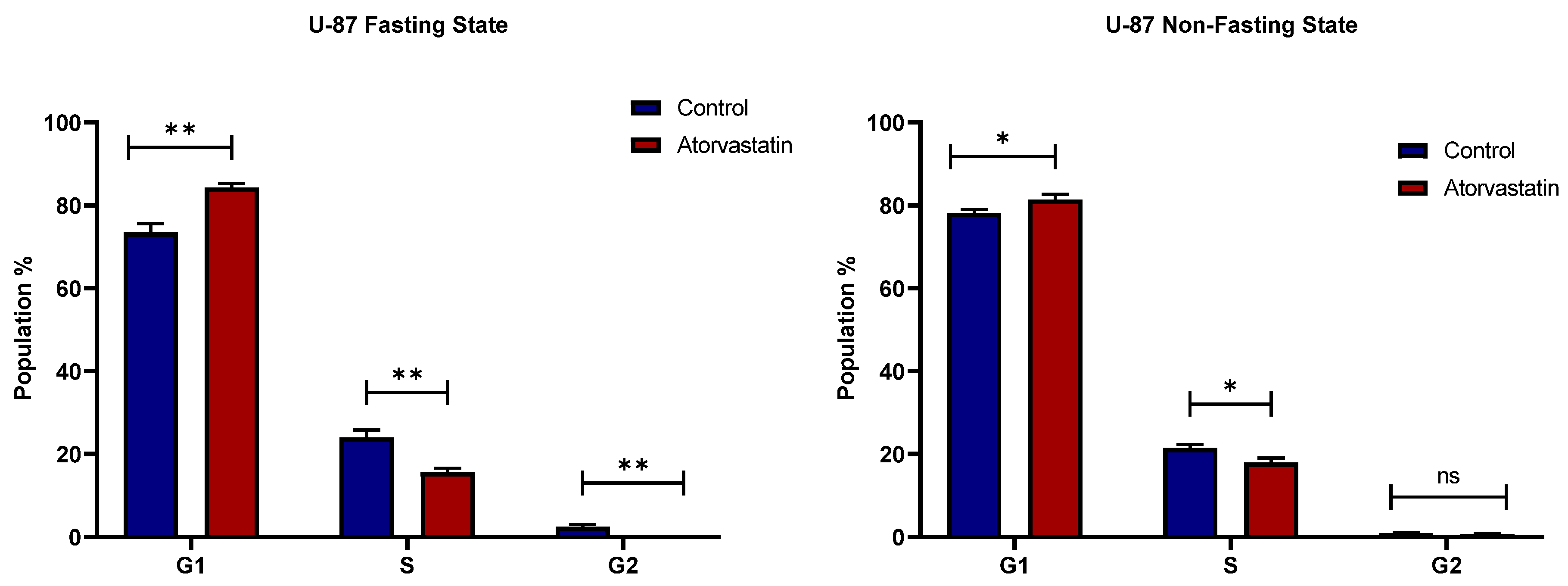
3.3. Cell Cycle
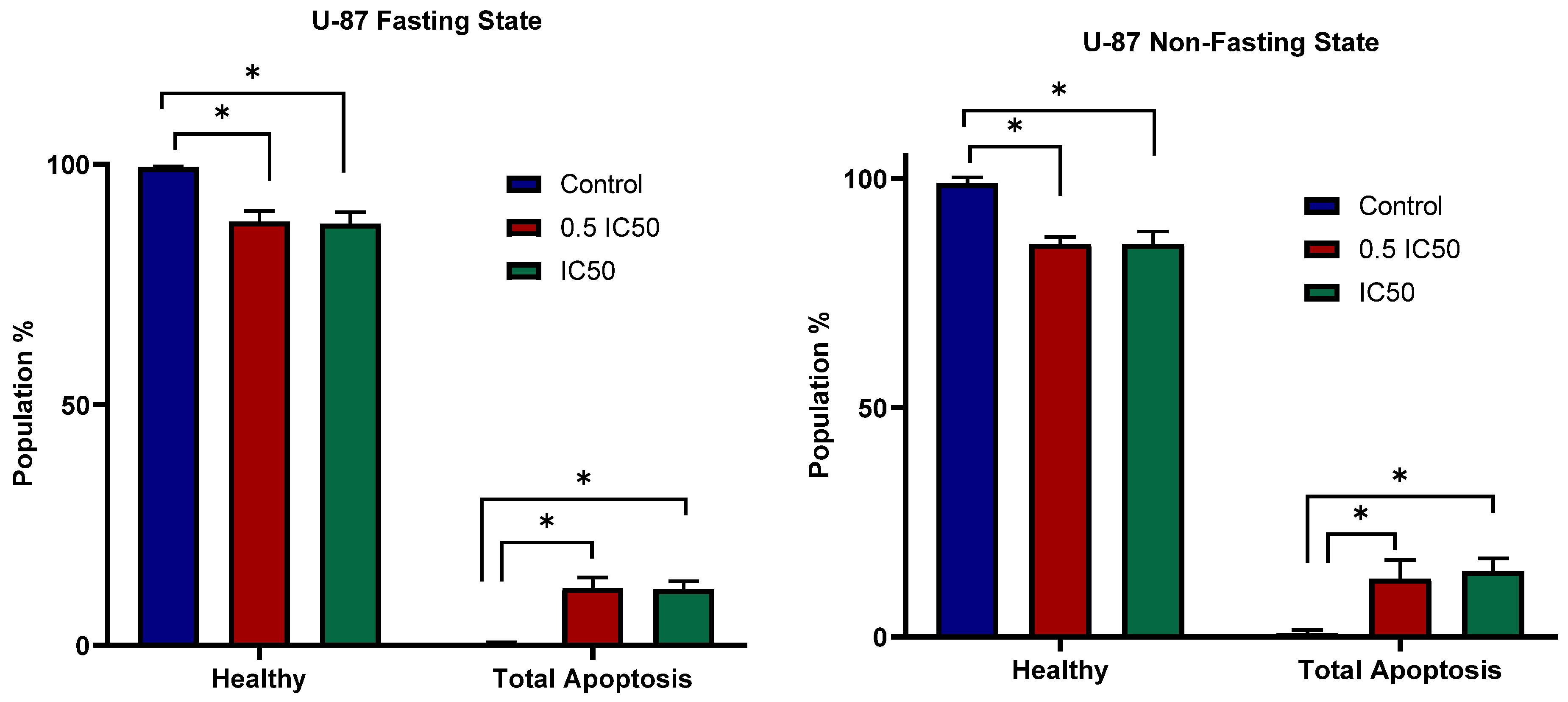
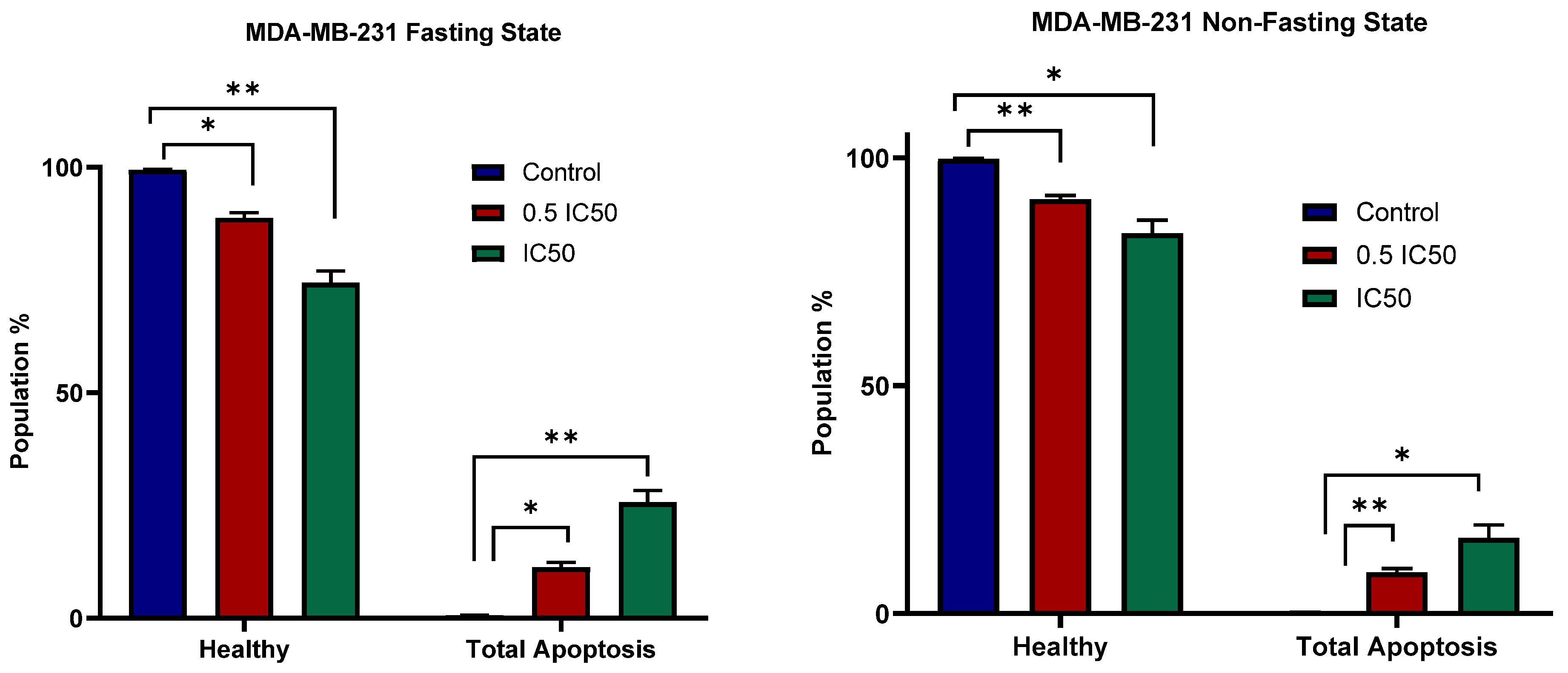
3.4. Apoptosis
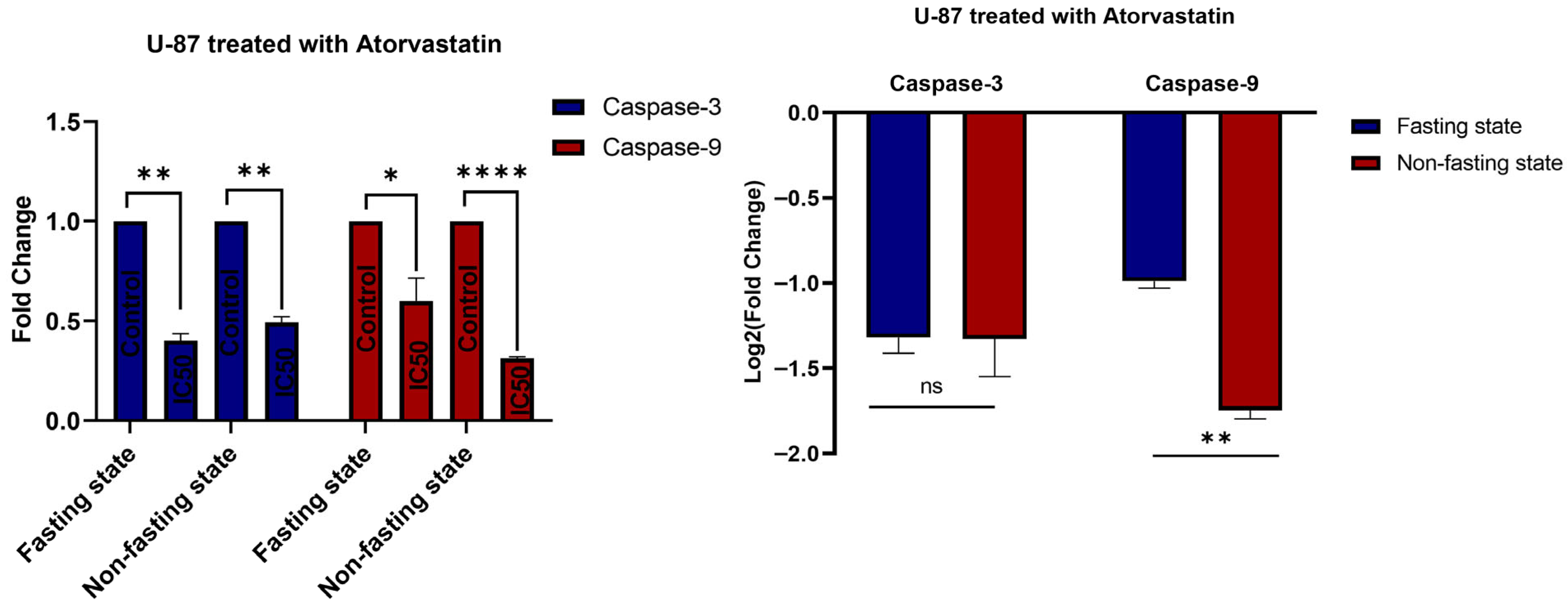
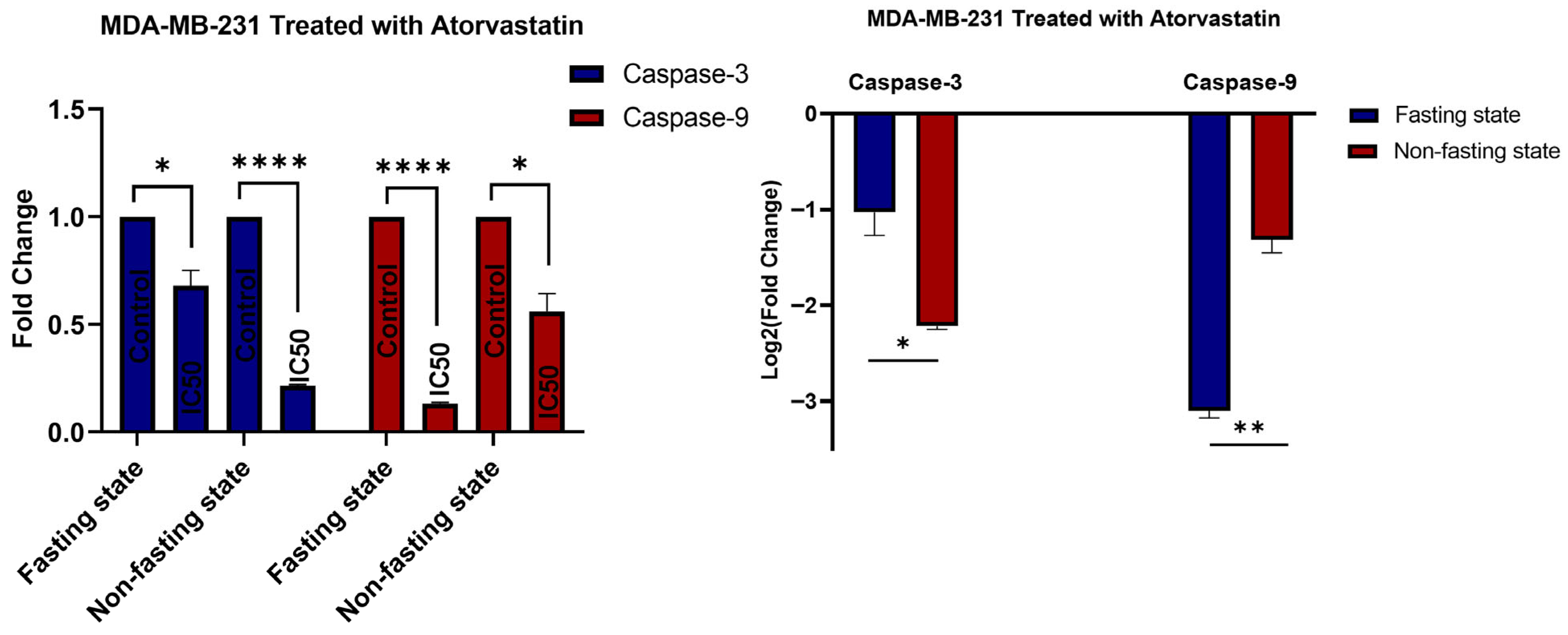
3.5. Gene Expression
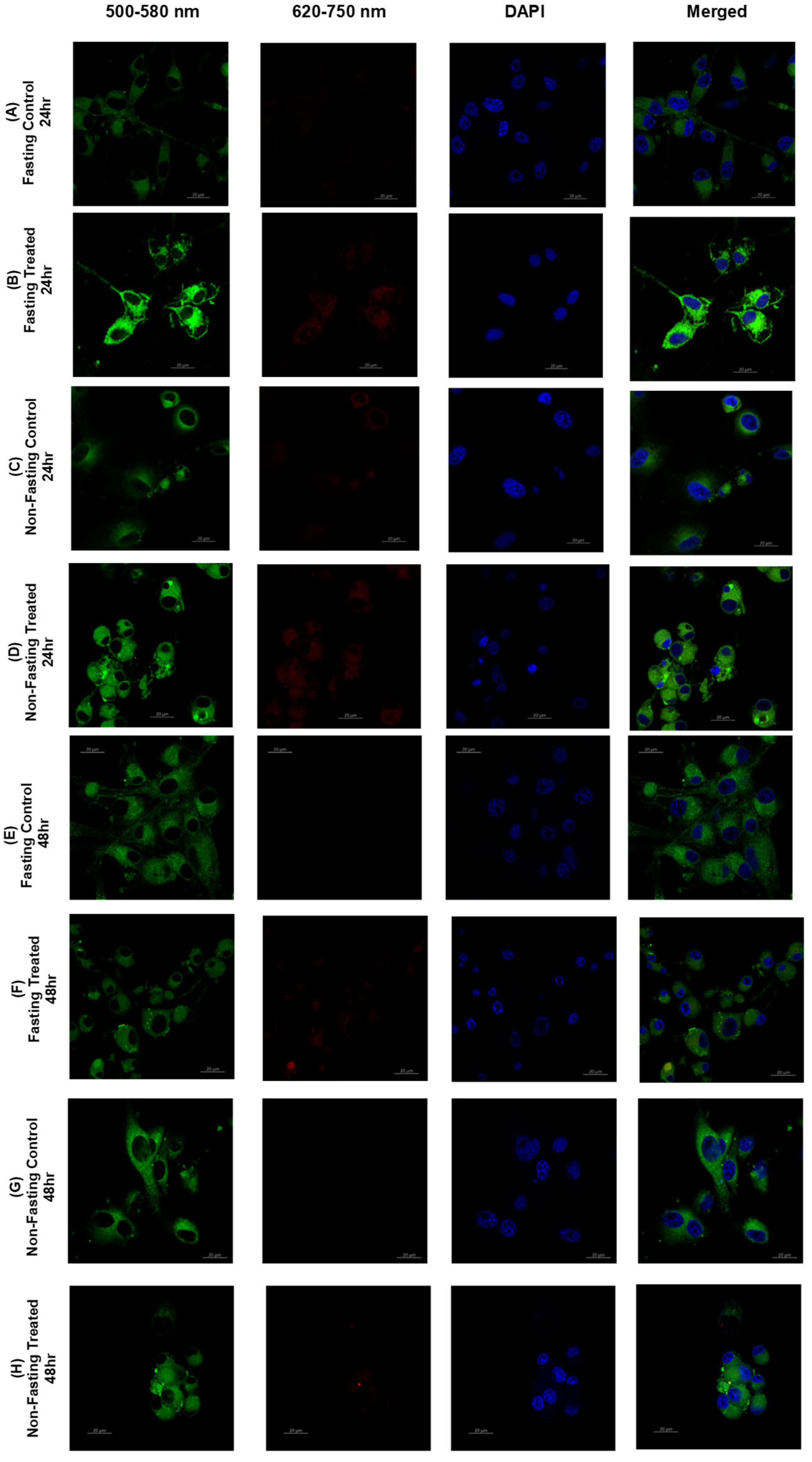
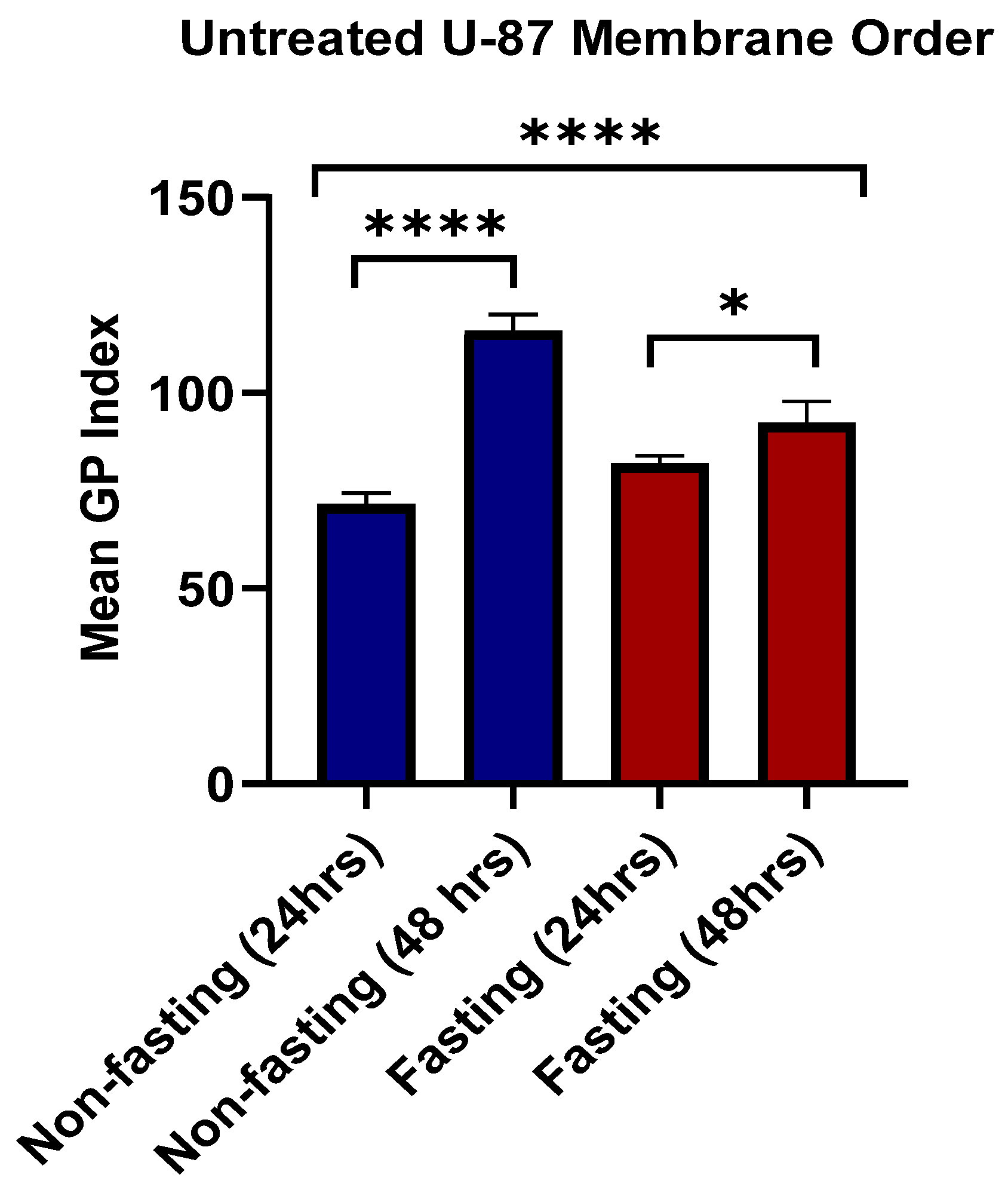
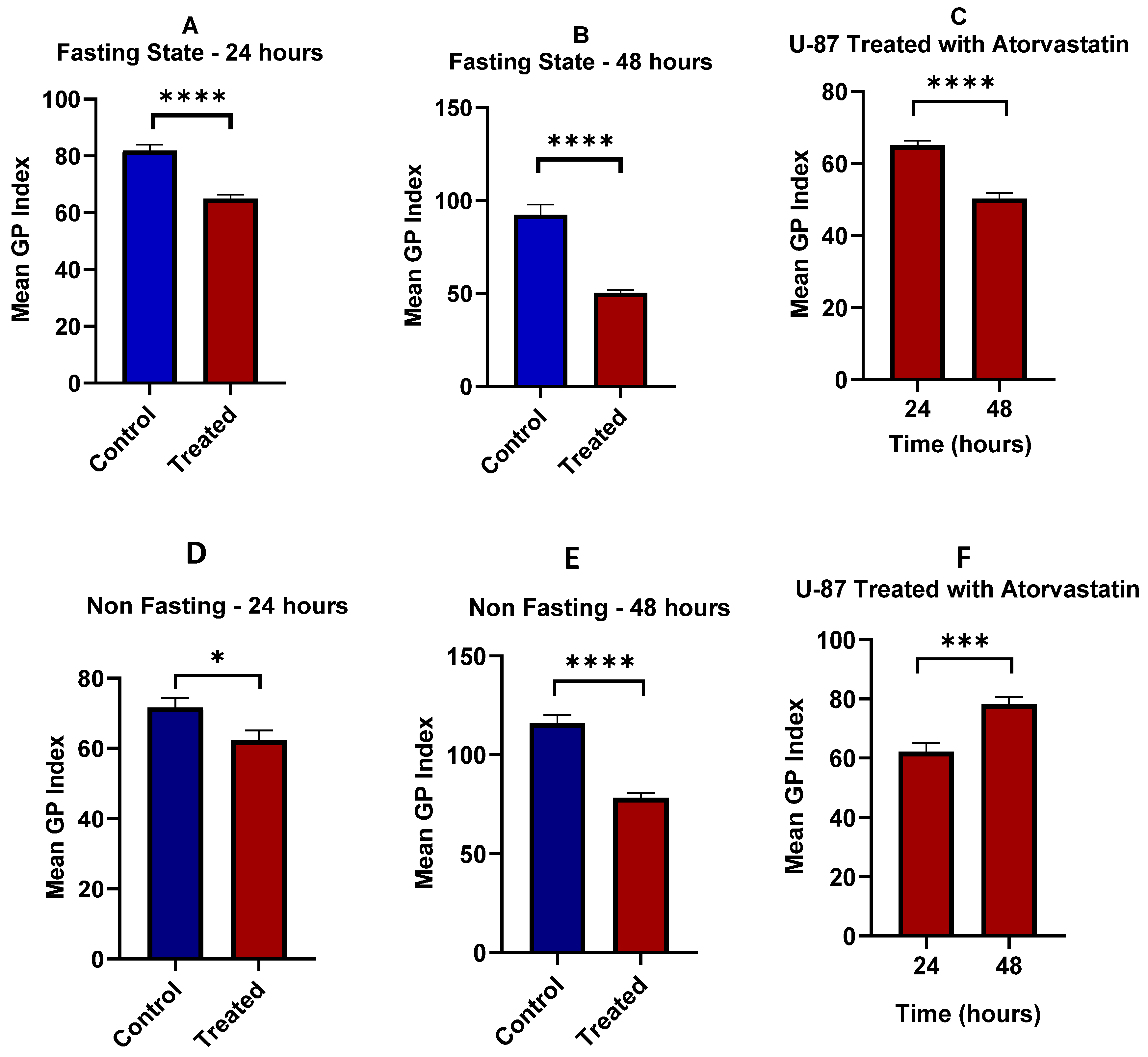
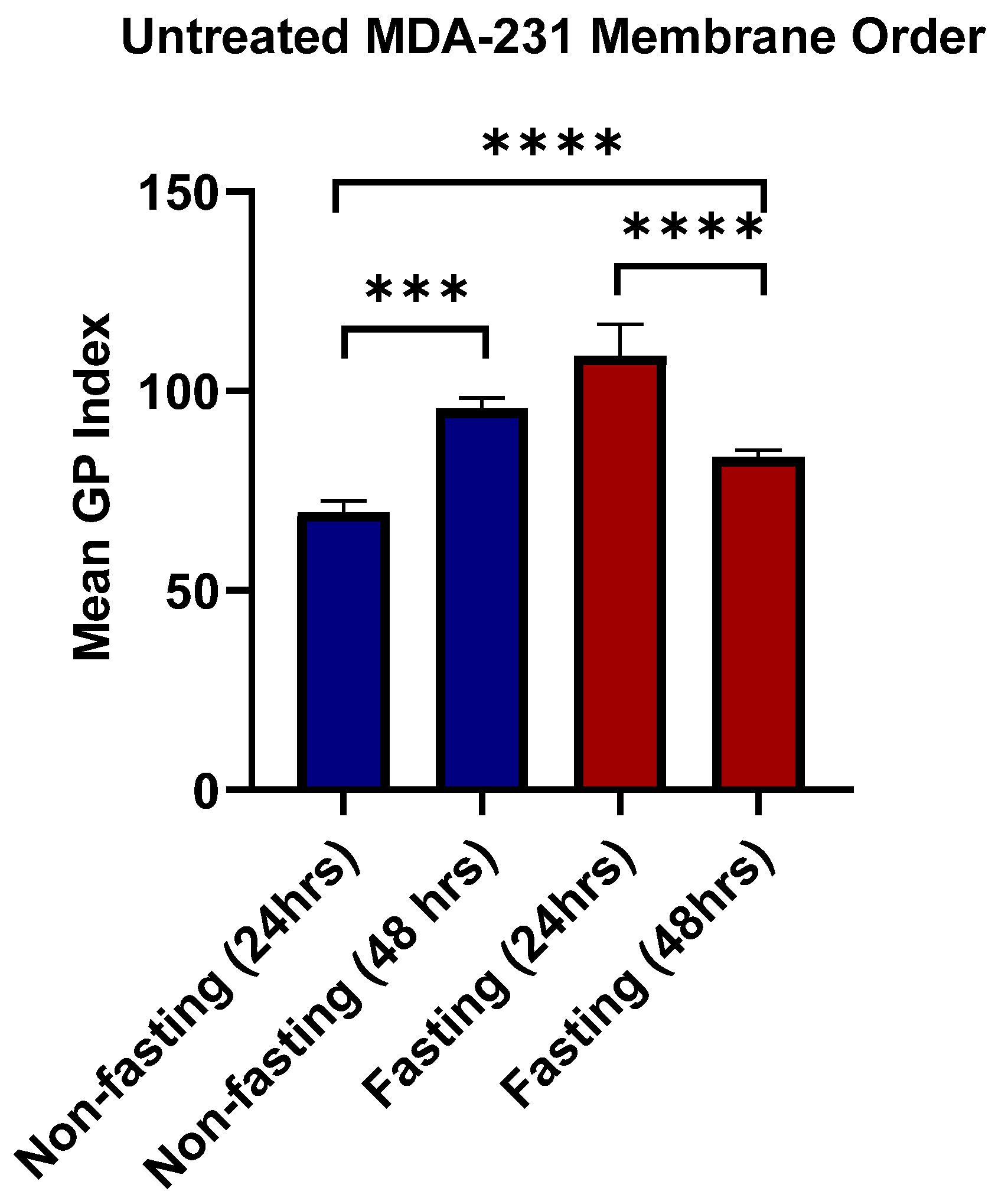
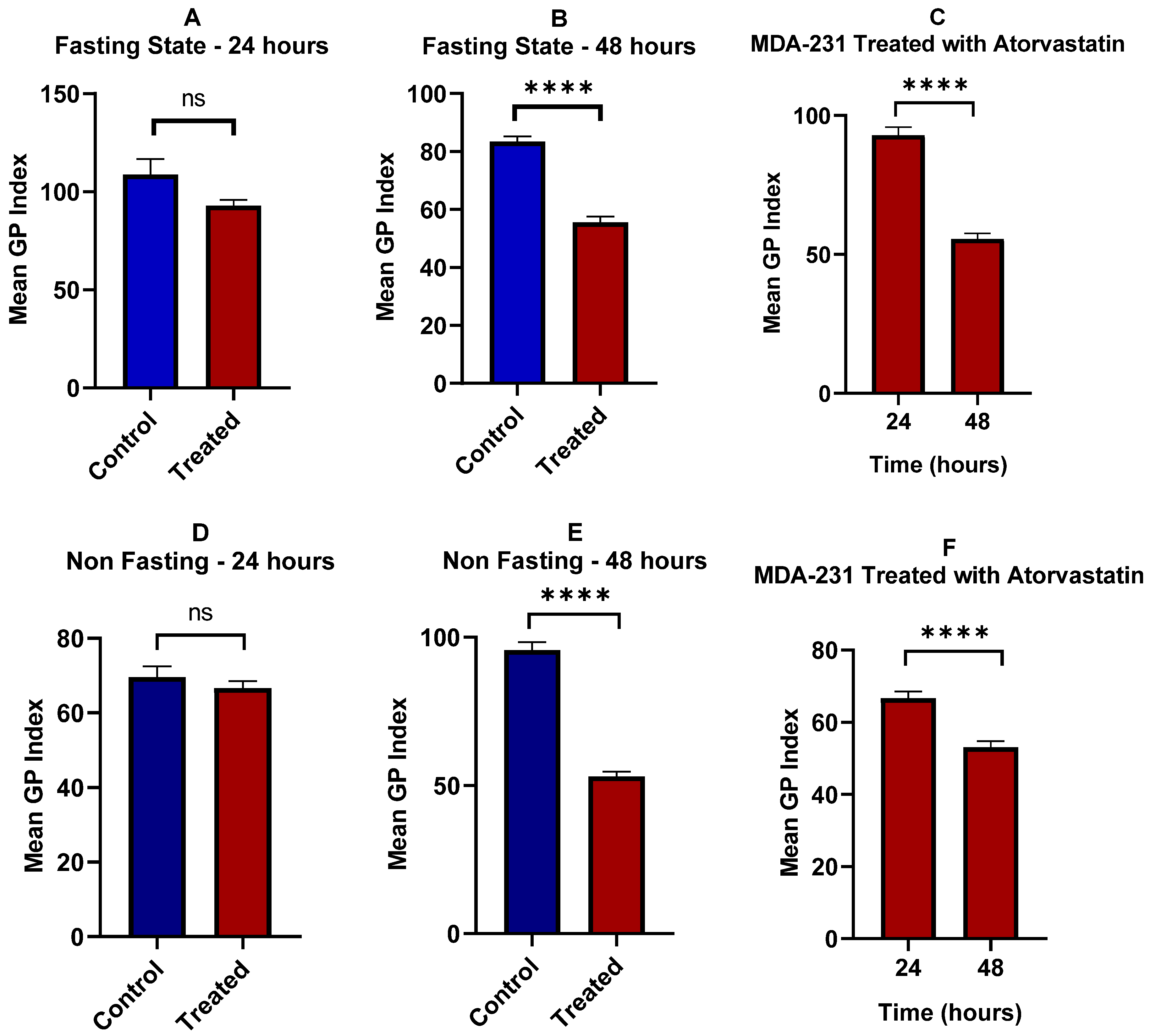
3.6. Lipid Raft
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seyfried, T.N. Confusion surrounds the origin of cancer. In Cancer as a Metabolic Disease: On the Origin, Management, and Prevention of Cancer; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 15–29. [Google Scholar]
- Fidler, I.J. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Lazebnik, Y. What are the hallmarks of cancer? Nat. Rev. Cancer 2010, 10, 232–233. [Google Scholar] [CrossRef] [PubMed]
- Blackadar, C.B. Historical review of the causes of cancer. World J. Clin. Oncol. 2016, 7, 54–86. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Chandel, N.S. Cancer metabolism: Looking forward. Nat. Rev. Cancer 2021, 21, 669–680. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Shelton, L.M. Cancer as a metabolic disease. Nutr. Metab. 2010, 7, 7. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Flores, R.E.; Poff, A.M.; D’agostino, D.P. Cancer as a metabolic disease: Implications for novel therapeutics. Carcinogenesis 2013, 35, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, H.G. Observations on the carbohydrate metabolism of tumours. Biochem. J. 1929, 23, 536–545. [Google Scholar] [CrossRef]
- Schiliro, C.; Firestein, B.L. Mechanisms of metabolic reprogramming in cancer cells supporting enhanced growth and proliferation. Cells 2021, 10, 1056. [Google Scholar] [CrossRef] [PubMed]
- Buono, R.; Longo, V.D. Starvation, stress resistance, and cancer. Trends Endocrinol. Metab. 2018, 29, 271–280. [Google Scholar] [CrossRef]
- Carnero, A.; Lleonart, M. The hypoxic microenvironment: A determinant of cancer stem cell evolution. Insid. Cell 2015, 1, 96–105. [Google Scholar] [CrossRef]
- Palm, W. Metabolic plasticity allows cancer cells to thrive under nutrient starvation. Proc. Natl. Acad. Sci. USA 2021, 118, e2102057118. [Google Scholar] [CrossRef]
- He, N.; Kim, N.; Jeong, E.; Lu, Y.; Mills, G.B.; Yoon, S. Glucose starvation induces mutation and lineage-dependent adaptive responses in a large collection of cancer cell lines. Int. J. Oncol. 2015, 48, 67–72. [Google Scholar] [CrossRef]
- Hirschey, M.D.; DeBerardinis, R.J.; Diehl, A.M.E.; Drew, J.E.; Frezza, C.; Green, M.F.; Jones, L.W.; Ko, Y.H.; Le, A.; Lea, M.A.; et al. Dysregulated metabolism contributes to oncogenesis. Semin. Cancer Biol. 2015, 35, S129–S150. [Google Scholar] [CrossRef]
- Mayers, J.R.; Torrence, M.E.; Danai, L.V.; Papagiannakopoulos, T.; Davidson, S.M.; Bauer, M.R.; Lau, A.N.; Ji, B.W.; Dixit, P.D.; Hosios, A.M.; et al. Tissue of origin dictates branched-chain amino acid metabolism in mutant Kras-driven cancers. Science 2016, 353, 1161–1165. [Google Scholar] [CrossRef]
- Munir, R.; Lisec, J.; Swinnen, J.V.; Zaidi, N. Lipid metabolism in cancer cells under metabolic stress. Br. J. Cancer 2019, 120, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.A.; Brault, C.; Peck, B.; Bensaad, K.; Griffiths, B.; Mitter, R.; Chakravarty, P.; East, P.; Dankworth, B.; Alibhai, D.; et al. SREBP maintains lipid biosynthesis and viability of cancer cells under lipid- and oxygen-deprived conditions and defines a gene signature associated with poor survival in glioblastoma multiforme. Oncogene 2015, 34, 5128–5140. [Google Scholar] [CrossRef] [PubMed]
- Kamphorst, J.J.; Cross, J.R.; Fan, J.; de Stanchina, E.; Mathew, R.; White, E.P.; Thompson, C.B.; Rabinowitz, J.D. Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proc. Natl. Acad. Sci. USA 2013, 110, 8882–8887. [Google Scholar] [CrossRef]
- Ackerman, D.; Simon, M.C. Hypoxia, lipids, and cancer: Surviving the harsh tumor microenvironment. Trends Cell Biol. 2014, 24, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Kuemmerle, N.B.; Rysman, E.; Lombardo, P.S.; Flanagan, A.J.; Lipe, B.C.; Wells, W.A.; Pettus, J.R.; Froehlich, H.M.; Memoli, V.A.; Morganelli, P.M.; et al. Lipoprotein lipase links dietary fat to solid tumor cell proliferation. Mol. Cancer Ther. 2011, 10, 427–436. [Google Scholar] [CrossRef]
- Zha, S.; Ferdinandusse, S.; Hicks, J.L.; Denis, S.; Dunn, T.A.; Wanders, R.J.; Luo, J.; De Marzo, A.M.; Isaacs, W.B. Peroxisomal branched chain fatty acid β-oxidation pathway is upregulated in prostate cancer. Prostate 2004, 63, 316–323. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, C.; Wang, D.; Zhang, Y.-F.; Lv, H.-X.; He, H.; Ren, Y.-Q.; Wang, J.; Zhou, F.-H. Proangiogenic potential of plasma exosomes from prostate cancer patients. Cell. Signal. 2024, 124, 111398. [Google Scholar] [CrossRef] [PubMed]
- Broadfield, L.A.; Pane, A.A.; Talebi, A.; Swinnen, J.V.; Fendt, S.-M. Lipid metabolism in cancer: New perspectives and emerging mechanisms. Dev. Cell 2021, 56, 1363–1393. [Google Scholar] [CrossRef]
- Jiang, W.; Hu, J.-W.; He, X.-R.; Jin, W.-L.; He, X.-Y. Statins: A repurposed drug to fight cancer. J. Exp. Clin. Cancer Res. 2021, 40, 241. [Google Scholar] [CrossRef]
- Kato, S.; Smalley, S.; Sadarangani, A.; Chen-Lin, K.; Oliva, B.; Brañes, J.; Carvajal, J.; Gejman, R.; Owen, G.I.; Cuello, M. Lipophilic but not hydrophilic statins selectively induce cell death in gynecological cancers expressing high levels of HMGCoA reductase. J. Cell. Mol. Med. 2009, 14, 1180–1193. [Google Scholar] [CrossRef] [PubMed]
- Dulak, J.; Jozkowicz, A. Anti-angiogenic and anti-inflammatory effects of statins: Relevance to anti-cancer therapy. Curr. Cancer Drug Targets 2005, 5, 579–594. [Google Scholar] [CrossRef]
- Raittinen, P.V.; Syvälä, H.; Tammela, T.L.; Häkkinen, M.R.; Ilmonen, P.; Auriola, S.; Murtola, T.J. Atorvastatin induces adrenal androgen downshift in men with prostate cancer: A post Hoc analysis of a pilot adaptive Randomised clinical trial. eBioMedicine 2021, 68, 103432. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Sun, Q.; Ran, L.; Wang, Y.; Qin, X.; Xu, X.; Tang, C.; Liu, L.; Zhang, G. pH-responsive selenium nanoplatform for highly efficient cancer starvation therapy by atorvastatin delivery. ACS Biomater. Sci. Eng. 2023, 9, 809–820. [Google Scholar] [CrossRef]
- Heikal, L.A.; Ashour, A.A.; Aboushanab, A.R.; El-Kamel, A.H.; Zaki, I.I.; El-Moslemany, R.M. Microneedles integrated with atorvastatin-loaded pumpkisomes for breast cancer therapy: A localized delivery approach. J. Control. Release 2024, 376, 354–368. [Google Scholar] [CrossRef]
- Shaghaghi, Z.; Alvandi, M.; Farzipour, S.; Dehbanpour, M.R.; Nosrati, S. A review of effects of atorvastatin in cancer therapy. Med. Oncol. 2022, 40, 27. [Google Scholar] [CrossRef]
- Hu, M.-B.; Zhang, J.-W.; Gao, J.-B.; Qi, Y.-W.; Gao, Y.; Xu, L.; Ma, Y.; Wei, Z.-Z. Atorvastatin induces autophagy in MDA-MB-231 breast cancer cells. Ultrastruct. Pathol. 2018, 42, 409–415. [Google Scholar] [CrossRef]
- Sheng, B.; Song, Y.; Zhang, J.; Li, R.; Wang, Z.; Zhu, X. Atorvastatin suppresses the progression of cervical cancer via regulation of autophagy. Am. J. Transl. Res. 2020, 12, 5252. [Google Scholar] [PubMed]
- Toepfer, N.; Childress, C.; Parikh, A.; Rukstalis, D.; Yang, W. Atorvastatin induces autophagy in prostate cancer PC3 cells through activation of LC3 transcription. Cancer Biol. Ther. 2011, 12, 691–699. [Google Scholar] [CrossRef]
- Yang, P.-M.; Liu, Y.-L.; Lin, Y.-C.; Shun, C.-T.; Wu, M.-S.; Chen, C.-C. Inhibition of autophagy enhances anticancer effects of atorvastatin in digestive malignancies. Cancer Res. 2010, 70, 7699–7709. [Google Scholar] [CrossRef]
- Wu, L.-M.; Wu, S.-G.; Chen, F.; Wu, Q.; Wu, C.-M.; Kang, C.-M.; He, X.; Zhang, R.-Y.; Lu, Z.-F.; Li, X.-H.; et al. Atorvastatin inhibits pyroptosis through the lncRNA NEXN-AS1/NEXN pathway in human vascular endothelial cells. Atherosclerosis 2020, 293, 26–34. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, M.; Xing, D.; Feng, Y. Atorvastatin enhances radiosensitivity in hypoxia-induced prostate cancer cells related with HIF-1α inhibition. Biosci. Rep. 2017, 37, BSR20170340. [Google Scholar] [CrossRef]
- Fujiwara, D.; Tsubaki, M.; Takeda, T.; Tomonari, Y.; Koumoto, Y.-I.; Sakaguchi, K.; Nishida, S. Statins induce apoptosis through inhibition of Ras signaling pathways and enhancement of Bim and p27 expression in human hematopoietic tumor cells. Tumor Biol. 2017, 39, 1010428317734947. [Google Scholar] [CrossRef] [PubMed]
- Brinkkoetter, P.-T.; Gottmann, U.; Schulte, J.; Van Der Woude, F.J.; Braun, C.; A Yard, B. Atorvastatin interferes with activation of human CD4+ T cells via inhibition of small guanosine triphosphatase (GTPase) activity and caspase-independent apoptosis. Clin. Exp. Immunol. 2006, 146, 524–532. [Google Scholar] [CrossRef]
- Chansrichavala, P.; Chantharaksri, U.; Sritara, P.; Ngaosuwankul, N.; Chaiyaroj, S.C. Atorvastatin affects TLR4 clustering via lipid raft modulation. Int. Immunopharmacol. 2010, 10, 892–899. [Google Scholar] [CrossRef]
- Zakyrjanova, G.F.; Matigorova, V.A.; Kuznetsova, E.A.; Dmitrieva, S.A.; Tyapkina, O.V.; Tsentsevitsky, A.N.; Andreyanova, S.N.; Odnoshivkina, J.G.; Shigapova, R.R.; Mukhamedshina, Y.O.; et al. Key genes and processes affected by atorvastatin treatment in mouse diaphragm muscle. Arch. Toxicol. 2025, 99, 2877–2901. [Google Scholar] [CrossRef] [PubMed]
- Murai, T. The role of lipid rafts in cancer cell adhesion and migration. Int. J. Cell Biol. 2011, 2012, 763283. [Google Scholar] [CrossRef]
- Gyoten, M.; Luo, Y.; Fujiwara-Tani, R.; Mori, S.; Ogata, R.; Kishi, S.; Kuniyasu, H. Lovastatin treatment inducing apoptosis in human pancreatic cancer cells by inhibiting cholesterol rafts in plasma membrane and mitochondria. Int. J. Mol. Sci. 2023, 24, 16814. [Google Scholar] [CrossRef]
- Ioudina, M.; Uemura, E.; Greenlee, H.W. Glucose insufficiency alters neuronal viability and increases susceptibility to glutamate toxicity. Brain Res. 2004, 1004, 188–192. [Google Scholar] [CrossRef]
- Ashrafi, G.; A Ryan, T. Glucose metabolism in nerve terminals. Curr. Opin. Neurobiol. 2017, 45, 156–161. [Google Scholar] [CrossRef] [PubMed]
- A Flavahan, W.; Wu, Q.; Hitomi, M.; Rahim, N.; Kim, Y.; E Sloan, A.; Weil, R.J.; Nakano, I.; Sarkaria, J.N.; Stringer, B.W.; et al. Brain tumor initiating cells adapt to restricted nutrition through preferential glucose uptake. Nat. Neurosci. 2013, 16, 1373–1382. [Google Scholar] [CrossRef]
- Mao, P.; Joshi, K.; Li, J.; Kim, S.-H.; Li, P.; Santana-Santos, L.; Luthra, S.; Chandran, U.R.; Benos, P.V.; Smith, L.; et al. Mesenchymal glioma stem cells are maintained by activated glycolytic metabolism involving aldehyde dehydrogenase 1A3. Proc. Natl. Acad. Sci. USA 2013, 110, 8644–8649. [Google Scholar] [CrossRef] [PubMed]
- Azzalin, A.; Brambilla, F.; Arbustini, E.; Basello, K.; Speciani, A.; Mauri, P.; Bezzi, P.; Magrassi, L. A new pathway promotes adaptation of human glioblastoma cells to glucose starvation. Cells 2020, 9, 1249. [Google Scholar] [CrossRef] [PubMed]
- Vogelhuber, W.; Spruß, T.; Bernhardt, G.; Buschauer, A.; Göpferich, A. Efficacy of BCNU and paclitaxel loaded subcutaneous implants in the interstitial chemotherapy of U-87 MG human glioblastoma xenografts. Int. J. Pharm. 2002, 238, 111–121. [Google Scholar] [CrossRef]
- Alhaddad, L.; Chuprov-Netochin, R.; Pustovalova, M.; Osipov, A.N.; Leonov, S. Polyploid/multinucleated giant and slow-cycling cancer cell enrichment in response to X-ray irradiation of human glioblastoma multiforme cells differing in radioresistance and TP53/PTEN status. Int. J. Mol. Sci. 2023, 24, 1228. [Google Scholar] [CrossRef]
- Lanfranchi, M.; Yandiev, S.; Meyer-Dilhet, G.; Ellouze, S.; Kerkhofs, M.; Dos Reis, R.; Garcia, A.; Blondet, C.; Amar, A.; Kneppers, A.; et al. The AMPK-related kinase NUAK1 controls cortical axons branching by locally modulating mitochondrial metabolic functions. Nat. Commun. 2024, 15, 2487. [Google Scholar] [CrossRef]
- Gallo, G. Neuronal glycolysis: Focus on developmental morphogenesis and localized subcellular functions. Commun. Integr. Biol. 2024, 17, 2343532. [Google Scholar] [CrossRef] [PubMed]
- Gupta, C.; Tikoo, K. High glucose and insulin differentially modulates proliferation in MCF-7 and MDA-MB-231 cells. J. Mol. Endocrinol. 2013, 51, 119–129. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, G.; Liu, Z.; Zheng, H.; Xu, Y.; Zhang, D.; Chen, Q.; Luo, D. High glucose enhances malignant progression of MDA-MB-231 cells through cumulative effect. Toxicol. Lett. 2024, 403, 17–31. [Google Scholar] [CrossRef]
- Ronaldson, P.T.; Brzica, H.; Abdullahi, W.; Reilly, B.G.; Davis, T.P. Transport properties of statins by organic anion transporting polypeptide 1A2 and regulation by transforming growth factor-β signaling in human endothelial cells. J. Pharmacol. Exp. Ther. 2020, 376, 148–160. [Google Scholar] [CrossRef]
- Burnett, J.R.; Wilcox, L.J.; Telford, D.E.; Kleinstiver, S.J.; Barrett, P.H.R.; Newton, R.S.; Huff, M.W. Inhibition of HMG-CoA reductase by atorvastatin decreases both VLDL and LDL apolipoprotein B production in miniature pigs. Arter. Thromb. Vasc. Biol. 1997, 17, 2589–2600. [Google Scholar] [CrossRef]
- Zipinotti dos Santos, D.; Santos Guimaraes, I.D.; Hakeem-Sanni, M.F.; Cochran, B.J.; Rye, K.A.; Grewal, T.; Hoy, A.J.; Rangel, L.B.A. Atorvastatin improves cisplatin sensitivity through modulation of cholesteryl ester homeostasis in breast cancer cells. Discov. Oncol. 2022, 13, 135. [Google Scholar] [CrossRef]
- Wang, L.; Shang, Z.; Zhou, Y.; Hu, X.; Chen, Y.; Fan, Y.; Wei, X.; Wu, L.; Liang, Q.; Zhang, J.; et al. Autophagy mediates glucose starvation-induced glioblastoma cell quiescence and chemoresistance through coordinating cell metabolism, cell cycle, and survival. Cell Death Dis. 2018, 9, 213. [Google Scholar] [CrossRef]
- Masui, K.; Tanaka, K.; Ikegami, S.; Villa, G.R.; Yang, H.; Yong, W.H.; Cloughesy, T.F.; Yamagata, K.; Arai, N.; Cavenee, W.K.; et al. Glucose-dependent acetylation of Rictor promotes targeted cancer therapy resistance. Proc. Natl. Acad. Sci. USA 2015, 112, 9406–9411. [Google Scholar] [CrossRef]
- Nooshabadi, V.T.; Khanmohammadi, M.; Shafei, S.; Banafshe, H.R.; Malekshahi, Z.V.; Ebrahimi-Barough, S.; Ai, J. Impact of atorvastatin loaded exosome as an anti-glioblastoma carrier to induce apoptosis of U87 cancer cells in 3D culture model. Biochem. Biophys. Rep. 2020, 23, 100792. [Google Scholar] [CrossRef]
- Gao, K.; Zhou, T.; Yin, Y.; Sun, X.; Jiang, H.; Li, T. Atorvastatin inhibits glioma glycolysis and immune escape by modulating the miR-125a-5p/TXLNA axis. Hereditas 2024, 161, 54. [Google Scholar] [CrossRef] [PubMed]
- Beckwitt, C.H.; Shiraha, K.; Wells, A. Lipophilic statins limit cancer cell growth and survival, via involvement of Akt signaling. PLoS ONE 2018, 13, e0197422. [Google Scholar] [CrossRef]
- Goodarzi, A.; Khanmohammadi, M.; Ai, A.; Khodayari, H.; Ai, A.; Farahani, M.S.; Khodayari, S.; Ebrahimi-Barough, S.; Mohandesnezhad, S.; Ai, J. Simultaneous impact of atorvastatin and mesenchymal stem cells for glioblastoma multiform suppression in rat glioblastoma multiform model. Mol. Biol. Rep. 2020, 47, 7783–7795. [Google Scholar] [CrossRef] [PubMed]
- Bakar-Ates, F.; Ozkan, E. Atorvastatin induces downregulation of matrix metalloproteinase-2/9 in MDA-MB-231 triple negative breast cancer cells. Med. Oncol. 2022, 40, 22. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Panda, S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 2016, 23, 1048–1059. [Google Scholar] [CrossRef]
- Nencioni, A.; Caffa, I.; Cortellino, S.; Longo, V.D. Fasting and cancer: Molecular mechanisms and clinical application. Nat. Rev. Cancer 2018, 18, 707–719. [Google Scholar] [CrossRef]
- Eriau, E.; Paillet, J.; Kroemer, G.; Pol, J.G. Metabolic reprogramming by reduced calorie intake or pharmacological caloric restriction mimetics for improved cancer immunotherapy. Cancers 2021, 13, 1260. [Google Scholar] [CrossRef]
- Aldape, K.; Brindle, K.M.; Chesler, L.; Chopra, R.; Gajjar, A.; Gilbert, M.R.; Gottardo, N.; Gutmann, D.H.; Hargrave, D.; Holland, E.C.; et al. Challenges to curing primary brain tumours. Nat. Rev. Clin. Oncol. 2019, 16, 509–520. [Google Scholar] [CrossRef]
- Brandhorst, S.; Choi, I.Y.; Wei, M.; Cheng, C.W.; Sedrakyan, S.; Navarrete, G.; Dubeau, L.; Yap, L.P.; Park, R.; Vinciguerra, M.; et al. A Periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab. 2015, 22, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Raffaghello, L.; Safdie, F.; Bianchi, G.; Dorff, T.; Fontana, L.; Longo, V.D. Fasting and differential chemotherapy protection in patients. Cell Cycle 2010, 9, 4474–4476. [Google Scholar] [CrossRef]
- Ahmadi, Y.; Fard, J.K.; Ghafoor, D.; Eid, A.H.; Sahebkar, A. Paradoxical effects of statins on endothelial and cancer cells: The impact of concentrations. Cancer Cell Int. 2023, 23, 43. [Google Scholar] [CrossRef]
- Ricco, N.; Kron, S.J. Statins in Cancer Prevention and Therapy. Cancers 2023, 15, 3948. [Google Scholar] [CrossRef]
- Gbelcová, H.; Rimpelová, S.; Knejzlík, Z.; Šáchová, J.; Kolář, M.; Strnad, H.; Repiská, V.; D’aCunto, W.C.; Ruml, T.; Vítek, L. Isoprenoids responsible for protein prenylation modulate the biological effects of statins on pancreatic cancer cells. Lipids Health Dis. 2017, 16, 250. [Google Scholar] [CrossRef]
- Garcia-Ruiz, C.; Morales, A.; Fernandez-Checa, J.C. Statins and protein prenylation in cancer cell biology and therapy. Anti-Cancer Agents Med. Chem. 2012, 12, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Longo, J.; van Leeuwen, J.E.; Mullen, P.J.; Ba-Alawi, W.; Haibe-Kains, B.; Penn, L.Z. Statin-induced cancer cell death can be mechanistically uncoupled from prenylation of RAS family proteins. Cancer Res. 2018, 78, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, G.J. Metabolic reprogramming: The emerging concept and associated therapeutic strategies. J. Exp. Clin. Cancer Res. 2015, 34, 111. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, S.; Yu, D. Metabolic reprogramming of chemoresistant cancer cells and the potential significance of metabolic regulation in the reversal of cancer chemoresistance. Metabolites 2020, 10, 289. [Google Scholar] [CrossRef]
- Evan, G.; Littlewood, T. A matter of life and cell death. Science 1998, 281, 1317–1322. [Google Scholar] [CrossRef]
- Olsson, M.; Zhivotovsky, B. Caspases and cancer. Cell Death Differ. 2011, 18, 1441–1449. [Google Scholar] [CrossRef]
- Fulda, S.; Debatin, K.-M. Caspase activation in cancer therapy. In Madame Curie Bioscience Database [Internet]; Landes Bioscience: Austin, TX, USA, 2013. [Google Scholar]
- Hashimoto, T.; Kikkawa, U.; Kamada, S. Contribution of caspase(s) to the cell cycle regulation at mitotic phase. PLoS ONE 2011, 6, e18449. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Strasser, A.; Vaux, D.L. Cell death in the origin and treatment of cancer. Mol. Cell 2020, 78, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Clay, D.E.; Fox, D.T. DNA damage responses during the cell cycle: Insights from model organisms and beyond. Genes 2021, 12, 1882. [Google Scholar] [CrossRef]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Solier, S.; Zhang, Y.-W.; Ballestrero, A.; Pommier, Y.; Zoppoli, G. DNA damage response pathways and cell cycle checkpoints in colorectal cancer: Current concepts and future perspectives for targeted treatment. Curr. Cancer Drug Targets 2012, 12, 356–371. [Google Scholar] [CrossRef]
- Marinac, C.R.; Natarajan, L.; Sears, D.D.; Gallo, L.C.; Hartman, S.J.; Arredondo, E.; Patterson, R.E. Prolonged nightly fasting and breast cancer risk: Findings from NHANES (2009–2010). Cancer Epidemiol. Biomark. Prev. 2015, 24, 783–789. [Google Scholar] [CrossRef]
- Marinac, C.R.; Sears, D.D.; Natarajan, L.; Gallo, L.C.; Breen, C.I.; Patterson, R.E. Frequency and circadian timing of eating may influence biomarkers of inflammation and insulin resistance associated with breast cancer risk. PLoS ONE 2015, 10, e0136240. [Google Scholar] [CrossRef]
- Kroemer, G.; Martin, S.J. Caspase-independent cell death. Nat. Med. 2005, 11, 725–730. [Google Scholar] [CrossRef]
- Lockshin, R.A.; Zakeri, Z. Caspase-independent cell death? Oncogene 2004, 23, 2766–2773. [Google Scholar] [CrossRef]
- Willimott, S.; Wagner, S.D. Post-transcriptional and post-translational regulation of Bcl2. Biochem. Soc. Trans. 2010, 38, 1571–1575. [Google Scholar] [CrossRef] [PubMed]
- Audic, Y.; Hartley, R.S. Post-transcriptional regulation in cancer. Biol. Cell 2004, 96, 479–498. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Emi, M.; Tanabe, K. Caspase-dependent and -independent cell death pathways after DNA damage (Review). Oncol. Rep. 2005, 14, 595–599. [Google Scholar] [CrossRef]
- Bhadra, K. A mini review on molecules inducing caspase-independent cell death: A new route to cancer therapy. Molecules 2022, 27, 6401. [Google Scholar] [CrossRef]
- Brown, D.A.; London, E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 2000, 275, 17221–17224. [Google Scholar] [CrossRef]
- Simons, K.; Ehehalt, R. Cholesterol, lipid rafts, and disease. J. Clin. Investig. 2002, 110, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Hume, D.A.; Weidemann, M.J. Role and regulation of glucose metabolism in proliferating cells. JNCI J. Natl. Cancer Inst. 1979, 62, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Robey, I.; Gatenby, R.A. Causes and consequences of increased glucose metabolism of cancers. J. Nucl. Med. 2008, 49, 24S–42S. [Google Scholar] [CrossRef]
- Anselmo, S.; Bonaccorso, E.; Gangemi, C.; Sancataldo, G.; Nibali, V.C.; D’angelo, G. Lipid rafts in signalling, diseases, and infections: What can be learned from fluorescence techniques? Membranes 2025, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhu, N.; Li, H.F.; Gu, J.; Zhang, C.J.; Liao, D.F.; Qin, L. The lipid rafts in cancer stem cell: A target to eradicate cancer. Stem Cell Res. Ther. 2022, 13, 432. [Google Scholar] [CrossRef]
- Li, B.; Qin, Y.; Yu, X.; Xu, X.; Yu, W. Lipid raft involvement in signal transduction in cancer cell survival, cell death and metastasis. Cell Prolif. 2021, 55, e13167. [Google Scholar] [CrossRef]
- Greenlee, J.D.; Subramanian, T.; Liu, K.; King, M.R. Rafting down the metastatic cascade: The role of lipid rafts in cancer metastasis, cell death, and clinical outcomes. Cancer Res. 2020, 81, 5–17. [Google Scholar] [CrossRef]
- Horváth, Á.; Erostyák, J.; Szőke, É. Effect of lipid raft disruptors on cell membrane fluidity studied by fluorescence spectroscopy. Int. J. Mol. Sci. 2022, 23, 13729. [Google Scholar] [CrossRef]
- Zhang, S.; Ren, X.; Zhang, B.; Lan, T.; Liu, B. A systematic review of statins for the treatment of nonalcoholic steatohepatitis: Safety, efficacy, and mechanism of action. Molecules 2024, 29, 1859. [Google Scholar] [CrossRef]
- Brown, A.J.; Coates, H.W.; Sharpe, L.J. Cholesterol synthesis. In Biochemistry of Lipids, Lipoproteins and Membranes; Elsevier: Amsterdam, The Netherlands, 2021; pp. 317–355. [Google Scholar]
- Krause, M.R.; Regen, S.L. The structural role of cholesterol in cell membranes: From condensed bilayers to lipid rafts. Accounts Chem. Res. 2014, 47, 3512–3521. [Google Scholar] [CrossRef]
- Brown, A.J. Cholesterol, statins and cancer. Clin. Exp. Pharmacol. Physiol. 2007, 34, 135–141. [Google Scholar] [CrossRef]
- Clendening, J.W.; Pandyra, A.; Li, Z.; Boutros, P.C.; Martirosyan, A.; Lehner, R.; Jurisica, I.; Trudel, S.; Penn, L.Z. Exploiting the mevalonate pathway to distinguish statin-sensitive multiple myeloma. Blood 2010, 115, 4787–4797. [Google Scholar] [CrossRef]
- Guerrero-Ochoa, P.; Rodríguez-Zapater, S.; Anel, A.; Esteban, L.M.; Camón-Fernández, A.; Espilez-Ortiz, R.; Gil-Sanz, M.J.; Borque-Fernando, Á. Prostate cancer and the mevalonate pathway. Int. J. Mol. Sci. 2024, 25, 2152. [Google Scholar] [CrossRef]
- Brault, C.; Schulze, A. The role of glucose and lipid metabolism in growth and survival of cancer cells. Metab. Cancer 2016, 207, 1–22. [Google Scholar]
- Xiao, X.; Luo, Y.; Peng, D. Updated understanding of the crosstalk between glucose/insulin and cholesterol metabolism. Front. Cardiovasc. Med. 2022, 9, 879355. [Google Scholar] [CrossRef]
- Kang, M.; Kang, J.H.; Sim, I.A.; Seong, D.Y.; Han, S.; Jang, H.; Lee, H.; Kang, S.W.; Kim, S.-Y. Glucose deprivation induces cancer cell death through failure of ROS regulation. Int. J. Mol. Sci. 2023, 24, 11969. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshaer, W.; Ijjeh, Y.; Alsarayreh, N.; Alqudah, D.A.; Rifai, A.; Abu-Siniyeh, A.; Alsalem, M. Combined Effects of Atorvastatin and Glucose Deprivation on Metabolic Stress and Lipid-Raft Disruption in Glioblastoma and Breast Cancer Cells. Pharmaceutics 2025, 17, 1275. https://doi.org/10.3390/pharmaceutics17101275
Alshaer W, Ijjeh Y, Alsarayreh N, Alqudah DA, Rifai A, Abu-Siniyeh A, Alsalem M. Combined Effects of Atorvastatin and Glucose Deprivation on Metabolic Stress and Lipid-Raft Disruption in Glioblastoma and Breast Cancer Cells. Pharmaceutics. 2025; 17(10):1275. https://doi.org/10.3390/pharmaceutics17101275
Chicago/Turabian StyleAlshaer, Walhan, Yousef Ijjeh, Nowar Alsarayreh, Dana A. Alqudah, Alaa Rifai, Ahmed Abu-Siniyeh, and Mohammad Alsalem. 2025. "Combined Effects of Atorvastatin and Glucose Deprivation on Metabolic Stress and Lipid-Raft Disruption in Glioblastoma and Breast Cancer Cells" Pharmaceutics 17, no. 10: 1275. https://doi.org/10.3390/pharmaceutics17101275
APA StyleAlshaer, W., Ijjeh, Y., Alsarayreh, N., Alqudah, D. A., Rifai, A., Abu-Siniyeh, A., & Alsalem, M. (2025). Combined Effects of Atorvastatin and Glucose Deprivation on Metabolic Stress and Lipid-Raft Disruption in Glioblastoma and Breast Cancer Cells. Pharmaceutics, 17(10), 1275. https://doi.org/10.3390/pharmaceutics17101275







