Emissive Pentacene-Loaded βcyclodextrin-Derived C-Nanodots Exhibit Red-Light Triggered Photothermal Effect
Abstract
1. Introduction
2. Materials and Methods
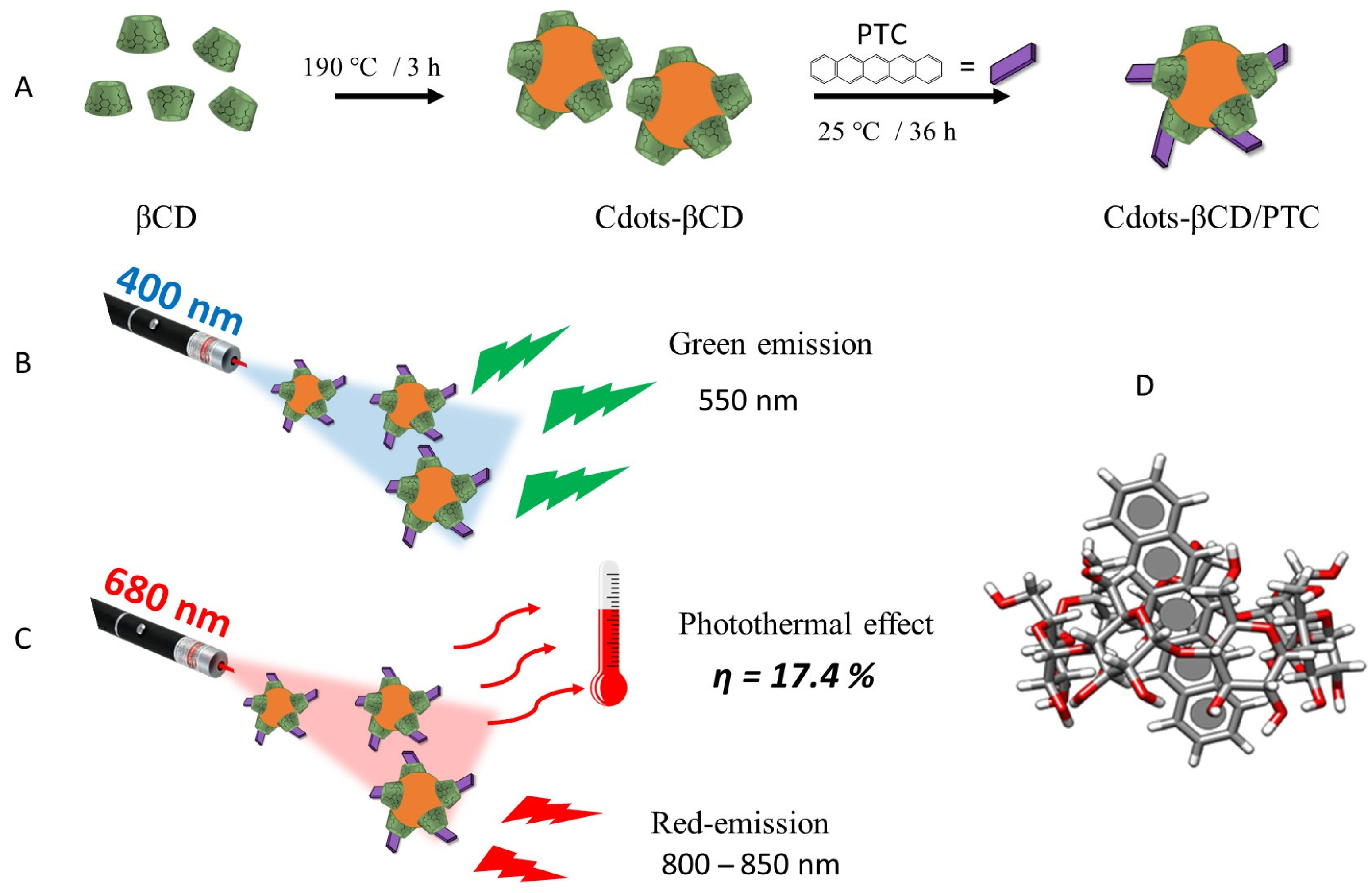
2.1. Preparation of Cdots-βCD
2.2. Preparation of Cdots-βCD/PTC Nanohybrid
2.3. Instrumentation
2.3.1. UV-Vis Spectroscopy
2.3.2. Fluorescence Spectroscopy
2.3.3. ATR-FTIR Spectroscopy
2.3.4. Atomic Force Microscopy (AFM)
2.3.5. DLS and Zeta Potential Measurements
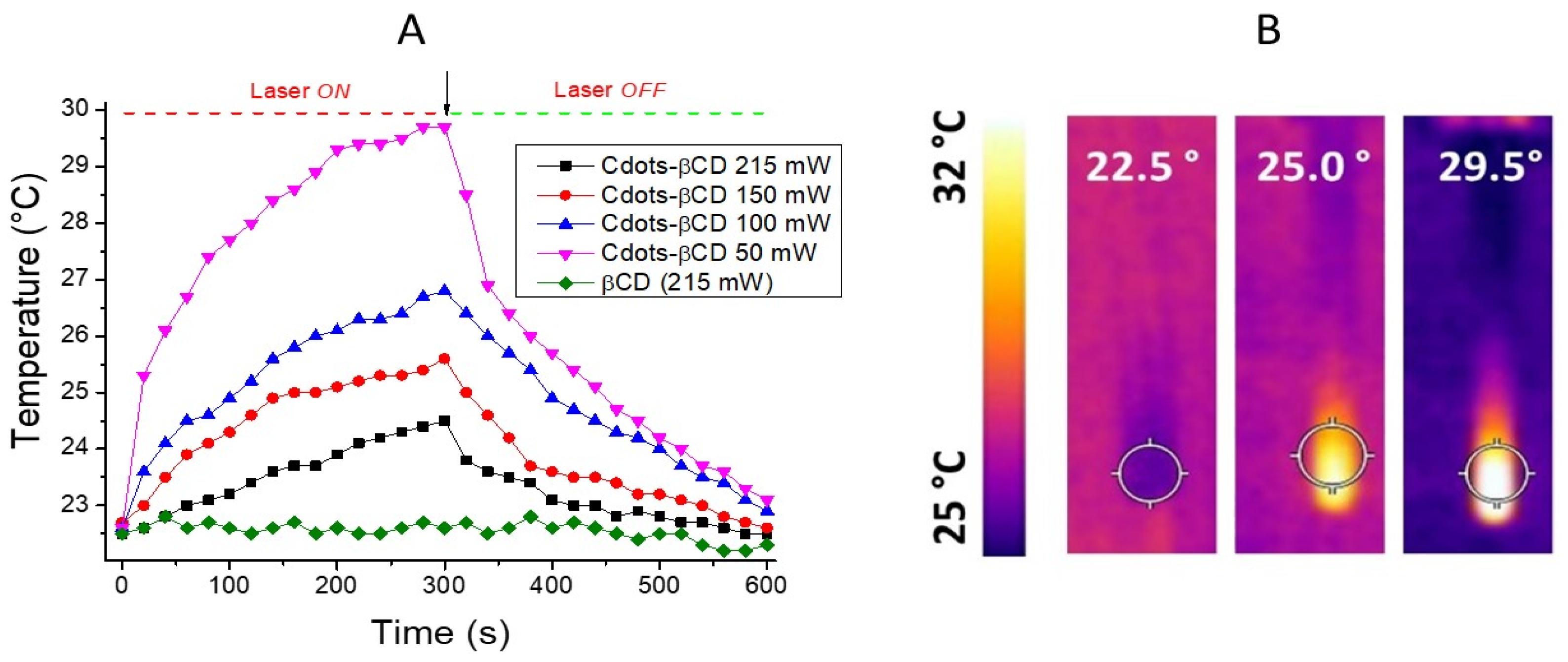
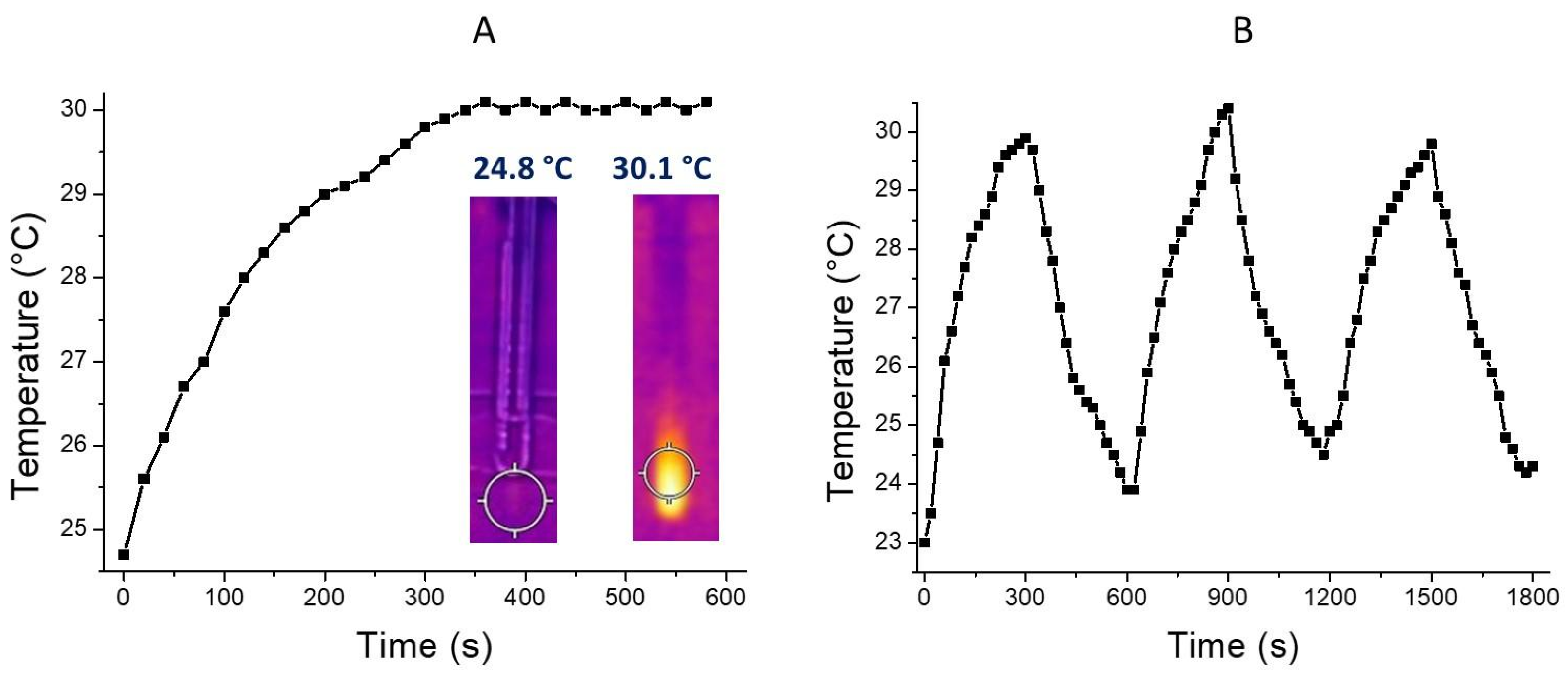
2.4. Photothermal Properties
2.5. Modeling Simulation
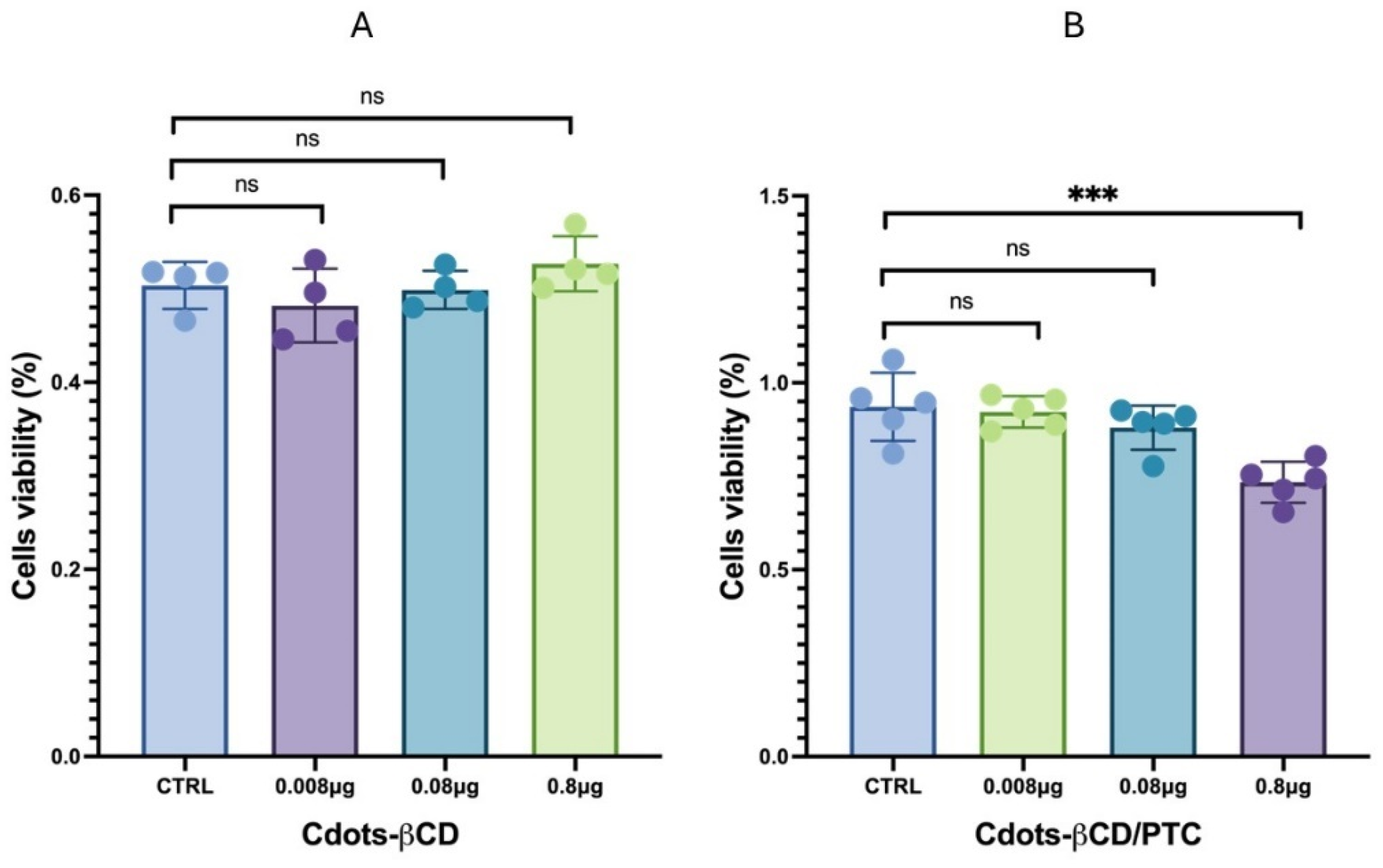
2.6. Cytotoxicity Tests
3. Results and Discussion
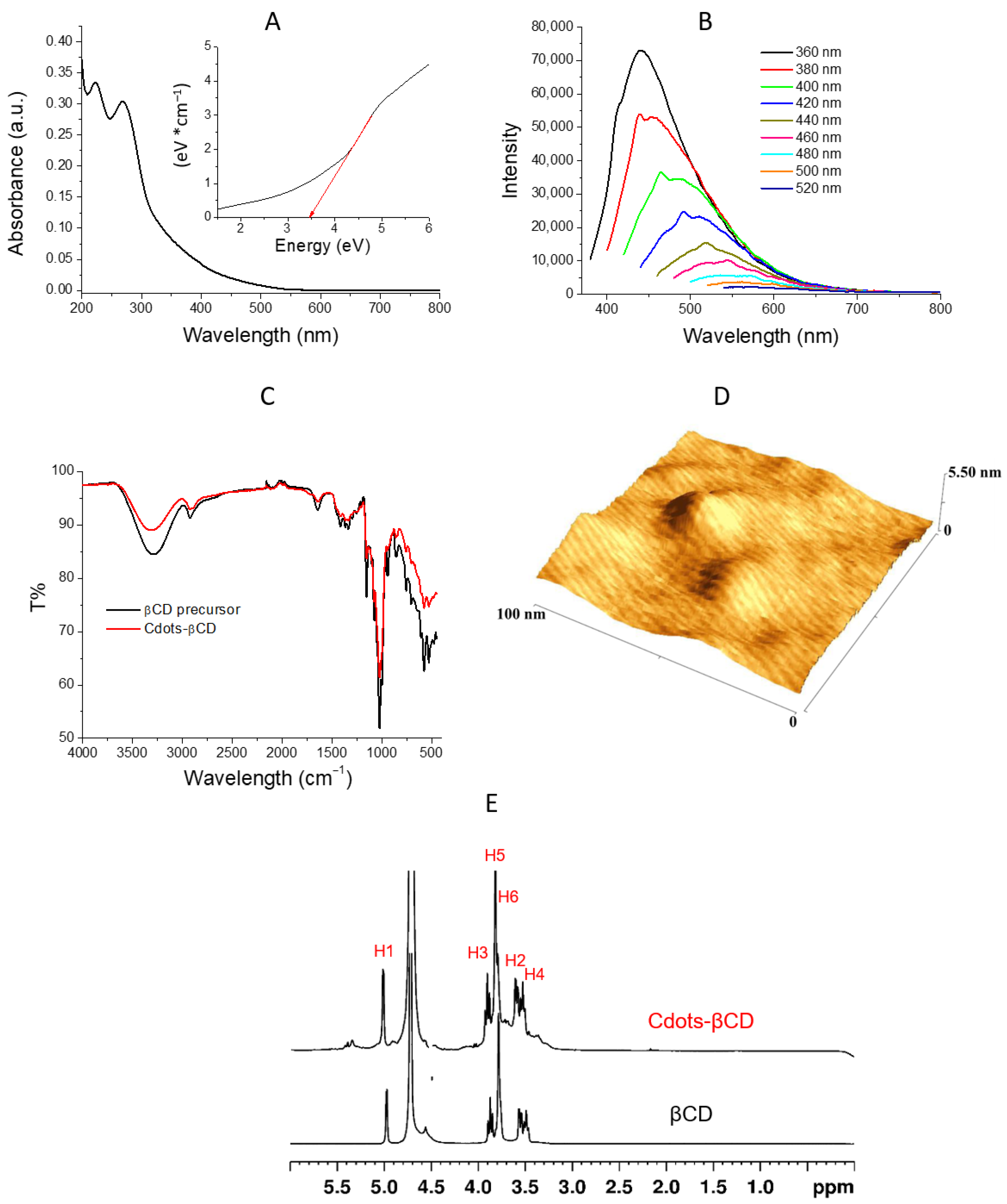
3.1. Cdots-βCD Preparation and Characterization
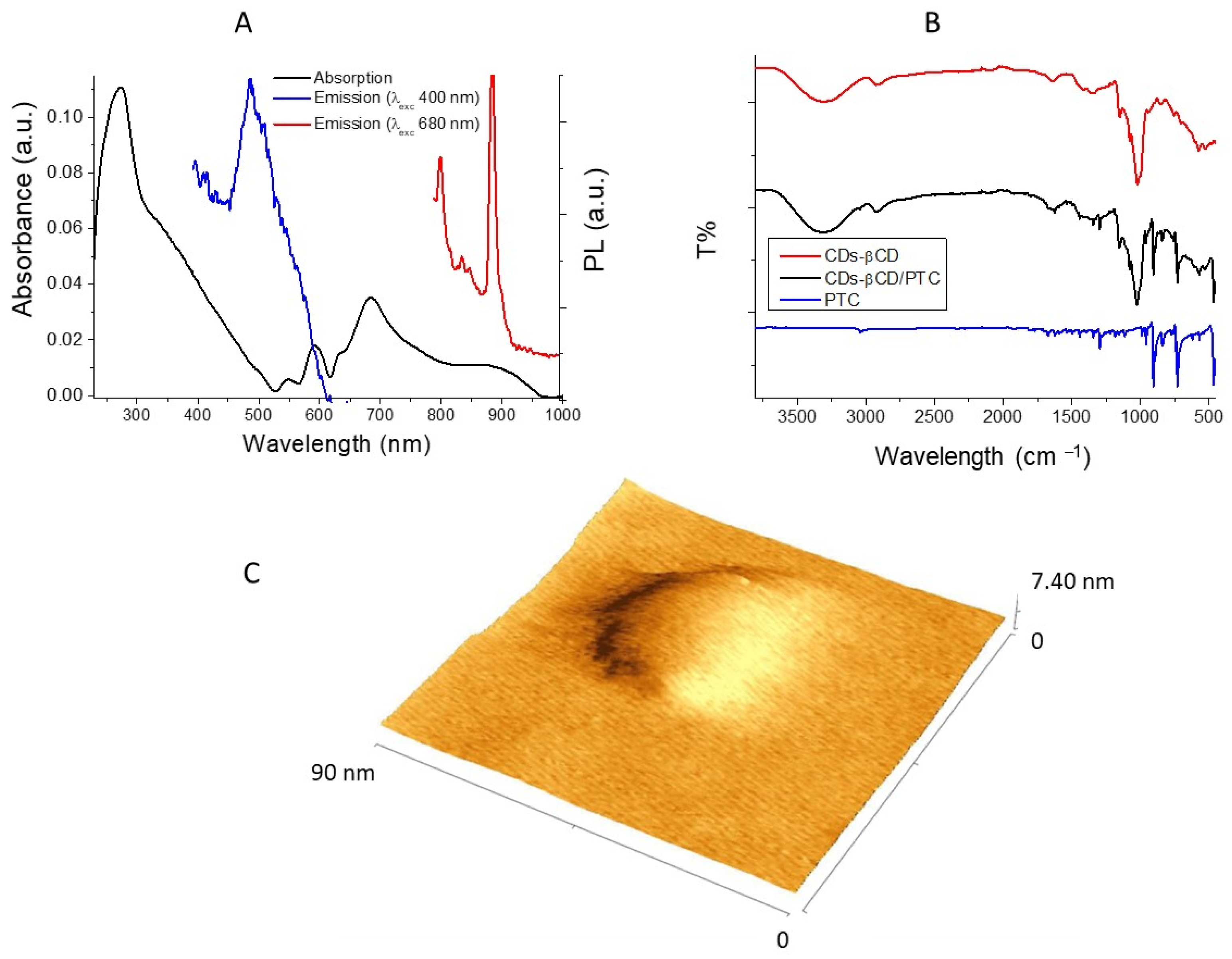
3.2. Cdots-βCD/PTC Preparation and Characterization
3.3. Cytotoxicity Tests Cdots-βCD and Cdots-βCD/PTC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| βCD | beta-cyclodextrin |
| Cdots | Carbon nanodots |
| PTC | Pentacene |
| CTRL | Control |
References
- Qin, W.; Wang, M.; Li, Y.; Li, L.; Abbas, K.; Li, Z.; Tedesco, A.C.; Bi, H. Recent advances in red-emissive carbon dots and their biomedical applications. Mater. Chem. Front. 2024, 8, 930–955. [Google Scholar] [CrossRef]
- Behi, M.; Gholami, L.; Naficy, S.; Palomba, S.; Dehghani, F. Carbon dots: A novel platform for biomedical applications. Nanoscale Adv. 2022, 4, 353–376. [Google Scholar] [CrossRef] [PubMed]
- Consoli, G.M.L.; Maugeri, L.; Forte, G.; Buongiorno, D.; Nicitra, E.; Aleo, D.; Spitaleri, F.; Ferreri, L.; Buscarino, G.; Campagna, T.; et al. Bioinspired β-Cyclodextrin-Derived Carbon Dots with Emissive and Photothermal Properties as Nanocarriers for Bioactive Agents. ACS Appl. Nano Mater. 2025, 8, 1078–1090. [Google Scholar] [CrossRef]
- Liu, C.; Fan, W.; Cheng, W.-X.; Gu, Y.; Chen, Y.; Zhou, W.; Yu, X.-F.; Chen, M.; Zhu, M.K.; Luo, Q.-Y. Red Emissive Carbon Dot Superoxide Dismutase Nanozyme for Bioimaging and Ameliorating Acute Lung Injury. Adv. Funct. Mater. 2023, 33, 2213856. [Google Scholar] [CrossRef]
- Consoli, G.M.L.; Giuffrida, M.L.; Zimbone, S.; Ferreri, L.; Maugeri, L.; Palmieri, M.; Satriano, C.; Forte, G.; Petralia, S. Green Light-Triggerable Chemo-Photothermal Activity of Cytarabine-Loaded Polymer Carbon Dots: Mechanism and Preliminary In Vitro Evaluation. ACS Appl. Mater. Interfaces 2023, 15, 5732–5743. [Google Scholar] [CrossRef] [PubMed]
- Forte, G.; Consoli, G.M.L.; Ferreri, L.; Maugeri, L.; Foti, A.; Satriano, C.; Fangano, G.; Petralia, S. Luminescent Pentacene-Loaded Carbon Nanodots with Red-Light Triggered Photothermal and Photosensitizing Properties. ChemPhotoChem 2025, 9, e202400167. [Google Scholar] [CrossRef]
- Consoli, G.M.L.; Maugeri, L.; Forte, G.; Buscarino, G.; Gulino, A.; Lanzano, L.; Bonacci, P.; Musso, N.; Petralia, S. Red light-triggerable nanohybrids of graphene oxide, gold nanoparticles and thermo-responsive polymers for combined photothermia and drug release effects. J. Mat. Chem. B 2024, 12, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Yoo, H.; Lee, E.K. New Opportunities for Organic Semiconducting Polymers in Biomedical Applications. Polymers 2022, 14, 2960. [Google Scholar] [CrossRef] [PubMed]
- Morin-Crini, N.; Fourmentin, S.; Fenyvesi, É.; Lichtfouse, E.; Torri, G.; Fourmentin, M.; Crini, G. 130 years of cyclodextrin discovery for health, food, agriculture, and the industry: A review. Environ. Chem. Lett. 2021, 19, 2581–2617. [Google Scholar] [CrossRef]
- Consoli, G.M.L.; Giuffrida, M.L.; Satriano, C.; Musumeci, T.; Forte, G.; Petralia, S. A novel facile one-pot synthesis of photothermally responsive carbon polymer dots as promising drug nanocarriers. Chem. Commun. 2022, 58, 3126–3129. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; Di Nola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Dauber-Osguthorpe, P.; Roberts, V.A.; Osguthorpe, D.J.; Wolff, J.; Genest, M.; Hagler, A.T. Structure and energetics of ligand binding to proteins: Escherichia coli dihydrofolate reductase-trimethoprim, a drug-receptor system. Proteins 1988, 4, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Accurate description of van der Waals complexes by density functional theory including empirical corrections. J. Comput. Chem. 2004, 25, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Jumardin, M.; Santoso, M.; Isnaeni, K. Synthesis of Carbon Dots (CDs) and Determination of Optical Gap Energy with Tauc Plot Method. Jambura Phys. J. 2021, 3, 73–86. [Google Scholar] [CrossRef]
- Li, M.Y.; Xiao, C.Q.; Xu, Z.-Q.; Yin, M.M.; Yiang, Q.Q.; Yin, Y.L.; Liu, Y. Role of surface charge on the interaction between carbon nanodots and human serum albumin. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 204, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Rachmawati, H.; Edityaningrum, C.A.; Mauludin, R. Molecular Inclusion Complex of Curcumin–β-Cyclodextrin Nanoparticle to Enhance Curcumin Skin Permeability from Hydrophilic Matrix Gel. AAPS PharmSciTech 2013, 14, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Gehlen, M.H. The centenary of the Stern-Volmer equation of fluorescence quenching: From the single line plot to the SV quenching map. J. Photochem. Photobiol. C 2020, 42, 100338. [Google Scholar] [CrossRef]
- Dramstad, T.A.; Wu, Z.; Gretz, G.M.; Massari, A.M. Thin Films and Bulk Phases Conucleate at the Interfaces of Pentacene Thin Films. J. Phys. Chem. C 2021, 125, 16803–16809. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maugeri, L.; Fangano, G.; Butera, E.; Forte, G.; Bonacci, P.G.; Musso, N.; Ruffino, F.; Ferreri, L.; Consoli, G.M.L.; Petralia, S. Emissive Pentacene-Loaded βcyclodextrin-Derived C-Nanodots Exhibit Red-Light Triggered Photothermal Effect. Pharmaceutics 2025, 17, 543. https://doi.org/10.3390/pharmaceutics17050543
Maugeri L, Fangano G, Butera E, Forte G, Bonacci PG, Musso N, Ruffino F, Ferreri L, Consoli GML, Petralia S. Emissive Pentacene-Loaded βcyclodextrin-Derived C-Nanodots Exhibit Red-Light Triggered Photothermal Effect. Pharmaceutics. 2025; 17(5):543. https://doi.org/10.3390/pharmaceutics17050543
Chicago/Turabian StyleMaugeri, Ludovica, Giorgia Fangano, Ester Butera, Giuseppe Forte, Paolo Giuseppe Bonacci, Nicolò Musso, Francesco Ruffino, Loredana Ferreri, Grazia Maria Letizia Consoli, and Salvatore Petralia. 2025. "Emissive Pentacene-Loaded βcyclodextrin-Derived C-Nanodots Exhibit Red-Light Triggered Photothermal Effect" Pharmaceutics 17, no. 5: 543. https://doi.org/10.3390/pharmaceutics17050543
APA StyleMaugeri, L., Fangano, G., Butera, E., Forte, G., Bonacci, P. G., Musso, N., Ruffino, F., Ferreri, L., Consoli, G. M. L., & Petralia, S. (2025). Emissive Pentacene-Loaded βcyclodextrin-Derived C-Nanodots Exhibit Red-Light Triggered Photothermal Effect. Pharmaceutics, 17(5), 543. https://doi.org/10.3390/pharmaceutics17050543







